Abstract
Megalencephalic leucoencephalopathy with subcortical cysts (MLC) is a rare congenital leucodystrophy caused by mutations in MLC1, a membrane protein of unknown function. MLC1 expression in astrocyte end-feet contacting blood vessels and meninges, along with brain swelling, fluid cysts and myelin vacuolation observed in MLC patients, suggests a possible role for MLC1 in the regulation of fluid and ion homeostasis and cellular volume changes. To identify MLC1 direct interactors and dissect the molecular pathways in which MLC1 is involved, we used NH2-MLC1 domain as a bait to screen a human brain library in a yeast two-hybrid assay. We identified the β1 subunit of the Na,K-ATPase pump as one of the interacting clones and confirmed it by pull-downs, co-fractionation assays and immunofluorescence stainings in human and rat astrocytes in vitro and in brain tissue. By performing ouabain-affinity chromatography on astrocyte and brain extracts, we isolated MLC1 and the whole Na,K-ATPase enzyme in a multiprotein complex that included Kir4.1, syntrophin and dystrobrevin. Because Na,K-ATPase is involved in intracellular osmotic control and volume regulation, we investigated the effect of hypo-osmotic stress on MLC1/Na,K-ATPase relationship in astrocytes. We found that hypo-osmotic conditions increased MLC1 membrane expression and favoured MLC1/Na,K-ATPase-β1 association. Moreover, hypo-osmosis induced astrocyte swelling and the reversible formation of endosome-derived vacuoles, where the two proteins co-localized. These data suggest that through its interaction with Na,K-ATPase, MLC1 is involved in the control of intracellular osmotic conditions and volume regulation in astrocytes, opening new perspectives for understanding the pathological mechanisms of MLC disease.
INTRODUCTION
Megalencephalic leucoencephalopathy with subcortical cysts (MLC, OMIM 604004) is a rare inherited, autosomal recessive form of spongiform leucodystrophy affecting children (1). MLC patients manifest macrocephaly, motor function deterioration, ataxia, spasticity, epileptic seizures and variable levels of mental impairment. The disease is also characterized by a slowly progressive clinical course that can be worsened by minor stress, particularly minor head trauma and common viral infections (1,2). White matter swelling and the presence of subcortical cysts in the fronto-parietal and temporal regions are the two main features characterizing MLC disease by magnetic resonance imaging (2). In brain biopsies of MLC patients, the white matter was vacuolated and liquid vacuoles were localized between the outer lamellae of the myelin sheaths, probably generated by their splitting along the intraperiod line or incomplete compaction (2). Alterations in blood–brain barrier structure and astrogliosis have also been reported (2,3). To date, the pathogenetic mechanisms underlying MLC disease are still unknown and no therapy is available for patients. The locus of the disease gene was mapped at the very end of the long arm of chromosome 22 and named MLC1 (4). Different types of pathological mutations, mostly missense mutations, have been found in this gene without any clear correlation with the severity of the phenotype (2,5). Furthermore, the involvement of a second MLC-associated gene has been hypothesized (6–8).
The MLC1 gene encodes a protein of still unknown function, containing eight putative transmembrane domains and short amino and carboxylic cytoplasmic domains. MLC1 proteic sequence shows the presence of an internal amino-acidic repeat that is also found in several ion channel proteins (2,9) but, with the exception of a low homology with a potassium Kv1.1 channel subunit, it does not show any similarities with known proteins. In the human brain, MLC1 protein is highly expressed in distal astrocytic processes in perivascular and subpial regions, in ependymal cells lining the ventricles and in Bergmann glia in the cerebellum (9–11). These observations led to hypothesize that brain damage in MLC patients might be generated by abnormalities in astrocyte function. In the mouse, expression of MLC1 in some neuronal populations of the central and peripheral nervous systems has been reported (9).
Altogether, the pathological alterations observed in MLC patients, the localization of MLC1 in astrocyte end-feet contacting brain barriers and its molecular structure suggest a role for MLC1 in astrocyte-mediated processes regulating fluid homeostasis and transport of ions or other substances between the central nervous system (CNS) tissue and the blood or cerebrospinal fluid. Astrocytes are the most abundant glial cell population of the CNS and play an essential role in the maintenance of neural tissue homeostasis through the regulation of ions, neurotransmitters and metabolites in the extracellular space (12). Recent findings from our and other groups indicate that MLC1 interacts with components of the dystrophin–glycoprotein-associated complex (DGC) (11,13,14). This multiprotein complex is expressed in brain astrocytes (15), where it is involved in electrolyte balance and water movement by targeting and stabilizing the inwardly rectifying K+ (Kir) channel Kir4.1 and the water channel aquaporin-4 (AQP4) at the astrocytic end-foot domains (16,17). Although it is conceivable that via association with DGC MLC1 might take part in water and ion homeostasis in the brain, no evidence supporting this hypothesis is available yet.
To shed light on MLC1 function in the brain, we searched for MLC1 intracellular interactors. By using the two-hybrid system assay, we identified the β1 subunit of the Na,K-ATPase pump, the ubiquitous enzyme responsible for the maintenance of the Na+ and K+ gradients across the plasma membrane, as an MLC1-interacting clone. Using biochemical assays and immunostainings, we further characterized the interaction between MLC1 and Na,K-ATPase β1 subunit in cultured astrocytes and whole-brain tissue. We found that, by binding to Na,K-ATPase, MLC1 is part of a multiprotein complex that includes Kir4.1 and the DGC proteins syntrophin and dystrobrevin and is functionally involved in the regulation of cell volume changes occurring after osmotic imbalance. These findings show for the first time the direct association of MLC1 with a known protein involved in intracellular osmotic control and volume regulation, thus providing new insights into potential mechanisms underlying MLC pathogenesis.
RESULTS
Yeast two-hybrid screening of a human fetal cDNA library identified the Na,K-ATPase β1 subunit as MLC1-interacting partner
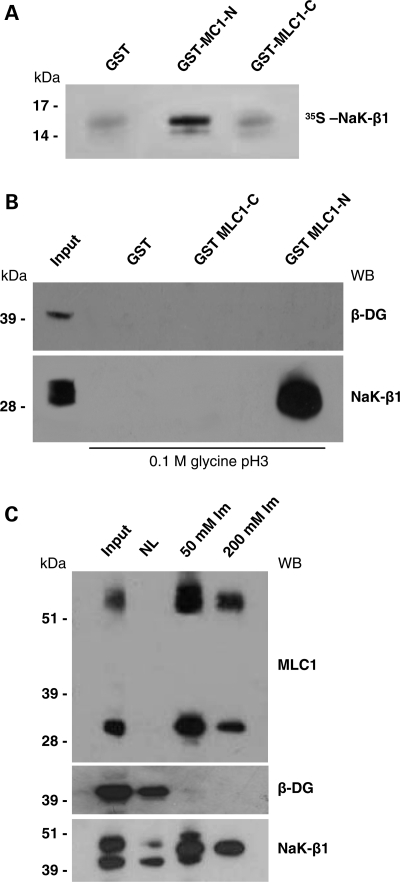
In order to isolate intracellular MLC1-interacting proteins in the human brain, we performed a yeast two-hybrid screening of the human fetal brain cDNA library using the MLC1-NH2 domain (from amino acid M1 to amino acid Y55 of the protein sequence) as bait. We used a yeast-mating strategy with a high level of stringency to isolate proteins that interact strongly with this bait. The human MLC1-NH2-domain-coding sequence obtained by RT–PCR from a human brain extract was fused in-frame to the DNA-binding domain of Gal4 (pGBKT7), transformed into yeast strain AH109 and used to screen a human fetal brain cDNA library subcloned into the Gal4 activation domain vector pGADT7-rec and pre-transformed into yeast strain Y187. Approximately 2 × 106 independent clones were screened for the expression of the reporter genes HIS3, ADE2 and Mel1/lacZ, and a total of 350 positive clones were found. Because these clones could contain more than one AD/library plasmid, we allowed segregation and recollected them on SD/-TLHA/X-α-Gal plates. We selected 144 diploids strongly activating Mel1/lacZ reporter genes, isolated their plasmid DNA and sorted them using PCR followed by restriction analysis. We rescued AD/library plasmids via transformation of Escherichia coli, obtaining 27 different types of insert. End sequencing and BLAST searching revealed that one of them encoded for the C-terminal half (residues 156–303) of the Na,K-ATPase β1 subunit isoform A (NaK-β1) (GenBank Accession no. NM_001677). The specificity of the direct interaction between MLC1 and NaK-β1 polypeptides was confirmed by glutathione-S-transferase (GST) pull-down experiments performed with recombinant GST-MLC1 N-terminal domain (GST-MLC1-NH2) and 35S-labelled NaK-β1 C-terminal domain (NaK-β1-COOH). In this experiment, we found that NaK-β1-COOH specifically interacted with the GST-MLC1-NH2 protein, although no interaction with GST-MLC1-COOH protein (residues 322–377) or with GST protein, used as negative control, was detected (Fig. 1A).
Figure 1.
MLC1-N terminal domain interacts with Na,K-ATPase β1 subunit (NaK-β1). (A) Purified GST-MLC1-NH2 terminal (GST-MLC1-N, amino acids 1–55), GST-MLC1-COOH terminal (GST-MLC1-C, amino acids 322–377) or glutathione-S-transferase (GST), pre-bound to glutathione-Sepharose beads, was incubated with in vitro-translated [35S] NaK-β1 C-terminal domain (amino acids 156–303). After extensive washings, bound radioactive proteins were separated by SDS–PAGE and detected by autoradiography. NaK-β1 C-terminal domain binds specifically to MLC1-N terminal domain. (B) Western blot (WB) analysis of proteins from primary cultures of rat astrocytes (Input) pulled down by agarose-bound GST-MLC1-N, GST-MLC1-C and GST, eluted with 0.1m glycine, pH 3, and revealed with an antibody against NaK-β1. NaK-β1 interacts with GST-MLC1-N-terminal but not with GST-MLC1-C-terminal and control GST alone. β-Dystroglycan (β-DG) is not detected among the pulled-down proteins. (C) Histidine (His)-tagged MLC1 protein co-fractionates with NaK-β1. WB analysis of His-MLC1-interacting proteins after elution from Ni-NTA agarose with 50 and 200 mm imidazole (Im). NaK-β1, but not β-DG, co-elutes with the 36 and 60 kDa MLC1 components. One representative experiment out of three is shown.
Interaction between MLC1 and NaK-β1 in in vivo systems
To verify whether MLC1-NH2 bound NaK-β1 in in vivo systems, we performed a pull-down assay using GST-MLC1-NH2 recombinant protein and protein extracts of rat primary astrocytes, which can be obtained in large numbers and with a high degree of purity from the newborn rat brain. As shown in Figure 1B, we observed a specific interaction between MLC1-NH2 domain and the endogenous astrocyte-derived NaK-β1. Neither GST-MLC1-COOH nor GST proteins interacted with NaK-β1. Since both our in-house-developed and the commercially available anti-MLC1 pAbs failed to immunoprecipitate MLC1, to further validate the observed interaction and verify whether the human full-length MLC1 protein bound NaK-β1 as well, we performed a co-purification assay that we set up previously to study MLC1 association with DGC proteins (14). A human astrocytoma cell line (U251) over-expressing histidine (His)-tagged MLC1 and the NiNTA-agarose resin specific for the binding of His tag were used to enrich His-MLC1 protein and its interactors from astrocyte protein extracts as described previously (11,14). Western blot (WB) of the samples obtained from NiNTA-agarose purification using His-MLC1-U251 cell protein extracts indicated that both the main MLC1 components expressed in astrocytes, the 60 and 36 kDa proteins (9,11), were enriched in the cellular extract eluted from His affinity column (Fig. 1C). We found that His-MLC1 co-purified with NaK-β1 but not with β-dystroglycan (β-DG), a protein previously shown not to interact directly with MLC1 (14) and therefore used as negative control (Fig. 1C). This experiment confirmed and extended the results of the two-hybrid system screening, indicating that the full-length MLC1 protein interacts with the β1 subunit of the Na,K-ATPase enzyme in a human cellular system.
Isolation of Na,K-ATPase and MLC1 in a multiprotein complex from the rat brain
The NaK-β1 subunit interacts with the catalytic α subunit to form the functional Na/K pump responsible for the maintenance of an electrochemical gradient across the cellular membrane. The Na,K-ATPase α subunit contains the binding site for ATP and an extracellular binding site of cardiac glycosides as ouabain (18). By exploiting the specificity and selectivity of the binding to the α catalytic subunit of the inhibitor ouabain, we set up an affinity chromatography procedure that was used previously to efficiently isolate the whole active Na,K-ATPase enzymatic complex and its interactors from different cell types (19,20). The use of ouabain affinity chromatography on rat cerebellum protein extracts has recently allowed the identification of a macromolecular complex where Na,K-ATPase interacts with the GLAST and GLT-1 glutamate transporters, thus acting as a functional unit to regulate glutamatergic neurotransmission (20).
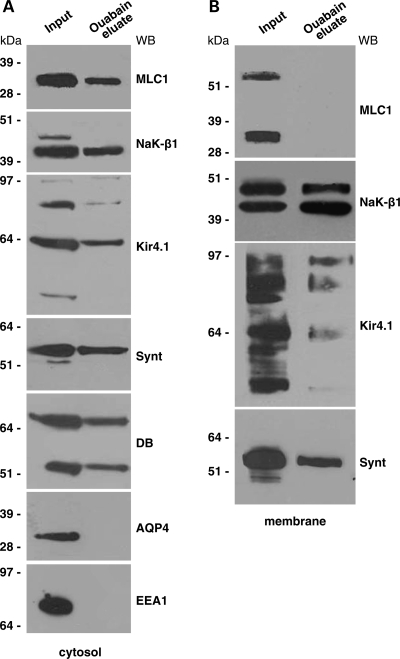
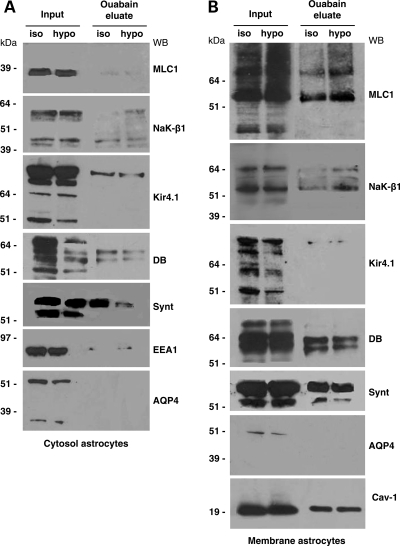
To ascertain whether, in our experimental systems, MLC1 interacted with the whole functional Na,K-ATPase enzymatic complex, the cytosolic and membrane protein fractions obtained from rat brain extracts were purified on ouabain affinity chromatography and, after elution, the proteins associated with Na,K-ATPase were revealed by WB. We found that in the brain cytosolic fraction, the 36 kDa MLC1 component was specifically eluted along with NaK-β1 by K+ and a high concentration of ouabain from the ouabain column (Fig. 2A). Since we previously reported that MLC1 interacts with the potassium channel Kir4.1 and with the DGC components syntrophin and dystrobrevin (14), we searched for the presence of these proteins in the ouabain eluate. Indeed, Kir4.1, syntrophin and dystrobrevin (both the α1 and α2 isoforms recognized by a specific monoclonal antibody) were detected within the Na,K-ATPase macromolecular complex (Fig. 2A). Despite the presence of syntrophin in the ouabain-eluted fraction, we did not find the water channel AQP4 (Fig. 2A), which was reported to bind syntrophin (21), to be functionally linked to Kir4.1 (17) and to interact with the Na,K-ATPase in rat atrocytes and brain (22). The early endosome antigen EEA1, the specific marker for early endosomes, which is abundantly expressed in brain cytosolic extract and partially co-localizes with MLC1 in immunofluorescences on cultured rat astrocytes (14), was not detected in the ouabain-eluate (Fig. 2A). When we analysed the ouabain-eluted fraction obtained from the rat brain membrane extract, we failed to reveal both the 60 and 30 kDa MLC1 membrane components (14; Fig. 2B), although NaK-β1 and the associated proteins Kir4.1 and syntrophin were present. It is possible that the amount of membrane MLC1 bound to the ouabain column is too low to be detected by WB or that several proteins known to interact with Na, K-ATPase in the brain (20) might interfere with its binding to MLC1.
Figure 2.
Co-purification of MLC1 and Na,K-ATPase by ouabain affinity chromatography. Fractions eluted from ouabain affinity chromatography of cytosol (A) and membrane (B) extracts from the rat brain. Chromatography fractions were analysed by SDS–PAGE and WB and probed with Abs against MLC1, Na,K-ATPase β1 (NaK-β1), early endosome antigen1 (EEA1), Kir4.1, syntrophin (synt), dystrobrevin (DB) and aquaporin 4 (AQP4). MLC1, NaK-β1, Kir4.1, synt and α1 and α2 dystrobrevin (DB) isoforms (87 and 55 kDa revealed by the monoclonal antibody), but not AQP-4 and EEA1, were detected in the ouabain eluate from the cytosolic extract of rat brain (A). No MLC1 was found in the membrane eluate, although NaK-β1, Kir4.1 and syntrophin were present (B).
Co-localization of MLC1 and NaK-β1 in cultured astrocytes and their re-distribution around intracellular vacuoles during hypo-osmotic shock
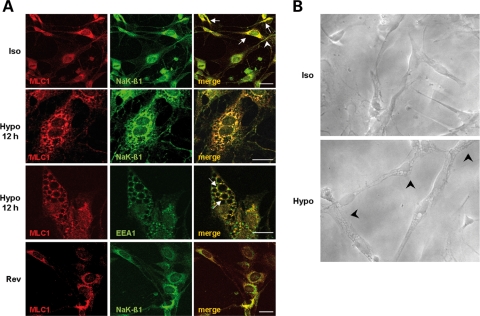
To identify the subcellular compartments where MLC1–NaK-β1 interaction occurs, we next performed immunolocalization experiments in rat primary astrocytes. Immunofluorescence stainings with an anti-MLC1 pAb raised against the intracellular MLC1-NH2 domain confirmed that MLC1 mainly localized in endoplasmic reticulum (ER) areas and endolysosomal compartments (Fig. 3A, Iso, red), as described previously (14). The distribution of NaK-β1, as revealed by an mAb recognizing the COOH domain of the protein, indicated that NaK-β1 was expressed both at the plasma membrane level and in discrete perinuclear areas and some cytoplasmic vesicles (Fig. 3A, Iso, green). The intracellular localization of the Na,K-ATPase enzyme in cytoplasmic structures has been described previously in different cell types and demonstrated to be due to its trafficking through the endolysosomal compartment (23–26). The merge of the immunostainings indicated that in most astrocytes, MLC1 and NaK-β1 co-localized primarily in cytoplasmic vesicles distributed around the nuclei, most likely in ER domains and endolysosomal organelles (Fig. 3A Iso, merge), thus suggesting a possible MLC1/Na,K-ATPase association during endocytosis processes or transport from ER to the plasma membrane (14).
Figure 3.
Co-immunolocalization of MLC1 and Na,K-ATPase β1 (NaK-β1) in control and in hypo-osmotic treated astrocytes. (A) Double-immunofluorescence staining of control rat astrocyte cultures with anti-MLC1 pAb (red) and anti-NaK-β1 mAb (green) shows co-localization of the two proteins in the perinuclear areas (Iso, merge, arrows) and rarely at the plasma membrane level (Iso, merge, arrowhead). After 12 h treatment of astrocytes with hypo-osmotic medium, double-immunofluorescence stainings with anti-MLC1 pAb (red) and anti-Na,K-β1 mAb (green) indicated a strong co-localization between MLC1 and NaK-β1 at the vacuolar delimiting and nuclear membranes (Hypo, merge). Similarly, in astrocytes subjected to 12 h hypo-osmotic treatment, anti-MLC1 pAb (red) and anti-EEA1 mAb (green) immunostaining revealed co-localization of the two molecules around vacuoles (Hypo, merge, arrows). In cells grown overnight in iso-osmotic culture medium after 12 h hypo-osmotic treatment, intracellular vacuolation is no more present and the co-localization of MLC1 (red) and NaK-β1 (green) is observed mostly in the perinuclear area (Rev, merge), similar to that observed in control, untreated cells (Iso, merge). Scale bars: 20 µm. (B) Phase contrast microscopy photographs of cultured rat astrocytes after overnight growth in iso-osmotic (Iso) or hypo-osmotic medium (Hypo). Note the changes in cell morphology after hypo-osmotic treatment. Astrocytes are slightly swelled, with enlarged nuclei and intracellular vacuoles (arrowheads). Original magnification: 400×.
In MLC patients, magnetic resonance imaging reveals diffuse signal abnormalities, swelling of the cerebral white matter and cysts, suggesting that MLC1 may have a role in the regulation of ion and water transport associated with cell volume regulation in the brain. Since Na,K-ATPase regulates cell swelling and regulatory volume decrease (RVD) occurring in astrocytes after exposure to hypo-osmotic medium (27,28), we cultured rat astrocytes in hypo-osmotic solution (29,30) for different time lengths (6 and 12 h) and analysed NaK-β1 and MLC1 intracellular distribution in comparison with untreated cells. Phase contrast microscopy analysis of control (Fig. 3B, Iso) and treated astrocytes (Fig. 3B, Hypo) indicated that the latter were slightly swelled with enlarged nuclei showing a 25–30% increase in size compared with controls, as assessed by confocal microscopy comparative analysis (data not shown), as described previously (27). We also observed the formation of intracellular vacuoles that increased in number and size over time and were more evident in cells subjected to 12 h hypo-osmotic treatment (Fig. 3B, Hypo, arrowheads).
The finding that osmotic imbalance can lead to the formation of intracellular vacuoles has been already reported in different cell types (31,32). Moreover, ER-derived watery vacuole formation induced by long-term hypo-osmotic shock was described previously in 3T3L1 cells (33). By performing immunofluorescence stainings of astrocytes subjected to 6 h hypo-osmotic treatment with anti-MLC1 and anti-NaK-β1 Abs, we observed that MLC1 was distributed around the newly formed vacuoles, most likely in the membranes bordering the vacuolar structures, where it partially co-localized with NaK-β1 (Fig. 1A, Supplementary Material). After the 12 h hypo-osmotic treatment, we observed an enlargement of the vacuoles and an almost complete co-localization between MLC1 and NaK-β1 in the vacuolar rims and around nuclear membranes (Fig. 3A, Hypo). The localization of NaK-β1 is in agreement with previous findings showing that Na,K-ATPase is constitutively expressed in the membranes of intracellular organelles, where it plays a role in the regulation of organelle pH and endocytosed membrane traffic (23,34–36).
Because the NaK-β1 distribution in astrocyte vacuole membranes induced by hypo-osmosis strongly resembled that described in the membranes of enlarged early endosomes in cells subjected to amine treatment (23,37) or induced by expression of a GTPase-defective mutant Rab5(Q79L) (38), we performed immunofluorescence stainings using anti-MLC1 pAb in combination with different Abs recognizing intracellular organelles of the endolysosomal pathway. In control astrocytes, MLC1 partially co-localized with EEA1 (not shown), as described previously (14). When astrocytes were grown in hypo-osmotic medium for 6 h, we observed several EEA1-positive enlarged organelles but only a few of them were also immunopositive for MLC1 (Fig. 1B, Supplementary Material). When cells were grown for 12 h in hypo-osmotic condition, the vacuoles increased in number and size and their limiting membranes were found immunopositive for both MLC1 and EEA1 (Fig. 3A, Hypo, arrows).
To further characterize hypo-osmosis-induced vacuoles, we also stained astrocytes with anti-Rab5 mAb in combination with anti-MLC1. Rab5 localizes to early endosomes (39) at the site of contact between endosomes about to fuse, and exerts its function on early endosome fusion and motility (40,41). When overexpressed, Rab5 induces the formation of enlarged endosomes (42). After 12 h hypo-osmotic treatment, we observed a high level of co-localization between MLC1 and Rab5 in the vacuolar limiting membranes and perinuclear area, confirming the early endosomal origin of the vacuolar structures forming in the astrocyte cytoplasm (Fig. 2A, Supplementary Material). Conversely, anti-CD63 Ab, an organelle marker recognizing late endosomal and multivesicular body compartments, did not stain the vacuolar structures forming during hypo-osmotic treatment (data not shown).
Because cell swelling and vacuole formation induced by hypo-osmotic treatment are reported to be reversible processes (43,44), astrocytes treated with hypo-osmotic solution were incubated for additional 12 h in iso-osmotic cell culture medium. After this time period, we observed an almost complete disappearance of intracellular vacuolation, and, similar to what observed in untreated astrocytes, MLC1 and NaK-β1 co-localized mostly in perinuclear areas (Fig. 3A, Rev). In the same experiments, we observed that MLC1 and EEA1 distribution also reverted to a condition almost identical to that of untreated astrocytes (data not shown).
To verify whether Kir4.1, the potassium channel found to be associated with MLC1 in astrocytes (13,14), was also present in the cytoplasmic vacuoles induced by 12 h hypo-osmotic shock, we performed double-immunostainings with anti-EEA1 and anti-Kir4.1 Abs in control and treated astrocytes. These stainings indicated a strong co-localization between the two proteins in the vacuolar membranes (Fig. 2B, Supplementary Material), suggesting the participation of Kir4.1 channel to the hypo-osmosis-induced changes observed in astrocytes.
Hypo-osmotic treatment increases the membrane component of MLC and its association with the Na,K-ATPase enzymatic complex in astrocytes
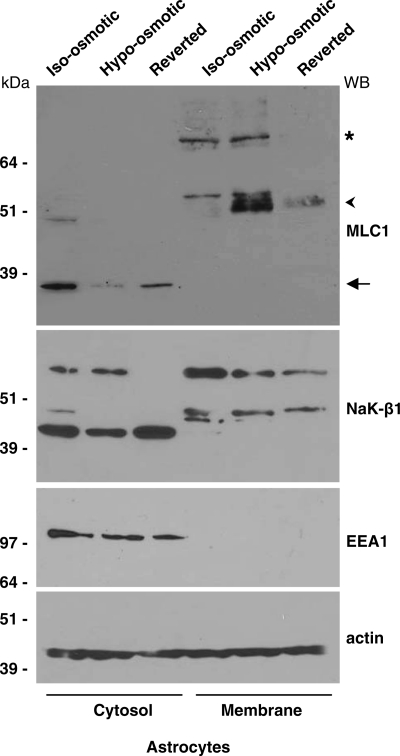
To investigate whether hypo-osmosis could also induce changes in the expression levels of the membrane and cytosolic MLC1 protein components, we performed WB analysis using extracts of control astrocytes, astrocytes exposed to hypo-osmotic solution for 12 h and astrocytes reverted to iso-osmotic condition after hypo-osmotic treatment. Figure 4 shows that the 60 kDa MLC1 membrane protein markedly increased during hypo-osmotic treatment. Conversely, the 36 kDa cytosolic MLC1 protein component was decreased. Both MLC1 components returned to control levels after the removal of the hypo-osmotic medium (Fig. 4). RT–PCR performed on control and hypo-osmotic astrocytes revealed no differences in MLC1 mRNA levels (data not shown), indicating that the higher levels of MLC1 membrane protein observed in WB experiments are due to an increase in protein stability and/or trafficking to the plasma membrane, and not to neosynthesis. No significant differences were observed in NaK-β1 protein levels, with the only exception of the highest molecular weight component, probably a multimeric protein aggregate that was found to decrease in the cytosol after the removal of hypo-osmotic medium. Similarly, no differences were detected in the protein levels of EEA1 and actin, used as internal loading control (Fig. 4), as well as of Kir4.1, dystrobrevin and syntrophin (data not shown). Since it has been proposed that the 60 kDa MLC1 component could represent the dimeric functional complex (9,14), these data suggest that MLC1 may be functionally involved in the processes regulating cell volume and vacuole formation under hypo-osmotic condition.
Figure 4.
Hypo-osmotic treatment of rat astrocytes increases the membrane components of MLC1. Rat astrocytes were incubated overnight with iso-osmotic or hypo-osmotic buffer or were put back in iso-osmotic cell culture medium for 12 h after hypo-osmotic treatment. Then, the cytosolic and membrane fractions were analysed by SDS–PAGE and WB. Hypo-osmotic treatment causes an increase in the membrane MLC1 components, the 60 kDa (arrowhead) and the higher molecular weight oligomers (asterisk), and a decrease in the cytosolic 36 kDa MLC1 component (arrow). NaK-β1 protein levels do not change, with the only exception of the higher molecular weight component, probably a multimeric proteic aggregate that is not present in the cytosol after the reversion treatment. No differences were detected in EEA1 and actin protein levels. Actin was also used as internal loading control.
To analyse the effects of hypo-osmotic treatment on MLC1 interaction with the whole Na,K-ATPase enzyme and the associated multiprotein complex observed after ouabain affinity chromatography on rat brain tissue (Fig. 2), we used protein extracts derived from the cytosol and membrane fractions of control and hypo-osmosis-treated astrocytes. WB analysis of ouabain-eluted proteins showed the presence of small amounts of the cytosolic 36 kDa MLC1 along with Na,K-ATPase complex components, Kir4.1, dystrobrevin and EEA1 without quantitative differences between normal and hypo-osmosis-treated astrocytes (Fig. 5A). Nonetheless, syntrophin appeared decreased in the ouabain eluate derived from hypo-osmosis-treated cells. In these experiments, we used a more sensitive in-house-generated antibody to detect dystrobrevin isoforms (45) (Fig. 5A and B). Similar to what observed in rat brain, AQP4 was not found in the ouabain eluate, although two bands of molecular weight ∼32 and 60 kDa, which represent the monomeric and dimeric forms of AQP4, respectively (46), were detected by the pAb used in the starting material (Fig. 5A and B, Input). When we analysed the fractions obtained from astrocyte membrane extracts, we found that in astrocytes, in contrast to what observed with rat brain membrane extracts, the 60 kDa and the higher molecular weight MLC1 oligomers (9,11,14) were eluted from the ouabain column along with Na,K-ATPase, Kir4.1, dystrobrevin and syntrophin (Fig. 5B). In the same eluted sample, we also found caveolin-1 (cav-1), the specific structural component of caveolae that has been reported to directly bind Na,K-ATPase (47,48) and MLC1 (14). Most importantly, after hypo-osmotic shock, the amount of the 60 kDa MLC1 and of the higher molecular weight oligomers eluted from the column increased along with NaK-β1, although no major differences were seen in the other proteins analysed (Fig. 5B). These data confirm that the interaction between MLC1 membrane components and NaK-β1is favoured by hypo-osmotic treatment, indicating that it may be functionally important in astrocytes during changes in homeostatic conditions. The presence of MLC1 in the Na,K-ATPase-associated protein complex in the eluates derived from astrocyte membranes but not in those derived from brain membrane extracts could be due to enrichment of the MLC1 60 kDa protein in purified astrocytes relatively to whole-brain-derived samples. Alternatively, because rat astrocytes prevalently express the α2 isoform of the Na,K-ATPase that shows a lower affinity for ouabain relative to the neuronal specific α1 isoform (49), we cannot exclude that, in the chromatography experiments performed with brain membrane extract, the competition between the two isoforms limits the binding of the α2 astrocytic-specific isoform to the ouabain column. Experiments are in progress to evaluate this possibility.
Figure 5.
MLC1 is a component of a multiprotein complex associated with Na,K-ATPase in astrocytes, and hypo-osmotic treatment increases its association with Na,K-ATPase. Analysis of the eluted fractions from ouabain affinity chromatography of cytosol (A) and membrane (B) extracts of cultured astrocytes grown for 12 h in iso-osmotic and hypo-osmotic mediums. Fractions were analysed by SDS–PAGE and WB and probed with Abs against MLC1, Na,K-ATPase β1 (NaK-β1), Kir4.1, dystrobrevin (DB), syntrophin (Synt), early endosome antigen1(EEA1), aquaporin-4 (AQP4) and caveolin-1 (Cav-1). Following hypo-osmotic shock, syntrophin decreases in the ouabain eluate of cytosol extract (A), whereas MLC1 and NaK-β1 increase in the ouabain eluate of membrane extract (B). AQP4 was not detected in the ouabain eluate of both cytosol and membrane fractions.
Association of MLC1 and Na,K-ATPase in hypertrophic astrocytes in the inflamed human brain
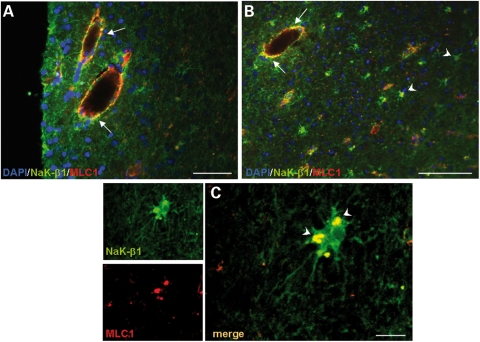
Altogether, the above results suggested that MLC1 may cooperate with Na,K-ATPase in processes regulating cell volume and osmotic balance in astrocytes. To investigate this issue further, we analysed MLC1/Na,K-ATPase relationship in an inflammatory condition of the CNS. Using immunohistochemistry, we analysed the localization of NaK-β1 and MLC1 in post-mortem brain samples from a patient with multiple sclerosis (MS), a disease characterized by chronic inflammation accompanied by reactive astrogliosis and astrocyte hyperthophy (50). As already reported (9–11), we found that, in the normal brain, MLC1 was expressed in astrocytes contacting blood vessels and that its expression increased in the MS brain in the perivascular areas of immunologically active, demyelinated lesions in the white matter (Fig. 3, Supplementary Material). We also observed that numerous small blood vessels, compared with the normal brain sample, were MLC1-immunopositive, probably reflecting the increased levels of expression of the MLC1 protein in MS brain (Fig. 3, Supplementary Material). Double-immunofluorescence stainings with anti-MLC1 and anti-NaK-β1 Abs on a brain section containing an active lesion with numerous perivascular inflammatory cell infiltrates revealed a partial co-localization of MLC1 with the NaK-β1 around blood vessels (Fig. 6A and B). Interestingly, in some hypertrophic astrocytes, the two molecules co-localized in cytoplasmic vesicles (Fig. 6C, arrowheads). Virtually, no co-localization between the two proteins was seen in normal brain tissue (not shown). These findings suggest that in MS lesions, MLC1–NaK-β1 interaction in activated astrocytes might be induced by osmotic changes caused by inflammation.
Figure 6.
Co-immunolocalization of NaK-β1 and MLC1 in multiple sclerosis (MS) brain lesions. (A and B) Immunofluorescence staining of MS brain tissue with anti-MLC1 pAb (red) and anti-Na,K-ATPase β1 (NaK-β1) mAb (green) shows that MLC1 and NaK-β1 immunoreactivities partially overlap around inflamed blood vessels (A and B, arrows) and in hypertrophic astrocytes in the lesioned periventricular white matter (B, arrowheads). (C) High-power magnification of a confocal image shows the overlapping vesicular localization of MLC1 and NaK-β1 in reactive astrocytes (arrowheads). Scale bars: A: 50 µm; B: 100 µm; C: 10 µm.
DISCUSSION
In this study, we demonstrate a direct association between MLC1, the protein product of the MLC gene whose mutations cause MLC disease, and the β1 subunit of Na,K-ATPase, the ubiquitous enzyme that is responsible for the maintenance of Na+ and K+ gradients across the plasma membrane. This molecular association was detected and characterized in vitro, in cultured astrocytes and in brain tissue. We also found that MLC1 along with Na,K-ATPase is a component of a macromolecular complex that encompasses the K+ channel Kir4.1 and the DGC proteins syntrophin and dystrobrevin and undergoes reversible dynamic association in early endosome-derived vacuoles that form in cultured astrocytes during hypo-osmotic shock. Because brain pathology in patients carrying MLC1 mutations is thought to be caused by alterations in the mechanisms regulating osmotic balance and cell volume changes, the demonstration that MLC1 associates with the Na,K-ATPase enzymatic complex represents the first experimental evidence of the hypothesized involvement of MLC1 in astrocyte functions controlling these processes.
We first identified the β1 subunit of Na,K-ATPase among the MLC1-interacting clones screened by the yeast two-hybrid assay from a human fetal brain cDNA library. The Na,K-ATPase enzyme is a heterodimer made of the assembly of α catalytic and β subunits (51), with some cell types, including astrocytes, expressing an additional regulatory γ subunit (52–54). By pumping three Na+ ions from the cytoplasm in exchange for two extracellular K+ ions coupled to the hydrolysis of one molecule of ATP, Na,K-ATPase produces ion gradients across the plasma membranes, thus maintaining the electrochemical gradient of the membrane (51,55). Cells use these ion gradients in many essential processes, such as osmoregulation, generation of plasma membrane potential and maintenance of intracellular pH and Ca2+ concentration, vectorial transport of many solutes and excitability in muscle fibres and neurons.
The β1 subunit is a highly glycosylated membrane protein of 303 amino acids that is responsible for the functional expression of the Na,K-ATPase pump. It contains a short NH2 cytoplasmic domain and a large extracellular portion that includes the C-terminal and, by binding the nascent α subunit, leads to the correct conformation of the whole enzymatic complex allowing its release from ER and targeting to the plasma membrane (56). By performing biochemical analyses on cultured rat and human astrocytes and brain tissue, we confirmed the specific direct interaction between the MLC1-NH2 terminal (1–55 amino acids) and the extracellular portion of the NaK-β1 that encompasses the COOH domain (156–303 amino acids). Although at the plasma membrane level the two interacting peptides show an opposite orientation (intracellular for the MLC1-NH2 terminal and extracellular for the COOH domain of NaK-β1), it can be hypothesized that the interaction described here can occur in other intracellular compartments where the proteins are correctly oriented in order to interact. This hypothesis is supported by the observation that in cultured astrocytes, MLC1 and NaK-β1 immunoreactivities co-localize in intracellular structures, like cytoplasmic vesicles and organelles. Interestingly, a similar structural interaction between the extracellular portion of the NaK-β1 subunit (229–298 amino acids) and the intracellular COOH domain of the large conductance Ca2+-activated K channel (BKCa channels or Slo1 protein) has been reported recently (57). Although also in this case the interacting regions of the two proteins are localized one inside and the other outside the cell membrane, the functional outcome of this interaction is supported by the finding that NaK-β1 plays a role in regulating the steady-state expression of BKCa channels on the cell surface (57). It has been suggested that the NaK-β1 subunit contributes to the targeting and stabilization of membrane proteins, particularly ion channels, to specific cellular domains to form a larger protein complex involved in ion homeostasis and signalling pathways (57), as indicated already by previous studies (58,59). Of note, in the mouse brain, the COOH domain of the NaK-β1 subunit was found to interact with MONAKA (60) and NKAIN family proteins (NKAIN 1, 2, 3, 4; 61), all recently cloned membrane proteins of unknown function that have been proposed to cooperate in the regulation of NaK-β1 function.
In line with the hypothesis that the extracellular domain of the β1 subunit of Na,K-ATPase could play a role in determining multiproteic interactions that are important for the different functions of the whole Na,K-ATPase enzymatic complex (see above), we show that MLC1, through its binding to NaK-β1, interacts with the whole Na,K-ATPase pump and is part of a multiproteic complex that includes the K+ channel Kir4.1, the DGC adaptor proteins syntrophin, dystrobrevin and caveolin-1 and could be assembled in specific cellular domains in response to functional demands. The presence of caveolin-1 among the proteins isolated by ouabain affinity chromatography supports the possible caveolar localization of the whole complex. Although in cultured astrocytes we observed very low levels of MLC1/NaK-β1 co-localization at the plasma membranes where caveolae are structured, we cannot exclude that in certain physiological conditions, by binding to the C-terminal of the NaK-β1, MLC1 can be recruited and stabilized to the caveolae to fulfil specific functions. After having exerted their functions, both proteins could be endocytosed in caveosomes, as reported previously for MLC1 (14), to be sorted to specific endolysosomal organelles for storing, recycling or degradation. This scenario could explain, at least in part, the intracellular vesicular localization of MLC1-NH2 and NaK-β1-COOH domains in astrocytes in vitro, indicating that their interaction may occur during intracellular sorting and trafficking route. It has been demonstrated that the presence of the Na,K-ATPase pump in early endosomes is functionally related to its activity of internal pH regulation (35,36,62). These organelles maintain a slightly acidic pH, which is directly responsible for their ability to ensure proper sorting of incoming receptors and ligands during endocytosis. Na,K-ATPase, which appears to be a functional component of the endosomal membrane, contributes to the early endosome pH control by inhibiting excessive acidification (23,37,62). This activity was found only in early endosomes, consistent with their limited acidification capacity relative to late endosomes and lysosomes (62). Our finding that MLC1 preferentially co-localizes with NaK-β1 in vacuolar structures of early endosome origin (EEA1- and Rab5-positive endosomes) that are generated in rat astrocytes during hypo-osmotic treatment supports the idea that the intracellular interaction between MLC1 and NaK-β1 occurs in early endosomes and may be functionally relevant during osmotic stress and cell swelling. The observations that in astrocytes MLC1/NaK-β1 co-immunostaining is maintained around the limiting membrane of vacuoles induced during stress condition and that the two molecules co-localize around blood vessels and in hypertrophic astrocytes in the inflamed MS brain but not in the non-pathological brain are consistent with the idea that this interaction is not constitutive but dynamic and might reflect specific functional requirements during osmotic stress or cell volume alterations. It is worth noting that alterations of Na,K-ATPase functionality in the brain have been observed in a fatal spongiform encephalopathy (63). Moreover, in mice, disruption of the β2 isofom of Na,K-ATPase, initially identified as the adhesion molecule on glia that mediates neuron–glia interaction (64), results in swelling and subsequent degeneration of astrocyte end-feet in the brainstem.
It has been suggested that brain pathology in patients carrying MLC1 mutations may be caused by alterations in the processes regulating osmotic balance and cell volume changes. MLC pathological features like fluid cysts, myelin vacuolation, enlargement of extracellular space and swelling of the white matter (1–3) are compatible with alterations in astrocyte-mediated processes controlling brain volume and ion and fluid exchanges. It is known that astrocytes, by contacting neurons and cells lining fluid-filled compartments, play a crucial role in regulating ion and water homeostasis through the selective transmembrane movements of organic and inorganic molecules and the balance of osmotic gradients which involve alterations of cell volume (reviewed in 12). Cell survival is ensured by avoiding excessive alterations of cell volume, a process that requires the strictly regulated and cooperative action of different ion and water channels (65), including the Na,K-ATPase pump (66,67). In astrocytes, Na,K-ATPase is involved in cell volume regulation and swelling induced by glutamate and hypo-osmotic treatment (27,67–71), water exchange (22) and the generation of calcium waves (72,73). Moreover, it plays a major role in K+ and glutamate uptake following neuronal excitation and takes part in astrocyte–neuron metabolic coupling (22,49,74,75). Our experiments suggest that the long-term exposure of astrocytes to hypo-osmotic solution may deregulate the pH control activity of the Na,K-ATPase, leading to organelle osmotic imbalance and swelling (23,37). The presence of MLC1 along with NaK-β1 in the membranes lining the swelled organelles indicates their possible cooperation in the molecular mechanism generating these structures. In support of this hypothesis, we also found that hypo-osmotic shock induced an increase in the amount of the MLC1 functional component (60 kDa) and favoured the association of MLC1 and NaK-β1 to the ouabain-eluted macromolecular complex. Collectively, these results suggest that, along with Na,K-ATPase, MLC1 is functionally involved in osmotic imbalance-induced volume alterations. Although we do not know yet the specific function of MLC1, we hypothesize that this protein works as an ion channel itself, or as a chaperone subunit of some other channel(s) cooperating with the Na,K-ATPase enzyme in astrocyte swelling and/or RVD occurring during hypo-osmotic shock.
Exposure of cells to hypo-osmotic extracellular fluid leads to water influx, along the osmotic gradient across the cell membrane and consequent cell swelling which involves the collaborative activity of different transporters, exchangers, water and ion channels, including AQPs, K+ channels and/or anion channels, KCl− co-transporter, K+/H+ and Cl−/HCO3− exchanger. Cell volume response of astrocytes in hypo-osmotic medium involves the net movement of osmoles by a mechanism that is dependent on cellular energy and tightly coupled to the Na,K-ATPase ion pump (27). It is noteworthy that Kir4.1, the K+ channel previously found to bind MLC1 (5,14), was also present in the macromolecular complex of the Na,K-ATPase, thus indicating that the two proteins may participate in the same process. In addition, the presence of syntrophin and dystrobrevin suggests that these scaffolding proteins may mediate the link between different components of the Na,K-ATPase transmembrane complexes. In glial cells, Kir channels, and particularly Kir4.1, control extracellular K+ homeostasis by uptake of K+ ions from the extracellular space and their release into the microvasculature. In addition, in glial cells, Kir4.1 has been implicated in K+-associated water influx and cell swelling in hypo-osmotic conditions, in vitro and in vivo systems (76,77). It has been shown that the activity of Kir4.1 on cell volume regulation is mediated by its association with the water channel AQP4, although this finding has not been reproduced in all studies (76). In our experiments, we could not detect AQP4 in the ouabain-eluted fraction, despite its recently reported interaction with the α subunit of Na,K-ATPase (22). It is possible that AQP4 is present in the Na,K-ATPase-associated macromolecular complex in lower amounts relative to other proteins and its detection may depend on the antibody used.
To understand whether the loss of MLC1 observed in MLC brain patients (5) may affect the functionality of the Na,K-ATPase/MLC1 macromolecular complex, we performed preliminary RNA-interfering experiments using lentiviral infection. Although MLC1 mRNA was downregulated to some extent (∼30% reduction), we did not observe a reduction of MLC1 protein levels, as assessed by WB and immunostainings (data not shown). We are currently exploring the possibility of using multiple combinations of different interfering RNA sequences to induce efficient MLC1 protein downregulation.
MLC1 patients show variability in both disease progression and severity of clinical symptoms (2,5,78). So far, about 50 different mutations distributed along the whole MLC1 gene have been identified with no evident correlation between genotype and phenotype (5). In fact, clinical phenotypes may be different in patients with the same mutation, whereas patients with genetically proved mutations may share similar clinical features with patients without mutations in the MLC1 gene (7,9). In exon 2, which encodes the N-terminal region and part of the first transmembrane domain of MLC1, different types of mutations have been identified: either deletion/insertion mutation types that cause frameshifts and the appearance of premature truncation codons (probably leading to protein loss of functions) or missense mutations that do not disrupt synthesis of full-length MLC1 protein (5,79). However, the observed clinical phenotypes are variable and do not correlate with the severity of the mutations, as observed for mutations affecting other regions of the protein (5). Interestingly, we have recently found that in the cytoplasmic N-terminal domain of MLC1, S27 can be phosphorylated in vitro by both protein kinase A and protein kinase C (14), suggesting a potential regulatory function of this domain in MLC1-binding properties. Based on these findings, we shall investigate further the association of MLC1 with Na,K-ATPase in human astrocytoma cell lines stably transfected with wild-type and mutated MLC1 in both normal and hypo-osmotic conditions, with particular attention to the phenotype of cells expressing mutations localized in the NH2 region of the protein that interacts with the β1 subunit of the Na,K-ATPase.
In conclusion, by showing a dynamic interaction between MLC1 and Na,K-ATPase β1, this study provides the first experimental evidence for an involvement of MLC1 in processes controlling cellular volume and ion homeostasis in astrocytes. These findings will pave the way for a better understanding of the pathogenetic mechanisms underlying MLC and thus for the development of the most appropriate strategies to cure this disease.
MATERIALS AND METHODS
Plasmid constructs
The coding region of the MLC1 N-terminal domain (MLC1-NH2; amino acids 1–55) obtained by RT–PCR from human brain tissue RNA samples, as described previously (11), was cloned into pGBKT7 vector (Clontech) to create pGBKT7/MLC1-NH2. PCR reactions were performed using the following primers: forward 5′-CAT GCC ATG GCA ACC CAG GAG CCA TTC AGA-3′ and reverse 5′-CGG AAT TCC ACA GAG AAG ACC CAC GT-3′. cDNA was subjected to PCR amplification for 35 cycles in the following conditions: 30 s/94°C, 30 s/50°C and 30 s/72°C for the first 5 cycles and 60 s/94°C, 30 s/65°C and 30 s/72°C for the remaining 30 cycles. After NcoI and EcoRI digestion, the MLC1-NH2 fragment was inserted into the NcoI–EcoRI site of pGBKT7 vector. The correct orientation of the cDNA insert was verified by sequencing service (M-Medical).
Yeast two-hybrid screen
Two-hybrid screening was carried out by yeast mating, using the Matchmaker Gal4 Two-Hybrid System 3 (Clontech) as reported previously (45). Briefly, pGBKT7/MLC1-NH2 was tested negative for auto-activation of reporter gene activity in the yeast two-hybrid reporter strains, Saccharomyces cerevisiae AH109 (MATa, trp1–901, leu2–3, 112, ura3–52, his3–200, gal4D, gal80D, LYS2::GAL1UAS–GAL1TATA –HIS3, GAL2UAS–GAL2TATA–ADE2, URA3::MEL1UAS–MEL1TATA–lacZ MEL1) and S. cerevisiae Y187 (MATα, ura3–52, his3–200, ade2–101, trp1–901, leu2–3, 112, gal4▵, gal80▵,met–, URA3::GAL1UAS–GAL1TATA–lacZ MEL1). The Gal4 DNA-binding domain construct pGBKT7/MLC1-NH2 was used to transform the MATa yeast strain AH109. AH109[pGBKT7/MLC1-NH2] was then employed as a bait strain to screen a human fetal brain cDNA library (Clontech), which was cloned into the activation domain vector pGADT7-Rec, and pre-transformed in the MATα yeast strain Y187. The yeast mating screening was performed according to the manufacturer's instructions. Diploids were selected by culture on minimal synthetic dropout medium lacking Trp, Leu, His and Ade (–TLHA), and including 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside (X-α-Gal) for 7–14 days. Single colonies of yeast obtained from the library screen growing on SD/-TLHA/X-α-Gal were re-streaked at least twice onto SD/-TL/X-α-Gal to allow segregation, and then transferred to SD/-TLHA/X-α-Gal to verify that they maintained the correct phenotype. Duplicates containing the same AD/library plasmids were eliminated by yeast colony PCR followed by restriction digestion with frequently cutting enzymes. AD/library plasmids from sorted colonies were isolated and rescued using E. coli strain DH5α on ampicillin-resistant plates. Unique inserts were sequenced and DNA and protein sequence analyses performed with the BLAST algorithm at the National Center for Biotechnology Information (NCBI). pGADT7-Rec plasmids encoding the library clones were tested for auto-activation of the reporter gene in yeast. Isolates growing in SD/-TLHA/X-α-Gal and developing blue staining without the presence of the MLC1-NH2 bait were excluded from further investigation. Activation of the reporter genes in the positive colonies was confirmed in the same experiments.
In vitro transcription and translation
In vitro transcription and translation of pGADT7/Na,K-ATPase β1 was carried out using the TNT T7 Quick Coupled Transcription/Translation System (Promega) in the presence of EasyTag L-[35S] Methionine (PerkinElmer) according to the manufacturer's protocol. Newly synthesized proteins were separated by SDS–PAGE and analysed with an Instant-Imager (Packard).
Recombinant protein preparation and pull-down assays
GST, GST-MLC1-N terminal (GST-MLC1-N) and GST-MLC1-C terminal (GST-MLC1-C) pre-bound to glutathione-Sepharose beads were used in in vitro protein-binding assays, as described previously (14). Briefly, primary rat astrocyte lysate obtained by lysis with 1% Triton X-100, 0.5% sodium deoxycholate, 150 mm NaCl, 150 mm Hepes (pH 7.4) and centrifugated at 16 000g at 4°C for 20 min, was pre-cleared by incubation with the GST-bound to glutathione-agarose and then incubated with agarose-bound GST-MLC1-N terminal or -C terminal peptides. Following exhaustive washes, protein-bound beads were eluted with 0.1 m glycine, pH 3. Aliquots (0.5 ml) of eluted proteins were precipitated with acetone (1:4 v/v) overnight at 4°C and analysed by SDS–PAGE and WB.
Biochemical enrichment of His-tagged proteins
Lysates obtained from an astrocytoma cell line stably overexpressing His-tagged MLC1 (U251-HisMLC1) were incubated overnight at 4°C with 100 µl (50% v/v suspension) of Ni-NTA Agarose (Qiagen, Hilden, Germany); after extensive washings, protein elution was carried out using imidazole, at a concentration of 50 and 200 mm (14). The eluted proteins were analysed by SDS–PAGE and WB.
Cell cultures and treatments
Astrocyte-enriched cultures (95% purity) were generated from 1–2-day-old newborn Wistar rats, as described previously (80). Cells were maintained in culture in DMEM medium (Euroclone, UK) supplemented with 10% FCS (Gibco, BRL, Gaithersburg, MD, USA) and antibiotics (penicillin/streptomycin, Euroclone) in 5% CO2 atmosphere. For cell treatments, primary cultured astrocytes were plated in polylysinated 60 mm diameter dishes, washed three times in PBS and treated for different time lengths with iso-osmotic medium (122 mm NaCl, 3.3 mm KCl, 0.4 mm MgSO4, 1.3 mm CaCl2, 1.2 mm KH2PO4, 10 mm d-glucose, 25 mm HEPES, pH 7.4) or in the same buffer in which NaCl concentration was half-reduced to 50 mm NaCl (hypo-osmotic buffer) as described previously (30,81). After stimulation, cells were washed three times in PBS, collected by scraping, and centrifuged at 2700g at 4°C for 20 min. Cell pellets were solubilized as described below. For immunofluorescence stainings, cells were treated with the same buffers. For the reversion experiments after 12 h of hypo-osmotic treatment, cells were put back in cell culture medium and left for additional 12 h before immunofluorescence or WB analysis.
Ouabain affinity chromatography
The ouabain affinity matrix was prepared according to a previously described procedure (20) with some modifications. For the cytosolic and membrane brain extracts, 0.5 g of epoxy-activated agarose (Sigma) with a 12 atom spacer was added to 7.5 ml of a solution containing 12.5 mm ouabain and 100 mm sodium carbonate, pH 8.5, at RT. The mixture was then incubated at 37°C for 20 h with gentle shaking after which the resin was washed with 10 ml of 100 mm sodium carbonate buffer, pH 8.5, followed by 10 ml of water. The resin was incubated with 1 m ethanolamine, pH 8.5, for 4 h at 37°C to block unreacted coupling sites. The column was washed with 200 mm Tris–HCl, pH 8.5, and stored in 50 mm imidazole containing 0.1% NaN3, pH 7.4. Before purification, the ouabain affinity column was washed with 50 bed volumes of 50 mm imidazole, pH 7.4, and equilibrated with two bed volumes of K+ loading buffer (60 mm KCl, 25 mm imidazole, 1 mm EDTA, 1 mm CaCl2 and 0.1% Triton X-100, pH 7.4). A mock elution was performed with 5–10 ml of elution buffer (12.5 mm ouabain, 25 mm imidazole, 150 mm NaCl and 1x protease inhibitor cocktail, pH 7.4), followed by 4x bed-volume wash with K+ loading buffer. The sample was incubated with the ouabain affinity matrix overnight at 4°C and the flow-through collected (ouabain unretained fraction). The column was then washed with 3x bed volumes of K+ loading buffer. One ml of elution buffer was then added to the column and after 50 min incubation at 4°C collected (ouabain-eluted fraction). Fractions were analysed by SDS–PAGE and WB. For hypo-osmotic and iso-osmotic astrocyte extracts, 1 g of epoxy-activated agarose was used for 15 ml solution of the cytosol and membrane fractions.
Immunofluorescence and confocal microscopy analysis
Astrocytes grown on polylysine-coated coverslips were incubated in control or hypo-osmotic solution for 6 or 12 h, then fixed for 10 min with 4% paraformaldehyde and washed with PBS. After 1 h of incubation with blocking solution (5% BSA in PBS), cells were incubated for 1 h at RT with the following primary antibodies (Abs) diluted in PBS, 0.025% Triton X100: affinity-purified anti-MLC1 pAb (1:50, Atlas AB, AlbaNova University Center, Stockholm, Sweden), anti-Na,K-ATPase β1 mAb (1:50, Millipore, Temecula, CA, USA), anti-EEA1 mAb (1:50, BD Transduction Laboratories, Lexington, KY, USA). A biotinilated secondary antibody (4.3 μg/ml, Biotin-SP-AffiniPure goat anti-rabbit IgG H+L; Jackson Immunoresearch Laboratories, West Grove, PA, USA) followed by incubation with 2 μg/ml streptavidin-TRITC (Jackson, UK), or a fluorescein-conjugated donkey anti-mouse (1:100, Jackson) was used. Coverslips were washed, sealed in Vectashield medium (Vector Lab, Burlingame, CA, USA) and analysed with a laser scanning confocal microscope (LSM 5 Pascal, Carl Zeiss, Jena, Germany).
Immunostaining of human brain tissue
Indirect immunofluorescence technique was used to detect MLC1 and NaK-β1 in autopsy brain tissues obtained from the UK MS Tissue Bank at Imperial College London. Post-mortem MS tissues were obtained via a UK prospective donor scheme with full ethical approval (08/MRE09/31). Brain tissue was fixed in 4% paraformaldehyde in PBS, cryoprotected in 30% sucrose for 1 week, frozen in dry ice-cooled isopentane and stored at −75°C. Air-dried, acetone-fixed (4°C), 10 μm thick cryosections were rehydrated with PBS and post-fixed sections were subjected to the antigen retrieval procedure with microwave in citrate buffer 10 mm (pH 6.0), as described previously (11). For double-immunofluorescence stainings, sections were incubated for 1 h with 20% of normal goat serum (NGS) (Jackson) and then overnight at 4°C with a mixture of rabbit anti-MLC1 pAb (1:250, ATLAS) and anti-Na,K-ATPase β1 mAb (1:200 Millipore) in PBS containing 2% BSA and 0.05% Triton X-100. After extensive washing, sections were incubated for 1 h at RT with a mixture of fluorescein-conjugated goat anti-mouse and rhodamine-conjugated goat anti-rabbit Abs diluted in PBS containing 10% NGS. Images were analysed with a fluorescence microscope (Leica DM-4000B Microsystems, Bannockburn, IL, USA) and with a laser scanning confocal microscope, as above.
Brain and astrocyte subcellular fractionation procedures and WB
Cytosolic and membrane fractions from rat brains and cultured astrocytes were extracted as described previously (14). In some experiments, cytosolic supernatant was ultracentrifuged at 100 000g for 2 h at 4°C. Protein samples were subjected to SDS–PAGE using gradient (4–12%)-pre-casted gels (Invitrogen) (14), transferred to a nitrocellulose membrane and immunoblotted overnight at 4°C with the following Abs: anti-MLC1 pAb (1:500, in-house generated), anti-Na,K-ATPase β1 mAb (1μg/ml, Millipore), anti-Kir4.1 pAb (1:400, Alomone, Israel), anti-AQP4 (4/18) mAb (1:500, Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA), anti-syntrophin mAb (1:2000, MA-1-745, Affinity BioReagents, Co., USA), anti-β-DG mAb (1:25, NCL-43 DAG, Novocastra Laboratories Ltd, Newcastle-upon-Tyne, UK), anti-EEA1 mAb (1:5000, BD Transduction Laboratories), anti-dystrobrevin mAb (1:750, BD Transduction Laboratories), anti-dystrobrevin pAb (1:500, in-house generated), anti-caveolin-1 pAb (1:1000, Santa Cruz Biotechnology) in PBS, 3% BSA followed by extensive washings and then incubated for 1 h with horseradish peroxidase-conjugated anti-mouse or anti-rabbit Abs (1:10 000; Thermo Scientific, MO, USA), for 1 h at RT. Immunoreactive bands were visualized using an enhanced chemiluminescence reagent (Pierce), according to the manufacturer's instructions and exposed on X-ray films.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by ELA Foundation (grants ELA 2006-001/4 and ELA 2009-002C5A to E.A.) and ISS-NIH Collaborative Programme on Rare diseases (grant 7/DR1 to P.M.).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Antonella Bernardo for providing astrocyte cell cultures. Brain tissue samples were kindly provided by the UK Multiple Sclerosis Tissue Bank (www.ukmstissuebank.imperial.ac.uk), funded by the MS Society of Great Britain and Northern Ireland (registered charity 207495).
Conflict of Interest statement. None declared.
REFERENCES
- 1.Van der Knaap M.S., Barth P.G., Stroink H., Van Nieuwenhuizen O., Arts W.F., Hoogenraad F., Valk J. Leukoncephalopathy with swelling and a discrepantly mild clinical course in eight children. Ann. Neurol. 1995;7:324–334. doi: 10.1002/ana.410370308. [DOI] [PubMed] [Google Scholar]
- 2.Leegwater P.A., Yuan B.Q., Van Der Steen J., Mulders J., Konst A.A., Boor P.K., Mejaski-Bosnjak V., Van Der Maarel S.M., Frants R.R., Oudejans C.B., et al. Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am. J. Hum. Genet. 2001;68:831–838. doi: 10.1086/319519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascual-Castroviejo I., Van der Knaap M.S., Pronk J.C., Garcia-Segura J.M., Gutierez-Molina M., Pascual-Pascual S.I. Vacuolating megalencephalic leukoencephalopathy: 24 year follow-up of two siblings. Neurologia. 2005;20:33–40. [PubMed] [Google Scholar]
- 4.Topku M., Gartioux C., Ribierre F., Yalçinkaya C., Tokus E., Oztekin N., Beckmann J.S., Ozguc M., Seboun E. Vacuolating megalencephalic leukoencephalopathy with subcortical cysts mapped to chromosome 22qtel. Am. J. Hum. Genet. 2000;66:733–739. doi: 10.1086/302758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boor P.K.I., de Groot K., Mejaski-Bosnjak V., Brenner C., van der Knaap M.S., Scheper G.C., Pronk J.C. Megalencephalic leukoencephalopathy with subcortical cysts: an update and extended mutation analysis of MLC1. Hum. Mutat. 2006;27:505–512. doi: 10.1002/humu.20332. [DOI] [PubMed] [Google Scholar]
- 6.Blattner R., Von Moers A., Leegwater P.A., Hanefeld F.A., Van Der Knaap M.S., Köhler W. Clinical and genetic heterogeneity in megalencephalic leukoencephalopathy with subcortical cysts (MLC) Neuropediatrics. 2003;34:215–218. doi: 10.1055/s-2003-42210. [DOI] [PubMed] [Google Scholar]
- 7.Patrono C., Di Giacinto G., Eymard-Pierre E., Santorelli F.M., Rodriguez D., De Stefano N., Federico A., Gatti R., Benigno V., Megarbané A., et al. Genetic heterogeneity of megalencephalic leukoencephalopathy and subcortical cysts. Neurology. 2003;61:534–537. doi: 10.1212/01.wnl.0000076184.21183.ca. [DOI] [PubMed] [Google Scholar]
- 8.van der Knaap M.S., Lai V., Köhler W., Salih M.A., Fonseca M.J., Benke T.A., Wilson C., Jayakar P., Aine M.R., Dom L., et al. Megalencephalic leukoencephalopathy with cysts without MLC1 defect. Ann. Neurol. 2010;67:834–837. doi: 10.1002/ana.21980. [DOI] [PubMed] [Google Scholar]
- 9.Teijido O., Martinez A., Pusch M., Zorzano A., Soriano E., Del Rio J.A., Palacin M., Estevez R. Localization and functional analyses of the MLC1 protein involved in megalencephalic leukoencephalopathy with subcortical cysts. Hum. Mol. Genet. 2004;13:2581–2594. doi: 10.1093/hmg/ddh291. [DOI] [PubMed] [Google Scholar]
- 10.Boor P.K.I., DeGroot K., Waisfisz Q., Kamphrost W., Oudejans C.B.M., Powers J.M., Pronk J.C., Shepert G.C., Van Der Knaap M.S. MLC1 a novel protein in distal astroglial processes. J. Neuropathol. Exp. Neurol. 2005;64:412–419. doi: 10.1093/jnen/64.5.412. [DOI] [PubMed] [Google Scholar]
- 11.Ambrosini E., Serafini B., Lanciotti A., Tosini F., Scialpi F., Psaila R., Di Girolamo F., Petrucci T.C., Aloisi F. Biochemical characterization of MLC1 protein in astrocytes and its association with the dystrophin–glycoprotein complex. Mol. Cell. Neurosci. 2008;37:480–493. doi: 10.1016/j.mcn.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Kimelberg H.K. Functions of mature mammalian astrocytes: a current view. Neuroscientist. 2010;16:79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- 13.Boor P.K.I., Nagtegaal M., Kamphorst W., van der Valk P., Pronk J.C., van Horssen J., Dinopoulos A., Bove K.E., Pascual-Castroviejo I., Muntoni F., et al. MLC1 is associated with the dystrophin–glycoprotein complex at astrocytic endfeet. Acta Neuropathol. 2007;114:403–410. doi: 10.1007/s00401-007-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanciotti A., Brignone M.S., Camerini S., Serafini B., Macchia G., Raggi C., Molinari P., Crescenzi M., Musumeci M., Sargiacomo M., et al. MLC1 trafficking and membrane expression in astrocytes: role of caveolin-1 and phosphorylation. Neurobiol. Dis. 2010;37:581–595. doi: 10.1016/j.nbd.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Waite A., Tinsley C.L., Locke M., Blake D.J. The neurobiology of the dystrophin-associated glycoprotein complex. Ann. Med. 2009;41:344–359. doi: 10.1080/07853890802668522. [DOI] [PubMed] [Google Scholar]
- 16.Neely J.D., Amiry-Moghaddam M., Ottersen O.P., Froehner S.C., Agre P., Adams M.E. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein; Proc. Natl Acad. Sci. USA; 2001. pp. 14108–14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connors N.C., Adams M.E., Froehner S.C., Kofuji P. The potassium channel Kir4.1 associates with the dystrophin–glycoprotein complex via alpha-syntrophin in glia. J. Biol. Chem. 2004;279:28387–28392. doi: 10.1074/jbc.M402604200. [DOI] [PubMed] [Google Scholar]
- 18.Hosoi R., Matsuda T., Asano S., Nakamura H., Hashimoto H., Takuma K., Baba A. Isoform-specific up-regulation by ouabain of Na+,K+-ATPase in cultured rat astrocytes. J. Neurochem. 1997;69:2189–2196. doi: 10.1046/j.1471-4159.1997.69052189.x. [DOI] [PubMed] [Google Scholar]
- 19.Yingst D.R., Yang S.Y., Schiebinger R. Purification of active Na+-K+-ATPase using a new ouabain-affinity column. Am. J. Physiol. 1998;275:C1167–C1177. doi: 10.1152/ajpcell.1998.275.4.C1167. [DOI] [PubMed] [Google Scholar]
- 20.Rose E.M., Koo J.C., Antflick J.E., Ahmed S.M., Angers S., Hampson D.R. Glutamate transporter coupling to Na,K-ATPase. J. Neurosci. 2009;24:8143–8155. doi: 10.1523/JNEUROSCI.1081-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amiry-Moghaddam M., Frydenlund D.S., Ottersen O.P. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004;129:999–1010. doi: 10.1016/j.neuroscience.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 22.Illarionova N.B., Gunnarson E., Li Y., Brismar H., Bondar A., Zelenin S., Aperia A. Functional and molecular interactions between aquaporins and Na,K-ATPase. Neuroscience. 2010;168:915–925. doi: 10.1016/j.neuroscience.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 23.Cain C.C., Murphy R.F. A chloroquine-resistant Swiss 3T3 cell line with a defect in late endocytic acidification. J. Cell. Biol. 1988;106:269–277. doi: 10.1083/jcb.106.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecuona E., Ridge K., Pesce L., Batlle D., Sznajder J.I. The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Mol. Biol. Cell. 2003;14:3888–3897. doi: 10.1091/mbc.E02-12-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecuona E., Minin A., Trejo H.E., Chen J., Comellas A.P., Sun H., Grillo D., Nekrasova O.E., Welch L.C., Szleifer I., et al. Myosin-Va restrains the trafficking of Na+/K+-ATPase-containing vesicles in alveolar epithelial cells. J. Cell. Sci. 2009;122:3915–3922. doi: 10.1242/jcs.046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertorello A.M., Komarova Y., Smith K., Leibiger I.B., Efendiev R., Pedemonte C.H., Borisy G., Sznajder J.I. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol. Biol. Cell. 2003;14:1149–1157. doi: 10.1091/mbc.E02-06-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson J.E., Sankar R., Holtzman D., James A., Fleischhacker D. Energy-dependent volume regulation in primary cultured cerebral astrocytes. J. Cell. Physiol. 1986;128:209–215. doi: 10.1002/jcp.1041280211. [DOI] [PubMed] [Google Scholar]
- 28.Fraser C.L., Swanson R.A. Female sex hormones inhibit volume regulation in rat brain astrocyte culture. Am. J. Physiol. 1994;267:C909–C914. doi: 10.1152/ajpcell.1994.267.4.C909. [DOI] [PubMed] [Google Scholar]
- 29.Kimelberg H.K. Occurrence and functional significance of serotonin and catecholamine uptake by astrocytes. Biochem. Pharmacol. 1986;35:2273–2281. doi: 10.1016/0006-2952(86)90451-x. [DOI] [PubMed] [Google Scholar]
- 30.Kimelberg H.K. Volume activated anion channel and astrocytic cellular edema in traumatic brain injury and stroke. Adv. Exp. Med. Biol. 2004;559:157–167. doi: 10.1007/0-387-23752-6_15. [DOI] [PubMed] [Google Scholar]
- 31.Chawla S., Skepper J.N., Fraser J.F., Huang C.L. Osmotic processes in vacuolation and detubulation of skeletal muscle. Cell Biol. Int. 2002;26:905–910. doi: 10.1006/cbir.2002.0944. [DOI] [PubMed] [Google Scholar]
- 32.Koffer A., Williams M., Johansen T. Vacuole formation in mast cells responding to osmotic stress and to F-actin disassembly. Cell Biol. Int. 2002;26:885–892. doi: 10.1006/cbir.2002.0940. [DOI] [PubMed] [Google Scholar]
- 33.Iwasa Y., Hirono C., Sugita M., Takemoto K., Shiba Y. External Cl(−)-dependent formation of watery vacuoles by long-term hypotonic shock in 3T3-L1 cells. Cell. Physiol. Biochem. 2001;11:311–320. doi: 10.1159/000047817. [DOI] [PubMed] [Google Scholar]
- 34.Cain C.C., Sipe D.M., Murphy R.F. Regulation of endocytic pH by the Na+,K+-ATPase in living cells; Proc. Natl Acad. Sci. USA; 1989. pp. 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grabe M., Oster G. Regulation of organelle acidity. J. Gen. Physiol. 2001;117:329–344. doi: 10.1085/jgp.117.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldmann T., Glukmann V., Medvenev E., Shpolansky U., Galili D., Lichtstein D., Rosen H. Role of endosomal Na+-K+-ATPase and cardiac steroids in the regulation of endocytosis. Am. J. Physiol. Cell. Physiol. 2007;293:C885–C896. doi: 10.1152/ajpcell.00602.2006. [DOI] [PubMed] [Google Scholar]
- 37.Cain C.C., Murphy R.F. Growth inhibition of 3T3 fibroblasts by lysosomotropic amines: correlation with effects on intravesicular pH but not vacuolation. J. Cell. Physiol. 1986;129:65–70. doi: 10.1002/jcp.1041290110. [DOI] [PubMed] [Google Scholar]
- 38.Wegner C.S., Malerød L., Pedersen N.M., Progida C., Bakke O., Stenmark H., Brech A. Ultrastructural characterization of giant endosomes induced by GTPase-deficient Rab5. Histochem. Cell Biol. 2010;133:41–55. doi: 10.1007/s00418-009-0643-8. [DOI] [PubMed] [Google Scholar]
- 39.Gorvel J.P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 40.Gillooly D.J., Raiborg C., Stenmark H. Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem. Cell. Biol. 2003;120:445–453. doi: 10.1007/s00418-003-0591-7. [DOI] [PubMed] [Google Scholar]
- 41.Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 42.Bucci C., Parton R.G., Mather I.H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 43.Henics T., Wheatley D.N. Vacuolar cytoplasmic phase separation in cultured mammalian cells involves the microfilament network and reduces motional properties of intracellular water. Int. J. Exp. Pathol. 1997;78:343–354. doi: 10.1046/j.1365-2613.1997.320367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kruczek C., Görg B., Keitel V., Pirev E., Kröncke K.D., Schliess F., Häussinger D. Hypoosmotic swelling affects zinc homeostasis in cultured rat astrocytes. Glia. 2009;57:79–92. doi: 10.1002/glia.20737. [DOI] [PubMed] [Google Scholar]
- 45.Macioce P., Gambara G., Bernassola M., Gaddini L., Torreri P., Macchia G., Ramoni C., Ceccarini M., Petrucci T.C. Beta-dystrobrevin interacts directly with kinesin heavy chain in brain. J. Cell Sci. 2003;116:4847–4856. doi: 10.1242/jcs.00805. [DOI] [PubMed] [Google Scholar]
- 46.Neely J.D., Christensen B.M., Nielsen S., Agre P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry. 1999;38:11156–11163. doi: 10.1021/bi990941s. [DOI] [PubMed] [Google Scholar]
- 47.Wang H., Haas M., Liang M., Cai T., Tian J., Li S., Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 2004;279:17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 48.Cai T., Wang H., Chen Y., Liu L., Gunning W.T., Quintas L.E., Xie Z.J. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J. Cell Biol. 2008;182:1153–1169. doi: 10.1083/jcb.200712022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng L., Huang R., Zhang S., Hertz L. Ouabain binding kinetics and FXYD7 expression in astrocytes and neurons in primary cultures: implications for cellular contributions to extracellular K+ homeostasis? Neuron. Glia Biol. 2010;26:1–9. doi: 10.1017/S1740925X10000013. [DOI] [PubMed] [Google Scholar]
- 50.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 52.Therien G., Pu H.X., Karlish S.J., Blostein R. Molecular and functional studies of the gamma subunit of the sodium pump. J. Bioenerg. Biomembr. 2001;33:407–414. doi: 10.1023/a:1010619623841. [DOI] [PubMed] [Google Scholar]
- 53.Gegelashvili M., Rodriguez-Kern A., Sung L., Shimamoto K., Gegelashvili G. Glutamate transporter GLAST/EAAT1 directs cell surface expression of FXYD2/gamma subunit of Na,K-ATPase in human fetal astrocytes. Neurochem. Int. 2007;50:916–920. doi: 10.1016/j.neuint.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Geering K. Functional roles of Na,K-ATPase subunits. Curr. Opin. Nephrol. Hypertens. 2008;17:526–532. doi: 10.1097/MNH.0b013e3283036cbf. [DOI] [PubMed] [Google Scholar]
- 55.Jaitovich A.A., Bertorello A.M. Na+, K+-ATPase: an indispensable ion pumping-signaling mechanism across mammalian cell membranes. Semin. Nephrol. 2006;26:386–392. doi: 10.1016/j.semnephrol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Ueno S., Takeda K., Izumi F., Futai M., Schwarz W., Kawamura M. Assembly of the chimeric Na+/K+-ATPase and H+/K+-ATPase beta-subunit with the Na+/K+-ATPase alpha-subunit. Biochim. Biophys. Acta. 1997;1330:217–224. doi: 10.1016/s0005-2736(97)00167-3. [DOI] [PubMed] [Google Scholar]
- 57.Jha S., Dryer S.E. The beta1 subunit of Na+/K+-ATPase interacts with BKCa channels and affects their steady-state expression on the cell surface. FEBS Lett. 2009;583:3109–3114. doi: 10.1016/j.febslet.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mauerer U.R., Boulpaep E.L., Segal A.S. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J. Gen. Physiol. 1998;111:161–180. doi: 10.1085/jgp.111.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Priebe L., Friedrich M., Benndorf K. Functional interaction between K(ATP) channels and the Na(+)-K(+) pump in metabolically inhibited heart cells of the guinea-pig. J. Physiol. 1996;492:405–417. doi: 10.1113/jphysiol.1996.sp021317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mao H., Ferguson T.S., Cibulsky S.M., Holmqvist M., Ding C., Fei H., Levitan I.B. MONaKA, a novel modulator of the plasma membrane Na,K-ATPase. J. Neurosci. 2005;25:7934–7943. doi: 10.1523/JNEUROSCI.0635-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorokhova S., Bibert S., Geering K., Heintz N. A novel family of transmembrane proteins interacting with beta subunits of the Na,K-ATPase. Hum. Mol. Genet. 2007;16:2394–2410. doi: 10.1093/hmg/ddm167. [DOI] [PubMed] [Google Scholar]
- 62.Fuchs R., Schmid S., Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification; Proc. Natl Acad. Sci. USA; 1989. pp. 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renkawek K., Renier W.O., de Pont J.J., Vogels O.J., Gabreëls F.J. Neonatal status convulsivus, spongiform encephalopathy, and low activity of Na+/K(+)-ATPase in the brain. Epilepsia. 1992;33:58–64. doi: 10.1111/j.1528-1157.1992.tb02283.x. [DOI] [PubMed] [Google Scholar]
- 64.Gloor S., Antonicek H., Sweadner K.J., Pagliusi S., Frank R., Moos M., Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J. Cell Biol. 1990;110:165–174. doi: 10.1083/jcb.110.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benfenati V., Ferroni S. Water transport between CNS compartments: functional and molecular interactions between aquaporins and ion channels. Neuroscience. 2010;168:926–940. doi: 10.1016/j.neuroscience.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 66.Friedrich B., Matskevich I., Lang F. Cell volume regulatory mechanisms. Contrib. Nephrol. 2006;152:1–8. doi: 10.1159/000096284. [DOI] [PubMed] [Google Scholar]
- 67.Lang F. Mechanisms and significance of cell volume regulation. J. Am. Coll. Nutr. 2007;26:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- 68.Schneider G.H., Baethmann A., Kempski O. Mechanisms of glial swelling induced by glutamate. Can. J. Physiol. Pharmacol. 1992;70:334–343. doi: 10.1139/y92-280. [DOI] [PubMed] [Google Scholar]
- 69.Hansson E. Metabotropic glutamate receptor activation induces astroglial swelling. J. Biol. Chem. 1994;269:21955–21961. [PubMed] [Google Scholar]
- 70.Bender A.S., Schousboe A., Reichelt W., Norenberg M.D. Ionic mechanisms in glutamate-induced astrocyte swelling: role of K+ influx. J. Neurosci. Res. 1998;52:307–321. doi: 10.1002/(SICI)1097-4547(19980501)52:3<307::AID-JNR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 71.Koyama Y., Ishibashi T., Okamoto T., Matsuda T., Hashimoto H., Baba A. Transient treatments with l-glutamate and threo-beta-hydroxyaspartate induce swelling of rat cultured astrocytes. Neurochem. Int. 2000;36:167–173. doi: 10.1016/s0197-0186(99)00109-6. [DOI] [PubMed] [Google Scholar]
- 72.Golovina V., Song H., James P., Lingrel J., Blaustein M. Regulation of Ca2+ signaling by Na+ pump alpha-2 subunit expression. Ann. NY Acad. Sci. 2003;986:509–513. doi: 10.1111/j.1749-6632.2003.tb07236.x. [DOI] [PubMed] [Google Scholar]
- 73.Hartford A.K., Messer M.L., Moseley A.E., Lingrel J.B., Delamere N.A. Na,K-ATPase alpha 2 inhibition alters calcium responses in optic nerve astrocytes. Glia. 2004;45:229–237. doi: 10.1002/glia.10328. [DOI] [PubMed] [Google Scholar]
- 74.Pellerin L., Magistretti P.J. Excitatory amino acids stimulate aerobic glycolysis in astrocytes via an activation of the Na+/K+ATPase. Dev. Neurosci. 1996;18:336–342. doi: 10.1159/000111426. [DOI] [PubMed] [Google Scholar]
- 75.Magistretti P.J. Neuroscience. Low-cost travel in neurons . Science. 2009;325:1349–1351. doi: 10.1126/science.1180102. [DOI] [PubMed] [Google Scholar]
- 76.Soe R., Macaulay N., Klaerke D.A. Modulation of Kir4.1 and Kir4.1-Kir5.1 channels by small changes in cell volume. Neurosci. Lett. 2009;457:80–84. doi: 10.1016/j.neulet.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 77.Hirrlinger P.G., Wurm A., Hirrlinger J., Bringmann A., Reichenbach A. Osmotic swelling characteristics of glial cells in the murine hippocampus, cerebellum, and retina in situ. J. Neurochem. 2008;105:1405–1417. doi: 10.1111/j.1471-4159.2008.05243.x. [DOI] [PubMed] [Google Scholar]
- 78.Gorospe J.R., Maletkovic J. Alexander disease and megalencephalic leukoencephalopathy with subcortical cysts: leukodystrophies arising from astrocyte dysfunction. Ment. Retard. Dev. Disabil. Res. Rev. 2006;12:113–122. doi: 10.1002/mrdd.20101. [DOI] [PubMed] [Google Scholar]
- 79.Tinsa F., Farid O., Douira W., Rodriguez D., Burglen L., Boussetta K., Bousnina S. Megalencephalic leukoencephalopathy with subcortical cysts in a Tunisian boy. J. Child Neurol. 2009;24:87–89. doi: 10.1177/0883073808324021. [DOI] [PubMed] [Google Scholar]
- 80.Agresti C., Aloisi F., Levi G. Heterotypic and homotypic cellular interactions influencing the growth and differentiation of bipotential oligodendrocyte-type-2 astrocyte progenitors in culture. Dev. Biol. 1991;144:16–29. doi: 10.1016/0012-1606(91)90474-h. [DOI] [PubMed] [Google Scholar]
- 81.Kimelberg H.K., O'Connor E. Swelling of astrocytes causes membrane potential depolarization. Glia. 1988;1:219–224. doi: 10.1002/glia.440010307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.