Using a co-dominant genotypic PCR marker we show for the first time that, in sugar beet, the GA and B-gene pathways are independent for bolt initiation. We show that vernalization permits GA-dependant stem elongation and that the B-allele influences subsequent flowering.
Abstract
Background and aims
Bolting, the first visible sign of reproductive transition in beets (Beta vulgaris), is controlled by the dominant bolting gene B (B allele), which allows for flowering under long days (LDs, >14 h light) without prior vernalization. The B-locus carries recessive alleles (bb) in sugar beet (Beta vulgaris L. spp. vulgaris), so that vernalization and LDs are required for bolting and flowering. Gibberellin growth hormones (GAs) control stem elongation and reproductive development, but their role during these processes in sugar beet is not defined. We aimed to investigate the involvement of GAs in bolting and flowering in sugar beet, and also its relationship with the vernalization requirement as defined by the B-gene.
Methodology
Plants segregating for the B allele were treated with exogenous GA4 under inductive (16 h light) and non-inductive (8 h light) photoperiods, with and without prior vernalization treatment. A co-dominant polymerase chain reaction (PCR) marker was used to genotype the B-gene locus. Bolting and flowering dates were scored, and bolt heights were measured as appropriate. Analysis of variance was used to determine the effects and interactions of GAs, the B allele and vernalization on bolting and flowering. The effects of the B allele on bolting were also verified in the field.
Principal results
Application of GAs or the B allele could initiate bolting independently. When the B allele was absent, the applied GAs promoted stem growth, but did so only in vernalized plants, irrespective of photoperiod. Under LDs, bolt height before flowering in plants carrying the B allele (BB; Bb) was not significantly influenced by GAs. The timing and frequency of flowering were influenced by the B allele without interactive effects from GAs.
Conclusions
In sugar beet, GA acts independently of the B allele and photoperiod to induce bolting. Vernalization enables GA action independently of the B allele; hence, the dominant B allele may not directly participate in vernalization-induced bolting.
Introduction
Sugar beet (Beta vulgaris spp. vulgaris) is an important source of sugar and is grown as a root crop in Europe, North America, the Middle East, Egypt, India, Chile, Japan and China. Effective control of bolting and flowering is essential for both the cultivation and breeding of sugar beet crops. High root yields depend on a prolonged vegetative growth phase that breeders have largely achieved by actively selecting against genotypes carrying the dominant bolting gene B (B allele; Abegg, 1936), thereby creating cultivars in which alleles at the B-gene locus are considered to be recessive. Sugar beet plants with the dominant B-allele reproduce in the first year of cultivation on exposure to long days (LDs, >12–14 h light), and without vernalization (prolonged exposure to cold temperature). In contrast, cultivars that lack the dominant B allele must first be vernalized (usually over winter), prior to LD exposure, a process termed photothermal induction (Owen et al., 1940; Owen, 1954). For simplicity, these sugar beet genotypes are generally referred to as annual and biennial types, respectively, and this terminology will be adopted here. In reality, the growth habit of B. vulgaris species is more complicated, ranging from annual to iteroparous perennial growth, as observed in B. vulgaris spp. maritima (Hautekeete et al., 2002). In both annual and biennial genotypes, the reproductive transition is marked by rapid elongation of the primary axis (bolting). The onset and development of the reproductive phase involve the distinct and sequential processes of bolting followed by flowering. The dependence of biennial plants on photothermal induction complicates bolting control in cultivated crops in which a large proportion of genetic variation is attributed to additive inheritance (Sadeghian and Johansson, 1993). Investigation of this process has been focused mainly on understanding contributions from vernalization (Schmid, 1974; Lexander, 1987; Crosthwaite and Jenkins, 1993; Sadeghian, 1993; Hohmann et al., 2005; Reeves et al., 2006; Milford et al., 2010) and, more recently, from photoperiod (Chia et al., 2008), including the interactive effects of vernalization (Van Dijk, 2009).
Breeding targets for the control of bolting and flowering in cultivated beet must meet the dual, but opposing, needs for high sugar yielding crops and seed production. The latter requires a short generation time and synchronized flowering. Transgenic approaches are an obvious and convenient option for crop improvement, and are made easier by the hybrid nature of sugar beet, which would allow the use of inducible transgenic targets, enabling bolting and flowering to be activated only in breeding lines. Efforts are under way to clone the B-gene locus (Gaafar et al., 2005), identify and map floral transcription factors (Reeves et al., 2006; Chia et al., 2008), and establish their relationships with bolting. The role of growth hormones has also been investigated (Khalil and Reda, 1980; Elliott et al., 1986; Wittenmayer and Schilling, 1998), with gibberellins (GAs) being most extensively studied in this respect (Garrod, 1974; Lenton et al., 1975; Lexander, 1987; Sadeghian et al., 1993). The discovery that GAs could induce flowering in many plant species under otherwise non-inductive conditions (Zeevaart, 1983) generated much interest among sugar beet breeders, who considered the possibility of accelerating breeding by substituting vernalization with GA application. However, in non-vernalized sugar beet, exogenous GAs failed to induce any significant bolting or flowering, although they affected cellular growth at the shoot apex (Sadeghian et al., 1993). In sugar beet, GAs are important for reproductive growth (Radley, 1975; Debenham, 1999; Sorce et al., 2002). Endogenous levels of GA are not limiting for bolting (Mutasa-Göttgens et al., 2009) and are also in the related B. vulgaris spp. maritima sufficient for vegetative bolt induction (Van Dijk, 2009).
The mechanism of GA participation in reproductive growth, in sugar beet, remains unclear, despite the early leads from the work of Gaskill (1957). Although Gaskill's study was limited by the small number of plants used (12), it nevertheless provided the first insight into the positive interaction between vernalization treatment and GA-induced bolting. Later, a clear link was established between vernalization requirement in biennial sugar beet and bolting behaviour in response to GAs (Margara, 1960). This work also revealed variation in the behaviour of annual types in response to GAs, which was found to have little effect on plants in the Beta section Corollinae, while in the section Patellaris, stem elongation was induced, with GAs being detrimental for flowering. Magara (1960) reported that, in B. maritima, GAs promoted both bolting and flowering in LDs, but only bolting in short days (SDs). It is logical, therefore, to assume that alleles at the B-gene locus (widely recognized for its major effects on bolting and vernalization requirement) might influence plant responses to the applied GAs. Here, we begin a systematic analysis of GA function during the reproductive transition by examining the relationship between GA signalling and the B-gene pathway. To achieve this, we determined the effect of the applied GA4 under conditions that were inductive (LDs—16 h light) and not inductive (SDs—8 h light) for bolting and flowering, using plants segregating for the B allele with and without prior vernalization. The alleles at the B-gene locus were determined by a co-dominant polymerase chain reaction (PCR) marker. Our objectives were to observe the effects of exogenous GAs, the B allele and vernalization on bolting and subsequent flowering, which, in sugar beet, is generally regarded as always proceeding from bolting.
Materials and methods
Plants
F2Bb
F2Bb is an F2 population (F1 sib-cross) segregating for the B allele and developed from an original cross between a biennial female parent plant derived from the sugar beet breeding line CZ259 (Lewellen, 2002) and an annual B. maritima pollinator (Broom's Barn accession 6952-5/IDDB 56771; originally from the former Yugoslavia).
NF transgenic line 2210
NF is an original breeding line from SES Vanderhave and was used to generate a GA-deficient line designated 2210 (Mutasa-Göttgens et al., 2009) which was used as a control for monitoring the potency of the applied GA4 solutions.
CZ259
This genotype is a bolting-susceptible biennial line, requiring only 8-week vernalization in controlled environment (CE) conditions (as below) to become fully vernalized. This genotype could be fully vernalized within 7 weeks outdoors in average daily temperatures of 6.8–7.5 °C, with an average vernalizing temperature of 3.8 °C
F2 populations 950619 and 960701
Selfed progeny from two F1 plants (940081/598 and 940081/604) derived from a cross between an annual parent and a biennial parent as described by El-Mezawy et al. (2002). Annual (BB and Bb) plants totalling 536 for 950619 and 365 for 960701 were selected using B-locus markers as detailed later. These populations were used to characterize the effects of the B allele on the time taken to bolt and the bolting frequency.
Glasshouse conditions
The glasshouse at Broom's Barn (52°16′N, 00°34′E) was maintained at 20–22 °C with a minimum 16-h photoperiod and light intensity as described by Mutasa-Göttgens et al. (2009).
CE room
The temperature was maintained at 22 °C and lights set to provide an 8-h photoperiod from a mixture of fluorescent tubes (125 W; GEC F125W/35) and incandescent bulbs (40 W; Phillips 60 mm, pearl). The last hour was programmed to provide light enriched with far-red irradiation (Osram 40 W bulbs). The average photosynthetically active radiation was measured at ∼262 µmol m−2 s−1 when all the lights were on and ∼17 µmol m−2 s−1 with tungsten lights only as used in the last hour of the light period.
Vernalization chamber
Vernalization was carried out in a Sanyo MLR350 Environment Test Chamber fitted with Daystar F36W-98 natural daylight fluorescent lights set to provide an 8-h SD photoperiod. To maintain low vernalizing temperatures and low light levels of 25–30 µmol m−2 s−1, we reduced the number of fluorescent tubes to three. The temperature was set at 4 °C for 9 days, followed by 6 °C for 18 days and a 7-day thermal buffer period at 15 °C immediately before plants were transferred to the SD CE room. Plants were vernalized under a non-inductive SD photoperiod to prevent undesired activation of bolting responses during vernalization.
Plant cultivation in the glasshouse and CEs
Seeds were broadcast in compost (Levington F2S, supplied by the Scotts Company UK Ltd, Suffolk, UK), supplemented with insecticide granules (Intercept 5GRTM with 5 % w/w imidacloprid, produced by Bayer, Germany and distributed by Scotts Company UK Ltd, Bramford, UK) and fertilizer (Osmocote Extract Standard 8-9M from Scotts International B.V., Heerlen, The Netherlands). Intercept was added at a rate of 8.4 g and osmocote was added at a rate of 183 g for every 30 L of compost. Emerging seedlings were transferred to 0.5-L pots in compost with fertilizer only and allowed to grow until the two-leaf stage in the glasshouse. This took between 18 and 24 days post-sowing, after which batches of plants were transferred to the SD CE room at 22 °C until 100 plants were obtained. Plants were allowed to reach at least five- to six-leaf stage in the SD CE room before GA application commenced.
Plants were watered regularly as required, with the water applied as a soil drench and at least 1 h prior to GA application or 1 day after GA application. The use of systemic pesticides was avoided in case they interfered with the GA treatment.
Plant cultivation in the field
Populations 950619 and 960701 were sown at Kiel University (54°20′N, 10°08′E) on 5 May 2003, grown in the greenhouse for 4 weeks under LD conditions with supplementary lighting (Son-T Agro 400 W, Koninklijke Philips Electronics N.V., Eindhoven, The Netherlands) to create a photoperiod of 16 h day−1 when it was required, and transplanted to the field on 3 June 2003.
Experimental procedures
F2Bb GA application experiments
Three different experiments were carried out as described subsequently.
Experiment 1: effects of applied GA in SD
This experiment was carried out before the genotypic marker (see below) was available. In this case, 100 F2Bb plants grown to the two-leaf stage in the glasshouse were transferred to the SD CE room and randomly split into two groups of 50 plants each. Leaf tissue was sampled for DNA extraction and later for genotyping. One group of 50 plants was used as controls and the other was treated with GA4 by applying 10 µL aliquots of a 20 µM aqueous solution directly to the shoot apex, equivalent to ∼13.3 ng of GA4 per application. GA was applied once every 3 days for 30 days and then once a week for a further 4 weeks. Each plant received a total of 14 GA applications, ∼186 ng of GA4, during the course of the experiment. The activity of the working stock solution was monitored by simultaneous application to the NF GA-deficient transgenic sugar beet line 2210. All plants were subsequently genotyped using the PCR marker (detailed below), before the final data analysis.
Experiment 2: effects of applied GA and vernalization in SD
In this experiment, 230 F2Bb plants were grown in the SD CE room and genotyped before being split into two groups. When plants reached the two- to four- true-leaf stage, one group of 100 plants was transferred to the vernalization chamber for 4 weeks as described above. Meanwhile, the non-vernalized plants remained in the SD CE room at 22 °C. At the end of the vernalization period, vernalized plants were returned to the SD CE room and each group of plants (vernalized and non-vernalized) was sub-divided into two groups of 50 plants each, one for GA application and the other as the control group, ensuring that at least 10 plants of the biennial genotype were assigned to each treatment. Although plants were at the same age, we tried to compensate for possible differences in developmental stage between the vernalized and non-vernalized plants by commencing GA treatment when plants had similar numbers of true leaves. Plants were treated with GA as described above, except that it was applied once a week for a total of 10 weeks. The experiment ended 20 weeks after sowing.
Experiment 3: effects of applied GA in LD
In this experiment, 100 F2Bb plants were grown in the LD (16 h light) CE room, genotyped and sub-divided for GA treatment. No vernalization treatment was given. When plants reached the six- to eight-true-leaf stage (∼6 weeks after sowing), GA4 was applied as above once a week for 10 weeks when the experiment ended.
In all three experiments, plants were scored for bolting date and, if they flowered, the date on which the floral bud was first observed. The total number of internodes extended at flowering was counted and the apical height was measured from the base of the rosette. The date on which the flowers subsequently opened was also recorded. If plants bolted, but did not produce a floral bud, internode numbers and apical heights were noted at the end of the experiment. If plants reverted from bolting to a vegetative rosette, then details of internode number and apical height were recorded as soon as the reversion was noted. Alternatively, apex height to root diameter ratios were used when scoring for bolts by simple visual inspection was considered inaccurate. Full details are given later.
During the experiments, some plants were lost due to pest and disease damage (mainly thrips and mildew, respectively). The number of plants that were finally used in each treatment is therefore indicated in the appropriate tables given in the Results section.
CZ259 GA application experiment
One hundred plants were grown in the glasshouse until they reached the two-leaf stage. They were then transferred to SDs in the CE room and allowed to reach six–eight leaves before half were vernalized for 8 weeks in the vernalization chamber. Non-vernalized control plants remained in the CE room at 22 °C under SDs. After vernalization, plants were returned to the SD CE room and, together with the non-vernalized plants, sub-divided for GA treatment (as above), except that GA was applied twice weekly for 8 weeks when the experiment was ended. The bolting dates (visually scored), number of extended internodes and apical heights at the time of floral bud appearance were recorded. At the end of the experiment (20 weeks after sowing), the total numbers of bolted and non-bolted plants were counted.
Genotyping the B-locus
The co-dominant PCR marker GJ1001c16 was used to distinguish plants that were homozygous (dominant and recessive) and heterozygous at the B-locus. PCR amplification was carried out using 50–100 ng of genomic DNA (extracted using the NucleoSpin 96 Plant DNA isolation kit supplied by Macherey and Nagel, Düren, Germany), template with primers A196 and A195 (available by Material Transfer Agreement (MTA) on request from CAU Kiel). The cycle conditions were 2 min at 94 °C, [30 s at 94 °C, 30 s at 59 °C, 25 s at 72 °C] × 30 and 5 min at 72 °C. Homozygous plants gave a 202-bp (recessive) or a 174-bp (dominant) product, whereas both were present in the heterozygous plants. The populations 950619 and 960701 were genotyped with two co-dominant markers tightly linked to the B-locus at R = 0.005 (GJ18T7b) or R = 0.007 (Y67L), respectively, and flanking the B-locus on either side. The indel marker GJ18T7b was assayed by PCR amplification with primers A015 and A016 (2 min at 94 °C, [30 s at 94 °C, 30 s at 54 °C, 30 s at 72°C] × 32, 5 min at 72°C) and electrophoresis on a 3 % MetaPhor high-resolution agarose gel (Biozym Scientific GmbH, Hessisch Oldendorf, Germany). Plants homozygous for the dominant or recessive allele at the B-locus in these populations give a 142- or 152-bp product, respectively, whereas heterozygous plants yield both. The cleaved, amplified, polymorphic sequence marker Y67L was assayed by PCR amplification with primers A019 and A020 (2 min at 94°C, [30 s at 94 °C, 30 s at 63 °C, 25 s at 72 °C] × 32, 5 min at 72 °C), HaeIII (Fermentas, St Leon-Rot, Germany) restriction enzyme digestion and standard agarose gel electrophoresis. In plants homozygous for the dominant allele, the 240-bp PCR product is not cleaved by HaeIII, whereas in plants homozygous for the recessive allele or heterozygous at the B-locus, the marker assay results in two fragments of 130 and 110 bp, or three fragments of 240, 130 and 110 bp, respectively.
Measurement of growth parameters
Bolting in field-grown plants (Kiel, F2 plant populations 960701 and 950619) was scored by visual inspection, when bolts were at least 5 cm tall as defined by Smit (1983). Plants were inspected every 2–4 days from 16 June to 17 July (42–73 days after sowing), when plants experienced LD photoperiods in the field, averaging 17.1 h day−1 from 3 June to 16 June 2003 and 17.0 h day−1 from 3 June to 17 July 2003. In all other plants (CE grown), bolting was scored when at least one internode was visibly extended from the rosette at the shoot apex. The final bolt height was measured and the total number of extended internodes was also recorded where appropriate. In cases where a visually discernible morphological change could be detected at the shoot apex but could not be identified as a bolt, we defined this as ‘bolt initiation’, although we did not distinguish between cell division/multiplication and cell elongation as the basis of this change. To enable an objective determination of bolt initiation, all leaves were removed by excising at the petiole base to expose the shoot tip. The height of the shoot apex was then measured from the lowest leaf scar on the root crown (for example, see Additional information, Fig. S1). The root diameter (average of two measurements at right angles at the widest part) of each plant was noted and the apical height to root diameter ratio was determined to standardize values across plants of different sizes within each treatment. A threshold ratio value (see Data analysis section) was then used to determine and score bolt initiation.
Flowering was scored when the first visible floral bud, firm to the touch, appeared. Flower opening time was scored when the first fully open, mature flower was observed.
Data analysis
The analyses of data for growth variables were carried out using analysis of variance (ANOVA) with GenStat 10 (VSN International, Hemel Hempstead, UK). For the analysis of count data, a log-linear model from the family of generalized linear model was used to test for significant association between treatments with GenStat 10.
Bolt initiation
A threshold value of apex height to root diameter ratio above which plants were considered as having ‘initiated bolting’ was calculated using ANOVA. Essentially, apex height to root diameter ratios were compared between all bolted and non-bolted plants, in each experiment, as determined by visual inspection. Where there was a significant difference in apex height to root diameter ratio between the two classes of plants, a threshold value delimiting a ‘bolting’ response was determined such that the two classes were separated from a value of least significant difference at 5 % probability level. This threshold value was then used as the baseline for calculating the total number of bolted plants and/or plants initiating bolts in each treatment.
Bolting
The number of plants that bolted in each treatment was counted and grouped according to genotype. The number and length of internodes were used as measures of stem extension. Analysis of variance was then applied to determine the effects from each treatment.
Results
Bolt initiation in SDs
In Experiment 1, we found that when F2Bb plants were subjected to GA treatment in SDs, if they did not bolt by extending at least one internode it was difficult to accurately score the GA-induced morphological changes at the shoot apex by visual inspection alone. Consequently, we measured apex height from the lowest leaf scar and expressed it as a ratio to average root diameter, on the widest part of the tap root. Our results showed significant differences (P < 0.01) between bolters and non-bolters in these ratio values for both the GA and control treatments. Mean and threshold values above which plants were considered to have bolted were therefore calculated using measurements from the bolted plants (Table 1). Threshold values (in this case, 2.41 and 2.95 for the control and GA treatments, respectively) were then used to partition plants into bolt-initiated and non-initiated groups, as given in Table 2. Analysis of these data showed that GAs had a significant effect on bolt initiation frequency, which increased from 31.8 to 68.9 % (P < 0.01). The GA treatment was the main source of variation (P < 0.001) in apex height to root diameter ratios and not the B-genotype (P > 0.8) (Table 3). There were no detectable interaction effects between GA and the B allele (P > 0.2). In this experiment, we were mainly concerned with examining the initial stages of the bolting process and therefore did not continue to monitor the progression of plants to bolting after the experiment was ended.
Table 1.
Mean apex height to root diameter ratio and associated critical threshold values of non-vernalized F2Bb plants under SD (8 h light) conditions
| Visual bolt score | Untreated controls |
GA treated |
|---|---|---|
| Apical height to root ratio | Apical height to root ratio | |
| Bolted | 3.50 (37) | 4.44 (37) |
| Not bolted | 1.75 (7) | 1.86 (8) |
| SEDa | 0.33 | 0.54 |
| Threshold ratioa | 2.41 | 2.95 |
Apex heights of bolted and non-bolted plants were measured from the lowest leaf scar on the root and divided by the root diameter, measured at the widest part of the root. A threshold apex height to root diameter ratio value above which plants were considered to have initiated bolting was then calculated using ANOVA. Where significant differences in ratio values were detected, a threshold value was determined to separate bolted and non-bolted plants and it was computed as the value of least significance at 5 % probability level. The number of plants classified by visual inspection as having bolted (including all those with discernible changes in apex morphology) or not bolted are shown in parentheses.
SED, standard error of difference.
aApical height to root ratio above which plants were considered to have bolted.
Table 2.
The effects of GA, genotype and their interaction on the initiation of changes in shoot apex morphology, as assessed by the ratio of apical height to root size, in the segregating F2/Bb plant population under SD conditions
| Change in apex morphology and B-genotype |
||||||
|---|---|---|---|---|---|---|
| Change |
No change |
|||||
| Treatment | BBa | Bba | bbb | BB | Bb | bb |
| Plus GA4 | 8 | 21 | 2 | 5 | 7 | 2 |
| No GA4 | 4 | 5 | 5 | 8 | 14 | 8 |
B-genotypes among the segregating population were determined using the co-dominant PCR marker GJ1001c16. At the end of the experiment, plants were partitioned for bolt initiation based on the critical threshold ratios for GA-treated and untreated plants (Table 1).
aAnnual types that normally require LDs to bolt.
bBiennial types that normally require vernalization and LDs to bolt.
Table 3.
The effects of GA, genotype and their interaction on the ratio of apical height to root size in the segregating F2/Bb plant population under SD (8 h light) conditions
| Source of variance | d.f. | Mean square | F | P |
|---|---|---|---|---|
| GA | 1 | 83.09 | 39.03 | <0.01 |
| Genotype | 2 | 0.39 | 0.18 | 0.83 |
| GA × genotype | 2 | 2.95 | 1.38 | 0.26 |
| Residual (random) | 83 | 2.13 | ||
| Total | 88 |
Non-vernalized F2Bb plants segregating for the dominant B-gene were grown in a SD CE room, treated with GA and scored for changes in shoot apex morphology as defined by threshold apex height to root diameter ratio for each genotype. Interactive effects between GA and genotype were then determined based on ANOVA using a log-linear model from the generalized linear model to test for significant association between treatments with GenStat 10 (VSN International, Hemel Hempstead, UK).
Vernalization effects in SDs
In Experiment 2, ‘true bolts’ as determined by visual scoring were measured. Here, we found that if vernalized F2Bb plants were kept under a non-inductive photoperiod (8 h light), the effects of vernalization on bolting were small but significant (P < 0.01). Across the plant population as a whole (221 plants, of which 33 were biennials), bolting increased from 3.4 to 8.2 % (Table 4) and the major difference between genotypes was that none of the biennials bolted without vernalization (Table 4). In contrast, GA alone had a greater effect and increased the bolting frequency from 3.4 to 45.3 % (P < 0.001). Again, this effect was detected only among the annual plants (Table 4). The combined effects of GA and vernalization resulted in a bolting frequency of 93.4 % (P < 0.05) and included all but one of the 10 biennial plants tested. The interactive effects between GA, vernalization and genotype were such that biennial genotypes bolted only if they were vernalized prior to GA application (Table 4). Such interactive effects of vernalization and GA were also observed in the biennial breeding line CZ259 (Table 5), where bolting increased from zero, in non-vernalized plants treated with GA, to 45 % (P < 0.01) if GA was applied after vernalization. Similar effects have been observed in three commercial cultivars and two other biennial breeding lines that we have examined (Mutasa-Göttgens, unpublished results).
Table 4.
The effect of vernalization ± GA application on bolting frequency among F2Bb plants in SD (8 h light) photoperiod
| Genotype | Treatment and associated responses |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vern. + GA |
Vern. − GA |
Non-vern. + GA |
Non-vern. − GA |
|||||
| Bolt | No bolt | Bolt | No bolt | Bolt | No bolt | Bolt | No bolt | |
| BB | 26 | 0 | 2 | 22 | 13 | 7 | 2 | 17 |
| Bb | 22 | 3 | 2 | 16 | 11 | 15 | 0 | 30 |
| bb | 9 | 1 | 0 | 7 | 0 | 7 | 0 | 9 |
| Total | 57 | 4 | 4 | 45 | 24 | 29 | 2 | 56 |
| Bolting (%) | 93.4c | 8.2a | 45.3b | 3.4 | ||||
Vernalized and non-vernalized F2Bb plants segregating for the dominant B allele were treated with GA and scored for bolting by visual inspection, to select plants with at least one extended internode. Interactive effects between GA, vernalization and genotype were then determined based on ANOVA as described in Table 3.
N.B. vernalization treatment was carried out in non-inductive SDs.
aSignificant effect of vernalization alone (P < 0.01).
bSignificant effect of GA application alone (P < 0.01).
cSignificant combined effects of vernalization and GA (P < 0.05).
Table 5.
The number of bolted and non-bolted biennial plants from the breeding line CZ259 (bb types) ± GA application in SDs (8 h light) with and without prior vernalization
| GA treatment | Vernalized |
Non-vernalized |
||
|---|---|---|---|---|
| Bolted | Not bolted | Bolted | Not bolted | |
| +GA | 9a | 11 | 0 | 25 |
| −GA | 0 | 17 | 0 | 24 |
Vernalized and non-vernalized CZ259 (easy bolting bb genotype) plants growing in a SD CE room were treated with GA and scored for bolting by visual scoring and as defined by the threshold apex height to root diameter ratio for each treatment. Interactive effects between GA and vernalization were then determined based on ANOVA as described for Table 3.
aBolting was significantly affected by GA (P < 0.01).
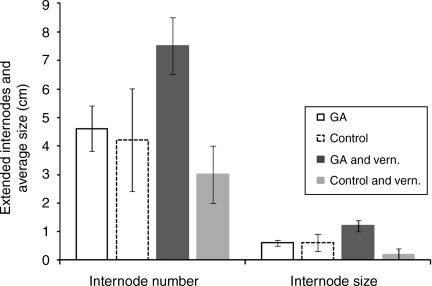
Among the bolted plants, in Experiment 2, the number and size of extended internodes were significantly larger only in vernalized GA-treated individuals. Thus, there were no significant differences between non-vernalized plants with or without GA treatment or vernalized plants without GA treatment (Fig. 1). Unvernalized plants extended approximately four internodes, with an average length of ∼0.8 cm, irrespective of GA treatment. However, when GA was applied to vernalized plants, the number of extended internodes increased to between seven and eight and the internode length to ∼1.5 cm (Fig. 1).
Fig. 1.
The effects of applied GA and vernalization on stem growth. The numbers of extended internodes were counted and the final stem heights used to calculate the average internode length in F2Bb plants treated with exogenous GA with and without prior vernalization. Plants were grown under SDs (8 h light) in the CE chamber at 22 °C. Significant GA effects were observed only in vernalized plants.
Full bolting responses, here defined as stem elongation leading to a floral transition resulting in the development of axillary and terminal floral spikes, were not observed under SD conditions in any treatment. In many of the plants, bolts terminated by reverting to a vegetative rosette. This clearly suggests that LDs are essential for the floral transition and could not be substituted by the application of GA and/or vernalization.
Effects of the B allele and GA on growth in LDs
Without vernalization, the biennial types were less likely to bolt than the annual types (P < 0.001), and 33 % of the F2Bb annual plants (both heterozygous and homozygous genotypes) also failed to bolt under LD conditions in the CE room (Table 6). Moreover, bolting did not necessarily commit plants to flowering, because 35 % of annual types that bolted reverted to a vegetative rosette growth habit. A significantly larger (P < 0.01) proportion of homozygous annual types progressed to flowering than their heterozygous counterparts. Representative phenotypes of F2Bb plants grown in inductive LDs, as described here, are shown in Fig. 2.
Table 6.
The total number of bolting and flowering plants in the non-vernalized F2Bb population growing under LDs (16 h light), in the CE room at 22 °C
| Genotype | Total plants | Bolts onlya | Bolts and flowers | Non-bolting |
|---|---|---|---|---|
| BB | 25 | 4 | 14 | 7 (28 %) |
| Bb | 50 | 22 | 10 | 18 (36 %) |
| Bb | 16 | 0 | 0 | 16 (100 %) |
The results are from the third experiment in which F2Bb plants were scored for bolting and flowering dates by visual inspection. Treatment and genotype effects were then determined based on ANOVA as described for Table 3.
aPlants reverted to a vegetative rosette perched on the bolted stem (Fig. 2); GA treatment had no major significant effects in these conditions (P > 0.19); no significant major interactive effects from B-gene and GA were detected (P > 0.22); the most significant effects were from the B-gene on the frequency of bolting (P < 0.01) and flowering (P < 0.01).
Fig. 2.
Typical phenotypes observed among F2Bb plants grown in the LD (16 h light) CE room. The plants are from left to right: non-bolted, representative of the biennial bb genotype; bolted with reversion to rosette growth, representative of the annual Bb genotype; and bolted with flowers, representative of the annual BB genotype. Such phenotypes were observed in LD conditions irrespective of GA treatment.
Analysis of apex height to root diameter ratios of plants that did not bolt in LDs, including all the biennial types (associated mean values are given in Table 7), provided good evidence (P < 0.05) that major inductive effects were from the B allele. No significant effects were detected from GA treatment (P > 0.19) and there were no significant effects of the interaction between the B allele and GA (P > 0.22). Among the bolted plants of annual types, the effects of GA and B-genotype on a range of parameters associated with bolting and flowering are shown in Additional information, Tables S1–S6. These results, summarized in Table 8, showed that there was a marginally significant effect of the B allele on bolting time (P = 0.052) but no evidence for significant effects of GA and its interaction with the B allele on bolting time (respectively, P > 0.73, P > 0.66). There was also no evidence for significant effects of applied GA, the B allele and their interaction on bolt height (respectively, P > 0.36, P > 0.13, P > 0.93), internode length (respectively, P > 0.63, P > 0.31, P > 0.32) or the total number of internodes extended before bud formation (respectively, P > 0.28, P > 0.81, P > 0.26), or on time taken to visible bud formation (respectively, P > 0.96, P > 0.17, P > 0.87).
Table 7.
Mean apex height to root diameter ratios in non-vernalized F2Bb plants which did not bolt ±GA4 application under LDs (16 h light)
| Genotype | −GA | +GA | Genotype mean |
|---|---|---|---|
| Bb | 0.95 | 1.24 | 1.11 |
| Bb | 1.53 | 1.55 | 1.54 |
| BB | 1.08 | 2.29 | 2.12 |
| GA treatment mean | 1.26 | 1.62 |
These are results from the third experiment in which F2Bb plants were grown in the LD CE room at 22 °C, genotyped at the B-gene locus using the co-dominant PCR marker and partitioned for GA treatment. Plants which, at the end of the experiment, were not regarded as having bolted by visual inspection were evaluated instead by comparing apex height to root ratio values as a measure of changes in shoot apex morphology ('bolt initiation'). Treatment and genotype effects were then determined using ANOVA as described for Table 3.
Major effects on apical height to root ratio values were detected only from the B-locus alleles (P < 0.05)
Table 8.
Reproductive growth parameters assessed for the effects of GA and B-genotype in LDs (16 h light) in the F2Bb plant population
| Growth parameter | Influencing factors |
||
|---|---|---|---|
| GA | Genotype | GA × genotype | |
| Bolt induction | No | Yes (P < 0.05) | No |
| Bolting frequency | No | Yes (P < 0.01) | |
| Bolting time | No | Yes (P = 0.052) | No |
| No. of internodes extendeda | No | No | No |
| Internode length | No | No | No |
| Bolt height | No | No | No |
| Flowering frequency | No | Yes (P < 0.01) | |
| Flowering timeb | No | No | No |
These are results from the third experiment in which F2Bb plants were grown in the LD CE room at 22 °C, genotyped at the B-gene locus using the co-dominant PCR marker and partitioned for GA treatment. Plants were then scored for bolting and flowering parameters as indicated. Treatment and genotype effects were then determined based on ANOVA for measurements other than counts, but based on ANOVA as described for Table 3.
aAs counted or measured from stem base to floral bud, when bud first appeared.
bTime from bolting to floral bud appearance.
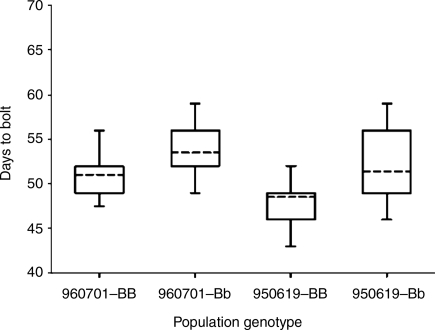
In LD conditions, the only significant effects, as determined by tests for association (χ2 analysis), were observed from the B allele on bolting (P < 0.01) and flowering (P < 0.01) frequencies. These genotype effects were associated with zygosity at the B-locus such that the homozygous dominant plants were more likely than the heterozygous plants to bolt and flower. In the heterozygous plants, there was a greater tendency for reversion to vegetative rosettes on the bolted stems, and the flowering frequency was reduced in this genotype. These observations support the idea that bolting does not commit the plants to flowering. Furthermore, bolting time was affected mostly by zygosity at the B-locus (see Additional information, Tables S1–S6), although it was only just significant (P = 0.052). To examine the effects of the dominant B allele further, we analysed the differences in the bolting time of homozygous and heterozygous annual plants raised from the F2 populations 950619 and 960701 and phenotyped in the field during spring and summer. In both populations, the homozygous annual plants bolted 3 days earlier (P < 0.01; Fig. 3).
Fig. 3.
The mean number of days to bolt for annual genotypes in populations 950619 and 960701. The lower boundary of the box indicates the 25th percentile; the upper boundary line of the box indicates the 75th percentile; the broken line in the box indicates the median; and the whiskers below and above the box indicate, respectively, the 5th and 95th percentiles. F2 plant populations segregating at the B-gene locus were grown in the field and scored for bolting when bolts were at least 5 cm tall as defined in Smit (1983). All bolting plants were genotyped and segregated into homozygous and heterozygous pools for analysis to determine the effects of the dominant B allele.
Discussion
Under SD conditions, GA promotes bolt initiation independently of the B allele
In earlier studies, attempts to investigate the effects of exogenous GA on sugar beet involved complex methods for measuring cell elongation in the shoot apex, and the results were difficult to interpret (Lexander, 1987; Sadeghian et al., 1993). Those experiments were conducted in inductive LDs where the effect of GA may have been masked by photoperiod effects. Here, we used non-inductive SDs and measurements of a simple apex height to root diameter ratio to distinguish responsive and non-responsive plants with respect to gross morphological changes at the shoot apex, defined here as ‘bolt initiation’. Our results clearly demonstrated that, in SDs and in the absence of vernalization, the applied GA4 was the major source of variation in shoot apex morphology, as determined using the apex height to root ratio values (P < 0.01) and hence, by our definition, the initiation of bolts. This was reflected in an ∼70 % bolt initiation frequency in GA-treated plants, irrespective of genotype, compared with ∼30 % in untreated plants (P < 0.01; Table 2). In these SD conditions, subsequent stem elongation (visible bolting) did not occur unless the plants had been vernalized previously (Fig. 1). We therefore suggest a role for GA in the early stages of bolting that is distinct from its later effects on promoting stem elongation, as indicated by an increased length and number of extended internodes, in vernalized plants. Whether or not the processes in the early bolt initiation stage mark the switch to reproductive development in sugar beet remains to be determined. This should become clearer once the appropriate molecular markers are developed to monitor the floral transition at the shoot apical meristem. It is not clear whether the B allele participates directly in this GA-dependent bolt initiation process, but our data suggest that it may not be involved as there were no effects from its interaction with GA either in SDs (P > 0.2) or LDs (P > 0.22). An inductive role for GA, as proposed here, may provide the physiological relevance of the observed increases in apical shoot GA content as plants approach bolting (Radley, 1975; Debenham, 1999; Sorce et al., 2002). However, the question still remains as to whether high levels of GA are the cause or the result of bolting.
Vernalization activates GA responses to promote stem growth in SDs
Under an inductive photoperiod, vernalization promotes stem elongation in biennials (Lexander, 1980; Longden, 1986), and here we show that in SDs vernalization also promotes morphological changes in the shoot apex (defined here as bolting initiation), in annual plants, even in the absence of applied GA. Thus, including bolt initiation in annual types, we observed a bolting frequency of 4.1 % (2 of 49 plants) in SDs, which increased to 9.5 % (4 of 42 plants) with vernalization treatment alone (Table 4). However, when GA was applied to vernalized plants, the combined effect of these treatments (across all plants including biennial types) resulted in a 90 % increase (P < 0.05) in bolting (including bolt initiation), compared with the increase of just 4.8 % (P < 0.01) and 41.8 % (P < 0.01) with vernalization or GA treatment alone (Table 4). Importantly, the applied GA did not promote stem growth of biennials unless they were vernalized, so that the three-way interaction of vernalization × GA × genotype was more significant (P < 0.01) than the vernalization × GA (P > 0.3) or vernalization × genotype (P > 0.3) interactions. Taken together, these data indicate that the obligate requirement for vernalization in biennial genotypes is associated with permitting GA-dependent stem elongation.
Vernalization and GA treatment do not substitute for LDs for flowering
For the duration of our experiments, we did not observe any flowering in SD-grown plants, even after vernalization and/or GA treatment. This supports the idea that LDs are necessary to promote flowering in sugar beet. Further, in the conditions described here, LDs could not be replaced by the applied GA or vernalization. In the related sea beet (B. vulgaris spp. maritima), adaptation to flowering in shorter day-length as a function of increased vernalization intensity has been demonstrated (Van Dijk, 2009) and, hence, it is reasonable to assume that the same may be true for sugar beet. In unrelated plant species, high light intensity is known to substitute for LD photoperiod (Evans, 1971) and, even though there is no direct evidence for this in sugar beet, it is worth noting that light intensities of ∼262 µmol m−2 s−1 in our CE rooms were significantly lower than the natural levels of ∼500 µmol m−2 s−1 which plants might experience in the field during the late spring/summer. In future, it will be important to investigate the effects of light intensity and quality on floral induction in sugar beet.
GA has no major significant effects on bolting and flowering times in LDs
The inductive effects of LDs on bolting in sugar beet are well recognized and known to be mediated through B allele function (Bell, 1946; Abe et al., 1997b). What is not clear is the role of GA or its interactions with the B allele. GA promotes cell elongation as required for bolting and is not normally considered to limit the ability of plants to bolt since, in LDs, the bolting frequency among plants with reduced levels of bioactive GA is not significantly affected (Mutasa-Göttgens et al., 2009). Based on apex height to root ratio values, our results demonstrate that, under inductive LDs, GA has no significant major effects on the induction of bolting (P > 0.19), which is instead influenced by the B allele (P < 0.05), without significant interactive effects between the two (P > 0.22). This supports our observations, based on SD data, that the B and GA pathways are independent.
In the BB and/or Bb plants within the F2Bb population, the B allele mainly affected the frequency of bolting (P < 0.01), and flowering (P < 0.01), and the time to bolt (P = 0.052), but not the time of flowering (P > 0.17). The effect of the B allele on time to bolting was also demonstrated by the earlier (∼3 days; P < 0.01) bolting of BB plants compared with Bb plants in both the 950619 and 960701 populations. However, we measured the time taken for the appearance of a floral bud, but this is not necessarily an accurate indication of the timing of floral transition. Therefore, we cannot conclude with certainty whether the B allele has a direct or an indirect role in the temporal control of the floral phase transitions during bolting in sugar beet. In inductive LDs neither the B allele nor GA affected the rate of flower development when measured as the time taken from floral bud formation to flower opening, and the final bolt height was not affected by GA. We know, however, from previous studies that although GA is not limiting for these processes, it has important roles in both (Mutasa-Göttgens et al., 2009). Here, we also detected gene dose effects from the dominant B allele, resulting in delayed bolting in the heterozygous annual types. Similar observations were reported by Abe et al. (1997a) and were attributed to photoperiods of <20 h light (Abe et al., 1997b). In our controlled environment conditions, we used 16 h light and additionally found that in these conditions, flowering was reduced, and to a greater extent than bolting.
Conclusions and forward look
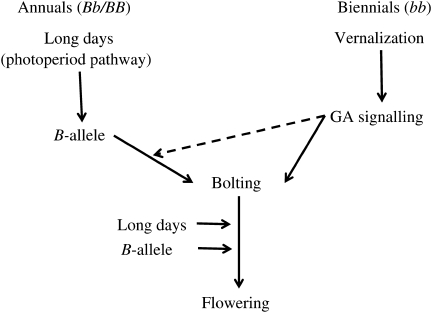
In the light of data presented here, we propose that either the GA or the B pathway can initiate bolting independently of photoperiod. In annual types carrying the dominant B allele, continued stem growth requires LDs and, in these conditions, the GA pathway is not limiting. In biennial types (including all cultivars), which do not carry the dominant B allele, it appears that vernalization is required for promotion of continued stem growth by GA. In this case, as the B-locus is in a homozygous recessive state, we can reasonably assume that the permissive effect of vernalization on GA-induced stem elongation is independent of B. We suggest, therefore, that in biennial sugar beet cultivars, while the B-gene locus determines the requirement for vernalization, the GA pathway is more likely than the B allele to condition the post-vernalization bolting responses. We believe that vernalization has an important role in the de-repression of processes that impact on GA-dependent growth in the shoot apex to trigger bolting and eventually leading to flowering. The precise nature of this interaction between vernalization and GA will become clearer when we have molecular markers to assay GA metabolism in the shoot meristem. Floral transition markers will also help to establish whether the initiation of bolting is associated with phase transitions in the meristem. However, we have seen that bolting and flowering processes can be uncoupled (as observed in bolted plants with vegetative rosettes instead of flowers), supporting the idea that the floral transition occurs after bolting. Finally, although we do not yet fully understand bolting mechanisms in sugar beet crops, knowledge that the GA-dependent stem elongation requires vernalization is an important step forward. We now propose a model for reproductive growth in sugar beet as shown in Fig. 4, in which B-gene and GA pathways converge on bolting, on cue from the photoperiod and vernalization pathways, respectively. This model separates bolt induction into the photoperiod-dependent pathway (B allele) or vernalization-dependent pathway (GA) and places flowering in the photoperiod pathway, downstream of bolting.
Fig. 4.
A simple model of events expected to result in phasic transitions in the apical shoot meristem of sugar beet during reproductive growth. Differences between annual types carrying the dominant B allele (BB; Bb) and biennial types (bb) are represented. Arrows point to the downstream pathway or process most significantly affected by the relevant upstream component. Essentially, bolting in annual types is dependent on the LD photoperiod pathway acting through the B allele, whereas in biennial types bolting requires vernalization to activate the GA signalling pathway. The B allele and GA pathways, therefore, converge on bolting, after which the plants proceed to flowering; a process that is favoured under LD conditions in both annual and biennial types. In SDs (8 h light) only, interactive effects exist between GA and the B-allele such that significantly more plants bolt when GA is applied (P < 0.01). This is indicated by the arrow with the broken line and is not the normal condition in sugar beet. N.B. The recessive bolting alleles (bb) at the B-gene locus are generally used by breeders as markers for vernalization requirement, in order to delay bolting in cultivated biennial sugar beet.
Additional information
The following additional information is available in the online version of this article.
Figure to show GA-induced gross morphological changes at the shoot apex and an illustration of how ‘apex height to root diameter ratio’ was measured to determine ‘bolt initiation’. Also included are tables summarizing measurements of growth parameters associated with reproductive growth comparing the effects of applied GA, day-length and plant genotype.
Sources of funding
Broom's Barn receives financial support from the UK beet industry, administered through the British Beet Research Organisation. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the UK. The project at Broom's Barn and Rothamsted was supported by grant no. 4788 (BBRO 06-20). The project in Kiel was funded by the Deutsche Forschungsgemeinschaft under grant no. DFG JU 205/14-1.
Contributions by the authors
E.S.M.-G., A.Q. and P.H. initiated the work, designed the experiments and wrote the manuscript. E.S.M.-G. and A.J. carried out the vernalization/GA application experiments and genotyped the F2Bb plants with the B-locus PCR marker. A.Q. and A.M. carried out the statistical analysis. Identification and development of B-locus flanking markers was conceived by A.M. and carried out in his group by W.Z. and G.S.-B. U.H. generated the F2 950619 and 960701 populations and phenotyped them for bolting in the field. G.S.-B. genotyped the same plants with the B-locus PCR marker.
Conflict of interest statement
None declared.
Acknowledgements
We thank Kevin Sawford (Broom's Barn), Monika Bruisch and Erwin Danklefsen (Kiel) for technical assistance with glasshouse, CE room and field experiments, and Monika Dietrich (Kiel) for technical assistance in the laboratory. We thank Steve Thomas and Andy Phillips for critical reviews of early drafts of the manuscript, and Keith Jaggard for comments on the final draft.
References
- Abe J, Guan GP, Shimamoto Y. A marker-assisted analysis of bolting tendency in sugar beet (Beta vulgaris L.) Euphytica. 1997;94:137–144. [Google Scholar]
- Abe J, Guan GP, Shimamoto Y. A gene complex for annual habit in sugar beet (Beta vulgaris L.) Euphytica. 1997;94:129–135. [Google Scholar]
- Abegg FA. A genetic factor for the annual habit in beets and linkage relationships. Journal of Agricultural Research. 1936;53:493–511. [Google Scholar]
- Bell GDH. Induced bolting and anthesis in sugar beet and the effect of selection of physiological types. Journal of Agricultural Science. 1946;36:167–183. [Google Scholar]
- Chia TY, Muller A, Jung C, Mutasa-Göttgens ES. Sugar beet contains a large CONSTANS-LIKE gene family including a CO homologue that is independent of the early-bolting (B) gene locus. Journal of Experimental Botany. 2008;59:2735–2748. doi: 10.1093/jxb/ern129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosthwaite SK, Jenkins GI. The role of leaves in the perception of vernalizing temperatures in sugar-beet. Journal of Experimental Botany. 1993;44:801–806. [Google Scholar]
- Debenham GB. UK: University of Nottingham; 1999. Bolting and flowering mechanisms in sugar beet, Beta vulgaris, spp. vulgaris (L.) PhD Thesis. [Google Scholar]
- El-Mezawy A, Dreyer F, Jacobs G, Jung C. High-resolution mapping of the bolting gene B of sugar beet. Theoretical and Applied Genetics. 2002;105:100–105. doi: 10.1007/s00122-001-0859-z. [DOI] [PubMed] [Google Scholar]
- Elliott MC, Hosford DJ, Smith JI, Lawrence DK. Opportunities for regulation of sugar beet storage root growth. Biologia Plantarum. 1986;28:1–8. [Google Scholar]
- Evans LT. Flower induction and the florigen concept. Annual Reviews of Plant Physiology. 1971;22:365–394. [Google Scholar]
- Gaafar RM, Hohmann U, Jung C. Bacterial artificial chromosome-derived molecular markers for early bolting in sugar beet. Theoretical and Applied Genetics. 2005;110:1027–1037. doi: 10.1007/s00122-005-1921-z. [DOI] [PubMed] [Google Scholar]
- Garrod JF. The role of gibberellins in early growth and development of sugar beet. Journal of Experimental Botany. 1974;25:945–954. [Google Scholar]
- Gaskill JO. A preliminary report on the use of gibberellic acid to hasten reproductive development in sugar beet seedlings. Journal of the American Society of Sugar Beet Technologists. 1957;9:521–528. [Google Scholar]
- Hautekeete NC, Piquot Y, Van Dijk H. Life span in Beta vulgaris ssp. maritima: the effects of age at first reproduction and disturbance. Journal of Ecology. 2002;90:508–516. [Google Scholar]
- Hohmann U, Jacobs G, Jung C. An EMS mutagenesis protocol for sugar beet and isolation of non-bolting mutants. Plant Breeding. 2005;124:317–321. [Google Scholar]
- Khalil S, Reda F. Pattern of growth regulating substances in the leaves of vernalized sugar-beet during flowering period. Biologia Plantarum. 1980;22:81–85. [Google Scholar]
- Lenton JR, Pocock TO, Radley ME. Endogenous gibberellins and bolting of sugar beet. vol 1. UK: Rothamsted Experimental Station; 1975. pp. 44–46. [Google Scholar]
- Lewellen RT. Registration of high sucrose, rhizomania resistant sugar beet germplasm line CZ25-9. Crop Science. 2002;42:320–321. doi: 10.2135/cropsci2002.3200. [DOI] [PubMed] [Google Scholar]
- Lexander K. Present knowledge of sugar beet bolting mechanisms. Proceedings of the 43rd Winter Congress of the International Institute of Sugar Beet Research; 1980. pp. 245–258. [Google Scholar]
- Lexander K. Characters related to the vernalization requirement of sugar beet. Manipulation of flowering. London, UK: Butterworths; 1987. pp. 147–158. [Google Scholar]
- Longden PC. Influence of the crop environment on the quality of sugar-beet seed. 49th Winter Congress; Brussels, Belgium: International Institute for Sugar Beet Research; 1986. pp. 1–16. [Google Scholar]
- Margara J. Recherches sur le déterminisme de l'élongation et de la floraison dans le genre Beta. Annales Amélioration des Plantes. 1960;10:362–471. [Google Scholar]
- Milford GFJ, Jarvis PJ, Walters C. A vernalization-intensity model to predict bolting in sugar beet. Journal of Agricultural Science. 2010;148:127–137. [Google Scholar]
- Mutasa-Göttgens E, Qi A, Mathews A, Thomas S, Phillips A, Hedden P. Modification of gibberellin signalling (metabolism & signal transduction) in sugar beet: analysis of potential targets for crop improvement. Transgenic Research. 2009;18:301–308. doi: 10.1007/s11248-008-9211-6. [DOI] [PubMed] [Google Scholar]
- Owen FV. The significance of single gene reactions in sugar beets. Proceedings of the American Society of Sugar Beet Technology. 1954;8:392–398. [Google Scholar]
- Owen FV, Carsner E, Stout M. Photothermal induction of flowering in sugar beet. Journal of Agricultural Research. 1940;61:101–124. [Google Scholar]
- Radley ME. Sugar beet. Growth substances and bolting. Report for 1974. UK: Rothamsted Experimental Station; 1975. p. 35. [Google Scholar]
- Reeves PA, He Y, Schmitz RJ, Amasino RM, Panella LW, Richards C. Evolutionary conservation of the FLC mediated vernalization response: evidence from the sugar beet (Beta vulgaris) Genetics. 2006;176:295–307. doi: 10.1534/genetics.106.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghian SY. Bolting in sugar beet: genetics and physiological aspects. Sweden: Swedish University of Agricultural Sciences; 1993. p. 49. [Google Scholar]
- Sadeghian SY, Johansson E. Genetic study of bolting and stem length in sugar beet (Beta vulgaris L.) using a factorial cross design. Euphytica. 1993;65:177–185. [Google Scholar]
- Sadeghian SY, Johansson E, Lexander K. A genetic analysis of the number of cells, length of cell, and gibberellic-acid sensitivity in sugar-beet and their relation to bolting mechanism. Euphytica. 1993;68:59–67. [Google Scholar]
- Schmid MG. Physiological studies on vernalized and unvernalized sugar beets. II. Enzyme activities in three sugar-beet populations differing in vernalization requirements, before, during and after cold treatment. Angewandte Botanik. 1974;48:339–352. [Google Scholar]
- Smit AL. Wageningen. Agricultural Research Reports, 914 PUDOC; 1983. Influence of external factors on growth and development of sugar-beet (Beta vulgaris L.) PhD Thesis. [Google Scholar]
- Sorce C, Stevanato P, Biancardi E, Lorenzi R. Physiological mechanisms of floral stem elongation (bolting) control in sugar beet (Beta vulgaris ssp. vulgaris L.) Agroindustria. 2002;1:87–91. [Google Scholar]
- Van Dijk H. Evolutionary change in flowering phenology in the iteroparous herb Beta vulgaris ssp. maritima: a search for the underlying mechanisms. Journal of Experimental Botany. 2009;60:3143–3155. doi: 10.1093/jxb/erp142. [DOI] [PubMed] [Google Scholar]
- Wittenmayer L, Schilling G. Behaviour of sugar-beet plants (Beta vulgaris L. ssp. vulgaris var. altissima [Doell]) under conditions of changing water supply: abscisic acid as indicator. Journal of Agronomy and Crop Science. 1998;180:65–72. [Google Scholar]
- Zeevaart JAD. Crozier A, ed. The biochemistry and physiology of gibberellins. 1983. Gibberellins and flowering; pp. 333–374. New York: Praeger. [Google Scholar]