We review strategies of sexual and asexual reproduction and persistence in plants of flood-prone Central Amazonia. Adaptations in response to the strong instability of these environments are highlighted together with the importance of river connectivity for species dispersal and persistence.
Abstract
Background
The Central Amazonian floodplain forests are subjected to extended periods of flooding and to flooding amplitudes of 10 m or more. The predictability, the length of the flood pulse, the abrupt transition in the environmental conditions along topographic gradients on the banks of major rivers in Central Amazonia, and the powerful water and sediment dynamics impose a strong selective pressure on plant reproduction systems.
Scope
In this review, we examine how the hydrological cycle influences the strategies of sexual and asexual reproduction in herbaceous and woody plants. These are of fundamental importance for the completion of the life cycle. Possible constraints to seed germination, seedling establishment and formation of seed banks are also covered. Likewise, we also discuss the importance of river connectivity for species propagation and persistence in floodplains.
Conclusions
The propagation and establishment strategies employed by the highly diversified assortment of different plant life forms result in contrasting successional stages and a zonation of plant assemblages along the flood-level gradient, whose species composition and successional status are continuously changing not only temporally but also spatially along the river channel.
Introduction
The Amazon basin covers two-fifths of South America and 5 % of the Earth's land surface. This area of ∼6.8 million km2 encloses the largest river system on the planet, responsible for unloading, annually, about 20 % of all fresh water carried to the oceans (Goulding et al., 2003). The level of water in the Amazon River channel varies seasonally throughout the year in response to the spatial and temporal distribution of rainfall in the headwaters. The marked seasonal change in water level of the rivers of the largest and most diverse of tropical rainforests imposes a wide range of hydrological, geomorphological and ecological processes (Junk et al., 1989; Alsdorf et al., 2000). Every year, the River Amazon and its tributaries, which together drain the basin, overflow and flood the adjacent forest, forming extensive wetlands. These wetlands cover about 300 000 km2 to create the largest flooded area in the world (Junk, 1993). Sites along the main river channel are highly unstable, being subjected to strong disturbance by sedimentation and erosion. Longstanding, more stable conditions predominate on adjacent terraces and uplands. In this regard, the distribution of species shows a well-defined zonation along the flood-level gradient (Salo et al., 1986; Puhakka et al., 1992; Wittmann et al., 2002, 2004).
The flood pulse exerts a strong influence on vegetation dynamics and composition, and on plant reproduction (Junk et al., 1989; Worbes et al., 1992; Wittmann et al., 2002). The vegetation associated with these water courses is subjected to an annual alternation between well-defined terrestrial and aquatic phases (Junk et al., 1989). The annual flood may last for more than 200 days and attain up to 10 m depth (Junk et al., 1989; Junk, 1993). The frequency, duration and intensity of flooding determine which species germinate, establish and reproduce along the flood-level gradient (Junk et al., 1989; Waldhoff et al., 1998; Wittmann and Parolin, 1999; Parolin, 2000; Piedade et al., 2000).
The present review examines available information on reproduction and propagation of species that colonize the floodplains of Central Amazonia. It aims to highlight how the regular and prolonged seasonal flood pulse influences the strategies of sexual and asexual reproduction that lead to the completion of plant life cycles and population maintenance. It is postulated that these strategies are set to minimize or even avoid the probable risk of extinction due to the strong instability of this type of environment. Possible constraints on germination, seedling establishment and formation of seed banks are also addressed. Similarly, we also discuss the importance of river connectivity for species propagation and persistence on floodplain forests.
Environmental characteristics, vegetation types and zonation in floodplains of Central Amazonia
Várzea and igapó floodplains
The Amazon wetlands are constituted by a complex network of lakes and floodplains that covers about 6 % of the Amazon basin or an area of 300 000 km2 (Junk, 1989). Although relatively small when compared with the dimensions of the Amazon forest, these floodplains cover a land area that is larger than the UK and about three times the size of Portugal.
These wetland physiognomies are classified according to the physicochemical properties and the quality of the flood water into two major types: the várzea (4 %; 200 000 km2) and the igapó (2 %; 100 000 km2). Várzea floodplains are periodically flooded by white-water rivers (Fig. 1A–C) with pH near neutrality. These are fertile areas because the rivers associated with them carry many suspended sediments, often originating from the Andes and pre-Andean slopes. The invading water deposits appreciable amounts of sediment on the river banks and on sand and mud flats near the river. This phenomenon promotes a cyclic land renewal and is responsible for the high fertility of várzea soils (Sioli, 1951; Irion et al., 1983). At the same time, this high rate of sedimentation, which can reach 300–1000 mm per year (Junk, 1989; Parolin, 2009), results in an additional disturbance to some components of the vegetation and strongly affects seedling establishment. In contrast, igapó wetlands are flooded by black-and-clear-water rivers that originate from the geologically old and eroded shields of Guiana and Central Brazil. These rivers drain areas of sandy soils (Fig. 2A and C); the water is acidic with a pH close to 4, poor in inorganic nutrients and rich in diluted organic material, particularly humic acids (Klinge and Ohly, 1964; Prance, 1978; Junk and Fürch, 1980; Furch, 1984; Junk, 1984).
Fig. 1.
Features of the várzea floodplain vegetation. (A) Várzea lakes colonized by macrophytes; (B) floating grasses on the edge of the flooded forest; and (C) interior of the forest during the terrestrial phase (the arrow points to a mark left in the tree that indicates the water level reached in the last flood); (D) extensive seedling bank in the interior of a várzea forest, about a month after water retreat; (E) colonization by seed-germinated plants of Paspalum fasciculatum; (F) colonization by vegetative regrowth of P. fasciculatum; (G) seeds of Pseudobombax munguba (circle) and other fruits (arrow) floating in water; (H) Clusiaceae seedling with new recently produced leaves and old leaves (arrow) which have survived the last flood (still covered with sediment); (I) seed germination in water of an unidentified legume.
Fig. 2.
Features of the igapó floodplain vegetation. (A) Aerial view of the igapó forest; (B) water-germinated seedling at the end of the flood period; (C) igapó lakes showing the absence of colonization by macrophytes; (D) colonization of the igapó shores by pioneer woody perennials during the period of water retreat; (E and F) seedlings that germinated in the igapó forest following the water retreat; (G) seedling bank of Astrocaryum jauari which have survived the last flood. The arrows in (B), (E) and (F) are pointing to seeds that contain large cotyledonary reserves.
The extent of flooding depends on precipitation, discharge and topography, and changes along the river course (Junk et al., 1989). The amplitude of water-level fluctuations varies extensively among the rivers of the Amazon basin and from year to year. Landforms are dependent on river size and channel characteristics, which in turn shape patterns of vegetation succession. Forests are eroded during the high-water period and replaced by successional vegetation, whereas depositional bars, which are exposed during the annual low-water season, are immediately colonized by pioneer species. In fact, várzea and igapó floodplains are composed of a mosaic of plant assemblages whose species composition and successional status are continuously changing not only temporally but also spatially along the river channel (Puhakka et al., 1992; Wittmann et al., 2004).
Distribution of plant assemblages in white-water (várzea) floodplains
A zonation of plant communities is created along the flood-level gradient with a clear replacement of species, which is dependent on the flooding conditions at each topographical position (Junk, 1989; Worbes et al., 1992; Ferreira, 2000; Wittmann et al., 2002, 2006). This vegetation zonation along the flood-level gradient on várzea floodplains is well characterized (Worbes et al., 1992; Wittmann et al., 2002, 2004, 2006). Wittmann et al. (2002) classified the Central Amazonian várzea forests as low várzea or high várzea, with few species that are found in both forest types. Low-várzea forests become established where the annual water column has an average height of >3 m, which corresponds to an inundation period of >50 days per year. Flood height and flood duration are the limiting factors and determine the position of the closed-forest border, which is generally located where the height of the water column reaches between 7.5 and 8 m or 228 days of flooding per year on average. Monospecific stands of pioneer short-lived (10 years) woody perennials like Alchornea castaneifolia (Euphorbiaceae) and Salix martiana (Salicaceae) tolerant to flood periods of up to 270 days occur just below the closed-forest border and dominate the river banks. They are followed along the flood-level gradient by typical early secondary tree species, such as the genus Cecropia which can reach ages of 10–30 years. Subsequently, late secondary species Crataeva benthamii (Capparaceae) and Pseudobombax munguba (Bombacaceae) can be found, which can be 20–100 years old. These are followed by well-developed low-várzea forests, where there are species with ages up to 400 years, such as Piranhea trifoliata (Euphorbiaceae). High-várzea forests are found on more stable sites, where the flood period is generally <50 days per year and the high-water level is <3 m. Brosimum lactescens (Moraceae), Theobroma cacao (Sterculiaceae) and Hura crepitans (Euphorbiaceae) are typical species of the high-várzea forests. Pseudobombax munguba, Euterpe oleracea (Arecaceae) and Astrocaryum chonta (Arecaceae) are important components of both the low- and high-várzea forests.
The lower levels of the flood-level gradient are colonized by herbaceous vegetation. Herbs and perennial grasses are typical of sites that are subjected to an average annual flooding of 8–9 months, whereas annual herbaceous plants colonize terrains that remain unflooded only for a few weeks each year. Sand shores below that level remain unflooded for only a few weeks every 2 or 3 years and are immediately colonized by terrestrial and semi-aquatic herbs from the sediment seed bank or deposited by the receding waters. Permanently flooded soils are predominantly colonized by macrophytes. Macrophytes, algae and periphyton are the dominant plant forms that grow during the aquatic phase of the annual cycle (Klinge et al., 1995; Piedade et al., 2001).
Distribution of plant assemblages in black-water (igapó) floodplains
The acidic pH and low nutrient levels restrict the presence of herbaceous vegetation in igapó floodplains (Fig. 2C and D). Furthermore, herbaceous plants would also be subjected to water deficiency during the terrestrial phase because of the high hydraulic conductivity and the low water-holding capacity of sandy soils (Junk and Piedade, 1997; Piedade et al., 2001). When clay prevails, herbaceous vegetation is denser but much smaller and less diversified than in the várzea (Junk and Piedade, 1997). Although a detailed classification of igapó vegetation types is still lacking, the few existing studies indicate that floristic composition and vegetation structure change along the flood-level gradient (Worbes, 1997; Ferreira, 1998, 2000; Ferreira and Prance, 1998; Piedade et al., 2005). A few generalist species such as Malouetia furfuracea (Apocynaceae) can occur along the entire flood gradient.
Species richness
Despite these extreme environmental conditions, which restrict the establishment of the majority of plant species, more than 900 tree species have been recorded in várzea forests (Ferreira and Prance, 1998; Wittmann et al., 2002, 2006). In fact, the Amazonian várzea forests are considered the most species-rich floodplain forests in the world (Wittmann et al., 2006). Although less well studied, the species richness of igapó forests is comparable to várzea forests. Species richness in várzea forests ranges from 20 to 150 tree species ha−1 (Campbell et al., 1992; Haugaasen and Peres, 2006) and from 30 to 137 tree species ha−1 (Ferreira, 1997; Ferreira et al., 2005) in igapó forests, with very low overlap in species composition between these two vegetation types. The large variability in species number is strongly linked to the flood-level gradient. The number of species is restricted in sites along the main river channel, which are highly unstable and exposed to prolonged periods of flooding. The much lower duration of the inundation period in adjacent terraces and uplands allows the establishment of many species of the surrounding terra firme forests. In these sites, the number of tree species is comparable to that in adjacent non-flooded terra firme forests, where the number of tree species typically ranges from 94 to 322 in 1-ha plots (Boom, 1986; Campbell et al., 1986; Lima Filho et al., 2001; Haugaasen and Peres, 2006).
Much less attention has been given to the floristic variation of the herbaceous vegetation. The few detailed studies suggest that the diversity of species is not as high in comparison to the woody vegetation (Kalliola et al., 1991; Junk and Piedade, 1993a,b). In the floodplain of Solimões/Amazon River near Manaus, a total of 388 herbaceous species belonging to 182 genera were collected and identified (Junk and Piedade, 1993a,b). The pioneer flora includes many widespread perennial herbs in várzea floodplains, whereas few herbaceous colonist species are found in black-water rivers (Kalliola et al., 1991; Junk and Piedade, 1997).
The role of the flood pulse and river connectivity for plant propagation
One of the attributes of the flood pulse is to homogenize the limnological, physical and chemical characteristics of contiguous water bodies by increasing hydrological connectivity (Thomaz et al., 2007), here defined as the water-mediated transport of matter, energy and organisms within or between the components of the hydrological cycle (Pringle, 2003).
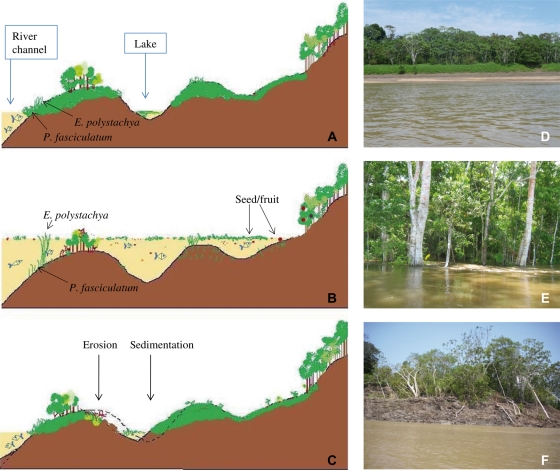
In the case of aquatic herbaceous vegetation, the flood pulse changes the availability of habitats and nutrients throughout the year, influencing the spatial distribution and the percentage of surface covered by their populations (Junk and Piedade, 1997). Floods that promote connectivity between river channels and other inland water bodies (Fig. 3A, B and E) favour the expansion of the coverage area of floating herbaceous vegetation, which is transported during the periods of rising and falling water, strongly influencing the input and output of energy, carbon and nutrients along the flooding gradient. In the high-water period, large numbers of aquatic herbs (emergent, rooted with floating leaves, free-submerged and free-floating) can be found colonizing the still waters of temporary and permanent lakes (Fig. 1A and B). They can cover more than 70 % of the open areas in várzea lakes (Bayley, 1989). In such environments, these species play an essential role in nutrient cycling and produce large amounts of organic matter (Piedade and Junk, 2000; Padial et al., 2009; Silva et al., 2009). Estimates of net primary productivity ranged from 2400 to 3500 g m−2 per year, depending on the duration of flooding (Silva et al., 2009). Higher values up to 10 000 g m−2 per year may be produced by some grasses like Echinochloa polystachya (Piedade et al., 1991). As the water recedes, the produced biomass dries and collapses. As a result, large amounts of dead biomass accumulate in the exposed sediments and their decomposition contributes to an increase in the nutritional status of the floodplains, and of the várzea forest (Piedade et al., 1997).
Fig. 3.
Ecological dynamics in the várzea floodplain. (A and D) Terrestrial phase, when the lakes show little or no connection with the main river channel; (B and E) aquatic phase, when the river overflows and the water invades the adjacent floodplain carrying large amounts of sediment, macrophytes, seedlings, seeds and fish; (C) water retreat, when sedimentation and (F) erosion of river banks are evident.
In contrast, the flood pulse imposes a strong restriction on the establishment of terrestrial plants, which are fixed to the substrate. However, the rise and fall of water is an important factor for seed dispersal of species that established in the floodplains and which adjust their phenology to the flood pulse (Kubitzki and Ziburski, 1994; Junk and Piedade, 1997; Haugaasen and Peres, 2005; Piedade et al., 2005). In addition, the bidirectionality of the water flow allows seeds to flow inland as the rivers overflow their banks but also to flow outwards when, later, the water retreats (Fig. 3A–C).
The flood pulse and its influence on colonization strategies
The periodic change between a terrestrial and an aquatic water phase is the most important factor affecting plant fitness in Amazonian wetlands. Usually, one or other of these phases is especially unfavourable or even critical for population maintenance. To sustain viable populations, it is essential that during the favourable phase of the hydrological cycle they recover from the population losses suffered during the unfavourable period (Junk and Piedade, 1997). The ‘flood pulse’, as termed by Junk et al. (1989), is an annual and predictable event, which can lead to changes in both biotic and biogeochemical processes in the floodplain (Junk, 1993; Neil et al., 2006), and it also reflects directly or indirectly in the successional processes, which are linked to the substantial environmental changes that occur during the hydrological cycle (Junk, 1989; Worbes et al., 1992; Ferreira and Prance, 1998; Wittmann et al., 2002; Lewis et al., 2006). Because it is a dominant driving force in the dynamics of vegetation in wetlands, it is expected that it is also reflected in the strategies of reproduction and establishment. As small differences in the magnitude of flooding can be extremely important, it is also possible that more than one strategy or multiple reproductive strategies are essential for maintenance of viable plant populations. Studies to shed light on the predominance of one strategy or of multiple strategies in a range of species of different life forms are needed (Fig. 1E, F and I).
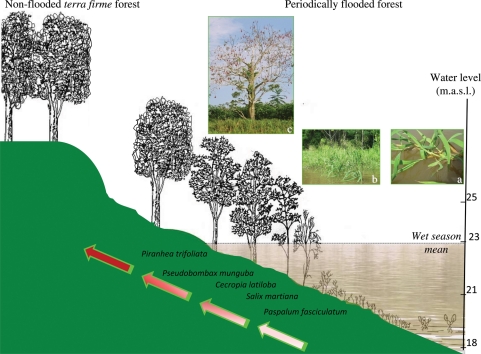
Different reproduction and establishment strategies lead to contrasting successional stages (Fig. 4), at least in várzea floodplains. Here, a clear zonation of plant communities is found along the flood-level gradient, as described earlier. Herbaceous plants, especially aquatic grasses, colonize the early successional stages in várzea floodplains, followed by pioneer woody perennials. In spite of the production of seeds, asexual reproduction systems are of particular relevance for both the herbaceous perennials and most pioneer woody perennials. The dominant herbaceous species are commonly E. polystachya and Paspalum fasciculatum (Poaceae), which spread predominantly by vegetative regrowth through the upper soil layers. These new culms also serve as mechanical shields not only accelerating the processes of sedimentation but also helping to stabilize the substrate by the retention of soil particles during the period of receding waters, preventing erosion and promoting the early stages of succession in várzea floodplains (Fig. 3C and D; Piedade et al., 2005). For this reason, E. polystachya and P. fasciculatum are important structural components of these environments, preceding the pioneer woody perennials such as S. martiana (Salicaceae) and A. castaneifolia (Euphorbiaceae) (Worbes et al., 1992). Seed reproduction is predominant among plants that colonize the uppermost parts of the floodplains.
Fig. 4.
Plant succession and propagation strategies in várzea floodplains. In terms of reproductive strategies, from the open waters towards the uppermost parts of the topographic gradient, there is an increase in sexual reproduction (represented by the colour intensity of the arrow) and dependence on dispersion mechanisms associated with long-lasting propagules such as dormant seeds. (a) Vegetative propagation in Paspalum repens; (b) P. repens with inflorescences; (c) P. munguba with fruits. Adapted from Junk (1997).
Annual herbaceous vegetation covers the lower portions of the floodplains during the low-water period, especially in the fertile várzeas. They propagated from seeds, whose longevity within the wet and anoxic sediments is unknown (Piedade and Junk, 2000). The available time to germinate and complete the life cycle can be only 1 month, the maximum period without water covering the substrate on which they have established. However, less intense floods may leave the substrate submerged for periods of several years, which would favour seeds that can survive for long periods and with prolonged dormancy. Moreover, the depth at which the seed is buried or covered by sediment deposition can be of great importance, because most species cannot grow or establish during the flooding period, and erosion and sedimentation are highly dynamic, especially in wetlands along the larger rivers (Fig. 3A–F). While at greater soil depths the seed is protected from daily fluctuations in temperature, this strategy also brings a reduction in germination rate (Grime et al., 1981). Such harsh environmental features would require these types of diaspore to develop specialized adaptations for flood tolerance and desiccation, in addition to dispersal mechanisms to aquatic environments (Fig. 1G). On the other hand, few arboreal species form permanent seed banks. The large majority germinate rapidly following the water retreat, when environmental conditions are more favourable to seedling establishment (Parolin et al., 2002; Ferreira et al., 2007; Oliveira-Wittmann et al., 2010).
Reproductive strategies of herbaceous species
Most studies with herbaceous plants have been performed in várzea floodplains, where large numbers of species and life forms are able to adjust their life cycles to the annual dynamics of the flood pulse (Piedade et al., 2001). Because of their relatively short life cycle and rapid growth, these light- and nutrient-demanding plants quickly colonize a wide range of habitats in the floodplain. Many species are distributed in the deeper parts of the aquatic–terrestrial transitional zone (ATTZ; Junk et al., 1989), thus forming a contact interface between the limnetic zone and the floodplain (Esteves, 1998). The ATTZ appears as a band of rather ephemeral substrate, which only becomes accessible when the water level is very low, thus restricting the period that these species have available to germinate and complete their life cycle. On the other hand, due to their small size, seeds of herbaceous species are transported in large quantities by the rivers (Fig. 3B) and can rapidly colonize recently exposed sediment banks (Junk and Piedade, 1997).
Seed production ensures genetic variability, as well as the colonization of new environments, whereas vegetative propagation helps to increase local densities of the species or restore populations wiped out by catastrophic events. Many species combine sexual reproduction with additional mechanisms of vegetative propagation to ensure reproductive success (Fig. 4). Thus, herbaceous plants of the Amazonian floodplains have developed multiple adaptations and strategies for propagation, establishment and growth (Fig. 1A, E and F). In this way, they maximize the likelihood of maintaining viable populations in dynamic environments subject to natural and anthropogenic disturbances. Most plants growing during the terrestrial phase produce a large number of diaspores (Junk and Piedade, 1994). In addition to sexual reproduction, free-floating macrophytes such as Salvinia spp. (Salviniaceae), Azolla spp. (Azollaceae), Ricciocarpus natans (Ricciaceae) and Najas spp. (Najadaceae) are able to reproduce via fragments of the parent plant while Limnobium stoloniferum (Hydrocharitaceae) reproduces by vegetative propagation. In Pistia stratiotes (Araceae), there are two peaks of sexual reproduction (Junk and Howard-Williams, 1984) in addition to vegetative propagation by runners. Ceratopteris pteridoides (Parkeriaceae) produces frond buds. Perennial grasses regenerate by sprouting new culms at the nodes.
Mechanisms that delay germination to favourable periods are probably present, although experimental evidence is lacking. Even semi-aquatic species such as E. polystachya, whose life cycle is associated with the aquatic phase of the hydrological cycle and has vegetative propagation as the preponderant mechanism of reproduction (Piedade et al., 1991), produce seeds in abundance. This is also the case for P. fasciculatum (Figs 3A and B, and 4). This species grows mainly during the terrestrial phase and the buds remain dormant when submerged, but is able to produce seeds in abundance in the high-water period (Conserva and Piedade, 2001). Other forms of propagation such as pseudo-viviparity and viviparity are possibly present but this needs confirming.
Most aquatic herbaceous plants have short life cycles and occupy the lowermost parts of the topographic gradient, which are exposed for a short time during the low-water period. This group of species is strongly dependent on seed banks in sediments (Junk, 2005). The few studies of the Amazon floodplains indicate that transient and persistent seed banks can be very large. Junk and Piedade (1997) reported up to 10 000 seedlings m−2 on exposed sediments of Lake Camaleão near Manaus. In a recent study, D'Angelo (2009) assessed seedling colonization and seed bank size on exposed sediments of a recently formed (15 years old) várzea island on the Solimões River in Central Amazonia. The seed bank comprised viable seeds of 31 herbaceous species belonging to seven families (Cyperaceae, Poaceae, Onagraceae, Scrophulariaceae, Pteridaceae, Loganiaceae and Sphenocleaceae). The highest number of seedlings was of sedges and grasses. The author concluded that there was good evidence for the presence of persistent seed banks (sensu Thompson and Grime, 1979). Many of the seeds that remain in the seed bank for long periods probably possess seed dormancy mechanisms. This seems to be a prerequisite in view of the need to synchronize germination with a particular low water level and the subsequent short time period (∼1 month) available for growth, reproduction and end of life. Although seed dormancy of many herbaceous species is physiological, in some sedges their delayed germination and persistence in the soil are related to their thick and impermeable seed coat (Leck and Schütz, 2005).
The diaspores are dispersed by water (hydrochory), sometimes associated with transport by air (anemochory) or by fishes (ichthyochory) (Gottsberger, 1978; Pires and Prance, 1985; Ziburski, 1991; Kubitzki and Ziburski, 1994; Piedade et al., 2005; Maia et al., 2007; Lucas, 2008; Oliveira-Wittmann et al., 2010). Most are small enough to be carried by the current without special organs for floatation (Junk and Piedade, 1997). Furthermore, drifting floating plants also carry seeds.
Reproductive strategies of trees
Beyond the herbaceous zone close to the river channel, woody vegetation is able to establish (Figs 3D and 4). Although sexual reproduction is the dominant reproductive strategy, this does not apply to species such as S. martiana that colonize the lower topographic positions typified by long periods of flooding and high sedimentation. They also depend on vegetative propagation (Oliveira, 1998), thereby offsetting the risk of reproduction by their seeds in the unstable environment (Fig. 4). Lateral branches of fallen trees of S. martiana can form a new independent root system, whereas the lower branches of Eugenia inundata (Myrtaceae) often become rooted (Worbes, 1997).
Despite the importance of asexual reproduction, S. martiana also exhibits continuous seed production. This will also contribute to colonization success and can be considered as an adaptation to a constantly changing environment (Parolin et al., 2002), where reliance on only one strategy could lead to failure. The establishment of S. martiana in the early successional stages is effective in reducing current and wave action. This favours the establishment of species such as Cecropia spp. and P. munguba, which are able to colonize the more stable environment and reproduce successfully only by seeds. Many species shows vigorous resprouting after damage, e.g. Cecropia latiloba (Cecropiaceae), Piranhea trifoliolata (Euphorbiaceae), Tabebuia barbata (Bignoniaceae) and Triplaris surinamensis (Polygonaceae) (Worbes, 1997).
Seed dispersal represents a critical stage in plant reproduction. Its effectiveness can determine the extent of colonization of unoccupied patches, range expansion and the connection of plant populations and the maintenance of gene flow in fragmented landscapes. The dispersal process is mediated by both abiotic and biotic vectors, which can vary greatly in effectiveness depending on the type of environment and the range of vectors available. Water is the principal seed dispersal vector (Fig. 1G) in Central Amazonian floodplains (Kubitzki and Ziburski, 1994; Piedade et al., 2005; Wittmann et al., 2007). The diaspores of most tree species of these seasonally flooded forests are capable of floating for prolonged periods or remain submerged over periods of weeks without losing viability (Kubitzki and Ziburski, 1994; Parolin and Junk, 2002). Because species differ in their buoyancy and mechanisms of dispersal (e.g. hairs, air pockets, fibrous arils), they probably also differ in their ability to be dispersed by water. Some species possess multiple mechanisms for dispersal, such as fleshy pulp and buoyancy, or explosive capsules and buoyancy (Kubitzki and Ziburski, 1994; Waldhoff et al., 1996). The efficiency of seed dispersal by water can be greatly improved by fruit-eating fishes, particularly for species whose seeds are non-buoyant. Frugivorous fishes are highly mobile, with long seed retention times, and many are migratory, leading to long-distance seed dispersal (Piedade et al., 2003, 2005; Correa et al., 2007; Anderson et al., 2009). Many frugivorous fishes consume fleshy fruits, normally without destroying the seeds during ingestion or digestion. Even among fruit crushers, such as the tambaqui (Colossoma macropomum), some seeds can pass through the gut intact (Goulding, 1980,1983). The proportion of intact seeds increases with body size (Kubitzki and Ziburski, 1994; Anderson et al., 2009). For fruits with small seeds, the pericarp may be well masticated, whereas the seeds within are swallowed whole (Goulding, 1980; Kubitzki and Ziburski, 1994; Correa et al., 2007). A few species with heavy fruits are dependent on ichthyochory for seed dispersal, e.g. C. benthamii and Crescentia amazonica (Bignoniaceae) (Kubitzki and Ziburski, 1994) and the palm tree Astrocaryum jauari (Piedade et al., 2003).
It should be noted that hydrochory does not preclude other vectors, such as birds, monkeys and bats, and secondary dispersers such as rodents (Haugaasen and Peres, 2007). Wind dispersal can be combined with water dispersal in some species. The widespread and dominant P. munguba produces anemochoric, light-weight seeds with a hairy (Fig. 1G) wad that keeps them afloat on the water surface for periods of minutes to several days (Kubitzki and Ziburski, 1994).
Given this strong dependency on water for seed dispersal, it is expected that the fruiting phenology in Amazon flooded forests should be synchronized with the annual flood cycle. Indeed, the fruiting peak of many igapó and várzea tree species occurs during the inundation period (Kubitzki and Ziburski, 1994; Parolin et al., 2002; Haugaasen and Peres, 2005). However, individual species differ in the timing and duration of the fruiting peak within the high-water season, which seems to be related to the germination biology of each particular species (Kubitzki and Ziburski, 1994).
The activation of physiological processes involved in seed germination requires an adequate supply of oxygen. Flooding restricts its availability to the embryo, preventing germination or imposing dormancy on the seeds of many species (Hook, 1984; Kozlowski, 1997; Kozlowski and Pallardy, 1997). However, in the Central Amazon, seeds of some tree species are able to germinate and form seedlings in water (Fig. 2B), as in Himatanthus sucuuba (Ferreira et al., 2007), S. martiana and P. munguba (Wittmann et al., 2007). This strategy favours rapid seedling establishment when the terrestrial period commences and deposits seedlings on wet banks (Ferreira et al., 2009). This is particularly important for species that inhabit the Amazon floodplains, as these have a relatively short time (about 3 months before the next flood) to germinate and establish (Figs 1I and 2E and F). Nevertheless, diaspores of many species that escape predation do not germinate in water and are ultimately deposited at the bottom of the inundated forests. Most seeds remain viable even after months of submergence and germinate readily following water retreat (Kubitzki and Ziburski, 1994; Piedade et al., 2003, 2005). One can therefore postulate a continuum of germination strategies that would have, at one end, species with buoyant seeds. They germinate rapidly as soon as they contact river water, which may enable the floating seedlings to establish as soon as they land on non-flooded substrates. At the other end of the continuum lie species with submerged seeds that undergo longer dormancy and germinate only when flood waters recede. However, all strategies benefit from long-distance dispersal by hydrographic corridors.
It is not known whether, along the flood-level gradient, the different species differ in the type of seed carbohydrate reserves. The type of reserves in the endosperm can be related to establishment success and onset of the autotrophic developmental stage which guarantees initial seedling survival (Buckeridge et al., 2004). Biochemical analysis of seeds and seedlings of H. sucuuba, a tree that colonizes both várzea floodplains and terra firme forests, showed significant differences in the proportion and types of accumulated carbohydrates in seeds of the two populations. Although, in várzea-originated seeds, the cell wall storage polysaccharides make up 90 % of the seed reserves on a percentage dry mass basis, in seeds of terra firme plants, the contribution of soluble sugars is ∼30 %. This suggests that distinct ecological functions are played by different carbohydrate reserves in this species during seed imbibition and seedling development. Várzea populations store a larger proportion of complex carbohydrates to use after germination for seedling development, whereas in terra firme populations, seeds allocate comparatively more readily respirable carbohydrates to support prompt germination (Ferreira et al., 2009).
In contrast to herbaceous plants, it seems that trees colonizing Amazonian floodplains do not form persistent seed banks. Cecropia is apparently the only colonizing tree that forms persistent seed banks in exposed floodplain sediments; its seeds remaining viable for about 3–5 years (Kubitzki and Ziburski, 1994). On the other hand, field observations suggest that igapó and várzea trees form extensive seedling banks (Figs 1D and H, and 2G), which could ensure long-term regeneration of existing tree stands.
The absence of permanent tree seed banks may prejudice the regeneration potential of disturbed sites, which would be more prone to year-to-year variation in fruit production. This can be aggravated by overexploitation of focal fish species and fishing practices that selectively harvest large individuals, skewing fish populations towards smaller size classes, which are mostly seed predators (Correa et al., 2007; Anderson et al., 2009).
Conclusions and forward look
Predictability and long-term duration of the flood pulse associated with an abrupt transition in the environmental conditions along the topographic gradient on the banks of major rivers in Central Amazonia, impose a strong selective pressure on plant populations and reproductive systems. Although the pattern of oscillation of the water level curve has a sinoidal shape, between-year variation may be of great importance in species composition (Piedade and Junk, 2000). As noted by Baldwin et al. (2001), in freshwater areas of estuarine regions, even small increases in the frequency and duration of flooding may reduce species diversity.
Land areas under the influence of annual flooding are exposed to heavy hydric and sediment dynamics, which determine the strategies of colonization and recolonization. For species that colonize the ATTZ, especially in lakes (Fig. 3A–F), seed dormancy and the successful establishment of seed banks seem to be of fundamental importance. In this environment, at the deeper vegetated part of the flooding gradient, seeds may remain in the dormant state for many years, despite varying amplitudes of water level and sediment load. When eventually the ground is ephemerally exposed, dormancy may break and germination takes place. Sedges (Cyperaceae) are particularly adapted to this type of habitat. Their short life cycles and between-year variation in dormancy may allow a better survival and specialization to an environment that is both harsh and unpredictable (Brown and Venable, 1986).
The shores along the river channels are subjected to strong disturbance due to sedimentation and erosion, which are enlarged by waves and wind. In such parts of the floodplains, the geomorphology is very dynamic and conditions are rough. Despite the highly destructive nature of the flooding events, they also favour pioneer species by removing competing plants and releasing space and resources on the floodplain, providing ideal open areas for regeneration (Barsoum, 2002). The várzea shores are colonized by pioneer woody perennials and herbaceous vegetation, whereas woody perennials predominate in the igapó floodplains (Figs 1B and 2D). In spite of the production of seeds, asexual reproduction systems are of particular relevance for both life forms, because they maximize the chances for quick and efficient establishment close to the river channels.
The higher parts of the plains, where more stable conditions predominate, are occupied by arboreal vegetation. Small-seeded trees inhabit intermediate flooding positions. Their seedlings tolerate the inundation in a dormant stage, since the water column is too high and growth in one single season not fast enough to avoid submersion. Large-seeded trees predominate at the higher positions of the flood gradient. They are inundated for shorter periods and have seedlings that grow fast in a strategy of escape from submersion (Parolin, 2002). When the water table reaches the seedlings, several of them already have a height that allows the leaves to remain above the water and remain photosynthetically active.
Flooding plays an important role in plant dispersal. Water is the main medium carrying the propagules of most herbaceous and woody plants of the flooded forests. The efficiency of seed dispersal by water can be greatly improved by fruit-eating fishes, particularly for non-buoyant seeds. Because water levels may increase up to 10 m, several isolated water bodies might coalesce, becoming connected during the flood period. Under such circumstances, seeds and other types of dispersal structures, such as fragments and culms of herbaceous plants, may be transported to places well distant from their site of origin. Thus, the connectivity, besides uniformizing to some extent the physical and chemical properties of the water bodies, is also a vehicle for uniformization of the wetland vegetation. Although the losses of seeds and other propagules may be large, given the stochastic nature of the flood disturbances, the semi-open nature of the systems ensures the expansion of distribution areas and the recolonization of disturbed sites.
The extreme seasonality in availability and scarcity of water, superimposed by the pluriannual cycles of flooding and extreme drought, make the Amazon floodplains unique environments in which to study effects of stress and disturbance on the vegetation and on plant adaptation. The critical importance of establishment and reproduction for completion of plant life cycles, the large diversity of trees and aquatic herbaceous plants, and the preliminary nature of existing studies make further research imperative. Future work can be expected to reveal the existence of novel adaptations that may help us to understand, preserve or recolonize these complex, fragile and important environments. Further studies on the following topics are currently needed: (i) identification of the dormancy mechanisms that ensure the viability of persistent seed banks and seed viability even after months of submergence; (ii) a comprehensive evaluation of the mechanisms that confer on many terrestrial species the ability to endure prolonged submergence and drought along with their role in the formation of persistent seedling banks; (iii) ecophysiology of reproduction, dispersal and establishment of aquatic herbaceous plants of different life forms; and (iv) comparative studies of the efficacy of sexual versus vegetative propagation for aquatic herbaceous plants and pioneer trees that rely on both mechanisms.
Sources of funding
This work contains parts of studies supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, the Programa de Apoio a Núcleos de Excelência—PRONEX/Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq/Fundação de Amparo à Pesquisa do Estado do Amazonas-FAPEAM, Brazil, and the scientific exchange project of the Instituto Nacional de Pesquisas da Amazônia, Brazil, and of the Max-Planck Institute, Germany.
Contributions by the authors
All authors have contributed to, seen and approved the manuscript.
Conflicts of interest statement
None declared.
Acknowledgement
We thank Dr Florian Wittmann for helpful comments and corrections to the manuscript.
References
- Alsdorf DE, Melack JM, Dunne T, Mertes LAK, Hess LL, Smith LC. Interferometric radar measurements of water level changes on the Amazon floodplain. Nature. 2000;404:174–177. doi: 10.1038/35004560. [DOI] [PubMed] [Google Scholar]
- Anderson JT, Rojas JS, Flecker AS. High-quality seed dispersal by fruit-eating fishes in Amazonian floodplain habitats. Oecologia. 2009;161:279–290. doi: 10.1007/s00442-009-1371-4. [DOI] [PubMed] [Google Scholar]
- Baldwin AH, Egnotovich MS, Clarke E. Hydrologic change and vegetation of tidal freshwater marshes: field, greenhouse and seed-bank experiments. Wetlands. 2001;21:519–531. [Google Scholar]
- Barsoum N. Relative contributions of sexual and asexual regeneration strategies in Populus nigra and Salix alba during the first years of establishment on a braided gravel bed river. Evolutionary Ecology. 2002;15:255–279. [Google Scholar]
- Bayley PB. Aquatic environments in the Amazon basin, with an analysis of carbon sources, fish production and yield. Canadian Special Publication of Fisheries and Aquatic Sciences. 1989;106:399–408. [Google Scholar]
- Boom BM. A forest inventory in Amazonian Bolivia. Biotropica. 1986;18:287–94. [Google Scholar]
- Brown JS, Venable DL. Evolutionary ecology of seed-bank annuals in varying environments. The American Naturalist. 1986;127:31–47. [Google Scholar]
- Buckeridge MS, Aidar MPM, Santos HP, Tine MA. Acúmulo de reservas. In: Ferreira AG, Borguetti F, editors. Germinação: do básico ao aplicado. Porto Alegre: Artmed; 2004. pp. 31–67. [Google Scholar]
- Campbell DG, Daly DC, Prance GT, Ubirajara NM. Quantitative ecological inventory of terre firme and varzea tropical forest on the Rio Xingu, Brazilian Amazon. Brittonia. 1986;38:369–393. [Google Scholar]
- Campbell DG, Stone JL, Rosas A. A comparison of the phytosociology and dynamics of three floodplain (várzea) forests of known ages, Rio Juruá, western Brazilian Amazon. Botanical Journal of the Linnean Society. 1992;108:213–237. [Google Scholar]
- Conserva AS, Piedade MTF. Ciclo de vida e ecologia de Paspalum fasciculatum Willd. Ex. Fluegge (Poaceae), na várzea da Amazônia Central. Acta Amazonica. 2001;31:205–219. [Google Scholar]
- Correa SB, Winemiller KO, López-Fernández H, Galetti M. Evolutionary perspectives on seed consumption and dispersal by fishes. Bioscience. 2007;57:748–756. [Google Scholar]
- D'Angelo SA. Colonização vegetal em áreas de sedimentação recente na várzea da Amazônia Central. Brazil: Instituto Nacional de Pesquisas da Amazônia; 2009. Masters Thesis. [Google Scholar]
- Esteves FA. Fundamentos de limnologia. Rio de Janeiro: Interciência Finep; 1998. [Google Scholar]
- Ferreira CS, Piedade MTF, Junk WJ, Parolin P. Floodplain and upland populations of Amazonian Himatanthus sucuuba: effects of flooding on germination, seedling growth and mortality. Environmental and Experimental Botany. 2007;60:477–483. [Google Scholar]
- Ferreira CS, Piedade MTF, Tine MAS, Rossatto DR, Parolin P, Buckeridge MS. The role of carbohydrates in seed germination and seedling establishment of Himatanthus sucuuba, an Amazonian tree with populations adapted to flooded and non-flooded conditions. Annals of Botany. 2009;104:1111–1119. doi: 10.1093/aob/mcp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LV. Effects of the duration of flooding on species richness and floristic composition in three hectares in the Jaú National Park in floodplain forests in Central Amazonia. Biodiversity and Conservation. 1997;6:1353–1363. [Google Scholar]
- Ferreira LV. Species richness and floristic composition in four hectares in the Jaú National Park in upland forest in Central Amazonia. Biodiversity and Conservation. 1998;10:1349–1364. [Google Scholar]
- Ferreira LV. Effects of flooding duration on species richness, floristic composition and forest structure in river margin habitat in Amazonian blackwater floodplain forests: implications for future design of protected areas. Biodiversity and Conservation. 2000;9:1–14. [Google Scholar]
- Ferreira LV, Almeida SS. Relação entre a altura de inundação, riqueza especıfica de plantas e o tamanho de clareiras naturais em uma floresta inundável de igapó na Amazônia Central. Revista Árvore. 2005;29:445–453. [Google Scholar]
- Ferreira LV, Prance GT. Structure and species richness of low diversity floodplain forest on the rio Tapajós, Eastern Amazonian. Biodiversity and Conservation. 1998;7:585–596. [Google Scholar]
- Furch K. Water chemistry of the Amazon basin: the distribution of chemical elements among freshwaters. In: Sioli H, editor. The Amazon: limnology and landscape ecology of a mighty tropical river and its basin. Dordrecht: Dr W. Junk; 1984. pp. 167–200. [Google Scholar]
- Gottsberger G. Seed dispersal by fish in the inundated regions of Humaitá, Amazonia. Biotropica. 1978;10:170–183. [Google Scholar]
- Goulding M. The fishes and the forest. Explorations in Amazonian natural history. Berkeley: University of California Press; 1980. [Google Scholar]
- Goulding M. Amazonian fisheries. In: Moran EF, editor. The dilemma of Amazonian development. Boulder: Westview Press; 1983. pp. 189–210. [Google Scholar]
- Goulding M, Barthem R, Ferreira E. The Smithsonian Atlas of the Amazon. Washington, DC: Smithsonian Institution Press; 2003. [Google Scholar]
- Grime JP, Mason G, Curtis AV, Rodman J, Band SR, Mowforth MAG, Neal AM, Shaw S. A comparative study of germination characteristics in a local flora. Journal of Ecology. 1981;69:1017–1059. [Google Scholar]
- Haugaasen T, Peres CA. Tree phenology in adjacent Amazonian flooded and unflooded forests. Biotropica. 2005;37:620–630. [Google Scholar]
- Haugaasen T, Peres CA. Floristic, edaphic and structural characteristics of flooded and unflooded forests in the lower Rio Purús region of central Amazonia, Brazil. Acta Amazonica. 2006;36:25–36. [Google Scholar]
- Haugaasen T, Peres CA. Vertebrate responses to plant phenology in Amazonian flooded and unflooded forest. Biodiversity and Conservation. 2007;16:4165–4190. [Google Scholar]
- Hook DD. Adaptations to flooding with fresh water. In: Kozlowski TT, editor. Flooding and plant growth. New York: Academic Press; 1984. pp. 265–294. [Google Scholar]
- Irion G, Adis J, Junk WJ, Wunderlich F. Sedimentological studies of the ‘Ilha da Marchantaria’ in the Solimões Amazon river near Manaus. Amazoniana. 1983;8:1–18. [Google Scholar]
- Junk WJ. Ecology of várzea, floodplain of Amazonian white-water rivers. In: Sioli H, editor. The Amazon: limnology and landscape ecology of a mighty tropical river and its basin. Dordrecht: Dr W. Junk; 1984. pp. 215–243. [Google Scholar]
- Junk WJ. Flood tolerance and tree distribution in Central Amazonian Floodplains. In: Nielsen LB, Nielsen IC, Baslev H, editors. Tropical forests: botanical dynamics, speciation and diversity. London: Academic Press; 1989. pp. 47–64. [Google Scholar]
- Junk WJ. Wetlands of tropical South-America. In: Whigham D, Hejny S, Dykyjová D, editors. Wetlands of the world. Dordrecht: Kluwer; 1993. pp. 679–739. [Google Scholar]
- Junk WJ. General aspects of floodplain ecology with special reference to Amazonian floodplains. In: Junk WJ, editor. The Central Amazon floodplain: ecology of a pulsing system. Ecological Studies. Vol. 126. Heidelberg: Springer; 1997. pp. 3–22. [Google Scholar]
- Junk WJ. Flood pulsing and the lineages between terrestrial, aquatic, and wetland systems. Verhandlungen der Internationale Vereinigung für Limnologie. 2005;29:11–38. [Google Scholar]
- Junk WJ, Fürch K. Química da água e macrófitas aquáticas de rios e igarapés na Bacia Amazônica e áreas adjacentes. Acta Amazonica. 1980;10:611–633. [Google Scholar]
- Junk WJ, Howard-Williams C. Ecology of aquatic macrophytes in Amazonia. In: Sioli H, editor. The Amazon: limnology and landscape ecology of a mighty tropical river and its basin. Dordrecht: Dr W. Junk; 1984. pp. 269–293. [Google Scholar]
- Junk WJ, Piedade MTF. Biomass and primary production of herbaceous plant communities in the Amazon floodplain. Hydrobiologia. 1993a;263:155–162. [Google Scholar]
- Junk WJ, Piedade MTF. Herbaceous plants of the Amazon floodplain near Manaus: Species diversity and adaptations to the flood pulse. Amazoniana. 1993b;XII:467–484. [Google Scholar]
- Junk WJ, Piedade MTF. Species diversity and distribution of herbaceous plants in the floodplain of the middle Amazon. Proceedings of the International Association of Theoretical and Applied Limnology. 1994;25:1862–1865. [Google Scholar]
- Junk WJ, Piedade MTF. Plant life in the floodplain with special reference to herbaceous plants. In: Junk WJ, editor. The Central Amazon floodplain. Berlin: Springer; 1997. pp. 147–181. Ecological Studies. [Google Scholar]
- Junk WJ, Barley PB, Sparks RE. The flood-pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences. 1989;106:110–127. [Google Scholar]
- Kalliola R, Salo J, Puhakka M, Rajasilta M. New site formation and colonizing vegetation in primary succession on the western Amazon floodplains. Journal of Ecology. 1991;79:877–901. [Google Scholar]
- Klinge H, Ohly H. Chemical properties of rivers in the Amazonian area in relation to soil conditions. Verhandlungen der Internationale Vereinigung für Limnologie. 1964;15:1067–1076. [Google Scholar]
- Klinge H, Adis J, Worbes M. The vegetation of a seasonal várzea forest in the lower Solimões river, Brazilian Amazonia. Acta Amazonica. 1995;25:201–220. [Google Scholar]
- Kozlowski TT. Tree Physiology Monograph No. 1. Victoria: Heron Publishing; 1997. Responses of woody plants to flooding and salinity. http://www.heronpublishing.com/tp/monograph/Kozlowsk.pdf . (March 2000) [Google Scholar]
- Kozlowski TT, Pallardy SG. Growth control in woody plants. San Diego: Academic Press; 1997. [Google Scholar]
- Kubitzki K, Ziburski A. Seed dispersal in flood plain forests of Amazonia. Biotropica. 1994;26:30–43. [Google Scholar]
- Leck MA, Schütz W. Regeneration of Cyperaceae, with particular reference to seed ecology and seed banks. Perspectives in Plant Ecology. Evolution and Systematics. 2005;7:95–133. [Google Scholar]
- Lewis WM, Jr, Hamilton SK, Lasi MA, Rodriguez M, Saunders JF., III Ecological Determinism on the Orinoco Floodplain. BioScience. 2006;50:681–692. [Google Scholar]
- Lima Filho DA, Matos FDA, Amaral IL, Revilla J, Coêlho LS, Ramos JF, Santos JL. Inventário florístico de floresta ombrófila densa de terra firme, na região do Rio Urucu-Amazonas, Brasil. Acta Amazonica. 2001;31:565–579. [Google Scholar]
- Lucas CM. Within flood season variation in fruit consumption and seed dispersal by two characin fishes of the Amazon. Biotropica. 2008;40:581–589. [Google Scholar]
- Maia L, Santos L, Parolin P. Germinação de sementes de Bothriospora corymbosa (Rubiaceae) recuperadas do trato digestório de Triportheus angulatus (sardinha) no lago Camaleão, Amazônia Central. Acta Amazonica. 2007;37:321–326. [Google Scholar]
- Neil C, Elsenbeer H, Krusche AV, Lehmann J, Markewitz D, Figueiredo RO. Hydrological and biogeochemical processes in a changing Amazon: results from small watershed studies and the large-scale biosphere-atmosphere experiment. Hydrological Processes. 2006;20:2467–2477. [Google Scholar]
- Oliveira AC. Aspectos da dinamica populacional de Salix martiana (Salicaceae) em areas de varzea da Amazonia central. Brazil: Instituto Nacional de Pesquisas da Amazônia; 1998. Masters Thesis. [Google Scholar]
- Oliveira-Wittmann A, Lopes A, Conserva AS, Piedade MTF. Germination and plant establishment in floodplain forests. In: Junk WJ, Piedade MTF, Parolin P, Wittmann F, Schöngart J, editors. Central Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management. Heidelberg: Springer, in press.; Ecological Studies, Vol. 210. [Google Scholar]
- Padial AA, Thomaz SM, Agostinho AA. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia. 2009;624:161–170. [Google Scholar]
- Parolin P. Seed mass in Amazonian floodplain forests with contrasting nutrient supplies. Journal of Tropical Ecology. 2000;16:417–428. [Google Scholar]
- Parolin P. Submergence tolerance vs. escape from submergence: two strategies of seedling establishment in Amazonian floodplains. Environmental and Experimental Botany. 2002;48:177–186. [Google Scholar]
- Parolin P. Submerged in darkness: adaptations to prolonged submergence by woody species of the Amazonian floodplains. Annals of Botany. 2009;103:359–376. doi: 10.1093/aob/mcn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin P, Junk WJ. The effect of submergence on seed germination in trees from Amazonian floodplains. Boletim do Museu Paraense Emilio Goeldi. Série Botanica. 2002;18:321–329. [Google Scholar]
- Parolin P, Armbrüster N, Wittmann F, Ferreira LV, Piedade MTF, Junk WJ. A review of tree phenology in central Amazonian floodplains. Pesquisas, Botânica. 2002;52:195–222. [Google Scholar]
- Piedade MTF, Junk WJ. Natural herbaceous plant communities in the Amazon floodplain and their use. In: Junk WJ, Ohly J, editors. The Central Amazon floodplain: actual use and options for a sustainable management. Leiden: Backhuys; 2000. pp. 129–290. [Google Scholar]
- Piedade MTF, Junk WJ, Long SP. The productivity of the C4 Grass Echinochloa polystachya on the Amazon Floodplain. Ecology. 1991;72:1456–63. [Google Scholar]
- Piedade MTF, Junk WJ, Long SP. Nutrient dynamics of the highly productive C4 macrophyte Echinochloa polystachya on the Amazon floodplain. Functional Ecology. 1997;11:60–65. [Google Scholar]
- Piedade MTF, Junk WW, Parolin P. The flood pulse and photosynthetic response of trees in a white water floodplain (várzea) of Central Amazon, Brazil. Verhandlungen der Internationale Vereinigung für Limnologie. 2000;27:1734–1739. [Google Scholar]
- Piedade MTF, Worbes M, Junk WJ. Geo-ecological controls on elemental fluxes in communities of higher plants in Amazonian floodplains. In: McClain ME, Victoria RL, Richey JE, editors. The biogeochemistry of the Amazon Basin. Oxford: Oxford University Press; 2001. pp. 209–234. [Google Scholar]
- Piedade MTF, Parolin P, Junk WJ. Estratégias de dispersão, produção de frutos e extrativismo da palmeira Astrocaryum jauari Mart. nos igarapés do Rio Negro: implicações para a ictiofauna. Ecología Aplicada. 2003;2:31–40. [Google Scholar]
- Piedade MTF, Junk WJ, Adis J, Parolin P. Ecologia, zonação e colonização da vegetação arbórea das Ilhas Anavilhanas. Pesquisas Botanica. 2005;56:117–144. [Google Scholar]
- Pires JM, Prance GT. The vegetation types of the Brazilian Amazon. In: Prance GT, Lovejoy TE, editors. Amazonia key environments. Oxford: Pergamon Press; 1985. pp. 109–145. [Google Scholar]
- Prance GT. The origin and evolution of the Amazon flora. Interciência. 1978;3:207–230. [Google Scholar]
- Pringle C. What is hydrologic connectivity and why is it ecologically important? Hydrological Processes. 2003;17:2685–2689. [Google Scholar]
- Puhakka M, Kalliola R, Rajasilta M, Salo J. River types, site evolution and successional vegetation patterns in Peruvian Amazonia. Journal of Biogeography. 1992;19:651–665. [Google Scholar]
- Salo JS, Kalliola R, Hakkinen I, Makinen Y, Niemela P, Puhakka M, Coley PD. River dynamics and the diversity of the Amazon lowland forest. Nature. 1986;322:254–258. [Google Scholar]
- Silva TSF, Costa M, Melack JM. Annual net primary production of macrophytes in the eastern Amazon floodplain. Wetlands. 2009;29:747–758. [Google Scholar]
- Sioli H. Sobre a sedimentação na várzea do Baixo Amazonas. Boletim Técnico do Instituto Agronomico do Norte. 1951;24:112–128. [Google Scholar]
- Thomaz SM, Bini LM, Bozelli RL. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia. 2007;579:1–13. [Google Scholar]
- Thompson K, Grime JP. Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. Journal of Ecology. 1979;67:893–921. [Google Scholar]
- Waldhoff D, Sant-Paul U, Furch B. Value of fruits and seeds from the floodplain forests of central Amazonia as food resource for fish. Ecotropica. 1996;2:143–156. [Google Scholar]
- Waldhoff D, Junk WJ, Furch B. Responses of three Central Amazonian tree species to drought and flooding under controlled conditions. International Journal of Ecology and Environmental Sciences. 1998;24:237–252. [Google Scholar]
- Wittmann AO, Piedade MTF, Parolin P, Wittmann F. Germination in four low-várzea tree species of Central Amazonia. Aquatic Botany. 2007;86:197–203. [Google Scholar]
- Wittmann F, Parolin P. Phenology of six tree species from Central Amazonian várzea. Ecotropica. 1999;5:51–57. [Google Scholar]
- Wittmann F, Anhuf D, Junk WJ. Tree species distribution and community structure of central Amazonian várzea forests by remote-sensing techniques. Journal of Tropical Ecology. 2002;18:805–820. [Google Scholar]
- Wittmann F, Junk WJ, Piedade MTF. The várzea forests in Amazonia: Flooding and the highly dynamic geomorphology interact with natural forest succession. Forest Ecology and Management. 2004;196:199–212. [Google Scholar]
- Wittmann F, Schöngart J, Montero JC, Motzer T, Junk WJ, Piedade MTF, Queiroz HL, Worbes M. Tree species composition and diversity gradients in white-water forests across the Amazon Basin. Journal of Biogeography. 2006;33:1334–1347. [Google Scholar]
- Worbes M. The forest ecosystem of the floodplains. In: Junk WJ, editor. The Central Amazon floodplain: ecology of a pulsing system. Vol. 126. Heidelberg: Springer; 1997. pp. 223–266. Ecological Studies. [Google Scholar]
- Worbes M, Klinge H, Revilla JD, Martius C. On the dynamics, floristic subdivision and geographical distribution of várzea forests in Central Amazonia. Journal of Vegetation Science. 1992;3:553–564. [Google Scholar]
- Ziburski A. Rauh W, editor. Dissemination, Keimung und Etablierung einiger Baumarten der Überschwemmungswälder Amazoniens. Tropische und Subtropische Pflanzenwelt. 1991;77:1–96. Akademie der Wissenschaften und der Literatur. [Google Scholar]