Abstract
Objectives. We investigated the contribution of gestational diabetes mellitus (GDM) to the historic epidemic of type 2 diabetes mellitus (T2DM) in Saskatchewan.
Methods. We constructed a population-level simulation model of the inter- and intragenerational interaction of GDM and T2DM for the period 1956 to 2006. The model was stratified by gender, ethnicity, and age; parameterized with primary and secondary data; and calibrated to match historic time series. Risk of diabetes was sigmoidally trended to capture exogenous factors.
Results. Best-fit calibrations suggested GDM may be responsible for 19% to 30% of the cases of T2DM among Saskatchewan First Nations people, but only for approximately 6% of cases among other persons living in Saskatchewan. The estimated contribution of GDM to the growth in T2DM was highly sensitive to assumptions concerning the post-GDM risk of developing T2DM.
Conclusions. GDM may be an important driver for the T2DM epidemic in many subpopulations. Because GDM is a readily identifiable, preventable, and treatable condition, investments in prevention, rapid diagnosis, and evidence-based treatment of GDM in at-risk populations may offer substantial benefit in lowering the T2DM burden over many generations. Model-informed data collection can aid in assessing intervention tradeoffs.
The rise of the global epidemic of type 2 diabetes mellitus (T2DM) has been particularly rapid and acute among disadvantaged and indigenous populations.1 In North America, for example, aboriginal peoples experience rates of diabetes several times higher than that among the general population.2 Although research has pointed to the influence of rapid environmental and behavioral changes,2 as well as possible genetic contributors,3 recent attention has also been directed at the possible role of diabetic pregnancies (gestational diabetes mellitus [GDM] and pre-existing maternal T2DM) in this epidemic. Previous research conducted among Saskatchewan First Nations people has provided indirect evidence to support a temporal contribution of GDM. Similar to many North American aboriginal peoples, Saskatchewan First Nations people suffer from high rates of GDM,4–8 with First Nations ethnicity being an independent predictor of GDM and with the magnitude of that risk exhibiting distinctive interactions between obesity and ethnicity.5 Among Saskatchewan First Nations people, rates of GDM and overweight or obesity appear to have risen many years prior to widespread appearance of T2DM.6 High-birthweight (HBW) rates, a frequent complication of GDM, have increased in Saskatchewan's predominantly First Nations communities over several decades.8,9 Similar to patterns seen among other Aboriginal groups,10 diabetic Saskatchewan First Nations adults are more likely to have been born with HBW than are their nondiabetic counterparts,8 and the HBW–T2DM relationship appears to have strengthened over time.8 In a reversal of the pattern seen in other Saskatchewan populations, Saskatchewan First Nations women also suffer from significantly higher rates of T2DM than do their male counterparts, with the disparity particularly pronounced in the childbearing years.11
GDM is associated with serious health consequences for both mother and offspring. There is substantial evidence suggesting that GDM predisposes women to T2DM,4,12–15 with approximately 4% to 10% of GDM cases proceeding on to T2DM within the first 9 months after pregnancy.16–18 Occurrence of GDM during a pregnancy is also a predictor for GDM in future pregnancies,17 as well as for other conditions such as cardiovascular disease.19 Finally, because GDM encourages fetal growth,14 women with GDM are more likely to require caesarean sections and are at greater risk of complications during birth.20
For children, the consequences of GDM are of equal or greater severity. GDM significantly elevates the risk of macrosomia and risk of fetal injury during delivery. Children of mothers with GDM also tend to have higher adiposity20 and abnormal glucose tolerance.21–26 Seminal work carried out in the Pima Indian population showed that the children of women with diabetes during pregnancy had increased rates of obesity14,27–29 and T2DM30–33 by adolescence and early adulthood. Observations from Manitoba, where aboriginal children with diabetes were more likely to have experienced a diabetic intrauterine environment,34 support this finding. Some of these effects appear to be independent of birthweight.27 Findings from animal models35 and from sibling studies,29 the absence of influence of paternal diabetes,36–38 and an apparent dose–response relationship between gestational glycemic control and risk of diabetes in one's offspring32 suggest that GDM plays a causal role.29,31,39 Evidence is mixed as to the degree to which infants of glycemically well-controlled mothers with GDM remain at risk.27,40
Although the concept of an intergenerational vicious cycle of diabetic pregnancies leading to progressively increasing rates of T2DM has been demonstrated in animal models35 and is now increasingly cited as a possible contributor to the diabetes epidemic,14,33,41,42 this effect has been challenging to observe and measure in diverse human populations. This may be in part because GDM was not broadly diagnosed until the 1980s and because of the long delays associated with intergenerational effects. Studies among the Pima have suggested that GDM has played a dominant role in elevating rates of T2DM among that population.33 Although these findings have been seminal for providing evidence that a vicious cycle is operating and suggest the importance of the subject, they require translation to understand the degree to which GDM contributes to T2DM rates among other populations. This translation process requires addressing a variety of contextual factors that distinguish the populations of interest, such as differences in age-specific fertility rates, birthweights, population-specific risk factors for GDM, weight profiles through life, and differences in health care systems. Establishing the existence and strength of a link between diabetic pregnancies and the epidemic of T2DM is important because it would contribute to our basic understanding of this devastating chronic disease, provide novel opportunities for primary prevention initiatives, and allow for targeted allocation of health care resources. While awaiting more definitive longitudinal studies, there are prospects for leveraging the large body of evidence regarding the linkages between diverse factors that shape the inter- and intragenerational influences of gestational diabetes on population health (e.g., changing fertility patterns, onset and progression of diabetes, macrosomia and overweight, mortality, and weight gain during and outside of pregnancy). Simulation modeling provides an attractive vehicle both for exploring contributions of GDM to the observed rates and changes in diabetes rates by ethnicity and gender and for lending insight into how focused interventions might reduce this burden. To this end, we investigated the contribution of GDM on the historic epidemic of T2DM in Saskatchewan. Saskatchewan offers a valuable opportunity to examine these factors because of the availability of a long and rich sequence of administrative data,43 the systematic series of studies that have already been conducted related to this subject, and the available interdisciplinary expertise.
METHODS
To better understand the historical impact of gestational diabetes on T2DM, we built a population-level system dynamics model depicting inter- and intragenerational interaction of GDM and T2DM. The model was constructed using the software package Vensim DSS version 5.6b.44 All simulations were performed with a 3-month time step by using Euler integration. Similar to traditional Markov modeling, the System Dynamics model simulated the transitions of individuals between different health states. However, as a dynamic model it also incorporated varying transition rates and featured an open population affected by births, deaths, and migration.
The model characterized the flow of the population among 7 subscripted compartments:
Normal/Underweight,
Overweight,
T2DM,
Normal/Underweight during pregnancy,
Overweight during pregnancy,
GDM,
GDM History.
The first 3 of these compartments applied to both males and females; the final 4 to women only.
Each of these compartments was further subdivided into finer subcategories through the use of subscripting. Specifically, subscripting was used to stratify individuals within a compartment by age (among 17 age categories), ethnicity (Saskatchewan First Nations people— Saskatchewan Ministry of Health beneficiaries registered under Section 6 of the Indian Act and assigned a 10-digit number in the Indian Registry—and other Saskatchewan populations), in utero exposure status (exposed, unexposed), and, where required, sex (male, female). At a given point in time, an individual in the model would thus be counted in exactly 1 of a total of 680 stocks (state variables).
When an individual underwent a change in health status, they transitioned from one stock to another. Such flows were associated with aging, onset and completion of pregnancy, birth, onset of GDM, development of overweight, development of T2DM, and death. Age-specific fertility rates were used to determine the relative distribution of pregnancies among age groups, but the total fertility rate was independently adjusted for each ethnic group to match the historic number of births for a given year.
The model further represented both net migration and the effects of Federal Bill C-3145 (which had the effect of reclassifying over 10 000 members of the other Saskatchewan population as Saskatchewan First Nations people). In both cases, only aggregate ethnic-specific data was available and calibration was used to distribute the total among age and sex groups.
While the model took into account a number of factors likely to have influenced the rise of T2DM in Saskatchewan, there were many dynamic factors not directly captured. For example, previous studies with Canadian aboriginal communities have documented important changes in the level of physical activity7,46–49 and dietary48–50 patterns over the period studied. To reduce the risk of attributing to GDM temporal trends caused by other factors, we accounted for the impact of other rising risk factors on the observed growth in T2DM incidence. Specifically, we used an implicit representation of the influence of diverse factors on T2DM risk by sigmoidally trending diabetes incidence rates over time. The magnitude, rapidity, and timing of the “S”-shaped rise in T2DM incidence rates were assumed to be the same across ethnic, sex, and age groups and were calibrated to best match historic data. By contrast, because the rise in GDM rates is believed to have significantly predated those of T2DM, and to limit the number of parameters being calibrated, we left GDM incidence rates untrended.
Parameter Estimation
Model parameters were estimated in 2 ways. Where suitable data was judged to be directly available, those estimates were used directly in the model. In cases where such data was limited or lacking, model parameters were adjusted (i.e., calibrated) so that the model results best-matched historic time series or data reported in the peer-reviewed literature.
Sources for direct parameter estimates.
Parameter estimates were drawn from a wide variety of sources, including sources in the secondary literature, clinical and survey data we collected, Saskatchewan and Health Canada vital statistics, and Canadian surveys.17,18,32,51–54 While most parameters were treated as constant, some, such as death rates and fertility rates, were trended according to historical time series.
Calibration.
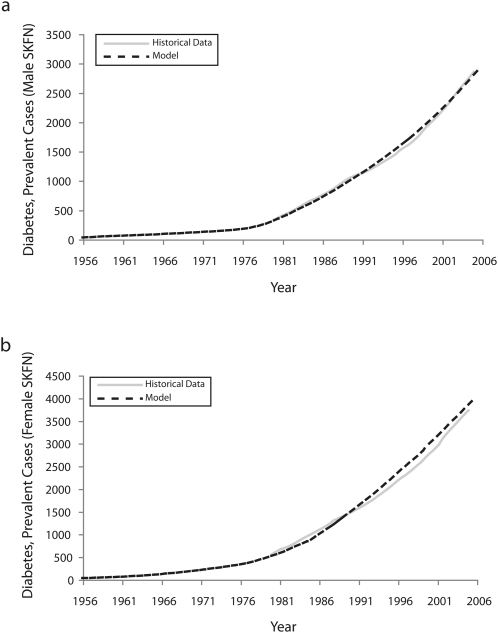
Although a wide variety of historic time series and data points were available through Saskatchewan public health reports, vital statistics, and other sources, such data frequently were too aggregate for use in direct estimation of model parameters. These data, however, did offer important indirect constraints on model parameters by providing reference modes55 to be matched by a model. Many parameters for which data estimates were less readily available were adjusted to yield a best match against a wide variety of historic time series. Calibration was performed for over 300 000 simulations by using a multistart linear Powell optimizer. Historic data series against which model output was compared are given in Table 1. As anticipated by its linear mathematical structure, the convergence of model calibration on very similar sets of values suggested that the best fit parameter values obtained via calibration were unique (details of model calibration available on request). Figure 1 illustrates how model output compared with reported diabetes rates for the study period. Even in the presence of unique solutions, the estimates of model parameters that emerged from calibration were conditional on model structure and therefore not guaranteed to be accurate. However, taken jointly with the model structure and the estimated values of other parameters, they specified at least 1 reasonably consistent dynamic hypothesis for processes underlying historically observed patterns.
TABLE 1.
Descriptions of Study Data Sources
| Description (Year) | Source |
| T2DM incidence cases (1980–2005) | Saskatchewan Health Administrative Data |
| T2DM prevalent cases (1980–2005) | Saskatchewan Health Administrative Data |
| T2DM deaths by age, sex, and ethnicity (1980–2005) and by ethnicity (OSK: 1956–2006; SKFN: 1956–2004) | Saskatchewan Health Administrative Data |
| GDM rates for mothers without diabetes mellitus by ethnicity (1998) | Dyck et al.5 |
| Total population size by ethnicity (1956–2004) and by age, sex, and ethnicity (OSK: 1980–2006; SKFN: 1976–2006) | Saskatchewan Vital Statistics (Saskatchewan Health; OSK populations)54; Vital Statistics of the Registered Indian Population of Saskatchewan (Health Canada; SKFN populations)53 |
| Macrosomia rates by ethnicity (OSK: 1992–2004; SKFN: 1985–2004) | Saskatchewan Vital Statistics (Saskatchewan Health; OSK populations)54; Vital Statistics of the Registered Indian Population of Saskatchewan (Health Canada; SKFN populations)53 |
| SKFN overweight rates by age and sex (1992–2005) | Dyck et al.6 and Katzmarzyk56 |
| Saskatchewan overweight rates by age (2001–2007) | Chad et al.57 |
| Historic deaths by ethnicity (1919–2006), age, and ethnicity (1956–1973) | Saskatchewan Vital Statistics (Saskatchewan Health)54 |
| SKFN deaths by age and sex (1976–1986) | Vital Statistics of the Registered Indian Population of Saskatchewan (Health Canada)53 |
Note. GDM = gestational diabetes mellitus; OSK = other Saskatchewan populations; SKFN = Saskatchewan First Nations people; T2DM = type 2 diabetes mellitus.
FIGURE 1.
Fitting of model to historic time series to show diabetes prevalence for (a) Saskatchewan First Nations (SKFN) males and (b) Saskatchewan First Nations females: 1980–2005.
The default model calibration tuned the annual risk of T2DM assumed to extend from a GDM pregnancy in Saskatchewan First Nations people to best match historic data. Because the calibrated value of this parameter suggested a rate well below that suggested by published studies of aboriginal populations,15 we also conducted a second model calibration in which this parameter remained fixed at a conservative value suggested by a previous study of development of diabetes among the Navajo following pregnancy.4
Initial State
An important goal of the project was to better understand the extent to which the GDM–T2DM link could explain the rise in GDM and T2DM in Saskatchewan. Because most elements of this rise took place over the past half century, we initialized the model to an estimate of the 1956 population.
The demographic structure of the 1956 population was estimated from the Census of Indian Population in the Report of the Department of Citizenship and Immigration 1956–1957 (for Saskatchewan First Nations people)52 and from the Vital Statistics of Saskatchewan (for other Saskatchewan populations).54
Lacking direct data on the weight characteristics of either the Saskatchewan First Nations people or other Sakatchewan populations in the 1950s, we made use of an approximation. On the basis of research that suggested low rates of weight change between the late 1950s and 1971 to 1973,56 we assumed that weight status in 1956 was equivalent to that from 1971 to 1973. We used aggregate sex-specific data for the population aged 20 to 64 years from Chad et al.57 (adjusting by the age-structure of the population) and age- and sex-specific unweighted estimates from Statistics Canada58 to arrive at rough estimates of weight status in 1956. Identical breakdowns by weight were assumed for the Saskatchewan First Nations people and other Saskatchewan populations in the initial year. Although we purposefully selected these data sources knowing that they would err on the side of assuming higher earlier rates of overweight and obesity (thereby attributing less of the rise of T2DM to GDM), we performed a sensitivity analysis in which we recalibrated the model against an initial state with half of the assumed level of overweight.
Finally, the T2DM prevalence in the 1956 populations of Saskatchewan First Nations people and other Saskatchewan residents was assumed to be equal and was estimated by extrapolating US National Health Interview Survey (NHIS) derived estimates back from 1960, using NHIS data from 1960 to 1981.56 These numbers were corrected for estimated differences between Saskatchewan and the US population by using respective information on the 1981 diabetes prevalence of each area.
Scenarios
Model scenarios for a given ethnicity were compared to a baseline (reference) scenario for that ethnicity in which the calibrated model was run forward from 1956 through 2006 using default parameter settings. Alternative scenarios were specified using alternative parameter settings and run over the same period of time. The results of these scenarios were then compared to the baseline scenario for the appropriate ethnicity.
Three scenarios were run. The first (baseline) performed the simulation for the default values of parameters. The second and third scenarios were counterfactual. The second scenario (no GDM) eliminated all impacts of GDM on both the mother and the child. The third scenario (no intergenerational effects of GDM) removed only the intergenerational impacts of GDM (but not of maternal T2DM), by eliminating the effects of GDM on the children's weight or risk of T2DM.
RESULTS
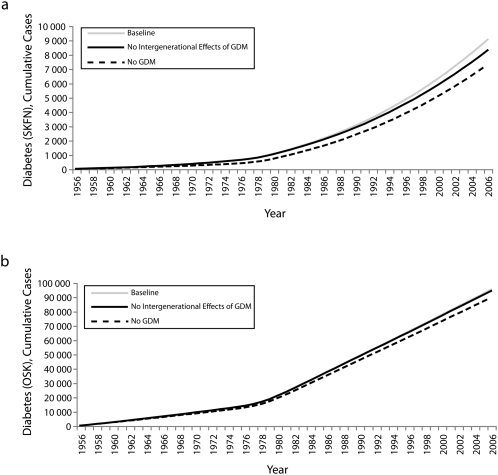
Scenario results are shown in Figure 2 for Saskatchewan First Nations people and other Saskatchewan populations, respectively. The baseline and no GDM scenarios represent the 2 extreme scenarios; the other scenario shows the results in between.
FIGURE 2.
Model results for cumulative cases of diabetes for the 3 scenarios for (a) Saskatchewan First Nations (SKFN) populations and (b) other Saskatchewan residents: 1980–2005.
The baseline scenario accurately captured many historical trends throughout the calibration and other data sets. Judging by matches in those years where data on diabetes incidence and prevalence were available and by early reports indicating extremely low diabetes rates amongst the Saskatchewan First Nations population in the 1930s,60 the baseline scenario captured quite well the observed rise in diabetes diagnoses (Figure 1). Although we lack historic data to confirm this, the calibrated model suggested that diabetes rates exhibited a gradual rise throughout the 1960s and 1970s in both the Saskatchewan First Nations people and other Saskatchewan residents, yielding the sizeable prevalent populations of diabetes cases reported in 1980 administrative data.
For Saskatchewan First Nations people, the elimination of all influences of GDM from both mother and child in the model resulted in an approximately 19% reduction in cumulative incidence of T2DM over the 50-year time period. The difference in diabetes burden between this scenario and the baseline widened rapidly especially early on, suggesting a possible strong early role for GDM in affecting T2DM rates amongst the Saskatchewan First Nations population. This gap then slowly enlarged over time as each successive generation emerged and was followed through the life course. These reductions of cumulative diabetes burden were much smaller in the other Saskatchewan populations (5.7%). In terms of prevalence, these results were similar, with the simulated elimination of GDM yielding decreases of 18.1% and 7.0% for Saskatchewan First Nations people and other Saskatchewan populations, respectively.
The elimination of all intergenerational impacts of GDM in Saskatchewan First Nations people yielded a modest but widening reduction in the prevalence of diabetes over time; the total reduction in prevalence was 7.33% by 2006. The timing of the growth of this impact was strongly shaped by the maturation times associated with successive generations, the levels of GDM seen, and birth rates. Continuation of the simulations suggested that the intergenerational impacts of GDM in the model would expand rapidly in future decades, as those exposed in utero became at highest risk of developing diabetes (data not shown). The small magnitude of this impact in other Saskatchewan populations (approximately 0.89% by 2006) reflected the lower risks apparently associated with GDM in that population and the lower birth rates involved.
Recalibration against an alternative initial state yielded virtually no change in the scenario outcomes. By contrast, we found that results were highly sensitive to calibration assumptions regarding the yearly risk of developing T2DM borne by Saskatchewan First Nations mothers with a history of GDM. The alternative calibration in which the associated parameter was left fixed at a conservative value yielded an approximately 50% higher estimated impact of GDM on cumulative T2DM cases (with GDM attribution increasing to 30.4%). The quality of the alternative calibration remained very similar to the original calibration, except for a modest decrease in the closeness of fit to historic data of model-estimated T2DM rates among Saskatchewan First Nations women in their reproductive years.
DISCUSSION
Building on epidemiological observations suggesting that a “vicious cycle”14,33,41,42 of T2DM among the Pima Indian population exists, we used computational modeling to examine the contribution of GDM to the T2DM epidemic amongst Canadian aboriginal peoples. Model results suggested that although GDM was likely a major contributor to the ongoing T2DM epidemic among Saskatchewan First Nations people, it may have had only a minor impact on the rise of T2DM among other Saskatchewan populations. Within the next few decades, the intragenerational impacts of GDM will be particularly dominant. In the multidecade timeframe, the intergenerational effects of GDM may be as large.
Although our results suggest that GDM may be a highly important causal factor in the T2DM epidemic for many subpopulations, it is also notable for being readily identifiable, preventable, treatable, and prevalent. Studies suggest that improvements in glucose control during GDM may reduce the risk of macrosomia and birth complications,61 childhood obesity,40 and birth complications. Pre- and postnatal exercise62 and dietary programs63,64 may offer benefits in lowering the occurrence and severity of GDM. Moreover, there is a great potential for behaviour change because pregnancy is associated with a short timeframe, close contact between women and the health care system, the presence of an inexpensive screening test, and a high level of motivation on the part of many expectant mothers for investments in health for the duration of a pregnancy.65 Despite the presence of barriers to breastfeeding among women with GDM,66,67 interventions promoting higher initiation and duration of breastfeeding also show promise. Although further research is required,67 breastfeeding appears to lower the risk of childhood obesity and T2DM among children,68 including those of diabetic mothers.67,69 In addition to these intergenerational effects, breastfeeding may offer benefits in delaying or reducing risk of T2DM66,67 among women with a history of GDM. Lactation heightens metabolism, appears to facilitate postpartum weight loss, and enhances short-term glucose tolerance, but evidence for a beneficial effect on long-term maternal glucose tolerance is limited.67
From a worldwide perspective, it is important to recognize that the high risk associated with GDM in Saskatchewan First Nations people may be shared by many other groups. GDM rates are rising across North America and are particularly high in some subpopulations.41,70,71 There is also growing evidence that exposure to a dysglycemic uterine environment elevates risk of obesity, prediabetes and diabetes among White populations.21,25,26,40,72–77
Our study had a number of important limitations. Most notable was the need to incorporate a more detailed representation of other factors contributing to the rise in obesity, such as changes in physical activity levels and dietary behavior. In light of recent findings suggesting a linkage between maternal visceral adiposity and risk of impaired glucose tolerance,78 the model could benefit from a more refined representation of weight and from distinguishing the weight status of women with a history of GDM. Finally, whereas studies have suggested significant intergenerational risks extending from dysglycemia below the GDM classification threshold,32 our model significantly underplays those risks. Future simulation studies could benefit from finer representation of degrees of maternal glycemic control.
An important finding from our work was the identification of priorities for additional study of both intragenerational and intergenerational effects of GDM. At the intragenerational level, our study suggests that improved estimates of the risks of T2DM borne by post-GDM Saskaetchewan First Nations mothers could greatly improve our understanding of the contribution of GDM to the T2DM epidemic. Our study also suggested the importance of better understanding the long-term effects of in utero exposure within White populations. Because we have made conservative assumptions in modeling the intergenerational effects of GDM among other Saskatchewan populations, our study may have underplayed the actual impact of GDM in that group.
Attribution is a critical consideration when examining counterfactual scenarios. As noted earlier, GDM is most likely diabetogenic in the association between GDM and T2DM in the mother and the offspring. Dose–response relationships and sibling studies of discordant siblings strongly suggest that occurrence of GDM plays an important causal role in worsening the risk of T2DM in one's children. In the mother, the evidence for the causal mechanisms of the interaction remains less complete, and some of the association between GDM and T2DM may not be causal. In light of this limited evidence, 2 points bear emphasis when considering scenario outcomes. First, it is best to view the model's estimate of the intragenerational contribution of gestational diabetes to T2DM as an upper bound on the historic contribution. Second, in light of the fact that GDM is a highly identifiable risk factor for T2DM, even for any degree to which GDM serves as more of a marker for T2DM risk than as a causative factor, the model estimates may still help to give some sense of the possible benefits of aggressive targeted interventions strategies informed by appearance of GDM.
Investments in preventive programs and rapid diagnosis and aggressive treatment among high-risk women may offer high returns in improved health not only throughout the mother's life but also for many generations hence.
Acknowledgments
Support of this work was provided through the University of Saskatchewan Summer Student Employment Program, from the Saskatchewan Health Research Foundation (SHRF) through the Obesity Team Research Grant (SHRF grant 1642), and from the National Science and Engineering Research Council's (NSERC's) Discovery Grant (RGPIN-327290-20).
The authors wish to express their appreciation to undergraduate Jing Bai for her assistance with data preparation.
Note. This study is based in part on nonidentifiable data provided by the Saskatchewan Ministry of Health. The interpretations and conclusions contained herein do not necessarily represent those of the Government of Saskatchewan, the Saskatchewan Ministry of Health, SHRF, or NSERC.
Human Participant Protection
No human participants were involved in this study.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Diabetes Care. 2004;27(5):1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Young TK, Reading J, Elias B, O'Neil JD. Type 2 diabetes mellitus in Canada's First Nations: status of an epidemic in progress. CMAJ. 2000;163(5):561–566 [PMC free article] [PubMed] [Google Scholar]
- 3.Barroso I. Genetics of type 2 diabetes. Diabet Med. 2005;22:517–535 [DOI] [PubMed] [Google Scholar]
- 4.Steinhart JR, Sugarman JR, Connell FA. Gestational diabetes is a herald of NIDDM in Navajo women. High rate of abnormal glucose tolerance after GDM. Diabetes Care. 1997;20(6):943–947 [DOI] [PubMed] [Google Scholar]
- 5.Dyck R, Klomp H, Tan LK, Turnell RW, Boctor MA. A comparison of rates, risk factors, and outcomes of gestational diabetes between aboriginal and non-aboriginal women in the Saskatoon health district. Diabetes Care. 2002;25(3):487–493 [DOI] [PubMed] [Google Scholar]
- 6.Dyck RF, Tan L, Hoeppner VH. Short report: body mass index, gestational diabetes and diabetes mellitus in three northern Saskatchewan aboriginal communities. Chronic Dis Can. 1995;16(1):24–26 [Google Scholar]
- 7.Rodrigues S, Robinson E, Gray-Donald K. Prevalence of gestational diabetes mellitus among James Bay Cree women in northern Quebec. CMAJ. 1999;160(9):1293–1297 [PMC free article] [PubMed] [Google Scholar]
- 8.Dyck RF, Klomp H, Tan L. From “thrifty genotype” to “hefty fetal phenotype”: the relationship between high birthweight and diabetes in Saskatchewan registered indians. Can J Public Health. 2001;92(5):340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyck RF, Tan L. Differences in high birthweight rates between northern and southern Saskatchewan: implications for aboriginal peoples. Chronic Dis Can. 1995;16(3):107–110 [Google Scholar]
- 10.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LTH, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ. 1994;308(6934):942–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyck R, Osgood N, Lin TH, Gao A, Stang MR. Epidemiology of diabetes mellitus among First Nations and non-First Nations adults. CMAJ. 2010;182(3):249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care. 2007;30(4):878–883 [DOI] [PubMed] [Google Scholar]
- 13.Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779 [DOI] [PubMed] [Google Scholar]
- 14.Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30(Suppl 2):S169–S174 [DOI] [PubMed] [Google Scholar]
- 15.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868 [DOI] [PubMed] [Google Scholar]
- 16.Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care. 2007;30(Suppl 2):S105–S111 [DOI] [PubMed] [Google Scholar]
- 17.Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care. 2007;30(5):1314–1319 [DOI] [PubMed] [Google Scholar]
- 18.Feig DS, Zinman B, Wang X, Hux JE. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ. 2008;179(3):229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078–2083 [DOI] [PubMed] [Google Scholar]
- 20.Saydah SH, Chandra A, Eberhardt MS. Pregnancy experience among women with and without gestational diabetes in the US, 1995 National Survey of Family Growth. Diabetes Care. 2005;28(5):1035–1040 [DOI] [PubMed] [Google Scholar]
- 21.Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes. Diabetes Care. 2008;31(2):340–346 [DOI] [PubMed] [Google Scholar]
- 22.Plagemann A. Glucose tolerance and insulin secretion in children of mothers with pregestational IDDM or gestational diabetes. Diabetologia. 1997;40(9):1094–1100 [DOI] [PubMed] [Google Scholar]
- 23.Weiss PA, Scholz HS, Haas J, Tamussino KF, Seissler J, Borkenstein MH. Long-term follow-up of infants of mothers with type 1 diabetes: evidence for hereditary and nonhereditary transmission of diabetes and precursors. Diabetes Care. 2000;23(7):905–911 [DOI] [PubMed] [Google Scholar]
- 24.Gautier J-F, Wilson C, Weyer C, et al. Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes. 2001;50(8):1828–1833 [DOI] [PubMed] [Google Scholar]
- 25.Sobngwi E. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet. 2003;361(9372):1861–1865 [DOI] [PubMed] [Google Scholar]
- 26.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care. 1995;18(5):611–617 [DOI] [PubMed] [Google Scholar]
- 27.Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR. Obesity in offspring of diabetic Pima Indian women despite normal birthweight. Diabetes Care. 1987;10(1):76–80 [DOI] [PubMed] [Google Scholar]
- 28.Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308(5):242–245 [DOI] [PubMed] [Google Scholar]
- 29.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–2211 [DOI] [PubMed] [Google Scholar]
- 30.Lindsay RS, Hanson RL, Bennett PH, Knowler WC. Secular trends in birthweight, BMI, and diabetes in the offspring of diabetic mothers. Diabetes Care. 2000;23(9):1249–1254 [DOI] [PubMed] [Google Scholar]
- 31.Dabelea D, Pettitt DJ. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab. 2001;14(8):1085–1091 [DOI] [PubMed] [Google Scholar]
- 32.Franks PW, Looker HC, Kobes S, et al. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes. 2006;55(2):460–465 [DOI] [PubMed] [Google Scholar]
- 33.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of Type II diabetes in American Indian children. Diabetologia. 1998;41(8):904–910 [DOI] [PubMed] [Google Scholar]
- 34.Young TK, Martens PJ, Taback SP, et al. Type 2 Diabetes mellitus in children: prenatal and early infancy risk factors among native Canadians. Arch Pediatr Adolesc Med. 2002;156(7):651–655 [DOI] [PubMed] [Google Scholar]
- 35.Aerts L, Van Assche FA. Animal evidence for the transgenerational development of diabetes mellitus. Int J Biochem Cell Biol. 2006;38(5-6):894–903 [DOI] [PubMed] [Google Scholar]
- 36.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. 1988;37(5):622–628 [DOI] [PubMed] [Google Scholar]
- 37.White P. Childhood diabetes: its course, and influence on the second and third generations. Diabetes. 1960;9(Sept–Oct):345–348 [DOI] [PubMed] [Google Scholar]
- 38.Pettitt DJ, Lawrence JM, Beyer J, et al. Association between maternal diabetes in utero and age at offspring's diagnosis of type 2 diabetes. Diabetes Care. 2008;31(11):2126–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salbe AD, Fontvieille AM, Pettitt DJ, Ravussin E. Maternal diabetes status does not influence energy expenditure or physical activity in 5-year-old Pima Indian children. Diabetologia. 1998;41(10):1157–1162 [DOI] [PubMed] [Google Scholar]
- 40.Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH. Gestational diabetes and the risk of offspring obesity. Pediatrics. 1998;101(2):e9. [DOI] [PubMed] [Google Scholar]
- 41.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM screening program. Diabetes Care. 2005;28(3):579–584 [DOI] [PubMed] [Google Scholar]
- 42.Pettitt DJ, Jovanovic L. The vicious cycle of diabetes and pregnancy. Curr Diab Rep. 2007;7(4):295–297 [DOI] [PubMed] [Google Scholar]
- 43.Downey W, Stang MR, Beck P, Osei W, Nichol JL. Health services databases in Saskatchewan. : Strom BL, Pharmacoepidemiology. 4th ed Mississauga, Ontario: John Wiley and Sons; 2005:295–310 [Google Scholar]
- 44.Ventana Systems, Inc Vensim DSS 5.6b. MA: Harvard; 2004 [Google Scholar]
- 45.Green J. Sexual equality and Indian government: an analysis of Bill C-31 Amendments to the Indian Act. Native Stud Rev. 1985;2(1):85–93 [Google Scholar]
- 46.Liu J, Young TK, Zinman B, Harris SB, Connelly PW, Hanley AJG. Lifestyle variables, non-traditional cardiovascular risk factors, and the metabolic syndrome in an aboriginal Canadian population. Obesity (Silver Spring). 2006;14(3):500–508 [DOI] [PubMed] [Google Scholar]
- 47.Hanley AJG, Harris SB, Gittelsohn J, Wolever TMS, Saksvig B, Zinman B. Overweight among children and adolescents in a Native Canadian community: prevalence and associated factors. Am J Clin Nutr. 2000;71(3):693–700 [DOI] [PubMed] [Google Scholar]
- 48.Kriska AM, Hanley AJG, Harris SB, Zinman B. Physical activity, physical fitness, and insulin and glucose concentrations in an isolated native Canadian population experiencing rapid lifestyle change. Diabetes Care. 2001;24(10):1787–1792 [DOI] [PubMed] [Google Scholar]
- 49.Young TK, Elias B, Reading J, O'Neil JD, Leader A, McDonald G. Chronic Diseases: Literature Review and Analysis of the First Nations and Inuit Regional Health Survey National Core Data. Winnipeg, Manitoba: Centre for Aboriginal Health Research; 1998 [Google Scholar]
- 50.Gittelsohn J, Wolever TMS, Harris SB, Harris-Giraldo R, Hanley AJG, Zinman B. Specific patterns of food consumption and preparation are associated with diabetes and obesity in a native Canadian community. J Nutr. 1998;128(3):541–547 [DOI] [PubMed] [Google Scholar]
- 51.Field E, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586 [DOI] [PubMed] [Google Scholar]
- 52.Department of Citizenship and Immigration Report of the Department of Citizenship and Immigration. Ottawa, Canada: King's Printer; 1956–1957 [Google Scholar]
- 53.First Nations and Inuit Branch, Health Canada Vital Statistics of the Saskatchewan Registered Indian Population. Ottawa, Canada: Health Canada [Google Scholar]
- 54.Saskatchewan, Department of Public Health Annual Report on Saskatchewan Vital Statistics. Saskatchewan, Canada: Queen's Printer [Google Scholar]
- 55.Sterman J. Business Dynamics: Systems Thinking and Modeling for a Complex World. Boston, MA: Irwin/McGraw-Hill; 2000 [Google Scholar]
- 56.Katzmarzyk PT. The Canadian obesity epidemic: an historical perspective. Obes Res. 2002;10(7):666–674 [DOI] [PubMed] [Google Scholar]
- 57.Chad K, Baxter-Jones A, Muhajarine N, Rodgers C, Humbert L, Ratcliffe-Smith D. Physical Activity/Active Living Research and Strategic Planning Project. Report to the Provincial Government of Saskatchewan. Saskatchewan, Canada; 2010 [Google Scholar]
- 58.Statistics Canada. Canadian Community Health Survey, Cycle 3.1, 2005. 2006-08-31 ed Ottawa, Ontario: Statistics Canada; 2006 [Google Scholar]
- 59.Flegal K. Prevalence and trends in overweight and diabetes. Presented at: The contributions of diet and inactivity to diabesity in America: an agenda for action. Alexandria, VA: March2001 [Google Scholar]
- 60.Chase LA. The trend of diabetes in Saskatchewan, 1905 to 1934. Can Med Assoc J. 1937;36:366–369 [PMC free article] [PubMed] [Google Scholar]
- 61.Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486 [DOI] [PubMed] [Google Scholar]
- 62.Weissgerber TL. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Appl Physiol Nutr Metab. 2006;31(6):661–674 [DOI] [PubMed] [Google Scholar]
- 63.Reece EA. Gestational diabetes: the need for a common ground. Lancet. 2009;373(9677):1789–1797 [DOI] [PubMed] [Google Scholar]
- 64.Major CA, De Veciana M, Henry MJ, Morgan MA. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet Gynecol. 1998;91(4):600–604 [DOI] [PubMed] [Google Scholar]
- 65.Dyck RF, Cassidy H. Position paper: preventing non-insulin-dependent diabetes among aboriginal peoples: is exercise the answer? Chronic Dis Can. 1995;16(4):175–177 [Google Scholar]
- 66.Taylor JS, Kacmar JE, Nothnagle M, Lawrence RA. A systematic review of the literature associating breastfeeding with type 2 diabetes and gestational diabetes. J Am Coll Nutr. 2005;24(5):320–326 [DOI] [PubMed] [Google Scholar]
- 67.Gunderson EP. Breastfeeding after gestational diabetes pregnancy: subsequent obesity and type 2 diabetes in women and their offspring. Diabetes Care. 2007;30(Suppl 2):S161–S168 [DOI] [PubMed] [Google Scholar]
- 68.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord. 2004;28(10):1247–1256 [DOI] [PubMed] [Google Scholar]
- 69.Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity. Diabetes Care. 2006;29(10):2231–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thorpe LE, Berger D, Ellis JA, et al. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. Am J Public Health. 2005;95(9):1536–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–S146 [DOI] [PubMed] [Google Scholar]
- 72.Aberg A. Association between maternal pre-existing or gestational diabetes and health problems in children. Acta Paediatr. 2001;90(7):746–750 [PubMed] [Google Scholar]
- 73.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birthweight, and adolescent obesity. Pediatrics. 2003;111(3):e221–e226 [DOI] [PubMed] [Google Scholar]
- 74.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment: The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21(Suppl 2):B142–B149 [PubMed] [Google Scholar]
- 75.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birthweight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296 [DOI] [PubMed] [Google Scholar]
- 76.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes. 1997;21(6):451–456 [DOI] [PubMed] [Google Scholar]
- 77.Vaarasmaki M, Pouta A, Elliot P, et al. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am J Epidemiol. 2009;169(10):1209–1215 [DOI] [PubMed] [Google Scholar]
- 78.Lim S, Choi SH, Park YJ, et al. Visceral fatness and insulin sensitivity in women with a previous history of gestational diabetes mellitus. Diabetes Care. 2007;30(2):348–353 [DOI] [PubMed] [Google Scholar]