Abstract
Background
Mannose-binding lectin (MBL) is an important component of innate immunity and activator of the lectin complement pathway. Within the MBL2 gene are seven 5′ “secretor” haplotypes that code for altered serum MBL levels and complement activation. However, recent evidence suggests that 3′ MBL2 haplotypes may also modify MBL function and circulating levels. Because MBL and the lectin complement pathway have been implicated in cardiovascular injury, we investigated whether MBL2 haplotypes are independently associated with an increased risk of postoperative myocardial infarction (PMI) in patients undergoing coronary artery bypass graft surgery.
Methods and Results
Genotyping of 18 polymorphic sites within the MBL2 gene was performed in a prospective, longitudinal multi-institutional study of 978 patients undergoing primary coronary artery bypass graft-only surgery with cardiopulmonary bypass between August 2001 and May 2005. After adjustment for multiple comparisons by permutation testing, multivariate, stepwise logistic regression, including a score test, was performed controlling for patient demographics, preoperative risk factors, medications, and intraoperative variables to determine if MBL2 secretor haplotypes are independent predictors of PMI in whites undergoing primary coronary artery bypass graft surgery. Neither the 5′ nor 3′ MBL2 haplotypes alone were associated with an increased incidence of PMI. However, the incidence of PMI in whites (n=843) expressing the combined MBL2 5′ LYQA secretor haplotype (CGTCGG) and 3′ haplotype (CGGGT) was significantly higher than in whites not expressing the haplotype (38% versus 10%; P<0.007). Moreover, the combined MBL2 LYQA secretor haplotype was an independent predictor of PMI in whites after primary coronary artery bypass graft surgery after adjustment for other covariates (P<0.02; adjusted OR: 3.97; 95% CI: 1.30 to 12.07). The combined MBL2 LYQA secretor haplotype in whites was also an independent predictor of postoperative CKMB levels exceeding 60 ng/mL (P<0.02; adjusted OR: 4.48; 95% CI: 1.95 to 16.80). Inclusion of the combined MBL2 LYQA secretor haplotype improved prediction models for PMI based on traditional risk factors alone (C-statistic 0.715 versus 0.705).
Conclusions
The combined MBL2 LYQA secretor haplotype is a novel independent predictor of PMI and may aid in preoperative risk stratification of whites undergoing primary coronary artery bypass graft surgery.
Keywords: genetics, myocardial infarction, immunology, inflammation, surgery
Human mannose-binding lectin (MBL) is an important component of the innate immune system and activator of the lectin complement pathway (LCP).1 A C-type collectin, MBL functions as a pattern recognition molecule that recognizes carbohydrate structures on microorganism surfaces (eg, bacteria, fungi, and parasites) and innate molecules mimicking carbohydrate structures.1-3 MBL opsonization in turn leads to activation of the LCP through MBL-associated serine proteases (MASP-1, -2, and -3). Although decreased MBL serum levels have been shown to be associated with an increased risk of infection in children and immunocompromised individuals, several lines of evidence suggest that increased MBL levels may be an important regulator of cardiovascular injury.4-15
Serum MBL levels and functional activity are partially regulated by genetic variation in the MBL2 gene.16-18 MBL2 gene sequencing reveals a recombination hotspot that divides MBL2 into 2 distinct haplotype blocks (a 5′ and 3′ block).16-18 Within exon 1 of MBL2 are 3 polymorphisms, known as the B, C, and D alleles, that code for structurally variant proteins.19-21 These alleles, along with 2 linked promotor single nucleotide polymorphisms (SNPs) (−550 H/L and −221 Y/X), as well as a 5′ UTR SNP (+4 P/Q), comprise 7 well-characterized “secretor haplotypes” (HYPA, LYPA, LYQA, LXPA, HYPD, LYPB, and LYQC), which partially account for alterations in complement activation and circulating MBL levels.22 However, recent evidence suggests that MBL serum levels do not fully correlate with the 5′ secretor haplotypes and that 3′ MBL2 haplotypes acting in concert with the 5′ secretor haplotypes also modify MBL function and circulating levels.16-18
Complement has long been known to be an important mediator of myocardial injury. Moreover, recent evidence specifically suggests that MBL and the LCP may play a pathophysiological role in cardiovascular injury.4-15 We thus sought to determine if MBL2 haplotypes are independently associated with an increased risk of postoperative myocardial infarction (PMI) in whites undergoing primary coronary artery bypass (CABG) surgery. We present data that suggest that the combined MBL2 5′ LYQA secretor haplotype (CGTCGG) and 3′ haplotype (CGGGT) is a novel independent predictor of PMI in whites undergoing CABG surgery.
Methods
Study Design
A prospective and longitudinal study of 978 patients undergoing primary CABG surgery with cardiopulmonary bypass between August 2001 and May 2005 at Brigham & Women’s Hospital, Boston, Mass, and the Texas Heart Institute, St. Luke’s Episcopal Hospital, Houston, Texas, was conducted. Institutional Review Board approval and written informed consent was obtained from each patient. Patients were excluded from the study if they were (1) less than 20 years old; (2) undergoing repeat CABG surgery; (3) undergoing CABG surgery with concomitant valve or other cardiac surgery; or (4) undergoing primary CABG surgery without cardiopulmonary bypass. Additionally, patients were excluded if they had a preoperative hematocrit <25% or if they had received leukocyte-rich blood products within the previous 30 days before surgery.
Study Data
Data on more than 700 perioperative variables, including patient demographics, preoperative risk factors, medications, and postoperative outcomes, were collected for each enrolled patient (<2% missing data). Because there is no agreed-on definition of PMI in the literature, 2 definitions of PMI were separately examined. A clinical diagnosis of PMI was made if reported by automated electrocardiographic interpretation (Mac 5500; GE Healthcare, Waukesha, Mich) or if serum myocardial enzymes were requested and reported as being diagnostic of PMI by the clinician. A protocol-specified diagnosis of PMI was made if the MB isoenzyme of creatine kinase drawn by the investigators within the first 3 postoperative days was >60 ng/mL.
MBL2 Genotype and Haplotype Analysis
Genomic DNA was isolated from whole blood samples. The isolated DNA was quality-tested and genotyped at the National Cancer Institute Genotyping Core Facility. All genotype assays contained negative and positive controls and 10% blinded duplicates. The overall genotype concordance among sample duplicates was 99% (discordance range per genotype: 0% to 2%). Haplotype tagging SNPs across the 10.0-kb locus that includes the MBL2 gene were selected on the basis of a previous resequencing analysis of this genomic region in samples of 102 healthy and unrelated individuals from 4 different ethnic groups (http://snp500cancer.nci.nih.gov) by use of the tagSNP program.17,23,24 SNPs in strong linkage to the secretor haplotypes and to variants that capture the 2 gene conversion elements in MBL2 were also included in the genotyping. Overall, a total of 18 SNPs across the entire locus were genotyped by Taqman assays (Applied Biosystems, Inc, Foster City, Calif) in the study population (Table 1). Assays were validated and optimized as described in the SNP500 Cancer web site (http://snp500cancer.nci.nih.gov).24 Haplotypes for study subjects were assigned by Phase (version 2.1).25
TABLE 1.
MBL2 Polymorphisms (chromosome: 10q11.2-q21) Used for Haplotyping
| SNP500Cancer ID |
dbSNP ID |
SNP Region and Alleles |
Minor Allele Frequency |
HWE P Value |
|---|---|---|---|---|
| MBL2–03 | rs5030737 | Ex1−34 C>T | 0.045 | 0.98 |
| MBL2–01 | rs1800450 | Ex1−27 G>A | 0.047 | 0.99 |
| MBL2–02 | rs1800451 | Ex1−18 G>A | 0.043 | 0.99 |
| MBL2–06 | rs1838066 | IVS2–250 T>C | 0.033 | 0.30 |
| MBL2–09 | rs930508 | IVS3–28 G>C | 0.132 | 0.63 |
| MBL2–08 | rs930507 | Ex4+5 C>G | 0.046 | 0.99 |
| MBL2–12 | rs7096206 | −289 G>C | 0.045 | 0.96 |
| MBL2–25 | rs7095891 | −65 T>C | 0.006 | 0.62 |
| MBL2–27 | rs10082466 | Ex4−1483 T>C | 0.030 | 0.56 |
| MBL2–28 | rs10824792 | Ex4−1067 G>A | 0.001 | 0.17 |
| MBL2–34 | rs2120132 | Ex4−901 G>A | 0.134 | 0.77 |
| MBL2–30 | rs2099902 | Ex4−710 G>A | 0.036 | 0.80 |
| MBL2–35 | rs2165810 | −2701 T>A | 0.037 | 0.78 |
| MBL2–37 | rs3925313 | −2477 T>C | 0.059 | 0.80 |
| MBL2–38 | rs1031101 | −1964 T>C | 0.164 | 0.17 |
| MBL2–53 | rs10450310 | 3238bp 3′ of STP T>C |
0.056 | 0.73 |
| MBL2–11 | rs11003125 | −618 G>C | 0.058 | 0.82 |
| MBL2–65 | rs12264958 | −2200 T>C | 0.058 | 0.79 |
HWE indicates Hardy-Weinberg equilibrium.
The National Cancer Institute SNP500 Cancer Database may be found at: http://snp500cancer.nci.nih.gov/home_1.cfm.
Measurement of Creatine Kinase MB
Creatine kinase MB levels were measured at a centralized location (Brigham & Women’s Hospital, Boston, Mass) from serum samples taken preoperatively (baseline) and on postoperative days 1, 2, and 3 using a sandwich immunoassay (Siemens ADVIA Centaur; Siemens Medical Solutions Diagnostics, Tarrytown, NY).
Statistics
Statistical analyses were performed using SAS, version 9.1 (SAS Institute, Cary, NC). Missing clinical data were handled using complete case analysis. Before evaluating the contribution of genetic factors, the relationship between traditional risk factors (ie, patient preoperative and intraoperative demographics, risk factors, medications) and PMI was first explored by multivariable logistic regression (clinical model). After testing for Hardy-Weinberg equilibrium (Fischer’s exact test), the association of each 5′ and 3′ MBL haplotype along with their interaction term with PMI was then tested using a 2-stage analysis approach: marker selection followed by modeling of genotype–phenotype relationships (clinical–genetic model). To control for multiple haplotype comparisons, only those MBL2 haplotypes significantly associated with PMI after permutation testing (P<0.05) were included in the overall model. Furthermore, to avoid assumptions regarding the modes of inheritance, all analyses were performed using an additive genetic model (homozygote major allele versus heterozygote versus homozygote minor allele) for each haplotype. All clinical predictor variables and haplotypes significant at a 2-tailed nominal P<0.15 in the univariate analysis were then entered into a multivariate stepwise logistic regression model to determine if MBL2 haplotypes were independently associated with an increased risk of PMI after primary CABG surgery. Only those variables significant at a 2-tailed nominal P<0.05 were retained within the model. Additionally, a second multivariate model was created using the score test, which controls for the effects of genetic linkage by comparing the effects of all haplotypes simultaneously within the model. To compare the additive value of the genotypic information to the traditional clinical model, a C-statistic was calculated for each multivariate model. Odds ratios and corresponding 95% CIs are reported with associated probability values.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the article as written.
Results
Within the total study population (n=978), there were 843 white and 135 nonwhite patients. Within the white subpopulation, the incidence of PMI ranged from 10% (n=88; clinical diagnosis) to 6% (n=53; protocol-specified diagnosis). All white patients with a protocol-specified diagnosis of myocardial infarction were also contained within the subpopulation of patients with a clinical diagnosis of myocardial infarction. White patient preoperative and intraoperative demographics are presented in Tables 2 and 3, respectively. Note that white patients having a PMI by clinical diagnosis were significantly more likely be receiving preoperative β-blocker (P=0.01), warfarin/heparin (P=0.04), vasodilator (P=0.002), nonaspirin platelet inhibitor (P=0.04), and nonstatin antihyperlipidemic therapy (P=0.04) compared with patients without a PMI. Additionally, white patients having a PMI by clinical diagnosis were significantly more likely to have longer cardiopulmonary bypass (P=0.004) and crossclamp (P=0.0001) times and a greater number of bypass grafts (P=0.007) compared with patients without a PMI.
TABLE 2.
Preoperative Demographic Variables and Risk Factors in White Patients (N=843) With and Without a PMI After Primary CABG Surgery
| Patient Demographics and Preoperative Risk Factors [% (n)] |
PMI (N=88) |
No PMI (N=755) |
P Value |
|---|---|---|---|
| Age, y | 64±11 | 65±10 | 0.48 |
| Female gender | 26 (23) | 21 (161) | 0.34 |
| Weight, kg | 87±16 | 88±19 | 0.61 |
| Height, cm | 172±10 | 173±10 | 0.12 |
| Body mass index, kg/m2 | 30±5.0 | 29±5.5 | 0.63 |
| Diabetes mellitus type I | 7 (6) | 10 (77) | 0.45 |
| Diabetes mellitus type II | 20 (18) | 21 (159) | 0.42 |
| Hyperlipidemia | 75 (65) | 76 (574) | 0.79 |
| History of smoking | 69 (61) | 71 (531) | 0.81 |
| Pulmonary disease | 8 (7) | 17 (123) | 0.04 |
| Renal disease | 7 (6) | 8 (58) | 0.99 |
| Hypertension | 81 (71) | 76 (573) | 0.42 |
| Prior myocardial infarction | 51 (45) | 45 (337) | 0.26 |
| Angina | 7(6) | 9 (68) | 0.69 |
| Anemia | 9 (8) | 8 (60) | 0.68 |
| Coagulopathy | 1 (1) | 2 (14) | 0.99 |
| Previous percutaneous transluminal coronary angioplasty |
20 (18) | 21 (157) | 0.99 |
| Atrial fibrillation | 8 (8) | 5 (41) | 0.33 |
| Other dysrhythmias | 2 (2) | 6 (42) | 0.19 |
| Valvular disease | 0 (0) | 0.3 (2) | 0.99 |
| Carotid disease | 2 (2) | 4 (33) | 0.57 |
| Left Ventricular ejection fraction <30% | 10 (88) | 19 (142) | 0.21 |
| Urgency of operation—elective | 36 (31) | 45 (339) | 0.17 |
| ≥3 coronary vessels with stenosis >50% |
14 (12) | 16 (120) | 0.76 |
| Peripheral vascular disease | 8 (6) | 10 (54) | 0.83 |
| Transient ischemic attack | 1 (1) | 3 (22) | 0.50 |
| Stroke | 6 (5) | 5 (35) | 0.60 |
| Gastrointestinal disease | 19 (17) | 15 (115) | 0.36 |
| History of malignancy | 16 (14) | 9 (70) | 0.06 |
| Hepatic disease | 8 (7) | 6 (42) | 0.34 |
| Preoperative medications | |||
| β-blockers | 89 (78) | 77 (582) | 0.01 |
| Aspirin | 81 (71) | 75 (565) | 0.29 |
| Antiarrhythmics | 2 (2) | 4(27) | 0.76 |
| Angiotensin-converting enzyme inhibitors | 49 (43) | 46 (347) | 0.65 |
| Ca2+ channel blockers | 8 (7) | 15 (110) | 0.10 |
| Diuretics | 22 (19) | 22 (166) | 0.99 |
| Intravenous inotropes | 3 (3) | 4 (27) | 0.99 |
| Nonaspirin platelet inhibitors | 26 (23) | 17 (127) | 0.04 |
| Vasodilators | 26 (23) | 13 (99) | 0.002 |
| Warfarin or heparin | 40(35) | 28 (214) | 0.04 |
| Statins | 68 (60) | 74 (560) | 0.25 |
| Nonstatin antihyperlipidemics | 13 (11) | 6 (47) | 0.04 |
| Intraaortic balloon pump | 5 (4) | 2 (16) | 0.15 |
Mean±SD.
TABLE 3.
Intraoperative Demographic Variables and Risk Factors in White Patients (N=843) With and Without a PMI After Primary CABG Surgery
| Intraoperative Demographics and Risk Factors [% (n)] |
PMI (N=88) |
No PMI (N=755) |
P Value |
|---|---|---|---|
| Crossclamp time, min | 84±29 | 73±35 | 0.004 |
| Total bypass time, min | 119±51 | 99±40 | 0.0001 |
| Average no. of bypass grafts | 4±0.9 | 3±0.9 | 0.007 |
| Lowest temperature, C° | 32.6±2.5 | 32.1±2.5 | 0.07 |
| Highest temperature, C° | 37.6±0.8 | 37.5±0.9 | 0.07 |
| Intraoperative intravenous inotropes | 43 (38) | 35 (261) | 0.13 |
| Intraoperative β-blockers | 22 (19) | 16 (123) | 0.23 |
| Intraoperative intraaortic balloon pump | 2 (2) | 2 (17) | 0.99 |
| Intraoperative antifibrinolytics | 90 (79) | 84 (637) | 0.21 |
Mean±SD.
The incidence of each MBL haplotype in whites (n=843) is presented in Table 4. All genotypes were in Hardy-Weinberg equilibrium (Fisher’s exact test) at the P>0.01 level (Table 1).
TABLE 4.
MBL2 Haplotype Incidence in Whites (n=843) With and Without a PMI After CABG Surgery
|
MBL2 Haplotype |
PMI (total N=88) |
No PMI (total N=755) |
||||
|---|---|---|---|---|---|---|
| 5′ Secretor Haplotype |
3′ Base Pair Sequence |
Heterozygotes [% (n)] |
Homozygotes [% (n)] |
Heterozygotes [% (n)] |
Homozygotes [% (n)] |
P Value (Fisher’s exact) |
| LXPA (CCCCGG) |
TGAAC | 8 (18) | 1 (3) | 82 (194) | 9 (21) | 0.50 |
| CGGGT | 15 (12) | 0 (0) | 85 (69) | 0 (0) | 0.18 | |
| TAAAC | 20 (4) | 0 (0) | 75 (15) | 5 (1) | 0.21 | |
| LYPB (CGCCAG) |
TGAAC | 0 (0) | 0 (0) | 100 (4) | 0 (0) | 0.99 |
| CGGGT | 6 (13) | 1 (2) | 86 (185) | 7 (15) | 0.09 | |
| TAAAC | 6 (2) | 0 (0) | 94 (30) | 0 (0) | 0.56 | |
| LYPA (CGCCGG) |
TGAAC | 0 (0) | 0 (0) | 100 (3) | 0 (0) | 0.99 |
| CGGGT | 10 (7) | 0 (0) | 90 (62) | 0 (0) | 0.99 | |
| TAAAC | 20 (1) | 0 (0) | 80 (4) | 0 (0) | 0.42 | |
| LYQC (CGTCGA) |
TGAAC | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| CGGGT | 17 (4) | 0 (0) | 78 (18) | 4 (1) | 0.35 | |
| TAAAC | 0 (0) | 0 (0) | 100 (1) | 0 (0) | 0.99 | |
| LYQA (CGTCGG) |
TGAAC | 0 (0) | 0 (0) | 100 (6) | 0 (0) | 0.99 |
| CGGGT | 38 (5) | 0 (0) | 54 (7) | 8 (1) | 0.007 | |
| TAAAC | 8 (23) | 1 (3) | 82 (226) | 8 (22) | 0.73 | |
| HYPA (GGCCGG) |
TGAAC | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| CGGGT | 0 (0) | 0 (0) | 100 (8) | 0 (0) | 0.99 | |
| TAAAC | 10 (43) | 2 (8) | 75 (339) | 14 (62) | 0.68 | |
| HYPD (GGCTGG) |
TGAAC | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| CGGGT | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.99 | |
| TAAAC | 8 (8) | 2 (2) | 87 (87) | 3 (3) | 0.11 | |
Neither the 5′ nor 3′ MBL2 haplotypes alone were associated with an increased incidence of PMI by clinical or protocol-specified diagnosis. However, the incidence of PMI by clinical diagnosis in whites (n=843) expressing the combined MBL2 5′ LYQA secretor haplotype (CGTCGG) and 3′ haplotype (CGGGT) was significantly higher than in whites not expressing the haplotype (38% versus 10%; P<0.007). Moreover, multivariate analysis controlling for patient demographics, preoperative risk factors, medications, and intraoperative variables revealed that the combined MBL2 LYQA secretor haplotype was an independent predictor of PMI by clinical diagnosis in whites after primary CABG surgery after adjustment for other covariates (Figure; P<0.02; adjusted OR: 3.97; 95% CI: 1.30 to 12.07). Furthermore, the multivariate score test, which controls for the effects of genetic linkage by comparing the effects of all haplotypes simultaneously within the model, still demonstrated that the combined MBL2 LYQA secretor haplotype was an independent predictor of PMI in whites after primary CABG surgery (P=0.01; adjusted OR: 4.72; 95% CI: 1.41 to 15.85).
Figure.
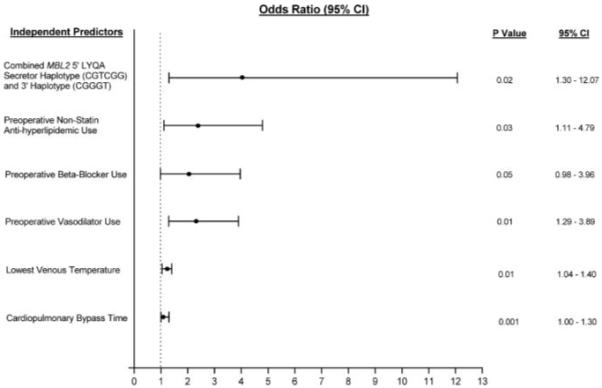
Multivariate analysis of PMI after primary CABG surgery controlling for patient demographics, perioperative risk factors, and medications. Significant, independent predictors of PMI, along with their associated ORs and 95% CIs are shown.
The combined MBL2 LYQA secretor haplotype in whites was also an independent predictor of postoperative creatine kinase MB levels exceeding 60 ng/mL (P<0.02; adjusted OR: 4.48; 95% CI: 1.95 to 16.80). Furthermore, inclusion of the combined MBL2 LYQA secretor haplotype improved prediction models for PMI based on traditional risk factors alone (C-statistic 0.715 versus 0.705).
Discussion
Increasing evidence suggests that MBL and the LCP play an important role in cardiovascular pathophysiology, including coronary artery disease, altered coronary artery reactivity, vascular restenosis, type I diabetes, and ischemia–reperfusion injury.4-15 We now demonstrate that white patients expressing the combined MBL2 5′ LYQA secretor haplotype (CGTCGG) and 3′ haplotype (CGGGT) have a significantly higher incidence of PMI by clinical or protocol-specified diagnoses after primary CABG surgery than patients who do not express this haplotype. Moreover, we demonstrate that the combined MBL2 LYQA secretor haplotype is a novel independent predictor of PMI, even after controlling for permutation testing, patient demographics, preoperative risk factors, medications, and intraoperative variables. Indeed, expression of the combined MBL2 LYQA secretor haplotype confers a greater than 3-fold risk of PMI by clinical or protocol-specified diagnosis in whites after primary CABG surgery (However, the large CI makes estimation of this effect imprecise).
Surgery invokes a systemic inflammatory response characterized by complement, leukocyte, and platelet activation, and the release of various proinflammatory cytokines. Although inflammation in general is thought to be an adaptive response, in certain individuals, the systemic inflammatory response to surgery may be severe enough to be associated with significant perioperative and long-term clinical morbidity, including impaired hemostasis, ventricular failure, myocardial infarction, stroke, and multisystem organ dysfunction. Recent evidence suggests that the degree and severity of surgical-induced inflammation may be significantly influenced by genotype.26,27 Thus, modulation of the perioperative immune response may represent one mechanism by which allotypic variation may influence the incidence of adverse postoperative outcomes.
MBL is a critical component of innate immunity and functions as a pattern recognition molecule that recognizes N-acetyl-glucosamine and mannose residues on microorganism surfaces or molecules that mimic these carbohydrate structures.1-3 Human MBL circulates in the blood (1 to 2 μg/mL plasma) as large homo-oligomers (200 to 650 kDa) consisting of 9 to 18 32 kDa monomers.1 Each monomer consists of 4 distinct domains: (1) a NH2-terminal region rich in cysteine residues, (2) a collagen-like domain composed of 18 to 20 tandem repeats of a Gly-X-Y sequence similar in overall structure to C1q, (3) a neck region, and (4) a COOH-terminal carbohydrate recognition domain that recognizes and binds to mannose and N-acetyl-glucosamine residues.1 Like C-reactive protein, MBL is an acute phase reactant (albeit much weaker) increasing in response to infection, surgery, or trauma.1,28 Additionally, human MBL2 gene transcription has been shown to be augmented by interleukin-6, dexamethasone, and heat shock and downregulated by interleukin-1.29
Serum MBL levels and functional activity are partially regulated by genetic variation in the MBL2 gene, which is divided into 2 distinct haplotype blocks (ie, a 5′ and 3′ block) by a recombination hotspot.16-18 Within the 5′ block are 7 well-studied “secretor haplotypes” (HYPA, LYPA, LYQA, LXPA, HYPD, LYPB, and LYQC), which partially account for alterations in complement activation and circulating MBL levels.22 However, recent evidence suggests that MBL serum levels do not fully correlate with the 5′ secretor haplotypes and that 3′ MBL2 haplotypes acting in concert with the 5′ secretor haplotypes also modify MBL function and circulating levels.16-18 This evidence is further supported by a recent study demonstrating that variant sites inside the 5′ and 3′ MBL2 haplotype blocks, including the 6 markers of the 7 secretor haplotypes, are in strong linkage disequilibrium.16 A functional genomic “interaction” between these 2 MBL2 haplotype blocks may thus explain in part our finding that the combined MBL2 5′ LYQA secretor haplotype (CGTCGG) and 3′ haplotype (CGGGT) is a novel independent predictor of PMI in whites undergoing primary CABG surgery.
In general, the LYQA haplotype is thought of as a “wild-type” or “normal” allele, coding for elevated serum MBL levels. However, increasing evidence suggests that MBL may play both protective and harmful roles depending on the clinical scenario. For example, decreased MBL serum levels have been shown to be associated with an increased risk of infection in children and immunocompromised individuals, including recurrent respiratory infections, meningococcal disease, and Pseudomonas aeruginosa infection in burn patients.30 Alternatively, MBL recognition of self-antigens has been demonstrated in several sterile inflammatory processes, including thoracoabdominal aortic aneurysms,8 IgA nephritis,31 rheumatoid arthritis,32 atherosclerosis,10 diabetes,7,12 cancer,33 and coronary artery disease.6,14 Moreover, inhibition of MBL was recently shown to reduce infarct size in an experimental model of myocardial infarction.15 Thus, the dichotomous nature of MBL to recognize both foreign- and/or self-antigens is currently the focus of intense research, because the balance of MBL and LCP activation may play an important role in various disease states.30
Although this prospective cohort study extends the results of previous studies suggesting that MBL and the LCP may play a pathophysiological role in cardiovascular disease, the present study is not without limitations. Despite the use of multivariate regression models to adjust for potential confounders that affect postoperative outcomes, immeasurable or unknown factors may still exist. Moreover, identification of a positive association between a specific genotype and clinical outcome does not necessarily imply causality. The identified genotype may actually be clinically “silent,” but be linked to one or more other allotypes that individually or collectively form a disease haplotype. Third, we do not yet have enough nonwhite patients to see if the combined MBL2 LYQA secretor haplotype is predictive of PMI in other ethnicities. Similarly, it is unclear if the combined MBL2 LYQA secretor haplotype is predictive of PMI in other types of cardiac or noncardiac surgery. Finally, the clinical relevance of a gene association study directly correlates with the quality of the phenotyping used. To this end, we have ongoing plans to measure perioperative serum MBL levels to determine whether (1) the combined MBL2 LYQA secretor haplotype is an independent predictor of perioperative MBL levels; and (2) whether perioperative MBL levels are predictive of PMI.
In conclusion, the combined MBL2 5′ LYQA secretor haplotype (CGTCGG) and 3′ haplotype (CGGGT) is a novel independent predictor of PMI by clinical or protocol-specified diagnosis in whites undergoing primary CABG surgery. Although further research is needed to determine the underlying mechanisms of this association, these data suggest that this haplotype may be clinically used to improve cardiac surgical patient risk stratification and resource utilization.
Acknowledgments
We acknowledge the efforts and contributions of the CABG Genomics research staff: James Gosnell, RN; Kujtim Bodinaku, MD; Jai Joshi, MD, MPH; Svetlana Gorbatov, MPH; Juliette Dean, RN; James Chen, RN; Jacques Estephan, RN; and Isabella Candelaria, BS.
Sources of Funding
These studies were supported in part by the NIH (HL-068774 and NCRR M01 02558), Society of Cardiovascular Anesthesiologists Research Starter Grant, and Siemens Medical Solutions Diagnostics, Tarrytown, NY.
Footnotes
Presented at the American Heart Association Scientific Sessions, Chicago, Ill, November 12–15, 2006.
Disclosures
C.D.C. received a grant from NIH (NCRR M01 02558); S.K.S. and P.J. received funding from Siemens Medical Solutions Diagnostics, Tarrytown, NY; A.A.F. received a Society of Cardiovascular Anesthesiologists Research Starter Grant; NIH (NCRR M01 02558); and S.C.B. received a grant from NIH (NHLBI HL-068774).
References
- 1.Takahashi K, Ip WE, Michelow IC, Ezekowitz RA. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard CD, Montalto MC, Reenstra WR, Buras JA, Stahl GL. Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am J Pathol. 2001;159:1045–1054. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montalto MC, Collard CD, Buras JA, Reenstra WR, McClaine R, Gies DR, Rother RP, Stahl GL. A keratin peptide inhibits mannose-binding lectin. J Immunol. 2001;166:4148–4153. doi: 10.4049/jimmunol.166.6.4148. [DOI] [PubMed] [Google Scholar]
- 4.Collard CD, Vakeva A, Morrissey MA, Agah A, Rollins SA, Reenstra WR, Buras JA, Meri S, Stahl GL. Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol. 2000;156:1549–1556. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aittoniemi J, Fan YM, Laaksonen R, Janatuinen T, Vesalainen R, Nuutila P, Knuuti J, Hulkkonen J, Hurme M, Lehtimaki T. The effect of mannan-binding lectin variant alleles on coronary artery reactivity in healthy young men. Int J Cardiol. 2004;97:317–318. doi: 10.1016/j.ijcard.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Best LG, Davidson M, North KE, MacCluer JW, Zhang Y, Lee ET, Howard BV, DeCroo S, Ferrell RE. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians: the Strong Heart Study. Circulation. 2004;109:471–475. doi: 10.1161/01.CIR.0000109757.95461.10. [DOI] [PubMed] [Google Scholar]
- 7.Bouwman LH, Eerligh P, Terpstra OT, Daha MR, de Knijff P, Ballieux BE, Bruining GJ, van der Slik AR, Roos A, Roep BO. Elevated levels of mannose-binding lectin at clinical manifestation of type 1 diabetes in juveniles. Diabetes. 2005;54:3002–3006. doi: 10.2337/diabetes.54.10.3002. [DOI] [PubMed] [Google Scholar]
- 8.Fiane AE, Videm V, Lingaas PS, Heggelund L, Nielsen EW, Geiran OR, Fung M, Mollnes TE. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation. 2003;108:849–856. doi: 10.1161/01.CIR.0000084550.16565.01. [DOI] [PubMed] [Google Scholar]
- 9.Rugonfalvi-Kiss S, Dosa E, Madsen HO, Endresz V, Prohaszka Z, Laki J, Karadi I, Gonczol E, Selmeci L, Romics L, Fust G, Entz L, Garred P. High rate of early restenosis after carotid eversion endarterectomy in homozygous carriers of the normal mannose-binding lectin genotype. Stroke. 2005;36:944–948. doi: 10.1161/01.STR.0000160752.67422.18. [DOI] [PubMed] [Google Scholar]
- 10.Keller TT, van Leuven SI, Meuwese MC, Wareham NJ, Luben R, Stroes ES, Hack CE, Levi M, Khaw KT, Boekholdt SM. Serum levels of mannose-binding lectin and the risk of future coronary artery disease in apparently healthy men and women. Arterioscler Thromb Vasc Biol. 2006:2345–2350. doi: 10.1161/01.ATV.0000240517.69201.77. [DOI] [PubMed] [Google Scholar]
- 11.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 12.Hansen TK, Gall MA, Tarnow L, Thiel S, Stehouwer CD, Schalkwijk CG, Parving HH, Flyvbjerg A. Mannose-binding lectin and mortality in type 2 diabetes. Arch Intern Med. 2006;166:2007–2013. doi: 10.1001/archinte.166.18.2007. [DOI] [PubMed] [Google Scholar]
- 13.Szeplaki G, Varga L, Laki J, Dosa E, Madsen HO, Prohaszka Z, Szabo A, Acsady G, Selmeci L, Garred P, Fust G, Entz L. Elevated complement C3 is associated with early restenosis after eversion carotid endarterectomy. Thromb Haemost. 2006;96:529–534. [PubMed] [Google Scholar]
- 14.Saevarsdottir S, Oskarsson OO, Aspelund T, Eiriksdottir G, Vikingsdottir T, Gudnason V, Valdimarsson H. Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J Exp Med. 2005;201:117–125. doi: 10.1084/jem.20041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 16.Bernig T, Breunis W, Brouwer N, Hutchinson A, Welch R, Roos D, Kuijpers T, Chanock S. An analysis of genetic variation across the MBL2 locus in Dutch Caucasians indicates that 3′ haplotypes could modify circulating levels of mannose-binding lectin. Hum Genet. 2005;118:404–415. doi: 10.1007/s00439-005-0053-5. [DOI] [PubMed] [Google Scholar]
- 17.Bernig T, Taylor JG, Foster CB, Staats B, Yeager M, Chanock SJ. Sequence analysis of the mannose-binding lectin (MBL2) gene reveals a high degree of heterozygosity with evidence of selection. Genes Immun. 2004;5:461–476. doi: 10.1038/sj.gene.6364116. [DOI] [PubMed] [Google Scholar]
- 18.Bernig T, Boersma BJ, Howe TM, Welch R, Yadavalli S, Staats B, Mechanic LE, Chanock SJ, Ambs S. The mannose-binding lectin (MBL2) haplotype and breast cancer: an association study in African American and Caucasian women. Carcinogenesis. 2007;28:828–836. doi: 10.1093/carcin/bgl198. Epub 2006 Oct 27. [DOI] [PubMed] [Google Scholar]
- 19.Sumiya M, Super M, Tabona P, Levinsky RJ, Arai T, Turner MW, Summerfield JA. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 20.Lipscombe RJ, Sumiya M, Hill AV, Lau YL, Levinsky RJ, Summerfield JA, Turner MW. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–715. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 21.Madsen HO, Garred P, Kurtzhals JA, Lamm LU, Ryder LP, Thiel S, Svejgaard A. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 22.Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO. Mannose-binding lectin and its genetic variants. Genes Immun. 2006;7:85–94. doi: 10.1038/sj.gene.6364283. [DOI] [PubMed] [Google Scholar]
- 23.Stram DO, Haiman CA, Hirschhorn JN, Altshuler D, Kolonel LN, Henderson BE, Pike MC. Choosing haplotype-tagging SNPS based on unphased genotype data using a preliminary sample of unrelated subjects with an example from the Multiethnic Cohort Study. Hum Hered. 2003;55:27–36. doi: 10.1159/000071807. [DOI] [PubMed] [Google Scholar]
- 24.Packer BR, Yeager M, Burdett L, Welch R, Beerman M, Qi L, Sicotte H, Staats B, Acharya M, Crenshaw A, Eckert A, Puri V, Gerhard DS, Chanock SJ. SNP500Cancer: a public resource for sequence validation, assay development, and frequency analysis for genetic variation in candidate genes. Nucleic Acids Res. 2006;34:D617–D621. doi: 10.1093/nar/gkj151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox AA, Shernan SK, Body SC, Collard CD. Genetic influences on cardiac surgical outcomes. J Cardiothorac Vasc Anesth. 2005;19:379–391. doi: 10.1053/j.jvca.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 27.Ziegeler S, Tsusaki BE, Collard CD. Influence of genotype on perioperative risk and outcome. Anesthesiology. 2003;99:212–219. doi: 10.1097/00000542-200307000-00032. [DOI] [PubMed] [Google Scholar]
- 28.Ytting H, Christensen IJ, Basse L, Lykke J, Thiel S, Jensenius JC, Nielsen HJ. Influence of major surgery on the mannan-binding lectin pathway of innate immunity. Clin Exp Immunol. 2006;144:239–246. doi: 10.1111/j.1365-2249.2006.03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arai T, Tabona P, Summerfield JA. Human mannan-binding protein gene is regulated by interleukins, dexamethasone and heat shock. Q J Med. 1993;86:575–582. [PubMed] [Google Scholar]
- 30.Casanova JL, Abel L. Human mannose-binding lectin in immunity: friend, foe, or both? J Exp Med. 2004;199:1295–1299. doi: 10.1084/jem.20040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hisano S, Matsushita M, Fujita T, Endo Y, Takebayashi S. Mesangial IgA2 deposits and lectin pathway-mediated complement activation in IgA glomerulonephritis. Am J Kidney Dis. 2001;38:1082–1088. doi: 10.1053/ajkd.2001.28611. [DOI] [PubMed] [Google Scholar]
- 32.Gupta B, Agrawal C, Raghav SK, Das SK, Das RH, Chaturvedi VP, Das HR. Association of mannose-binding lectin gene (MBL2) polymorphisms with rheumatoid arthritis in an Indian cohort of casecontrol samples. J Hum Genet. 2005;50:583–591. doi: 10.1007/s10038-005-0299-8. [DOI] [PubMed] [Google Scholar]
- 33.Baccarelli A, Hou L, Chen J, Lissowska J, El Omar EM, Grillo P, Giacomini SM, Yaeger M, Bernig T, Zatonski W, Fraumeni JF, Jr, Chanock SJ, Chow WH. Mannose-binding lectin-2 genetic variation and stomach cancer risk. Int J Cancer. 2006;119:1970–1975. doi: 10.1002/ijc.22075. [DOI] [PubMed] [Google Scholar]


