SUMMARY
Ischemic pain – examples include the chest pain of a heart attack and the leg pain of a 30 second sprint – occurs when muscle gets too little oxygen for its metabolic need. Lactic acid cannot act alone to trigger ischemic pain because the pH change is so small. Here we show that another compound released from ischemic muscle, ATP (adenosine tri-phosphate), works together with acid by increasing the pH sensitivity of ASIC3 (acid sensing ion channel #3), the molecule used by sensory neurons to detect lactic acidosis. Our data argue that ATP acts by binding to P2X receptors that form a molecular complex with ASICs; the receptor on sensory neurons appears to be P2X5, an electrically quiet ion channel. Coincident detection of acid and ATP should confer sensory selectivity for ischemia over other conditions of acidosis.
Keywords: ASIC, P2X, ischemic pain, cardiovascular reflexes, pressor reflex, angina, intermittent claudication, sickle cell anemia, muscle pain, metaboreception, ergoreception
INTRODUCTION
Musculoskeletal pain is among the most common reasons that patients seek medical care, yet its molecular basis is poorly understood compared to skin pain (Mense, 2008). Muscle pain caused by ischemia – when skeletal or cardiac muscle get insufficient oxygen for their metabolic needs – includes the physiological pain of anaerobic exercise and the pathological pains of angina, intermittent claudication, and sickle cell anemia (Fu and Longhurst, 2009). Ischemia is detected by sensory neurons called metaboreceptors, which innervate muscle and evoke both protective cardiovascular reflexes and muscle pain (Craig, 2002; Kaufman and Hayes, 2002).
In classic experiments using the submaximal effort tourniquet technique, no pain occurred when a tourniquet stopped blood flow to a subject’s arm, but striking and persistent pain occurred when the occluded arm then exercised lightly (MacWilliam and Webster, 1923; Smith et al., 1966). Similarly, reflex control of local blood pressure in response to ischemia required muscle contraction (Alam and Smirk, 1937). These experiments show that metaboreceptors do not directly sense low oxygen, but instead detect chemicals released into (or depleted from) the extracellular media by working, oxygen-starved muscle. Thomas Lewis called this contraction-induced chemical “Factor P” and argued that it triggers ischemic pain in both skeletal and cardiac muscle (Lewis, 1932). Factor P clearly is not a single chemical as there is evidence for contributions from a wide variety (Longhurst et al., 2001; Meller and Gebhart, 1992; Mense, 1993).
Lactic acid, produced in and released from muscle during anaerobic metabolism, is a possible mediator of ischemic pain but the signal it generates is small: extracellular pH drops from 7.4 to 7.0 during the tourniquet test (Issberner et al., 1996) and to pH 6.9 during minutes of extreme cardiac ischemia (Cobbe and Poole-Wilson, 1980; Street et al., 2001). This is far smaller than pH changes that trigger pain in skin (Steen et al., 1995). Moreover, pH 7.0 can be reached during metabolic acidosis (Rose and Post, 2001), which does not evoke sensations of angina or other ischemic pain. Finally, patients with McArdle’s disease, who have diminished ability to make lactic acid, generate a substantial neural response to ischemic exercise (Vissing et al., 2001). Despite these arguments against a role for acid in metaboreception and ischemic pain, two experiments compellingly argue for it: 1) the onset and intensity of pain in the tourniquet test tracks the drop in pH (Issberner et al., 1996); 2) strong buffering of extracellular pH greatly diminishes the ability of metaboreceptors to detect ischemia in vivo (Pan et al., 1999).
If indeed acid helps to trigger ischemic pain, its detector must be able to respond to pH 7.0 and it also must be able to distinguish ischemic acidosis from metabolic acidosis. We have previously argued that the sensor for ischemic acidosis in rats is ASIC3 – acid-sensing ion channel #3 (Benson and McCleskey, 2007). ASICs are trimeric proteins that open a Na+-selective pore when there is a drop in extracellular pH, thereby activating neurons in response to local acidification (Deval et al., 2008; Holzer, 2009; Jasti et al., 2007; Lingueglia, 2007; Wemmie et al., 2006). Cardiac and skeletal muscle metaboreceptors in rats express ASIC3 at extraordinarily high levels on their cell bodies (Benson et al., 1999; Sutherland et al., 2001) and on their sensory endings (Molliver et al., 2005). ASIC3 is much more sensitive than other rat ASICs: at physiological ionic conditions, it is closed at pH 7.4, starts to open by pH 7.1, and is fully activated near pH 6.5. Over this range of pH, which closely flanks the range that occurs during muscle ischemia, the ASIC3 activation curve has a Hill slope of 4, essentially four times as sensitive as a pH meter (Sutherland et al., 2001; Waldmann et al., 1997; Yagi et al., 2006). ASIC3 sensitivity is increased by lactate (Immke and McCleskey, 2001), making the channel better at detecting lactic acid than other acids. Thus, metaboreceptors express at very high levels an ion channel that is well-tuned to detect subtle lactic acidosis. Mice lacking ASIC3 (but not ASIC1) fail to exhibit a pathological pain condition caused by injections of acid into muscle (Sluka et al., 2003; Sluka et al., 2007); thus, ASIC3 responds to muscle acidity in vivo and affects nociception.
How might metaboreceptors distinguish ischemic acidosis from metabolic acidosis? We considered whether other compounds that appear during ischemia might work synergistically with acid upon ASIC3. Screening a variety of compounds for their ability to potentiate ASIC gating in sensory neurons, we found one, extracellular ATP at micromolar concentrations, that greatly increased ASIC currents evoked at the pH values that occur during muscle ischemia. Extracellular ATP is of particular interest in ischemia because it rapidly rises from undetectable levels to tens of micromolar when an occluded forearm exercises, but not when it rests (Forrester, 1972; Hellsten et al., 1998); extracellular ATP also rises in ischemic heart (Borst and Schrader, 1991). Synergistic action of acid and ATP has been seen in measurements of calcium transients in isolated sensory neurons (Light et al., 2008), in whole animal cardiovascular reflexes to muscle ischemia (Hayes et al., 2008; McCord et al., 2009), and in a whole animal ischemic pain model (Seo et al., 2010). Our study provides a molecular mechanism for these physiological observations. We demonstrate that muscle metaboreceptors use ASICs to integrate acid and ATP signals and that the ATP binding site is an ion channel in the P2X family; the data further suggest that the phenomenon involves a protein assembly of P2X and ASIC channels.
RESULTS
Extracellular ATP persistently increases ASIC sensitivity on putative metaboreceptive sensory neurons
Whole cell patch clamp recordings were performed on cell bodies of sensory neurons that had innervated rat thigh muscle. Neurons were fluorescently labeled by a retrogradely transported dye (DiI) injected into the muscle about 1 week before dissection of dorsal root sensory ganglia (Fig. 1A inset). Roughly 60% of these neurons, and virtually all of those with larger diameters, exhibited large inward Na+ currents when exposed to pH 6.9 (Fig. 1A,B); such high acid sensitivity is typical only of ASIC3. Sensory neurons that project to rat muscle and express high levels of ASIC3 have the following properties: 1) they can innervate muscle arterioles; 2) most express CGRP (a vasodilatory peptide) and TrkA (the receptor for nerve growth factor); 3) relatively few express TRPV1 and virtually none express P2X3, two other nociceptive ion channels (Molliver et al., 2005). Such high expression of ASIC3 is seen in virtually all sensory neurons that innervate rat heart, an organ from which ischemic pain is the only conscious sensation (Benson et al. 1999; Sutherland et al. 2001). These expression patterns are among the evidence that ASIC-positive muscle afferents are metaboreceptors.
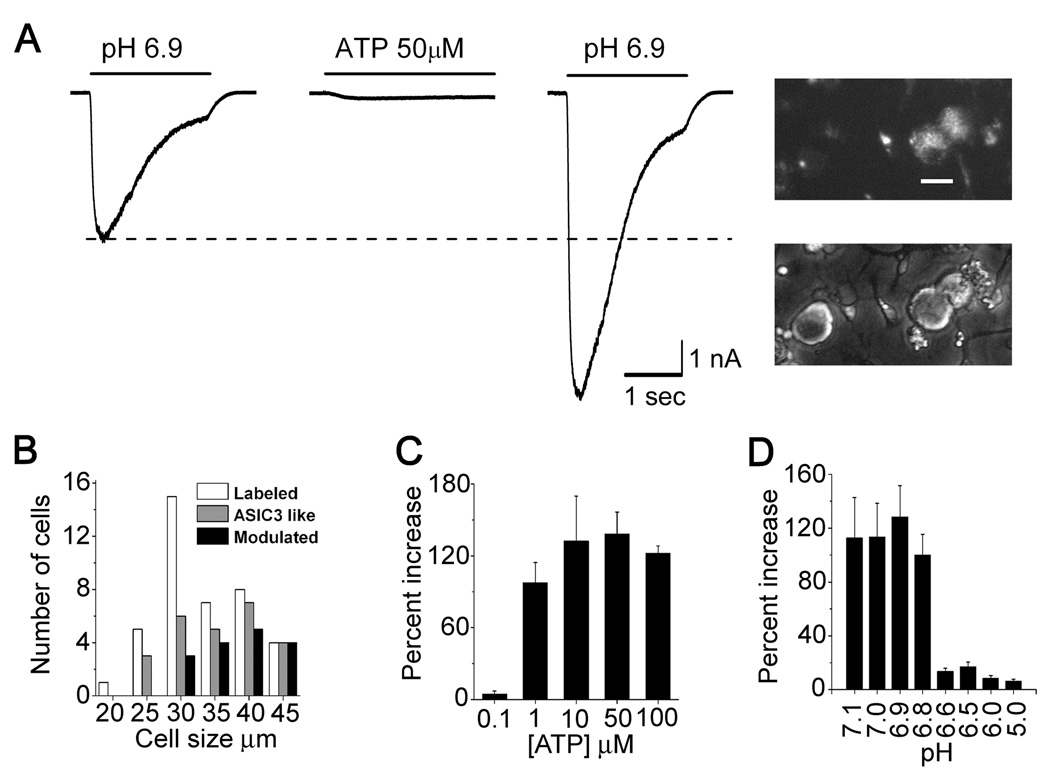
Figure 1. Extracellular ATP increased acid sensitivity of ASIC channels in a subset of rat sensory neurons.
(A) Whole cell patch clamp currents evoked by changing external pH from 7.4 to 6.9 on a DiI-labeled sensory neuron that innervated skeletal muscle. The smaller pH-evoked current (left) was before and the larger (right) was 15 seconds following washout of extracellular 50 µM ATP that had been applied for 25 seconds; the first 3 seconds of ATP application is shown between the two pH-evoked currents. Scale: 1 nA, 1 sec. Inset: fluorescence (upper) and phase micrographs showing dissociated sensory neurons, two of which are fluorescent because of retrograde transport of DiI injected into thigh muscles. Scale bar: 30 µm.
(B) Distribution of muscle afferents by cell diameter (white bars). Note that larger cells were more likely to express ASIC3-like currents (grey) and that currents in 35% of ASIC3+ cells were not sensitized by ATP (black).
(C) Mean (+/− s.e.m.) percent increase (100% = 2-fold increase) in ASIC3-like current (pH 6.9) at different ATP concentrations, each applied for 1 minute (n ≥ 5 for each concentration).
(D) Mean percent increase in ASIC3-like current evoked at the indicated pH. 50 µM ATP was applied for 1 minute at pH 7.4. ASICs were evoked by brief (2 sec) steps to the indicated pH before and 20 seconds after removal of ATP. Currents at the peak of the ASIC activation curve (ca. pH 6) were almost unchanged, indicating that ATP does not increase the total number of available ASIC molecules. (n ≥ 5 for each pH).
After evoking a large inward Na+ current with a step in pH from 7.4 to 6.9 (Fig. 1A, left trace), we returned to pH 7.4 and applied 50 µM ATP for 25 seconds, which evoked a modest inward current in this neuron (middle trace). Upon removal of ATP, we retested ASIC amplitude and found it much increased (right trace). Typically, it took about a minute in ATP for ASIC current to reach maximum amplitude (Supplementary Fig. S1). Whether or not ATP remained in the external media, ASIC currents remained elevated for about 10 minutes during which time they gradually returned toward baseline level (Supplementary Fig. S1, S3). Subsequent ATP applications had little effect on ASIC current, indicating that the decline to baseline is an adaptation. We will call this long-lived effect of extracellular ATP on ASIC channels “sensitization”. Using the same protocol, other compounds that appear in the extracellular space during muscle ischemia—bradykinin, serotonin, adenosine, histamine—had no such effect on acid-evoked current (not shown).
The mean increase in ASIC current was 2.3 fold (± 0.15 s.e.m.). A majority (64%) of putative muscle metaboreceptors were sensitized by ATP; larger neurons (>35 µm soma diameter) were more likely to respond (Fig. 1B), suggesting that ATP acts on neurons that have myelinated axons. No effect was seen with 0.1 µM ATP, whereas 1 µM sensitized ASICs to essentially the same degree as higher concentrations (Fig. 1C); sensitization appeared slower with the lower concentrations of ATP but eventually reached the same final level (Supplementary Fig. S1).
Although ATP increased ASIC currents evoked at the modest pH levels (7.1 to 6.8) that occur during heart attack and anaerobic muscle exercise(Cobbe and Poole-Wilson, 1980; Street et al., 2001) it did not strongly alter currents evoked at pH 6.5 and below (Fig. 1D), values that are at the peak of the ASIC3 activation curve (Sutherland et al., 2001). This argues that ATP does not alter the total number of ASIC channels but, instead, increases their effective acid sensitivity.
The binding site is an ATP-gated ion channel
The ASIC channel cannot itself be the ATP binding site because about a third of neurons with strong ASIC currents were not sensitized by ATP (Fig. 1B). We therefore considered the cell membrane purine receptors, of which there are three families: P1, P2X, and P2Y (Burnstock, 2007). Unlike the ATP-gated P2 receptors, the primary ligand for P1 receptors is adenosine, which did not affect ASIC current (not shown). The slow time course of ASIC sensitization is similar to channel modulation by G-protein coupled receptors (Hille, 2001), so the G-protein coupled P2Y receptors seemed likely. This was disproven because ATP was not mimicked by either ADP or UTP (Fig. 2), agonists that activate P2Y receptors as well as or better than ATP (Burnstock, 2007).
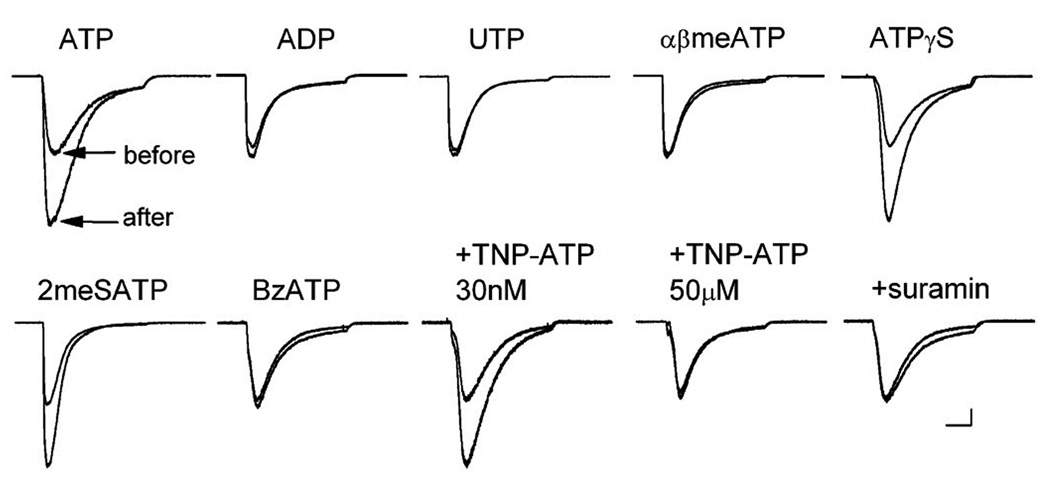
Figure 2. Pharmacological profile rules out P2Y and dominant P2X receptors.
Each pair of acid-evoked currents (pH step from 7.4 to 6.9, 4 sec) is from a single representative sensory neuron (n ≥ 5 cells tested for each condition). Currents were evoked either before or 30 sec after a 1 minute application of the indicated compound. Purinergic agonists were applied at 50 µM for 1 minute except BzATP (100 µM). Antagonists (TNP-ATP, concentration indicated; suramin, 100 µM; PPADS, 4 µM) were applied together with ATP. If ASIC current did not increase in presence of an antagonist, ATP was reapplied without antagonist and then current increased (not shown). Scale bars: 1 sec, 0.5 nA. Insensitivity to either ADP or UTP rules out P2Y receptors; insensitivity to αβmethylene-ATP rules out P2X3, P2X2/3, and P2X1. Inhibition by suramin and PPADS (not shown) is inconsistent with P2X4 and P2X7.
Sensory neurons express mRNA for six of the seven P2X receptors (all but P2X7) (Collo et al., 1996; Kobayashi et al., 2005). Despite this heterogeneity of mRNA, the bulk of ATP-gated current in sensory neurons is carried by channels that contain P2X3 receptors, either homomeric P2X3 or heteromers of P2X2 and P2X3 (Cook et al., 1997; Lewis et al., 1995). These channels are activated by αβmethylene-ATP, which failed to affect ASIC current. Thus, the channel responsible for most ATP-gated current on sensory neurons does not sensitize ASICs.
We tested several other P2X agonists, finding that two general P2X activators, ATPγS and 2methylthio-ATP, mimicked ATP but benzoyl-ATP, a specific P2X7 agonist, did not. Three antagonists were tested. PPADS (not shown) and suramin, both of which block all homomeric P2X channels except P2X4, blocked ASIC sensitization by ATP. TNP-ATP (trinitrophenol-ATP) blocked at high, non-specific concentrations but not at a low concentration (30 nM) that is specific for P2X3 and P2X1 receptors. This pharmacological profile matches P2X2 and P2X5, but not other functional homomeric P2X channels (North and Surprenant, 2000).
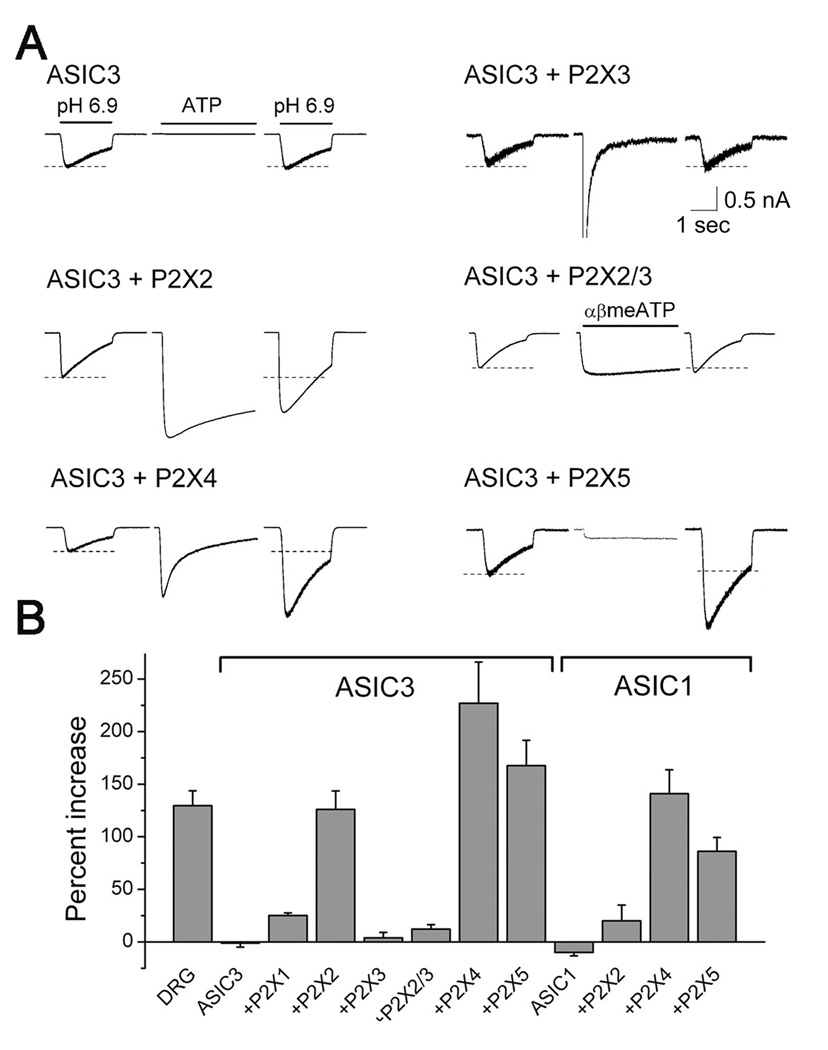
To test if sensitization indeed is mediated by P2X receptors, we expressed them together with ASICs in three mammalian cell lines (CHO, COS, HEK293). Expressed alone, ASIC3 was unaffected by application of ATP (Fig. 3A). As expected from the previous pharmacology of native neurons, expression of P2X1 (not shown) or P2X3 resulted in large ATP-evoked currents but no subsequent change in ASIC3 current. In contrast, expression of P2X2, P2X4, or P2X5 all supported robust potentiation of ASIC3 current by ATP. Activation of P2X2/3 heteromers did not increase ASIC3 current, indicating that P2X3 serves as a dominant negative over P2X2. P2X receptors could also mediate sensitization of ASIC1 channels (Fig. 3B). Evidently, ATP sensitizes ASICs by binding to certain P2X ion channels.
Figure 3. Reconstitution finds three P2X receptors that can mediate ASIC sensitization.
(A) Each trio of traces is from a single COS or CHO cell transfected with the indicated cDNAs. The ASIC3 currents, evoked by 2 second steps to pH 6.9 before ATP application and after removal, flank a trace showing the first 3 seconds of a 30 second ATP application. αβ-methylene-ATP was used on the P2X2/P2X3 co-transfection to assure only activation of the heteromer and not any P2X2 homomers. Scale bars: 1 sec; 0.5 nA. See supplemental Figure S3 for time course of sensitization in CHO cells. (B) Mean percent ASIC current increase (n = 7–83 cells, each from dishes not previously exposed to ATP) in sensory neurons (DRG) or cell lines transfected with ASIC3 or ASIC1 together with the indicated P2X receptor(s). P2X2, P2X4, and P2X5 can mediate sensitization of ASIC3; ASIC1 also responds to ATP.
The time course of ASIC sensitization in the cell lines varied with the cell type. After a brief ATP application, ASIC currents remained elevated above baseline in COS and CHO cells for as long as we recorded (~ 1 hour. Supplementary Fig. S3). HEK293 cells were more like sensory neurons, with ASIC currents returning to their original levels over about 10 minutes after ATP application (not shown).
Mechanism of P2X-ASIC sensitization: evidence for proximity
The slow onset and persistence of sensitization would occur if activation of the P2X receptor created or depleted some second messenger that alters ASIC activity, but we failed to find evidence to support this. First, flux through the P2X receptor does not appear to create a signal because sensitization occurred equally well if P2X flux was inward or outward (Supplementary Fig. S6A). Second, changes in intracellular Ca2+ are irrelevant because sensitization is neither triggered by intracellular Ca2+ release nor inhibited by complete Ca2+ chelation (intracellular 10 mM BAPTA) or depletion of Ca2+ stores (Supplementary Fig. S6B). Third, phosphorylation by ATP is irrelevant because a non-hydrolyzable ATP analog (AMP-PNP) could substitute for ATP in either the intracellular (5 mM in patch pipet, no added Mg2+) or extracellular (50 µM, no Mg2+) media (Supplementary Figs. S6C,F). Further negative results were obtained upon modulation of G proteins, phosphatases, and a number of specific signaling pathways summarized in Supplementary Fig. S6 and Tables S6.
Failing to find evidence for a second messenger created when ATP activates P2X receptors, we asked whether ASIC and P2X receptors might be directly coupled. Attempts to co-immunoprecipitate ASIC and P2X receptors with two different protocols led to apparent artifact: ASIC3 expressed in COS7 cells immunoprecipitated with co-expressed P2X2 and P2X5 channels, but also with SK channels (small conductance Ca2+-activated potassium channels) and with P2X3, which does not mediate ASIC sensitization. This suggests that ASIC3 formed artifactual aggregates with other membrane proteins when solubilized under the conditions we used. We next used Főrster resonance energy transfer (FRET) to test whether the two proteins are within molecular proximity in intact, live cell membranes(Ciruela, 2008). FRET measurements relate the fluorescence energy of CFP that gets transferred to YFP because of their overlapping emission and absorption spectra and close proximity. Constructs were prepared that fused P2X5 and ASIC3 proteins to cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP), respectively, and these were transfected into CHO cells. P2X5-CFP and ASIC3-YFP generated normal currents and normal ATP-sensitization of ASIC3 (Supplementary Fig. S4C). We quantified FRET using “donor-dequenching”, an increase in CFP (donor) fluorescence that occurs when YFP (acceptor) is destroyed using high intensity light.
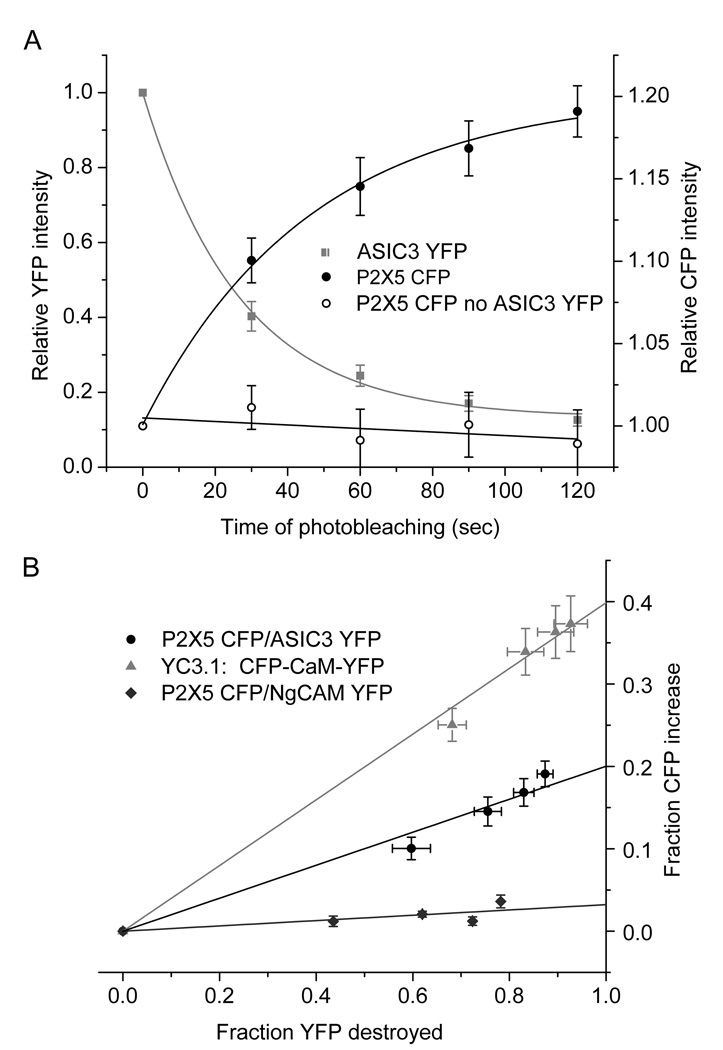
Fluorescence of ASIC3-YFP was almost fully eliminated by exposing transfected CHO cells to four 30 second intervals of bright 514 nm light. Fluorescence of CFP and YFP was measured after each of these bleaching intervals (Fig. 4A) and P2X5-CFP fluorescence (solid circles) increased in parallel to the loss of ASIC3-YFP fluorescence (squares), thereby confirming FRET between the two fluorophores. No FRET occurred in cells transfected with YFP that was not fused to ASIC3 (open circles) or if YFP was fused to a different membrane-spanning protein, NgCAM (triangles, Fig. 5B). The 20% FRET signal between P2X5 and ASIC3 was about half the signal obtained from yellow CaMeleon (YC3.1, red circles), in which YFP and CFP are fused to either end of calmodulin, which is about 45 angstroms in length. Application of ATP did not clearly alter the FRET signal between P2X5 and ASIC3, although there appeared to be a slight decrease (Supplementary Fig. S4D). We also detected FRET between P2X5 and ASIC3 using optics for total internal reflection (TIRF), which selectively detects surface membrane fluorescence and thereby confirms that P2X5 and ASIC3 co-localize on the surface membrane (Supplementary Fig. S4). There appear to be hotspots of colocalized P2X5 and ASIC3 fluorescence in the TIRF images. In summary, we conclude that P2X5 and ASIC3 molecules can be no further apart than 100 angstroms, the upper limit for FRET(Ciruela, 2008). For perspective, the width of the barrel structure in the fluorescent proteins is 40 angstroms(Ormo et al., 1996) and the greatest distance between adjacent subunits in ASIC and P2X channels are, respectively, 85 and 75 angstroms (Jasti et al., 2007; Kawate et al., 2009).
Figure 4. FRET between P2X5-CFP and ASIC3-YFP argues for co-localization.
(A) Photodestruction of ASIC3-YFP resulted in an increase of P2X5-CFP fluorescence. Relative CFP (closed black circles, right axis) and YFP (grey squares, left axis) intensities measured on an epifluorescence microscope were plotted as a function of time of photobleaching of YFP in CHO cells co-expressing P2X5-CFP and ASIC3-YFP. When only P2X5-CFP was expressed (open circles), there was no increase in CFP fluorescence. CFP excitation and emission wavelengths centered at 436 and 485 nm; YFP at 514 and 535 nm; photobleaching at 514 nm.
(B) The increase in CFP fluorescence was proportional to the fraction of YFP that was photodestroyed. P2X5-CFP appeared closely associated with ASIC3-YFP with a FRET efficiency of approximately 20% (black circles). FRET did not occur between P2X2-CFP and NgCAM-YFP (dark grey diamonds). Yellow CaMeleon 3.1 (YC3.1) served as a positive FRET control, exhibiting a 40% FRET signal (light grey triangles). See supplemental figure S4.
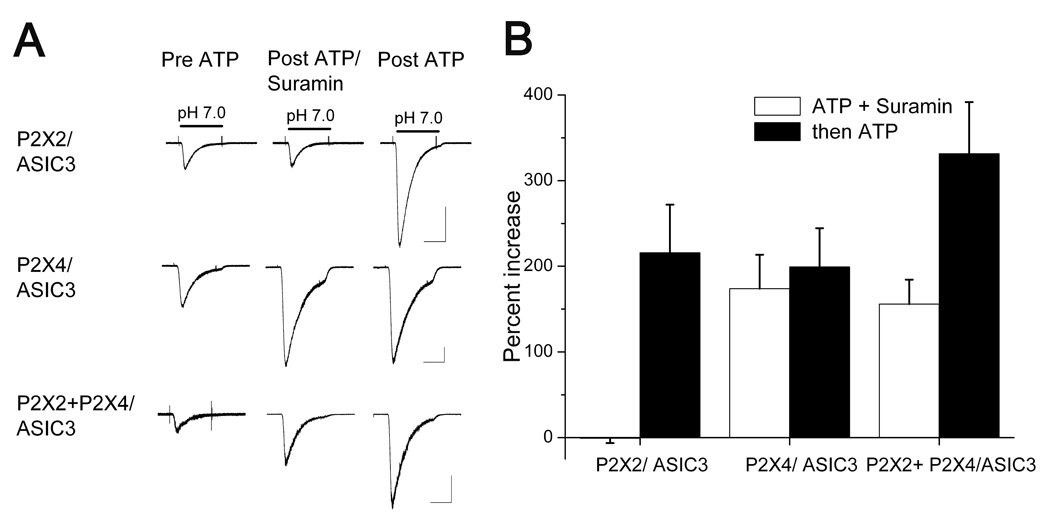
Figure 5. Different P2X receptors independently sensitize ASIC3.
(A) CHO cells transfected with the indicated combination of channels. Each trio of traces shows ASIC3 currents evoked prior to ATP, after 20 seconds in 5 µM ATP plus 150 µM suramin, and after a subsequent 20 second application of 5 µM ATP alone. Suramin blocked ASIC sensitization by P2X2 (upper traces) but not by P2X4 (middle traces). After 20 seconds in ATP plus suramin, subsequent ATP application had no further effect on P2X4 cells. Cells transfected with both P2X2 and P2X4 exhibited sensitization in suramin, like P2X4, and also after suramin, like P2X2 (lower traces).
(B) Summary data (n ≥ 5 in each condition) shows that CHO cells that co-express P2X2 and P2X4 exhibited ASIC sensitization in response to the first application (ATP plus suramin) roughly equivalently to P2X4-only cells, and to the second application (ATP only) roughly equivalently to P2X2-only cells. This is consistent with independent actions of P2X2 and P2X4.
Mechanism of P2X-ASIC sensitization: proximity appears necessary
P2X5 and ASIC3 appear to be close to each other on the membrane, but is this proximity necessary for sensitization? If so, individual P2X receptors should operate independently of each other, affecting only the ASICs to which they connect. We tested for independence by co-expressing two pharmacologically distinct P2X receptors, P2X2 and P2X4, and asking if sensitization of ASICs by one of them could occlude sensitization by the other. Suramin blocks activation of P2X2 and blocked sensitization of ASIC3 in P2X2-expressing CHO cells; after removal of suramin a subsequent ATP application sensitized the ASIC current (Fig. 5A, upper traces). Suramin does not block P2X4 and did not block ASIC3 sensitization in P2X4-expressing CHO cells; a second ATP application had no further effect (Fig. 5A, middle). In CHO cells expressing both P2X2 and P2X4 receptors, the response was a hybrid: sensitization occurred in the presence of suramin, as expected of P2X4 receptors, and it increased further in the absence of suramin, as expected of P2X2 receptors (Fig. 5A, lower traces). We find this result compelling because P2X2/P2X4 co-expression was the only condition in which we ever saw a second ATP application have an effect after the first application had reached its peak sensitization of ASIC current. Summary data (Fig. 5B) suggests that P2X2 and P2X4 effects are additive. Such independence is expected if individual P2X and ASIC molecules must be associated for successful sensitization.
If P2X and ASIC channels communicate through a direct connection rather than a 2nd messenger, ASIC sensitization should increase with P2X density until it is roughly similar to ASIC density; in contrast to such linearity, 2nd messenger systems exhibit amplification. Consistent with a direct connection, we found, in COS cells transfected with ASIC3 and various levels of P2X2, that sensitization increased in proportion to the number of active P2X2 receptors (grey bars, Fig. 6D; Supplementary Fig. S6G). In summary, FRET, occlusion experiments, and amplitude dependence together suggest that P2X and ASIC channels form some sort of assembly that is necessary for ASIC sensitization by P2X.
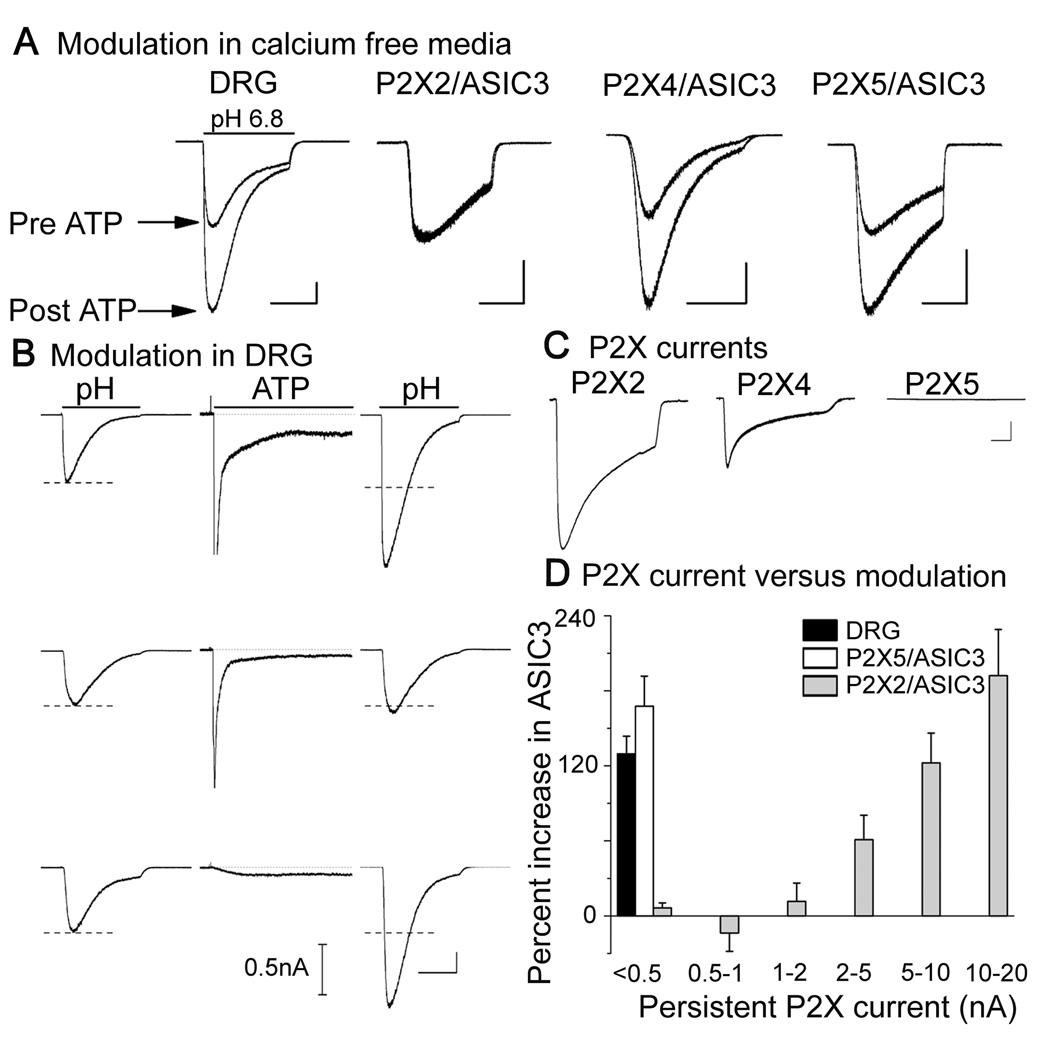
Figure 6. Only P2X5 mimics the response typical of sensory neurons.
(A) ASIC currents before and after exposure to ATP when extracellular Ca2+ was chelated during ATP application (1 mM EGTA, no added Ca2+ or Mg2+. Bath solution was returned to 2 mM Ca2+ and 1 mM Mg2+ prior to evoking ASIC currents). Sensitization did not occur in P2X2-transfected CHO cells when ATP was applied in the absence of external Ca2+ (2nd set of traces), but did in sensory neurons (DRG) and in CHO cells transfected with P2X4 or P2X5. Scale bars: 1 sec; 1 nA. For signaling pathways not implicated in sensitization see supplemental Figure S6, supplemental Table S6.
(B) Three different sensory neurons that either exhibited ATP sensitization (top and bottom cells) or did not (middle). Nothing about the waveform of ATP-evoked current (middle trace in each trio) could predict whether sensitization occurred. pH steps were from 7.4 to 6.8; ATP was 50 µM (4 sec is shown of a 30 sec application). Scale bars: 1 sec; 1 nA (ASIC currents) or 0.5 nA (P2X currents).
(C) Representative amplitudes of three kinds of P2X currents each transfected with 10 µg/ml of the indicated cDNA. As previously shown by Collo et al.(Collo et al., 1996), P2X5 makes little current. Scale bars: 1 sec; 1 nA.
(D) Plot of average percent increase of ASIC current vs. the amplitude of sustained ATP-evoked current in DRG sensory neurons and COS cells transfected with ASIC3 and either P2X2 or P2X5. ASIC sensitization by ATP occurred in native neurons and P2X5-expressing COS cells although there was little sustained ATP-evoked current. In contrast, sensitization required high P2X2 currents (>2 nA) and increased linearly with P2X amplitude above 2 nA.
P2X5, an electrically quiet channel, mimics sensitization in sensory neurons
Figure 3 showed that three subtypes of homomeric P2X receptor are capable of sensitizing ASICs: P2X2, 4, and 5. Which ones are used by native sensory neurons? We argue against P2X4 because it is insensitive to suramin and PPADS, compounds that blocked sensitization in the sensory neurons that we tested (n = 6 and 7 respectively). There is no known pharmacological distinction between P2X2 and P2X5, but three results described below argue in favor of P2X5 and against P2X2: 1) Ca2+ independence of sensitization; 2) the size of ATP-evoked currents that accompany sensitization; 3) co-expression of P2X and ASIC immunoreactivities in sensory neurons.
ASIC sensitization in sensory neurons did not require either intracellular or extracellular Ca2+: ATP increased ASIC current in neurons despite strong Ca2+ chelators inside the cell (10 mM BAPTA) and outside (1 mM EGTA, no added Ca2+ and Mg2+) during the ATP application (Fig. 6A, left traces). In contrast, sensitization did not occur in COS and CHO cells expressing P2X2 if external Ca2+ was chelated during the ATP application (2nd pair of traces). P2X4 and P2X5 were like the native response, insensitive to extracellular Ca2+ chelation. We do not understand why ASIC sensitization via P2X2 depends on external Ca2+, but the observation clearly argues that P2X2 did not mediate the responses that we observed in sensory neurons.
P2X4 was ruled out by suramin and P2X2 was ruled out by external Ca2+, leaving only P2X5 satisfying these pharmacological criteria. Transfected P2X5 makes currents about 100-fold smaller than other P2X receptors (Collo et al., 1996), as illustrated Fig. 6C. Is the relevant P2X receptor on sensory neurons electrically quiet like P2X5? Consider the P2X kinetics of the three sensory neurons in Fig. 6B (middle traces). The top two neurons each had large, transient currents; the top also had a substantial sustained current whereas the other two had small sustained current. ATP increased ASIC current in the top and bottom neurons but not the middle one. Such data convinced us that: a) the P2X receptors carrying the large, transient currents are irrelevant to ASIC sensitization, and b) sensitization occurs even in neurons that exhibit sustained P2X current that seems negligibly small.
In contrast to typical sensory neurons, COS and CHO cells that expressed P2X2 (Fig. 6D, grey bars) and P2X4 (not shown) had no ATP-induced sensitization of ASICs unless they also had clear ATP-evoked current. P2X2 currents had to be 1–2 nA (a large current) before sensitization was detectable, and the extent of sensitization increased roughly in proportion to P2X2 amplitude out to 20 nA. Unlike P2X2 and P2X4 but like most sensory neurons, COS and CHO cells expressing P2X5 exhibited strong sensitization with only miniscule sustained ATP-evoked current (black and white bars). We suggest that the relevant P2X channel on sensory neurons shares an unusual property with P2X5 channels: both are electrically quiet.
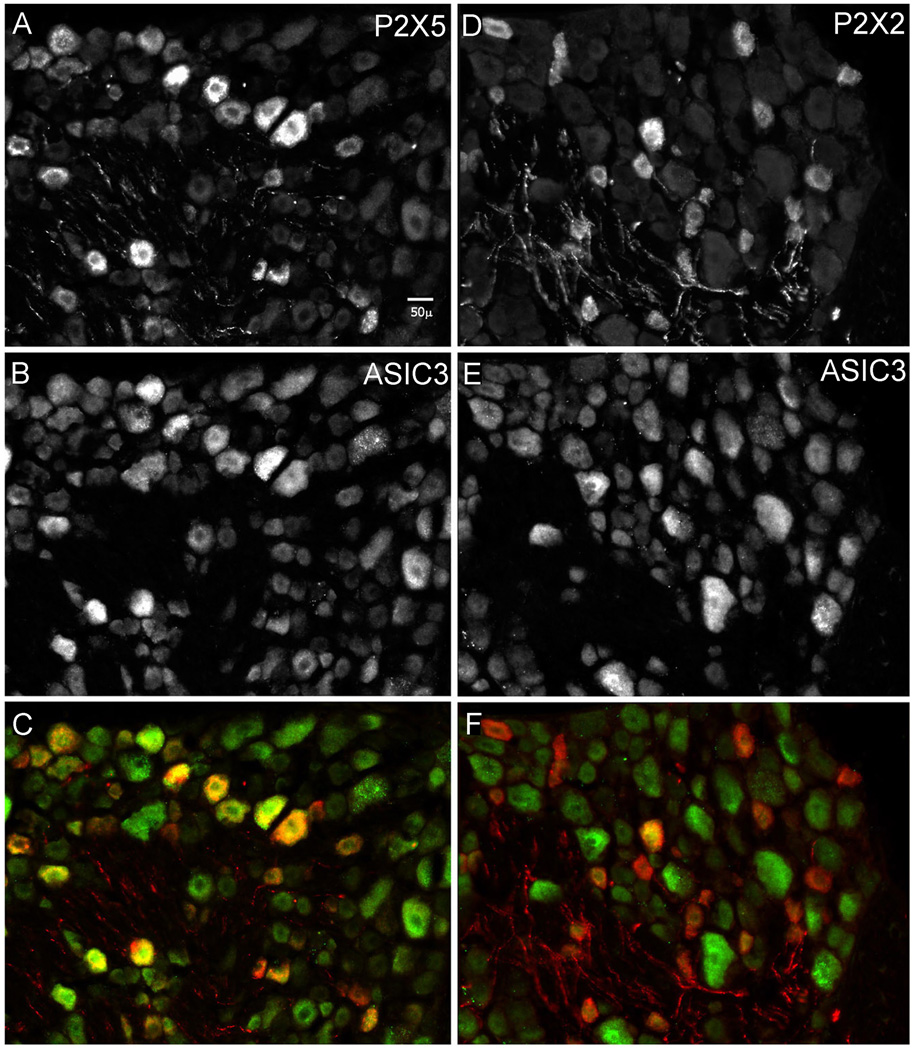
P2X5 mRNA has been demonstrated in sensory neurons (Collo et al., 1996; Kobayashi et al., 2005), but its protein has not. In order to observe the protein and to see if it is expressed together with ASIC3 in sensory neurons, we raised and characterized a P2X5 antisera (Supplementary Figs. S7. Table S7) and compared the distributions in sensory ganglia of P2X5-immunoreactivity (P2X5-IR) to those of ASIC3 (Molliver et al., 2005) and P2X2 (Vulchanova et al., 1996). Neurons labeled for P2X5 were often also labeled for ASIC3 (Fig. 7, left panel), whereas little overlap existed for P2X2 and ASIC3 (right). A blinded count was performed and decoded (n = 3 rats; at least 6 DRG sections from each; 1276 total sensory neurons counted). P2X5 antisera labeled 25% of sensory neurons and just over 50% of these also stained brightly for ASIC3, twice what would occur by chance (26% of all neurons scored bright for ASIC3). P2X2 antisera labeled a similar fraction (26%) of neurons as P2X5, but only 11% of them were positive for ASIC3. We conclude that P2X5 often co-expresses with ASIC3 in sensory neurons whereas P2X2 does not. In summary, four results argue that P2X5 is the most common ATP binding site mediating ASIC sensitization on rat sensory neurons: block by suramin and PPADS, Ca2+ independence, low P2X current amplitude, and co-expression.
Figure 7. P2X5 immunoreactivity co-expresses with ASIC3.
Sensory neurons in two sections taken from an L5 dorsal root ganglion. Left: section labeled and imaged for antisera to P2X5 (A), and ASIC3 (B). Right: section labeled and imaged for antisera to P2X2 (D), and ASIC3 (E). Bottom images (C and F) superimpose the two above (P2X in red, ASIC in green). Secondary antibodies: anti-guinea pig-cy3 (for P2X), anti-rabbit cy5 (for ASIC). Counts of all labeled cells were made blindly in well stained sections from 3 rats (Supplementary Table S7). For antibody validation see supplemental Figure S7.
DISCUSSION
This study was stimulated by the paradox that acid appears incapable of triggering ischemic pain by itself (Meller and Gebhart, 1992) yet buffering acid severely decreases the ability of metaboreceptors to detect ischemia (Pan et al., 1999). In the clinic, the question is this: if acid triggers ischemic muscle pain at a pH that drops only to 7.0–6.9, then why do patients suffering from metabolic acidosis not experience angina or other ischemic pain when their whole body pH drops to such levels? Our data demonstrate a molecular mechanism through which ATP, which is released from oxygen-deprived, contracting muscle (Forrester, 1972), increases the ability of ASICs to respond to a subtle decrease in pH. ATP-induced sensitization of its ASICs should render a sensory neuron more sensitive to ischemic acidosis, which is accompanied by extracellular ATP, than to metabolic acidosis, which is not. The importance of coincident detection of multiple ischemic signals and a role for extracellular ATP has been noted in physiological studies of cardiac and muscle metaboreception (Hayes et al., 2008; Light et al., 2008; Longhurst et al., 2001; Seo et al., 2010). A previous study on lactate (Immke and McCleskey, 2001) and the present one on ATP demonstrate that ASICs act as a molecular integrator of three ischemic signals: acid, lactate, and ATP. The major results and conclusions of our study are as follows.
Evoked at pH 7 on most muscle metaboreceptive neurons, ASIC currents gradually increased by two-fold, on average, when challenged with micromolar extracellular ATP for tens of seconds. Surprisingly, currents remained high long after ATP was removed. ASIC currents evoked at the peak of their activation curve did not increase, arguing that ATP increases the effective sensitivity of ASICs and not the number of functional ASIC channels.
The ATP binding site is another ion channel, a P2X receptor, even though calcium flux and the net direction of flux through the P2X receptor are both irrelevant to sensitization. The dominant P2X receptor on sensory neurons, P2X3, cannot mediate the effect but three others can: P2X2, 4, and 5. Only P2X5 successfully mimicked all properties of the native response in rat sensory neurons, including co-expression with ASIC3. This is the first function on sensory neurons proposed for P2X5, which, though it is an ion channel, makes little electrical current.
There was a strong FRET signal between fluorophores fused to transfected P2X and ASIC3 channels, and dissimilar P2X receptors expressed on the same cell failed to occlude each other’s ability to sensitize ASIC3. These results argue that ASIC and P2X channels co-assemble in some way and that an individual P2X only sensitizes an ASIC within its local assembly.
Lactate and ATP both increase the apparent acid sensitivity of ASICs, but the molecular mechanisms differ fundamentally. ATP requires expression of another protein, a P2X receptor, whereas lactate does not. Lactate acts by binding to and diminishing the free concentration of extracellular Ca2+ ions (Immke and McCleskey, 2001). The proton binding sites that gate ASICs also appear to bind Ca2+, so diminishing their occupancy by Ca2+ decreases the concentration of protons necessary for activation (Babini et al., 2002; Immke and McCleskey, 2003). The different mechanisms for sensitization by lactate and ATP should have several functional consequences: 1) every neuron expressing ASICs will respond to lactate but only those also expressing the appropriate P2X can respond to ATP; 2) lactate acts immediately and reversibly whereas ATP acts slowly and persistently; 3) ATP and lactate actions on ASIC can be additive (data not shown).
Detection of the coincident appearance of acid, lactate and ATP by ASICs could allow metaboreceptors to sense a subtle decrease in extracellular pH while also discerning that its cause is muscle ischemia. Such coincidence detection is likely a common mechanism to obtain sensory specificity; for example, the ion channel TrpA1 is considered a selective trigger for inflammatory pain because it integrates multiple chemicals that are generated during inflammation (Bautista et al., 2006).
Unanswered questions
Despite progress toward understanding the P2X-ASIC mechanism, our data fails to explain the slow onset kinetics, the persistence of sensitization after removal of ATP, and the apparent ATP-independent mechanism of adaptation. Data supporting a direct P2X-ASIC interaction joins a list of other examples in which P2X receptors alter activity of different ligand-gated ion channels: acetylcholine receptors(Khakh et al., 2005; Khakh et al., 2000; Nakazawa, 1994; Zhou and Galligan, 1998), 5HT3 channels(Boue-Grabot et al., 2003), and GABA channels(Boue-Grabot et al., 2004). However, in these previous cases, ATP rapidly and reversibly inhibits the other channels whereas ATP sensitizes ASICs slowly and this persists after removal of ATP. Given the tens of seconds of ATP exposure necessary for sensitization, it is unlikely that ASICs simply sense the conformation change of P2X opening, which occurs in milliseconds. Are there alternative mechanisms? One possibility is that the ASIC senses some slow conformation change in the nearby P2X. For example, P2X inactivation can be slow in onset and traps an ATP molecule on the P2X receptor for an even longer time period. The relevance of P2X inactivation might be explored with ATP analogs that vary in their off rate from the desensitized receptor (Pratt et al., 2005) and with P2X mutants that have altered desensitization (Fabbretti et al., 2004). Another slow P2X phenomenon is a non-selective conductance induced when some P2X receptors are exposed to ATP for long times (Chaumont and Khakh, 2008); we have some preliminary data showing a correlation between ASIC sensitization and such a non-selective conductance (not shown, see (Birdsong, 2008). Slow effects between different membrane proteins might also occur through lipid interactions: deformation of the lipid bilayer within several nanometers of membrane proteins creates a large attractive force between near neighbors and is proposed to be sufficient to induce cooperative gating between membrane proteins (Ursell et al., 2007). A related mechanism is a slow conformation spread through a lattice of receptors (Bray and Duke, 2004).
ASIC currents remained elevated for about 10 minutes after a transient ATP application to sensory neurons, and for as long as we could record (~ 1 hour) in transfected CHO and COS cell lines (Supplementary Fig. S3). We have not explained why a transient appearance of extracellular ATP has such a prolonged effect, nor do we understand why the time course differs in different cell types. One should consider the possibility that P2X activation can trigger a cytosolic signaling cascade. Our data in Fig. 5 may rule out second messengers that could diffuse between different assemblies of P2X-ASIC channels, but it does not rule out the possibility of a second messenger that remains restricted to the vicinity of a P2X-ASIC assembly. The literature now has several examples in which ion channels play dual roles as current carriers and as triggers for cytosolic signaling cascades (Hayashi et al., 1999; Runnels et al., 2001). Indeed, P2X5 has been suggested to trigger a phosphorylation cascade in muscle stem cells (Ryten et al., 2002).
Other remaining biophysical questions include: is the apparent increase in ASIC sensitivity due to an actual change in proton binding affinity; do P2X and ASIC channels connect to each other directly, or through intermediate proteins (Lingueglia, 2007; Wemmie et al., 2006), or through lipid forces (Ursell et al., 2007); if there are protein-protein interactions, are they through intracellular domains or extracellular (Petroff et al., 2008); why does sensitization via P2X2 depend on extracellular Ca2+ whereas P2X4 and P2X5 do not show any Ca2+ sensitivity; why does adaptation of ASIC sensitization proceed even after ATP is removed and why does it proceed to completion on some cell types (neurons, HEK) but not others (CHO, COS)?
The physiological relevance of ASIC sensitization by ATP deserves further exploration. As noted above, synergism between acid and ATP during ischemia in whole animal models has been shown to be relevant to vascular reflexes (Hayes et al., 2008; McCord et al., 2009) and ischemic pain (Seo et al., 2010). An excised organ model (Wenk and McCleskey, 2007) would complement these studies by allowing extensive pharmacological manipulation. Finally, although ASIC3 and muscle ischemia has been our focus, we found that ASIC1 can also be sensitized through P2X receptors (Fig. 3). ASIC1 contributes to damage during brain ischemia (Xiong et al., 2004), so sensitization by extracellular ATP might be relevant in stroke.
EXPERIMENTAL PROCEDURES
Electrophysiology
As the effect of ATP on ASICs is essentially irreversible, ATP was generally applied only to a single cell on any coverslip, after which the coverslip was discarded and the recording chamber washed. Whole cell patch clamp recordings were done at room temperature (~23°C) on rat dorsal root ganglion neurons and mammalian cell lines (CHO, COS, HEK293). Holding potential, unless noted, was −70 mV. For sensory neurons, the typical whole cell patch pipet solution contained (mM): 135 methane sulphonic acid, 150 KOH, 10 KCl, 8 NaCl, 1 MgCl2, 10 MOPS, 5 EGTA, pH 7.0. In some experiments, EGTA was dropped to 0.5 mM or substituted by 10 mM BAPTA-K4 with no apparent difference in results. KCl substituted for K-methanesulfonate without different results. Some experiments used 0.3 Na3GTP and 2 Mg-ATP and this did not affect results provided we took care not to expose the exterior of the cell at any time to the pipet solution. For cell lines, the typical pipet solution contained (mM): 130 K-methanesulfonate, 10 KCl, 4 NaCl, 10 MOPS, 10 EGTA, adjusted to pH 7.0 with KOH. KCl could substitute for K-methanesulfonate. Standard extracellular solution contained (mM): 140 NaCl, 5 KCl, 2 mM CaCl2, 1 MgCl2, 10 HEPES, 10 MES; pH was adjusted with either HCl or NMG (N-Methyl-D-Glucamine). External solutions were exchanged on cells within 20 msec using computer actuated solenoid valves controlling flow through an array of small (1 mm diameter, 10 µl volume) tubes positioned within several hundred micrometers of the cell. Experiments were done 18–48 hours after dissociation or transfection.
Dissociated neuron labeling and culture
Sensory neurons innervating muscle were labeled by retrograde transport of a lipophilic fluorescent dye, DiI (Molecular Probes, Eugene, Oregon. 5% DMSO), injected into quadriceps muscle as described(Honig and Hume, 1986). Dorsal root ganglion neurons were dissociated, plated on laminin-coated plastic or glass coverslips, and maintained at room temperature and air in L15 media and nerve growth factor as described(Eckert et al., 1997). Experiments were done within 48 hours of dissociation.
Cell line transfection
CHO, COS, or HEK293 cells were used for transfection experiments. CHO cells were preferred because they are round, have no endogenous ASICs (unlike COS), and (unlike HEK) ASICs remained sensitized for as long as recordings proceeded (eg. Supplementary Fig. S2). Cells were transiently transfected using electroporation. After suspending cells with trypsin and quenching with F-12 medium, cells were transferred to a 15mL conical vial and pelleted (800rpm, 2 min). Supernatant was removed and cells were resuspended in 400–700µL of cold HBS electroporation buffer (500mL: 4.09g NaCl, 2.975g Hepes, 0.1g Na2CO3, pH adjusted to 7.4 with NaOH). 0.4 to 10µg of DNA in multiple plasmids containing ASIC, P2X, and pCMV-DsRed-Express (Clontech) were added to a 0.4cm electroporation cuvette (Invitrogen), to which 100µL of resuspended CHO cells was added. Cells were electroporated for 1 second in a BioRad Gene Pulser with voltage set at 0.360V and capacitance set at 0.075mF. Cells were immediately transferred to room temperature F-12 medium in 35mm culture dishes containing 7 glass cover slips and incubated at 37 degrees Celsius in 5% CO2. Cells were used between 18 and 48 hours of plating. Transfected cells were visualized using a rhodamine filter set.
Plasmids
P2X and ASIC clones were driven by CMV-containing promoters in JPA or PCI vectors. pCMV DsRed-express was purchased from Clontech. G. Banker provided vectors for making CFP and YFP fusion proteins (JPA5-CFP and YFP) and the NgCAM fusion (JPA5-NgCAM CFP and YFP). W. Almers provided YC3.1 and mCherry C1. To fluorescently tag ASIC and P2X constructs, two EcoRI restriction sites were PCR amplified onto the N and C termini of each parent construct. The PCR amplified product was then ligated into JPA5-CFP or JPA5-YFP cloning vector into an EcoRI site.
FRET imaging
Whole cell FRET was performed on an Olympus IX71 inverted microscope with a mercury arc lamp for fluorescence excitation. Images were acquired on a cooled Princeton instruments NTE/CCD camera controlled with Metamorph 6.2r6 software. Excitation of CFP and FRET used a 436/20 nm bandpass filter (Chroma Technologies) after reflection off a 450 nm long pass dichroic mirror. YFP was selectively excited and bleached using a 514/10 nm filter. Simultaneous imaging of CFP and FRET/YFP emission was obtained by projecting onto half of the CCD array each image from a Dual View beam splitter equipped with a 520 nm dichroic mirror, a 485/25 bandpass filter (CFP fluorescence), and a 535/25 bandpass filter (YFP and FRET). Two images were obtained after each photobleaching interval: one with 436 nm excitation for CFP and FRET, and one with 514 nm for direct YFP excitation. Fluorescence intensities were obtained by circling the cell, averaging the pixel intensities, and subtracting the average background pixel intensities; intensities are expressed as the fractional value obtained before any YFP bleaching. Methods for FRET using total internal reflection microscopy are in the Supplement together with the TIRF data.
Antisera
P2X2 and P2X5 antisera were raised in guinea pigs against peptides from the ectodomains corresponding to P2X2 residues 222–233 (GTSDNHFLGKM-NH2) and P2X5 residues 293–302 (KHTHSISSGY-NH2). ASIC3 antisera were raised in rabbits against peptide 491–505 (EELNGHRTHVPHLSL-NH2), near the carboxyl terminus of the molecule. Antisera were validated by homologous and heterologous absorption on tissues(Vulchanova et al., 1996) and on cultured COS cells 24 hours following transfection with receptor-containing vectors (see Supplement). The new rabbit anti-ASIC3 antibody was compared to a previously validated guinea pig anti-ASIC3 (Molliver et al., 2005) and found to have essentially identical staining pattern in DRG sections (not shown).
Fixed DRG preparation and neuron counting
Young adult Sprague-Dawley rats (150g) were deeply anesthetized and perfused transcardially with calcium-free Tyrode's solution, then approximately 300 ml fixative (4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffered saline (PBS), pH 6.9), and finally about 300 ml 10% sucrose in PBS. Lumbar dorsal root ganglia and spinal cords were immediately dissected and rapidly frozen on chucks for cryosectioning. Fourteen µm sections were cut on a cryostat and mounted on gelatin-coated glass slides. Slides were blocked and washed 3 × 10 min. in antibody diluent (PBS containing 0.3% Triton X-100, 1% normal donkey serum, 1% bovine serum albumin, 0.01% sodium azide), then incubated in primary antisera solution overnight at 4° C. Primary antisera dilutions were: P2X5 – 1:750, P2X2 – 1:1000, ASIC3 – 1:1000. Slides were then washed 3 × 15 minutes in PBS and incubated in secondary antibodies diluted 1:200 in antibody diluent for 120 minutes at room temperature. Secondary antibodies were (CY5-donkey anti-rabbit and CY3-donkey anti-guinea pig; Jackson Immunoresearch, West Grove PA). Slides were washed 3 × 15 min. in PBS, coverslips were mounted with glycerol-based antifade medium and photographed for Cy3 and Cy5. To count the total number of cells/field, micrographs were made by adding dim birefringent visible light to the green epifluorescence emission channel. To assure that cell diameters were not underestimated, only neurons containing distinct nuclei were evaluated. Printed micrographs were coded for blind analysis and the number, size, and relative brightness (1–3) of immunopositive neurons was recorded. Sizes were determined using a reference figure and the method of area equivalent diameters(Weibel, 1979). Size and number of double-labeled P2X- and ASIC3-positive neurons were determined by comparing separate channel views of the same double-labeled DRG field.
HIGHLIGHTS
Transient micromolar ATP doubles ASIC currents on metaboreceptors for minutes
ATP acts through P2X receptors but flux through P2X channels appears irrelevant
FRET and P2X receptor independence argue that P2X and ASIC form a complex
Only P2X5 mimics all aspects of the native response on sensory neurons
Supplementary Material
ACKNOWLEDGEMENTS
We thank Chris Bond for help in preparing molecular constructs, Lori Vaskalis for help in preparing figures, Michel Lazdunski for ASIC clones, Mark Voigt for P2X clones, and Gary Banker for providing constructs for fluorescent proteins. LAN received a scholarship from CAPES-Brasil.
SOURCES OF FUNDING
The work was supported by the NIH (NS37010 and HL64840) and the American Heart Association (0750053Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
LF discovered ATP sensitization of ASICs on sensory neurons and did most of the experiments with neurons. WTB, LN and VS did the electrophysiology experiments with transfected cell lines. FGW did the immunocytochemistry in the Elde lab. MK and WTB did FRET experiments in the Almers lab. JM-H made molecular constructs and prepared plasmids in the Adelman lab. LF, WTB, and EWM designed experiments and wrote the manuscript.
References
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-Evoked Currents in Cardiac Sensory Neurons: A Possible Mediator of Myocardial Ischemic Sensation. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Benson CJ, McCleskey EW. In: ASICs function as lactic acid sensors during cardiac ischemia. In Molecular Sensors for Cardiovascular Homeostasis. Wang DH, editor. Springer; 2007. [Google Scholar]
- Birdsong WT. Dissertation. Portland, OR, USA: Oregon Health & Sciences University; 2008. Acid Sensing Ion Channels: Coincident Detection of Ischemia by a Channel-Channel Interaction. [Google Scholar]
- Borst MM, Schrader J. Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ Res. 1991;68:797–806. doi: 10.1161/01.res.68.3.797. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot E, Barajas-Lopez C, Chakfe Y, Blais D, Belanger D, Emerit MB, Seguela P. Intracellular cross talk and physical interaction between two classes of neurotransmitter-gated channels. J Neurosci. 2003;23:1246–1253. doi: 10.1523/JNEUROSCI.23-04-01246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boue-Grabot E, Emerit MB, Toulme E, Seguela P, Garret M. Crosstalk and co-trafficking between rho1/GABA receptors and ATP-gated channels. J Biol Chem. 2004;279:6967–6975. doi: 10.1074/jbc.M307772200. [DOI] [PubMed] [Google Scholar]
- Bray D, Duke T. Conformational spread: the propagation of allosteric states in large multiprotein complexes. Annu Rev Biophys Biomol Struct. 2004;33:53–73. doi: 10.1146/annurev.biophys.33.110502.132703. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Chaumont S, Khakh BS. Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements but not Panx-1 channels. Proc Natl Acad Sci U S A. 2008;105:12063–12068. doi: 10.1073/pnas.0803008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F. Fluorescence-based methods in the study of protein-protein interactions in living cells. Curr Opin Biotechnol. 2008;19:338–343. doi: 10.1016/j.copbio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Cobbe SM, Poole-Wilson PA. The time of onset and severity of acidosis in myocardial ischaemia. J Mol Cell Cardiol. 1980;12:745–760. doi: 10.1016/0022-2828(80)90077-2. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. Embo J. 2008;27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert SP, Taddese A, McCleskey EW. Isolation and culture of rat sensory neurons having distinct sensory modalities. J Neurosci Methods. 1997;77:183–190. doi: 10.1016/s0165-0270(97)00125-8. [DOI] [PubMed] [Google Scholar]
- Fabbretti E, Sokolova E, Masten L, D'Arco M, Fabbro A, Nistri A, Giniatullin R. Identification of negative residues in the P2X3 ATP receptor ectodomain as structural determinants for desensitization and the Ca2+-sensing modulatory sites. J Biol Chem. 2004;279:53109–53115. doi: 10.1074/jbc.M409772200. [DOI] [PubMed] [Google Scholar]
- Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol. 1972;224:611–628. doi: 10.1113/jphysiol.1972.sp009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LW, Longhurst JC. Regulation of cardiac afferent excitability in ischemia. Handb Exp Pharmacol. 2009:185–225. doi: 10.1007/978-3-540-79090-7_6. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Kaufman MP. Role played by P2X and P2Y receptors in evoking the muscle chemoreflex. J Appl Physiol. 2008;104:538–541. doi: 10.1152/japplphysiol.00929.2007. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine Concentrations in the Interstitium of Resting and Contracting Human Skeletal Muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3 edn. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol. 2009:283–332. doi: 10.1007/978-3-540-79090-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig MG, Hume RI. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986;103:171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neuroscience Letters. 1996;208:191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Hayes SG. The Exercise Pressor Reflex. Clinical Autonomic Research. 2002;12:429. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Fisher JA, Nashmi R, Bowser DN, Lester HA. An angstrom scale interaction between plasma membrane ATP-gated P2X2 and alpha4beta2 nicotinic channels measured with fluorescence resonance energy transfer and total internal reflection fluorescence microscopy. J Neurosci. 2005;25:6911–6920. doi: 10.1523/JNEUROSCI.0561-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature. 2000;406:405–410. doi: 10.1038/35019066. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol. 2005;481:377–390. doi: 10.1002/cne.20393. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Lewis T. Pain in muscular ischaemia: its relation to anginal pain. Archives of Internal Medicine. 1932;49:713–727. [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Longhurst JC, Tjen-A-Looi SC, Fu L-W. Cardiac Sympathetic Afferent Activation Provoked by Myocardial Ischemia and Reperfusion. Mechanisms and Reflexes. Annals of the New York Academy of Sciences. 2001;940:74–95. doi: 10.1111/j.1749-6632.2001.tb03668.x. [DOI] [PubMed] [Google Scholar]
- MacWilliam JA, Webster WJ. SOME APPLICATIONS OF PHYSIOLOGY TO MEDICINE: 1.--SENSORY PHENOMENA ASSOCIATED WITH DEFECTIVE BLOOD SUPPLY TO WORKING MUSCLES. Br Med J. 1923;1:51–53. doi: 10.1136/bmj.1.3237.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Tsuchimochi H, Kaufman MP. Acid-sensing ion channels contribute to the metaboreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2009;297:H443–H449. doi: 10.1152/ajpheart.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience. 1992;48:501–524. doi: 10.1016/0306-4522(92)90398-l. [DOI] [PubMed] [Google Scholar]
- Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- Mense S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl Int. 2008;105:214–219. doi: 10.3238/artzebl.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J Neurosci. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- Pan HL, Longhurst JC, Eisenach JC, Chen SR. Role of protons in activation of cardiac sympathetic C-fibre afferents during ischaemia in cats. J Physiol. 1999;518(Pt 3):857–866. doi: 10.1111/j.1469-7793.1999.0857p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff EY, Price MP, Snitsarev V, Gong H, Korovkina V, Abboud FM, Welsh MJ. Acid-sensing ion channels interact with and inhibit BK K+ channels. Proc Natl Acad Sci U S A. 2008;105:3140–3144. doi: 10.1073/pnas.0712280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt EB, Brink TS, Bergson P, Voigt MM, Cook SP. Use-Dependent Inhibition of P2X3 Receptors by Nanomolar Agonist. J Neurosci. 2005;25:7359–7365. doi: 10.1523/JNEUROSCI.5189-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. Professional: McGraw-Hill; 2001. [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Ryten M, Dunn PM, Neary JT, Burnstock G. ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J Cell Biol. 2002;158:345–355. doi: 10.1083/jcb.200202025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Roh DH, Yoon SY, Kang SY, Moon JY, Kim HW, Han HJ, Chung JM, Beitz AJ, Lee JH. Peripheral Acid-Sensing Ion Channels and P2X Receptors Contribute to Mechanical Allodynia in a Rodent Thrombus-Induced Ischemic Pain Model. J Pain. 2010 doi: 10.1016/j.jpain.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Egbert LD, Markowitz RA, Mosteller F, Beecher HK. An experimental pain method sensitive to morphine in man: the submaximum effort tourniquet technique. J Pharmacol Exp Ther. 1966;154:324–332. [PubMed] [Google Scholar]
- Steen KH, Issberner U, Reeh PW. Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neuroscience Letters. 1995;199:29–32. doi: 10.1016/0304-3940(95)12002-l. [DOI] [PubMed] [Google Scholar]
- Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiol. 2001;537:993–998. doi: 10.1111/j.1469-7793.2001.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell T, Huang KC, Peterson E, Phillips R. Cooperative gating and spatial organization of membrane proteins through elastic interactions. PLoS Comput Biol. 2007;3:e81. doi: 10.1371/journal.pcbi.0030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing J, MacLean DA, Vissing SF, Sander M, Saltin B, Haller RG. The exercise metaboreflex is maintained in the absence of muscle acidosis: insights from muscle microdialysis in humans with McArdle's disease. J Physiol. 2001;537:641–649. doi: 10.1111/j.1469-7793.2001.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Weibel E. Stereological Methods Vol. 1: Practical Methods for Biological Morphometry. Vol 1. London: Academic Press; 1979. [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wenk HN, McCleskey EW. A novel mouse skeletal muscle-nerve preparation and in vitro model of ischemia. J Neurosci Methods. 2007;159:244–251. doi: 10.1016/j.jneumeth.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained Currents Through ASIC3 Ion Channels at the Modest pH Changes That Occur During Myocardial Ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- Zhou X, Galligan JJ. Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J Physiol. 1998;513(Pt 3):685–697. doi: 10.1111/j.1469-7793.1998.685ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.