Abstract
Analyses of microvascular networks with traditional tracer filling techniques suggest that the blood and lymphatic systems are distinct without direct communications, yet involvement of common growth factors during angiogenesis and lymphangiogenesis suggest that interactions at the capillary level are possible. In order to investigate the structural basis for lymphatic/blood endothelial cell connections during normal physiological growth, the objective of this study was to characterize the spatial relations between lymphatic and blood capillaries in adult rat mesenteric tissue. Using immunohistochemical methods, adult male Wistar rat mesenteric tissues were labeled with antibodies against PECAM (an endothelial marker) and LYVE-1, Prox-1, or Podoplanin (lymphatic endothelial markers) or NG2 (a pericyte marker). Positive PECAM labeling identified apparent lymphatic/blood endothelial cell connections at the capillary level characterized by direct contact or direct alignment with one another. In PECAM labeled networks, a subset of the lymphatic and blood capillary blind ends were connected with each other. Intravital imaging of FITC-Albumin injected through the femoral vein did not identify lymphatic vessels. At contact sites, lymphatic endothelial markers did not extend along blood capillary segments. However, PECAM positive lymphatic sprouts, structurally similar to blood capillary sprouts, lacked observable lymphatic marker labeling. These observations suggest that non-lumenal lymphatic/blood endothelial cell interactions exist in unstimulated adult microvascular networks and highlight the potential for lymphatic/blood endothelial cell plasticity.
Keywords: Microcirculation, Angiogenesis, Lymphangiogenesis, Endothelial Cell
INTRODUCTION
Understanding microvascular dysfunction associated with lymphatic disorders, including cancer metastasis, requires investigation of the spatial and temporal interrelationships between angiogenesis and lymphangiogenesis. Previous analyses of microvascular networks using intravital microscopy or contrast media methods suggested that lymphatics form networks distinct and separate from blood vessels (Clark and Clark, 1937; Hauck, 1973; Schmid-Schönbein et al., 1977) with little evidence for direct communication between the two systems. Yet the requirement of common growth factors during lymphangiogenesis and angiogenesis (Karpanen and Makinen, 2006) suggest that at the tissue level, cell interactions between the two systems may exist. This concept is further supported by the phenotypic similarities between lymphatic and blood endothelial cells during quiescent and pathological conditions (Karpanen and Makinen, 2006; Stacker et al., 2002).
In order to investigate the structural basis for association between lymphatic and blood endothelial cells during normal physiological growth, the objective of this study was to examine the spatial relationships between lymphatic and blood capillaries in adult rat mesenteric tissue. Using intravital microscopy and immunohistochemistry to label vessel-specific endothelial markers, we demonstrate apparent non-lumenal lymphatic/blood endothelial cell connections at the capillary level.
MATERIAL AND METHODS
Tissue Collection and Immunohistochemistry
All experimental protocols were reviewed and approved by the University of California- San Diego or Tulane Animal Use and Care Committee.
For the structural analysis of lymphatic/blood endothelial cell connections, the mesentery was exteriorized from male Wistar rats (350 +/− 25g) and sectors, defined as the thin translucent connective tissue in between the mesenteric arterial/venous vessels feeding the bowel, were blindly harvested for the ileum section of the small intestine. Sectors were included in the structural analysis if it passed one criteria: it contained both lymphatic and blood microvascular networks regardless of blood/lymphatic network overlap. The tissue sectors that we analyzed did not require apparent contact between the two vessel types only that both vessel types were present in the tissue. The rationale for this single criterion was that the objective of this study was to characterize the spatial relations between lymphatic and blood capillaries. The tissues were immediately placed in 10mM phosphate buffered saline (PBS), mounted on positively charged glass slides, and fixed for 1 hour in 4% paraformaldehyde at 4°C. The rat mesentery was selected for this study because it allows for complete visualization of intact microvascular and lymphatic networks down to the single cell level, a requirement to detect lymphatic/blood endothelial cell connections. After fixation, the mesenteric tissues were transferred to a 10 mM PBS solution at 4°C. Three to four sectors harvested from animals (n = 5) were labeled with an antibody against PECAM (CD31) according to the following protocol: 1) 1 hour incubation at room temperature with 1:200 mouse monoclonal biotinylated CD31 antibody (BD PharMingen) diluted in antibody buffer (0.1% Saponin in PBS + 2% BSA); 2) 1 hour incubation at room temperature with streptavidin-peroxidase secondary antibody solution (VECTASTAIN Elite ABC; Vector Laboratories) for 1 hour at room temperature; and 3) 15-20 minute incubation at room temperature with Vector Nova Red (Vector Laboratories) substrate. All labeling steps were followed by at least three 10 minute rinses with PBS + 0.1% Saponin, except after the Nova Red developing step, which was followed by 5 minute incubation in distilled water.
To confirm examples of lymphatic/blood blind-ended vessel spatial alignment in other tissues, the intestinal muscular coat from the ileum (n = 4 animals) and the cremaster muscle (n = 2 animals) were harvested from adult Wistar rats (316 g – 385 g) and labeled for PECAM in a manner similar to the mesenteric tissue with increased antibody incubation and washing durations. Muscles were fixed for 2-3 hours in 4% paraformaldehyde at 4°C. The primary antibody was applied for 3 days at 4°C and streptavidin-peroxidase secondary antibody solution (VECTASTAIN Elite ABC; Vector Laboratories) was applied for 2 hours at room temperature.
For the phenotypic characterization of lymphatic/blood endothelial cell connections, mesenteric sectors were harvested from adult male Wistar rats were fixed in 100% methanol at 4°C for 30 minutes and then immunofluorescently labeled according to the following procedure: 1) 1 hour incubation at room temperature with 1:40 mouse monoclonal biotinylated CD31 (Serotec) plus 1:100 rabbit polyclonal Prox-1 (Novus), 1:100 rabbit polyclonal LYVE-1 (AngioBio), or 1:100 rabbit polyclonal Neuron-glia antigen 2 (NG2; gift from Dr. Stallcup at the Burnham Institute, La Jolla, CA) antibodies diluted in antibody buffer (0.1% Saponin in PBS + 2% BSA+ 5% NGS); 2) 1 hour incubation at room temperature with 1:500 streptavidin-CY2 (Jackson ImmunoResearch Laboratories) plus 1:100 goat anti-rabbit IgG-Cy3 (Jackson ImmunoResearch Laboratories) diluted in antibody buffer for 1 hour at room temperature. For PECAM plus Podoplanin labeling, tissues were sequentially labeled according to the following steps: 1) 1 hour incubation at room temperature with 1:100 mouse anti-rat Podoplanin (AngioBio) diluted in antibody buffer; 2) 1 hour incubation at room temperature with 1:50 goat anti-mouse IgG-Cy3 (Jackson ImmunoResearch Laboratories) diluted in antibody buffer; 3) 1 hour incubation at room temperature with 1:40 mouse monoclonal biotinylated CD31 (Serotec) diluted in antibody buffer; 4) 1 hour incubation at room temperature with 1:500 streptavidin-CY2 (Jackson Immuno) diluted in antibody buffer. All labeling steps were followed by at least three ten minute rinses with PBS + 0.1% Saponin. Positive immunostaining along microvessels was confirmed by comparison to the appropriate controls: unstained tissue, secondary antibody alone, and secondary antibody plus rabbit or mouse IgG (Supplemental Figure A).
Image Acquisition
Transmitted light images were captured with a digital camera (FUJIFILM FinePix S1 Pro) mounted on an inverted microscope (Olympus IX70) with a Cooke 5X/numerical aperture (NA)=0.15 dry, Olympus 20X/NA=0.65 dry, or Olympus 60X/NA=1.25 oil immersion objective. Muscle images were captured using the same microscope with a CCD camera and Scion frame grabber. Fluorescent images were captured with a digital camera (Pixelfly) mounted on an inverted microscope (Olympus IX71) with an Olympus 20X/NA=0.80 oil immersion objective. Network montages were generated by overlaying sequential images using custom software. Images of connections in Figure 3 were taken with a confocal microscope (Zeiss LSM 510 META) using a Zeiss 63X/NA=1.4 oil immersion objective.
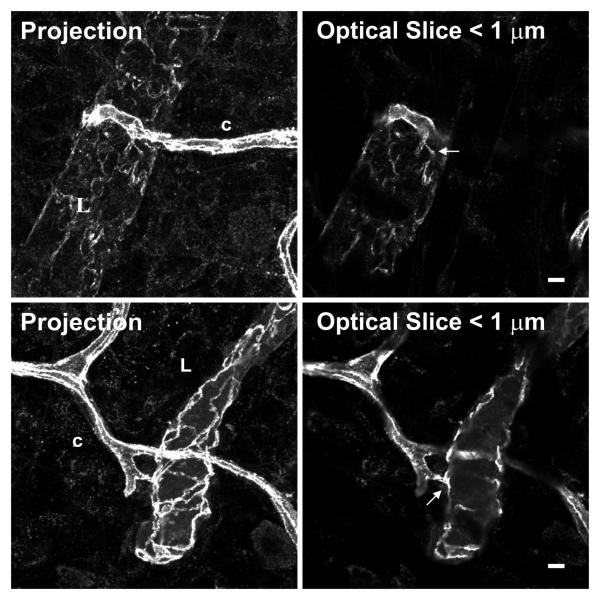
Figure 3.
Confocal projections and single optical slices of direct lymphatic blood/vessel connections immunolabeled for PECAM. In optical slices < 1μm thick, continuous PECAM labeling is observed at sites of lymphatic/blood endothelial cell connection. Scale bar, 5 μm.
Identification of Apparent Lymphatic/Blood Endothelial Cell Connections at the Capillary Level
For each PECAM labeled tissue, sectors were qualitatively evaluated for the presence of both lymphatic and blood microvascular networks regardless of blood/lymphatic network overlap within the sector. Tissue sectors with both lymphatic and blood vessels (n=15), were further examined for the presence of apparent connections. Apparent connections were defined to be between endothelial cells along a terminating, blind-ended lymphatic vessel and blood vessel or a terminating, blind-ended blood vessel and a lymphatic vessel. Apparent endothelial cell connections were identified based on 2 classifications: direct contact and direct alignment. Direct contact between two vessels had 1) overlap of PECAM staining, 2) PECAM staining in the same focal plane, and 3) shared cellular or staining morphology. A direct alignment consisted of two vessels positioned in parallel within a distance of one microvessel diameter (~ 5 μm). The 5X objective was used for initial identification and the 20X objective was used for confirmation and classification of cell connection type.
The mesenteric tissue was selected because it has been reported to be approximately 15-30 μm thick (Gahm and Witte, 1986) and allows for essentially 2-dimensional observation of intact microvascular vascular networks down to the single cell level. Given the thin and transparent nature of the mesentery, all blind ends can be observed per sector. This observation method is supported by previous analysis of microvascular structures in the rat mesentery (Anderson et al., 2008; Murfee et al., 2006; Ponce et al., 2002).
Intravital Observation and Acquisition of Lymphatic/Blood Vessel Connections
A bolus of 2.5 ml FITC-Albumin (10mg/ml, SigmaAldrich) was injected via the femoral vein in adult male Wistar rats. Immediately following the injection, the mesenteric tissue was exteriorized and the animal was prepared for intravital microscopy. BSI-TRITC (1:5 dilution in sterile physiological saline; 1mg/ml, SigmaAldrich) was topically applied to the exteriorized mesenteric tissue and allowed to incubate for 20 minutes. Intravital Images were captured with a digital camera (Pixelfly) mounted on an upright fluorescence microscope (Nikon Eclipse LV100). Images were taken with a Nikon 4X/numerical aperture (NA)=0.10 dry or Nikon 20X/NA=0.50 water immersion.
RESULTS
Along the hierarchy of the adult rat mesenteric microvascular networks, both lymphatic and blood endothelial cells were labeled by the antibody against PECAM (Figure 1). Lymphatic vessels were generally distinguishable from microvessels by their larger dimension, irregular vessel diameter, and decreased PECAM labeling intensity. PECAM expression in all vessel segments was confirmed by labeling of lymphatic blind ends.
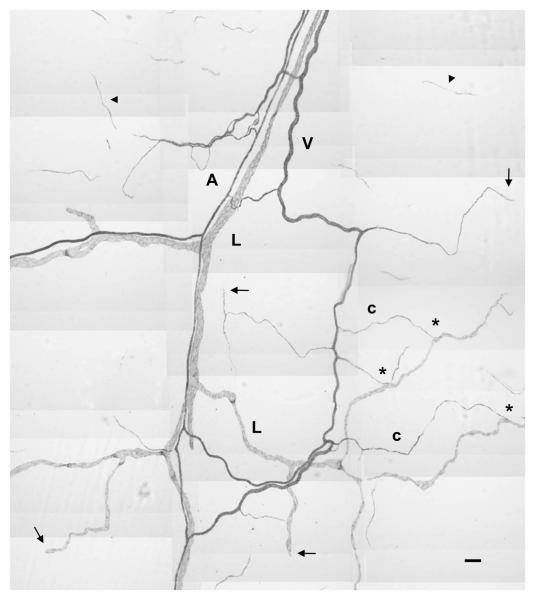
Figure 1.
Montage of adult rat mesenteric lymphatic and blood microvascular networks immunolabeled for PECAM expression. Positive PECAM staining serves to identify lymphatic (L) and blood microvessels, including arterioles (A), venules (V) and capillaries (c). Endothelial cell expression of PECAM along the hierarchy of the respective networks is confirmed by continuous staining from the tissue feeding and draining vessels to lymphatic and blood capillary blind-ended vessel segments (arrows). Examples of a lymphatic/blood vessel connections are indicated by the “*”. Arrowheads indicate the endothelial cell structures disconnected from the nearby microvascular network. Scale bar, 100 μm.
In all PECAM labeled mesentery tissues with both lymphatic and microvascular networks, we observed apparent endothelial cell connections between lymphatic and blood vessels at the capillary level (Figure 2). A subset of all blind vessel endings, including lymphatic and blood capillary vessels, was connected. We observed both lymphatic capillary blind ends connecting with blood vessels and blood capillary blind ends connecting with lymphatic vessels. Approximately half of the connections were direct contacts, versus alignments (see Methods for definitions). Direct endothelial cell connections between blood and lymphatic endothelial cells is supported by continuous PECAM junctional labeling at sites of connections in < 1 μm optical sections using confocal microscopy (Figure 3). Apparent endothelial cell connections between the two vessel systems have also been identified by positive VE-cadherin labeling (Figure 4).
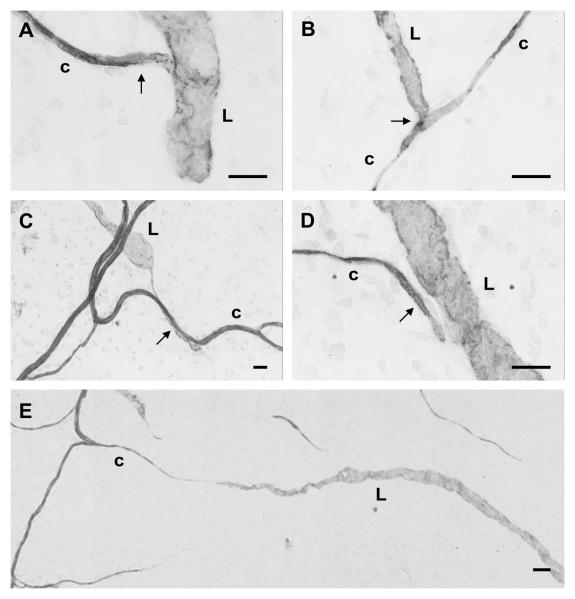
Figure 2.
Examples of lymphatic/blood vessel connections (arrows) classified as either direct contact (A, B, C) or direct alignment (D). Connections were defined as involving either a blind-ended lymphatic vessel (L) or blood capillary (c) or both. Apparent cases in which lymphatic and capillary blind ends are involved in directed growth toward each other (E) were not included in the connection analysis, but suggest the occurrence of common guidance signals. Scale bars, 20 μm (A, B, C, D), 100 μm (E).
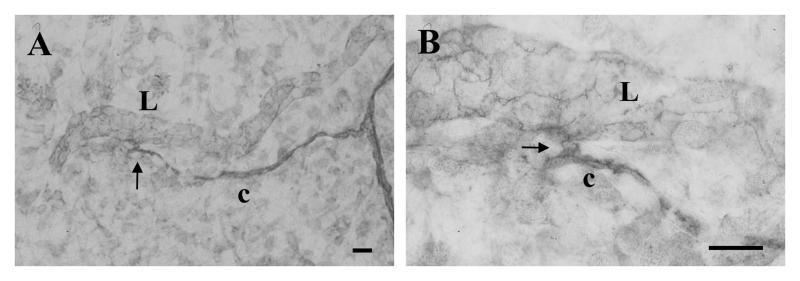
Figure 4.
Example of an apparent lymphatic/blood vessel connection arrow identified with VEcadherin between a blood capillary (c) and a blind ended lymphatic vessel (L). (B) shows a magnification of the connection present in (A). Scale bars, 20 μm.
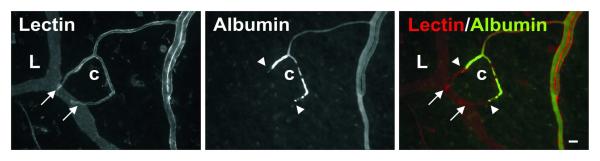
The locations of lymphatic/blood endothelial cell contact were distal to the vascular lumens identified via intra-lumenal injection of FITC labeled albumin (Figure 5). Similar to PECAM labeling, intra-vital labeling with BSI-lectin identified all lymphatic and blood vessels including endothelial cell connections.
Figure 5.
Topical BSI-lectin labeling and intralumenal FITC-albumin injection labeling of two blood capillary (c) blind ends connecting to a lymphatic capillary (L). Injected FITC-albumin identifies the blood capillary sprout lumens, which terminate (arrowheads) proximal to lymphatic/blood endothelial cell direct contact (arrows). Scale bar, 20 μm
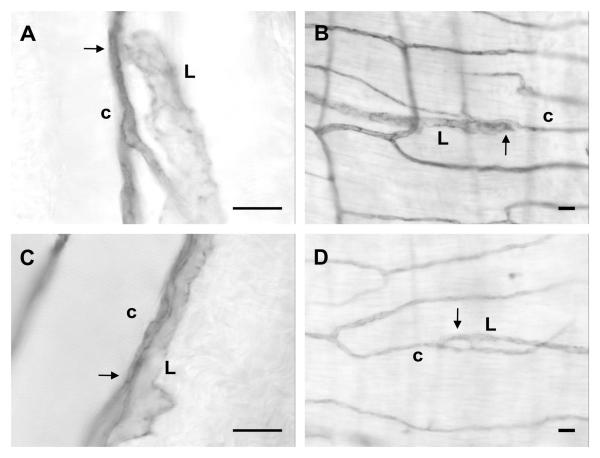
In order to examine whether apparent connections between lymphatic and blood endothelial cells along capillaries were present in organs other than the mesenteric connective tissue, we harvested, whole mounted, and labeled the ileum muscle coat and cremaster muscle from adult rats. Examples of endothelial cell connections similar to that in the mesentery were observed in each muscle specimen (Figure 6).
Figure 6.
Example of endothelial contact between lymphatic (L) and blood capillary (c) vessels in cremaster muscle (A, C) and the muscle coat around the ileum portion of the small intestine (B, D). Lymphatic and blood capillary lineage was confirmed by tracing along the respective networks (not shown). Scale bars, 20 μm.
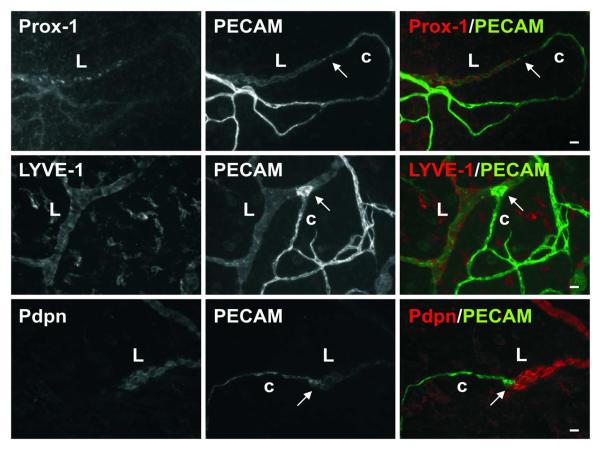
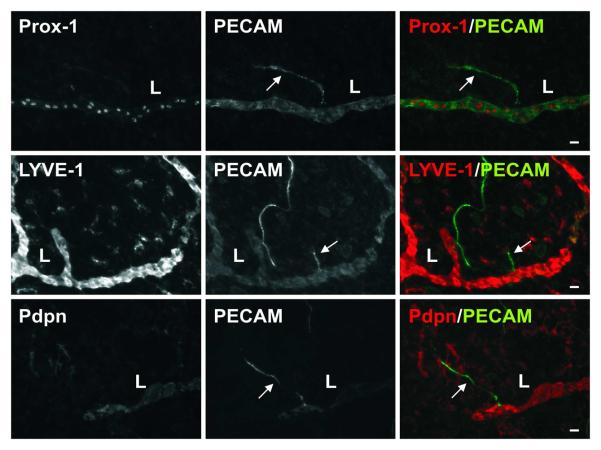
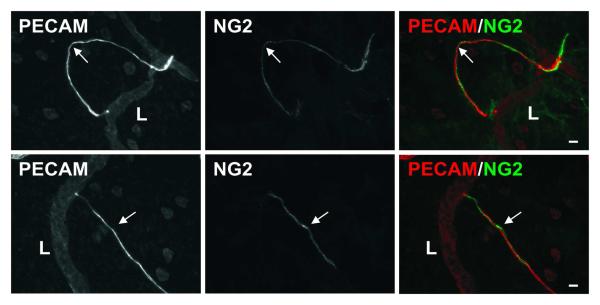
At locations of direct lymphatic/blood endothelial cell contact in the mesenteric microvascular networks, lymphatic marker (LYVE-1, Prox1, and Podoplanin) expression did not extend along the blood capillary segment (Figure 7). However, the potential for lymphatic endothelial cell plasticity and the ability for lymphatic endothelial cells to connect with cells that are phenotypically different are supported by the presence of a subset of lymphatic blind-ended vessel segments that are similar in morphology to blood capillary sprouts and negative for LYVE-1, Prox-1, and Podoplanin (Figure 8). Evidence for these blind-ended lymphatic endothelial structures to share phenotypic similarities with blood endothelial cells is further supported by their ability to recruit NG2 positive pericytes (Figure 9). NG2 positive pericyte coverage surrounded the capillaries but does not extend along immediate upstream lymphatic vessel segments.
Figure 7.
An example of direct endothelial contact between lymphatic (L) and blood capillaries (c) labeled for lymphatic marker, Prox-1, LYVE-1, or Podoplanin (Pdpn), plus PECAM. Lymphatic marker expression serves to delineate the two systems at the site of direct contact (arrows). Scale bars, 20 μm.
Figure 8.
Representative lymphatic vessels labeled for lymphatic marker, Prox-1, LYVE-1, or Podoplanin (Pdpn), plus PECAM. The lymphatic markers did not label a subset of blind-ended lymphatic endothelial cell segments (arrows) structurally similar to blood capillary sprouts. These blind-ended lymphatic endothelial cell segments originated from initial lymphatics (L) with positive lymphatic marker labeling. Scale bars, 20 μm.
Figure 9.
Representative lymphatic sprouts labeled for NG2 plus PECAM. Blind-ended endothelial cell segments (arrows), which originate from lymphatic vessels (L) and are structurally similar to blood capillary sprouts are NG2 positive, indicating the presence of vascular pericytes. NG2 labeling does not extend along the upstream lymphatic vessel. Scale bars, 20 μm.
DISCUSSION
The primary finding in this study is that lymphatic and blood endothelial cells make apparent contact at the capillary level in adult rat microvascular networks. Based on intravital microscopy (Clark and Clark, 1937; Hauck, 1973) and contrast filling studies from either the vascular or the lymphatic side (Schmid-Schönbein et al., 1977; Skalak et al., 1984; Unthank and Bohlen, 1988), there is no evidence that the two systems form connections with an open lumen capable of permitting fluid exchange. Instead, our results suggest the existence of cellular contact between lymphatic and blood endothelial cells along blind-ended capillary vessels. We observe examples of lymphatic/blood capillary alignment in the whole mount preparations of ileum muscle coat and cremaster muscle, suggesting that lymphatic/blood vessel connections at the capillary level are not tissue-specific. Together with our observation of a subset of lymphatic blind-ended endothelial cell segments with similar endothelial cell structures associated with capillary sprouts and a lack of lymphatic marker expression emphasize the need to investigate the common cellular and molecular mechanisms involved in lymphatic and blood vessel growth and the functional significance of these cell connections.
Lymphatic/venous communication at the macrocirculation level outside the entry points to the subclavian and thoracic ducts have been previously reported especially in situations of increased pressure due to vessel occlusion (Miller, 1982; Yoffrey and Courtice, 1970). In the heart, lymphatic/venous anastomoses between vessels in the microcirculation with diameters 30 μm – 50 μm were observed at 7 and 14 days post lymphatic occlusion (Eliska and Eliskova, 1975). In the context of these findings, our observations suggest that physical lymphatic/blood vessel connections at the capillary level are pre-established in unstimulated tissues.
Patent connections between the lymphatic and blood vessel systems in peripheral tissues have recently been proposed to exist in mice lacking certain signaling proteins. Intravenous injection of FITC-labeled dextran identified abnormal lymphatic/blood vessel lumenal connections in SLP-76−/− or Syk−/− mice (Abtahian et al., 2003). In Fiaf−/− (fasting-induced adipose factor) mice, intravenous injection of BSI lectin served to identify lymphatic/blood vessel connections during postnatal development (Backhed et al., 2007). Fiaf is believed to be an upstream regulator of Prox-1. Johnson et al. (2008) demonstrated that lymphatic endothelial cell identity depends on Prox-1 activity and further found that Prox-1 conditional mutants displayed lumenal connections between the lymphatic and blood vascular systems as determined by dextran infusion and the presence of blood-filled lymphatics. In the context of these studies, our observations suggest sites of pre-existing lymphatic/blood vessel connections at the capillary level that can be influenced by local epigenetic stimuli.
The presence of lymphatic/blood endothelial connections highlights the possibility for shared patterning signals between lymphatic and blood microvascular systems in the adult. Molecular players, such as vascular endothelial growth factors and angiopoietins, are involved in angiogenesis and play similar roles in lymphangiogenesis (Karpanen and Makinen, 2006). Recently, macrophages have been implicated in both angiogenesis and lymphangiogenesis via paracrine mechanisms (El-Chemaly et al., 2009; Maruyama et al., 2005). During inflammation induced lymphangiogenesis, macrophages have also been shown to be able to differentiate into lymphatic endothelial cells (El-Chemaly et al., 2009). Currently the role of macrophages at sites of apparent lymphatic/blood endothelial connections in our unstimulated tissues remains unclear (Online Supplemental Figure B). Further investigation during inflammatory conditions regarding the mechanistic role of shared molecular and cellular players will provide valuable insight to the formation and functionality of these connections.
The observation of blood vessel-like lymphatic blind-ended segments originating from lymphatic capillaries supports the ability for cells of different phenotypes to interact with each other and therefore, the presence of direct contact lymphatic/blood endothelial cell connections. While a dynamic time-lapse study is required to confirm that the blind-ended vessels analyzed in this study are in fact sprouting, previous characterizations of angiogenesis in this rat mesenteric tissue suggest that these structures are indicative of a vessel growth process (Anderson et al., 2004; Murfee et al., 2006). Additionally, we observed a lack of evidence for apoptosis along blind-ended vessels in rat mesenteric tissue as determined by the TUNEL technique (data not shown). Initial lymphatics are generally considered to be void of vascular pericytes. Our observations of a subset of blind-ended lymphatic endothelial cell segments lacking lymphatic marker expression and able to recruit vascular pericytes highlight the potential for the local environment to influence endothelial cell plasticity.
In summary, our work provides anatomical evidence for cell-cell contact between lymphatic and blood vessels at the capillary level. While the functionality of these apparent connections during tissue remodeling inflammation and other remodeling scenarios requires further investigation, our work offers a novel perspective for understanding the common cell lineage and overlapping mechanisms associated with angiogenesis and lymphangiogenesis.
Supplementary Material
Acknowledgments
Grant support: Supported by NHLBI Grant HL10881 (GWSS) and by the Board of Regents of the State of Louisiana LEQSF(2009-12)-RD-A-19 (WLM).
LITERATURE CITED
- Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CR, Hastings NE, Blackman BR, Price RJ. Capillary Sprout Endothelial Cells Exhibit a CD36low Phenotype. American Journal of Pathology. 2008;173:1220–1228. doi: 10.2353/ajpath.2008.071194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CR, Ponce AM, Price RJ. Absence of OX-43 antigen expression in invasive capillary sprouts: identification of a capillary sprout-specific endothelial phenotype. Am J Physiol Heart Circ. Physiol. 2004;286:H346–H353. doi: 10.1152/ajpheart.00772.2003. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Ponce AM, Price RJ. Immunohistochemical identification of an extracellular matrix scaffold that microguides capillary sprouting in vivo. J. Histochem Cytochem. 2004;52:1063–1072. doi: 10.1369/jhc.4A6250.2004. [DOI] [PubMed] [Google Scholar]
- Backhed F, Crawford PA, O'Donnell D, Gordon JI. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci. 2007;104:606–611. doi: 10.1073/pnas.0605957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204(10):2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castenholz A, Hauck G, Rettberg U. Light and electron microscopy of the structural organization of the tissue-lymphatic fluid drainage system in the mesentery: an experimental study. Lymphology. 1991;24:82–92. [PubMed] [Google Scholar]
- Clark E, Clark E. Observations on living mammalian lymphatic capillaries and their relation to blood vessels. Am J Anat. 1937;60:253–298. [Google Scholar]
- El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, Wu H, Nathan SD, Cuttitta F, McCoy JP, Gochuico BR, Moss J. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. PNAS. 2009;106:3958–3963. doi: 10.1073/pnas.0813368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliska O, Eliskova M. Contribution to the solution of the question of lympho-venous anastomoses in heart of dog. Lymphology. 1975;8:11–15. [PubMed] [Google Scholar]
- Gahm T, Witte S. Measurement of the optical thickness of transparent tissue layers. J Microsc. 1986;141:101–110. doi: 10.1111/j.1365-2818.1986.tb02704.x. [DOI] [PubMed] [Google Scholar]
- Hauck G. Functional aspects of the topical relationship between blood capillaries and lymphatics of the mesentery. Pflugers Arch. 1973;339:251–256. doi: 10.1007/BF00587376. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22:3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpanen T, Makinen T. Regulation of lymphangiogenesis—from cell fate determination to vessel remodeling. Exp Cell Res. 2006;312:575–583. doi: 10.1016/j.yexcr.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Li M, Cursiefan C, Jackson DG, Keino H, Tomita M, Rooijen NV, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. The Journal of Clinical Investigation. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ. Lymphatics of the Heart. Raven Press; New York: 1982. pp. 181–185. [Google Scholar]
- Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005;12(2):151–160. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation. 2006;13:261–273. doi: 10.1080/10739680600559153. [DOI] [PubMed] [Google Scholar]
- Ponce AM, Price RJ. Angiogenic stimulus determines the positioning of pericytes within capillary sprouts in vivo. Microvascular Research. 2003;65:45–48. doi: 10.1016/s0026286202000146. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein GW, Zweifach BW, Kovalcheck S. The application of stereological principles to morphometry of the microcirculation in different tissues. Microvasc Res. 1977;14:303–317. doi: 10.1016/0026-2862(77)90028-0. [DOI] [PubMed] [Google Scholar]
- Skalak TC, Schmid-Schönbein GW, Zweifach BW. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc Res. 1984;28:95–112. doi: 10.1016/0026-2862(84)90032-3. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- Unthank JL, Bohlen HG. Lymphatic pathways and role of valves in lymph propulsion from small intestine. Am J Physiol Gastrointest Liver Physiol. 1988;254:G389–G398. doi: 10.1152/ajpgi.1988.254.3.G389. [DOI] [PubMed] [Google Scholar]
- Yoffrey JM, Courtice FC. Lymphatics, Lymph and the Lympomyeloid Complex. Academic Press; London: 1970. pp. 27–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.