Abstract
Major experimental and theoretical studies on microcirculation and hemorheology are reviewed with the focus on mechanics of blood flow and the vascular wall. Flow of the blood formed elements (red blood cells (RBCs), white blood cells or leukocytes (WBCs) and platelets) in individual arterioles, capillaries and venules, and in microvascular networks is discussed. Mechanical and rheological properties of the formed elements and their interactions with the vascular wall are reviewed. Short-term and long-term regulation of the microvasculature is discussed; the modes of regulation include metabolic, myogenic and shear-stress-dependent mechanisms as well as vascular adaptation such as angiogenesis and vascular remodeling.
Keywords: Blood flow, biofluidmechanics, cell mechanics, vascular regulation, computational model
1. INTRODUCTION
A great many advances have taken place in the field of microcirculation in recent years and a number of excellent reviews have examined its specific aspects (Bevan et al 1991, Michel & Curry 1999, Schmid-Schonbein & Granger 2003). In this review we will focus on the issues of mechanics of the systemic microcirculation; we will discuss both the relevant experimental observations and mathematical models.
The microcirculation represents the smallest blood vessels in the body and it consists of the capillary network, the smallest vessels of 4-8 μm inner diameter (i.d.), the arterioles, vessels up to ~ 100 μm i.d. in the arterial system, and the venules, vessels somewhat larger in the venous system. The microcirculation is responsible for regulation of blood flow in individual organs and for exchange between blood and tissue. Approximately 80% of the total pressure drop between the aorta and the vena cava occurs in these vessels. These features differentiate the microcirculation from the larger vessels of the macrocirculation, which serve as conduits to and from the heart and peripheral organs and as high and low pressure reservoirs essential to cardiac function. Another distinction is that microcirculatory vessels are embedded within an organ while most macrocirculatory vessels are not. This allows for communication between the parenchymal tissue and these vessels. The deleterious consequences of diseases such as hypertension, sickle cell anemia and diabetes exclusively, or to great extent, afflict the microcirculation. Major blood loss due to injury or other causes, if not rapidly replenished, can lead to irreversible malfunctions in the microcirculation (circulatory shock) and death. Microcirculatory disorders are major contributors to morbidity and mortality and constitute a significant fraction of total health costs to society. Both basic and clinical research has focused on the means of preventing or ameliorating the damaging effects of these disorders on normal bodily function. For example, the critical importance of timely replenishment of blood loss, whether due to injury or surgery, has led to large-scale efforts over the past 20 years to develop blood substitutes. Despite the research and development efforts of many laboratories, both public and private, and the expenditure of hundreds of millions of dollars, no substitute has yet been developed that can carry out the essential functions that whole blood performs in the circulatory system and most especially in the microcirculation. Clearly there is a need for a better understanding of how the special characteristics of blood and its flow properties make it such an effective means for delivery and exchange in the microcirculation.
Blood is a concentrated suspension of formed elements that includes red blood cells (RBCs) or erythrocytes, white blood cells (WBCs) or leukocytes, and platelets. The suspending fluid, the blood plasma, is an aqueous solution containing numerous chemical species, from ions, mainly Na+, K+, Ca2+ and Cl-, to macromolecules, ranging up to 500 kilodalton molecular weight. Red blood cells are biconcave discs with typical dimensions of 6-8 μm in diameter and 2 μm thick; in mammals the cells are non-nucleated and they consist of a concentrated hemoglobin solution enveloped by a highly flexible membrane. There are several classes of WBCs, e.g., granulocytes, which include neutrophils, basophils and eosinophils, monocytes, lymphocytes, macrophages, and phagocytes. They vary in size and properties e.g., a typical inactivated neutrophil is approximately spherical in shape with a diameter ~8 μm. Platelets are discoid particles with a diameter ~2 μm. Normal blood has a volume concentration of RBCs (hematocrit) ~40-45 %; under physiological conditions WBCs occupy ~1/600 of total cell volume and platelets occupy ~1/800 of total cell volume.
Out of the need for a detailed understanding of the flow properties of blood has arisen the field of hemorheology, the science of the deformation and flow of blood and its formed elements. This field includes investigation of the bulk properties of blood, determined in viscometric experiments in macroscopic samples, and of its microscopic properties in vitro and in vivo. The latter includes studies of the interactions among the cellular components of the blood and between these cellular components and endothelial cells that line blood vessels. The term microhemorheology is sometimes used to emphasize this scale of interest.
The rheological properties of blood are dependent on shear rate and its time history and the dimensions and geometry of the system in which it is contained. The principal rheological properties of blood, including its non-Newtonian characteristics, have been known for many years from viscometric studies that have utilized rotational viscometers and small glass tubes with length to diameter ratio of 102 to 103. Such studies have yielded valuable information on the properties of blood under certain well-defined conditions. For a number of reasons this information is not sufficient to understand the flow behavior of blood in the microcirculation. First, it is to be expected that the rheological properties of blood in a network, such as the microcirculation with its myriad vessel segments of different lengths, diameter, and shear rates, cannot be adequately predicted from viscometry in much simpler systems. Second, in the microcirculation the luminal surface of the vessel wall is coated with a fibrous material that retards, to varying degree depending on flow rate, the flow of blood in the immediate vicinity of the wall. Additionally, endothelial cells that line the walls of venules are endowed with receptors that interact with ligands on the WBC and allow the latter to adhere to the wall, leading in some cases to their transmigration into the tissue. Also of considerable importance is the fact that the arterioles, the microcirculatory vessels that regulate blood flow, are endowed with sensor mechanisms that monitor shear stress at the blood-vessel wall interface and apparently also monitor the circumferential stress in the wall. These mechanisms provide input to contractile elements in the vessel wall that respond in such a manner as to maintain these forces constant. Chronic changes in these forces over a period of days and weeks lead to adaptive changes in the vessel wall and in the network organization (angiogenesis and vascular remodeling). Since these forces are transmitted to the vessel wall by the blood in the vessel lumen, it is evident that the mechanics of this coupling would be significantly influenced by the rheological properties of the blood.
The history of experimental studies on microvessels goes back to the 17th century with the advent of the microscope. This led to Malpighi’s discovery of the capillary system while Van Leeuvenhoek described the complex branching network of microcirculatory vessels in the tail of the eel and measured the velocity of red cells in precapillary vessels to be on the order of 2 mm/s. To better understand the factors that determine flow in the blood vessels, in 1830 a French physician, Poiseuille, performed his now-classic experiments on the hydrodynamics of tube flow (Sutera & Skalak 1993). The principles revealed in those studies form the basis of much of our current understanding of blood flow in the larger vessels and in the microcirculation. In the 1930s a Swedish physiologist, Fahraeus, investigated the unique properties of blood flow in small glass tubes and set the foundation for subsequent research on microvascular flow and hemorheology (Goldsmith et al 1989). A renewed interest in the field since the 1960s has led to significant advances in the mechanics of the microcirculation (Chien 1987, Cokelet 1987, Fung & Zweifach 1971, Skalak et al 1989). Recent experimental description of the microcirculation has progressed greatly in large part because of developments in intravital microscopy and image analysis, the development of fluorescent probes for in vivo measurements, and new techniques for measuring molecular concentrations with high spatial and temporal resolution. As methodologies for direct studies in the microcirculation progress, comparisons between in vitro and in vivo rheological findings are providing new insights to the unique features of the microcirculation. Recent reviews give an account of experimental and theoretical developments in the field of microvascular hemorheology (Baskurt & Meiselman 2003, Mchedlishvili & Maeda 2001, Pries et al 1996, Secomb 2003), flow mechanics (Schmid-Schonbein 1999), and mathematical models (Secomb 2003).
2. RHEOLOGICAL PROPERTIES OF THE FORMED ELEMENTS
Rheological properties of the formed elements in blood have been recently reviewed (Waugh & Hochmuth 2001). The main function of RBCs is to carry oxygen from the lungs to the tissues and to carry carbon dioxide from the tissues to the lungs. The RBC membrane consists of a plasma membrane, which includes the lipid bilayer and its associated proteins, and an underlying cytoskeleton. The major cytoskeletal elements are spectrin, actin, and protein 4.1. These proteins form a viscoelastic network that is chiefly responsible for maintaining the structural integrity of the cell. The lipid bilayer, on the other hand, is responsible for near-conservation of the membrane surface area. Recent research is focused on the molecular properties of the cytoskeletal network (Discher 2000) and cytoskeleton-membrane interactions (Gov et al 2003). Taking a continuum perspective, the constitutive properties of the membrane have been modeled by relationships proposed by Evans and Skalak (Evans & Skalak 1980). The membrane envelops a concentrated solution of an oxygen-carrier protein called hemoglobin. Under physiological conditions, the solution is Newtonian, with a viscosity ~6 cPs. In many animal species, including human, RBCs have a tendency to aggregate in the presence of certain macromolecules, such as fibrinogen, forming one dimensional rouleaux or three-dimensional aggregates. The molecular models of RBC aggregation include bridging, due to the adsorption of macromolecules onto adjacent cell surfaces (Chien & Lang 1987), and depletion, due to the oncotic gradient arising from the exclusion of macromolecules near the cell surface (Neu & Meiselman 2002). Disaggregation is determined mainly by mechanical shear forces; the force required is ~1 dyn/cm2. Under physiological conditions, RBCs do not interact with the endothelium via specific receptors; however, receptor-ligand interactions are present under pathological conditions (Shiu & McIntire 2003).
White blood cells are part of the immune system. Some of them circulate in the blood stream and get activated by inflammation or by the presence of foreign particles or molecules; this activation results in a complex cascade of adhesion to the vascular endothelium and, ultimately, migration of the cell through the vascular wall and into the tissue. Other WBCs are lodged in the interstitial space between tissue cells. White blood cells consist of a plasma membrane, an underlying cortical layer whose primary component is actin, a three-dimensional cytoskeleton as part of the cytoplasm, and a nucleus. The nucleus is significantly stiffer than the rest of the cell. When WBCs are activated, e.g., in the presence of an antigen, their shape, cytoskeletal structure, and mechanical properties change dramatically. Rheological properties of WBCs have been described by viscoelastic models and by a model of a compound drop comprised of a viscous nucleus enveloped by two layers of less viscous fluid representing the cytoplasm and the plasma membrane/cortical layer (Tran-Son-Tay et al 1998). White blood cells interact with other cells, particularly endothelial cells, via their adhesion molecules and cell surface receptors. The nanomechanics of these interactions has been elucidated by numerous experimental studies in the last decade (Kamm 2002, Ley 2003, Zhu et al 2000). Specifically, the roles of adhesion receptors L-, P-, and E-selectin and of integrins have been investigated.
Unlike RBCs and WBCs, platelets are not cells, but rather non-nucleated disk-shaped cell fragments that are mainly involved in blood coagulation. Platelets express numerous surface receptors and adhesion molecules that mediate platelet-platelet interactions as well as interactions with endothelium and plasma components. Like WBCs, platelets possess a membrane and three-dimensional cytoskeleton. Activated platelets change their shape, cytoskeletal structure, and mechanical properties (Hellums 1994); these changes play an important role in blood coagulation.
3. FLOW OF RBC SUSPENSIONS IN NARROW TUBES – FAHRAEUS AND FARHAEUS-LINDQVIST EFFECTS
Due to the scale and complexity of the microcirculation, it has proven helpful to employ in vitro studies of blood in rotational viscometers and small glass tubes to infer the rheological properties of blood in microvessels. Red blood cells in many animal species, including humans, have a tendency to form aggregates that can reversibly disaggregate under the influence of shear forces. Red blood cell aggregation is a major determinant of the shear thinning property of blood; the other determinant is RBC deformation under local fluid forces. Fahraeus and many subsequent investigators performed experiments with blood flow in long glass tubes; they found that the dynamic or “tube” hematocrit (measured by stopping the flow and emptying the tube content) is consistently smaller than the “discharge” hematocrit measured in the discharge reservoir. This effect is referred to as the Fahraeus effect (Goldsmith et al 1989). In addition, the apparent viscosity of blood measured in long tubes < ~ 200 μm in diameter shows a precipitous decrease with decreasing diameter, reaching a minimum at diameters of ~ 5-7 μm, corresponding to the diameter of capillary blood vessels. This trend is known as the Fahraeus-Lindqvist effect (Pries et al 1992). The apparent or effective viscosity, μa, is defined as
| (1) |
where Δp is the pressure drop, D is the tube i.d., L is its length, and Q is the volumetric flow rate. For a Newtonian fluid Eq.1 becomes the Poiseuille law. These effects are similar to those observed in other concentrated suspensions of deformable particles. Simple qualitative physical arguments show that these effects are interrelated. The experimentally observed migration of RBCs away from the wall and the formation of a cell-depleted layer near the wall result in the mean RBC velocity being higher than the mean blood velocity. A mass-balance analysis shows that the Fahraeus effect is a consequence of this phenomenon. Further, because the viscosity of the cell-depleted layer is lower than the viscosity of the concentrated RBC core suspension, the apparent viscosity of blood is lower than the bulk viscosity of the uniform suspension as measured in tubes of large diameter or in rotational viscometers. However, while this qualitative reasoning is widely accepted, quantitative description of these effects has been difficult to achieve. Indeed, at a normal hematocrit of ~ 40%, the thickness of the cell-depleted layer is only several microns and is flow dependent. Since this thickness is on the order of RBC dimensions, the problem falls between the continuum and discrete descriptions. Nevertheless, two-phase continuum models considering a core of a Newtonian viscous fluid, representing the concentrated RBC core suspension and an annual concentric layer of a less viscous Newtonian fluid, representing the cell-depleted layer, are in reasonable quantitative agreement with experimental data on the apparent viscosity (Fahraeus-Lindqvist effect) of blood flow in tubes > ~ 30 μm in diameter (Secomb 2003). Similar agreement is obtained for tube hematocrit predictions (Fahraeus effect). In an attempt to reconcile the continuum model with data on both the apparent viscosity and tube hematocrit, the model was applied to calculate the thickness of the cell-depleted layer and an additional variable, the effective viscosity of the cell-depleted layer (Sharan & Popel 2001). It was argued that the presence of the “rough” interface between the RBC core and the cell-depleted layer should lead to additional viscous dissipation. This dissipation was estimated to contribute up to a factor of two to the effective viscosity. The calculated cell-depleted layer thickness was in good agreement with available experimental data in tubes > ~ 20 μm in diameter. Models considering discrete RBCs would be needed to describe the rheology in smaller-diameter tubes.
As the tube diameter decreases to the dimensions of an individual RBC, the apparent viscosity achieves a minimum and then increases as tube diameter decreases further. This inversion of the Fahraeus-Lindqvist effect corresponds to the flow regime in capillaries, where RBCs move in single-file flow, one after another. This geometry makes it possible to formulate a closed model of capillary blood flow. Significant advances have been made on this problem, starting with the classic studies on the pressure-driven flow of tightly-fitting deformable particles in a tube (Lighthill 1968), and continuing with extensive studies of R. Skalak and coworkers (Secomb 2003, Skalak et al 1989), which include more realistic constitutive models of the RBC.
Studies of RBC aggregation in tube flow have demonstrated a complex interplay between the increased local viscosity of an RBC aggregate and the tendency of that aggregation to increase the thickness of the cell-depleted layer (Reinke et al 1987). While RBC aggregation tends to create a more blunted velocity profile, which tends to increase apparent viscosity, it also leads to greater axial migration, which results in a larger cell-depleted layer at the wall. Thus, while some aggregation generally increases apparent viscosity, sufficiently high levels of aggregation can have the opposite effect. Red blood cell aggregation also affects distributions in WBCs and platelets (Goldsmith et al 1999). We will see below that many of these phenomena are qualitatively similar under in vivo conditions in the microcirculation. However, as noted above, there are a number of additional factors that arise in the microcirculation that limit direct applicability of in vitro findings.
4. MICROVASCULAR ARCHITECTURE AND ORGANIZATION
The flow properties of blood found in vivo depend importantly upon the geometry of the microvascular network. A microvascular network can consist of several vessels or several thousand vessels, and it can be supplied by one or more feed arterioles or arteries and drained by one or more collecting venules or veins. The networks are typically three dimensional, except in special tissues such as mesentery. Angioarchitecture and hemodynamics of microvascular networks have been characterized, to a some degree, in many organs and tissues including skeletal muscle, heart, brain, kidney, liver, mesentery, bone, eye, cochlea and tumors. These characteristics are highly specialized to meet the needs of the organ or tissue. Figure 1 shows two examples of microvascular architecture in skeletal muscle. The vasculature is much more abundant in kidney and brain for example which receive 22% and 15% of the resting cardiac output (5 L/min in humans), respectively, but constitute only 0.4% and 2% of body weight, respectively. Despite these specializations for unique requirements, certain common features are found in all vascular beds.
Figure 1.
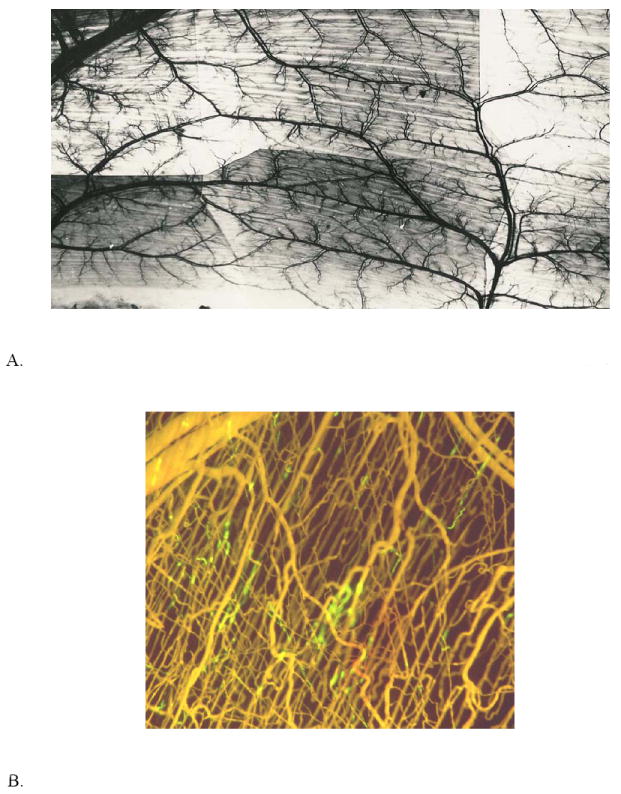
A. India ink injected microvascular network of cat sartorius muscle. Area shown is approximately 3 cm × 5 cm. (Courtesy of I. Torres Filho). B. Microvascular cast of hamster cremaster muscle. Microfil was infused following perfusion with fluorescein conjugated to albumin (FITC-BSA). Microfil displaced FITC-BSA except in segments indicated by green fluorescence. Area shown is approximately 1.5 mm × 1.5 mm. (Courtesy of S. Segal).
The arterial vascular network consists of a series of approximately cylindrical segments with decreasing diameter and length and increasing numbers of parallel channels as one moves from the aorta to the capillary beds of individual organs. The thickness of the vessel wall varies with vessel diameter. The vessel wall, from outside inward, consists of consecutive layers of connective tissue, vascular smooth muscle, internal elastic lamina or basement membrane, and endothelial cells. The width of each layer, except the basement membrane and endothelium, decreases with decreasing vessel diameter. When an arteriole is dilated, its lumen is approximately circular; however, during arteriolar constriction the lumen may change to have an irregular star-shaped cross section, primarily due to bulging endothelial cells. Sympathetic nerve fibers, which are particularly abundant in arterioles, are adjacent to smooth muscle cells and release a neurotransmitter (norepinephrine) from varicosities in the nerve to cause constriction of the vessel. Vascular smooth muscle and endothelial cells communicate with adjacent cells through gap junctions that allow the spread of electrical depolarization and provide a means of coordinating constrictor and dilator responses of adjacent vascular segments by decremental conduction with a length constant of about 1 mm (Dora et al 2000).
The arteriolar network in many organs is organized in two general patterns, an arcade arrangement in larger vessels (> ~ 25 μm) and a sequential branching pattern in the more distal vessels. Quantitative description of the tree-like morphology of distal arteriolar networks shows that vessel number, and their diameter and length follow power-law relationships (Ellsworth et al 1987, Koller et al 1987). The implications are that the networks can be described as fractal structures, i.e., they exhibit self-similarity. Fractal properties of vascular networks in different organs and tissues have been extensively explored (Baish & Jain 2000, Bassingthwaighte et al 1994, Popel 1987).
The interplay between increasing vessel numbers and decreasing vessel diameter and length leads to a pressure gradient through the arterial network that is significant in the small arteries (resistance arteries) and is most pronounced in the arterioles where the ratio of wall thickness to lumen diameter is also greatest. This anatomical structure enables regulation of blood flow primarily by means diameter change (vasodilation or vasoconstriction). These diameter changes lead to changes in hydrodynamic resistance, defined as the ratio of pressure drop to volumetric flow rate, roughly in accordance with Poiseuille’s law, e.g., a two-fold uniform dilation in a vascular segment leads to an approximately 16-fold change in resistance in that segment. This dependence is modified somewhat by the Fahraeus-Lindqvist effect. It is not surprising that the region of the arteriolar network where the pressure gradient is steepest is also the region where the greatest active changes in diameter typically occur (Dodd & Johnson 1991).
In skeletal muscle, capillary networks consist of numerous parallel channels with typically three to six capillaries arising from each precapillary arteriole. Additional branching occurs along the length of the capillary network and diameter is somewhat greater in the distal portion of the capillary network. The pressure drop through the capillary network varies from 5 to 20 mm Hg in different vascular beds under normal conditions and depends on the number, length and diameter of capillaries as described above for arterioles. The mean capillary transmural pressure in most organs is ~ 20 mm Hg but varies from ~ 45 mm Hg in the renal glomerulus to ~ 4 mm Hg in the liver. Capillaries lack smooth muscle and thus cannot actively dilate or contract. However, there is evidence that the surface area available for exchange in the capillaries in some vascular beds may be regulated to a degree by precapillary sphincters or by terminal arterioles independently of overall flow regulation. On the luminal side, the endothelium is endowed with a glycocalyx surface layer that contains proteoglycans, glycoproteins, and glycosaminoglycans (Desjardins & Duling 1990, Henry & Duling 1999, Pries et al 2000). The glycocalyx plays an important role in modulating hemodynamic resistance and hematocrit in the capillary, as discussed below in section 5.2. On the abluminal side, part of the endothelium is covered by pericytes, and both are embedded within a basement membrane or basal lamina, a dense specialized form of the extracellular matrix.
The capillaries provide a large surface area for exchange. In the peripheral circulation the surface area is estimated to total 70 m2 in humans. The capillaries are specialized to provide minimal diffusion distance from blood to tissue and maximal opportunity for exchange of certain molecules while providing a barrier to others (Michel & Curry 1999). Small arterioles and venules also contribute to exchange of some molecular species. The specialized morphology of this region makes possible, among other things, exchange of nutrients and waste products between blood and tissue, maintenance of fluid balance, communication between endocrine glands and target organs, bulk transfer between organs, and immunological defense. Fluid transfer occurs across the capillary wall by bulk flow through specialized channels (aquaporins) and gaps between adjacent endothelial cells. In capillaries and venules, macromolecular transfer takes place through specialized channels, which are larger than aquaporins or endothelial gap junctions. In brain and kidney specialized extra-luminal structures (tight junctions) prevent or modulate movement of solutes and solvent molecules. The glycocalyx also affects vascular permeability. Effective fluid balance between blood and tissue requires regulatory mechanisms that maintain hydrostatic pressure within narrow limits in the capillaries.
The immediate post-capillary venules (6-8 μm i.d.) collect blood from several capillaries, and like capillaries, are simple endothelial tubes surrounded by a basement membrane, which together with the surrounding parenchymal cells, provide mechanical support for the endothelium. Composition of the walls of the larger venules is similar to, but thinner than, their arteriolar counterparts. However, in skeletal muscle tissue, the smooth muscle layer is absent in venules < ~50 μm i.d. The venous network resembles the arteriolar network in branching architecture except that the venous vessels are more numerous and individual segments are correspondingly shorter and wider. In some vascular beds arterial and venous vessels are side by side through a portion of the network, allowing for exchange of vasoactive agents directly between the vessels. Additional information on microvascular network angioarchitecture and hemodynamics can be found in Popel (1987) and Popel & Johnson (1986).
5. BLOOD FLOW IN INDIVIDUAL MICROVESSELS
A wide variety of methods are available to measure blood flow in the microcirculation. Centerline velocity and velocity profiles in arterioles and venules have been measured using temporal or spatial correlations in the intensity of light under either trans- or epi-illumination. Fluorescently labeled RBCs, platelets, or microspheres as marker particles have also been used. Recently, Particle Image Velocimetry (PIV) has been adopted from fluid mechanics and applied to measurements of velocity profiles in microvessels (Nakano et al 2003, Smith et al 2003). Local hematocrit, a key factor in oxygen delivery, has been assessed by the RBC-dependent absorption or scattering of transmitted light or by measuring the concentration of fluorescent marker particles. Unfortunately, direct measurements of pressure gradients and therefore wall shear stress in microvascular segments are scarce; these measurements require simultaneous insertion of two micropipettes in a vessel segment and are technically very difficult. Summaries of velocity and pressure measurements in different tissues and organs can be found in reviews by Popel (1987) and Zweifach & Lipowsky (1984). Table 1 illustrates typical hemodynamic parameters for several selected microvessels in skeletal muscle. Note that both the Reynolds and Womersley numbers are small and, therefore, inertia does not play a significant role. The cell-depleted layer near the wall has also been observed in vivo in arterioles and venules. Its width, which varies slightly with vessel diameter (Bishop et al 2001c, Tateishi et al 1994), is typically a few microns and depends on blood flow rate, hematocrit, and the degree of RBC aggregation.
Table 1.
Typical microcirculatory parameters in resting cat sartorius muscle for several representative vessels
| Diameter, μm | Mean velocity, mm/s | Re | α | Pseudoshear rate, s-1 | *Wall shear stress, dyn/cm2 | **In vitro μa, cPs, at Hd=0.45 | **In vivo μa, cPs, at Hd=0.4 | |
|---|---|---|---|---|---|---|---|---|
| Arterioles | 60 | 12 | 0.2 | 0.8 | 200 | 60 | 2.6 | 2.8 |
| 15 | 7 | 0.03 | 0.2 | 470 | 140 | 1.8 | 4.9 | |
| Capillaries | 5 | 0.2 | 0.0003 | 0.07 | 40 | 12 | 1.8 | 15 |
| Venules | 18 | 0.2 | 0.001 | 0.2 | 10 | 3 | 1.9 | 4.2 |
| 72 | 2.4 | 0.05 | 0.9 | 30 | 10 | 2.7 | 2.9 | |
Notation: Re is the Reynolds number, Re=ρVmD/μb, where Vm is the mean velocity, μb=3.84 cPs is the bulk viscosity of blood at 45% hematocrit; α is the Womersley number, α=D(ρω/μb)1/2, where ω is the characteristic frequency.
Estimated as τw=8μbVm
Estimated from (Pries et al 1996)
Before we turn to the specific descriptions of blood flow in microvessels, it is appropriate to mention general theoretical work on blood flow mechanics. A multicomponent model of blood flow has been developed, based on principles of continuum mechanics and nonequilibrium thermodynamics, to describe relationships between gradients of state variables (e.g., local hematocrit) and the corresponding fluxes (Regirer 1990). Practical application of this model to specific problems requires the knowledge of a large number of the phenomenological coefficients, most of which remain unknown; however, the model is valuable in providing the general view of the physical interactions in flow of concentrated RBC suspensions. On the other extreme are the models of flow of discrete formed elements. Modeling flow with a large number of discrete particles has become possible due to the progress in numerical techniques and computer hardware capabilities (Hu et al 2001). However, numerical modeling of the three-dimensional motion of RBCs has remained a difficult problem and only limited success has been achieved. The main difficulty stems from enforcing the local near-conservation of the RBC plasma-membrane surface area. Motion of a single RBC in simple shear flow was modeled using the immersed boundary method (Eggleton & Popel 1998) and boundary integral method (Pozrikidis 2004). Considering the difficulty in simulating single RBCs, it does not appear that the current techniques could be readily extended to multiparticle systems, with hundreds or thousands of particles present.
White blood cell deformation in unbounded shear flow has been modeled using a variety of rheological models and numerical techniques. For example, a WBC represented as a composite viscous drop in an extensional flow was modeled using a variation of the immersed boundary method (Tran-Son-Tay et al 1998). A version of this method was also utilized in modeling platelet aggregation (Wang & Fogelson 1999).
5.1 Flow in arterioles
Flow velocity in arterioles varies during the cardiac cycle. Pulsatile flow is increasingly attenuated in the more distal vessels. Measurements of velocity in arterioles under 60 μm i.d. demonstrate blunted velocity profiles, with some degree of asymmetry. The degree of blunting increases in the smaller vessels (Ellsworth & Pittman 1986, Nakano et al 2003, Tangelder et al 1986). In a vessel of radius R, axisymmetric velocity profiles, v(r,t), can be described in cylindrical coordinates (r, θ, z) by the empirical relationship
| (2) |
where f(t) is a periodic function of time phase-shifted with respect to the cardiac cycle and K represents the degree of blunting (a value of 2 reflects a parabolic profile). For example, in 17-32 μm arterioles a range of K values of 2.4-4 was reported at normal flow rates (Tangelder et al 1986). Blunting would be expected when the size of the red cell becomes a significant fraction of vessel diameter. However, in many cases the velocity profile is nearly parabolic. It is to be expected that blunting would occur even in larger arterioles at lower shear rates due to RBC aggregate formation. Measurements in arterioles at low flow rates, however, are not available.
If the thickness of the arterial glycocalyx and its associated layer of adsorbed proteins is negligible compared to both the width of the cell-depleted layer and the vessel diameter, theoretical description of flow in sufficiently long arteriolar segments should be similar to that described above for long tubes. Namely, for vessels larger that 30 μm i.d., a two-phase continuum model, consisting of a concentrated RBC suspension in the core and an annular concentric layer representing the cell-depleted layer, qualitatively describes axisymmetric velocity profiles and the Fahraeus and Fahraeus-Lindqvist effects. However, comprehensive quantitative comparisons between the models and experimental velocity profiles are lacking. Comparisons between the measured and calculated values of apparent viscosity and of vessel hematocrit in microvessels are indirect. One such comparison (Pries et al 1994), based on minimization of errors between velocity and hematocrit measurements in hundreds of vessels in the rat mesentery and their calculated values using a network model, are described below in section 6. Note that for arterioles having diameters less than 20–25 μm, where the applicability of continuum models is questionable (Cokelet 1999), there is no alternative theory. As noted above, computational models that account for interactions between discrete RBCs in these vessels have not been developed.
Flowing WBCs do not significantly affect arteriolar flow because of their small concentration. White blood cells typically do not adhere to the arteriolar endothelium. However, they can plug capillaries and they strongly interact with venular endothelium. These issues are discussed below in section 5.3.
5.2 Flow in capillaries
Since, as described above, capillary diameter is often smaller than the size of the undeformed RBC, RBCs deform into axisymmetric parachute-like shapes or asymmetric crepe-like shapes when they traverse the capillaries. The cellular flow in capillaries can be measured in terms of the velocity of individual RBCs, the number of RBCs per unit time (RBC flux), or the transit time through the network. Red blood cell flux is important physiologically as an indicator of oxygen delivery. Time-averaged RBC flux can be expressed as the product of RBC velocity and RBC density. The latter is closely related to the instantaneous volume concentration of red cells in the vessel, or dynamic hematocrit, which is analogous to tube hematocrit in vitro. Duling and coworkers observed that the capillary hematocrit was flow dependent and its values were significantly smaller than the systemic hematocrit or even the corresponding in vitro tube hematocrit. This observation led them to hypothesize the existence of a shear-rate dependent 1-μm-thick capillary glycocalyx. Subsequent studies validated this hypothesis and probed the mechanical and electromechanical properties of the glycocalyx and its associated macromolecules, which is often referred to, collectively, as the endothelial surface layer (ESL) (Desjardins & Duling 1990, Henry & Duling 1999, Pries et al 2000, Vink & Duling 2000). There is some evidence that the thickness of the layer depends on local shear forces, falling from ~ 1 μm at red-cell velocities ~0.1 mm/s to less than 0.2 μm at 0.4 mm/s. Whereas RBCs are small and flexible enough to be excluded by the capillary ESL, the relatively larger and stiffer WBCs overwhelm the restoring forces in the ESL, which is apparently “stripped down” as a WBC traverses a capillary (Vink & Duling 1996). In the wake of a WBC, the capillary ESL appears to recover within seconds. Studies show that the structure of the ESL is physiologically regulated and might be partially shed during inflammation and ischemia (Mulivor & Lipowsky 2004).
Experimental studies of capillary flow have provided fertile ground for mathematical modeling of RBC motion in capillaries for over three decades. Numerous models have been developed and both analytical and numerical solutions have been derived (Secomb 2003). In most models the RBC membrane is treated as an elastic membrane or thin shell undergoing finite deformations (Pozrikidis 2003). Most of the hydrodynamic resistance to flow is contributed by the thin plasma film between the cell and the vessel wall; this region is typically treated using lubrication theory approximations. Both axisymmetric and non-axisymmetric problems have been considered as well as flow in “corrugated” non-cylindrical capillaries, where the corrugations are meant to simulate the effect of the protrusions of endothelial cells into the lumen. More recent studies focus on modeling the effect of the endothelial glycocalyx in capillaries. A mechano-electrochemical model considers a multicomponent mixture of an incompressible fluid, an anionic porous deformable matrix, and mobile ions (Damiano & Stace 2002). In this model a significant part of the resistance of the glycocalyx to mechanical deformation is determined by electrical charges located primarily on the glycosaminoglycan (GAG) chains. In another model, oncotic pressure generated by plasma proteins adsorbed to the ESL is implicated as a key contributor to the stiffness of the ESL (Pries et al 2000). These two models take a continuum approach. A microstructural model has been developed in which the glycocalyx is considered as a discrete matrix of core proteins that determine resistance to fluid flow and deformation; this is in contrast to the assumption that the side GAG chains of the core proteins are the major determinant (Feng & Weinbaum 2000, Weinbaum et al 2003). The authors argue that the structural integrity of the glycocalyx is consistent with the known flexural rigidity of core proteins. Estimates by several authors also show that the elastic recoil force exerted by an arrested RBC is sufficient to deform the glycocalyx in accordance with experimental observations that RBC width increases in flow arrest (Secomb 2003).
Models of RBC flow in capillaries in the presence of the glycocalyx are based on these developments (Feng & Weinbaum 2000, Secomb et al 2002). They predict a significant increase in capillary resistance in vivo relative to blood-perfused glass tubes in vitro. In particular, blood flow resistance, or apparent viscosity, is predicted to be eight-fold greater in a 6-μm-diameter capillary with a 1-μm-thick glycocalyx than in a glass capillary tube of the diameter. (Secomb et al 2002). This difference reduces to approximately a factor of two in a 6-μm-diameter capillary if a 0.5-μm-thick glycocalyx were considered (Damiano 1998), which is consistent with the thickness reported by Vink & Duling (1996). The model of Secomb et al (2002) also identifies the role of the glycocalyx in “softening” the effect nonuniformities in capillary cross section have on RBC deformations and, possibly, in extending RBC life span.
Capillary plugging by WBCs, especially activated WBCs, can lead to significant flow changes in the microvasculature (Eppihimer & Lipowsky 1996, Schmid-Schonbein 1999). This effect is important under pathological conditions such as inflammation or ischemia-reperfusion.
5.3 Flow in venules
Due to the relatively lower shear rates in the venular network (where pseudoshear rates, defined as the ratio of mean velocity to vessel diameter, have been reported in cat sartorius muscle in the range of 5 to 23 s-1 under resting flow conditions) flow resistance is dependent on flow rate in species where RBC aggregation occurs. Interestingly, RBC aggregation seems to arise in athletic species (e.g., human, dog, horse) but appears to be absent from sedentary species (e.g., sheep, cow) (Popel et al 1994). Venous vascular resistance in skeletal muscle tissue was found to be inversely proportional to flow rate in normally aggregating cat blood but this nonlinearity was essentially abolished when the muscle was perfused with RBCs suspended in Dextran-40, which eliminates aggregation (Cabel et al 1997). The venous resistance doubled when flow was reduced five-fold from the control level. Similarly, resistance fell by 60% when flow was increased four-fold from the control level. This property may be important in maintaining normal capillary hydrostatic pressure with large increases in blood flow, as typically occurs in exercising skeletal muscle (Bishop et al 2001d).
Due to the frequent branching in the venular network, there is a continuous input of RBCs and RBC aggregates into the peripheral layer of the flow stream. This, coupled with the short segment length of individual microvessels, where the diameter to length ratio is only 1:3.5 in rat spinotrapezius muscle (Bishop et al 2001b, Bishop et al 2001c), tends to attenuate the formation of a cell-depleted layer adjacent to the vessel wall. In the presence of aggregation (in these experiments induced by high molecular weight Dextran-500 since rat RBCs do not naturally aggregate), there is a significant increase in the RBC axial migration rate. Red blood cell aggregates were directly observed in venules at pseudoshear rates < 70 s-1 (Bishop et al 2004). One consequence of RBC aggregation that might further enhance apparent viscosity is the tendency of aggregates to marginalize WBCs into the cell-depleted layer and thereby increase the effective local viscosity in this region. (Pearson & Lipowsky 2000). Velocity profiles extracted from RBC marker particles exhibit blunting at low flow rate, and are sometime non-axisymmetric (Bishop et al 2001a, Ellsworth & Pittman 1986). The values of K in Eq. 2 vary between 2 at pseudoshear rates > ~ 50 s-1 and 3 at pseudoshear rates ~ 5 s-1 (Bishop et al 2001a). Estimates suggest that this degree of blunting may double venous resistance. Recent experiments used fluorescent microspheres of ~ 0.5 μm in diameter to obtain velocity profiles in venules of the mouse cremaster muscle (Smith et al 2003). Even though mouse RBCs do not aggregate, the velocity profiles exhibit significant blunting. Moreover, the measurements reveal an ESL thickness ranging between 0.3-0.5 μm (Damiano et al 2004; Smith et al 2003), and a nearly vanishing plasma velocity inside the ESL, i.e., this layer so effectively retards plasma flow that it can be essentially excluded from the total cross-sectional area available to blood flow in the microcirculation. Even though with increasing vessel diameter the ratio of ESL thickness to vessel diameter becomes relatively small in post-capillary venules (ESL thickness appears to be independent of vessel diameter in venules < ~ 50 μm i.d.), there is evidence that the effect on the vessel resistance remains significant for vessel diameters up to 50 μm. This effect will be further discussed in section 6.
Flow in venules with diameter > ~ 25 μm can be described by a two-phase continuum model (Cokelet 1999), similar to the ones formulated for arteriolar flow, or a model with a continuous variation of local hematocrit (Long et al 2004) with two notable exceptions: (a) flow in the core is modeled as non-Newtonian, shear-thinning, e.g., using Casson’s or Quemada’s rheological model, or a model for thixotropic fluid that includes time-dependent effects reflecting aggregations/disaggregation kinetics and (b) flow may consist of several streams with different hematocrits and different viscosities, giving rise to non-axisymmetric velocity profiles (Das et al 1997, Das et al 1998). Detailed velocity measurements using microspheres in mouse venules permitted estimates of the shear rate profile, from which the shear stress profile was estimated assuming the viscosity at the ESL interface was equal to the measured plasma viscosity (Long et al 2004). Based on a continuum model that assumed a time-averaged viscosity profile exists that reflects a time-averaged hematocrit profile (Damiano et al 2004, Long et al 2004), other characteristics of the flow were also estimated. Validation of these estimates was provided by glass tube experiments in vitro and hemodilution experiments in vivo (Long et al 2004). For venules < ~ 20 μm i.d., where discrete RBCs must be taken into account, no theory is available.
Adhesion molecules dependent WBC-endothelial interactions occur predominantly on the venous side of the microcirculation and a vast amount of experimental work has been devoted to the subject, which includes direct observations of WBCs rolling on, adhering to, and transmigrating across venular endothelium, and numerous in vivo and in vitro investigations into the molecular mechanisms of these interactions (Schmid-Schonbein & Granger 2003). The space limitations of this review preclude us from describing this experimental work in detail. Thus, we limit ourselves to a brief discussion of the key theoretical work on the subject. The first step in the leukocyte adhesion cascade is leukocyte margination from the free stream towards the vascular wall. This process was modeled in 2-D by considering a circular rigid WBC and a train of rigid disk-shaped RBCs (Sun et al 2003). A lattice Boltzmann method was used in which both fluid and solid particles were regarded as discrete packets moving along a prescribed grid of nodes. The computer simulations suggested a role for RBCs in the margination of WBCs. Once the WBC begins to interact directly with the endothelium, these interactions are governed by receptor-ligand forces as well as fluid mechanical forces. The forces result in the WBC rolling along the endothelium or adhering and remaining in a stationary or quasi-stationary position. Since WBCs are deformable, both rolling and adhesion are accompanied by the cell deformation. These processes have been modeled using various rheological models of the WBC, kinetic models of receptor-ligand interactions, and a variety of numerical methods (Das et al 2000, Dong et al 1999, Dong & Lei 2000, King & Hammer 2001, Krishmatullin & Truskey 2004, N’Dri et al 2003, Sugihara-Seki & Schmid-Schonbein 2003, Sun et al 2003). WBCs possess small rod-like protrusions (microvilli) and the receptor-ligand interactions occur through the molecules on the tips of the microvilli. The presence of the endothelial glycocalyx or ESL poses the question: how do the ligands (adhesion molecules) interact with their receptors through the thick ESL whose dimensions are significantly larger than the size of the interacting molecules? This dilemma formed the basis of a microstructural model in which hydro-elastic penetration of the microvilli into the glycocalyx was considered (Weinbaum et al 2003); the theory showed that such penetration is consistent with the parameters of the system. An alternative or complementary explanation is that WBC begin rolling in venules as they crawl out of capillaries where they strip down the ESL (Smith et al 2003).
6. FLOW IN MICROVASCULAR NETWORKS
As noted in section 4, there are certain anatomic and topographic features of microvascular networks that appear to be common to a variety of organs and tissues. A functional feature commonly present in vascular beds is the substantial heterogeneity in the distributions of RBC velocity and concentration between vascular segments of similar size in the microcirculation. In skeletal muscle, which has been studied most extensively, the coefficient of variation (Standard Deviation/mean) of RBC velocity in capillaries, arterioles, and venules typically varies between 50% and 100% (Duling & Damon 1987). These heterogeneities are the hallmark of the microcirculation and have significant impact on its exchange function. They can be interpreted as fractal properties of the microcirculation (Beard & Bassingthwaighte 2000). The heterogeneity of RBC velocities are determined by several factors, among them the topology and geometry of the microvascular pathways resulting in different effective hydrodynamic resistances along these pathways. The source of hematocrit heterogeneity is uneven separation of RBCs and plasma at microvascular bifurcations. It has been shown experimentally that daughter branches with higher bulk flow draw a disproportionately larger number of RBCs. When flow in a daughter branch is sufficiently small, the branch might receive few or no RBCs, which in the extreme results in a plasmatic vessel. The relationship between the ratio of RBC flux into a daughter branch and the total RBC flux in the parent vessel of a bifurcation versus the ratio of the corresponding bulk flows is called “the bifurcation law.” Support for the bifurcation law has been obtained empirically in vitro and in vivo (Pries et al 1996) and in some cases, can be established on theoretical grounds (Enden & Popel 1994). At the network level, the tendency of vascular segments with lower blood flow to have lower vessel hematocrit results in a lower mean hematocrit calculated over segments of similar dimensions. By analogy with the Fahraeus effect for a single segment, this effect was termed the “network Fahraeus effect” (Pries et al 1996). In essence, the low-flow vessels in a network can be likened to the cell-depleted (high-drag) layer in an individual microvessel. The apparent viscosity of blood in a vascular segment is a function of segment diameter, the local hematocrit and, in some situations, wall shear rate, e.g., if RBC aggregation is significant. Theoretical modeling of blood flow in a microvascular network involves several steps. First, the geometry of the network is specified, i.e., segment interconnections and their lengths and diameters (and cross-sectional shapes if not cylindrical) are measured. Pressure-flow relationships are expressed in terms of Eq. 1 with the apparent viscosity μa specified. In addition, the bifurcation law describes bulk flow versus the RBC flux relationship at every bifurcation. Therefore, for a network containing N segments the problem consists of 2N nonlinear algebraic equations with respect to the unknown flow rate Q and discharge hematocrit H for each segment. Alternatively, the flow can be expressed in terms of pressures at the ends of the segment according to Eq. 1. At the inlet and outlet nodes of the network, either flow or pressure can be specified. Solving the system of algebraic equations results in a prediction of the distribution of flow, pressure, and hematocrit throughout the network. This scheme has been applied to predict the distribution of hemodynamic parameters in a rat mesentery network consisting of several hundred segments, for which detailed morphologic and hemodynamic measurements are available (Pries et al 1996, Pries et al 1994). Using the values of μa based on in vitro measurements resulted in significant discrepancies between the predicted and measured values; minimization of these discrepancies yielded the values of “in vivo” apparent viscosity that were higher than the corresponding in vitro values; the difference was attributed to several sources including one due to the ESL, which effectively decreases the effective luminal diameter available to blood flow in the microcirculation by retarding plasma flow near the endothelial-cell surface. These calculations suggested that for a given discharge hematocrit, the total network hydrodynamic resistance considerably higher than the resistance based on in vitro values of μa (Pries et al 1996). This disparity persisted in both arterioles and venules as large as 30 to 40 μm i.d. Thus far, the rat mesentery is the only tissue for which theoretical predictions have been compared with experimental measurements on a segment-by-segment basis; it remains to be seen whether the formulated in vivo apparent viscosity and bifurcation laws are quantitatively the same for other tissues. The emerging understanding of the dynamic nature of the ESL and its dependence on such factors as plasma composition suggests that this structure may be an important determinant of local blood flow regulation in a network. Below we consider other regulatory factors such as wall shear stress, oxygen level, and arteriolar wall hoop stress.
7. REGULATION OF BLOOD FLOW IN MICROVASCULAR NETWORKS
7.1 Short-term regulation
In the preceding section, flow in microvascular networks is described for a fixed network geometry. However, blood vessels can change their diameter passively or actively through alteration of the contractile state of smooth muscle in the vascular wall. These diameter changes lead to alterations in resistance within the vascular segment, which varies in approximately inverse proportion to the fourth power in vessel diameter (see Eq. 1). This strong dependence of vascular resistance on vessel diameter is the basis for blood flow regulation. Most of these diameter changes occur in arterioles and small arteries. Large changes in vascular resistance and blood flow are common in many tissues, e.g., heart and skeletal muscle in transition from rest to exercise. These changes occur on a time scale of seconds or minutes; they may also include recruitment and derecruitment of small blood vessels, mostly capillaries. Under physiological conditions, these changes are part of the exquisite matching between the supply of oxygen and nutrients by blood and the demand of tissues. In pathological conditions, this matching between supply and demand can become abrogated and lead to tissue hypoxia and other metabolic deficiencies.
The regulatory mechanisms that induce active changes in the contractile state of vascular smooth muscle include neural, hormonal, metabolic, autacoidal (secreted) and mechanical factors. Regulation of vascular tone is a major problem of cardiovascular physiology and a vast experimental literature exists on the various aspects of this topic. Here we briefly discuss the mechanisms where mechanics plays a significant role. When vascular smooth muscle is inactivated, vessels respond passively to changes in transmural pressure, i.e. their diameter increases with increasing pressure and decreases with decreasing pressure. When smooth muscle is physiologically active, arterioles and small arteries possess the unique ability to respond actively to changes in transmural pressure, i.e. they constrict when pressure is increased and dilate when pressure is decreased. This is the myogenic mechanism of vascular-tone regulation. Contrary to the passive behavior, therefore, the slope of the pressure-diameter relationships for arterioles and small arteries is negative under active regulation. It has been shown empirically that this response tends to maintain a constant hoop stress. Recent studies have elucidated molecular mechanisms underlying vascular-tone regulation. These are associated with smooth muscle intracellular calcium concentration, the function of ion channels, and the role of cell signaling via its mechanical receptors integrins (Davis et al 2001). While it is expected that the instantaneous control of blood flow would be linked to the metabolic needs of the tissue, the myogenic mechanism is a principal determinant of blood flow in many organs such as skeletal muscle, brain and myocardium. It is responsible for the phenomenon of blood flow autoregulation in which blood flow is maintained nearly constant over a range of arterial pressure variations.
The response of resistance vessels to changes in wall shear stress through an effect on the endothelium that releases nitric oxide (NO) is another important regulatory mechanism determined by mechanical factors. This effect is generally present in arterial vessels and provides a mechanism for dilating the supply arteries to an organ when the arterioles within the organ dilate in response to a rise in vasodilator signals within the organ, which leads to a further increase in blood flow (a positive feedback mechanism). Molecular mechanisms of shear-stress dependent vasodilation has been studied extensively (Busse & Fleming 2003). A recently proposed local regulatory mechanism places RBC in the center stage; when RBC deforms as it traverses the microvessels, it sheds adenosine triphosphate (ATP), which is a potent vasodilator (Ellsworth 2004). It should be noted that in addition to stimulating secretion of NO and affecting the ESL, fluid shear stress causes other significant changes in endothelial cells, including possible changes in the plasma membrane viscosity (Butler et al 2001, Haidekker et al 2000) and the cytoskeleton (Helmke & Davies 2002), which in turn might affect the micromechanics of blood flow.
Computational models have explored aspects of vascular regulation, as individual mechanisms, and as an integrative system. The negative slope of the pressure-diameter relationship associated with the myogenic mechanism may lead to mechanical instability and self-sustained oscillations, either periodic or deterministically chaotic (Regirer & Shadrina 2002, Ursino et al 1998). These predictions are consistent with experimentally observed spontaneous oscillations of vascular diameter, called vasomotion. The physiological significance of vasomotion has not been definitively established. However, non-mechanical electrophysiological factors have also been implicated in spontaneous oscillations (Bartlett et al 2000). An electrophysiological model of the smooth muscle cell with an explicit representation of ionic channels, pumps, and other cellular transport entities demonstrates the existence of complex oscillations in intracellular Ca2+ and membrane potential that would lead to smooth muscle contractions (Parthimos et al 1999). This modeling approach should eventually allow the response to pharmacological interventions to be understood at the cellular and molecular levels. A similar approach can be developed for endothelial cells. Such models could then be combined and extended to provide a detailed molecular-level model of the vascular wall.
In recent years several integrative models of microvascular flow regulation have been developed that take into account metabolic, myogenic and shear-dependent mechanisms. In one such model, propagated vasodilation was also included as a way of facilitating information transfer between vessels in a network (Pries et al 2003). The model reveals complex interactions between these different mechanisms and shows how these interactions lead to a coordinated network response to metabolic and mechanical stimuli. The model describes steady state and was designed specifically for angioadaptation or long-term regulation; however, the concepts are also applicable to modeling short-term regulation. Significant progress has been achieved in making very difficult measurements of microvascular parameters in the beating heart. In addition to the metabolic, myogenic, and shear-stress dependent factors, models of the coronary microcirculation have also included the effects on transmural pressure induced by changes in the myocardial tissue pressure (Cornelissen et al 2002). These studies integrate complex relationships between different mechanisms of local microvascular control and could serve as a guide for formulating quantitative hypotheses and designing further experiments.
7.2 Long-term regulation: Vascular remodeling and angiogenesis
Long-term changes of vascular network structure occur on a time scale of days or weeks or more. Vascular networks may adapt in response to such factors as prolonged changes of arterial pressure, ischemia, hypoxia, glucose level, and tumor growth. Adaptation includes structural changes of the vascular wall as well as changes in network topology that occur through growth of new vessels (angiogenesis) or remodeling of existing vessels (including vascular rarefaction or retraction). Systematic modeling studies of microvascular network adaptation have been carried out based on geometric and hemodynamic measurements in rat mesenteric networks (Pries et al 2003). The authors hypothesized rules for diameter adaptation in response to changes in metabolic stimuli, intravascular pressure, wall shear stress, and information transfer from segment to segment. These rules were then applied to match the computer simulated and experimentally observed (static) network structure. They obtained good quantitative agreement under specific adaptation rules. In particular, wall shear stress, hoop stress and local oxygen level appear to play major roles; note that these same factors are also major players in short-term regulation. Their results suggest that increasing shear stress causes capillary channels with high flow to develop into arteriolar arcade vessels, increasing hoop stress causes growth of vascular smooth muscle, and local hypoxia causes formation of new vessels. At the cellular and molecular levels, processes that govern vascular remodeling, growth, and retraction involve myriad of molecular species. These include heparan sulfate proteoglycans, integrins, components of the extracellular matrix, intracellular signaling molecules, growth factors, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), tissue transforming factor (TGF), and growth-factor receptors. Understanding the molecular mechanisms of mechanical force transduction, or mechanotransduction, is one of the challenging problems in vascular biology. During angiogenesis by capillary sprouting, endothelial cells undergo proliferation, migration, differentiation, and apoptosis. New sprouts are stabilized by recruiting stromal cells that differentiate into pericytes or smooth muscle cells. During vascular remodeling, microvessels recruit circulating progenitor endothelial cells as well as progenitor cells or fibroblasts residing in the surrounding tissue, and transform them into smooth muscle cells. The complexity of the relationships between these molecular and cellular processes, most of which are poorly understood, preclude formulating global molecularly detailed models at the present time. However, general principles for simulating microvascular adaptation and remodeling are emerging (Peirce et al 2004).
8. ARE THERE GENERAL OPTIMALITY PRINCIPLES THAT GOVERN BLOOD FLOW IN THE MICROCIRCULATION?
Understanding the relations between structure and function has been at the center of biological science; the monograph by D’Arcy Thompson “On Growth and Form,” first published in 1917, is an example of conceptual thinking on the subject. In the context of vascular networks, Murray in 1926 questioned whether vascular networks grow to optimize a certain quantity. He showed that at a vascular bifurcation, minimization of a “cost function” equal to the sum of the viscous dissipation and the energy required to maintain the vascular volume yields the ratio of the diameters of parent to daughter branches Dparent/Ddaughter=2⅓; this relationship is referred to as Murray’s law. One of the consequences of Murray’s law is a cubic relationship between blood flow and vessel diameter in a network, i.e., when blood flow, Q, is plotted on a logarithmic scale against vessel diameter, D, for different vascular segments in a network, the slope of the relationship is approximately equal to 3 (not to be confused with the 4th-power relationship in Poiseuille’s law). Numerous studies explored the question of optimality and showed agreement with the cubic law in some cases and disagreement in others. Analysis of microvascular data on vessel diameters at bifurcations showed that while the cost function considered in Murray’s law reaches a minimum when the exponent is equal to 3, the deviation from this minimum value is less than 5% within a wide range of the exponent values, between 2 and 10, and increases outside this range (Sherman et al 1989). Thus, adherence to Murray’s law (or similar minimization principles) could not be expected since moderate deviations from the minimum are not costly and other factors might affect the structure of the network. Another consequence of Murray’s law is that wall shear stress should be constant throughout the vascular network. However, a combination of experiment and theory shows that wall shear stress tightly correlates with intravascular pressure along the network and therefore decreases gradually from the arterial to the venous ends of the network (Pries et al 2003). The search for universal principles of vascular network design continues (West et al 1997). However, at present the existence of such universal principles has not been ascertained.
9. UNRESOLVED PROBLEMS, FUTURE STUDIES
Despite the plethora of experimental and theoretical studies of hemorheological aspects of the microcirculation, there is a need for better and more complete models of blood rheology in vivo to adequately understand the normal microcirculation and to elucidate changes in various diseases. From an experimental standpoint, measurements of pressure gradient, wall shear stress, and velocity profiles in arterioles and venules need to be developed or improved, the role of RBC aggregation under physiological and pathological conditions has to be clarified, and interactions between flowing formed elements in microvessels and between the formed elements and the endothelium are complex and require further investigation. From a theoretical standpoint, the mechanics of cell-cell interactions have not been adequately described, and blood-flow models that account for discrete RBCs, WBCs, and platelets in individual arterioles and venules and in whole microvascular networks are incomplete.
This review of experimental and theoretical developments in the hemorheology of the microcirculation has outlined major findings in mechanics of blood flow and its regulation. The brevity of the review precludes a comprehensive coverage. In particular, we have not discussed such relevant topics as transendothelial and interstitial water transport and endothelial and smooth muscle cell mechanotransduction. New experimental techniques make it possible to investigate molecular mechanisms of microcirculatory phenomena and the corresponding in silico models that simulate those phenomena. One of the major problems in developing modeling methodologies is structuring models at the different scales of biological organization, from molecular to tissue levels (multiscale models). Such hierarchical modeling, which systematically covers this range of scales, and which is accompanied by databases of the necessary cell- and tissue-dependent parameters, represents the emerging concept of the Physiome Project (Hunter & Borg 2003, Popel et al 1998). These developments would not only lead to a deeper quantitative understanding of physiology of the microcirculation and its changes in disease states, but would also facilitate the design of drugs, drug-delivery vehicles, enable control of microvascular network growth and remodeling, particularly for tissue-engineered constructs.
Acknowledgments
We thank S.A. Regirer for his valuable discussions of the outline of the chapter, E.R. Damiano for his critical reading of the manuscript and numerous suggestions, and S. Kim and F. Mac Gabhann for critical comments. The work was supported by NIH grant HL52684.
LITERATURE CITED
- Baish JW, Jain RK. Fractals and cancer. Cancer Res. 2000;60:3683–8. [PubMed] [Google Scholar]
- Bartlett IS, Crane GJ, Neild TO, Segal SS. Electrophysiological basis of arteriolar vasomotion in vivo. J Vasc Res. 2000;37:568–75. doi: 10.1159/000054090. [DOI] [PubMed] [Google Scholar]
- Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29:435–50. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte JB, Liebovitch LS, West BJ. Fractal Physiology. New York; Oxford University Press: 1994. [Google Scholar]
- Beard DA, Bassingthwaighte JB. The fractal nature of myocardial blood flow emerges from a whole-organ model of arterial network. J Vasc Res. 2000;37:282–96. doi: 10.1159/000025742. [DOI] [PubMed] [Google Scholar]
- Bevan J, Halpern W, Mulvany M, editors. The Resistance Vasculature. Totowa, N.J.: Humana Press; 1991. p. 476. [Google Scholar]
- Bishop JJ, Nance PR, Popel AS, Intaglietta M, Johnson PC. Effect of erythrocyte aggregation on velocity profiles in venules. Am J Physiol Heart Circ Physiol. 2001a;280:H222–36. doi: 10.1152/ajpheart.2001.280.1.H222. [DOI] [PubMed] [Google Scholar]
- Bishop JJ, Nance PR, Popel AS, Intaglietta M, Johnson PC. Erythrocyte margination and sedimentation in skeletal muscle venules. Am J Physiol Heart Circ Physiol. 2001b;281:H951–8. doi: 10.1152/ajpheart.2001.281.2.H951. [DOI] [PubMed] [Google Scholar]
- Bishop JJ, Popel AS, Intaglietta M, Johnson PC. Effects of erythrocyte aggregation and venous network geometry on red blood cell axial migration. Am J Physiol Heart Circ Physiol. 2001c;281:H939–50. doi: 10.1152/ajpheart.2001.281.2.H939. [DOI] [PubMed] [Google Scholar]
- Bishop JJ, Popel AS, Intaglietta M, Johnson PC. Rheological effects of red blood cell aggregation in the venous network: a review of recent studies. Biorheology. 2001d;38:263–74. [PubMed] [Google Scholar]
- Bishop JJ, Nance PR, Popel AS, Intaglietta M, Johnson PC. Relationship between erythrocyte aggregate size and flow rate in skeletal muscle venules. Am J Physiol Heart Circ Physiol. 2004;286:H113–20. doi: 10.1152/ajpheart.00587.2003. [DOI] [PubMed] [Google Scholar]
- Busse R, Fleming I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci. 2003;24:24–9. doi: 10.1016/s0165-6147(02)00005-6. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol. 2001;280:C962–9. doi: 10.1152/ajpcell.2001.280.4.C962. [DOI] [PubMed] [Google Scholar]
- Cabel M, Meiselman HJ, Popel AS, Johnson PC. Contribution of red blood cell aggregation to venous vascular resistance in skeletal muscle. Am J Physiol. 1997;272:H1020–32. doi: 10.1152/ajpheart.1997.272.2.H1020. [DOI] [PubMed] [Google Scholar]
- Chien S. Red cell deformability and its relevance to blood flow. Annu Rev Physiol. 1987;49:177–92. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- Chien S, Lang LA. Physicochemical basis and clinical implications of red cell aggregation. Clin Hemorhelogy. 1987;7:71–91. [Google Scholar]
- Cokelet GR. Rheology and tube flow of blood. In: Skalak R, Chien S, editors. Handbook of Bioengineering. New York: McGraw Hill; 1987. pp. 14.1–.7. [Google Scholar]
- Cokelet GR. Poiseuille Award Lecture. Viscometric, in vitro and in vivo blood viscosity relationships: how are they related? Biorheology. 1999;36:343–58. [PubMed] [Google Scholar]
- Cornelissen AJ, Dankelman J, VanBavel E, Spaan JA. Balance between myogenic, flow-dependent, and metabolic flow control in coronary arterial tree: a model study. Am J Physiol Heart Circ Physiol. 2002;282:H2224–37. doi: 10.1152/ajpheart.00491.2001. [DOI] [PubMed] [Google Scholar]
- Damiano ER. The effect of the endothelial-cell glycocalyx on the motion of red blood cells through capillaries. Microvasc Res. 1998;55:77–91. doi: 10.1006/mvre.1997.2052. [DOI] [PubMed] [Google Scholar]
- Damiano ER, Long DS, Smith ML. Estimation of velocity profiles using velocimetry data from parallel flows of linearly viscous fluids: Application to microvascular hemodynamics. J Fluid Mech. 2004 in press. [Google Scholar]
- Damiano ER, Stace TM. A mechano-electrochemical model of radial deformation of the capillary glycocalyx. Biophys J. 2002;82:1153–75. doi: 10.1016/S0006-3495(02)75474-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Enden G, Popel AS. Stratified multiphase model for blood flow in a venular bifurcation. Ann Biomed Eng. 1997;25:135–53. doi: 10.1007/BF02738545. [DOI] [PubMed] [Google Scholar]
- Das B, Johnson PC, Popel AS. Effect of nonaxisymmetric hematocrit distribution on non-Newtonian blood flow in small tubes. Biorheology. 1998;35:69–87. doi: 10.1016/S0006-355X(98)00018-3. [DOI] [PubMed] [Google Scholar]
- Das B, Johnson PC, Popel AS. Computational fluid dynamic studies of leukocyte adhesion effects on non-Newtonian blood flow through microvessels. Biorheology. 2000;37:239–58. [PubMed] [Google Scholar]
- Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, et al. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol. 2001;280:H1427–33. doi: 10.1152/ajpheart.2001.280.4.H1427. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol. 1990;258:H647–54. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- Discher DE. New insights into erythrocyte membrane organization and microelasticity. Curr Opin Hematol. 2000;7:117–22. doi: 10.1097/00062752-200003000-00008. [DOI] [PubMed] [Google Scholar]
- Dodd LR, Johnson PC. Diameter changes in arteriolar networks of contracting skeletal muscle. Am J Physiol. 1991;260:H662–70. doi: 10.1152/ajpheart.1991.260.3.H662. [DOI] [PubMed] [Google Scholar]
- Dong C, Cao J, Struble EJ, Lipowsky HH. Mechanics of leukocyte deformation and adhesion to endothelium in shear flow. Ann Biomed Eng. 1999;27:298–312. doi: 10.1114/1.143. [DOI] [PubMed] [Google Scholar]
- Dong C, Lei XX. Biomechanics of cell rolling: shear flow, cell-surface adhesion, and cell deformability. J Biomech. 2000;33:35–43. doi: 10.1016/s0021-9290(99)00174-8. [DOI] [PubMed] [Google Scholar]
- Dora KA, Damon DN, Duling BR. Microvascular dilation in response to occlusion: a coordinating role for conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2000;279:H279–84. doi: 10.1152/ajpheart.2000.279.1.H279. [DOI] [PubMed] [Google Scholar]
- Duling BR, Damon DH. An examination of the measurement of flow heterogeneity in striated muscle. Circ Res. 1987;60:1–13. doi: 10.1161/01.res.60.1.1. [DOI] [PubMed] [Google Scholar]
- Eggleton CD, Popel AS. Large deformation of red blood cell ghosts in a simple shear flow. Physics of Fluids. 1998;10:1834–45. doi: 10.1063/1.869703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Liu A, Dawant B, Popel AS, Pittman RN. Analysis of vascular pattern and dimensions in arteriolar networks of the retractor muscle in young hamsters. Microvasc Res. 1987;34:168–83. doi: 10.1016/0026-2862(87)90051-3. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Pittman RN. Evaluation of photometric methods for quantifying convective mass transport in microvessels. Am J Physiol. 1986;251:H869–79. doi: 10.1152/ajpheart.1986.251.4.H869. [DOI] [PubMed] [Google Scholar]
- Enden G, Popel AS. A numerical study of plasma skimming in small vascular bifurcations. J Biomech Eng. 1994;116:79–88. doi: 10.1115/1.2895708. [DOI] [PubMed] [Google Scholar]
- Eppihimer MJ, Lipowsky HH. Effects of leukocyte-capillary plugging on the resistance to flow in the microvasculature of cremaster muscle for normal and activated leukocytes. Microvasc Res. 1996;51:187–201. doi: 10.1006/mvre.1996.0020. [DOI] [PubMed] [Google Scholar]
- Evans EA, Skalak R. Mechanics and Thermodynamics of Biomembranes. Boca Raton: CRC Press; 1980. [Google Scholar]
- Feng J, Weinbaum S. Lubrication theory in highly compressible porous media: the mechanics of skiing, from red cells to humans. Journal of Fluid Mechanics. 2000;422:281–317. [Google Scholar]
- Fung YC, Zweifach BW. Microcirculation: Mechanics of blood flow in capillaries. Annu Rev Fluid Mech. 1971;3:189–210. [Google Scholar]
- Goldsmith HL, Bell DN, Spain S, McIntosh FA. Effect of red blood cells and their aggregates on platelets and white cells in flowing blood. Biorheology. 1999;36:461–8. [PubMed] [Google Scholar]
- Goldsmith HL, Cokelet GR, Gaehtgens P. Robin Fahraeus: evolution of his concepts in cardiovascular physiology. Am J Physiol. 1989;257:H1005–15. doi: 10.1152/ajpheart.1989.257.3.H1005. [DOI] [PubMed] [Google Scholar]
- Gov N, Zilman AG, Safran S. Cytoskeleton confinement and tension of red blood cell membranes. Phys Rev Lett. 2003;90:228101. doi: 10.1103/PhysRevLett.90.228101. [DOI] [PubMed] [Google Scholar]
- Haidekker MA, L’Heureux N, Frangos JA. Fluid shear stress increases membrane fluidity in endothelial cells: a study with DCVJ fluorescence. Am J Physiol Heart Circ Physiol. 2000;278:H1401–6. doi: 10.1152/ajpheart.2000.278.4.H1401. [DOI] [PubMed] [Google Scholar]
- Hellums JD. 1993 Whitaker Lecture: biorheology in thrombosis research. Ann Biomed Eng. 1994;22:445–55. doi: 10.1007/BF02367081. [DOI] [PubMed] [Google Scholar]
- Helmke BP, Davies PF. The cytoskeleton under external fluid mechanical forces: hemodynamic forces acting on the endothelium. Ann Biomed Eng. 2002;30:284–96. doi: 10.1114/1.1467926. [DOI] [PubMed] [Google Scholar]
- Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277:H508–14. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- Hu HH, Patankar NA, Zhu MY. Direct numerical simulations of fluid-solid systems using the arbitrary Lagrangian-Eulerian technique. J Comp Phys. 2001;169:427–62. [Google Scholar]
- Hunter PJ, Borg TK. Integration from proteins to organs: the Physiome Project. Nat Rev Mol Cell Biol. 2003;4:237–43. doi: 10.1038/nrm1054. [DOI] [PubMed] [Google Scholar]
- Kamm RD. Cellular fluid mechanics. Annu Rev Fluid Mech. 2002;34:211–32. doi: 10.1146/annurev.fluid.34.082401.165302. [DOI] [PubMed] [Google Scholar]
- King MR, Hammer DA. Multiparticle adhesive dynamics: hydrodynamic recruitment of rolling leukocytes. Proc Natl Acad Sci U S A. 2001;98:14919–24. doi: 10.1073/pnas.261272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller A, Dawant B, Liu A, Popel AS, Johnson PC. Quantitative analysis of arteriolar network architecture in cat sartorius muscle. Am J Physiol. 1987;253:H154–64. doi: 10.1152/ajpheart.1987.253.1.H154. [DOI] [PubMed] [Google Scholar]
- Krishmatullin D, Truskey G. 3D numerical simulation of receptor-mediated leukocyte adhesion to surfaces: effects of cell deformability and viscoelasticity. submitted 2004 [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–8. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Lighthill M. Pressure-forcing of tightly fitting pellets along fluid-filled elastic tubes. J Fluid Mech. 1968;34 [Google Scholar]
- Long DS, Smith ML, Pries AR, Ley K, Damiano ER. Micro-viscometry reveals reduced blood viscosity and altered shear rate and shear stress profiles in microvessels after hemodilution. Submitted. 2004 doi: 10.1073/pnas.0402937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchedlishvili G, Maeda N. Blood flow structure related to red cell flow: determinant of blood fluidity in narrow microvessels. Jpn J Physiol. 2001;51:19–30. doi: 10.2170/jjphysiol.51.19. [DOI] [PubMed] [Google Scholar]
- Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–61. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- Mulivor AW, Lipowsky HH. Inflammation and ischemia induced shedding of the venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004 doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- Nakano A, Sugii Y, Minamiyama M, Niimi H. Measurement of red cell velocity in microvessels using particle image velocimetry (PIV) Clin Hemorheol Microcirc. 2003;29:445–55. [PubMed] [Google Scholar]
- N’Dri NA, Shyy W, Tran-Son-Tay R. Computational modeling of cell adhesion and movement using a continuum-kinetics approach. Biophys J. 2003;85:2273–86. doi: 10.1016/s0006-3495(03)74652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu B, Meiselman HJ. Depletion-mediated red blood cell aggregation in polymer solutions. Biophys J. 2002;83:2482–90. doi: 10.1016/S0006-3495(02)75259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthimos D, Edwards DH, Griffith TM. Minimal model of arterial chaos generated by coupled intracellular and membrane Ca2+ oscillators. Am J Physiol. 1999;277:H1119–44. doi: 10.1152/ajpheart.1999.277.3.H1119. [DOI] [PubMed] [Google Scholar]
- Pearson MJ, Lipowsky HH. Influence of erythrocyte aggregation on leukocyte margination in postcapillary venules of rat mesentery. Am J Physiol Heart Circ Physiol. 2000;279:H1460–71. doi: 10.1152/ajpheart.2000.279.4.H1460. [DOI] [PubMed] [Google Scholar]
- Peirce SM, Van Gieson EJ, Skalak TC. Multicellular simulation predicts microvascular patterning and in silico tissue assembly. Faseb J. 2004 doi: 10.1096/fj.03-0933fje. in press. [DOI] [PubMed] [Google Scholar]
- Popel AS. Network models of peripheral circulation. In: Skalak R, Chien S, editors. Handbook of Bioengineering. New York: McGraw Hill; 1987. pp. 20.1–.4. [Google Scholar]
- Popel AS, Greene AS, Ellis CG, Ley KF, Skalak TC, Tonellato PJ. The Microcirculation Physiome Project. Ann Biomed Eng. 1998;26:911–3. doi: 10.1114/1.112. [DOI] [PubMed] [Google Scholar]
- Popel AS, Johnson PC, Kameneva MV, Wild MA. Capacity for red blood cell aggregation is higher in athletic mammalian species than in sedentary species. J Appl Physiol. 1994;77:1790–4. doi: 10.1152/jappl.1994.77.4.1790. [DOI] [PubMed] [Google Scholar]
- Pozrikidis C. Shell theory for capsules and cells. In: Pozrikidis C, editor. Modeling and Simulation of Capsules and Biological Cells. Boca Raton: Chapman & Hall/CRC; 2003. pp. 35–101. [Google Scholar]
- Pozrikidis C. Axisymmetric motion of red blood cells through capillaries. Physics of Fluids. 2004 in press. [Google Scholar]
- Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol. 1992;263:H1770–8. doi: 10.1152/ajpheart.1992.263.6.H1770. [DOI] [PubMed] [Google Scholar]
- Pries AR, Reglin B, Secomb TW. Structural response of microcirculatory networks to changes in demand: information transfer by shear stress. Am J Physiol Heart Circ Physiol. 2003;284:H2204–12. doi: 10.1152/ajpheart.00757.2002. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. Biophysical aspects of blood flow in the microvasculature. Cardiovasc Res. 1996;32:654–67. [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–66. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gessner T, Sperandio MB, Gross JF, Gaehtgens P. Resistance to blood flow in microvessels in vivo. Circ Res. 1994;75:904–15. doi: 10.1161/01.res.75.5.904. [DOI] [PubMed] [Google Scholar]
- Regirer SA. Diffusion of blood cells. In: Chernii GG, Regirer SA, editors. Contemporary Problems of Biomechanics. Boca Raton: CRC Press; 1990. pp. 75–98. [Google Scholar]
- Regirer SA, Shadrina N. A simple model of a vessel with a wall sensitive to mechanical stimuli. Biophysics. 2002;47:845–50. [PubMed] [Google Scholar]
- Reinke W, Gaehtgens P, Johnson PC. Blood viscosity in small tubes: effect of shear rate, aggregation, and sedimentation. Am J Physiol. 1987;253:H540–7. doi: 10.1152/ajpheart.1987.253.3.H540. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW. Biomechanics of microcirculatory blood perfusion. Annu Rev Biomed Eng. 1999;1:73–102. doi: 10.1146/annurev.bioeng.1.1.73. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW, Granger D, editors. Molecular Basis of Microcirculatory Disorders. Springer France Editions; 2003. p. 640. [Google Scholar]
- Secomb TW. Mechanics of red blood cells and blood flow in narrow tubes. In: Pozrikidis C, editor. Modeling and Simulation of Capsules and Biological Cells. Boca Raton: Chapman & Hall/CRC; 2003. pp. 163–96. [Google Scholar]
- Secomb TW, Hsu R, Pries AR. Blood flow and red blood cell deformation in nonuniform capillaries: effects of the endothelial surface layer. Microcirculation. 2002;9:189–96. doi: 10.1038/sj.mn.7800132. [DOI] [PubMed] [Google Scholar]
- Sharan M, Popel AS. A two-phase model for flow of blood in narrow tubes with increased effective viscosity near the wall. Biorheology. 2001;38:415–28. [PubMed] [Google Scholar]
- Sherman TF, Popel AS, Koller A, Johnson PC. The cost of departure from optimal radii in microvascular networks. J Theor Biol. 1989;136:245–65. doi: 10.1016/s0022-5193(89)80162-6. [DOI] [PubMed] [Google Scholar]
- Shiu Y-T, McIntire LV. In vitro studies of erythrocyte-vascular endothelium interactions. Ann Biomed Eng. 2003;31:1299–313. doi: 10.1114/1.1630320. [DOI] [PubMed] [Google Scholar]
- Skalak R, Ozkaya N, Skalak TC. Biofluid Mechanics. Annu Rev Fluid Mech. 1989;21:167–204. [Google Scholar]
- Smith ML, Long DS, Damiano ER, Ley K. Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys J. 2003;85:637–45. doi: 10.1016/s0006-3495(03)74507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara-Seki M, Schmid-Schonbein GW. The fluid shear stress distribution on the membrane of leukocytes in the microcirculation. J Biomech Eng. 2003;125:628–38. doi: 10.1115/1.1611515. [DOI] [PubMed] [Google Scholar]
- Sun C, Migliorini C, Munn LL. Red blood cells initiate leukocyte rolling in postcapillary expansions: a lattice Boltzmann analysis. Biophys J. 2003;85:208–22. doi: 10.1016/S0006-3495(03)74467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutera SP, Skalak R. The history of Poiseuille’s law. Annu Rev Fluid Mech. 1993;25:1–19. [Google Scholar]
- Tangelder GJ, Slaaf DW, Muijtjens AM, Arts T, oude Egbrink MG, Reneman RS. Velocity profiles of blood platelets and red blood cells flowing in arterioles of the rabbit mesentery. Circ Res. 1986;59:505–14. doi: 10.1161/01.res.59.5.505. [DOI] [PubMed] [Google Scholar]
- Tateishi N, Suzuki Y, Soutani M, Maeda N. Flow dynamics of erythrocytes in microvessels of isolated rabbit mesentery: cell-free layer and flow resistance. J Biomech. 1994;27:1119–25. doi: 10.1016/0021-9290(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Tran-Son-Tay R, Kan HC, Udaykumar HS, Damay E, Shyy W. Rheological modelling of leukocytes. Med Biol Eng Comput. 1998;36:246–50. doi: 10.1007/BF02510753. [DOI] [PubMed] [Google Scholar]
- Ursino M, Colantuoni A, Bertuglia S. Vasomotion and blood flow regulation in hamster skeletal muscle microcirculation: A theoretical and experimental study. Microvasc Res. 1998;56:233–52. doi: 10.1006/mvre.1998.2106. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–9. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278:H285–9. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- Wang N-T, Fogelson AL. Computational methods for continuum models of platelet aggregation. J Comp Phys. 1999;151:649–75. [Google Scholar]
- Waugh R, Hochmuth RM. Mechanics and deformability of hematocytes. In: Bronzino JD, editor. Biomedical Engineering Handbook. Boca Raton: CRC Press; 2001. pp. xxx–xxx. [Google Scholar]
- Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100:7988–95. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–6. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- Zhu C, Bao G, Wang N. Cell mechanics: mechanical response, cell adhesion, and molecular deformation. Annu Rev Biomed Eng. 2000;2:189–226. doi: 10.1146/annurev.bioeng.2.1.189. [DOI] [PubMed] [Google Scholar]
- Zweifach BW, Lipowsky HH. Pressure and flow relations in blood and lymph microcirculation. In: Renkin EM, Michel CC, editors. Handbook of Physiology The Cardiovascular System. Bethesda: American Physiological Society; 1984. pp. 251–307. [Google Scholar]


