Abstract
Whereas pneumonia is the most common cause of death and disability worldwide, most cases of pneumonia spontaneously resolve. Mechanisms that promote pneumonia resolution remain to be determined. Resolvin E1(RvE1) is an endogenous mediator that displays proresolving actions in sterile inflammation. In this study, we developed a new model of aspiration pneumonia to evaluate the effect of RvE1 on acute lung injury caused by acid aspiration and subsequent bacterial challenge. Mice received hydrochloric acid into the left lung followed by the enteric pathogen Escherichia coli. I.v. administration of RvE1 (~0.005 mg/kg) prior to acid injury selectively decreased lung neutrophil accumulation by 55% and enhanced clearance of E. coli. RvE1 significantly decreased lung tissue levels of several proinflammatory chemokines and cytokines, including IL-1β, IL-6, HMGB-1, MIP-1α, MIP-1β, keratinocyte-derived chemokine, and MCP-1, in a manner independent of the anti-inflammatory mediators IL-10 and lipoxin A4. In addition, animals treated with RvE1 had a marked improvement in survival. These findings in experimental aspiration pneumonia have uncovered protective roles for RvE1 in pathogen-mediated inflammation that are both anti-inflammatory for neutrophils and protective for host defense, suggesting that RvE1 represents the first candidate for a novel therapeutic strategy for acute lung injury and pneumonia that harnesses natural resolution mechanisms.
More than any other infection, acute pneumonia causes the greatest morbidity and mortality (1). To address the important global unmet need for treatment (2), several new antibiotics were developed over the last several decades, yet pneumonia mortality has not decreased (1, 3). One explanation for the persistent adverse outcomes for pneumonia is the pathogen-initiated inflammatory response that can spiral out of control and lead to acute lung injury (ALI) or the acute respiratory distress (ARDS) (4). Whereas pathogen-mediated inflammation is essential for host defense, unrestrained activation of leukocytes and lung tissue resident cells can lead to excess tissue injury (5) and in the case of pneumonia, subsequent hypoxemia. New insights are needed to provide new therapeutic approaches.
Aspiration pneumonia is one of the leading causes of pneumonia and ALI/ARDS (4). Originally described in women during labor and in perioperative patients (6, 7), aspiration occurs when gastric or oropharyngeal contents inadvertently gain access into the lower respiratory tract. Below a threshold pH, gastric acid causes airway injury and can predispose to bacterial pneumonia owing to transient disruption in mucosal host defense mechanisms (8–11).
No matter the cause, in most instances, pneumonia spontaneously resolves (12), suggesting the existence of endogenous, host-protective signaling pathways. Despite carefully detailing the remarkable histopathologic events that return the architecture of the lung from complete consolidation to a seemingly normal state (13), there is only a limited understanding of the mechanisms underlying this process of resolution from lung infection.
Eicosapentaenoic acid (EPA) and docosahexaenoic acid are ω-3 polyunsaturated fatty acids (PUFA) that are essential fatty acids with beneficial properties in a wide range of human inflammatory disorders (14). In ALI/ARDS, fish oil-based nutrition can significantly improve oxygenation and decrease the length of stay in an intensive care unit, time on mechanical ventilation, occurrence of new organ failure, and mortality (15–18). Recently, a novel lipid mediator was identified in resolving inflammatory exudates that was enzymatically derived from EPA and termed resolvin E1 (RvE1) (19). This mediator decreases polymorphonuclear neutrophil (PMN) migration, production of proinflammatory cytokines, and inflammation in several disease models, including periodontitis and allergic airway inflammation (19–25). Whereas RvE1 carries potent anti-inflammatory properties in sterile airway inflammation (25), its effect on lung infection has yet to be determined.
In this study, using a new experimental murine model of aspiration pneumonia, RvE1 displayed both anti-inflammatory and proresolving actions to decrease PMN infiltration and enhance microbial clearance from the lung.
Materials and Methods
Materials
RvE1 and EPA were obtained from Cayman Chemical (Ann Arbor, MI). Hydrochloric acid (0.1N hydrochloric acid [HCl],pH 1.0, endotoxin free)was purchased from Sigma-Aldrich (St. Louis, MO). Escherichia coli strain ATCC 19138 was obtained from the American Type Culture Collection (Manassas, VA). Trypticase soy agar plate with 5% sheep blood was obtained from BD Biosciences (Franklin Lakes, NJ).
Mice
Six-to-8-wk old male C57BL/6J mice were obtained from Charles River Laboratories Japan (Yokohama, Japan). Mice were given free access to water and standard rodent chow and were housed in pathogen-free cages. All animal experiments were approved by the Animal Care and Use Committees of Harvard Medical School and Keio University School of Medicine.
Model of acid-induced lung injury
Mice were anesthetized by an i.p. injection of ketamine (80 mg/kg)/xylazine (8 mg/kg). Mice received 100 ng RvE1 or the same volume (100 µl) of saline as a vehicle via tail vein injection, followed 30 min later by instillation of 25 µl HCl (pH 1.0) into the left lung as described previously (26).
Twelve hours after acid instillation, the mice were anesthetized using i.p. pentobarbital sodium (50 mg/kg) and euthanized. The trachea was exposed, and a 20-gauge angiocatheter was inserted into the trachea and secured. The lungs were lavaged with two separate 0.7-ml volumes of ice-cold PBS. The bronchoalveolar lavage (BAL) fluid was pooled, centrifuged at 400 × g for 10 min at 4°C to pellet the cell fraction and the supernatant was stored at −80° C until mediator analysis. The cell pellet was resuspended in cold saline, and total cell counts were determined using a hemacytometer. Differential cell counts were performed using cytocentrifuge smears stained with Diff-Quik (Sysmex, Kobe, Japan).
Development of a new murine model of aspiration pneumonia
Aspiration pneumonia was modeled by the sequential administration of HCl followed by enteric bacteria—namely, E. coli. HCl (25 µl, 0.1 N, pH 1.0) was instilled into the left lung as described (26), and followed 12, 24, or 48 h later by E. coli. After acid-induced ALI (vide supra), mice were reanesthetized and 12, 24, or 48 h later saline (0.9%) containing 1–2 × 105 CFU E. coli was instilled (25 µl) into the left lung. Some animals received PBS instead of HCl followed 12 h later by E. coli inoculation. The concentration of viable bacteria was determined by enumerating CFUs from serial dilutions grown on blood agar plates. Left lungs were collected 24 h after E. coli inoculation, homogenized in ice cold sterile water containing protease inhibitor mixture (Roche, Indianapolis, IN) and 0.4 mM PMSF, and serially diluted; aliquots were plated on blood agar. After incubation for 24 h at 37°C, colonies were counted and results expressed as CFU per lung. A bacterial growth index (BGI) was calculated as the ratio of lung CFU to the original inoculum instilled. Select animals were given RvE1 (100 ng), its parent fatty acid EPA (100 ng), or vehicle (0.9% saline) by tail vein 30 min before initiation of the aspiration pneumonia protocol. Left lungs were collected 24 h after E. coli inoculation, and BGI was calculated. Mediator levels in lung homogenates were determined at pivotal checkpoints in this model, namely at baseline, after acid injury (12 h) and after pneumonia (24 h later) (vide infra). To examine whether RvE1 improved the prognosis in this model, RvE1 (100 ng) or vehicle (0.9% saline) was administered i.v. 30 min before HCl instillation, or 2 h after E. coli inoculation. The survival rate was monitored for 1 wk after the initiation of aspiration pneumonia. To determine whether RvE1 had direct antibiotic actions, E. coli was plated on blood agar with 0, 0.1, 1, 10, 100 nM RvE1, incubated for 24 h at 37°C, and colonies were counted.
Measurements of inflammatory mediators and myeloperoxidase
Lung tissue homogenates were generated from lungs collected at baseline, 12 h after HCl instillation, or 24 h after E. coli inoculation by exposing murine lung to lysis buffer (0.5% Triton X-100, 150 mM NaCl, and 15 mM Tris-base) for 30 min at 4°C. After centrifugation, supernatants were collected for myeloperoxidase (MPO), cytokine, chemokine, and lipoxin A4 (LXA4) measurements. IL-1β, IL-6, keratinocyte-derived chemokine (KC), IL-10, MIP-1α, MIP-1β, MIP-2 and MCP-1 were determined using a multiplex cytokine bead array system (Bio-Plex; Bio-Rad, Hercules, CA). HMGB1 was measured using a monoclonal Ab to HMGB1 (Shino-Test, Tokyo, Japan). Sensitive and specific ELISAs were used in tandem to detect IL-17 (R&D Systems, Minneapolis, MN), IL-23 (eBiosciences, San Diego, CA), IFN-γ (R&D Systems), LXA4 (Oxford Biomedical Research, Oxford, MI), and MPO (Hycult Biotechnology, Uden, the Netherlands). LTB4, KC and 8-isoprostane were measured in bronchoalveolar lavage fluid (BALF) cell-free supernatants by individual ELISAs (Cayman Chemical, Minneapolis, MN; and R&D Systems).
NF-κB (p65) DNA-binding activity in the lung
For determination of NF-κB (p65) DNA-binding activity in the lung, left lungs were collected and homogenized 6 h after E. coli inoculation. Nuclear protein was extracted using CelLytic NuCLEAR Extraction Kit (Sigma-Aldrich) according to the manufacturer’s protocol. The protein concentration of the nuclear extracts was determined using a BCA Protein Assay Kit (Pierce, Rockford, IL). NF-κB (p65) DNA-binding activity was examined using the TransAM ELISA kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. In brief, 10 µg of nuclear protein was subjected to the binding of NF-κB to an immobilized consensus sequence (5′-GGGACTTTCC-3′) in a 96-well plate, and the primary and secondary Abs were added. After the colorimetric reaction, the samples’ absorbance was measured in a spectrophotometer at 450 nm with a reference wavelength of 655 nm. Recombinant NF-κB p65 (active motif) was used as a protein standard. The DNA binding specificity was assessed using wild-type and mutated oligonucleotides.
Mass spectrometry
Lipids present in lung homogenates were extracted with methanol as described (27). The methanolic extract was then diluted with 10 volumes of water, acidified with HCl to a pH of 3.5, and applied to Sep-Pak C18 cartridges(Waters, Milford, MA)for solid phase extraction. To correct for losses during sample preparation, deuterated internal standard (1 ng of LTB4-6, 7, 14, 15-d4;Biomol) was added to the murine lung homogenates before extraction. For liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis, a triple quadruple linear ion trap mass spectrometer (4000Q-TRAP; Applied Biosystems, Foster City, CA) equipped with an Acquity ultraperformance liquid chromatography bridged ethyl hybrid C18 column (1.7 µm, 1.0 × 150 mm; Waters) was used. LC-MS/MS analyses were conducted in negative ion mode, and eicosanoids were identified and quantified by multiple reaction monitoring using transitions for RvE1 (349 > 195 m/z), 18-hydroxy-eicosapentaenoic acid (18-HEPE) (317 > 215 m/z) and EPA (301 > 257 m/z). Calibration curves (1–1000 pg) and liquid chromatography retention times for each compound were established with synthetic standards. In some animals, left lungs were collected 5 min after i.v. injection of 100 ng RvE1, and the lung tissue levels of RvE1 were determined by LC-MS/MS analysis.
Histopathologic analysis
Lung tissue was fixed by inflation with 4% paraformaldehyde at a transpulmonary pressure of 25 cm H2O and embedded in paraffin. For histologic analysis, lungs were collected 24 h after E. coli inoculation, and paraffin-embedded 5-µm sections of lungs were cut and stained with H&E for light microscopy. To detect phosphorylated NF-κB (p65), lungs were collected 6 h after E. coli inoculation and immunohistochemical analysis was performed according to the manufacturer’s protocol. Paraffin-embedded sections were dehydrated and pretreated with citrate buffer to expose antigenic epitopes. After blocking endogenous peroxidase activity, sections were incubated with a rabbit polyclonal anti-phospho-NF-κB p65 (Ser276) Ab (Cell Signaling, Danvers,MA) for 60 min at room temperature followed by a biotinylated secondary Ab. Biotinylated immune complexes were visualized using a streptavidin-based detection kit. Sections were counter-stained with hematoxylin.
Statistical analysis
All data are expressed as mean ± SEM. Comparisons between groups were conducted using ANOVA and Student t test as appropriate. Survival curves after E. coli inoculation were estimated using the Kaplan-Meier method and compared using the log-rank test. A level of p < 0.05 was considered to indicate statistical significance. Statistics were performed using Graphpad Prism 4.0 for Windows (San Diego, CA).
Results
Acid injury transiently impairs airway host defense
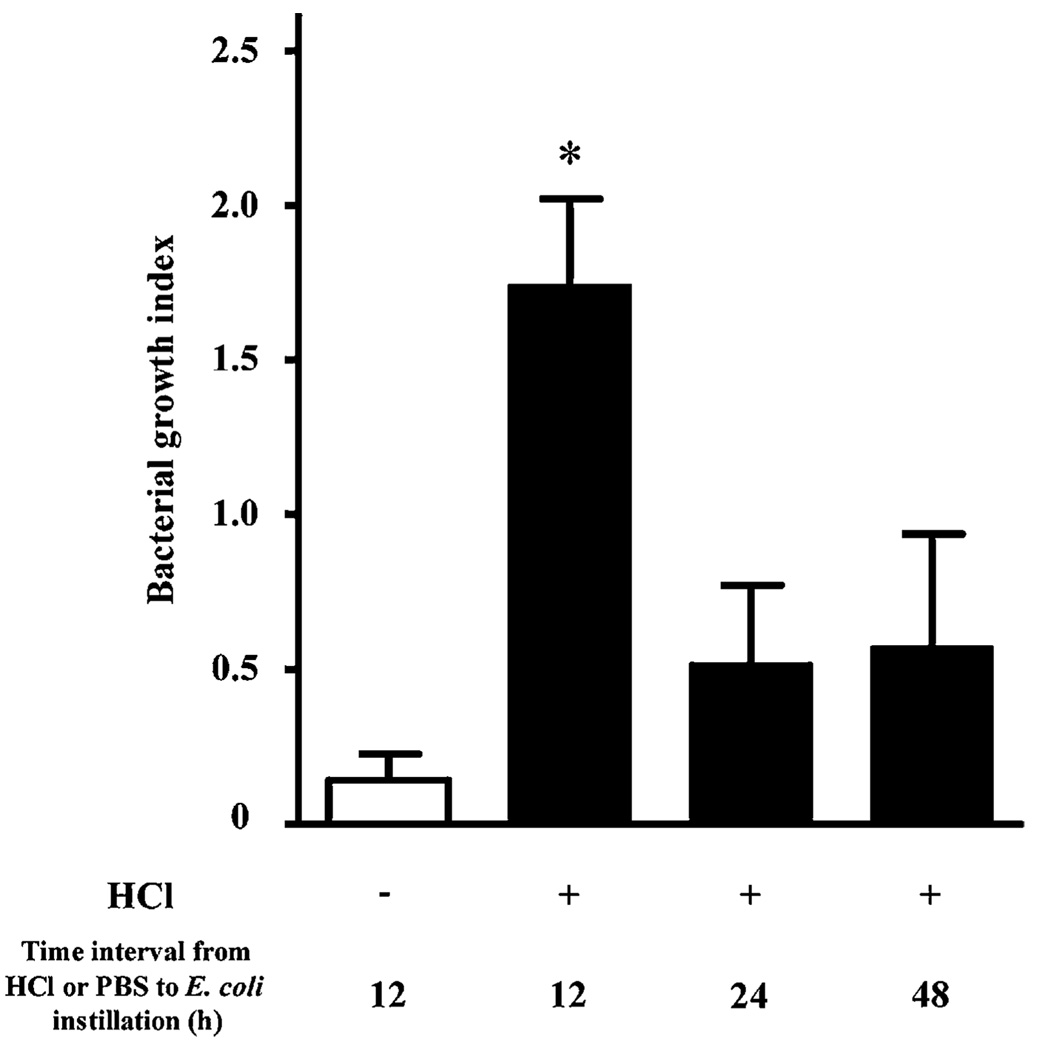
In order to model aspiration pneumonia and more severe forms of ALI, acid (0.1 N HCl, pH 1.0) was administered by intratracheal instillation into the left mainstem bronchus and 12, 24, or 48 h later the acid-injured mice were exposed to the enteric pathogen E. coli by intratracheal instillation into the same airway (see Materials and Methods). To facilitate investigation of host defense mechanisms for spontaneous pneumonia resolution, the left lung was chosen for selective injury and infection, because it is smaller than the murine right lung. The size of the inoculum of E. coli was optimized so that uninjured animals would spontaneously resolve the pneumonia. The left lung was harvested 24 h after E. coli instillation, and a BGI was determined by CFU assay (viable bacteria in lung homogenate/inoculum). In mice receiving PBS (pH 7.4) followed by 1–2 × 105 E. coli, the lung’s BGI 24 h later was 0.14 ± 0.08, indicating that >80% of the bacteria were spontaneously eliminated from lungs within 24 h after inoculation (Fig. 1). Acid-injured animals had significantly more difficulty clearing the bacterial challenge. Despite the presence of increased numbers of PMNs in the airway 12 h after acid injury (26), the BGI was >1.5 [1.74 ± 0.28], which was consistent with a failure to effectively clear E. coli at this time point. In contrast, the BGI was ~0.5 when bacteria were introduced 24 h or longer after acid (Fig. 1). These results provide a time course for acid mediated disruption of airway mucosal host defense mechanisms that required >12 h to restore the capacity to clear subsequent bacterial challenge.
FIGURE 1.
Acid injury transiently disrupts airway host defense to bacterial challenge. Mice received an inoculum of E. coli (1–2 × 105 CFU in 25 µl) into the left lung 12, 24, or 48 h after instillation of HCl (0.1 N, pH 1.0, 25 µl) and a BGI was determined with lung homogenates. Some animals received PBS (−) instead of HCl (+) followed 12 h later by E. coli inoculation. BGI was calculated as the ratio of lung CFU to the original inoculum. *p < 0.05 versus PBS (−) animals. Values represent the mean ± SEM (n > 4).
RvE1 enhances bacterial clearance in a model of aspiration pneumonia
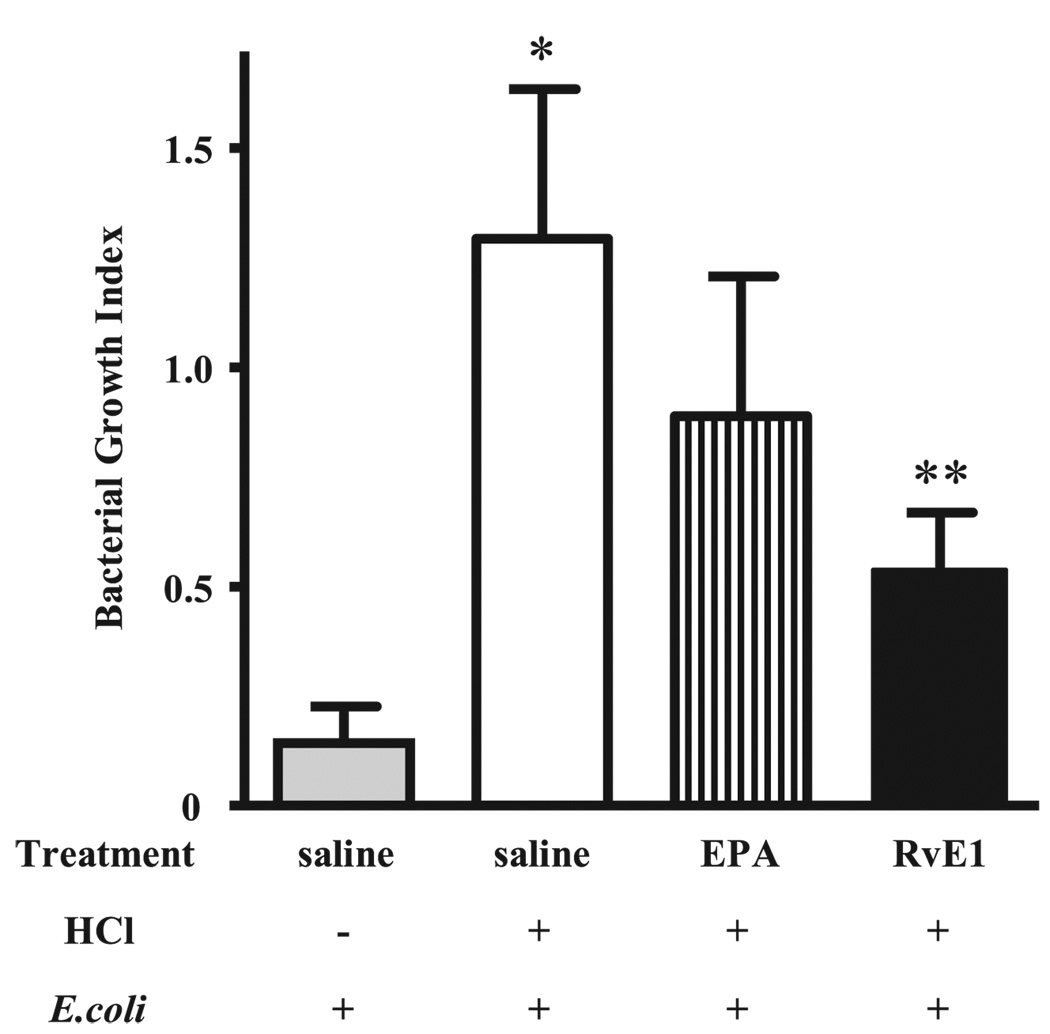
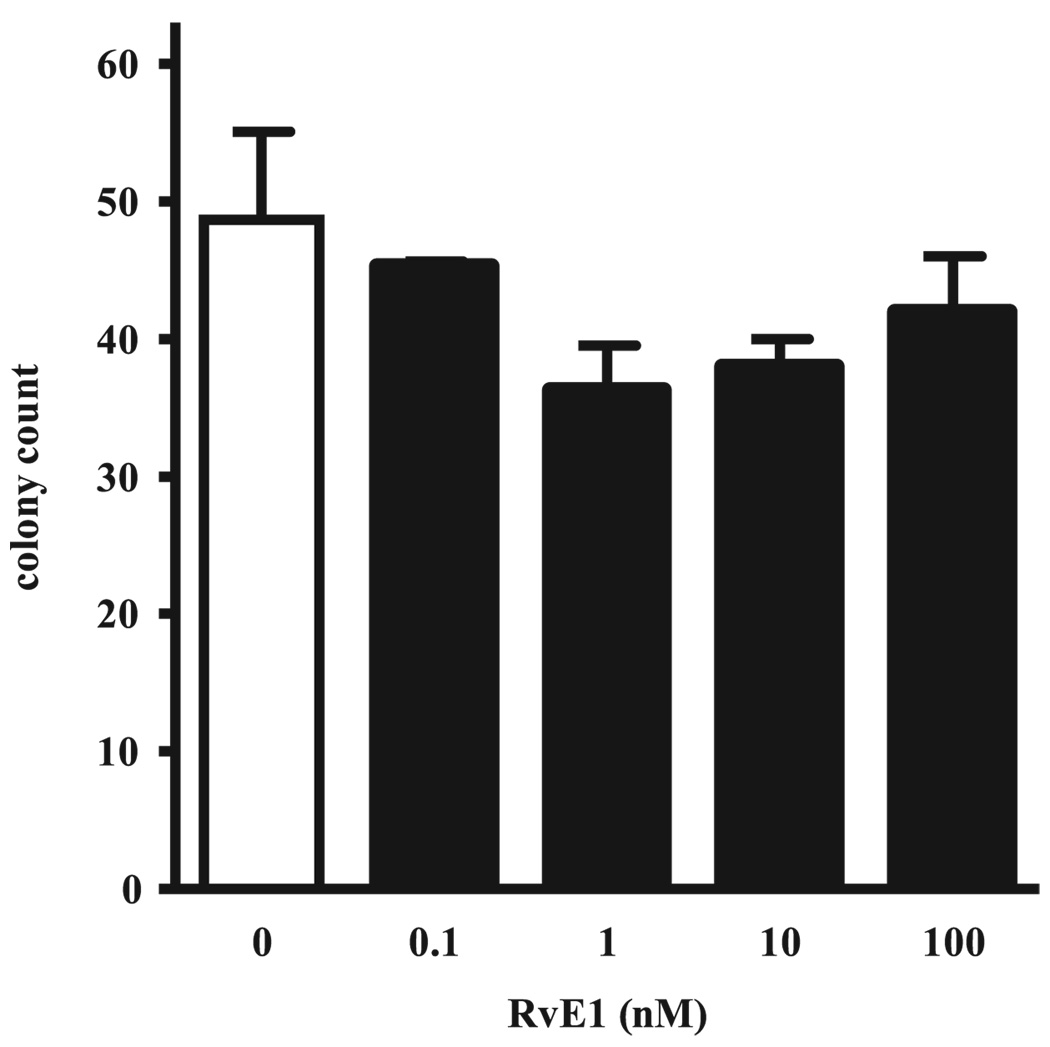
In view of potent anti-inflammatory actions of RvE1 for PMNs (20), we next determined the effect of RvE1 on host defense in this model of aspiration pneumonia. RvE1 is enzymatically derived from the ω-3 fatty acid EPA, so one dose of RvE1 (100 ng, ~0.005 mg/kg), its parent fatty acid EPA (100 ng), or vehicle (0.9% saline) was given i.v. (100 µl) 30 min before acid injury, and 1–2 × 105 E. coli were administered by intratracheal instillation into the left lung 12 h later (see Materials and Methods). Lungs were harvested 24 h after E. coli instillation and CFUs were determined. The BGI in lungs from this cohort of animals receiving HCl before bacteria increased from 0.14 ± 0.08 to 1.29 ± 0.33 (p < 0.05; Fig. 2). Of interest, the BGI in animals treated with EPA decreased below 1.0, indicating that EPA enhanced clearance of E. coli from the lung. With RvE1, animals displayed a marked decrease in lung homogenate BGI to 0.54 ± 0.13 (p < 0.05) that was superior to EPA and consistent with a significant improvement in host defense. To determine whether this route of administration resulted in detectable levels of RvE1 in lung tissue, murine lungs were collected 5 min after i.v. injection of RvE1, and lipid extracts were prepared for analysis by LC-MS/MS. After i.v. administration, RvE1-treated mice had 35.7 ± 15.7 pg RvE1 per lung (n = 5). For control animals receiving only vehicle (0.9% saline), quantities of RvE1 in lung tissues were below the limits of detection (5 pg) by mass spectrometry. Although RvE1 was not detectable in lung tissues 24 h after induction of aspiration pneumonia, the RvE1 biosynthetic precursors EPA and 18-HEPE were present at this time point (1834 ± 450 pg EPA per lung and 22 ± 9 pg 18-HEPE per lung, respectively; n = 3). RvE1 did not directly kill or limit the growth of E. coli when coincubated with the bacteria in vitro for 24 h at 37°C (Fig. 3)
FIGURE 2.
RvE1 enhances bacterial clearance from the lung after acid injury. Mice were given 0.9% saline, EPA (100 ng), or RvE1 (100 ng) i.v. followed 30 min later by intratracheal instillation of acid (+) or PBS (−) into the left lung. Twelve hours after HCl instillation, mice were next given 1–2 × 105 CFU of E. coli into the left lung. After 24 h, the left lungs were collected and homogenized, and a BGI was calculated. *, p < 0.05 for saline/HCl/E. coli versus saline/PBS/E. coli; **p < 0.05 for RvE1/HCl/E. coli versus saline/HCl/E. coli. Values are the mean ± SEM (n > 12).
FIGURE 3.
RvE1 does not directly impair the growth of E. coli in vitro. E. coli was plated on blood-agar with 0, 0.1, 1, 10, 100 nM RvE1, incubated for 24 h at 37°C, and colonies were counted. Values are the mean ± SEM (n = 3).
RvE1 blocks leukocyte recruitment to the lung in aspiration pneumonia
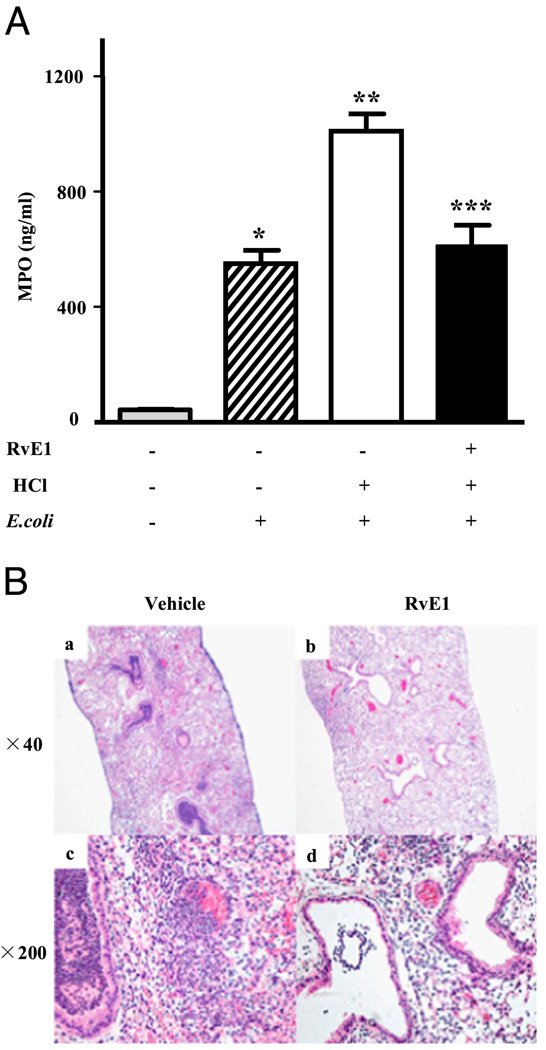
To assess PMN accumulation in lung tissue, MPO levels were determined. E. coli instillation into the left mainstem bronchus increased lung MPO levels from 44.0 ± 1.7 ng/ml (saline control) to 550.2 ± 44.6 ng/ml (p < 0.05; Fig. 4A). Acid injury 12 h before E. coli infection further increased lung tissue MPO to 1010.0 ± 59.6 ng/ml (p < 0.05), which represented an ~2-fold increase compared with lungs from animals receiving E. coli alone. RvE1 given before acid injury dramatically decreased MPO levels. This biochemical evidence for RvE1-mediated decreases in PMN infiltration was confirmed by histologic examination of lung tissue (Fig. 4B). The numbers of PMNs in large and small airways were markedly decreased with RvE1, and decrements in interstitial edema formation in the presence of RvE1 were noted (Fig. 4B).
FIGURE 4.
RvE1 blocks leukocyte accumulation after aspiration pneumonia. A, MPO levels in the supernatant of lung homogenates. Left lungs were harvested and homogenized 24 h after E. coli inoculation. MPO levels were determined by ELISA. *p < 0.05 versus vehicle/PBS/saline, **p < 0.05 versus vehicle/PBS/E. coli and ***p < 0.05 versus vehicle/HCl/E. coli. Values are the mean ± SEM (n > 12). B, H&E-stained sections of lung tissue obtained from mice treated with vehicle or RvE1 24 h after airway E. coli inoculation. Original magnification, ×40 (A and B) and ×200 (C and D).
RvE1 decreases proinflammatory mediators in aspiration pneumonia
To investigate potential anti-inflammatory and proresolving mechanisms for RvE1 in experimental aspiration pneumonia, the levels of several inflammatory mediators that have been assigned important roles in the pathogenesis of ALI/ARDS (28) were determined in the supernatants of lung homogenates. Administering HCl alone led only to a modest increase in select proinflammatory mediators in the lung (Table I). Alternatively, administration of E. coli alone markedly increased IL-1β, MIP-1α, MIP-1β, KC, MIP-2, and MCP-1 (Table I). Levels of the anti-inflammatory mediator IL-10 were decreased (Table I). This broad proinflammatory response in the lung to E. coli was distinct from acid injury alone. The new experimental model of aspiration pneumonia with both acid injury and bacterial instillation induced significant additional increments in the production of several proinflammatory mediators (Table I). RvE1 markedly decreased IL-1β, IL-6, HMGB-1, MIP-1α, MIP-1β, KC, and MCP-1 without evident increases in IL-10 (Table I). Of note, levels of the counter-regulatory lipid mediator LXA4 were increased in the setting of aspiration pneumonia and not further increased with RvE1. These findings indicate that RvE1 dampens the inflammatory responses to microbial invasion of the lower respiratory tract and promotes resolution of aspiration pneumonia via mechanisms that are independent from the anti-inflammatory IL-10 or LXA4 signaling pathways.
Table I.
RvE1 regulates mediator generation by lung tissue after aspiration pneumonia
| Mediator | Control | Acid Injury | Pneumonia | Aspiration Pneumonia | |||
|---|---|---|---|---|---|---|---|
| RvE1 | − | + | − | + | − | − | + |
| HC1 | − | − | + | + | − | + | + |
| E. coli | − | − | − | − | + | + | + |
| Cytokines | |||||||
| IL-1β | 0.529 ± 0.030 | 0.452 ± 0.017 | 0.281 ± 0.018 | 0.250 ± 0.012 | 1.355 ± 0.217† | 3.998 ± 0.919‡ | 1.752 ± 0.240§ |
| IL-6 | 0.266 ± 0.020 | 0.233 ± 0.008 | 0.356 ± 0.038 | 0.227 ± 0.011 | 0.353 ± 0.067 | 2.502 ± 0.481‡ | 0.933 ± 0.241§ |
| IL-10 | 0.109 ± 0.012 | 0.087 ± 0.005 | 0.046 ± 0.003 | 0.046 ± 0.003* | 0.053 ± 0.004† | 0.065 ± 0.005 | 0.056 ± 0.004 |
| IL-17 | 0.054 ± 0.006 | 0.057 ± 0.004 | 0.068 ± 0.009 | 0.086 ± 0.003 | 0.073 ± 0.009 | 0.069 ± 0.009 | 0.064 ± 0.021 |
| IL-23 | 4.487 ± 0.787 | 3.673 ± 0.318 | 2.596 ± 0.058 | 2.316 ± 0.196* | 3.673 ± 0.318 | 2.216 ± 0.229 | 1.452 ± 0.156 |
| IFN-γ | 0.079 ± 0.008 | 0.086 ± 0.018 | 0.067 ± 0.006 | 0.092 ± 0.007* | 0.126 ± 0.018 | 0.094 ± 0.010 | 0.079 ± 0.007 |
| HMGB-1 | 5.259 ± 0.363 | 4.270 ± 0.291 | 4.665 ± 0.335 | 4.605 ± 0.298 | 6.412 ± 0.400 | 8.395 ± 0.531‡ | 6.365 ± 0.772§ |
| Chemokines | |||||||
| MIP-1α | ND | ND | 0.004 ± 0.006 | ND | 2.234 ± 0.892 | 6.308 ± 1.057‡ | 3.015 ± 0.502§ |
| MIP-1β | 0.403 ± 0.020 | 0.333 ± 0.007 | 0.211 ± 0.006 | 0.190 ± 0.011 | 1.020 ± 0.165† | 5.223 ± 0.762‡ | 2.229 ± 0.282§ |
| KC | 0.020 ± 0.003 | 0.024 ± 0.012 | 0.447 ± 0.103 | 0.259 ± 0.037 | 1.613 ± 0.216† | 7.810 ± 2.319 | 0.259 ± 0.037 |
| MIP-2 | 0.021 ± 0.000 | 0.027 ± 0.002 | 0.211 ± 0.048 | 0.093 ± 0.010 | 3.364 ± 0.468† | 6.636 ± 1.332‡ | 4.531 ± 0.895 |
| MCP-1 | 0.322 ± 0.010 | 0.299 ± 0.018 | 0.422 ± 0.061 | 0.335 ± 0.027* | 1.112 ± 0.267† | 9.092 ± 1.520‡ | 4.128 ± 0.484§ |
| Lipid mediator | |||||||
| LXA4 | 0.843 ± 0.067 | 0.450 ± 0.036 | 0.639 ± 0.072 | 0.661 ± 0.070 | 0.796 ± 0.124 | 3.054 ± 0.454 | 2.907 ± 0.773 |
Values are in ng/lung (mg/lung for HMGB1) and represent the mean ± SEM (n = 9–13).
p < 0.05 for acid injury-RvE1 (+) versus control;
p < 0.05 for pneumonia versus control;
p < 0.05 for aspiration pneumonia-RvE1 (−) versus control and acid injury-RvE1 (−);
p < 0.05 for aspiration pneumonia-RvE1 (+) versus aspiration pneumonia-RvE1 (−).
RvE1 inhibited the translocation and activation of NF-κB (p65) induced by aspiration pneumonia
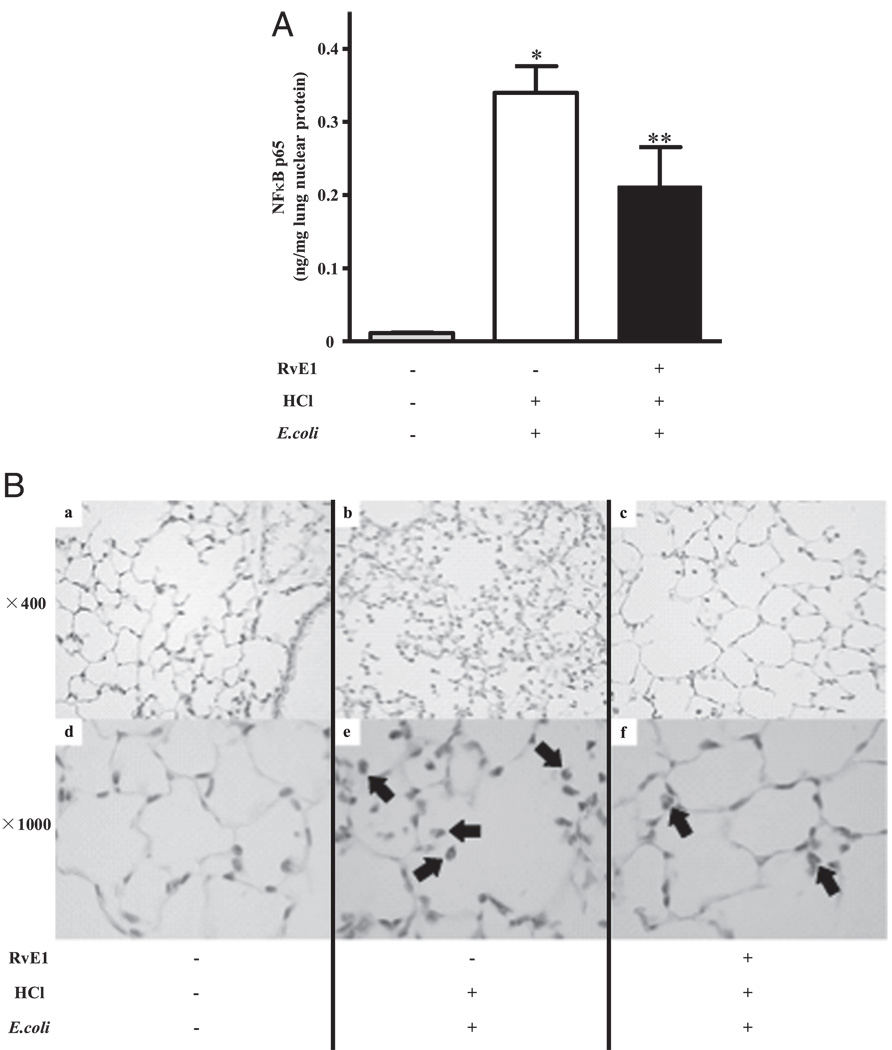
The pathophysiology of acid-initiated lung injury is linked to increased oxidative stress and NF-κB activation (29), so we next determined the effect of RvE1 on these host responses. Levels of the sensitive oxidative stress biomarker 8-isoprostane were significantly increased in BALF after acid injury (25.8 ± 5.0 versus 11.0 ± 0.1 pg/ml with PBS, mean ± SEM for n ≥ 3; p < 0.05), but there was no significant decrease in RvE1-treated animals (38.8 ± 7.7 pg 8-isoprostane/ml). To elucidate the mechanism for the anti-inflammatory effect of RvE1, the translocation of NF-κB (p65) into the nucleus was determined. NF-κB (p65) was almost undetectable in noninjured lungs (0.01 ± 0.001 ng/µg nuclear protein; Fig. 5A). HCl alone did not induce significant translocation of NF-κB (0.01 ± 0.007 ng/µg nuclear protein); however, aspiration pneumonia induced marked translocation of NF-κB (p65) into the nucleus (0.34 ± 0.04 ng/µg nuclear protein). RvE1 inhibited this translocation by ~40% (p < 0.05; n = 6 each). Immunohistochemical analysis showed positive staining for phosphorylated NF-κB p65 predominantly in PMNs (Fig. 5B).
FIGURE 5.
RvE1 inhibited the activation of NF-κB p65. Left lung was harvested 6 h after E. coli inoculation. A, Left lung was homogenized and nuclear protein was extracted. Translocation of NF-κB p65 into the nucleus was determined using ELISA. *p < 0.05 versus vehicle/HCl (−)/E. coli (−); **p < 0.05 versus vehicle /HCl (+)/E. coli (+). B, Immunohistochemical analysis to detect phosphorylated NF-κB p65 was performed using a rabbit polyclonal anti-phospho-NF-κB p65 (Ser276) Ab. Arrows show positive staining of NF-κB. Original magnification, ×400 (A–C) or ×1000 (D–F).
RvE1 blocks PMN recruitment into acid-injured lung
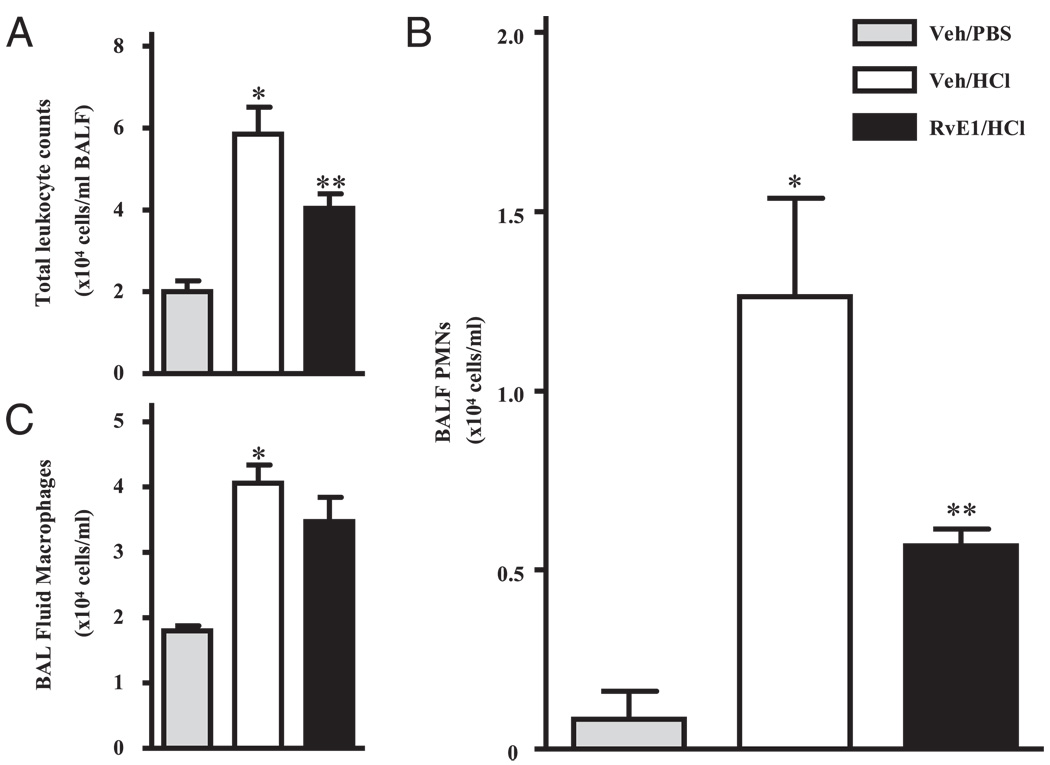
To determine whether RvE1 directly regulated leukocyte recruitment in response to ALI, animals were given either RvE1 (100 ng) or vehicle (0.9% saline) i.v, followed 30 min later by either sterile PBS (pH 7.4) or HCl (0.1 N; pH 1.0) instilled into the left mainstem bronchus (see Materials and Methods). Within 12 h, acid initiated significant inflammation (Fig. 6A–C). Total leukocyte count in BAL fluids from animals given PBS was 2.00 ± 0.26 × 104/ml (mean ± SEM) with >90% macrophages and only 0.08 ± 0.08 × 104 PMNs/ml (Fig. 6A–C). After acid instillation, the number of PMNs and macrophages in BAL fluids significantly increased to 1.26 ± 0.27 × 104/ml and 4.05 ± 0.28 × 104/ml, respectively (p < 0.05). RvE1 (100 ng) markedly blocked PMN infiltration by 55% compared with vehicle treated animals (Fig. 6B). RvE1 did not significantly affect the number of BALF macrophages (Fig. 6C). To determine whether RvE1 decreased PMN numbers by indirect means, concentrations of the potent PMN chemoattractants LTB4 and KC were measured in BAL fluids. Intrapulmonary acid instillation induced a substantial increase in both LTB4 and KC (Table II), but the levels of these mediators in BALF were not significantly altered by RvE1, suggesting a direct action for RvE1 on PMNs.
FIGURE 6.
RvE1 blocks acid-induced PMN recruitment into the lung. Mice received vehicle (Veh; 0.9% saline) or RvE1 (100 ng) i.v. followed 30 min later by intratracheal instillation of PBS (pH 7.4) or HCl (pH 1.0) into the left lung. After 12 h, BALF was collected. A, Total leukocytes in BAL fluids were enumerated, and the number of (B) PMNs and (C) macrophages were determined. *p < 0.01 versus vehicle/PBS; **p < 0.05 versus vehicle/HCl. Values are mean ± SEM (n = 3–4).
Table II.
Concentrations of neutrophil chemoattractants in BAL
| Mediator | Veh + PBS | Veh + HC1 | RvE1 + HC1 |
|---|---|---|---|
| LTB4 | 5.3 ± 0.9 | 86.3 ± 10.7* | 97.8 ± 14.2 |
| KC | 5.0 ± 1.4 | 33.2 ± 7.4* | 33.2 ± 5.7 |
Values are in pg/ml and represent the mean ± SEM (n = 3–4).
p < 0.05 versus veh + PBS cohort.
Veh, vehicle.
RvE1 improved survival after aspiration pneumonia
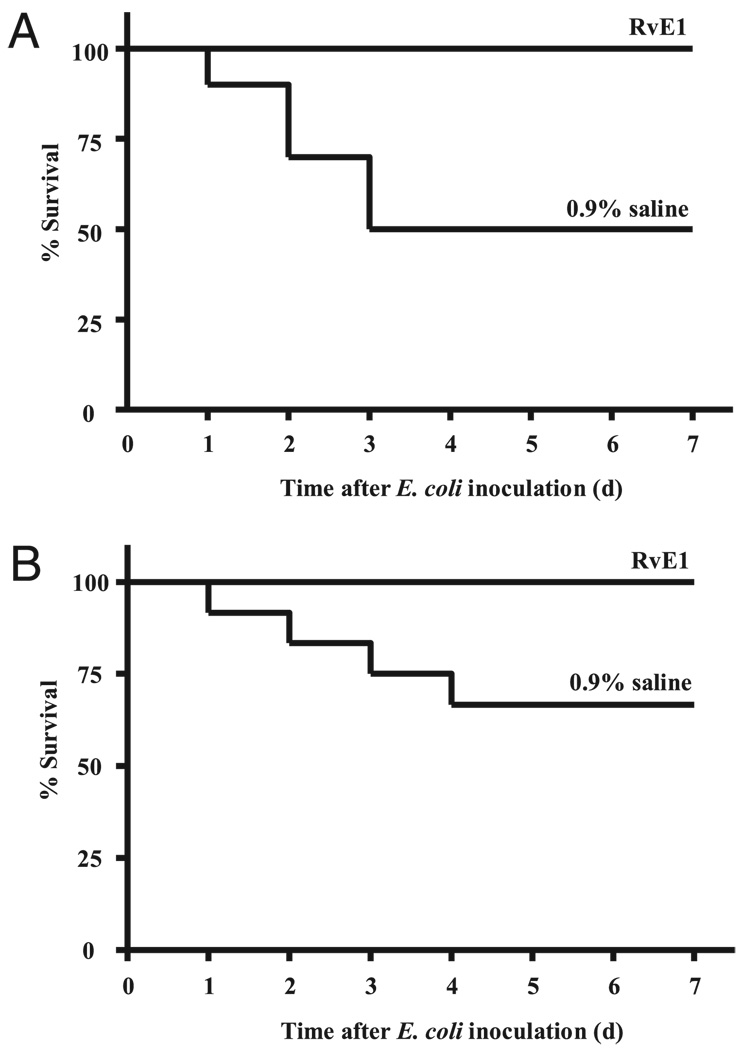
Because aspiration pneumonia can lead to increased mortality from ALI/ARDS or sepsis (30), the animals’ survival was closely monitored after experimental aspiration pneumonia in the presence or absence of RvE1. Despite introduction of the acid and bacteria to only the left lung, induction of aspiration pneumonia resulted in an early mortality rate of 50% within 3 d (Fig. 7A). In sharp contrast, all of the animals treated with RvE1 (100 ng, i.v.) before aspiration pneumonia survived during the study period. Of note, RvE1 significantly improved survival rate even when administered 2 h after E. coli inoculation (Fig. 7B). Because the survival rate with both vehicle and RvE1 was 100% during the first 24 h, potential early differences between RvE1 and vehicle cohorts could not be detected. Therefore, in a separate set of experiments, we increased the dose of E. coli instilled by 2 logs to 1 × 107 CFU. At this higher dose, the survival advantage with RvE1 (100 ng) was no longer evident, because all animals died within the first 24 h, suggesting that the protective mechanisms of RvE1 are more operative during the resolution of this infectious challenge, rather than the initial, acute responses to microbial invasion. No complications of RvE1 were observed during the 7-d period of observation.
FIGURE 7.
RvE1 improves the survival rate after aspiration pneumonia. Animals were treated with a single dose of RvE1 (100 ng) or vehicle (0.9% saline) i.v. A, Thirty minutes before initiation of ALI and aspiration pneumonia or (B) 2 h after E. coli inoculation. Mice were observed every 24 h for 1 wk. The percent survival was determined for each cohort of 10 mice on a daily basis.
Discussion
Aspiration of gastric acid is a frequent clinical event and can lead to both increased susceptibility to pneumonia and ALI/ARDS (4, 30). In this study, the EPA-derived lipid mediator RvE1 both dampened acid-initiated ALI and enhanced pneumonia host defense in mice. Despite decreasing lung PMN infiltration after ALI, RvE1 promoted the clearance of E. coli pneumonia, decreased proinflammatory cytokines and chemokines, and enhanced survival from the microbial challenge. The anti-inflammatory and protective actions of this endogenous lipid mediator for bacterial pneumonia distinguish it from immunosuppressive agents that increase, rather than decrease, the risk of infection.
Enteric feeding with EPA can markedly reduce ALI/ARDS morbidity and mortality (15), in part via marked decreases in lung PMNs (16). In addition, increased fish intake by subjects in the Physicians’ Health Study is associated with a lower risk of pneumonia (31), and murine diets rich in ω-3 fatty acids decrease the severity of experimental bacterial pneumonia (32, 33). The potential for lung protection by EPA-derived mediators is underscored by fat-1 transgenic mice that are protected from ALI (34). These transgenic mice express the Caenorhabditis elegans ω-3 desaturase fat-1 gene and can endogenously generate ω-3 PUFAs from ω-6 PUFAs (35). Mechanisms for the actions of EPA to decrease the severity of ALI in these human and animal experimental systems have not been previously established. In this study, EPA dampened the severity of bacterial pneumonia when it was directly infused i.v.; however, the EPA-derived lipid mediator RvE1 displayed even more potent protective actions for pneumonia. Tissue from fat-1 mice or nontransgenic mice that were fed diets enriched with EPA have increased EPA availability and generate RvE1 in detectable amounts upon provocative challenge (36, 37). In results presented in this study, RvE1 was provided by i.v. administration, which led to detectable picogram quantities in lung tissues. The presence of these pharmacologic properties emphasizes the potent bioactivity of this natural compound. Moreover, it is notable that the pharmacologically active dose of RvE1 administered i.v. was 100 ng per mouse, or ~0.005 mg/kg, providing compelling evidence of this compound’s potent anti-inflammatory and proresolving actions. Thus, even if only present in low amounts in lung tissues, enzymatic conversion of EPA to RvE1 would serve to limit overexuberant tissue responses to injury or infection and could be responsible for some of the observed beneficial properties of EPA in ALI/ARDS and pneumonia.
Acid aspiration evokes inflammatory responses by injured mucosal epithelial cells and activated PMNs. Excessive or inappropriate PMN activation can cause bystander tissue damage and contribute to the pathogenesis of ALI/ARDS (38). Inhibition of PMN function in animal studies attenuates lung injury induced by acid aspiration (39, 40). In this study, intratracheal acid instillation induced PMN recruitment that was blocked by i.v. RvE1. LTB4 and KC (murine functional analog of human IL-8) are potent PMN chemoattractants and activators (39, 41). Both LTB4 and KC increased in BALF after ALI and remained elevated to the same magnitude with RvE1, despite decreases in PMN accumulation. There are several distinct possible explanations for these interesting findings, including an intravascular rather than an airway target for the i.v. RvE1 and direct regulatory actions for RvE1 on PMNs that are downstream from LTB4 and KC receptor signaling. Cell type-specific actions for RvE1 have been linked to distinct patterns of receptor expression. RvE1 can interact with the LTB4 receptor BLT1 as a receptor-level antagonist and partial agonist to dampen PMN migration and activation at sites of inflammation (42). With similar levels of airway LTB4 after aspiration pneumonia, this receptor level antagonism for RvE1 at BLT1 would serve to functionally inhibit LTB4-mediated activation of PMNs. RvE1 can also interact with ChemR23 on macrophages to promote the clearance of apoptotic PMNs and microbial debris (20, 43), and ChemR23-deficient mice display a proinflammatory phenotype (44). In addition, RvE1 interacts with ChemR23 on mucosal epithelial cells to promote clearance of PMNs from apical surfaces in a CD55-dependent manner (45), and RvE1 prevents destruction of periodontal tissues in experimental periodontitis (24). These findings of direct actions for RvE1 on leukocytes and mucosal epithelial cells to regulate their function are in accordance with results presented in this study that RvE1 inhibited PMN, but not macrophage accumulation in the lung during ALI, and protected the lung from aspiration pneumonia by increased clearance of E. coli infection.
Lipid mediators interact with peptide mediators of inflammation in complex regulatory signaling networks (46). RvE1 is a potent regulator of TNFα and NF-κB signaling (20). In our model of aspiration pneumonia, RvE1 decreased production of select proinflammatory mediators, including IL-1β, IL-6, MIP-1α, MIP-1β, KC, and MCP-1. Of interest, data from clinical trials in the early phase of ARDS have identified significantly higher BALF levels of IL-1β and IL-6 in nonsurvivors (47). These cytokines can bind to receptors on the surface of gram-negative bacteria to favor growth of the bacteria (48, 49). Although RvE1 did not carry direct antimicrobial actions, it enhanced microbial clearance of a modest dose of E. coli from the lung and survival, perhaps in part by regulating levels of these cytokines. MCP-1 and IL-8 are also present in BALF from patients at risk and with established ARDS (50, 51). In murine tissues, increased MCP-1 leads to PMN accumulation and distant organ damage by increasing the IL-8 homolog KC, which is a direct and potent PMN agonist (52). Of interest, HMGB-1 is an abundant nuclear protein that can serve as a cytokine when released into the extracellular milieu to play important roles in sepsis and ALI (53). In this study, RvE1 decreased levels of HMGB-1, likely also contributing to its improvement in bacterial clearance and survival.
In summary, early addition of the EPA-derived mediator RvE1 “jump started” resolution to dampen PMN recruitment in acid-induced ALI and promote host defense to bacterial challenge in a new model of aspiration pneumonia. The combination of anti-inflammatory and anti-infective actions for RvE1 define it as a proresolving mediator and distinguish it from immunosuppressive agents that block inflammation but predispose the host to an increased risk of infection. RvE1 regulated PMN accumulation and a broad array of proinflammatory cytokines and chemokines, suggesting agonist properties for RvE1 at cell type specific receptors. These results with RvE1 support the notion that understanding host responses that promote the resolution of pathogen-mediated inflammation may provide insights into natural counter-regulatory mechanisms that can be leveraged into new therapeutic strategies to improve host defense and lessen the severity of pneumonia.
Acknowledgments
This work was supported by National Institutes of Health Grants HL68669 and P50-DE016191 (to B.D.L.), Grant-in-Aid for Young Scientists (B) by the Ministry Of Education, Culture, Sports, Science and Technology (to K.F.), Precursory Research for Embryonic Science and Technology by Japan Science and Technology Agency (to M.A.), and Core Research for Evolutional Science and Technology by Japan Science and Technology Agency (to H.A.).
Abbreviations used in this paper
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- BGI
bacterial growth index
- EPA
eicosapentaenoic acid
- 18-HEPE
18-hydroxy-eicosapentaenoic acid
- HCl
hydrochloric acid
- KC
keratinocyte-derived chemokine
- LXA4
lipoxin A4
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- MPO
myeloperoxidase
- PUFA
polyunsaturated fatty acids
- PMN
polymorphonuclear neutrophil
- RvE1
resolvin E1
- Veh
vehicle
Footnotes
Disclosures
B. D. L. is a coinventor on a pending patent on RvE1 in airway diseases that is owned by Brigham and Women’s Hospital, has been licensed for clinical development, and is the subject of a consultancy agreement.
References
- 1.Mizgerd JP. Lung infection—a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott JA, Brooks WA, Peiris JS, Holtzman D, Mulhollan EK. Pneumonia research to reduce childhood mortality in the developing world. J. Clin. Invest. 2008;118:1291–1300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Weiss SJ. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 6.Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am. J. Obstet. Gynecol. 1946;52:191–205. doi: 10.1016/s0002-9378(16)39829-5. [DOI] [PubMed] [Google Scholar]
- 7.Vandam LD. Aspiration of gastric contents in the operative period. N. Engl. J. Med. 1965;273:1206–1208. doi: 10.1056/NEJM196511252732207. [DOI] [PubMed] [Google Scholar]
- 8.Ishizuka S, Yamaya M, Suzuki T, Nakayama K, Kamanaka M, Ida S, Sekizawa K, Sasaki H. Acid exposure stimulates the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells: effects on platelet-activating factor receptor expression. Am. J. Respir. Cell Mol. Biol. 2001;24:459–468. doi: 10.1165/ajrcmb.24.4.4248. [DOI] [PubMed] [Google Scholar]
- 9.Mitsushima H, Oishi K, Nagao T, Ichinose A, Senba M, Iwasaki T, Nagatake T. Acid aspiration induces bacterial pneumonia by enhanced bacterial adherence in mice. Microb. Pathog. 2002;33:203–210. doi: 10.1006/mpat.2002.0529. [DOI] [PubMed] [Google Scholar]
- 10.Rotta AT, Shiley KT, Davidson BA, Helinski JD, Russo TA, Knight PR. Gastric acid and particulate aspiration injury inhibits pulmonary bacterial clearance. Crit. Care Med. 2004;32:747–754. doi: 10.1097/01.ccm.0000114577.10352.46. [DOI] [PubMed] [Google Scholar]
- 11.van Westerloo DJ, Knapp S, van’t Veer C, Buurman WA, de Vos AF, Florquin S, van der Poll T. Aspiration pneumonitis primes the host for an exaggerated inflammatory response during pneumonia. Crit. Care Med. 2005;33:1770–1778. doi: 10.1097/01.ccm.0000172277.41033.f0. [DOI] [PubMed] [Google Scholar]
- 12.Mizgerd JP. Acute lower respiratory tract infection. N. Engl. J. Med. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farver CF. Bacterial Diseases. In: Zander DS, Farver CF, editors. Pulmonary Pathology. Philadelphia: Churchill Livingstone Elsevier; 2008. pp. 167–203. [Google Scholar]
- 14.Calder PC, Yaqoob P. Ω-3 polyunsaturated fatty acids and human health outcomes. Biofactors. 2009;35:266–272. doi: 10.1002/biof.42. [DOI] [PubMed] [Google Scholar]
- 15.Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M Enteral Nutrition in ARDS Study Group. Effect of enteral feeding with eicosapentaenoic acid, γ-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit. Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, γ-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit. Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- 17.Pontes-Arruda A, Aragão AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, γ-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit. Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 18.Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and γ-linolenic acid in ventilated patients with acute lung injury. Crit. Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 19.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from ω-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the ω-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun YP, Uddin J, Petasis NA, Serhan CN. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 22.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from ω-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 25.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-γ and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 27.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J. Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 29.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N. Engl. J. Med. 2001;344:665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 31.Merchant AT, Curhan GC, Rimm EB, Willett WC, Fawzi WW. Intake of n-6 and n-3 fatty acids and fish and risk of community-acquired pneumonia in US men. Am. J. Clin. Nutr. 2005;82:668–674. doi: 10.1093/ajcn.82.3.668. [DOI] [PubMed] [Google Scholar]
- 32.Pierre M, Husson MO, Le Berre R, Desseyn JL, Galabert C, Béghin L, Beermann C, Dagenais A, Berthiaume Y, Cardinaud B, et al. Ω-3 polyunsaturated fatty acids improve host response in chronic Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L1422–L1431. doi: 10.1152/ajplung.00337.2006. [DOI] [PubMed] [Google Scholar]
- 33.Tiesset H, Pierre M, Desseyn JL, Guéry B, Beermann C, Galabert C, Gottrand F, Husson MO. Dietary (n-3) polyunsaturated fatty acids affect the kinetics of pro- and anti-inflammatory responses in mice with Pseudomonas aeruginosa lung infection. J. Nutr. 2009;139:82–89. doi: 10.3945/jn.108.096115. [DOI] [PubMed] [Google Scholar]
- 34.Mayer K, Kiessling A, Ott J, Schaefer MB, Hecker M, Henneke I, Schulz R, Günther A, Wang J, Wu L, et al. Acute lung injury is reduced in fat-1 mice endogenously synthesizing n-3 fatty acids. Am. J. Respir. Crit. Care Med. 2009;179:474–483. doi: 10.1164/rccm.200807-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 36.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, et al. Increased dietary intake of ω-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous ω-3 fatty acids are protected from colitis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham E. Neutrophils and acute lung injury. Crit. Care Med. 2003;31(4) Suppl:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 39.Folkesson HG, Matthay MA, Hébert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J. Clin. Invest. 1995;96:107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight PR, Druskovich G, Tait AR, Johnson KJ. The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology. 1992;77:772–778. doi: 10.1097/00000542-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J. Clin. Invest. 1989;84:1609–1619. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 43.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 46.Serhan CN, Haeggström JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 47.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 48.Meduri GU, Kanangat S, Stefan J, Tolley E, Schaberg D. Cytokines IL-1beta, IL-6, and TNF-α enhance in vitro growth of bacteria. Am. J. Respir. Crit. Care Med. 1999;160:961–967. doi: 10.1164/ajrccm.160.3.9807080. [DOI] [PubMed] [Google Scholar]
- 49.Porat R, Clark BD, Wolff SM, Dinarello CA. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991;254:430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 50.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Grant IS, Pollok AJ, Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 1993;341:643–647. doi: 10.1016/0140-6736(93)90416-e. [DOI] [PubMed] [Google Scholar]
- 51.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 52.Frink M, Lu A, Thobe BM, Hsieh YC, Choudhry MA, Schwacha MG, Kunkel SL, Chaudry IH. Monocyte chemoattractant protein-1 influences trauma-hemorrhage-induced distal organ damage via regulation of keratinocyte-derived chemokine production. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1110–R1116. doi: 10.1152/ajpregu.00650.2006. [DOI] [PubMed] [Google Scholar]
- 53.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Respir. Crit. Care Med. 2004;170:1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]