Abstract
BACKGROUND AND PURPOSE
Vasoactive intestinal peptide is expressed in the respiratory tract and induces its effects via its receptors, VPAC1 and VPAC2. RO5024118 is a selective VPAC2 receptor agonist derived via chemical modification of an earlier VPAC2 agonist, RO0251553. In the present studies, we characterized the pharmacological activity of RO5024118.
EXPERIMENTAL APPROACH
Stability of RO5024118 to human neutrophil elastase was assessed. Bronchodilatory activity of RO5024118 was investigated in guinea pig and human isolated airway smooth muscle preparations and in a guinea pig bronchoconstriction model. Pulmonary anti-inflammatory activity of RO5024118 was investigated in a lipopolysaccharide mouse model and in a porcine pancreatic elastase (PPE) rat model.
KEY RESULTS
RO5024118 demonstrated increased stability to neutrophil elastase compared with RO0251553. In human and guinea pig isolated airway preparations, RO5024118 induced bronchodilatory effects comparable with RO0251553 and the long-acting β-agonist salmeterol and was significantly more potent than native vasoactive intestinal peptide and the short-acting β-agonist salbutamol. In 5-HT-induced bronchoconstriction in guinea pigs, RO5024118 exhibited inhibitory activity with similar efficacy as, and longer duration than, RO0251553. In a lipopolysaccharide-mouse model, RO5024118 inhibited neutrophil and CD8+ cells and myeloperoxidase levels. In rats, intratracheal instillation of PPE induced airway neutrophilia that was resistant to dexamethasone. Pretreatment with RO5024118 significantly inhibited PPE-induced neutrophil accumulation.
CONCLUSIONS AND IMPLICATIONS
These results demonstrate that RO5024118 induces dual bronchodilatory and pulmonary anti-inflammatory activity and may be beneficial in treating airway obstructive and inflammatory diseases.
Keywords: chronic obstructive pulmonary disease, airway inflammation, VPAC2 receptors, VIP, bronchodilator
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow limitation that is not fully reversible and airway inflammation that is comprised of CD8+ T cells, neutrophils and macrophages. Current COPD therapy is limited to bronchodilators and inhaled corticosteroids as the mainstay of therapy; however, unlike in asthma, inhaled corticosteroids are often ineffective in alleviating lung inflammation and do not prevent the progressive development of airway obstruction (Belvisi et al., 2004; Birrell et al., 2005; Pahl and Szelenyi, 2007). Hence, there is a need to identify more effective treatments for this disease.
The human airway is innervated with neurons that express and release both pro- and anti-inflammatory neuromediators. Vasoactive intestinal peptide (VIP) is one of the most abundant neuropeptides found in the airway (Groneberg et al., 2001), which, upon release, relaxes airway smooth muscle and exerts anti-inflammatory effects in the lungs via its two receptors, VPAC1 and VPAC2 (Palmer et al., 1986; Delgado et al., 2000; Gomariz et al., 2001; Voice et al., 2002; Groneberg et al., 2006; receptor nomenclature follows Alexander et al., 2009). Studies utilizing VPAC2 gene deletion and over-expression in mice have supported a role of this receptor in modulating local immune mechanisms in the airway (Voice et al., 2002; 2003;). Further, RO0251553, a synthetic peptide analogue of VIP that is highly selective for the VPAC2 receptor, was shown to suppress airway oedema, hyperreactivity and inflammatory cell influx in a guinea pig model of allergic lung inflammation (O'Donnell et al., 1994a,b; Dewit et al., 1998). RO0251553 was also evaluated clinically as a bronchodilator and was shown to have a rapid onset of action with improvement of forced expiratory volume in one second comparable with formoterol. However, the short duration of action of RO0251553 in comparison with formoterol, most likely due to degradation by peptidases such as neutrophil elastase that are prevalent in the inflamed airway, limited the potential clinical use of this molecule (Linden et al., 2003).
RO5024118 (Figure 1) is a successor to RO0251553, and we show here it to be also highly selective for the VPAC2 receptor. In the present preclinical pharmacological studies, we demonstrate that RO5024118 has greater stability than RO0251553 and exhibits dual bronchodilatory and anti-inflammatory activity in several in vitro and in vivo assays. RO5024118 represents a potential novel treatment for airway obstructive and inflammatory diseases.
Figure 1.
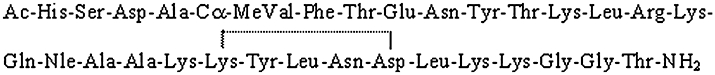
The amino acid sequence for RO5024118. This structure differs from that of RO 0251553 by the CαMeVal residue at position 5. The line from Lys21 to Asp25 represents a lactam link between the side chains.
Methods
Animals
All animal care and experimental procedures were approved by the Roche Animal Care and Use Committee and conducted in accordance with the Institute of Laboratory Animal Resources guide for the Care and Use of Laboratory Animals (1996). Male C57B/6 mice (25–30 g) were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). Male CD® rats (250–300 g) and male Hartley guinea pigs (300–400g) were purchased from Charles River (Raleigh, NC, USA). Animals were housed under controlled temperature (22 ± 2°C), humidity (50% ± 19%) and lighting (6:30 am–6:30 pm) in solid bottom cages. Standard rodent diet and reverse osmosis filtered water were provided ad libitum during acclimation periods and experiments.
In vitro assays
Measurement of cAMP (VPAC selectivity)
A human T-lymphoid cell line, Sup-T1, which endogenously expresses VPAC2 receptors (Menghang et al., 1996), was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in growth medium [RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 25 mM HEPES buffer and 10% fetal bovine serum (Gemini Bioproducts)] at densities between 0.2 and 2 × 106 cells·mL−1 in a 37°C CO2 incubator. Cells were placed into 96-well plates at a density of 4 × 104 cells per well in 150 µL growth medium, and test compounds [RO5024118, RO0251553, native VIP and a VPAC1 selective agonist, RO4983407 ([Ala11,22,28]VIP analogue; Nicole et al., 2000)] were added to designated wells. cAMP levels were determined as described in the cAMP enzyme immunoassay kit (Amersham Biosciences, Piscataway, NJ, USA). The VPAC2 agonist activity of each compound was calculated by fitting the 7-concentration dose–response data to a sigmoidal dose–response equation using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
A human colon carcinoma cell line, HT-29, which endogenously expresses VPAC1 (Summers et al., 2003), was obtained from ATCC and maintained in growth medium as described above. Cells were trypsinized from monolayer culture and placed in 96-well plates at a density of 4 × 104 cells per well in 100 µL of growth medium. After 48 h, the medium was removed and replaced with 150 µL of fresh medium containing two phosphodiesterase inhibitors [375 µM of isobutyl methyl xanthine (IBMX Tocris Bioscience, Ellisville, Missouri, USA) and 15 µM of RO201724 (Roche, Nutley, NJ, USA)], to prevent the degradation of cAMP. After 2 min at room temperature, test compounds were added to designated wells. The methods for cAMP detection and calculations were the same as those described for VPAC2.
Stability to human neutrophil elastase
Test compounds (RO5024118, RO0251553, and native VIP) were exposed in vitro to human neutrophil elastase (EMD4 Biosciences, Gibbstown, NJ, USA), and time-dependent degradation was measured with reversed phase HPLC electrospray ionization (ESI) mass spectrometry (MS). The relative abundance of a peptide was calculated from the measured integrated ion currents (LC ESI MS) of the respective triple charged ions. The half-life of each peptide was calculated, assuming that the degradation rate follows first order kinetics, using the equation t(½) = 0.693/k derived from the equation –kt = ln(A0/At), where k = rate constant, A0 = initial peptide abundance, At = peptide abundance at time of measurement and t = time of measurement. Values were normalized against the half-life of native VIP.
Guinea pig isolated trachea
Male Hartley guinea pigs were killed (overdose of sodium pentobarbitone), tracheas removed, cleaned of excess connective tissue and divided into four rings. Each ring, approximately 2–3 mm in length, was transferred to an organ bath containing 10 mL of a modified Krebs solution (in mM: 11.1 d-glucose, 1.08 MgSO4, 0.75 KH2PO4, 4.7 KCl, 118.07 NaCl, 2.54 CaCl2, 25 NaHCO3, pH 7.4, 37°C) and continuously gassed with O2/CO2 95:5% v/v. Tissues were suspended by a wire hook attached to Grass force transducers and allowed to equilibrate under a baseline tension of ∼1–1.5 g with readjustment of tension and washing every 15 min for a 90 min period. In preliminary studies, a concentration curve to carbachol was constructed (0.1 nM to 1 µM) to establish a sub-maximal response (approximately 70% maximum response; 2.36 ± 0.38 g; n= 7) that was used in subsequent experiments. To test the relaxation induced by test compounds (RO5024118, RO0251553, native VIP and salbutamol), tissues were first contracted with the sub-maximal dose of carbachol (3 nM) and allowed to stabilize for 15–20 min, after which cumulative concentration–response curves were constructed to the test compound. Intervals of 10 min between successive concentrations were used. At the completion of the experiment, 10 µM of papaverine was added to relax the tissues completely and provide a standard to which the relaxation of each tissue could be compared. Appropriate curve-fitting to a sigmoidal model was used to calculate EC50 values (GraphPad Prism software).
Human isolated bronchi
Macroscopically normal sections of three human lungs, taken from an area as far as possible from the malignancy, were obtained with full informed consent from patients undergoing surgery for lung cancer but without a history of chronic airway disease. Sections were immediately placed into oxygenated (O2/CO2 95:5% v/v) modified Krebs-Henseleit (Krebs) buffer solution (in mM: 117.5 NaCl, 5.60 KCl, 1.18 MgSO4, 2.50 CaCl2, 1.28 NaH2P04, 25.00 NaHCO3 and 5.5 d-glucose) containing the cyclooxygenase inhibitor indomethacin (10 µM), and transported to the laboratory. None of the patients had been chronically treated with theophylline, β2-adrenoceptor agonists, corticosteroids or anticholinergic drugs. Preoperative lung function parameters were generally normal. Bronchi with intact epithelium, typically 3–5 mm in diameter, were dissected free from connective tissue and cut into rings. Bronchial rings were transferred to 10 mL organ baths containing Krebs buffer (pH 7.4, 37°C) containing indomethacin (10 µM) and continuously gassed with O2/CO2 95:5% v/v. Rings were suspended by a wire hook attached to an isometric force displacement transducer (Fort 10 WPI, Basile Instruments, Italy) and allowed to equilibrate under a baseline tension of ∼1–1.5 g with readjustment of tension and washing every 15 min for a 90 min period. The tissue responsiveness was assessed using acetylcholine (ACh) 100 µM. When the ACh response reached a plateau, rings were washed three times and allowed to equilibrate for 30 min. In preliminary experiments, after washout, a concentration curve to histamine (1 nM to 1 mM) was constructed to establish a sub-maximal response (approximately 70% maximum response; 0.7 ± 0.06 g; n= 6) for subsequent studies. To test the relaxation induced by the test compound (RO5024118, RO0251553, native VIP, salbutamol and salmeterol), the bronchial rings were contracted with histamine at the sub-maximal concentration (10 µM) and allowed a 15 min stabilization period, after which cumulative concentration–response curves were constructed to the test compound. Intervals of 20 min between successive concentrations were used. At the completion of the experiment, 10 µM of papaverine was added to relax the tissues completely and provide a standard to which the relaxation of each tissue could be compared. Appropriate curve-fitting to a sigmoidal model was used to calculate EC50 values (GraphPad Prism software).
In Vivo Assays
5-HT-induced bronchoconstriction in conscious guinea pigs
To assess the effects of test compounds on 5-HT-induced bronchoconstriction, guinea pigs were exposed to aerosol RO5024118 (0.001%–0.1%, w/v), RO0251553 (0.1%; Tocris Bioscience, MO, USA) or vehicle (0.9% saline) for 20 min generated by a Pari IS 2 nebulizer. Immediately or 2 h following exposure to test compounds, 5-HT-induced bronchoconstriction was assessed in unrestrained guinea pigs by barometric plethysmography using whole body plethysmographs (WBP) from Buxco Electronics, Inc. (Wilmington, NC, USA). Enhanced pause (Penh) was used as an index of bronchoconstriction, as described previously (Hamelmann et al., 1997). Response to 5-HT (175 µg·mL−1) was obtained by administering nebulized aerosol for 1 min followed by a 7 min lung function measurement to determine Penh. This concentration of 5-HT was chosen because it was previously shown to produce a sub-maximal bronchoconstriction response in the guinea pig (Battram et al., 2006). Baseline Penh was obtained prior to 5-HT challenge by administering aerosol 0.9% saline followed by a 7 min lung function measurement.
5-HT-induced bronchoconstriction in anaesthetized guinea pigs
Airway reactivity to 5-HT was also measured using the Buxco FinePointe resistance–compliance invasive WBP system. Guinea pigs were exposed to aerosol RO5024118 (0.0003%–0.03%, w/v) or vehicle (0.9% saline) for 20 min generated by a Pari IS 2 nebulizer. Immediately following exposure to test compounds, guinea pigs were anaesthetized with sodium pentobarbital (80–100 mg·kg−1, i.p.; Henry Schein, Melville, NY, USA) and through a mid-line neck incision, the trachea was cannulated with a 15-gauge tubing adapter. Guinea pigs were then placed in the WBP and mechanically ventilated using a tidal volume of 1 mL·100 g−1 body weight and a frequency of 150 breaths/min, with a positive end-expiratory pressure of 2 cm H2O. The depth of anaesthesia was checked prior to mechanical ventilation by examining the reflex response to pressure on the hind paw applied by a haemostat. Supplemental anaesthesia was administered and the depth assessment repeated when needed. Guinea pigs were then allowed to stabilize for 5 min. Once stabilized, they were challenged with aerosol 0.9% saline followed by increasing concentrations of 5-HT (3, 10, 30 µg·mL−1; Sigma-Aldrich, St. Louis, MO, USA) obtained via 20 s exposure with a nebulizer connected in-line with the respirator pump to deliver a small amount of aerosol directly into the airway. Measurements of airway reactivity were recorded for 1.5 min at 1 s intervals beginning at the start of nebulization. Flow signal was collected using the plethysmograph and pressure signal was collected from a sidearm of the tracheal catheter. The flow and pressure signals were processed together to determine lung resistance (RL) and dynamic compliance (Cdyn) using a software analyser provided in the FinePointe resistance–compliance software. Changes in RL and Cdyn following 5-HT challenge were calculated as a percentage change from saline control (baseline).
Lipopolysaccharide (LPS)-induced lung injury in mice
To assess the ability of RO5024118 to prevent LPS-induced lung inflammation, mice were exposed to aerosol vehicle (0.9% saline) or RO5024118 (0.00003–0.1%, w/v) for 20 min immediately prior to an aerosol exposure to LPS (500 µg·mL−1 in sterile saline; Sigma, St. Louis, MO, USA) for 20 min. Bronchoalveolar lavage (BAL) was performed 24 h following LPS challenge in anaesthetized mice [ketamine/xylazine (80–120 mg·kg−1/2–4 mg·kg−1, i.p.; Henry Schein, Melville, NY, USA)]. Lungs were lavaged with 2 × 1 mL sterile Hank's balanced salt solution (HBSS; Gibco, Grand Island, NY, USA). All mice were killed by cervical dislocation following the lavage procedure. Samples were then centrifuged and supernatant collected. Red blood cells were lysed from the resulting pellet, and the cells remaining were reconstituted with 5 mL HBSS. Samples were recentrifuged, and the resulting pellet resuspended in 0.5 mL of HBSS. Total cell number was determined from an aliquot of the cell suspension using a haemocytometer or Coulter counter (Beckman-Coulter, Miami, Fl.). For cytological preparations, the cells were fixed on cytocentrifuge slides (Thermo Electron Corp., Pittsburg, PA, USA) and stained with a modified Wright's stain (LeukoStat stain; Fisher Scientific, Inc. Kalamazoo, MI, USA). Differential counts on at least 300 cells were made using standard morphological criteria. Myeloperoxidase (MPO) levels were measured in mouse BAL supernatant using a commercially available ELISA kit (Hycult Biotechnology, Cell Science, Canton, MA, USA) to assess the effects of RO5024118 on neutrophil activation following LPS challenge. Flow cytometry was used to assess the effects of RO5024118 on lymphocyte subsets in the BAL fluid following LPS challenge. Briefly, anti-mouse CD4, CD8, CD11b, Gr-1 and CD45 were added to BAL cells and then analysed using FACSAria flow cytometer (BD Bioscience, CA, USA) and FlowJo software. Absolute cell numbers for lymphocyte subsets were the product of the frequency and total cells counted.
Porcine pancreatic elastase (PPE)-induced lung injury in rats
Rats were exposed to aerosol vehicle (0.9% saline), RO5024118 (0.001% and 0.0003%, w/v), or dexamethasone 21 sodium phosphate (0.1% w/v; Sigma, St. Louis, MO, USA) for 20 min generated by a Pari IS 2 nebulizer. Immediately after drug exposure, rats were anaesthetized with ketamine/xyzaline (40–60 mg·kg−1; 1.5–2.5 mg·kg−1) i.p. With the aid of a laryngoscope, a MicroSprayer (Penn-Century, Inc., PA, USA) was inserted into the trachea. PPE (Sigma, St. Louis, MO, USA) was then administered intratracheally at a dose of 60 U·kg−1 in a dose volume of 1 mL·kg−1 with a high-pressure syringe attached to the MicroSprayer. Rats were placed on heating pads and continuously monitored until recovered from the anaesthesia. BAL was performed 24 h following instillation of PPE. The BAL procedure and cell differential technique were similar to those described above for the mouse except that lungs were lavaged with 3 × 3 mL sterile HBSS.
Data analysis
Statistical differences of groups of in vitro and in vivo data were determined by a one- or two-way analysis of variance followed by Bonferroni post-test or by Student's t-test where appropriate. P < 0.05 was considered statistically significant.
Materials
Vasoactive intestinal peptide was supplied by Tocris Bioscience (Ellisville, MO, USA) and all RO compounds were from Roche, Nutley, NJ, USA RO5024118 was synthesized according to Bolin et al. (2008). Other compounds (salbutamol, salmeterol, carbachol, papaverine, indomethacin, histamine) were from Sigma Chemical (St. Louis, MO, USA).
Results
The structure of RO5024118 is shown in Figure 1. This compound contains a Cα-methylated valine residue in the amino acid position 5 instead of the valine present in the predecessor compound RO0251553.
In Vitro assays
VPAC receptor selectivity
RO5024118, RO0251553 and VIP induced a dose-dependent increase in cellular cAMP levels in Sup-T1 cells, expressing the VPAC2 receptor, with EC50 values of 3.1, 6.5 and 7.9 nM, respectively (Figure 2A). RO4983407, a selective VPAC1 agonist, had minimal effects on cAMP production in these cells up to 10 µM (Figure 2A). RO5024118 and RO0251553 had minimal effects on cAMP levels in HT-29 cells, expressing the VPAC1 receptor, when tested up to 10 µM (Figure 2B). Both VIP and RO4983407 induced a dose-dependent increase in cAMP levels in HT-29 cells with EC50 values of 0.47 nM and 0.48 nM, respectively (Figure 2B).
Figure 2.
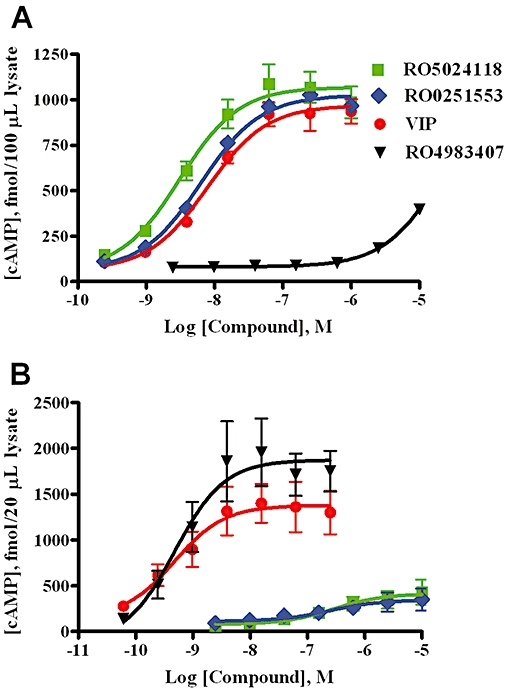
Effects of RO5024118, RO0251553, native vasoactive intestinal peptide (VIP) and RO4983407 on cAMP levels in Sup-T1 cells, expressing the VPAC2 receptor (A) and HT-29 cells, expressing the VPAC1 receptor (B). In the absence of ligands, the basal cytoplasmic cAMP levels were 67.5 ± 7.5 fmol per 100 µL of Sup-T1 lysate and 62 ± 26 fmol per 20 µL of HT-29 lysate. Data are mean ± SEM of 3–4 experiments.
Specificity for VPAC2 receptors was confirmed by evaluating RO5024118 in a broad screening panel of 140 cell-surface receptors, ion channels and enzymes (MDS Pharma Service, PA, USA) in which no or weak activity was observed (IC50 > 10 µM).
Stability to human neutrophil elastase
Figure 3 demonstrates the enhanced relative stability of RO5024118 towards neutrophil elastase degradation over time, compared with native VIP and RO0251553. The relative half-life was found to be increased approximately eightfold when compared with RO0251553 and approximately 31-fold when compared with native VIP.
Figure 3.
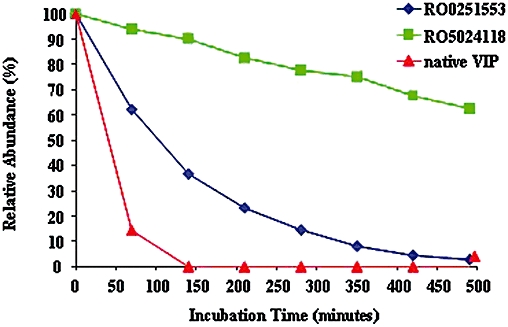
Comparison of the stability of RO5024118 to human neutrophil elastase with vasoactive intestinal peptide (VIP) and RO0251553. Stability of the substrates was evaluated by HPLC followed by mass spectrometry. Survival of each peptide is shown as % relative abundance of the unchanged substrate.
Guinea pig isolated trachea
In carbachol pre-contracted guinea pig tracheal rings, RO5024118 and RO0251553 evoked a concentration-dependent relaxation with EC50 values of 0.75 ± 0.42 nM and 0.89 ± 0.39, respectively (Figure 4). The effects of RO5024118 and RO0251553 were not significantly different from each other. RO5024118 was significantly more potent and efficacious than salbutamol (EC50 value of 1.75 ± 1.25 nM) and VIP (EC50 value of 230 ± 226 nM; Figure 4).
Figure 4.
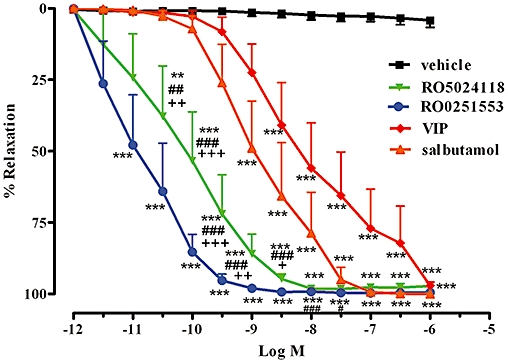
Effects of RO5024118, RO0251553, salbutamol and native vasoactive intestinal peptide (VIP) on guinea pig tracheal rings, pre-contracted with carbachol. Data are mean ± SEM of 6–7 tissues per group. **P < 0.01, ***P < 0.001 for test compounds versus vehicle; ##P < 0.01, ###P < 0.001 for RO5024118 versus VIP.; ++P < 0.01 RO5024118 versus salbutamol.
Human isolated bronchial smooth muscle
In human isolated bronchial rings, pre-contracted with histamine, RO5024118 evoked a concentration-dependent relaxation with an EC50 value of 35.7 ± 17 nM (Figure 5A). RO5024118 was significantly more potent than RO0251553 (EC50 value of 144 ± 50 nM). The efficacies of RO5024118 and RO0251553 were not significantly different from each other (Figure 5A). RO5024118 was significantly more potent than native VIP (EC50 value of 322 ± 128 nM; Figure 5A). Further, RO5024118 was significantly more potent than the short-acting β-agonist salbutamol (EC50 value of 153 ± 74 nM; Figure 5B) and exhibited comparable potency to the long-acting β-agonist salmeterol (EC50 value of 39.2 ± 32 nM; Figure 5B).
Figure 5.
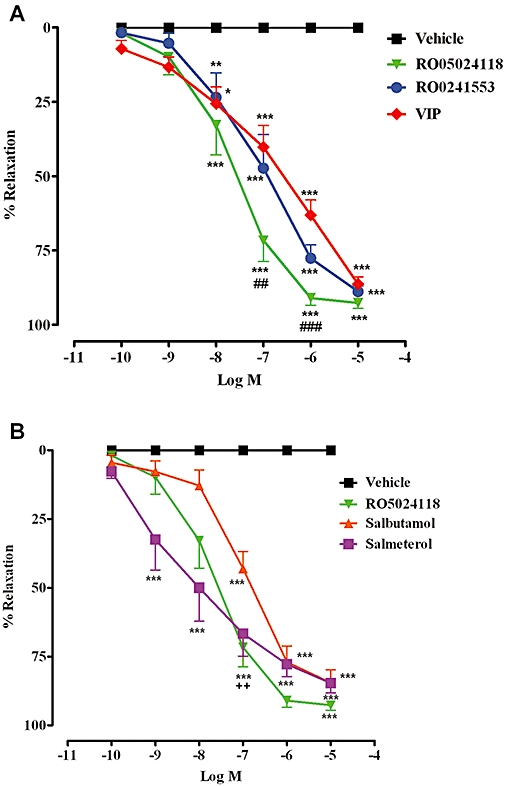
Effects of cumulative concentrations of RO5024118, RO0251553 and native vasoactive intestinal peptide (A) and salbutamol and salmeterol (B) on human bronchial rings, pre-contracted with histamine. Data are mean ± SEM of 6 rings per group. **P < 0.01, ***P < 0.001 for test compounds versus vehicle; ##P < 0.01, ###P < 0.001 RO5024118 versus VIP; ++P < 0.01 RO5024118 versus salbutamol.
In Vivo assays
5-HT-induced bronchconstriction in conscious guinea pigs
To investigate the bronchodilatory activity of RO5024118 in vivo, RO5024118 was evaluated in an established conscious guinea pig bronchoconstriction model (Battram et al., 2006). Pretreatment with a 20 min aerosol of RO5024118 (0.001–0.03% w/v) immediately prior to 5-HT challenge significantly inhibited the bronchoconstriction response in a concentration-dependent manner (Figure 6A). Separate experiments were performed using this bronchoconstriction model to compare the efficacy and duration of action of RO5024118 with RO0251553 at a single high concentration of 0.1% w/v. An aerosol of RO0251553 immediately prior to 5-HT challenge inhibited the bronchoconstriction with comparable effectiveness to RO5024118 (Figure 6B). When administered 2 h prior to 5-HT challenge, RO5024118 retained significant inhibitory activity, whereas RO0251553 was without effect (Figure 6C).
Figure 6.
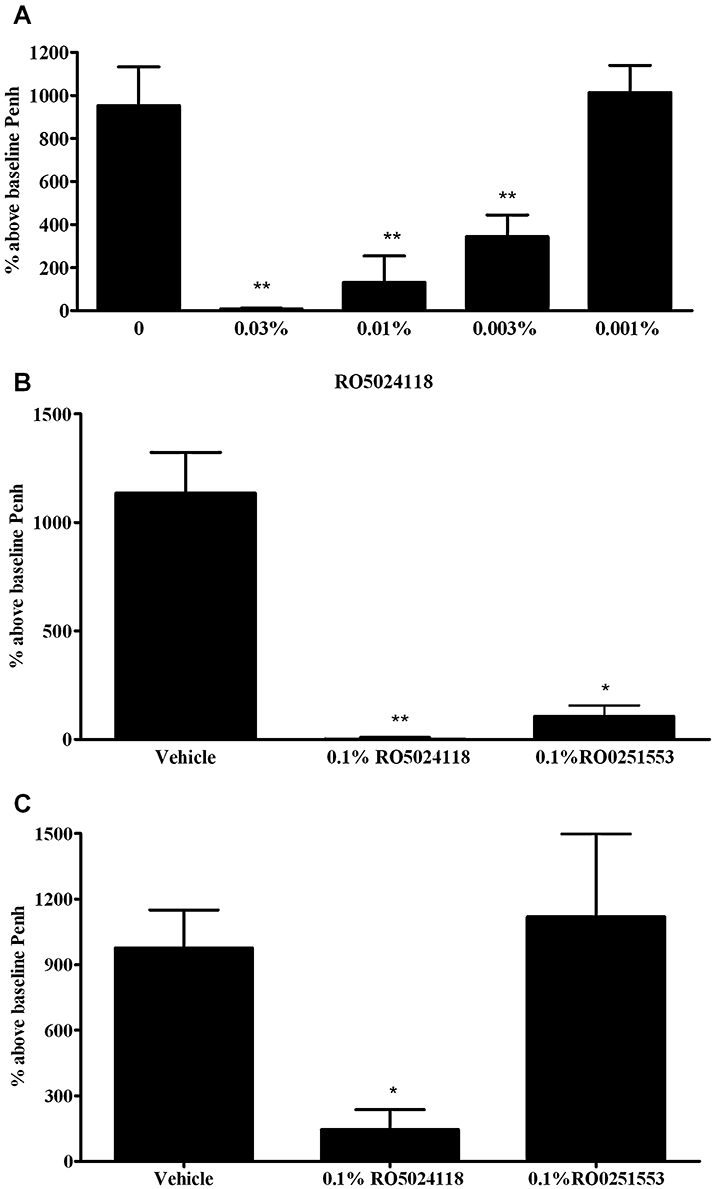
Effects of immediate pretreatment with RO5024118 (0.001–0.03% w/v, A) on 5-HT-induced bronchoconstriction in conscious guinea pigs and in comparison with RO0251553 at 0.1% immediately (B) and 2 h prior to (C) 5-HT. Data are mean ± SEM of 4–12 animals per group. *P < 0.05, **P < 0.01 versus vehicle.
5-HT-induced bronchconstriction in anaesthetized guinea pigs
RO5024118 was also evaluated for its ability to inhibit changes in airway function to increasing concentrations of 5-HT in anaesthetized, mechanically ventilated guinea pigs to confirm the findings in conscious animals. Pretreatment with a 20 min aerosol of RO5024118 (0.0003–0.03% w/v) immediately prior to 5-HT challenge significantly inhibited increases in lung resistance (Figure 7A) and decreases in lung compliance (Figure 7B) in a concentration-dependent manner.
Figure 7.
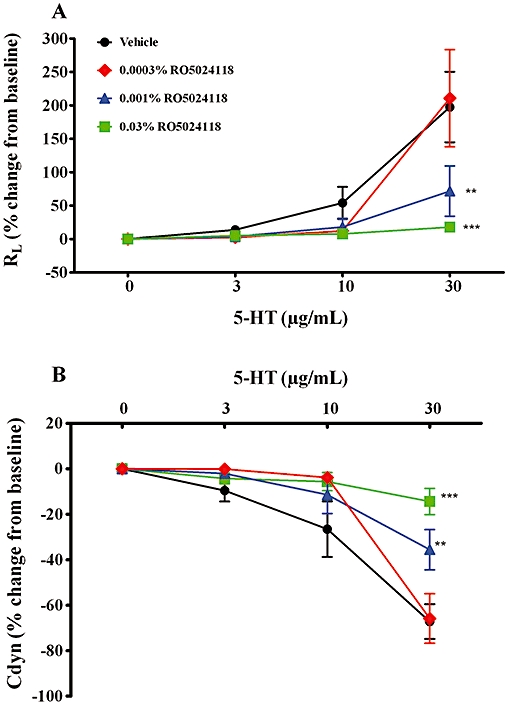
Effects of immediate pretreatment of RO5024118 (0.003–0.03% w/v, A) on 5-HT-induced changes in lung resistance (A) and dynamic compliance (B) in anaesthetized, mechanically ventilated guinea pigs. Data are mean ± SEM of 5–7 animals per group. **P < 0.01, ***P < 0.001 versus vehicle.
LPS-induced lung inflammation in mice
To demonstrate anti-inflammatory activity in vivo, RO5024118 was evaluated in the LPS-mouse model of airway inflammation. Exposure of mice to LPS aerosol induced an influx of inflammatory cells into the airway, composed predominantly of neutrophils and lymphocytes (Figure 8B and D). The numbers of macrophages were not significantly different following LPS exposure (Figure 8C). Pretreatment with a 20 min aerosol exposure of RO5024118 (0.00003–0.1% w/v) inhibited LPS-evoked influx of total cells and neutrophils into the BAL fluid in a concentration-dependent manner (Figure 8A and B). Percentage inhibitions at each concentration are shown in Table 1. The effects of the two high (0.1% and 0.03%) and the two low (0.0001% and 0.00003%) concentrations were not statistically significant from vehicle, suggesting a bell-shaped curve response. Significant reductions in lymphocytes were observed following RO5024118 treatment but were not concentration-dependent (Figure 8D). The inhibition of lymphocytes in BAL fluid by 0.01%, 0.03% and 0.1% of RO5024118 was determined to be primarily due to a reduction of CD8+ T cells rather than CD4+ T cells (Figure 9). MPO levels in the BAL fluid were significantly inhibited by RO5024118, to approximately the same extent at all concentrations used (Figure 10).
Figure 8.
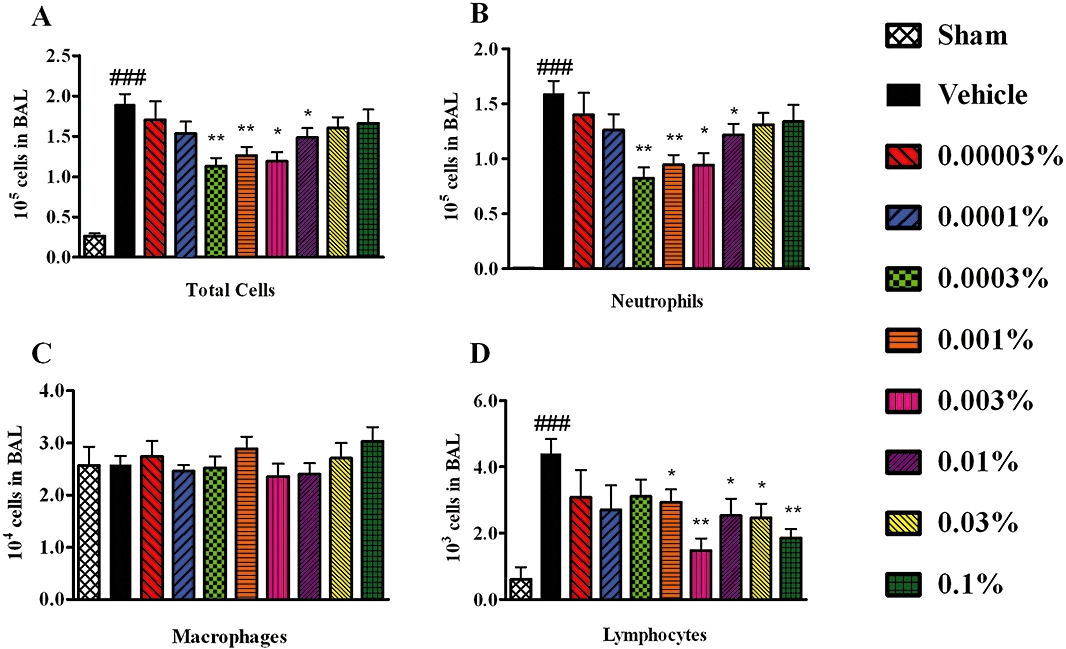
Effects of RO5024118 (0.00003–0.1% w/v) on total cells (A), neutrophils (B), macrophages (C) and lymphocytes (D) in the bronchoalveolar lavage (BAL) fluid of mice 24 h after exposure to LPS. Data are mean ± SEM of five experiments. **P < 0.01 versus vehicle; ###P < 0.001 versus sham.
Table 1.
Percentage inhibition of total cells, neutrophils and lymphocytes with RO5024118 in the bronchoalveolar lavage fluid of mice 24 h after exposure to lipopolysaccharide
| % inhibition | |||
|---|---|---|---|
| RO5024118 (% w/v) | Total cells | Neutrophils | Lymphocytes |
| 0.00003 | 10.1 ± 11.2 | 12.1 ± 11.6 | 29.8 ± 17.1 |
| 0.0001 | 19.1 ± 7.1 | 20.8 ± 8.1 | 38.4 ± 15.1 |
| 0.0003 | 41.6 ± 5.3 | 48.2 ± 5.9 | 41.6 ± 5.3 |
| 0.001 | 33.4 ± 5.4 | 40.7 ± 5.6 | 33.1 ± 8.6 |
| 0.003 | 37.1 ± 5.4 | 40.9 ± 6.5 | 66.4 ± 7.8 |
| 0.01 | 21.8 ± 6.0 | 23.5 ± 6.3 | 42.2 ± 11.2 |
| 0.03 | 15.4 ± 6.8 | 17.8 ± 6.6 | 44.0 ± 9.6 |
| 0.1 | 12.4 ± 5.8 | 15.9 ± 6.2 | 57.7 ± 7.5 |
Percentage inhibition calculated relative to vehicle. Data are mean ± SEM of five experiments.
Figure 9.
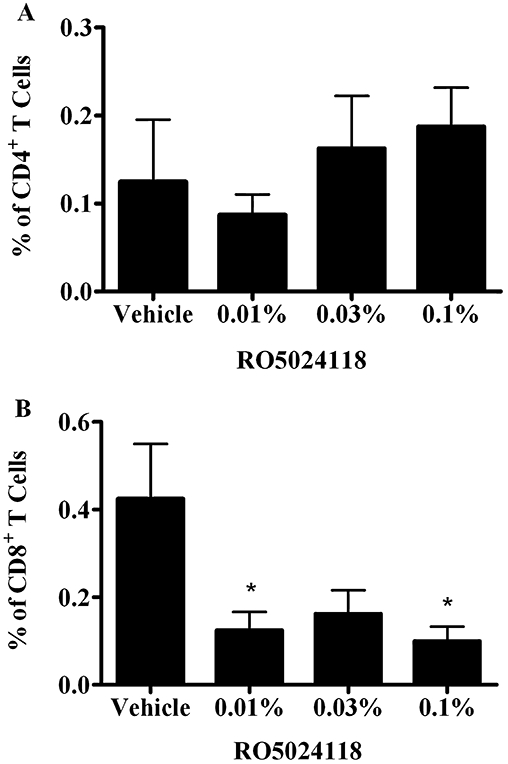
Effects of RO5024118 (0.01–0.1%) on % of CD4+ T cells (A) and CD8+ T cells (B) in the bronchoalveolar lavage fluid of mice 24 h after exposure to lipopolysaccharide. Data are mean ± SEM of eight animals per group. *P < 0.05 versus vehicle.
Figure 10.
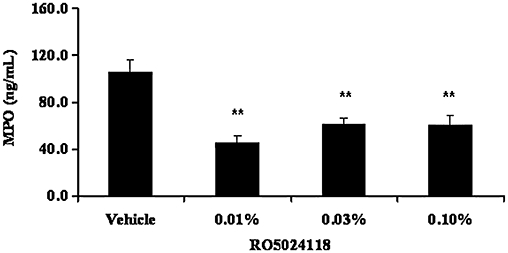
Effects of RO5024118 (0.01–0.1% w/v) on MPO levels in the bronchoalveolar lavage fluid of mice 24 h after exposure to lipopolysaccharide. Data are mean ± SEM of eight animals per group. **P < 0.01 versus vehicle.
PPE-induced acute lung inflammation in rats
Inhaled corticosteroids are often ineffective in COPD. Therefore, we evaluated the anti-inflammatory activity of RO5024118 in a rat steroid-resistant model of neutrophilic airway inflammation, induced by intratracheal PPE (Birrell et al., 2005). Pretreatment with 0.1% aerosol dexamethasone had no effect on PPE-induced accumulation of neutrophils in BAL fluid (Figure 11, Table 2). In contrast, pretreatment with a 20 min aerosol exposure of RO5024118 at 0.0003% (w/v) significantly inhibited PPE-induced increases in neutrophils and total cells in the BAL fluid. Macrophages tended to decrease at this dose, but not significantly. RO5024118 at 0.001% also significantly inhibited neutrophils and tended to decrease total cell accumulation although not significantly (Figure 11, Table 2).
Figure 11.
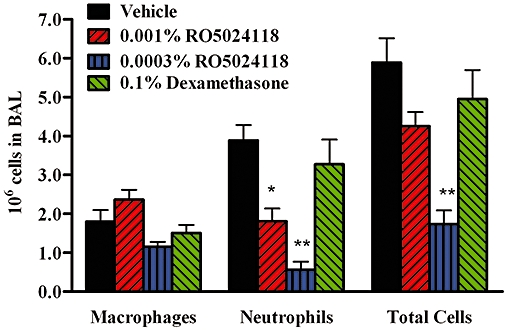
Effects of RO5024118 (0.0003–0.001% w/v) on inflammatory cells in the bronchoalveolar lavage (BAL) fluid of rats 24 h after intratracheal instillation of porcine pancreatic elastase. Data are mean ± SEM of four experiments. *P < 0.05, **P < 0.01 versus vehicle.
Table 2.
Percentage inhibition of cells with RO5024118 or dexamethasone in the airway of rats 24 h after exposure to porcine pancreatic elastase
| % inhibition | ||
|---|---|---|
| RO5024118 (% w/v) | Neutrophil | Total cells |
| 0.001 | 53.4 ± 7.6 | 27.8 ± 5.7 |
| 0.0003 | 85.6 ± 4.7 | 70.5 ± 5.1 |
| Dexamethasone (0.1%) | 15.7 ± 14.2 | 16.0 ± 11 |
Percentage inhibition calculated relative to vehicle. Data are mean ± SEM of four experiments.
Discussion
The present studies have demonstrated that RO5024118 is a highly selective agonist at the VPAC2 receptor and exhibits increased stability to human neutrophil elastase degradation, relative to its predecessor RO0251553 and native VIP. In vitro, it is a potent relaxant of guinea pig and human airway smooth muscle. In vivo, RO5024118 inhibits acute bronchospasm in guinea pigs and in a mouse model of LPS-induced airway inflammation, inhibits neutrophil and CD8+ T-cell accumulation, as well as MPO release. Finally, we showed inhibition of lung inflammation in a steroid-resistant rat model. Taken together, these results demonstrate that RO5024118, by selectively activating the VPAC2 receptor, evokes dual bronchodilatory and anti-inflammatory activity in the lungs.
RO5024118 is a successor to RO0251553, a potent, stable analogue of VIP that is highly selective for the VPAC2 receptor (Bolin et al., 1995; Gourlet et al., 1997). RO5024118 contains a Cα-methylated valine derivative in the amino acid position 5 instead of the normal valine present in the predecessor compound RO0251553. This modification was designed based on the suggested neutrophil elastase cleavage site of RO0251553 in an effort to increase stability to this enzyme. In vitro studies using cell lines expressing VPAC1 and VPAC2 receptors confirmed the previously reported VPAC2 selectivity of RO0251553 and demonstrated that VPAC2 selectivity was retained in the second generation compound, RO5024118. Maintaining VPAC2 selectivity was a goal of these chemistry approaches, on the basis of several reports of a pulmonary bronchodilatory and anti-inflammatory role of this receptor, including early studies with RO0251553 (O'Donnell et al., 1994a,b; Dewit et al., 1998; Voice et al., 2002; 2003;).
Under physiological conditions, VIP is mainly cleaved by neutral endopeptidase in the airway, whereas under inflammatory conditions, mast cell tryptase and chymase play a dominant role in the degradation of VIP (Groneberg et al., 2006). In the case of COPD where neutrophils are the predominant inflammatory cells, neutrophil elastase dominates the degradation of VIP (Ohbayashi, 2002). Thus, the stability of RO5024118 to neutrophil elastase was compared with native VIP and RO0251553. RO5024118 was shown to be significantly more stable to enzymatic degradation by neutrophil elastase. This prolonged half-life of RO5024118 observed in vitro may translate into longer duration of action in COPD lungs compared with native VIP and RO0251553. Indeed, RO0251553 has been evaluated clinically as a bronchodilator and was shown to have a rapid onset of bronchodilation with improvement of forced expiratory volume in one second, comparable with formoterol. However, its short duration of action, 5 h compared with 12 h for formoterol, was probably due to degradation by peptidases (Linden et al., 2003).
The bronchodilatory effects of RO5024118 were evaluated in vitro and in vivo. In experiments conducted on isolated human and guinea pig airway smooth muscle, RO5024118 exhibited similar activity to its predecessor molecule, RO0251553, and was significantly more potent than VIP and the short-acting β-agonist salbutamol and exhibited similar potency to the long-acting β-agonist salmeterol. Interestingly, although RO5024118 and VIP induced increases in cAMP levels in a similar concentration range at human VPAC2 receptors (EC50 of 3.1 vs. 7.9 nM, respectively), differences were observed in the potency of RO5024118 compared with VIP in these airway relaxation experiments (EC50 of,35.7 vs. 322 nM in the human and 0.75 vs. 230 nM in guinea pig tissues, respectively). This is likely to be due to the enhanced stability of RO5024118 to neutral endopeptidase release from the epithelium of these isolated tissues. Other studies have shown that decreasing the neutral endopeptidase activity in isolated airway tissues by treating with inhibitors or removing the epithelium leads to enhanced VIP-induced relaxation (Farmer and Togo, 1990; Tam et al., 1990; Groneberg et al., 2001).
The potent bronchodilatory activity observed in vitro was also observed in vivo. 5-HT-induced bronchospasm in conscious guinea pigs was chosen to test the bronchodilatory effects of RO5024118 because Battram et al. (2006) have successfully demonstrated efficacy with both short- and long-acting β-agonists in this model. Pretreatment with RO5024118 potently inhibited 5-HT-induced bronchoconstriction. Further, comparison with equivalent (and maximal) concentrations of RO0251553 demonstrated comparable efficacy. Limited studies were performed to compare the duration of action of RO5024118 and RO0251553 in this guinea pig in vivo model because the levels of neutrophil elastase in naïve animals is low and therefore the applicability of such an animal model to the clinical situation in COPD lungs must be interpreted with caution (Johnson et al., 2005). RO5024118 retained bronchodilatory activity for 2 h, whereas RO0251553 did not. As Penh analysis was used as an index of bronchoconstriction in this conscious guinea pig model, we performed experiments using invasive resistance–compliance plethysmograpy to confirm the bronchodilatory activity of RO5024118. In the invasive model, pretreatment with RO5024118 also potently inhibited 5-HT-induced changes in RL and Cdyn. These data demonstrated that RO5024118 is a potent bronchodilator in human and guinea pig airways and suggest that RO5024118 may be an effective treatment for respiratory diseases such as COPD where bronchodilators are recommended to relieve obstructive symptoms. Furthermore, the increased stability of RO5024118 to neutrophil elastase combined with evidence of increased duration of action in vivo suggest that RO5024118 may exhibit an increased duration of action, relative to RO0251553 in clinical settings.
In addition to its role as a bronchodilator, VIP also functions as an anti-inflammatory/immunological modulator. VPAC1 and VPAC2 receptors are expressed on many immune cells, including T cells and macrophages. When activated, these receptors regulate immune cell functions such as proliferation, differentiation, survival, migration and generation of cytokines (Voice et al., 2002). Therefore, the anti-inflammatory properties of RO5024118 were explored in lung. The mouse LPS-induced lung inflammation model was chosen because it is a simple, acute mechanistic assay that exhibits some of the key pathological features of COPD such as macrophage activation, elevated levels of TNF-α, and neutrophil influx and activation (Corteling et al., 2002). Furthermore, the effects of a VPAC2 receptor agonist have not been previously explored in this model. Pretreatment with RO5024118 significantly inhibited neutrophil and CD8+ T-cell influx in the mouse airway following LPS exposure. Interestingly, we found that RO5024118 inhibited LPS-induced lung neutrophil influx in a bell-shaped manner, with the higher and the lower concentrations inducing less inhibitory activity. The reasons for this remain unclear; however, this phenomenon may be due to increased bronchodilatory activity with higher concentrations of RO5024118 (as seen in the data with guinea pigs in vivo), which translates into increased exposure to inhaled LPS via relaxation and opening of central and peripheral airways. Lower concentrations of RO5024118 could equate to less compound exposure and, are therefore, simply less effective. Interestingly, Riesenfeld et al. (2010) recently reported a similar exacerbation of allergic evoked lung inflammation in mice following treatment with high concentrations of the inhaled bronchodilator salmeterol. This is unlikely to represent an issue in clinical settings where inflammation is already present. RO5024118 also significantly inhibited MPO levels in BAL fluid suggesting an effect on both recruitment and activation of neutrophils. In COPD patients, there is an abnormal inflammatory response consisting of increased neutrophils, macrophages and CD8+ T cells, which play a role in producing deleterious structural changes in the airways. Neutrophils are believed to play a dominant role in COPD due to the secretion of serine proteases that contribute to mucus hyper-secretion and alveolar wall destruction. CD8+ T cells are present in greater numbers than CD4+ T cells in the lung parenchyma and peripheral and central airways of COPD patients, and they have been shown to have the capacity to produce cytokines that may contribute to emphysema (Cosio et al., 2002; Maeno et al., 2007). In addition, there is correlation between the number of T cells, the amount of alveolar wall destruction and the severity of airway obstruction (Barnes, 2004). Therefore, the ability of RO5024118 to decrease neutrophil and CD8+ T-cell accumulation and mediator release in the mouse model of LPS-induced airway inflammation suggest that RO5024118 may represent a novel clinical intervention in COPD patients.
Lung inflammation and compromised lung function in COPD patients are reported to be resistant to steroid treatment (Barnes, 2000; Soriano et al., 2007). Therefore, the effects of RO5024118 were evaluated in a rat model of steroid-resistant inflammation. The effects of a VPAC2 receptor agonist in this model have not previously been investigated. Birrell et al. (2005) have shown that intratracheal instillation of PPE in the rat induces acute neutrophilic inflammation followed by enlargement of the airspaces and compromised lung function, characteristic features of COPD. In the present studies, treatment with RO5024118 attenuated PPE-induced acute neutrophil inflammation whereas dexamethasone did not, suggesting that RO5024118 may be an effective therapy for airway inflammation in patients who are steroid-resistant. Interestingly, we observed a trend towards a similar relationship between RO5024118 concentration and neutrophilic inflammation as seen in the mouse LPS model (see above). Corticosteroid-sensitive, NF-κB pathway activation is known to play a key role in the induction of pro-inflammatory gene expression, leading to mediator release and recruitment and activation of inflammatory cells. However, Birrell et al. (2006) have shown that PPE-induced inflammation is NF-κB-independent, which may explain the lack of efficacy of dexamethasone. These data suggest that the anti-inflammatory activity of RO5024118 is irrespective of NF-κB pathway activation as efficacy was observed in both NF-κB-dependent (mouse LPS) and -independent (rat PPE) inflammatory responses. Further studies are needed to investigate the anti-inflammatory mechanisms of RO5024118.
In conclusion, these studies demonstrate that RO5024118 retains the high affinity and specificity for VPAC2 receptors of the predecessor molecule RO0251553, while demonstrating increased stability to human neutrophil elastase. RO5024118 inhibited acute bronchospasm in guinea pigs, decreased neutrophil and CD8+ T-cell accumulation and mediator release in a mouse model of LPS-induced airway inflammation, and attenuated elastase-induced lung inflammation in a steroid-resistant rat model. These results suggest that RO5024118 may be beneficial in treating airway obstructive and inflammatory diseases such as COPD via dual bronchodilator and anti-inflammatory mechanisms. In addition, these results suggest that RO5024118 may be an effective therapy for airway inflammation in patients who are steroid-resistant.
Acknowledgments
We would like to thank Nelson Montero, John O'Neill and Joyce Arlauskas of Laboratory Animal Resources for their excellent technical assistance and Dr Grace Ju for critical review of the manuscript.
Glossary
Abbreviations
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- PPE
porcine pancreatic elastase
- VIP
vasoactive intestinal peptide
- VPAC
VIP-pituitary adenylate cyclase activating peptide
- VPAC1/VPAC2
high affinity G-coupled receptors for VIP
Conflict of interest
SAT, LMR, NT, JDV, DL, TAL, AM, DR, HM, LS, MH and AH are employees of Roche pharmaceuticals.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:342–344. doi: 10.1164/ajrccm.161.2.16125_2. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- Battram C, Charlton SJ, Cuenoud B, Dowling MR, Fairhurst RA, Fozard JR, et al. In Vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy ethyl]-8-hydroxy-1hquinolin- 2-one (indacaterol), a novel inhaled β2 adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317:762–770. doi: 10.1124/jpet.105.098251. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Hele DJ, Birrell MA. New anti-inflammatory therapies and targets for asthma and chronic obstructive pulmonary disease. Expert Opin Ther Targets. 2004;8:265–285. doi: 10.1517/14728222.8.4.265. [DOI] [PubMed] [Google Scholar]
- Birrell MA, Wong S, Hele DJ, McCluskie K, Hardaker E, Belvisi MG. Steroid-resistant inflammation in a rat model of chronic obstructive pulmonary disease is associated with a lack of nuclear factor-κB pathway activation. Am J Respir Crit Care Med. 2005;172:74–84. doi: 10.1164/rccm.200409-1257OC. [DOI] [PubMed] [Google Scholar]
- Birrell MA, Wong S, Hardaker EL, Catley MC, McCluskie K, Collins M, et al. IκB kinase-2 independent and –dependent inflammation in airway disease models: relevance of IKK-2 inhibition to the clinic. Mol Pharmacol. 2006;69:1791–1800. doi: 10.1124/mol.105.019521. [DOI] [PubMed] [Google Scholar]
- Bolin DR, Khan W, Michel H. Preparation of novel analogs of vasoactive intestinal peptide. 2008. Patent WO2008003612 A2.
- Bolin DR, Michalewsky J, Wasserman MA, O'Donnell M. Design and development of a vasoactive intestinal peptide analog as a novel therapeutic for bronchial asthma. Biopolymers. 1995;37:57–66. doi: 10.1002/bip.360370203. [DOI] [PubMed] [Google Scholar]
- Corteling R, Wyss D, Trifilieff A. In vivo models of lung neutrophil activation. Comparison of mice and hamsters. BMC Pharmacology. 2002;2:1–8. doi: 10.1186/1471-2210-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio MG, Majo J, Cosio MG. Inflammation of the Airways and Lung Parenchyma in COPD. Chest. 2002;121:160S–165S. doi: 10.1378/chest.121.5_suppl.160s. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gomariz RP, Martinez C, Abad C, Leceta J. Anti-inflammatory properties of the type 1 and type 2 vasoactive intestinal peptide receptors: role in lethal endotoxic shock. Eur J Immunol. 2000;30:3236–3246. doi: 10.1002/1521-4141(200011)30:11<3236::AID-IMMU3236>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Dewit D, Gourlet P, Amraoui Z, Vertongen P, Willems F, Robberecht P, et al. The vasoactive intestinal peptide analogue RO25-1553 inhibits the production of TNF and IL-12 by LPS-activated monocytes. Immunol Lett. 1998;60:57–60. doi: 10.1016/s0165-2478(97)00129-6. [DOI] [PubMed] [Google Scholar]
- Farmer SG, Togo J. Effects of epithelium removal on relaxation of airway smooth muscle induced by vasoactive intestinal peptide and electrical field stimulation. Br J Pharmacol. 1990;100:73–78. doi: 10.1111/j.1476-5381.1990.tb12054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr Pharm Des. 2001;7:89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vertongen P, Vandermeers A, Vandermeers-Piret M-C, Rathe J, Neef PD, et al. The long-acting vasoactive intestinal polypeptide agonist RO 25-1553 is highly selective of the VIP2 receptor subclass. Peptides. 1997;18:403–408. doi: 10.1016/s0196-9781(96)00322-1. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Springer J, Fisher A. Vasoactive Intestinal Polypeptide as Mediator of Asthma. Pulm Pharmacol Ther. 2001;14:391–401. doi: 10.1006/pupt.2001.0306. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Rabe KF, Fisher A. Novel concepts of neuropeptide-based drug therapy: vasoactive intestinal polypeptide and its receptors. Eur J Pharmacol. 2006;533:182–194. doi: 10.1016/j.ejphar.2005.12.055. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, et al. Noninvasive measurements of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- Johnson FJ, Reynolds LJ, Toward TJ. Elastolytic activity and alveolar epithelial type-1 cell damage after chronic LPS inhalation: effects of dexamethasone and rolipram. Toxicol Appl Pharmacol. 2005;207:257–265. doi: 10.1016/j.taap.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Linden A, Hansson L, Andersson A, Palmqvist M, Arvidsson P, Lofdahl C-G, et al. Bronchodilation by an inhaled VPAC2 receptor agonist in patients with stable asthma. Thorax. 2003;58:217–221. doi: 10.1136/thorax.58.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T Cells Are Required for Inflammation and Destruction in Cigarette Smoke-Induced Emphysema in Mice. J Immunol. 2007;178:8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- Menghang X, Sreedharan SP, Goetzl EJ. Predominant expression of type II vasoactive intestinal peptide receptors by human T lymphoblastoma cells: transduction of both Ca2+ and cyclic AMP signals. J Clin Immunol. 1996;16:21–30. doi: 10.1007/BF01540969. [DOI] [PubMed] [Google Scholar]
- Nicole P, Lins L, Rouyer-Fessard C, Drouot C, Fulcrand P, Thomas A, et al. Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a highly selective VPAC1 receptor agonist. J Biol Chem. 2000;275:24003–24012. doi: 10.1074/jbc.M002325200. [DOI] [PubMed] [Google Scholar]
- O'Donnell M, Garippa RJ, Rinaldi N, Selig WM, Simko B, Renzetti L, et al. Ro 25-1553: a novel, long-acting vasoactive intestinal peptide agonist. Part I: in vitro and in vivo bronchodilator studies. J Pharmacol Exp Ther. 1994a;270:1282–1288. [PubMed] [Google Scholar]
- O'Donnell M, Garippa RJ, Rinaldi N, Selig WM, Tocker JE, Tannu SA, et al. Ro 25-1553: a novel long-acting vasoactive intestinal peptide agonist. Part II: effect on in vitro and in vivo models of pulmonary anaphylaxis. J Pharmacol Exp Ther. 1994b;270:1289–1294. [PubMed] [Google Scholar]
- Ohbayashi H. Neutrophil elastase inhibitors as treatment for COPD. Expert Opin Investig Drugs. 2002;11:965–980. doi: 10.1517/13543784.11.7.965. [DOI] [PubMed] [Google Scholar]
- Pahl A, Szelenyi I. Chronic obstructive pulmonary disease (COPD) Drugs Future. 2007;32:799–807. [Google Scholar]
- Palmer JB, Cuss FM, Barnes PJ. VIP and PHM and their role in nonadrenergic inhibitory responses in isolated human airways. J Appl Physiol. 1986;61:1322–1328. doi: 10.1152/jappl.1986.61.4.1322. [DOI] [PubMed] [Google Scholar]
- Riesenfeld EP, Sullivan MJ, Thompson-Figueroa JA, Haverkamp HC, Lundblad LK, Bates JHT, et al. Inhaled salmeterol and/or fluticasone alters structure/function in a murine model of allergic airways disease. Respir Res. 2010;11:22–33. doi: 10.1186/1465-9921-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano JB, Sin DD, Zhang X, Camp PG, Anderson JA, Anthonisen NR, et al. A pooled analysis of FEV1 decline in COPD patients randomized to inhaled corticosteroids or placebo. Chest. 2007;131:682–689. doi: 10.1378/chest.06-1696. [DOI] [PubMed] [Google Scholar]
- Summers MA, O'Dorisio MS, Cox MO, Lara-Marquez M, Goetzl EJ. A lymphocyte generated fragment of vasoactive intestinal peptide with VPAC1 agonist activity and VPAC2 antagonist effects. J Pharmacol Exp Ther. 2003;306:638–645. doi: 10.1124/jpet.103.050583. [DOI] [PubMed] [Google Scholar]
- Tam EK, Franconi GM, Nadel JA, Caughey GH. Protease inhibitors potentiate smooth muscle relaxation induced by vasoactive intestinal peptide in isolated human bronchi. Am J Respir Cell Mol Biol. 1990;2:449–452. doi: 10.1165/ajrcmb/2.5.449. [DOI] [PubMed] [Google Scholar]
- Voice JK, Dorsam G, Chan RC, Grinninger C, Kong Y, Goetzl EJ. Immunoeffector and immunoregulatory activities of vasoactive intestinal peptide. Regul Pept. 2002;109:199–208. doi: 10.1016/s0167-0115(02)00182-9. [DOI] [PubMed] [Google Scholar]
- Voice JK, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl EJ. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell targeted type II VIP receptor transgenic mice. J Immunol. 2003;170:308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]


