Abstract
BACKGROUND AND PURPOSE
Uterine spontaneous contraction and pacemaking are poorly understood. This study investigates the role of the mitochondrial Ca2+ store in uterine activity.
EXPERIMENTAL APPROACH
We investigated the effects of mitochondrial and sarco-endoplasmic reticulum (SER) inhibitors on contraction, membrane potential (Vm) and cytosolic Ca2+ concentration ([Ca2+]c) in longitudinal smooth muscle of the mouse uterus.
KEY RESULTS
The mitochondrial agents rotenone, carbonylcyanide-3-chlorophenylhydrazone (CCCP), 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157) and kaempferol decreased the force of contractions. The ATP synthase inhibitor oligomycin had no significant effect. The effects of these agents were compared with those of SER inhibitors cyclopiazonic acid (CPA), 2-amino ethoxyphenylborate (2-APB) and caffeine. All agents, except CPA and oligomycin, decreased contractile force. CPA and CCCP transiently increased contraction frequency, which returned to control levels, whereas rotenone, CGP37157, kaempferol and 2-APB decreased frequency and caffeine had no significant effect. Application of the mitochondrial agents when CPA functionally inhibited stores did not change contraction frequency but, with the exception of kaempferol, decreased force. CCCP caused depolarization and maintained increase in [Ca2+]c or depolarization/transient hyperpolarization and transient increase in [Ca2+]c for oestrus and di-oestrus tissues respectively. Rotenone caused hyperpolarization and maintained increase in [Ca2+]c. CGP37157 and kaempferol caused hyperpolarization but no measurable change in [Ca2+]c. Application of a range of K+ channel blockers indicated a role of Ca2+-activated K+ (KCa) channels in the CCCP- and CGP37157-induced actions.
CONCLUSIONS AND IMPLICATIONS
Mitochondria have a modulatory role on uterine contractions, with mitochondrial inhibition reducing contraction amplitude and pacemaker frequency by changes in Vm, [Ca2+]c and/or Ca2+ influx.
Keywords: uterus, mitochondria, spontaneous contractions, pacemaking
Introduction
The basis for the pacemaker mechanism that times and initiates uterine action potentials and the resultant influx of Ca2+ remains to be elucidated (Mironneau, 1973; Parkington and Coleman, 2001; Wray et al., 2003; Young, 2007). One possible contributing mechanism is that provided by intracellular Ca2+ stores. The sarco-endoplasmic reticulum (SER) has been shown to be involved in pacemaking in other smooth muscles (van Helden, 1993; Liu et al., 1995; Hashitani et al., 1996; Sergeant et al., 2001; Lang et al., 2006; Berridge, 2008). However, studies investigating this possibility in uterus have demonstrated that blockade of the SER Ca2+ ATPase (SERCA) with cyclopiazonic acid (CPA) does not inhibit spontaneous contractions and/or action potentials in human (Kupittayanant et al., 2002), guinea pig (Coleman et al., 2000) or rat uterine smooth muscle (Shmigol et al., 1999). Based on these results, it has been suggested that SER Ca2+ stores are not directly involved in uterine pacemaking, but are involved in Ca2+ buffering (Shmigol et al., 1999). However, SER Ca2+ stores are likely to have a modulatory role, and this mechanism may underlie the stimulatory actions of hormones such as oxytocin (see Berridge, 2008).
Importantly, other organelles within the cell, such as mitochondria, also take up, store and release Ca2+. Mitochondrial Ca2+ uptake occurs through the mitochondrial Ca2+ uniporter (MCU) in the inner membrane of these organelles. The MCU is actually an ion channel and not a carrier, as had been previously thought (Kirichok et al., 2004). Ca2+ entry through the MCU is driven by the mitochondrial membrane potential (Vm) and is activated by extra-mitochondrial Ca2+ (Szabadkai et al., 2006). Mitochondrial Ca2+ release is provided by both the Na+/Ca2+ exchanger (NCX) and the H+/Ca2+ exchanger (HCX). Significantly, mitochondria have been shown to play a role in pacemaker function in other smooth muscle tissues where Ca2+ stores are known to underlie pacemaker activity, including gastrointestinal interstitial cells of Cajal (ICCs) (Ward et al., 2000), guinea pig gallbladder (Balemba et al., 2008) and ICCs of rabbit urethra (Sergeant et al., 2008). The mechanisms for this are only now being unravelled. It may arise through inhibition of physical interactions between the SER and mitochondria, which have been well documented (Rizzuto et al., 1998; Arnaudeau et al., 2001; Csordas et al., 2006; Pizzo and Pozzan, 2007) and include Ca2+ shuttling between the two organelles (Ishii et al., 2006). It does not result from ATP depletion, as the effects occur independent of the presence of an ATP synthase inhibitor (Ward et al., 2000).
Mitochondria are abundant in gastrointestinal pacemaker cells [i.e. ICCs (Komuro, 2006)] and ICC-like cells have been reported in rat and human uteri (Ciontea et al., 2005; Duquette et al., 2005), but whether these cells are involved in uterine pacemaking remains unclear (Allix et al., 2008). Uterine ICC-like cells also exhibit abundant mitochondria (Ciontea et al., 2005) and hence investigations into the role of mitochondria may provide new insights into uterine contraction and pacemaker mechanisms. The present study has investigated this in mouse uterus. We provide evidence that mitochondria have a modulatory role in both pacemaking and contractile activity.
Methods
Animals
All animal care and experimental procedures complied with, and were approved by, the Animal Care and Ethics Committees at the University of Newcastle (ethics approval number 452/1209) and Monash University (SoBSA/P/2007/106). Female Swiss virgin mice (6–10 weeks old) were maintained in a controlled environment in a 12/12 h light/dark cycle, at a temperature of approximately 20°C, with free access to food and water. Fifty-three mice were killed by isoflurane inhalation (5–10% in air) followed by exsanguination.
Contractility experiments
Uteri were dissected out, trimmed of connective tissue, opened along the mesometrial border, and transected medially to provide four preparations per mouse. These strips were equilibrated for 10 min in 3 mL organ baths with physiological saline solution (PSS) at 37°C containing (mM): NaCl 120, KCl 5, CaCl2 2.5, MgCl2 2, NaHCO3 25, NaH2PO4 1, glucose 10 and bubbled with carbogen (95% O2, 5% CO2). Tissues were placed under 5 mN tension and the differential force and frequency of spontaneous contractions in the longitudinal muscle layer recorded. KCl (40 mM) was then added for 20 s to confirm tissue viability, followed by a further 10 min equilibration, after which time test drugs were applied. The force of contraction (mN) was recorded isometrically via a Grass FT.03 transducer connected to a MacLab 4e recording system.
Concentration–response curves
Log concentration–response curves were obtained by the cumulative addition of mitochondrial inhibitors every 5 min. Four mitochondrial inhibitors were used: carbonylcyanide-3-chlorophenylhydrazone (CCCP), rotenone, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157) and oligomycin.
Single concentration experiments
A single concentration of SER or mitochondrial drugs was applied for 30 min, applying one or combinations of drugs, such as CGP37157 and kaempferol or CCCP and oligomycin.
CPA and mitochondrial drugs
Cyclopiazonic acid was added to the bath for 15 min to empty the SER store, and then the mitochondrial agents CCCP, rotenone, CGP37157 or kaempferol were added to each CPA-containing bath for 30 min.
K+ channel blockers and CCCP
K+ channel blockers including iberiotoxin (IbTX), charybdotoxin (CTX), apamin (Apa), tetraethylammonium (TEA), 4-aminopyridine (4-AP) and glibenclamide were added to the PSS, followed by the addition of CCCP (5 min later) for 30 min.
Measurements
When constructing concentration–response curves, the differential force of contractions (i.e. from base to peak) was measured by averaging the maximum amplitude of the three last contractions for each concentration. In all other experiments contractions were analysed by averaging the force and frequency of contractions during the last 3 min of equilibration (control) and during the last 3 min of drug application (test). When K+ channel blockers and CCCP were applied, contractions were measured between 10 and 15 min after addition of CCCP; this was done because, in some cases, the tissue started to slightly recover from the actions of the blockers after 15 min. All measurements were expressed relative to control (=100%), which was measured during the last 5 min of equilibration.
Simultaneous recording of Vm or relative [Ca2+]c and tension in uterine strips
A vaginal smear from each mouse was examined before experiments commenced and an animal in pro-oestrus/oestrus was usually chosen, although tissues from a small number of mice in di-oestrus or met-oestrus were also examined. In most cases, experiments were performed on small longitudinal uterine strips, with some circular muscle left attached to better represent the tissues used for the contractility studies. However, in a small number of cases, strips of isolated circular muscle were examined (see Results). Strips were pinned to one end of a recording chamber and the other end was attached to a force transducer. Strips were placed under 0.1 mN tension in the longitudinal direction and continuously superfused with carbogen-bubbled PSS at 35°C. Vm was recorded from the longitudinal and, in a few cases (where stated), from the circular smooth muscle using intracellular glass microelectrodes filled with 1 M KCl and having resistances of 90–120 MΩ (Parkington and Coleman, 2001). Relative changes in [Ca2+]c were measured using the ratiometric excitation dye fura-2, with tissues alternately illuminated with 340 and 380 nm at 100 Hz (Parkington et al., 1999). The changes in [Ca2+]c are expressed as % basal value for each tissue. The persistence of spontaneous activity in these small strip preparations was variable, as was the case in ∼70% of tissues studied. Therefore in a small number of cases (where noted), contractile activity was induced by including oxytocin (0.5 nM) in the superfusate.
Statistical analyses
Responses are expressed as percentage of control, with data presented as the mean ± standard error of mean (SEM). Mean log concentration–response curves were analysed by fitting data to a four-parameter logistic equation, using non-linear regression with GraphPad Prism 4.02 (GraphPad Software, San Diego, CA, USA) to determine pIC50 values (−log concentration giving half-maximal inhibition). For paired statistical analyses Student's two-tailed paired t-test was applied, using PASW Statistics 17 software, comparing the treatment with the corresponding control, or with CPA. A two-tailed unpaired t-test was applied for non-paired comparisons. n represents the number of replicates in different mice. All data fitted the criteria for normality assessed by Kolmogorov–Smirnov test using PASW Statistics 17 software.
Materials
Dimethyl sulphoxide (DMSO), caffeine, CCCP, rotenone, oligomycin, CPA, 2-amino ethoxyphenylborate (2-APB), kaempferol, IbTX, CTX, Apa, TEA, 4-AP and glibenclamide were purchased from Sigma (St Louise, MI, USA). CGP37157 was purchased from Calbiochem (San Diego, CA, USA). All drugs were prepared as stock solutions in DMSO, except TEA, IbTX and CTX, which were dissolved in water; and caffeine, which was made up directly to final concentration in PSS. The nomenclature used here follows Alexander et al. (2009).
Results
Investigations on uterine contractility, made in large tissue preparations containing both longitudinal and circular smooth muscle, demonstrated that rhythmic contractions occurred in 92% of these tissues providing a mean force in the longitudinal direction of 19 ± 1 mN (n= 40). The force of spontaneous contractions (when corrected for wet weight) was not significantly different between the upper (ovarian end) and lower (cervical end) segments (data not shown). There was also no significant difference in the amplitude or frequency of contractions for tissue taken from mice in oestrus (n= 6), met-oestrus (n= 20) or di-oestrus (n= 5). In some cases the frequency of spontaneous contractions decreased at times longer than 1.5 h of recording and hence experiments were completed within the first hour and usually within the first 30 min of establishing these large tissue preparations at 37°C.
In small strips of longitudinal muscle with adherent circular muscle attached, 61% exhibited spontaneous contractions, and their resting potential was −63 ± 1 mV (n= 28). These latter strips were used (except where noted) for the Vm and Ca2+ imaging studies made on uterine longitudinal smooth muscle.
Cumulative concentration–response curves
Cumulative concentration–response experiments were performed to determine the effects of specific mitochondrial drugs on the force of spontaneous contractions of longitudinal smooth muscle in large tissue preparations. Response curves were determined for: CCCP (n= 5–10), a protonophore uncoupler that abolishes the mitochondrial Vm; rotenone (n= 3–9), a respiratory chain inhibitor (complex I); and CGP37157 (n= 4–9), a NCX blocker. These agents decreased the force of contractions in a concentration-dependent manner (Figure 1). The order of potency in inhibiting responses was rotenone (pIC50 6.28 ± 0.10), CCCP (pIC50 5.78 ± 0.09) and CGP37157 (pIC50 4.22 ± 0.09). However, ATP production is also inhibited by protonophores (Wray, 1990) and may be reduced by respiratory chain inhibitors and this could underlie the decrease in contraction amplitude. To determine whether the effects of the other mitochondrial inhibitors were due to a lack of ATP, the F1/Fo ATP synthase inhibitor oligomycin was tested but it had no significant effect on contractile force (Figures 1 and 2) or frequency (Table 1) during the experiment (i.e. over 30–60 min).
Figure 1.
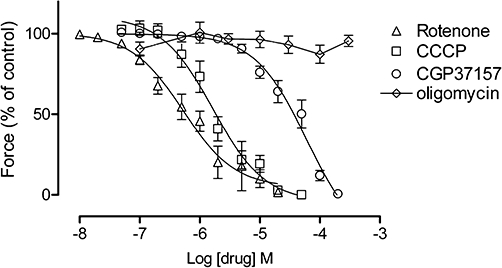
The effects of mitochondrial inhibitors on uterine longitudinal smooth muscle. Shown are concentration–response curves for rotenone (n= 3–9), CCCP (n= 5–10), CGP37157 (n= 4–9) and oligomycin (n= 4–7) (mean ± SEM). Data points for rotenone, CCCP and CGP37157 fitted a sigmoidal curve, while oligomycin did not. Error bars that are not shown lie within the dimensions of the symbols. CCCP, carbonylcyanide-3-chlorophenylhydrazone; CGP37157, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one.
Table 1.
Contraction frequency
| Drugs | Control (contract per 5 min) | 0–5 min | 25–30 min | n |
|---|---|---|---|---|
| DMSO 0.1% | 3.8 ± 0.8 | 4.1 ± 0.9 | 4.1 ± 0.7 | 6 |
| CPA (10 µM) | 4.0 ± 1.1 | 16.2 ± 3.4a | 6.8 ± 0.9 | 6 |
| 2-APB (10 µM) | 5.1 ± 1.2 | 4.1 ± 0.8 | 2.8 ± 0.5a | 7 |
| Caffeine (0.3 mM) | 4.5 ± 0.6 | 3.5 ± 1.1 | 3.2 ± 1.0 | 4 |
| CCCP (2 µM) | 4.5 ± 0.6 | 9.7 ± 1.7a | 3.7 ± 1.1 | 6 |
| Rotenone (1 µM) | 4.9 ± 0.7 | 6.2 ± 1.6 | 1.9 ± 0.4a | 7 |
| CGP37157 (10 µM) | 3.5 ± 0.5 | 2.2 ± 0.5a | 1.3 ± 0.6a | 7 |
| Kaempferol (10 µM) | 4.2 ± 0.6 | 3.6 ± 0.8 | 2.8 ± 0.8a | 5 |
| Oligomycin (3 µM) | 9.3 ± 1.5 | 9.8 ± 1.9 | 8.85 ± 0.6 | 3 |
Values are mean ± SEM.
significantly different from control P < 0.05 (Student's two-tailed paired t-test).
2-APB, 2-amino ethoxyphenylborate; CCCP, carbonylcyanide-3-chlorophenylhydrazone; CGP37157, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one; CPA, cyclopiazonic acid; DMSO, dimethyl sulphoxide.
Effect of mitochondrial and SER agents on spontaneous contractions
Single concentration experiments were performed to determine the relative effects of the different mitochondrial and SER drugs on the differential force of spontaneous contractions in longitudinal smooth muscle of large tissue preparations. Intermediate drug concentrations were chosen to avoid total inhibition with drugs applied for 30 min, allowing evaluation of drug effects on frequency. As shown in Figure 2, the mitochondrial agents CCCP (2 µM, n= 6), rotenone (1 µM, n= 6), CGP37157 (10 µM, n= 4), kaempferol (10 µM, n= 5; a flavonoid that activates the mitochondrial Ca2+ uniporter, i.e. MCU) and the combination of 10 µM CGP37157 and 10 µM kaempferol (n= 5) all inhibited the force of uterine contractions (Figure 2). The inositol 1,4,5-trisphosphate receptor (IP3R)/store-operated Ca2+ entry (SOCE) inhibitor, 2-APB (10 µM, n= 7) decreased the force of uterine contractions. Caffeine, which inhibits phosphodiesterase (Beavo and Reifsnyder, 1990), activates ryanodine receptors (RyRs) (Herrmann-Frank et al., 1999) and inhibits IP3Rs (Berridge, 1991), diminished force at a concentration of 0.3 mM (n= 4). CPA (10 µM, n= 5), which inhibits the SERCA (Goeger et al., 1988), did not significantly alter the differential force of the transient contractions but greatly augmented resting tension during the first 5 min (313% of control) with the resting tension then decreasing but remaining elevated (131% of control) for the rest of the 30 min recording period (P < 0.05 for both, n= 6; Figure 3A,C). In contrast, CCCP (Figure 3B,C) and the other mitochondrial inhibitors (data not shown) did not have a significant effect on resting tension.
Figure 2.
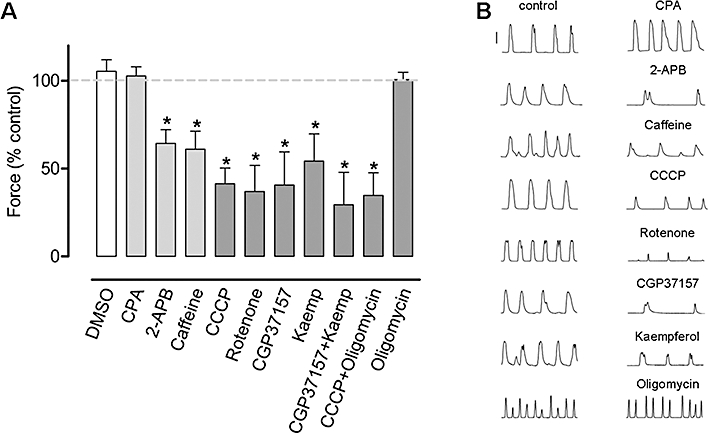
The effect of various SER and mitochondrial drugs on the force of spontaneous contractions of uterine longitudinal smooth muscle. (A) The SERCA inhibitor CPA (10 µM, n= 5) did not inhibit contractile force whereas the IP3R/SOCE blocker 2-APB (10 µM, n= 7) and caffeine (0.3 mM, n= 4) decreased contractile force. The mitochondrial agents CCCP (2 µM, n= 6), rotenone (1 µM, n= 6), CGP37157 (10 µM, n= 5) and kaempferol (Kaemp; 10 µM, n= 5) and a combination of 2 µM CCCP and 3 µM oligomycin (n= 4), all inhibited contractile force. Oligomycin (3 µM, n= 4) and the vehicle DMSO (0.1%, n= 6) did not affect force of contraction. Columns represent the mean ± SEM. *Significantly different from control, Student's two-tailed paired t-test, P < 0.05. Dashed line represents 100% control. (B) Representative paired traces (control vs. test) for the different agents. Vertical bar is 10 mN. 2-APB, 2-amino ethoxyphenylborate; CCCP, carbonylcyanide-3-chlorophenylhydrazone; CGP37157, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one; CPA, cyclopiazonic acid; DMSO, dimethyl sulphoxide; IP3R, inositol 1,4,5-trisphosphate receptor; SER, sarco-endoplasmic reticulum; SERCA, sarco-endoplasmic reticulum Ca2+ ATPase; SOCE, store-operated Ca2+ entry.
Figure 3.
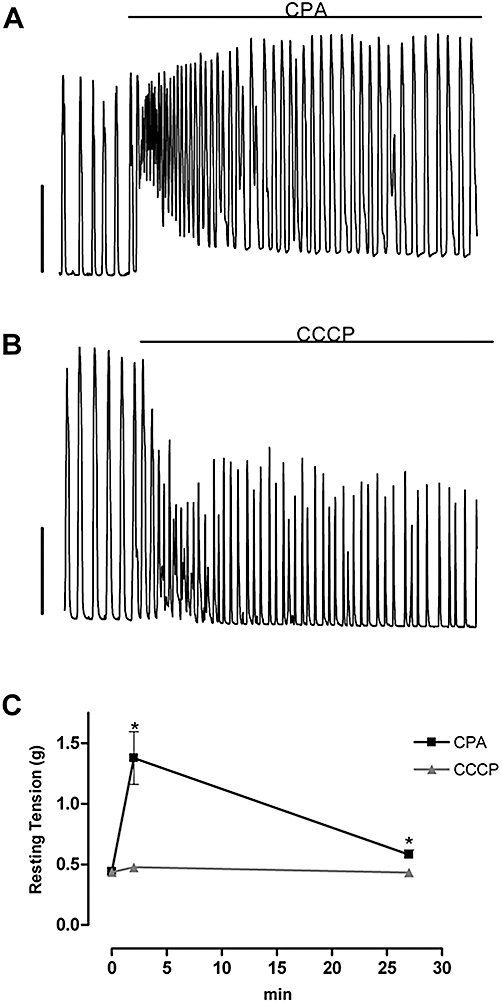
CPA but not CCCP increased resting tension in uterine longitudinal smooth muscle. (A) CPA (10 µM) increased resting tension, while (B) CCCP (2 µM) did not. Vertical bars in (A) and (B) represent 10 mN. Time scale is as in (C). (C) Mean effect of CPA (n= 6) and CCCP (n= 6) on resting tension. *Significantly different from control, Student's two-tailed paired t-test, P < 0.05. Error bars that are not shown lie within the dimensions of the symbols. CCCP, carbonylcyanide-3-chlorophenylhydrazone; CPA, cyclopiazonic acid.
Effect of mitochondrial and SER agents on pacemaking
We also analysed whether these drugs affected uterine pacemaking in these single concentration experiments (Table 1). CCCP (2 µM) significantly increased contraction frequency in the first 5 min to 216% of control, the frequency then decreased to near control levels (25–30 min). Rotenone (1 µM), CGP37157 (10 µM) and kaempferol (10 µM) all significantly reduced contraction frequency to 39%, 37% and 67% of control, respectively, after 25–30 min. CPA (10 µM) significantly increased contraction frequency in the first 5 min to 405% of control, which returned to a near normal rate after 25–30 min. 2-APB significantly reduced contraction frequency to 55% of control after 25–30 min.
Effect of CCCP on Vm and [Ca2+]c in oestrus and dioestrus longitudinal smooth muscle
These experiments aimed to characterize the actions of CCCP on Vm and cytosolic Ca2+ concentration and to determine whether there was a different response between longitudinal smooth muscle from mice in oestrus or di-oestrus. Vm and ratiometric [Ca2+]c photometry experiments were made on small longitudinal muscle strips with adherent circular muscle. CCCP (2 µM) induced a slow depolarization with a peak amplitude of 9 ± 1 mV (n= 11, P < 0.0001). Seven of these longitudinal strips were from animals in oestrus with the peak CCCP-induced depolarization reaching 11 ± 1 mV, but this was not sustained and Vm returned towards the resting value within ∼10 min (Figure 4A). Also in longitudinal strips from mice in oestrus, [Ca2+]c remained elevated during the presence of CCCP (Figure 4B, n= 7). The Vm response to CCCP in longitudinal strips from four di-oestrus mice was more complex, with an initial depolarization followed by hyperpolarization of 4 ± 1 mV and then depolarization that stabilized at 7 ± 2 mV from resting (P= 0.018; Figure 4C). Washout of CCCP from these tissues caused a prompt and striking hyperpolarization with a very slow recovery that delayed resumption of spontaneous activity for 15–20 min. In separate experiments CCCP when applied to longitudinal strips from mice in di-oestrus transiently increased [Ca2+]c but this was not sustained (Figures 4D, n= 3). In general, CCCP increased or induced contraction initially, but contractility generally ceased within 5 min. Brief application of high K+ solutions in the presence of CCCP caused a transient increase in [Ca2+]c and contraction, although the latter was weaker than K+ contractions recorded in the control period (i.e. 68 ± 8% of control; n= 6). CCCP also reduced the amplitude of the action potential by 76 ± 5% mV (n= 5).
Figure 4.
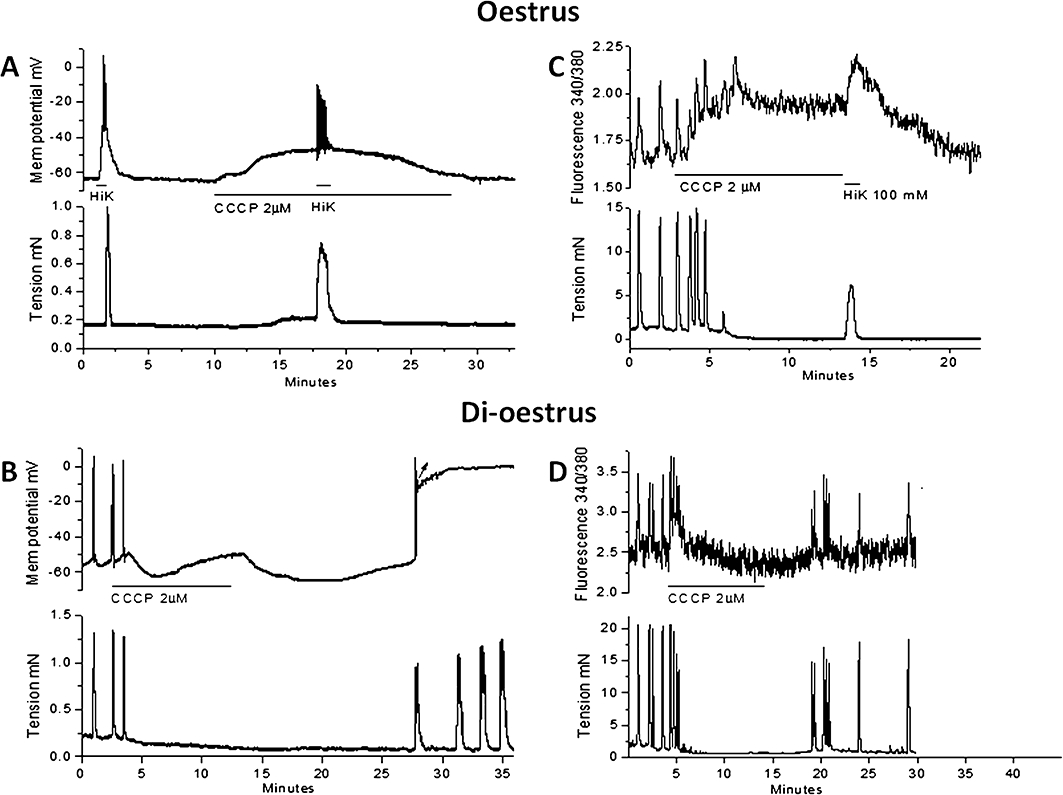
The effect of CCCP on Vm and relative [Ca2+]c in uterine longitudinal smooth muscle from oestrus and di-oestrus mice. (A) In an oestrus tissue, CCCP (2 µM) caused depolarization and transient generation of action potentials with Vm gradually returning to near control levels. Brief application of high K+ (HiK) solution after ∼8 min CCCP caused transient depolarization and contraction. (B) In an oestrus tissue, CCCP (2 µM) increased relative [Ca2+]c that persisted for the duration of CCCP application and could be further increased by high K+ solution. (C) In a di-oestrus tissue, CCCP (2 µM) caused depolarization and action potential activity continuing until the onset of a transient hyperpolarization. This was followed by depolarization but now without action potentials. (D) In a di-oestrus tissue, CCCP (2 µM) caused a transient increase in relative [Ca2+]c that rapidly returned to control levels during CCCP application. [Ca2+]c, cytosolic calcium; CCCP, carbonylcyanide-3-chlorophenylhydrazone; Vm, membrane potential.
In three of the longitudinal strips in oestrus, all circular muscle was removed and the 8 ± 2 mV depolarization evoked by CCCP was not accompanied by contraction due to these strips exhibiting more negative resting Vms. However, substantial contraction could still be evoked by brief depolarization with high K+ PSS (100 mM K+, isosmotic Na+ substitution, data not shown). Studies were also made on longitudinal muscle strips (with adherent circular muscle) that were quiescent but exhibited spontaneous activity when 0.5 nM oxytocin was included in the superfusate. This depolarized the smooth muscle cells to threshold for the initiation of bursts of action potentials and contractions. CCCP (2 µM) applied in the maintained presence of the oxytocin caused hyperpolarization to 3 ± 2 mV negative of the pre-oxytocin resting Vm (n= 4).
Effect of rotenone, CGP37157 and kaempferol on Vm and [Ca2+]c
Experiments were performed to characterize the actions of rotenone, CGP37157 and kaempferol on Vm and [Ca2+]c. The experiments differed in that oestrus and di-oestrus longitudinal smooth muscle data were pooled, as they generally responded to these specific drugs in a similar manner and were not significantly different (at least for the sample size we used). Rotenone (1 µM), unlike CCCP, did not induce a transient depolarization but evoked hyperpolarization of 9 ± 3 mV (n= 5; Figure 5A), which was accompanied by a sustained increase in [Ca2+]c of 161 ± 5% of control (n= 3; Figure 5B) and an inhibition of contractions. CGP37157 induced hyperpolarization of 4 ± 1 mV that was accompanied by a cessation of action potential and contractile activity (n= 5; Figure 5C). CGP37157 caused a decrease in action potential amplitude to 56 ± 5% of control, as measured just before cessation of action potentials due to the hyperpolarization (n= 3). Kaempferol caused 5 ± 1 mV hyperpolarization, cessation of action potentials and contractions (n= 4; Figure 5E), but had no effect on action potential amplitude (98 ± 3% of control; n= 3). CGP37157 or kaempferol did not significantly change [Ca2+]c or the response to high K+ solution (Figures 5D,F, n= 4 for both).
Figure 5.
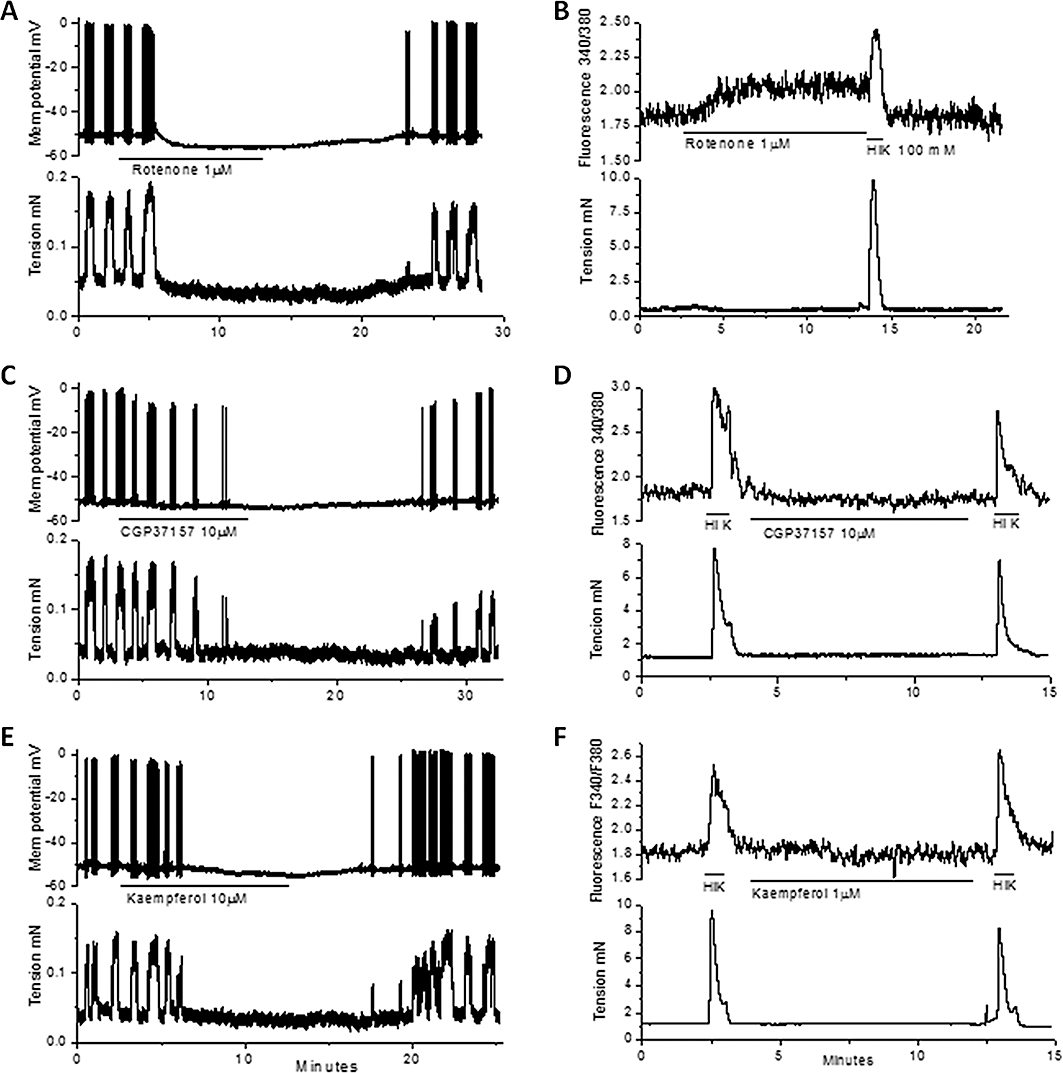
The effects of rotenone, CGP37157 and kaempferol on Vm and relative [Ca2+]c on uterine longitudinal smooth muscle. (A, C, E) rotenone (1 µM), CGP37157 (10 µM) and kaempferol (10 µM) all caused hyperpolarization and cessation of action potentials. (B, D, F) rotenone (1 µM) caused a sustained increase in [Ca2+]c while CGP37157 (10 µM) or kaempferol (10 µM) were without effect. A high K+-containing (HiK) solution was transiently applied to the tissues in these experiments. [Ca2+]c, cytosolic calcium; CGP37157, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one; Vm, membrane potential.
Effects of SER agents on Vm and tension
The effects that CPA and caffeine had on Vm and [Ca2+]c in the longitudinal smooth muscle of small strip preparations were investigated to allow comparison with the mitochondrial drug actions. CPA (10 µM) caused substantial depolarization of 22 ± 3 mV which induced the firing of action potentials and contraction (n= 7; Figure 6A). The depolarization was significantly reduced to 4 ± 1 mV (n= 3) when voltage-gated L-type Ca2+ channels (i.e. CaV1.x) were blocked with nifedipine (3 µM). This resulted in a marked reduction in the amplitude of contraction and rendered it transient, returning to resting tension within 3 min despite the continued presence of CPA (data not shown). Application of 2-APB (10 µM), induced rapid hyperpolarization of 6 ± 1 mV (n= 4). Caffeine 0.3 mM had no measurable effect on Vm (n= 3), but increasing the concentration to 1 mM caused hyperpolarization of 6 ± 2 mV and reversible cessation of action potentials and contraction (n= 5; Figure 6B).
Figure 6.
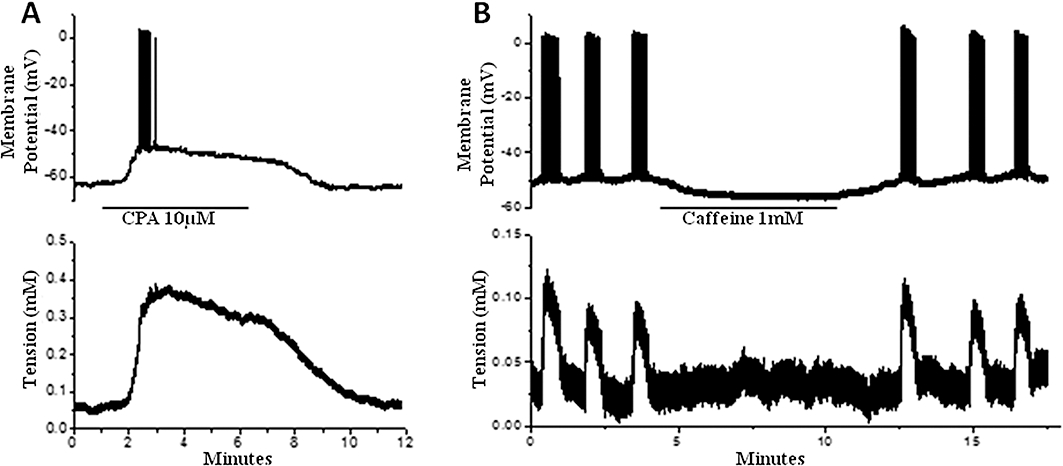
The effect of CPA and caffeine on uterine longitudinal smooth muscle. (A) CPA (10 µM) caused depolarization and maintained contraction. Action potentials were initially induced but this rapidly waned during the maintained depolarization. (B) Caffeine caused hyperpolarization and cessation of spontaneous action potentials and associated contractions. CPA, cyclopiazonic acid.
Interactions between SER and mitochondria
To evaluate possible interactions between the SER and mitochondria, the SERCA store inhibitor CPA was applied to large tissue preparations 15 min before addition of the mitochondrial inhibitors CCCP, rotenone, CGP37157 or kaempferol with the mixture applied for the following 30 min. CPA (10 µM) by itself did not decrease the amplitude of contractions; however, contraction amplitude was significantly decreased by addition of CCCP (2 µM, n= 5), rotenone (1 µM, n= 8), CGP37157 (10 µM, n= 7) but not kaempferol (10 µM, n= 7) (Figure 7A). This parallels the responses to these mitochondrial agents without CPA with the exception of kaempferol, which significantly decreased contraction amplitude when applied alone (Figure 2). Measurement of Vm in small strip preparations showed that CCCP (2 µM) in the presence of CPA (10 µM) induced further depolarization of the longitudinal muscle but now only partially inhibited action potential firing and resultant transient contractions (Figure 7B). Action potential firing recommenced when high K+ solution was added, despite the continued presence of CPA and CCCP.
Figure 7.
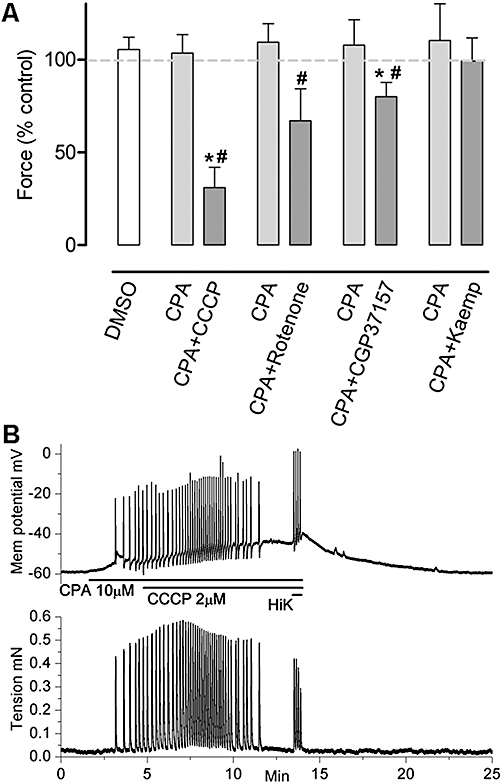
The effects of CPA and subsequent addition of specific mitochondrial drugs on the force of spontaneous contractions in uterine longitudinal smooth muscle. (A) While CPA (10 µM, n= 5–8) alone did not decrease the force of contractions, contraction amplitude was significantly decreased by addition of CCCP (2 µM, n= 5), rotenone (1 µM, n= 8), CGP37157 (10 µM, n= 7) but not kaempferol (Kaemp; 10 µM, n= 7). Columns represent the mean ± SEM. *Significantly different from control, #significantly different from CPA, Student's two-tailed paired t-test, P < 0.05. Dashed trace represents 100% control. (B) Depolarization and firing of action potentials and associated transient contractions caused by CPA (10 µM) was not obviously altered by addition of CCCP (2 µM). Action potential activity ceased after ∼10 min but was transiently re-initiated by brief application of high K+ solution. CCCP, carbonylcyanide-3-chlorophenylhydrazone; CGP37157, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one; CPA, cyclopiazonic acid; DMSO, dimethyl sulphoxide.
The effects of combining SER and mitochondrial drugs on contraction frequency are presented in Table 2. In the continued presence of 10 µM CPA (15 min), addition of 2 µM CCCP, 1 µM rotenone, 10 µM CGP37157 or 10 µM kaempferol for an additional 30 min now failed to significantly reduce contraction frequency to below control levels (Table 2). This is to be compared with the significant decreases caused by the same concentrations of rotenone, CGP37157 or kaempferol when applied alone (Table 1).
Table 2.
Contraction frequency
| Drugs | Control (contract per 5 min) | CPA 10–15 min | CPA + Mito drug 0–5 min | CPA + Mito drug 25–30 min | n |
|---|---|---|---|---|---|
| CPA + CCCP | 6.0 ± 1.5 | 10.8 ± 1.9a | 10.0 ± 2.2 | 8.6 ± 2.4 | 5 |
| CPA + rotenone | 6.4 ± 1.2 | 9.6 ± 0.8a | 7.6 ± 1.2 | 5.4 ± 2.2 | 5 |
| CPA + CGP37157 | 6.8 ± 1.2 | 10.2 ± 1.0a | 9.2 ± 1.1a | 6.0 ± 1.5 | 5 |
| CPA + kaempferol | 7.8 ± 1.8 | 10.1 ± 1.1 | 8.4 ± 1.0 | 6.8 ± 1.0 | 5 |
Values are mean ± SEM.
Significantly different from control, P < 0.05 (Student's two-tailed paired t-test).
CCCP, carbonylcyanide-3-chlorophenylhydrazone; CGP37157, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one; CPA, cyclopiazonic acid; Mito, mitochondrial.
Effects of K+ channel blockers on responses to mitochondrial agents
The possibility that K+ channels were involved in the CCCP, rotenone, CGP37157 and kaempferol responses was investigated using agents that blocked specific K+ channels. A combination of 50 nM IbTX, an inhibitor of large-conductance Ca2+-activated K+ channels (Maxi KCa), 100 nM CTX, an inhibitor of Maxi KCa and the intermediate-conductance Ca2+-activated K+ channel (IKCa), and 100 nM Apa, an inhibitor of the small-conductance Ca2+-activated K+ channel (SKCa), added together 5 min before addition of CCCP (2 µM), partially reversed the CCCP-induced inhibition of contractile force (n= 5, P < 0.05) (Figure 8A,B). Consistent with this, the non-selective K+ channel inhibitor TEA (15 mM) also reduced the inhibition of contractile force by 2 µM CCCP (n= 7, P < 0.05; Figure 8A,B). However, a contributing factor to TEA action could be a direct effect of TEA to enhance contractions (Figure 8Bb). Application of 4-AP (1 mM; n= 4; Figure 8A), a blocker of voltage-dependent K+ channels or glibenclamide (10 µM; n= 3; data not shown), a blocker of KATP channels (ATP-activated K+ channels), did not significantly alter the CCCP-induced inhibition of contractile force, indicating that these K+ channels do not play a substantial role in the CCCP-induced actions. The inhibitory effect of CGP37157 (10 µM) on contractile force was significantly counteracted by combined application of 50 nM IbTX, 100 nM CTX and 100 nM Apa or 15 mM TEA (Figure 8A,D). In contrast, these agents did not significantly counteract the inhibition caused by rotenone (1 µM) or kaempferol (10 µM), at least for the sample size used (Figure 8A,C,E).
Figure 8.
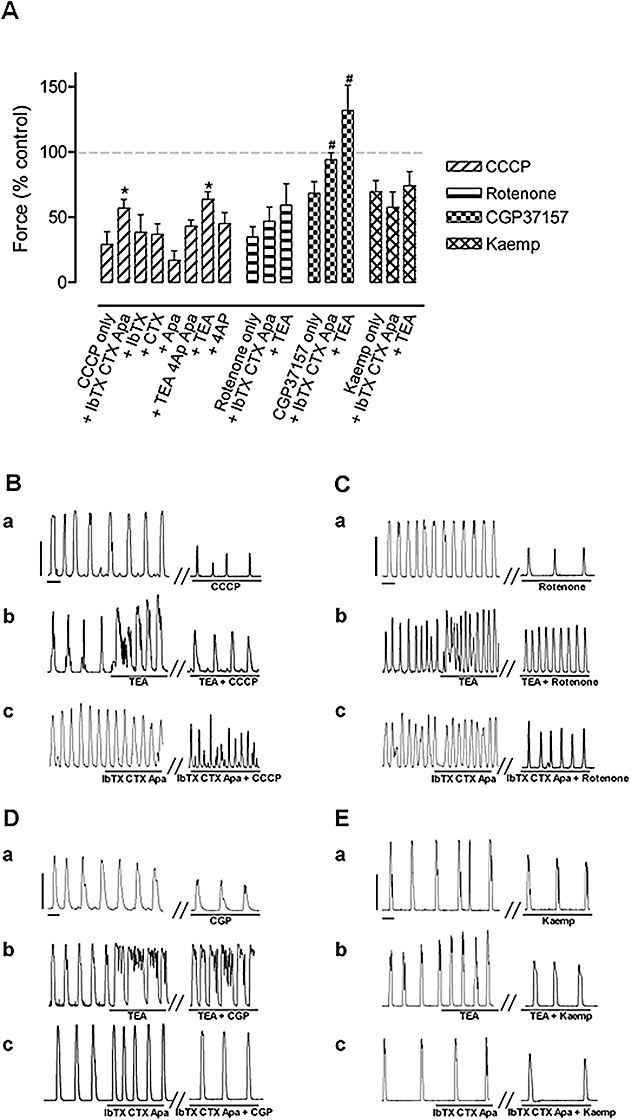
The effect of K+ channel blockers on CCCP, rotenone, CGP37157 and kaempferol-induced inhibition of contractions. (A) The effects of mitochondrial agents alone and in the presence various K+ channel blockers on the force of spontaneous uterine contractions. The effects of CCCP and CGP37157 on contractile force were significantly counteracted by the combined application of IbTX, CTX and apamin (Apa) or TEA, whereas the effects of rotenone and kaempferol (Kaemp) were not. Concentrations used: CCCP, 2 µM; IbTX, 50 nM; CTX, 100 nM; apamin, 100 nM; TEA, 15 mM; 4-AP, 1 mM; rotenone, 1 µM; CGP37157, 10 µM; kaempferol, 10 µM. Columns are the mean ± SEM. (B–E) Representative traces showing the effect of CCCP (B), rotenone (C), CGP37157 (D) and kaempferol (E) alone (a), together with 15 mM TEA (b) or 50 nM IbTX, 100 nM CTX and 100 nM apamin (c). The vertical and horizontal scale bars (B–E) correspond to 10 mN and 1 min, respectively, and apply to all panels. Student's two-tailed unpaired t-test was applied with *significant difference (P < 0.05) from the response to CCCP; n = 4–7 and #significant difference (P < 0.05) from the response to CGP37157. 4-AP, 4-aminopyridine; CCCP, carbonylcyanide-3-chlorophenylhydrazone; CGP37157, 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one; CTX, charybdotoxin; IbTX, iberiotoxin; TEA, tetraethylammonium.
In electrophysiological studies of strips from oestrus mice, TEA (15 mM) caused depolarization of 8 ± 3 mV (n= 9), and this was associated with an increase in action potential activity and an increase in [Ca2+]c. CCCP (2 µM) abolished this activity but spontaneous activity resumed within 5–10 min following washout of CCCP (Figure 9). The hyperpolarization that occurred in di-oestrus strips in the presence of CCCP and following washout of CCCP was prevented by TEA (compare Figure 4C with Figure 9A).
Figure 9.
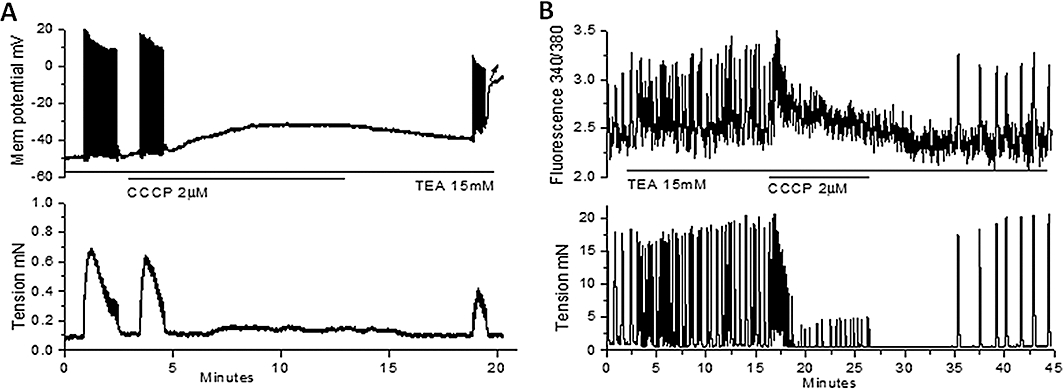
The effect of CCCP in the presence of TEA on Vm and relative [Ca2+]c in uterine longitudinal smooth muscle from di-oestrus mice. (A) CCCP (2 µM) applied in the presence of TEA (15 mM) caused depolarization (without the transient hyperpolarization observed previously in control; Figure 4C) and cessation of action potentials and associated contractions. (B) CCCP (2 µM) applied in the presence of TEA (15 mM) caused a transient increase in relative [Ca2+]c, which declined to near control levels during the 10 min application of CCCP. [Ca2+]c, cytosolic calcium; CCCP, carbonylcyanide-3-chlorophenylhydrazone; TEA, tetraethylammonium; Vm, membrane potential.
Discussion and conclusions
This study has investigated the actions of drugs that target mitochondria and SER Ca2+ stores on spontaneous contractions in uterine smooth muscle from non-pregnant mice in various phases of the oestrous cycle.
The effect of mitochondrial disruption on uterine longitudinal smooth muscle
The effects of the mitochondrial targeting agents CCCP, rotenone, CGP37157 and kaempferol indicate that mitochondria play a role in modulating uterine contractions. The F1/Fo ATP synthase inhibitor oligomycin had no effect over a wide concentration range for at least 30 min, a time over which CCCP, rotenone and CGP37157 markedly reduced contraction amplitude. This indicates that these actions did not occur through lack of ATP within the uterine cells and is consistent with the view that mitochondria can modulate [Ca2+]c without compromising ATP production (Chalmers and McCarron, 2008). Thus, the finding that the inhibition of contractile force was not due to ATP depletion suggests that mitochondrial Ca2+ handling has a role in determining uterine contractility. Mitochondrial Ca2+ has been shown to be essential for maintaining motility and tone in other smooth muscle driven organs, including gastrointestinal tract, gallbladder and prostate gland (Ward et al., 2000; Balemba et al., 2008; Chalmers and McCarron, 2008; Jawien et al., 2008; Exintaris et al., 2009).
The putative role of mitochondria was investigated in most detail using the mitochondrial inhibitor CCCP. Abolishing mitochondrial function, as achieved by CCCP, would be expected to increase [Ca2+]c upon release of the mitochondrial Ca2+ store. In this regard, CCCP induced a transient increase in the frequency of contractions. Studies on small strips indicated that this initial enhancement correlated with a transient depolarization and an increase in [Ca2+]c. Interestingly, CCCP caused different responses in these strips dependent on whether the uterine tissues were from oestrus or di-oestrus mice, with depolarization and a maintained increase in [Ca2+]c occurring in oestrus tissues, and depolarization with a transient hyperpolarization and transient increase in [Ca2+]c occurring in di-oestrus tissues. Some tissues were quiescent but depolarized and exhibited contractile activity when oxytocin was added. Subsequent addition of CCCP abolished this activity and caused a net hyperpolarization. CCCP decreased the amplitude of spontaneous and K+ contractions to ∼30% and 70% of control respectively. The fact that spontaneous contractions were more affected than the K+ contraction could relate to other actions of CCCP including a reduction in the amplitude of action potentials that generate each spontaneous contraction.
Inhibition of the mitochondrial respiratory chain by rotenone, mitochondrial Ca2+ release by CGP37157 or activation of mitochondrial Ca2+ influx by kaempferol all decreased the force and frequency of spontaneous contractions and caused membrane hyperpolarization. The reason for the decrease in contraction frequency might relate to the hyperpolarization caused by these agents, as a larger voltage excursion would now be necessary to reach threshold this possibly slowing the pacemaker rate. The hyperpolarizing influence could also reduce the number of action potentials recruited once pacemaker threshold was reached this reducing the amplitude of contractions. In contrast, CCCP caused both depolarization and hyperpolarization, with the longer-term action to leave Vm slightly depolarized, this possibly explaining the near normal pacemaker rate in CCCP.
Carbonylcyanide-3-chlorophenylhydrazone (for tissues from mice in oestrus) or rotenone, but not CGP37157 or kaempferol, caused a sustained increase in [Ca2+]c. Surprisingly, mitochondrial disruption by CCCP or rotenone did not cause a basal increase in tension, whereas inhibition of SER function by CPA did. CCCP generated depolarization of ∼10 mV, which could open voltage-dependent Ca2+ channels leading to a sustained increase in [Ca2+]c. However, such channels could not have been opened for rotenone, which caused hyperpolarization of ∼9 mV. Therefore, disruption of mitochondrial function by these agents either causes maintained release of mitochondrial Ca2+ and/or activates Ca2+ entry that, at least in the case of rotenone, is independent of voltage-dependent Ca2+ channels. Should the latter prove true, then it would indicate that mitochondrial inhibition influences membrane channels in a manner that has parallels to SER store-operated Ca2+ entry.
Effect of SER inhibitors on uterine contractions
2-APB, an IP3R/SOCE inhibitor (Bootman et al., 2002), has been reported to decrease the force of rat uterine contractions (Ascher-Landsberg et al., 1999). We found it had this action on mouse uterine contractile force but it also decreased the frequency of contraction probably due to the 2-APB-induced hyperpolarization.
Caffeine at low concentration (0.3 mM) decreased the force of contractions without significantly altering contraction frequency. Higher concentrations (e.g. 1 mM) abolished action potentials and resultant contractions, an action most likely due to the membrane hyperpolarization caused by 1 mM caffeine. These actions were unlikely to be due to ryanodine receptors, as significant activation of these receptors by caffeine requires higher concentrations (e.g. 10 mM) (Herrmann-Frank et al., 1999). In this regard, studies by others indicate that ryanodine receptors do not have a significant role in uterine contractions (Ashoori et al., 1985; Wray et al., 2003; Matthew et al., 2004a,b;). Whether the caffeine-induced inhibition was due to blockade of IP3Rs (Berridge, 1991) is unclear, as inhibition could also result through phosphodiesterase inhibition to increase cAMP (Beavo and Reifsnyder, 1990) or other actions such as a possible inhibitory effect of caffeine on Ca2+ currents, as has been demonstrated in intestinal smooth muscle (Zholos et al., 1991).
Cyclopiazonic acid is widely used to disable SER Ca2+ stores by inhibiting the SERCA and hence refilling of the SER Ca2+ store (Kosterin et al., 1996; Lang et al., 2007; Taggart and Wray, 1998). We found that CPA caused depolarization, increased the frequency of contractions, increased [Ca2+]c and the resting tension in uterine tissues. This generally fits with findings of others in various species (Coleman et al., 2000; Wray et al., 2001; 2003; Kupittayanant et al., 2002; Matthew et al., 2004b) including a study on mouse uterus (Matthew et al., 2004a). Importantly, the increase in resting tension was generated primarily by Ca2+ entry. This occurred through voltage-dependent Ca2+ channels, as inhibition of these channels with nifedipine markedly reduced the CPA-induced depolarization and increase in resting tension, the latter returning to near baseline within 3 min despite the continued presence of CPA.
Effect of mitochondrial inhibitors when Ca2+ stores were disabled by CPA
Inhibition of SER Ca2+ store function by CPA abolished the inhibition of contractile force by kaempferol but not that in response to CCCP, rotenone or CGP37157. CPA also prevented the decrease in pacemaker frequency caused by rotenone, CGP37157 and kaempferol. This may relate to CPA having a depolarizing action that counteracts the hyperpolarization caused by rotenone, CGP37157 and kaempferol. This would reduce the voltage excursion necessary for the pacemaker depolarization to reach threshold and hence increase contraction frequency. CPA action to set Vm to more positive levels might also explain the observation that action potential firing was less inhibited by CCCP when CPA was present.
Role of K+ channels on the effects of the mitochondrial inhibitor CCCP
We found evidence that inhibitors of KCa channels were effective in restoring the CCCP- and CGP37157-induced inhibition in contraction force but not the inhibition induced by rotenone or kaempferol (at least for the sample size of our study). The finding for CCCP is in accordance with the observation that CCCP increased [Ca2+]c and is consistent with the finding that the CCCP-induced hyperpolarization in di-oestrus longitudinal smooth muscle was inhibited by TEA. In contrast, the KCa dependence of CGP37157 is surprising. CGP37157 should prevent mitochondrial Ca2+ release and hence should not increase [Ca2+]c and this was found to be the case. Therefore, it should not cause hyperpolarization or show KCa dependence. The fact that it does suggests that the disruption in mitochondrial Ca2+ homeostasis has other consequences such as a localized increase in [Ca2+]c in microdomains near KCa channels (Rizzuto et al., 1993; Zhuge et al., 2002), but this needs further investigation. The presence of KCa channels fits with other reports that these channels are important regulators of uterine relaxation (Brainard et al., 2007; Parkington and Coleman, 1988; Wray, 2007). However, the finding that interfering with mitochondrial function alters contraction frequency by activating K+ channels remains an extreme circumstance and may not be relevant to normal uterine pacemaking.
Conclusions
This study provides insight into the role of mitochondria in the regulation of uterine contractions. Interfering with mitochondrial function by a range of agents, such as CCCP, rotenone, CGP3715 and kaempferol caused activation of ionic currents, resulting in a change in Vm, a decrease in contraction amplitude and, in cases where there was hyperpolarization (i.e. rotenone, CGP3715, kaempferol), a decrease in pacemaker frequency. The findings indicate that mitochondrial Ca2+ stores are not directly involved in the pacemaker mechanism, as they are in the gastrointestinal tract (Ward et al., 2000). However, mitochondrial disruption will have a modulatory role on uterine pacemaking due to effects on ion channels and uptake/release of Ca2+.
Acknowledgments
We are grateful to Mr Peter Dosen for his expert technical assistance and to the National Health and Medical Research Council and Hunter Medical Research Institute for funding this research.
Glossary
Abbreviations
- [Ca2+]c
cytosolic calcium
- 2-APB
2-amino ethoxyphenylborate
- 4-AP
4-aminopyridine
- Apa
apamin
- CCCP
carbonylcyanide-3-chlorophenylhydrazone
- CGP37157
7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one
- CPA
cyclopiazonic acid
- CTX
charybdotoxin
- Maxi
KCa, large-conductance Ca2+-activated K+ channels
- IbTX
iberiotoxin
- ICC
interstitial cell of Cajal
- IP3R
inositol 1,4,5-trisphosphate receptor
- KATP
ATP-activated K+ channels
- MCU
mitochondrial Ca2+ uniporter
- NCX
Na+/Ca2+ exchanger
- SER
sarco-endoplasmic reticulum
- SERCA
sarco-endoplasmic reticulum Ca2+ ATPase
- SOCE
store-operated Ca2+ entry
- TEA
tetraethylammonium
Conflicts of interest
None to declare.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allix S, Reyes-Gomez E, Aubin-Houzelstein G, Noel D, Tiret L, Panthier JJ, et al. Uterine contractions depend on KIT-positive interstitial cells in the mouse: genetic and pharmacological evidence. Biol Reprod. 2008;79:510–517. doi: 10.1095/biolreprod.107.066373. [DOI] [PubMed] [Google Scholar]
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca(2+) to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- Ascher-Landsberg J, Saunders T, Elovitz M, Phillippe M. The effects of 2-aminoethoxydiphenyl borate, a novel inositol 1,4, 5-trisphosphate receptor modulator on myometrial contractions. Biochem Biophys Res Commun. 1999;264:979–982. doi: 10.1006/bbrc.1999.1602. [DOI] [PubMed] [Google Scholar]
- Ashoori F, Takai A, Tomita T. The response of non-pregnant rat myometrium to oxytocin in Ca-free solution. Br J Pharmacol. 1985;84:175–183. [PMC free article] [PubMed] [Google Scholar]
- Balemba OB, Bartoo AC, Nelson MT, Mawe GM. Role of mitochondria in spontaneous rhythmic activity and intracellular calcium waves in the guinea pig gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2008;294:G467–476. doi: 10.1152/ajpgi.00415.2007. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Caffeine inhibits inositol-trisphosphate-induced membrane potential oscillations in Xenopus oocytes. Proc Biol Sci. 1991;244:57–62. doi: 10.1098/rspb.1991.0051. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol. 2007;18:332–339. doi: 10.1016/j.semcdb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers S, McCarron JG. The mitochondrial membrane potential and Ca2+ oscillations in smooth muscle. J Cell Sci. 2008;121(Pt. 1):75–85. doi: 10.1242/jcs.014522. [DOI] [PubMed] [Google Scholar]
- Ciontea SM, Radu E, Regalia T, Ceafalan L, Cretoiu D, Gherghiceanu M, et al. C-kit immunopositive interstitial cells (Cajal-type) in human myometrium. J Cell Mol Med. 2005;9:407–420. doi: 10.1111/j.1582-4934.2005.tb00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HA, Hart JD, Tonta MA, Parkington HC. Changes in the mechanisms involved in uterine contractions during pregnancy in guinea-pigs. J Physiol. 2000;523(Pt. 3):785–798. doi: 10.1111/j.1469-7793.2000.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette RA, Shmygol A, Vaillant C, Mobasheri A, Pope M, Burdyga T, et al. Vimentin-positive, c-kit-negative interstitial cells in human and rat uterus: a role in pacemaking? Biol Reprod. 2005;72:276–283. doi: 10.1095/biolreprod.104.033506. [DOI] [PubMed] [Google Scholar]
- Exintaris B, Nguyen DT, Lam M, Lang RJ. Inositol trisphosphate-dependent Ca stores and mitochondria modulate slow wave activity arising from the smooth muscle cells of the guinea pig prostate gland. Br J Pharmacol. 2009;156:1098–1106. doi: 10.1111/j.1476-5381.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeger DE, Riley RT, Dorner JW, Cole RJ. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988;37:978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Hashitani H, van HeIden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol. 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann-Frank A, Luttgau HC, Stephenson DG. Caffeine and excitation-contraction coupling in skeletal muscle: a stimulating story. J Muscle Res Cell Motil. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- Ishii K, Hirose K, Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006;7:390–396. doi: 10.1038/sj.embor.7400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawien J, Bian Z, Sheikine Y, Olofsson PS, Pang Y, Edholm T, et al. Abrogation of mitochondrial transcription in smooth muscle cells impairs smooth muscle contractility and vascular tone. J Physiol Pharmacol. 2008;59:239–252. [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576(Pt. 3):653–658. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterin SA, Babich LG, Shlykov SG, Rovenets NA. [Mg2+,ATP-dependent transport of Ca2+ in the endoplasmic reticulum of myometrial cells] Biokhimiia. 1996;61:73–81. [PubMed] [Google Scholar]
- Kupittayanant S, Luckas MJ, Wray S. Effect of inhibiting the sarcoplasmic reticulum on spontaneous and oxytocin-induced contractions of human myometrium. BJOG. 2002;109:289–296. doi: 10.1111/j.1471-0528.2002.01110.x. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Nguyen DT, Matsuyama H, Takewaki T, Exintaris B. Characterization of spontaneous depolarizations in smooth muscle cells of the guinea pig prostate. J Urol. 2006;175:370–380. doi: 10.1016/S0022-5347(05)00003-0. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Hashitani H, Tonta MA, Suzuki H, Parkington HC. Role of Ca2+ entry and Ca2+ stores in atypical smooth muscle cell autorhythmicity in the mouse renal pelvis. Br J Pharmacol. 2007;152:1248–1259. doi: 10.1038/sj.bjp.0707535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LW, Thuneberg L, Huizinga JD. Cyclopiazonic acid, inhibiting the endoplasmic reticulum calcium pump, reduces the canine colonic pacemaker frequency. J Pharmacol Exp Ther. 1995;275:1058–1068. [PubMed] [Google Scholar]
- Matthew A, Kupittayanant S, Burdyga T, Wray S. Characterization of contractile activity and intracellular Ca2+ signalling in mouse myometrium. J Soc Gynecol Investig. 2004a;11:207–212. doi: 10.1016/j.jsgi.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Matthew A, Shmygol A, Wray S. Ca2+ entry, efflux and release in smooth muscle. Biol Res. 2004b;37:617–624. doi: 10.4067/s0716-97602004000400017. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973;233:127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkington HC, Coleman HA. Ionic mechanisms underlying action potentials in myometrium. Clin Exp Pharmacol Physiol. 1988;15:657–665. doi: 10.1111/j.1440-1681.1988.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Coleman HA. Excitability in uterine smooth muscle. Front Horm Res. 2001;27:179–200. doi: 10.1159/000061026. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Tonta MA, Davies NK, Brennecke SP, Coleman HA. Hyperpolarization and slowing of the rate of contraction in human uterus in pregnancy by prostaglandins E2 and f2alpha: involvement of the Na+ pump. J Physiol. 1999;514(Pt. 1):229–243. doi: 10.1111/j.1469-7793.1999.229af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP(3) in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol Cell Physiol. 2001;280:C1349–1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Bradley E, Thornbury KD, McHale NG, Hollywood MA. Role of mitochondria in modulation of spontaneous Ca2+ waves in freshly dispersed interstitial cells of Cajal from the rabbit urethra. J Physiol. 2008;586:4631–4642. doi: 10.1113/jphysiol.2008.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol AV, Eisner DA, Wray S. The role of the sarcoplasmic reticulum as a Ca2+ sink in rat uterine smooth muscle cells. J Physiol. 1999;520(Pt. 1):153–163. doi: 10.1111/j.1469-7793.1999.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Bianchi K, De Stefani D, Leo S, Wieckowski MR, et al. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Wray S. Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activation: gestational dependence in isolated rat uterus. J Physiol. 1998;511(Pt. 1):133–144. doi: 10.1111/j.1469-7793.1998.133bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525(Pt. 2):355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. The effects of metabolic inhibition on uterine metabolism and intracellular pH in the rat. J Physiol. 1990;423:411–423. doi: 10.1113/jphysiol.1990.sp018030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. Insights into the uterus. Exp Physiol. 2007;92:621–631. doi: 10.1113/expphysiol.2007.038125. [DOI] [PubMed] [Google Scholar]
- Wray S, Kupittayanant S, Shmygol A, Smith RD, Burdyga T. The physiological basis of uterine contractility: a short review. Exp Physiol. 2001;86:239–246. doi: 10.1113/eph8602114. [DOI] [PubMed] [Google Scholar]
- Wray S, Jones K, Kupittayanant S, Li Y, Matthew A, Monir-Bishty E, et al. Calcium signaling and uterine contractility. J Soc Gynecol Investig. 2003;10:252–264. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Young RC. Myocytes, myometrium, and uterine contractions. Ann N Y Acad Sci. 2007;1101:72–84. doi: 10.1196/annals.1389.038. [DOI] [PubMed] [Google Scholar]
- Zholos AV, Baidan LV, Shuba MF. The inhibitory action of caffeine on calcium currents in isolated intestinal smooth muscle cells. Pflugers Arch. 1991;419:267–273. doi: 10.1007/BF00371106. [DOI] [PubMed] [Google Scholar]
- Zhuge R, Fogarty KE, Tuft RA, Walsh JV., Jr Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca(2+) concentration on the order of 10 microM during a Ca(2+) spark. J Gen Physiol. 2002;120:15–27. doi: 10.1085/jgp.20028571. [DOI] [PMC free article] [PubMed] [Google Scholar]


