Abstract
Objective
To compare the trajectories of cognitive decline between groups with, and without, the later development of psychotic symptoms during Alzheimer disease (AD) or Mild Cognitive Impairment (MCI).
Design
We examined cognitive function in a new analysis of an existing data set, The Cardiovascular Health Study (CHS), an epidemiologic, longitudinal follow-up study. Our analyses examined 9 years of follow-up data.
Setting
Community.
Participants
We examined subjects who were without dementia at study entry, received a diagnosis of AD or MCI during follow up and had been rated on the Neuropsychiatric Inventory for the presence of psychosis; 362 for the Modified Mini-Mental State Examination (3MS) analysis and 350 for the Digit Symbol Substitution Test (DSST) analysis had sufficient follow-up data and APOE genotyping.
Measurements
The 3MS and DSST were administered annually and analyzed using mixed effects models including APOE4 status.
Results
Mean 3MS and DSST scores did not differ between AD with psychosis and without psychosis groups at baseline. 3MS and DSST scores decreased more rapidly in subjects who ultimately developed psychosis.
Conclusions
Individuals who ultimately develop psychosis have more rapid cognitive deterioration during the earliest phases of AD than individuals with AD not developing psychosis. The genetic and other neurobiologic factors leading to the expression of AD+P may exert their effects via acceleration of the neurodegenerative process.
Keywords: Alzheimer’s disease, MCI (mild cognitive impairment), Psychosis
Introduction
Psychotic symptoms, delusions and hallucinations, are common in Alzheimer Disease (AD), occurring in approximately 40% of individuals over the course of the illness.1 Psychotic symptoms in AD (AD with psychosis, AD+P) cause significant distress for patients and family members.2 AD+P is a predictor of worse functional outcome, higher likelihood of institutionalization, and, in those with hallucinations, higher mortality rate.3 Importantly, a number of studies indicate that the occurrence of psychosis in AD is familial, with an estimated heritability of 61%,4,5,6 indicating a distinct neurobiology of this phenotype.7
Numerous studies have shown that greater cognitive impairment is the most consistent clinical correlate of the development of psychotic symptoms during AD than in individuals who have AD without psychosis (AD−P).1 A few studies8,9,10 have prospectively examined cognitive course after clinical diagnosis of a cognitive disorder and prior to the onset of psychotic symptoms, finding that more severe cognitive dysfunction preceded the onset of psychosis by at least 1–2 years. In these studies, the rate of global cognitive decline did not differ between the groups in the two years prior to psychosis onset. However, the association between greater cognitive impairment and subsequent AD+P was strongest in those entering the study in the early stages of AD.8
These findings suggest the hypothesis that the cognitive course of individuals destined to develop AD+P diverges from that of individuals destined for AD−P in the earliest stages of disease, prior to the manifestation of sufficient clinical symptoms of cognitive impairment to lead to presentation to an Alzheimer Disease Center. This could result, for example, if genetic variation leading to AD+P interacts with the neurodegenerative process, resulting in a more rapid cognitive decline preceding clinical symptoms, an interpretation consistent with the recent recognition that both cognitive deficits11 and amyloid deposition12 can occur years in advance of the clinical manifestation of AD.
To our knowledge, no studies have examined whether cognitive function in pre-clinical and early disease stages differs between those destined to and destined not to develop AD+P. To address this question, a large cohort with data on both cognitive and neuropsychiatric measures on individuals followed prospectively to AD onset is necessary. For this reason, we utilized data from the Cardiovascular Health Study (CHS), a population based study that, for over a decade, collected cognitive data on individuals preceding the onset of AD.
Methods
Cardiovascular Health Study Overview
Detailed descriptions of the methods and assessments used in the CHS and CHS Cognition Study have been published elsewhere.13,14,15 The CHS was designed to investigate risk factors for cardiovascular disease in individuals over 65 years of age. Four sites in the United States (Forsyth Co., NC; Washington Co., MD; Sacramento Co., CA; and Pittsburgh, PA) recruited 5,201 participants in 1989–90. In 1998–9, the ancillary Cognition Study recruited 3,608 participants from the original study to determine the prevalence of cognitive and neurological disorders in the CHS cohort. All participants signed informed consent and protocols were approved by each of the four sites’ university institutional review boards. APOE4 genotyping was only conducted on consenting participants.
Subjects
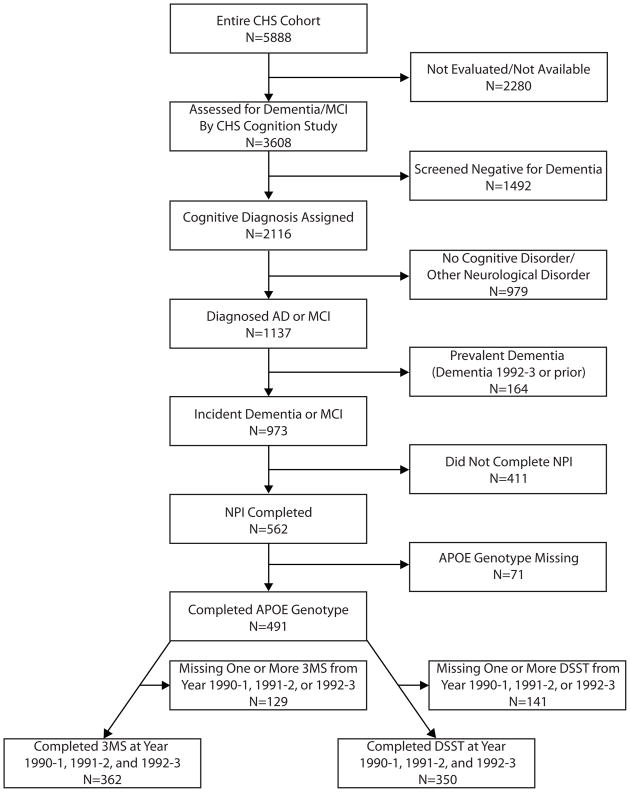
A flow chart of the subjects who were included in the current analyses is shown in Figure 1. Participants were excluded from the analyses if they: were diagnosed with prevalent dementia at study entry; were not diagnosed with an incident cognitive disorder (AD, mixed AD and Vascular dementia, or MCI); did not complete ratings of psychosis on the Neuropsychiatric Inventory (NPI); or were not genotyped for APOE4. To establish a minimum longitudinal data set from which cognitive trajectories could adequately be characterized, participants were excluded if they did not complete the 3MS at all three of the first three assessment points (1990–1 through 1992–3 inclusive), and were excluded from analysis of the DSST if they did not complete the DSST at all three of the same time points.
Figure 1. Flow Chart of Inclusion/Exclusion Criteria and Number of Subjects Included in Analyses.
CHS, Cardiovascular Health Study; AD, Alzheimer Disease; MCI, Mild Cognitive Impairment; NPI, Neuropsychiatric Inventory; 3MS, Modified Mini-Mental Status Examination; APOE, Apolipoprotein-ε; DSST, Digit Symbol Substitution Test.
Assessments
Cognition was examined using the Modified Mini-Mental Status Examination (3MS),16 a measure of global cognition, and the Digit Symbol Substitution Test (DSST), in general a measure of attention,17 from 1990–1 and thereafter at annual visits, prospectively examining cognitive function.
In 1998–9, presence of dementia or MCI was evaluated in participants who had completed an MRI in the CHS and consented to an additional evaluation and neuropsychological assessments.18 Participants at risk for cognitive disorder were systematically assessed by a team of psychiatrists and neurologists for dementia or MCI and assigned diagnoses using DSM-IV and NINCDS-ADRDA/AIREN criteria (see 13 for details). At the Pittsburgh site, all participants were evaluated regardless of classification as high or low risk. Across the four sites, 2116 participants were assigned a diagnosis of either prevalent dementia (onset 1992–3 or prior), incident dementia (onset 1993–4 or after), MCI, or normal cognition. Dementia was further classified as either AD, Vascular Dementia, mixed dementia (AD and Vascular Dementia), or other (e.g., Dementia with Lewy Bodies, Parkinson’s Dementia). Individuals with AD or mixed dementia (AD and Vascular) were included in our analyses, while those with Vascular Dementia, probable Dementia with Lewy Bodies, or Parkinson’s Dementia were excluded. APOE genotyping was conducted on participants as previously described.13
Participants were additionally evaluated in 1998–9 for presence of neuropsychiatric symptoms using the Neuropsychiatric Inventory (NPI).19 The NPI is a structured interview of an informant in close contact with the subject on various neuropsychiatric domains including delusions and hallucinations. Of 346 (95.6%) participants with data on informant relationship, 94% had a close relative serve as informant: spouse, sibling, child or other close relation (e.g., daughter-in-law). Informants were asked if there have been any delusions/hallucinations present in the past month or since the onset of memory problems. Delusions were defined as a fixed false belief. Hallucinations were defined as perceptions with no basis in reality. For our analyses, participants were classified as Ever Psychotic if informants answered yes to any of the delusions in the past month, hallucinations in the past month, delusions since the onset of memory problems, or the hallucinations since the onset of memory problems items on the NPI and Never Psychotic if they answered no to all of these items. One participant was included in the Never Psychotic group for whom there was insufficient data on the since the onset of memory problems items, but the answer for both the delusions and hallucinations in the past month items was no.
Statistical Analysis
Baseline comparisons of race, gender, and APOE4 status were conducted using Pearson’s Chi-squared; age, education, and baseline 3MS and DSST scores were compared with One-Way Analysis of Variance (ANOVA) using SPSS software (SPSS, Inc., Chicago, IL). The trajectory of DSST and 3MS data over time was examined between the groups with mixed-effect modeling using linear terms with SAS version 9 software (SAS Institute, Inc., Cary, NC). Age at baseline, race, gender, education, APOE4 grouping and psychotic grouping were included as fixed effects and intercept and time were treated as random effects. We used an unstructured correlation matrix to minimize the assumptions of the dataset. This approach uses more parameters, thus taking additional penalties and resulting in a more conservative approach.
Upon visual inspection of the data, we re-examined the trajectory of 3MS data using both linear and quadratic terms as the 3MS data appeared to have a quadratic fit. Goodness of fit of competing statistical models was evaluated using chi-square distributions (−2log likelihood). Confirmatory analyses were performed in a smaller cohort restricting to only individuals completing all 9 assessments in both the DSST and 3MS analyses, in a larger cohort including those with at least the first two 3MS or DSST present and with and without APOE4 genotype, and by examining dementia and MCI groups separately. Pearson Chi-Square was used to test for between groups differential dropout from the 3MS and DSST secondary analyses and completion rates of 3MS and DSST assessments.
Results
Demographics and clinical characteristics for the 362 participants are shown in Table 1. Of these, 74 (20.4%) were classified as Ever Psychotic and 288 (79.6%) were classified as Never Psychotic. The Ever Psychotic group was more likely to be Caucasian, female, to complete fewer DSST assessments, and to have positive APOE4 status; mean 3MS and DSST scores at baseline (1990–1) were similar for the two groups (See Table 1).
Table 1.
Demographic and Clinical Characteristics.
| Ever Psychotic (N=74) | Never Psychotic (N=288) | χ2/F | df | P-value | |
|---|---|---|---|---|---|
| Mean (SD) or N (%) | Mean (SD) or N (%) | ||||
| Range | Range | ||||
| Age at Baseline (years) | 73.4 (4.8) | 73.4 (4.7) | 0.015‡ | 1, 360 | 0.903 |
| 65–84 | 65–88 | ||||
| Education (years) | 13.0 (4.7) | 13.7 (4.9) | 1.291‡ | 1, 360 | 0.257 |
| 4–21 | 0–21 | ||||
| 3MS at Year 1990–1 | 89.4 (6.2) | 89.5 (6.9) | 0.013‡ | 1, 360 | 0.908 |
| 71–100 | 60–100 | ||||
| DSST at Year 1990–1§ | 37.0 (14.1) | 37.0 (12.7) | 0.285‡ | 1, 348 | 0.587 |
| 10–83 | 0–83 | ||||
| 3MS Assessments | 7.9 (1.6) | 8.2 (1.4) | 2.203‡ | 1, 360 | 0.139 |
| Completed Per Subject | 3–9 | 3–9 | |||
| DSST Assessments | 7.2 (1.9) | 7.9 (1.6) | 9.553‡ | 1, 348 | 0.002 |
| Completed Per Subject§ | 3–9 | 3–9 | |||
| Gender | |||||
| Female | 53 (72) | 168 (58) | 4.372† | 1 | 0.045 |
| Male | 21 (28) | 120 (42) | |||
| Race | |||||
| Caucasian | 74 (100) | 257 (89) | 8.71† | 3 | 0.033 |
| African American | 0 (0) | 28 (10) | |||
| Asian/Other | 0 (0) | 3 (1) | |||
| APOE Status | |||||
| APOE4 Positive | 31 (42) | 82 (28) | 4.938† | 1 | 0.034 |
| APOE4 Negative | 43 (58) | 206 (72) |
N=350, 71 were Ever Psychotic and 279 were Never Psychotic
Pearson’s Chi-square test. χ2 values are presented
One-Way Analysis of Variance. F Values are presented.
3MS, Modified Mini-Mental Status Examination; DSST, Digit Symbol Substitution Test; APOE4, Apolipoprotein-ε 4 allele
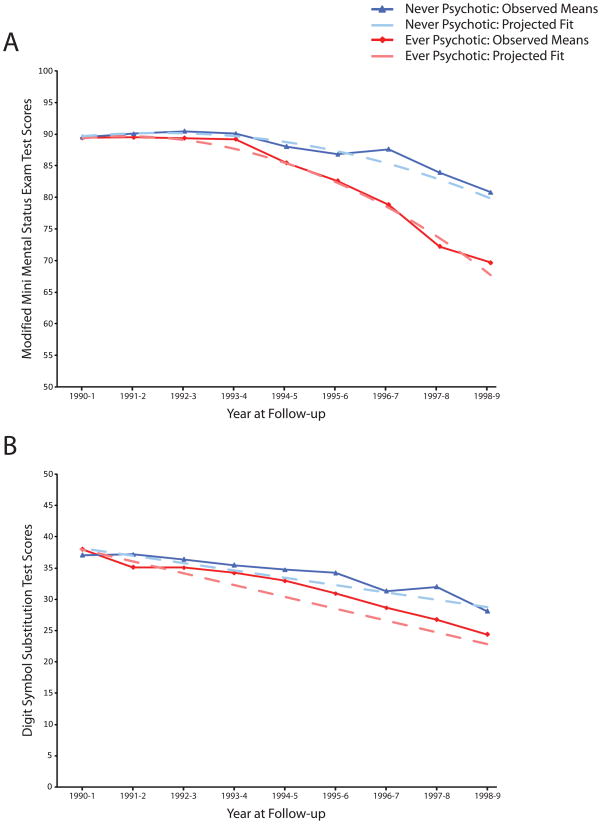
In the mixed-effect model using only linear terms, there was a significant main effect of psychosis with lower 3MS score (F=15.32, df=1, 2228, p<0.001). There was also a significant linear (F=25.37, df=1, 2228, p<0.001) interaction of time * psychosis such that the Ever Psychotic group had a more rapid rate of cognitive decline. In the mixed-effect model using linear and quadratic terms, the main effect of psychosis was no longer significant, the linear interaction of time * psychosis only approached conventional levels of significance, but an interaction of time2 * psychosis was significant such that the Ever Psychotic group’s deterioration accelerated over time (See Table 2 and Figure 2, Panel A). The chi-square distribution for the mixed-effect model including both linear and quadratic terms (19400.0) was significantly lower than the model with only linear terms (19672.9), the difference being 272.9 distributed as chi-square of 1 df and indicating a better statistical fit.
Table 2.
Results for Mixed Effect Model of 3MS. N=362
| Effect | Model Parameters† | SE | DF | t | p |
|---|---|---|---|---|---|
| Age at Baseline | −0.02260 | 0.05557 | 1, 2226 | −0.41 | 0.6843 |
| Race | −5.8816 | 0.9375 | 1, 2226 | −6.27 | <.0001 |
| Gender | −0.6640 | 0.5296 | 1, 2226 | −1.25 | 0.2101 |
| Education | 0.6120 | 0.05389 | 1, 2226 | 11.36 | <.0001 |
| APOE4 | −0.2736 | 0.5697 | 1, 2226 | −0.48 | 0.6310 |
| Psychotic Ever | 1.8446 | 2.0965 | 1, 2226 | 0.88 | 0.3790 |
| Time | 3.3312 | 0.5618 | 1, 360 | 5.93 | <.0001 |
| Time2 | −0.4268 | 0.03809 | 1, 360 | −11.20 | <.0001 |
| Time * Psychotic Ever | −1.0505 | 0.6269 | 1, 2226 | −1.68 | 0.0939 |
| Time2 * Psychotic Ever | 0.1808 | 0.04234 | 1, 2226 | 4.27 | <.0001 |
SE = Standard Error; DF = degrees of freedom; APOE4 = Apolipoprotein-ε 4 allele
Model parameters are unstandardized estimates
Figure 2. Means of Cognitive Test Scores by Presence of Psychosis.
Panel A: Observed means with quadratic fit lines of Modified Mini-Mental State Examination (3MS) test scores. Quadratic fit lines were generated from mixed-effect model using linear and quadratic terms including age at baseline, race, gender, education, APOE4 grouping and psychotic grouping as fixed effects and intercept and time as random effects (N=362). Panel B: Observed means with linear fit lines of Digit Symbol Substitution Test (DSST) test scores. Linear fit lines were generated from mixed-effect model using linear terms including age at baseline, race, gender, education, APOE4 grouping and psychotic grouping as fixed effects and intercept and time as random effects (N=350).
When restricting to a smaller cohort including only individuals who completed all 9 assessment points and using only linear terms, the main effect of psychosis (F=12.04, df=1, 1645, p<0.001) and the linear interaction of time * psychosis (F= 21.63, df=1, 1645, p<0.001) remained significant. When using linear and quadratic terms in this group the main effect of psychosis and linear interaction of time * psychosis were still not significant, but the interaction of time2 * psychosis remained significant (F=9.27, df=1, 1643, p=0.002). In this confirmatory analysis, the chi-square distribution was again significantly lower in the mixed-effect model including both linear and quadratic terms (13829.9) than in the model with only linear terms (14032.0), the difference being 272.9 distributed as chi-square of 1 df.
Analysis of DSST scores displayed a similar effect in the mixed model including linear terms (Table 3; Figure 2, Panel B). Although the main effect of psychosis with lower DSST score was not significant, there was a significant linear interaction of time * psychosis such that the Ever Psychotic group had a more rapid rate of DSST score decline. Using this model but restricting only to those completing all 9 DSST assessments, the effect of psychosis and interaction of time * psychosis were no longer significant.
Table 3.
Results for Mixed Effect Model of DSST. N=350
| Effect | Model Parameters† | SE | DF | t | p |
|---|---|---|---|---|---|
| Age at Baseline | −0.4102 | 0.1034 | 1, 2018 | −3.97 | <.0001 |
| Race | −9.5978 | 1.7744 | 1, 2018 | −5.41 | <.0001 |
| Gender | −0.5616 | 0.9927 | 1, 2018 | −0.57 | 0.5716 |
| Education | 1.1248 | 0.1019 | 1, 2018 | 11.04 | <.0001 |
| APOE4 | 0.3349 | 1.0654 | 1, 2018 | 0.31 | 0.7533 |
| Psychotic Ever | −1.8555 | 1.5983 | 1, 2018 | −1.16 | 0.2458 |
| Time | −1.8790 | 0.1717 | 1, 348 | −10.95 | <.0001 |
| Time * Psychotic Ever | 0.6945 | 0.1899 | 1, 2018 | 3.66 | 0.0003 |
SE = Standard Error; DF = degrees of freedom; APOE4, Apolipoprotein-ε 4 allele
Model parameters are unstandardized estimates
Considering that the Ever Psychotic group completed fewer DSST assessments on average (results above), we examined whether the differences between the original analyses of effects on 3MS and DSST and the analyses restricted to completers might reflect differential drop-out from the more challenging DSST during follow-up due to the greater impairments associated with the Ever Psychotic group. In the completer analyses we included 235 participants who completed 3MS assessments at all 9 time-points out of 362 in the original analysis and 188 participants who completed all 9 DSST assessments out of 350 in the original analysis. A similar proportion of participants in each psychosis group completed all of the 3MS assessments and were included in the completer analysis (χ2=2.72, df=1, p=0.099). However, a significantly greater proportion of participants in the Ever Psychotic group were excluded from the DSST completer analysis because they missed one or more assessments (χ2=5.93, df=1, p=0.015).
The above analyses were confirmed in an enlarged cohort, created by only requiring individuals to have completed the 3MS or DSST at the first two visits, and including individuals without an available APOE4 genotype. The main effects in the primary analyses strengthened. In the 3MS model (N=418) including only linear terms, the effect of psychosis (F=15.46, df=1, 2566, p<0.001) and linear effect of time * psychosis (F=25.42, df=1, 2566, p<0.001) remained significant. Using linear and quadratic terms the quadratic effect of time2 * psychosis remained significant (F=20.35, df=1, 2564, p<0.001); the linear effect of time * psychosis strengthened but still only approached conventional levels of significance (F=3.29, df=1, 2564, p=0.070). In the DSST model (N=408), the effect of time * psychosis also remained significant (F=14.74, df=1, 2372, p<0.001).
We further evaluated whether the association between psychosis and cognitive decline was present in subject groups with MCI and with dementia. The dementia group (N=170) included 56 individuals from the Ever Psychotic group and the MCI group (N=192) included 18 from the Ever Psychotic group. For 3MS in the dementia group the effects of psychosis (F=5.72, df=1, 1041, p=0.017) and time * psychosis (F=7.39, df=1, 1041, p=0.007) remained significant in the model including only linear terms. When restricting to MCI cases there was not a significant effect of time * psychosis. In the model including linear and quadratic terms limited to dementia cases, the effect of time2 * psychosis remained significant (F=4.23, df=1, 1039, p=0.0399). When restricting to MCI cases, there was not a significant effect of time2 * psychosis. For analysis of the DSST in dementia cases, the linear interaction of time * psychosis remained significant (F=7.60, df=1, 922, p=0.006). In the analysis of the DSST in MCI cases, there was a not a significant effect of time * psychosis.
Conclusions
We found that the trajectory of cognitive decline in individuals destined to express psychotic symptoms during dementia diverges from that of non-psychotic individuals with dementia in the earliest disease stages. Individuals who developed AD+P, compared to those who developed AD without psychosis, reached late life with similar performance on two cognitive measures, the 3MS and the DSST. However, they subsequently deteriorated more rapidly. Because the risk for psychosis in AD has been shown to be substantially heritable,4,5 this suggests that genetic variations resulting in AD+P are likely to exert their effects via accelerating the earliest progression of neurodegeneration.
Although numerous studies have established the association of lower cognitive performance with AD+P later in disease course,1 we examined cognitive performance in the prodromal and early stages of dementia. A few prior studies have examined this relationship in clinical samples of individuals with prevalent cognitive disorders (AD or MCI), but prior to the onset of psychotic symptoms.8,9,10 These studies found more severe global cognitive burden measured on the MMSE was already present one to two years prior to psychosis onset, with Weamer et al.8 finding the effect was strongest in those individuals in early disease stages as defined by an MMSE score ≥ 20 at baseline. The rate of cognitive decline on the MMSE did not predict subsequent onset of psychosis in Weamer et al.8 or Paulsen et al 9, although Paulsen et al.9 found more rapid decline on other neuropsychological tests (Mattis Dementia Rating Scale Total Score and Attention and Construction Subscale Score; verbal fluency) to be associated with increased risk of psychosis onset. Our findings extend the findings of these prior studies, indicating that the acceleration in cognitive decline ultimately leading to the expression of psychosis in AD subjects occurs in the earliest disease stages in individuals with incident dementia. The acceleration of global cognitive decline in AD+P was evident from the outset of measurable decline in our group (Figure 2).
The more rapid decline in 3MS in AD+P subjects remained significant when restricting to those with all data present, while findings related to DSST decline no longer held. This is possibly due to biases inherent in data collection in this population, i.e., individuals with greater degrees of cognitive impairment have more difficulty completing complex neuropsychiatric measures like the DSST. If this is the case, those who declined more rapidly would be more likely to become impaired enough during follow-up that they were no longer able to complete the measure. Non-random exclusion of the most severely impaired individuals may have limited our ability to detect a potentially true association between psychosis and decline on the DSST. Our findings that a greater proportion of individuals in the Ever Psychotic group missed at least one DSST assessment, and that this group missed more DSST assessments on average would support this interpretation.
Some other limitations should be considered in interpreting these results. Individuals with MCI may develop pathologies other than AD, but in a follow-up of this cohort 3–4 years later, the most common diagnosis of those with MCI in 1998–9 was AD.20 Missed assessments and dropout also could have biased the results, although the concurrence of multiple analyses and similar rate of missed assessments for the 3MS makes this less likely for this measure. Also, our cohort had a low rate of missed assessments for a longitudinal study of this magnitude, with most participants completing over 85% of annual assessments.
The timing of the separation of the cognitive courses of AD+P and AD−P individuals may be a cue to pathologic mechanisms contributing to psychosis risk in AD. It has been appreciated for a number of years that the strongest correlate of cognitive impairment in individuals with AD is loss of synapses across neocortical regions,21 with excitatory synapses onto dendritic spines particularly affected.22 Evidence now indicates that self-aggregation of Aβ into soluble low-n oligomers is a primary source of synaptotoxicity in AD.23 In vitro observations of the deleterious effects of soluble Aβ oligomers on synapses have been complemented by findings from animal and human postmortem studies. This includes evidence from animals transgenic for mutant human APP that deficits in synaptic structure and function precede deposition of insoluble Aβ into plaques and correlate with cortical soluble Aβ levels, but do not correlate with plaque numbers or APP levels.23,24 Similarly, in some transgenic animals there is evidence for early synapse loss that exceeds neuronal loss.25 Human studies indicate that cortical synapse loss is an early pathologic event and that cognitive impairments and synapse loss correlate most strongly with soluble Aβ, even in subjects with early disease.26
Thus, to the extent that the risk for AD+P may be genetically determined, it is likely that the effect of genetic variation is to accelerate the process of synaptic loss in early disease stages. This interpretation is consistent with our prior post-mortem magnetic resonance spectroscopy study, which provided biochemical evidence consistent with increased synaptic disruption (albeit in end stage disease) across multiple neocortical regions in subjects with AD+P.27 Similarly, reductions in densities of pre-synaptic axon boutons and post-synaptic dendritic spines are among the most consistently replicated findings in subjects with schizophrenic psychosis.28 Whether increased synapse loss in AD+P is mediated via processes which increase the accumulation of soluble Aβ itself, or via processes which increase the synaptotoxicity of soluble Aβ’s downstream mediators (including microtubule associated protein tau), is not known. However, most studies have found no consistent association between AD+P risk and indexes of insoluble Aβ in end stage illness.29 Future examinations of potential early neurobiological differences between AD−P and AD+P, such as differential accumulation of Aβ, are warranted.
Acknowledgments
Supported in part by USPHS grant AG05133 and AG027224 from the National Institute of Aging. The research reported in this article was also supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke and grant AG15928 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm
Footnotes
Previously presented at the 63rd Annual Scientific Convention and Program of the Society of Biological Psychiatry, Washington, DC, May 1–3, 2008.
Analyses performed by: Patricia R. Houck, M.S.H., Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA
James E. Emanuel, B.S., Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA
The authors have no conflicts of interest to report.
Reference List
- 1.Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- 2.Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46:210–15. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 3.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacanu SA, Devlin B, Chowdari KV, et al. Heritability of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:624–627. doi: 10.1176/appi.ajgp.13.7.624. [DOI] [PubMed] [Google Scholar]
- 5.Sweet RA, Nimgaonkar VL, Devlin B, et al. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002;58:907–911. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- 6.Hollingworth P, Hamshere ML, Holmans PA, et al. Increased familial risk and genomewide significant linkage for Alzheimer’s disease with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:841–848. doi: 10.1002/ajmg.b.30515. [DOI] [PubMed] [Google Scholar]
- 7.Sweet RA, Nimgaonkar VL, Devlin B, et al. Psychotic symptoms in Alzheimer disease: evidence for a distinct phenotype. Mol Psychiatry. 2003;8:383–392. doi: 10.1038/sj.mp.4001262. [DOI] [PubMed] [Google Scholar]
- 8.Weamer EA, Emanuel JE, Varon D, et al. The relationship of excess cognitive impairment in MCI and early Alzheimer’s disease to the subsequent emergence of psychosis. Int Psychogeriatr. 2008:1–8. doi: 10.1017/S1041610208007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulsen JS, Salmon DP, Thal L, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable Alzheimer’s disease. Neurology. 2000;54:1965–1971. doi: 10.1212/wnl.54.10.1965. [DOI] [PubMed] [Google Scholar]
- 10.Wilkosz PA, Miyahara S, Lopez OL, et al. Prediction of Psychosis Onset in Alzheimer Disease: The Role of Cognitive Impairment, Depressive Symptoms, and Further Evidence for Psychosis Subtypes. Am J Geriatr Psychiatry. 2006;14:352–356. doi: 10.1097/01.JGP.0000192500.25940.1b. [DOI] [PubMed] [Google Scholar]
- 11.Elias MF, Beiser A, Wolf PA, et al. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 12.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 13.Lopez OL, Kuller LH, Fitzpatrick A, et al. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 15.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 16.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 17.Wechsler Adult Intelligence Scale Manual. New York: Psychological Corporation; 1955. [Google Scholar]
- 18.Lopez OL, Becker JT, Jagust WJ, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77:159–165. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 20.Lopez OL, Kuller LH, Becker JT, et al. Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol. 2007;64:416–420. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- 21.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 22.Grutzendler J, Helmin K, Tsai J, et al. Various dendritic abnormalities are associated with fibrillar amyloid deposits in Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:30–39. doi: 10.1196/annals.1379.003. [DOI] [PubMed] [Google Scholar]
- 23.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 24.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 25.Hsia AY, Masliah E, McConlogue L, et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 27.Sweet RA, Panchalingam K, Pettegrew JW, et al. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002;23:547–553. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 28.Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sweet RA, Hamilton RL, Lopez OL, et al. Psychotic symptoms in Alzheimer’s disease are not associated with more severe neuropathologic features. Int Psychogeriatr. 2000;12:547–558. doi: 10.1017/s1041610200006657. [DOI] [PubMed] [Google Scholar]