Abstract
Urethral closure mechanisms during abrupt elevation of intravesical pressure (Pves) were investigated. During sneezing, the middle urethral closing response was observed and it still remained after opening the abdomen. The middle urethral response was almost completely abolished after bilateral transection of somatic nerves innervating the external urethral sphincter and the pelvic floor muscles, while bilateral transection of both pelvic nerves and hypogastric nerves had no effects. Somatic nerve transection resulted in fluid leakage from the urethral orifice during sneezing. Passive increments of Pves for 120 seconds by elevating a saline reservoir connected to the bladder also induced the middle urethral closing response in rats with spinal cord transection at T8–T9. The response was totally abolished by cutting pelvic nerves bilaterally, and partially reduced after bilateral transection of pudendal nerves, nerves to pelvic floor muscles or hypogastric nerves. Electrical stimulation of abdominal muscles (ESAM) for 1 second elevated Pves in a stimulus-dependent manner in the spinal cord-transected rats, and the Pves rise was almost lost when the abdomen was opened. The Pves inducing fluid leakage from the urethral orifice was lowered in rats when pelvic nerves or somatic nerves were cut bilaterally, while transection of bilateral hypogastric nerves showed smaller effects. These results indicate that at least two kinds of urinary continence reflexes close the middle urethra during abrupt elevation of Pves; one reflex observed during sneeze is preprogrammed so as to close the urethra automatically irrespective of bladder afferent activity, and the other reflex is triggered by bladder afferent excitation. During momentary stress events such as sneezing (<0.15 seconds) and ESAM (1 second), the striated muscles mainly contribute to the urethral closure, while during events for a relatively long period like passive Pves elevation for 120 seconds, both striated and smooth muscles are involved in the prevention of stress urinary incontinence.
Introduction
Stress urinary incontinence (SUI), defined as the involuntary loss of urine during elevation of abdominal pressure in the absence of bladder contractions, generally occurs as a result of defects in the various passive and reflex mechanisms that maintain urethral closure in the presence of elevated abdominal pressure. However, in animal studies, urethral closing mechanisms under stress conditions that passively elevates abdominal pressure have not been investigated in detail except for a few pioneering studies examining urethral functions during sneezing in dogs1–3. Therefore, the SUI research in Pittsburgh was performed to clarify the urethral functions during the events that elevate intravesical pressure (Pves) in order to fully elucidate mechanisms preventing SUI. We have demonstrated that at least two kinds of nerve-mediated urethral closing reflexes contribute to the prevention of SUI in rats, and the findings in Pittsburgh have provided great insights to elucidate pathophysiology of SUI. In this review, roles of urinary continence reflexes and contribution of striated and smooth muscles to reflex urethral closure mechanisms are discussed based on our observations.
Sneeze reflex
The guarding reflex to maintain urinary continence has been well documented, however basic animals research on functions of reflex urethral closure during abrupt elevation of abdominal pressure during stress conditions had hardly been performed especially in small animals like rats. Therefore, we initiated measurements of urethral closing responses during stress conditions such as sneezing using micro-tip transducers to clarify mechanisms preventing SUI4–6. When sneeze was induced in rats by stimulation of nostril inner surface with their whisker, urethral closing responses were observed in the proximal and middle urethra5. The time course of proximal urethral responses was quite the same as that of Pves, and these changes were almost abolished after opening the abdomen, suggesting that elevated abdominal pressure was passively and similarly transmitted to the bladder and the proximal urethra. In contrast, middle urethral responses, which showed the different time course from Pves, were clearly observed even after opening the abdomen, implying participation of active urethral closing mechanisms. This middle urethral closure was almost abolished after bilateral transection of somatic nerves such as pudendal nerves and nerves to pelvic floor muscles (iliococcygeus/pubococcygeus muscles), whereas bilateral transection of both pelvic nerves and hypogastric nerves had no effects5, indicating that striated muscles (external urethral sphincter and pelvic floor muscles) participate in the reflex urethral closing mechanism (Fig. 1). Furthermore, somatic nerve transection induced fluid leakage from the urethral orifice during sneezing5,6, confirming that the reflex urethral closure prevents urinary incontinence during sneezing. In addition, the recent findings that overdistention of the rat vagina caused the impairment of the nerve-mediated active middle urethral closure mechanism and induced the urinary leakage during sneezing also support the idea4.
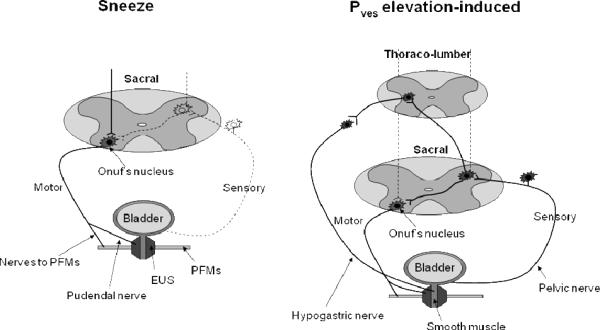
Fig. 1.
Hypothetical schemas of two urethral continence reflexes preventing stress urinary incontinence. One is observed during sneezing (left) and the other is detected when the intravesical pressure (Pves) is elevated passively (right) in rats. Solid and dashed lines show active and ineffective pathways, respectively. EUS: external urethral sphincter, PFMs: pelvic floor muscles (iliococcygeus/pubococcygeus muscles).
Bladder afferent-dependent reflex
As sneeze is merely one of events causing a Pves rise, urethral responses under other stress conditions were also studied after suppression of reflex bladder contractions by spinal cord transection at T8–T9 in rats. Abruptly raising Pves for 120 seconds by elevating the saline reservoir connected to the bladder induced urethral closing responses in a Pves-dependent manner in a restricted portion of the middle urethra7. The passive Pves elevation–induced urethral responses were totally abolished by cutting pelvic nerves bilaterally7, indicating that the reflex is triggered by bladder afferent activity. The middle urethral responses partially reduced after bilateral transection of pudendal nerves, nerves to pelvic floor muscles, or hypogastric nerves7, suggesting involvement of both striated and smooth muscles in the urethral closing mechanisms (Fig. 1).
To clarify the contribution of the reflex to total urethral resistance, leak point pressure (LPP), which is defined as Pves inducing fluid leakage from the urethral orifice, was also measured using electrical stimulation of abdominal muscles (ESAM). ESAM for 1 second elevated Pves in a stimulus-dependent manner, and the Pves rise was almost lost when the abdomen was opened8. The LPP was lowered in rats whose pelvic nerves or somatic nerves were cut bilaterally, while transection of bilateral hypogastric nerves showed smaller effects8. Based on these findings it is concluded that the bladder afferent-induced reflex urethral closing mechanisms prevent the urinary leakage during a stress condition momentarily induced by ESAM.
Roles of two reflexes
During sneezing, passive Pves elevation or ESAM, the nerve-mediated urethral closure is active to raise the urethral resistance as demonstrated by nerve transection techniques5–8 (Fig. 1). In addition, duloxetine, which is effective for the treatment of SUI9,10, increases the urethral closing reflexes during sneezing11 and passive Pves elevation8, and it also raises the urethral resistance (LPP) during sneezing11 and ESAM8, supporting the idea that the nerve-mediated mechanisms prevent urine leakage.
Intriguingly, the sneeze-induced urethral reflex is clearly different from other reflexes in terms of the participation of bladder afferent information (Fig. 1, Table 1). Pelvic nerve transection abolishes the passive Pves elevation-induced reflex7 and reduces the urethral resistance during ESAM8, while pelvic and hypogastric nerve transection does not affect the reflex urethral contractions during sneezing5. The intense middle urethral closure is detected during sneezing even when only a little increment of Pves is observed after opening the abdomen5. In addition, the middle urethra starts to close before Pves elevation by sneezing in some rats5. These findings suggest that the sneeze-induced urethral reflex would be pre-programmed so as to close the urethra automatically when sneeze occurs. It might be reasonable to consider that as sneeze is a very brief and sudden event within 0.15 second in rats5, the passive Pves elevation-induced urethral reflex might be too late to prevent the leakage of urine through the urethra, therefore, the pre-programmed reflex might be required. As the urethra also starts to close before Pves elevation during coughing in humans12–14, the cough-induced reflex might also be pre-programmed.
Table 1.
Characteristics of urinary continence reflexes observed under various conditions in rats
| Events | Sneeze | ESAM | Passive Pves elevation |
|---|---|---|---|
| Pre-programmed | Bladder afferent-dependent | Bladder afferent-dependent | |
| Pves elevation time | <0.15s | <1 s | 120 s |
|
| |||
| Contribution | |||
| Smooth muscle | − | ± | + |
| Striated muscle | ++ | ++ | + |
ESAM: electrical stimulation of abdominal muscles
Pves: Intravesical pressure
Striated versus smooth muscles
It is controversial which muscle component is mainly related to the urethral closure, striated or smooth muscles15, because the number of reports clarifying their functions under stress conditions is limited. During both sneezing and ESAM, the striated muscles rather than urethral smooth muscles are mainly involved in the prevention of urine leakage as shown by nerve transection techniques5,8 (Table 1). However, during passive Pves elevation the urethral smooth muscle is also involved in reflex urethral closing responses7 (Table 1). One possible explanation of these differences regarding the smooth muscle contribution might be following: as the smooth muscle is not suitable to respond quickly, it does not function practically during momentary-induced stress events such as sneezing (<0.15 seconds)5 and ESAM (1 second)8, but it does in events for a relatively long period like passive Pves elevation (120 seconds)7. In addition, an ex vivo study for investigating biomechanical properties of the isolated rat urethra16 shows that the smooth muscle rather than the striated muscle greatly contributes to resistance of intraluminal pressure15. Basically the passive Pves elevation-induced urethral reflex might function to keep the urethral resistance sustained at a high level when the increasing urine volume distends the bladder wall in the long urinary storage period, and the smooth muscle seems suitable for this purpose. Then the prevention of urine leakage during abrupt elevation of abdominal pressure might require the additional reflex, and the striated muscle is adopted for this second purpose.
We believe that the understandings of the multiple urethral reflexes could provide the foundation for the development of novel pharmacotherapies for SUI. Based on the findings discussed in this review, argumentation of the striated muscle function to raise the urethral closure during momentary stress events as well as enhancement of the smooth muscle function to increase the urethral baseline tone would be promising approaches for the treatment of SUI8,11,17.
References
- 1.Heidler H, Casper F, Thuroff JW. Role of striated sphincter muscle in urethral closure under stress conditions: an experimental study. Urol Int. 1987;42:195–200. doi: 10.1159/000281894. [DOI] [PubMed] [Google Scholar]
- 2.Thuroff JW, Bazeed MA, Schmidt RA, Tanagho EA. Mechanisms of urinary continence: an animal model to study urethral responses to stress conditions. J Urol. 1982;127:1202–6. doi: 10.1016/s0022-5347(17)54297-4. [DOI] [PubMed] [Google Scholar]
- 3.Thuroff JW, Casper F, Heidler H. Pelvic floor stress response: reflex contraction with pressure transmission to the urethra. Urol Int. 1987;42:185–9. doi: 10.1159/000281892. [DOI] [PubMed] [Google Scholar]
- 4.Kamo I, Kaiho Y, Canon TW, Chancellor MB, de Groat WC, Prantil RL, Vorp DA, Yoshimura N. Functional analysis of active urethral closure mechanisms under sneeze induced stress condition in a rat model of birth trauma. J Urol. 2006;176:2711–5. doi: 10.1016/j.juro.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 5.Kamo I, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol. 2003;285:R356–65. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- 6.Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:359–63. doi: 10.1007/s00192-004-1263-4. [DOI] [PubMed] [Google Scholar]
- 7.Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol. 2004;287:F434–41. doi: 10.1152/ajprenal.00038.2004. [DOI] [PubMed] [Google Scholar]
- 8.Kamo I, Hashimoto T. Involvement of reflex urethral closure mechanisms in urethral resistance under momentary stress condition induced by electrical stimulation of rat abdomen. Am J Physiol Renal Physiol. 2007;293:F920–6. doi: 10.1152/ajprenal.00466.2006. [DOI] [PubMed] [Google Scholar]
- 9.Cannon TW, Yoshimura N, Chancellor MB. Innovations in pharmacotherapy for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:367–72. doi: 10.1007/s00192-003-1093-9. [DOI] [PubMed] [Google Scholar]
- 10.Thor KB. Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: implications for treating stress urinary incontinence. Urology. 2003;62:3–9. doi: 10.1016/s0090-4295(03)00754-4. [DOI] [PubMed] [Google Scholar]
- 11.Miyazato M, Kaiho Y, Kamo I, Chancellor MB, Sugaya K, de Groat WC, Yoshimura N. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2008;295:F264–71. doi: 10.1152/ajprenal.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thind P, Lose G, Colstrup H. Initial urethral pressure increase during stress episodes in genuine stress incontinent women. Br J Urol. 1992;69:137–40. doi: 10.1111/j.1464-410x.1992.tb15483.x. [DOI] [PubMed] [Google Scholar]
- 13.Thind P, Lose G, Jorgensen L, Colstrup H. Variations in urethral and bladder pressure during stress episodes in healthy women. Br J Urol. 1990;66:389–92. doi: 10.1111/j.1464-410x.1990.tb14960.x. [DOI] [PubMed] [Google Scholar]
- 14.van der Kooi JB, van Wanroy PJ, De Jonge MC, Kornelis JA. Time separation between cough pulses in bladder, rectum and urethra in women. J Urol. 1984;132:1275–8. doi: 10.1016/s0022-5347(17)50122-6. [DOI] [PubMed] [Google Scholar]
- 15.Jankowski RJ, Prantil RL, Chancellor MB, de Groat WC, Huard J, Vorp DA. Biomechanical characterization of the urethral musculature. Am J Physiol Renal Physiol. 2006;290:F1127–34. doi: 10.1152/ajprenal.00330.2005. [DOI] [PubMed] [Google Scholar]
- 16.Jankowski RJ, Prantil RL, Fraser MO, Chancellor MB, De Groat WC, Huard J, Vorp DA. Development of an experimental system for the study of urethral biomechanical function. Am J Physiol Renal Physiol. 2004;286:F225–32. doi: 10.1152/ajprenal.00126.2003. [DOI] [PubMed] [Google Scholar]
- 17.Kaiho Y, Kamo I, Chancellor MB, Arai Y, de Groat WC, Yoshimura N. Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2007;292:F639–46. doi: 10.1152/ajprenal.00226.2006. [DOI] [PubMed] [Google Scholar]