Abstract
Background & Aims
Chronic visceral pain is frequent, extremely debilitating, and generally resistant to pharmacological treatment. It has been shown that chronic visceral inflammation, through altered afferent visceral sensory input, leads to plastic changes in the central nervous system that ultimately sustain pain. Therefore approaches aiming at modulation of brain activity are attractive candidates to control visceral pain.
Methods
Here we report findings of a phase II, sham-controlled clinical trial assessing the clinical and neurophysiological effects of a 10-day course of daily sessions of slow frequency, repetitive transcranial magnetic stimulation (rTMS) targeting the right secondary somatosensory cortex (SII) in patients with chronic pancreatitis and severe visceral pain.
Results
Our results show a significant reduction in pain after real rTMS that lasted for at least 3 weeks following treatment. These clinical changes were correlated with increases in glutamate and N-acetyl aspartate (NAA) levels - neurometabolites associated with cortical activity and brain damage - as measured by in vivo single-voxel proton magnetic resonance spectroscopy (1H-MRS). Adverse effects in the real rTMS group were mild and short-lasting.
Conclusions
Our results support preliminary findings showing that modulation of right SII with rTMS is associated with a significant analgesic effect and that this effect is correlated with an increase in excitatory neurotransmitter levels such as glutamate and NAA.
Keywords: Visceral pain, chronic pain, neuromodulation, brain stimulation, transcranial magnetic stimulation, brain metabolites, magnetic resonance spectroscopy
Introduction
Visceral pain in chronic pancreatitis is exceedingly refractory to medical and surgical treatments(Zorn et al 1994). Most patients experience debilitating pain despite the use of high doses of morphine and other narcotics. Videothoracoscopic splanchnicectomy leads to short-term pain relief only in a small fraction of patients (Maher et al 2001), and even total pancreatectomy fails to relieve pain in up to 30% of patients (Rattner et al 1996). In fact, in a recent study, Rattner and colleagues showed that distal pancreatectomy for pain relief had poor results in 6 patients (out of 19 patients) (Rattner et al 1996). We hypothesized, that a major reason for this treatment-resistance is that chronic visceral inflammation, through altered afferent visceral sensory input, leads to plastic changes in the spinal cord and brain (Giamberardino et al 1997), and that these neuroplastic changes may ultimately be responsible for sustaining visceral inflammation as well as pain (Patrizi et al 2006). If so, it is not surprising that treatments aimed at controlling visceral inflammation or activity in the peripheral nervous system provide at best limited, transient relief. Modifying activity in appropriate central nervous system targets might be necessary to control chronic visceral pain, and in a small pilot study, we found that modulation of activity in the right, but not the left, secondary somatosensory (SII) cortex with slow-frequency, but not with high-frequency, repetitive transcranial magnetic stimulation (rTMS) resulted in a significant reduction in pain (Fregni et al 2005).
Here we report the results of a phase II, sham-controlled clinical trial of rTMS in chronic pancreatitis. In addition to the assessment of the clinical impact on visceral pain, we aimed to gain insights into the mechanisms of action of the stimulation by using single-voxel proton magnetic resonance spectroscopy (1H-MRS) to assess brain chemistry and metabolic activity before and after treatment, and relate the findings to the level of pain.
Methods
Participants
Patients were prospectively and sequentially selected from the Pancreas Center at Beth Israel Deaconess Medical Center with some individuals referred from the Pancreas Clinic at Brigham and Women’s Hospital. They were regarded as suitable to participate in the study if they carried the diagnosis of chronic pancreatitis and fulfilled the following criteria: (i) age between 18 and 65 years; (ii) presence of daily abdominal pain for at least three months attributed to their chronic pancreatitis; and (iii) average pain scores (VAS) in the baseline period higher than 4 out of 10. The diagnosis of chronic pancreatitis was based on the existence of chronic abdominal pain and at least one of the four following criteria: (1) calcifications throughout the pancreas on plain abdominal radiograph; (2) endoscopically derived pancreatogram showing beading and irregularity of the pancreatic duct characteristic of chronic pancreatitis; (3) abnormal secretin pancreatic function test with a measured bicarbonate level of less than 70 mEq/L; or (4) tissue diagnosis of chronic pancreatitis from a surgical specimen. Patients were excluded if they had: (i) undergone a prior neurosurgical procedure; (ii) a past history of seizures or unexplained spells of loss of consciousness; (iii) previous severe head injury; (iv) metal in the head, i.e. shrapnel, surgical clips, or fragments from welding; (v) signs of increased intracranial pressure; (vi) previous stroke; (vii) abnormal neurological examination; (viii) implanted pacemaker, medication pump, vagal stimulator, deep brain stimulator or ventriculo-peritoneal shunt; (ix) pregnancy; (x) history of substance abuse; or (xi) known complications of chronic pancreatitis requiring interventions including pseudocysts, pancreatic duct obstruction or cancer.
We screened on average 2 patients per week during the period of two years and enrolled seventeen patients (mean age of 43.5 ± 11.9 years, mean ± SD, 14 females) to participate in the study. The main reasons for exclusion were: history of substance abuse, complications of chronic pancreatitis, limitations for rTMS use (such as history of seizures), and inability to commit to 10 daily rTMS sessions over 2 consecutive weeks. The general clinical characteristics of the patients are summarized in table 1 and supplementary table 1. The study was approved by the Beth Israel Deaconess Medical Center institutional review board, and participants were enrolled after granting written informed consent following a full explanation of the purpose of the study and procedures. Patients stayed in the Harvard-Thorndike Clinical Research Center (CRC) throughout the study allowing for intensive nursing support, careful oversight of medication and nutritional intake, and detailed pain measurements. This trial was registered at ClinicalTrials.gov as NCT00130052.
Table 1.
Demographic characteristics
| Sham rTMS | Real rTMS | |
|---|---|---|
| Mean age (years (SD) | 46.71 (13.03) | 41.11 (11.27) |
| Females (total) | 6(8) | 8(9) |
| Duration of disease (n) | ||
| > 5 years | 7 | 5 |
| 2–5 years | 1 | 4 |
| < 2 years | 0 | 0 |
| Duration of opioid analgesics use (continuous) (n) | ||
| >5 years | 5 | 3 |
| 2–5 years | 1 | 4 |
| <2 years | 2 | 2 |
| Previous nerve procedure for pain treatment (n)* | ||
| Yes | 1 | 2 |
| No | 7 | 7 |
| Surgical treatment for chronic pain and pancreatitis** (n) | ||
| Yes | 4 | 6 |
| No | 4 | 3 |
n – number of subjects
bilateral thoracoscopic splanchnicectomy
four patients underwent whipple procedure, three patients underwent bilateral thoracoscopic splanchnicectomy, three patients had surgical sphincteroplasty and eight patients had no surgical procedure.
Transcranial Magnetic Stimulation (TMS) treatment
Patients were randomized (using a computer generated list with blocks of 4) to receive 10 sessions of real or sham repetitive TMS administered five days per week (weekdays only) for two consecutive weeks (see figure 1). Stimulation was delivered with a Magstim Super Rapid magnetic stimulator (Magstim Company Ltd, Wales, UK) and a figure 8-shaped coil with each loop measuring 7 cm in diameter. A specially designed sham coil was used for sham stimulation (Magstim Company Ltd, Wales, UK). The sham and the real TMS coils looked identical and were matched for weight and acoustic artifact. This sham coil induces a similar tapping sensation and generates the same clicking noise as the real TMS coil, but without induction of a significant magnetic field and secondary current. Repetitive TMS was applied by positioning the stimulation coil over the secondary somatosensory cortex (SII) on the subject’s scalp guided by each participant’s anatomical brain magnetic resonance image (MRI) using a frameless stereotactic system. The frameless stereotactic system (Brainsight, Rogue Research, Montreal, QC, Canada) allowed accurate localization of the cortical region to be targeted by TMS on each subject’s own anatomical MRI and to monitor targeting throughout each TMS session and across sessions in order to assure reliability. The methodology has been previously described and the efficacy in assuring a constant, reliable targeting of a given brain region has been well documented (Gugino et al 2001). The secondary somatosensory cortex SII was defined by macroanatomical landmarks based on each participant’s high-resolution, 3D anatomical brain MRI. The targeted brain region was defined as the cortical area adjacent to the junction of the rostral end of the post-central gyrus and the Sylvian fissure. Each TMS session consisted of 1600 pulses of TMS applied at 1Hz frequency and 70% of maximal output stimulator intensity. These parameters were approved by the IRB and FDA, and proved to be safe in our pilot study (Fregni et al 2005) and in a detailed review of the literature on rTMS to non-motor brain areas (Machii et al 2006). We chose not to set the stimulation intensity as percent of each patient’s motor threshold since there is no evidence of a correlation of motor threshold intensity and the effects of rTMS to non-motor brain regions (Machii et al 2006). These stimulation parameters suppress activity in the targeted brain region in most subjects as evidenced by studies in humans and animal models (Chen et al 1997; Maeda et al 2000; Valero-Cabre et al 2007; Valero-Cabre et al 2005).
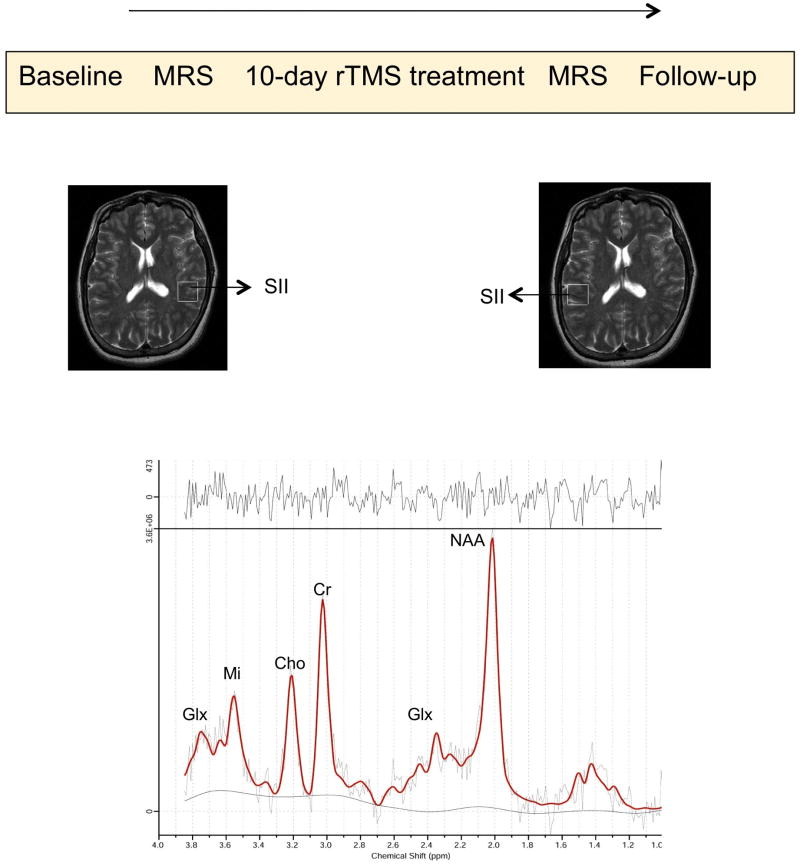
Figure 1.
Diagram showing order of intervention and assessments and sagital brain slice showing the voxel in left and right SII. The graph at the bottom represents the peaks of brain metabolites.
Clinical Evaluation
Pain intensity was assessed using visual analogue scales (VAS) where 0 represents no pain and 10 the most intense pain imaginable. The pain evaluation was carried out by a blinded rater and was conducted according to the following schedule (figure 1): (i) baseline evaluation: for 3 consecutive weeks participants recorded pain logs daily and kept a diary of pain medication intake including chronic pain medications and those taken on as needed basis; (ii) treatment evaluations: participants were asked to fill out daily pain logs following each TMS session, and keep a diary of pain medication during the CRC stay for the TMS course, and (iii) follow-up evaluation 3 weeks after treatment. In addition to the pain measurements, at the end of each study week, Beck Depression Inventory (BDI), VAS for anxiety, and Adverse Event Monitoring Tests were completed by a blinded rater.
Adverse event monitoring and reporting
Participants were carefully observed for seizures or seizure-like activity during and for 30 minutes after rTMS. In addition, we assessed adverse events using a structured questionnaire listing the most common adverse effects associated with rTMS (headache, neck pain, scalp burning, hearing difficulties, cognitive changes, changes in concentration or mood changes), as well as open-ended questions.
Magnetic Resonance Spectroscopy
Participants underwent two magnetic resonance imaging (MRI) studies (before and after rTMS treatment) using a 3-T whole-body scanner and a transmit-receive head coil (General Electric Medical Systems, Milwaukee, Wisconsin) capable of providing a homogeneous radiofrequency (RF) field and spectroscopic measurements from brain tissue. We were interested in studying brain metabolites at the site of TMS and in the homologous area in the contralateral (unstimulated) hemisphere. Therefore, proton magnetic resonance (MR) spectra were acquired from two volumes of interest centered in the macroanatomically-defined right and left secondary somatosensory cortex (SII). The volume of interest comprised a 2 × 2 × 2 cm3 cube positioned in the cortical area adjacent to the junction of the rostral end of the post-central gyrus and the Sylvian fissure. Figure 1 shows the placement of this volume of interest in the horizontal plane in a representative patient.
MR Spectra were acquired with a single voxel double spin-echo point-resolved spectroscopy (PRESS) pulse sequence (Bottomley et al 2005). The PRESS acquisition parameters were an echo time of 35 ms, repetition time of 3000 ms with 128 averages and a spectral bandwidth of 5000 Hz. The spectral acquisition time was 4 min and 20 s for each voxel, including time for setup.
Proton spectra were analyzed by measuring heights of specified peaks by an investigator (X.W.) blinded to each participant’s treatment assignment (real or sham). The peak heights ratio method is precise and accurate if the peak height is directly proportional to the peak area (Ross and Bluml 2001). We measured concentrations of N-acetyl aspartate (NAA), choline (Cho), glutamate (Glu), glutamine (Gln), myo-inositol (Mi) and total creatine (Cr).
Statistical Analysis
Analyses were done with Stata® statistical software (version 8.0, College Station, Texas). We initially performed a mixed ANOVA model. Dependent variables were pain measurement as indexed by VAS (or the other clinical outcomes such as mood assessment or other outcomes measuring pain) or brain metabolites (as indexed by MRS). Independent variables were condition of stimulation (sham vs. real rTMS) and time (baseline vs. after treatment). In addition, hemisphere (left vs. right SII) was included as an independent variable for the model focusing on brain metabolites as the main variable. We also added the random variable subject ID to control for within subject variability (factors: hemisphere and time). When appropriate, post-hoc comparisons were performed using Bonferroni corrections for frequent comparisons.
Regarding the assessment of brain metabolites, we used 2-tailed paired t tests for post-hoc analyses to detect changes in brain chemicals between baseline values and those after treatment. Our main outcome was to detect changes in glutamate and NAA levels, thus we used a Bonferroni-adjusted significance level of P = .025 for hypothesis testing. For all other analyses, we considered them as exploratory secondary analyses; therefore no correction for multiple comparisons was performed. In addition, also as secondary analyses, we computed Pearson correlation coefficients to assess the relationship between brain metabolites concentrations and clinical changes in pain. Given the large number of statistical comparisons performed in assessing these correlations, the results of the secondary analyses were considered exploratory and we did not apply Bonferroni adjustments (significance level set at p = .05).
Finally, for the analysis of adverse effects, we compared the frequency of events in each group by constructing a table and measuring associations between cells using Fisher’s exact test.
Results
We enrolled seventeen subjects (mean age of 43.5 ± 11.9 years, mean ± SD, 14 females) to participate in the study. Most of the subjects had at least 5 years of disease (12/17), and also most of them were using continuous opioid analgesics for at least 2 years (13/17). Finally most of them had surgical procedures for pain treatment (10/17).
Clinical Outcome
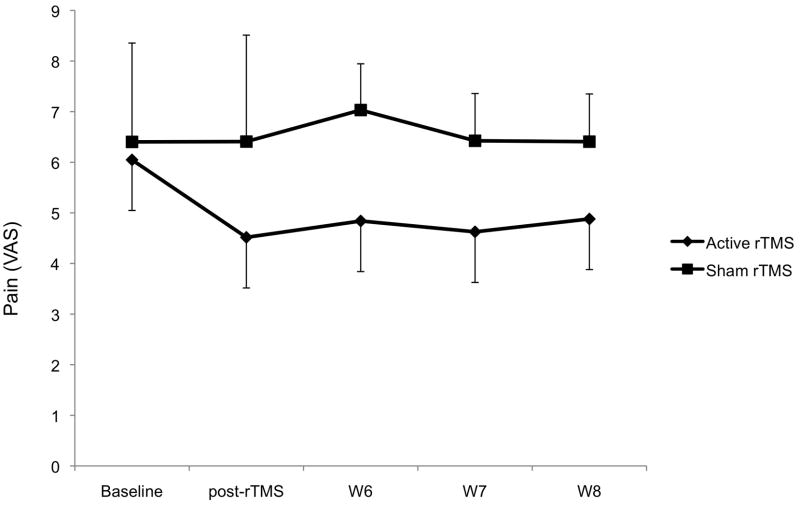
We initially assessed the effects of rTMS during the treatment. The ANOVA model disclosed a significant interaction term time*condition of stimulation (F(1, 15)=0.014). There was a significantly larger improvement in the real as compared to sham stimulation group (p=0.015). For the period during and 1 week after, real stimulation induced a mean decrease in pain levels of 27.2% (± 24.5%), while after sham stimulation there was a small increase in pain levels of 1.1% (± 17.1%) (see figure 2). Pain levels at the end of 2 weeks of rTMS were not different than those at the end of the three-week follow-up (p=0.99 and p=0.71 for the real and sham rTMS groups, respectively). This indicates that the analgesic effects of a 2-week course of real rTMS is sustained over the entire course of treatment.
Figure 2.
Pain changes throughout the trial. There was a significant difference between active and sham rTMS treatment. Error bars represent the standard error of the mean (S.E.M.).
One question is whether the effects of rTMS are modified by previous surgical procedures. This analysis is important as surgical procedures might result in a change in peripheral sensory processing that might affect central and peripheral sensitization. In our sample of patients, four patients underwent whipple procedure, three patients underwent bilateral thoracoscopic splanchnicectomy, three patients had surgical sphincteroplasty and seven patients had no surgical procedure. Given each procedure, there was no trend to favor one procedure against the other regarding pain relief, neither when comparing each surgical procedure vs. not having a surgical procedure (p>0.2 for these two analyses). The small sample size might also explain the lack of significant results.
Adverse Effects
As summarized in table 2, patients in the real rTMS group complained more often of headache and neck pain (41 vs. 19 and 18 vs. 3, mean number of reports, headache and neck pain for real vs. sham rTMS group, respectively) (p=0.01, Fisher’s exact test). However, subjects who reported headache and neck pain described them as mild and short lived with symptoms typically resolving 1 or 2 hours after the end of the stimulation. Importantly, there were no seizures or other serious adverse effects in any of the treatment groups.
Table 2.
Adverse effects during rTMS treatment
| Sham rTMS | Real rTMS | |
|---|---|---|
| Headache | 19 | 41 |
| Neck | 3 | 18 |
| Scalp | 0 | 5 |
| Seizure | 0 | 0 |
| Hearing | 0 | 2 |
| Cognition | 13 | 15 |
| Concentration | 30 | 29 |
| Mood | 18 | 21 |
Numbers represent mean number of adverse effects for each group of treatment
Confounding factors – mood and anxiety
We assessed whether improvements in pain were due to an effect of rTMS on mood and anxiety. We conducted a mixed ANOVA in which the dependent variable was mood as indexed by BDI and anxiety as indexed by VAS. The interaction term was not significant for either model (F (1,22)=0.23, p=0.64, F(1,22)=1.71, p=0.20; for mood and anxiety assessment, respectively) (table 3). This result indicates that mood and anxiety did not change differentially between the sham and real TMS groups and therefore were not confounding the observed effects on visceral pain.
Table 3.
Confounders (mood and anxiety)
| Sham rTMS | Real rTMS | |
|---|---|---|
| Beck Depression Inventory | ||
| Baseline | 22.11 (10.71) | 23.05 (12.55) |
| After Treatment | 21.69 (12.19) | 18.31 (10.54) |
| Anxiety (VAS) | ||
| Baseline | 6.07 (1.66) | 4.26 (2.70) |
| After Treatment | 7.13 (2.90) | 3.35 (2.27) |
Mean mood and anxiety scores before and after the treatment (numbers between parenthesis represent the standard deviation)
Magnetic Resonance Spectroscopy (MRS) Results - Baseline levels of brain metabolites
At baseline, there was no difference in metabolite levels in either hemisphere comparing participants in the real and sham stimulation groups (supplementary table 2). Additionally, there were no significant correlations between baseline pain scores and any of the metabolites analyzed (N-acetyl aspartate, creatine, choline, glutamate, glutamine, myo-inositol) in right or left SII (p>0.5 for all the correlations). Similarly, we found no significant correlations between interhemispheric differences across right and left SII for any of the metabolites studied and baseline pain scores.
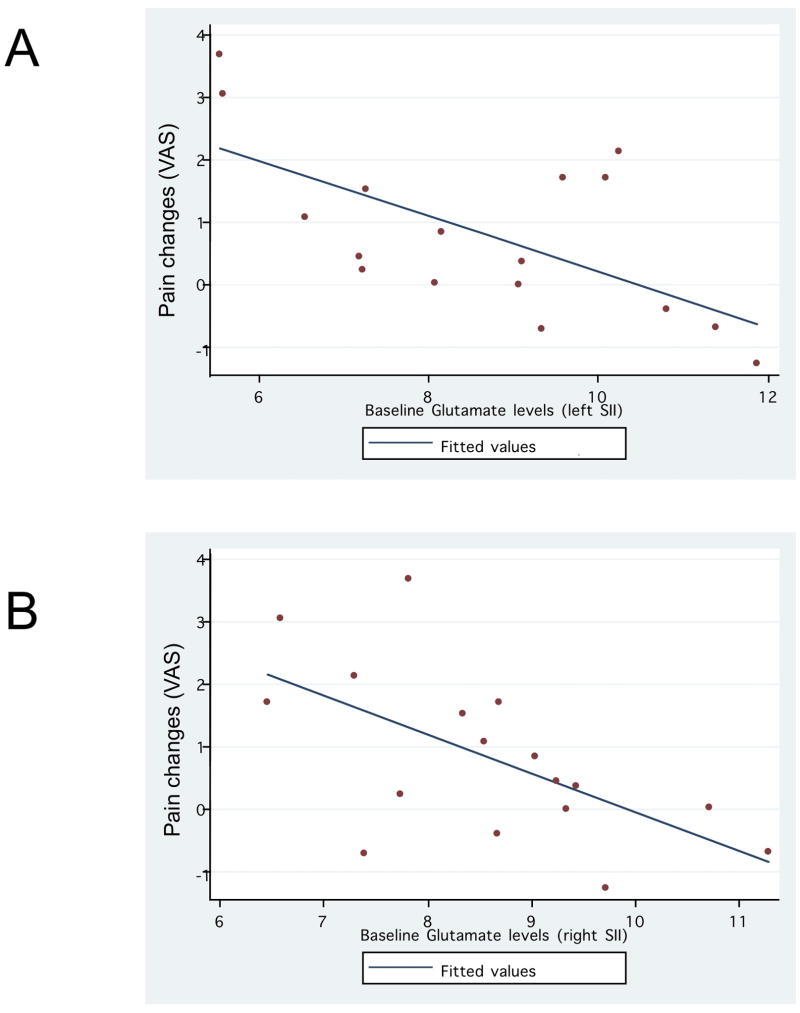
Finally we assessed whether baseline metabolite levels were correlated with a reduction in pain severity over the course of the study regardless of the treatment group. This analysis (figure 3A) showed a significant negative correlation between pain response and baseline glutamate level (r=−0.60, p=0.01 for right SII and r=−0.63, p=0.0066 for left SII) and a trend for the correlation with baseline NAA for the right hemisphere (r=−0.41 and p=0.09) (figure 3B). Thus, the lower the baseline glutamate level (and possibly also the baseline NAA) in the right SII, the greater the reduction in pain over the course of the study.
Figure 3.
Correlation between pain changes after treatment and baseline glutamate levels at right and left SII.
Magnetic Resonance Spectroscopy (MRS) Results - Changes in glutamate and NAA levels
We assessed changes in glutamate levels with a model in which the dependent variable was glutamate levels and the independent variables were condition of stimulation (real vs. sham), hemisphere (left vs. right SII) and time (baseline vs. post-treatment). The three-way interaction term (condition*hemisphere*time) was significant (F(11,48)=2.68, p=0.0092). We therefore performed pair-wise comparisons. For the sham group, there were no significant changes in glutamate levels when comparing post-treatment vs. baseline for either left or right SII (p>0.5 for both comparisons) (supplementary table 3). However, for the real rTMS group, there was a significant increase in glutamate levels in both hemispheres after stimulation (p=0.0038 for the left SII and p=0.01 for right SII).
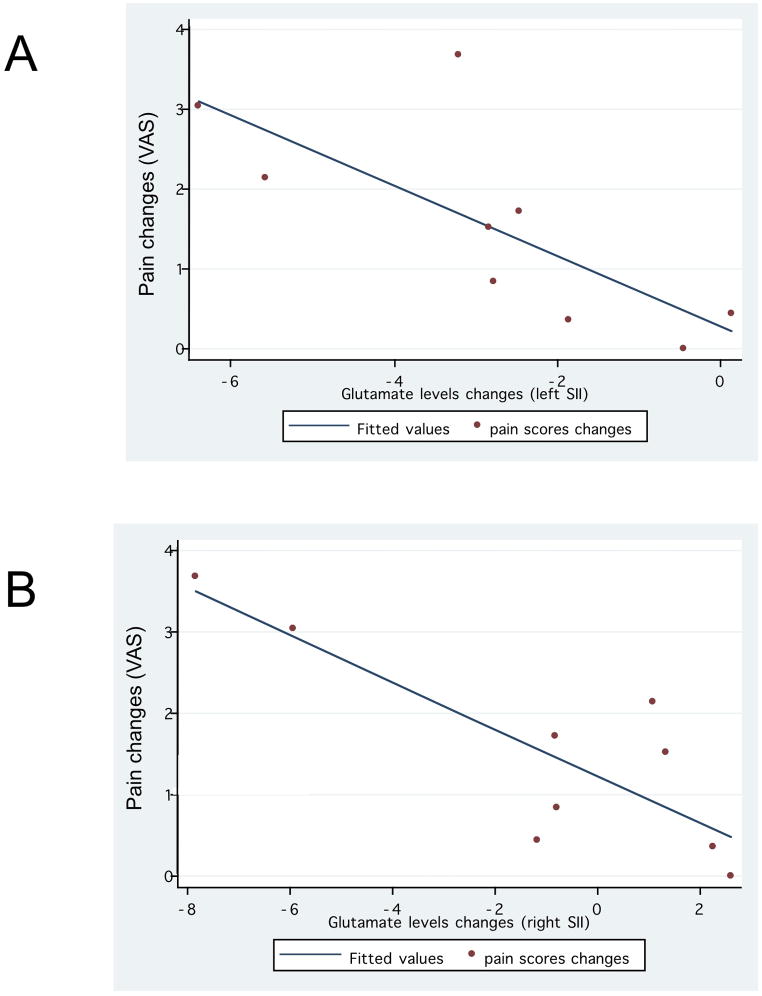
We then compared changes in glutamate levels with changes in pain severity and found a significant positive correlation for both hemispheres in the real rTMS group (r=0.74 and p=0.021 for left SII; and r=0.82 and p=0.006 for right SII, figure 4A and 4B).
Figure 4.
Correlation between glutamate levels changes in the left and right SII and pain changes in the active rTMS group only.
The same analysis was performed for NAA. First, our model showed a significant three-way interaction term (condition*hemisphere*time -F(11,48)=5.13, p<0.0001). We then performed pair-wise comparisons. For the sham group, there were no significant changes in NAA levels when comparing post-treatment vs. baseline for either left or right SII (p>0.5 for both comparisons). However, for the real group, there was a significant increase in NAA levels after stimulation in both hemispheres (p=0.0062 for the left SII – and p=0.0075 for right SII for NAA levels, see supplementary table 3). We then compared NAA levels with clinical changes, and found a trend for a positive correlation between changes in pain severity and changes in NAA levels for both hemispheres (r=0.66 and p=0.051 for right SII; and r=0.60 and p=0.08 for left SII).
Magnetic Resonance Spectroscopy (MRS) Results - Other metabolites
Analysis of other metabolites – creatine, myo-inositol, choline and glutamine – using similar models to those described for glutamate and NAA revealed an interaction term (condition*hemisphere*time), which was not significant (p>0.5 for all the models). Supplementary table 3 shows the absolute values for each time-point.
Discussion
The findings from our preliminary study show that rTMS of SII is an efficacious approach to relieve chronic visceral pain as compared to sham stimulation. This beneficial effect of brain stimulation to decrease chronic visceral pain supports the study hypothesis that pain in chronic pancreatitis is, at least in part, due to brain activity dysfunction or alternatively SII stimulation can elicit top down mechanisms responsible to activate the descending pain inhibitory system.
Clinical impact: The analgesic effect of rTMS in chronic pancreatitis
Our study provides sham-controlled preliminary evidence of the therapeutic efficacy of non-invasive rTMS of the right SII in patients with visceral pain due to chronic pancreatitis. The present findings confirm results of our small pilot data (Fregni et al 2005) and are consistent with the notion that non-invasive neuromodulation of specific cortical targets can have therapeutic utility in chronic pain (Jensen et al 2007; Khedr et al 2005; Lefaucheur et al 2004). Although adverse effects were larger in the real rTMS group, the encountered side-effects have all been previously reported (Machii et al 2006) and all were mild and short-lived. Compared to other treatments for chronic visceral pain, such as opioid analgesics, rTMS treatment has an evident better tolerability profile.
One important issue that needs to be discussed here is the role of SII in visceral pain processing. The secondary somatosensory (SII) cortex is a critical brain region involved in sensorimotor integration, integration of information from the two body halves, and also pain-related attention, learning and memory, including visceral input processing. In a magnetoencephalography (MEG) study, Schnitzler et al (1999) showed that visceral afferents from the oesophagus primarily project to the SII cortex (Schnitzler et al 1999). Activation of frontoparietal opercular areas containing SII cortex has also been observed during the mechanical stimulation of the oesophagus through PET (Aziz et al 1997) (Aziz et al 2000) and fMRI studies (Seitz et al 1998). Furthermore, rectal stimulation also results in bilateral activation of the inferior SI and SII (Song et al 2006). In addition it has been shown that bilateral painful rectal distenction leads to activation of the primary (S1) and secondary (S2) somatosensory cortices, the motor cortex, the frontal inferior gyrus, the thalamus, the insula, the striatum and the cerebellum as shown by fMRI and confirmed by DTI assessment showing connections between these areas to demonstrate the visceral pain network (Moisset et al 2010). In addition a recent EEG study comparing healthy vs. patients with chronic pancreatitis has shown that chronic pancreatitis patients have decreased latencies of the early event-related potentials (as compared with healthy subjects) located consistently in the bilateral insula, anterior cingulate gyrus, and in the bilateral secondary somatosensory area (Dimcevski et al 2007). Besides visceral processing, SII is also associated with non-visceral nociceptive processing such as painful stimuli in the hand (Hsiao et al 2008).
Although we chose SII as the target in our study, it is possible that pain would also be decreased after stimulation of other targets including the primary somatosensory cortex (although a recent study showed no significant effects for stimulation of this area for central pain (Hirayama et al 2006)) and primary motor cortex (a major area used in brain stimulation studies that has been shown consistently good results (see (Lima and Fregni 2008)). In addition we chose right (not left SII) as in our previous pilot study only right-1Hz stimulation was associated with a significant pain improvement with a mean decrease of pain of 62% (+/−26%) (Fregni et al 2005). In fact, in our previous pilot study we also showed that left SII stimulation with either 1Hz and 20Hz caused pain worsening (mean worsening of 45%, +/−33%).
Along these lines, it should be considered the distant network effects of right SII stimulation. Because this area is highly connected with adjacent areas associated with somatosensory processing such as SI, thalamic areas and insula, it is possible that effects observed in this study were due to secondary modulation of these areas. One interesting aspect was that the most common adverse effect in this study was pain – similar to stimulation of the insular cortex observed in other studies (Afif et al 2008); therefore it should be considered the alternative explanation that the analgesic effects observed here are due to secondary modulation of other areas associated with the visceral pain related neural network. Further studies should also investigate other targets by measuring and modulating activity in other cortical and subcortical areas.
Different modalities of neuromodulation such as other techniques of noninvasive brain stimulation (electrical alternating and direct current stimulation – tACS and tDCS) in combination with other treatments such as psychotherapy and pharmacological treatment might also result in effective treatments for chronic visceral pain. Non-invasive brain stimulation might serve as proof-of-principle to guide implantation of epidural or subdural electrodes for minimally invasive cortical stimulation, which would allow more continuous treatment and potentially lead to longer lasting benefits. In addition, other conditions such as irritable bowel syndrome and functional dyspepsia might benefit from treatment with neuromodulation and therefore should be explored in future brain stimulation trials.
One important insight here is that visceral pain leads to significant plastic changes that are responsible to maintain pain levels despite the activation of visceral nociceptors. In fact other studies have shown that activity changes in brain areas, such as the cingulate cortex leads to a change in visceral processing that can result in pain (Wu et al 2008; Yan et al 2009). In this context, it is important to explore other factors that can increased plasticity in pain-related neural networks, for instance, whether certain psychological or genetic traits are associated with increased plasticity and therefore increased likelihood of chronic pain development. Therefore novel treatments should also focus on factors that can promote increased plasticity of pain-related brain areas.
Our results need also to be discussed in view of other theories that explain the lasting nature of pain in chronic pancreatitis. A recent theory hypothesizes that pain in chronic pancreatitis might be sustained by changes in pancreatic nerve fibers due to continuous local inflammation. Some inflammatory mediators such as Interleukin (IL) 1 and SP can induce an increase in neurothrophic nerve factors that may lead to an enlargement of pancreatic nerves and promote peripheral sensitization (Maher et al 2001). In this context, the beneficial effects of rTMS might be conceptualized from two different perspectives: (i) rTMS may decrease cortical processing of increased afferent signals from pancreatic areas; and (ii) rTMS might also modulate the immune system (Fregni et al 2007). However in the latter case we would expect that potential changes in nerve size and shape would be seen only in the long term.
Insights into mechanisms of action of rTMS using MR Spectroscopy to measure brain metabolites
MR spectroscopy is a useful tool to measure brain metabolites in vivo. In this study, we found changes in two metabolites: glutamate and NAA. Glutamate is the major excitatory transmitter in the brain and can be modulated by rTMS as shown by a recent study in which stimulation of the prefrontal cortex with high-frequency rTMS increased glutamate levels. (Luborzewski et al 2006). N-Acetyl-Aspartate (NAA), on the other hand, is a marker of neuronal functionality. It is localized within neurons, being involved in synaptic processes, and can be considered a neuronal and axonal marker (Miller 1991). It may be decreased with degenerative states such as Alzheimer’s disease (Miller et al 1993; Renshaw and Cohen 1993).
In our study, we initially measured baseline metabolite levels and did not find a significant correlation between baseline levels of brain metabolites and baseline pain scores. One explanation is that glutamate levels are not associated with pain processing or pain level as indexed by VAS does not represent level of pain-related brain areas processing activity. Another potential explanation is that chronic pain is related to disturbance in an extensive neural network, rather than a single brain cortical node. Therefore, the lack of MRS findings at baseline may also simple reflect the fact that the dysfunction is distributed cortico-subcortically across the hemispheres and not localized in a single cortical area.
In contrast, we found that that real rTMS induces a significant increase in glutamate and NAA levels in both right and left SII, and these changes are correlated with clinical changes in visceral pain. In addition, glutamate levels in left and right SII seem to be a predictor of response to rTMS treatment. Although in our study rTMS was applied only to right SII, we observed changes also in the left SII that were potentially induced directly by transcallosal connections or indirectly via thalamic circuitry as suggested by previous studies (Blankenburg et al 2008; Krubitzer et al 1998).
These results provide novel insights into the effects of a neuromodulation approach for the treatment of chonic visceral pain. 1-Hz rTMS led to an increase in glutamate – a major excitatory neurotransmitter (Behar and Rothman 2001) – rather than a decrease as might be expected given findings suggesting that slow-frequency rTMS suppresses cortical excitability (Chen et al 1997; Maeda et al 2000; Valero-Cabre et al 2007; Valero-Cabre et al 2005). It is possible that in our patients 1Hz rTMS might have induced an increase of the local excitability due to homeostatic mechanisms (Siebner et al 2004) – i.e., normally inhibitory 1-Hz rTMS might paradoxically induce an increase rather than a decrease of local activity due to the low levels of baseline activity and thus induce a homeostatic response to maintain activity in a desirable range (Fierro et al 2005; Fregni et al 2006; Siebner et al 2004; Silvanto et al 2008). In patients with chronic visceral pain, baseline activity in right and left SII might be decreased as a consequence of altered activity in the cortico-subcortical visceral sensation network. Consistent with this homeostatic notion, we found that decreased baseline levels of glutamate were associated with a greater likelihood of response to rTMS.
N-acetyl aspartate (NAA) levels showed changes similar to those of glutamate. NAA is converted to aspartate, which acts as an excitatory amino acid neurotransmitter. Therefore changes in NAA would be expected to parallel changes in glutamate. Decreases in NAA have been documented in various conditions involving neuronal cell damage and loss, including stroke, multiple sclerosis, Alzheimer’s disease, epilepsy, and several neurodegenerative disorders (Tsai and Coyle 1995). In addition, a recent study has shown that the concentration of NAA was negatively correlated with pain intensity in patients with chronic pain and NAA was significantly different between patients with and those without pain (Pattany et al 2002). Our results are consistent with these previous findings and extend it to SII.
Limitations
Although we show significant correlations between pain changes and MRS results. The results have to be interpreted cautiously. Because we were interested to investigate the effects of rTMS, we did this analysis only in patients who received active rTMS; therefore our analysis concerned a small sample. In this scenario, it is possible that a few patients might be driving the results. These results need consequently to be confirmed in larger studies. In addition, because we did not investigate other areas in our MRS assessments (for instance we did not measure biochemical changes in the primary (S1) somatosensory cortices, the motor cortex, the frontal inferior gyrus, the thalamus, the insula, the striatum and the cerebellum), it is not clear whether the effects seen in SII are due to general or specific effects related to modulation of pain networks. Future studies need to investigate other areas associated with the visceral pain neural network. Another limitation pertains to TMS blinding. Although participants were blinded to the assigned treatment and we used a sham coil for sham rTMS that has the same appearance and produces similar sounds as the real TMS coil; real rTMS induces a scalp sensation that is different than sham rTMS. Therefore, even considering that patients were naïve to TMS, it is possible that patients became aware of their assignment during the study. This limitation needs to be considered when analyzing our results. Finally, although in our pilot study (Fregni et al 2005), we were able to determine that low frequency rTMS of right SII is the optimal condition when compared to high-frequency rTMS of left and right SII and low-frequency rTMS of left SII; there are other parameters such as number of sessions, intensity of stimulation and optimal timing of stimulation that were not tested in this study and therefore it is possible that other parameters of stimulation could have been more effective in our study and should be further explored in future trials.
Because this was an initial study that had the goal also to provide mechanistic insights, we decided to increase the internal validity and thus use very strict inclusion criteria. As a consequence, despite screening a relatively large number of patients, only a small number of patients qualified for the trial. Therefore, given the small number of patients, our results should be viewed with some caution.
Conclusions
Our results support the notion that noninvasive neuromodulation of activity in SII can have a beneficial therapeutic effect in pain from chronic pancreatitis. Furthermore our findings show that modulation of SII with non-invasive brain stimulation can change brain metabolites and these changes can predict the effects of SII stimulation. Further studies are needed to explore other areas related to pain and the effects of other neuromodulatory tools for the treatment of chronic visceral pain; duration of effects beyond 4 weeks of treatment; predictors of response to rTMS treatment and the potential effects of this treatment on pancreatic function (Fregni et al 2007). Finally our results encourage further studies exploring the effects of brain stimulation in other visceral pain syndromes.
Supplementary Material
Acknowledgments
Grant support: This work was supported by a grant from NIH (DK071851-01 SDF) and the Harvard-Thorndike Clinical Research Center at Beth Israel Deaconess Medical Center integrated in the Harvard Clinical and Translational Science Center, supported by grants M01-RR-01066 and UL1 RR025758 from the National Center for Research Resources, National Institutes of Health. APL is supported by a grant from the National Institutes of Health (K24 RR018875). F.F. is supported by grant from NIH R21DK081773.
Footnotes
Conflict of interest: The authors have no conflicts of interest.
Authors’ contribution
F.F. wrote the first draft of this manuscript
F.F., A.P.-L., R.L. and S.F. designed the study
F.F., K.P., D.D. and X.W. collected and analyzed the data
F.F., A.P-L and S.F. interpreted the results
All the authors revised the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afif A, Hoffmann D, Minotti L, Benabid AL, Kahane P. Middle short gyrus of the insula implicated in pain processing. Pain. 2008;138:546–555. doi: 10.1016/j.pain.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Andersson JL, Valind S, Sundin A, Hamdy S, Jones AK, et al. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology. 1997;113:50–59. doi: 10.1016/s0016-5085(97)70079-9. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Thompson DG, Ng VW, Hamdy S, Sarkar S, Brammer MJ, et al. Cortical processing of human somatic and visceral sensation. J Neurosci. 2000;20:2657–2663. doi: 10.1523/JNEUROSCI.20-07-02657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar KL, Rothman DL. In vivo nuclear magnetic resonance studies of glutamate-gamma-aminobutyric acid-glutamine cycling in rodent and human cortex: the central role of glutamine. J Nutr. 2001;131:2498S–2504S. doi: 10.1093/jn/131.9.2498S. discussion 2523S–2494S. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, et al. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley MJ, Stier G, Pennacchini D, Legube G, Simon B, Akhtar A, et al. NMR structure of the first PHD finger of autoimmune regulator protein (AIRE1). Insights into autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) disease. J Biol Chem. 2005;280:11505–11512. doi: 10.1074/jbc.M413959200. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Dimcevski G, Sami SA, Funch-Jensen P, Le Pera D, Valeriani M, Arendt-Nielsen L, et al. Pain in chronic pancreatitis: the role of reorganization in the central nervous system. Gastroenterology. 2007;132:1546–1556. doi: 10.1053/j.gastro.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Fierro B, Brighina F, Vitello G, Piazza A, Scalia S, Giglia G, et al. Modulatory effects of low- and high-frequency repetitive transcranial magnetic stimulation on visual cortex of healthy subjects undergoing light deprivation. J Physiol. 2005;565:659–665. doi: 10.1113/jphysiol.2004.080184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Otachi P, Thut G, Rigonatti SP, et al. Homeostatic effects of plasma valproate levels on corticospinal excitability changes induced by 1Hz rTMS in patients with juvenile myoclonic epilepsy. Clin Neurophysiol. 2006 doi: 10.1016/j.clinph.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Fregni F, DaSilva D, Potvin K, Ramos-Estebanez C, Cohen D, Pascual-Leone A, et al. Treatment of chronic visceral pain with brain stimulation. Ann Neurol. 2005;58:971–972. doi: 10.1002/ana.20651. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A, Freedman SD. Pain in chronic pancreatitis: a salutogenic mechanism or a maladaptive brain response? Pancreatology. 2007;7:411–422. doi: 10.1159/000108958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamberardino MA, Valente R, Affaitati G, Vecchiet L. Central neuronal changes in recurrent visceral pain. Int J Clin Pharmacol Res. 1997;17:63–66. [PubMed] [Google Scholar]
- Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual-Leone A, et al. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol. 2001;112:1781–1792. doi: 10.1016/s1388-2457(01)00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain. 2006;122:22–27. doi: 10.1016/j.pain.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hsiao FJ, Chen WT, Liao KK, Wu ZA, Ho LT, Lin YY. Oscillatory characteristics of nociceptive responses in the SII cortex. Can J Neurol Sci. 2008;35:630–637. doi: 10.1017/s0317167100009434. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Hakimian S, Sherlin LH, Fregni F. New Insights Into Neuromodulatory Approaches for the Treatment of Pain. J Pain. 2007 doi: 10.1016/j.jpain.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76:833–838. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Clarey JC, Tweedale R, Calford MB. Interhemispheric connections of somatosensory cortex in the flying fox. J Comp Neurol. 1998;402:538–559. [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Zerah F, Bendib B, Cesaro P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004;75:612–616. doi: 10.1136/jnnp.2003.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329–2337. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- Luborzewski A, Schubert F, Seifert F, Danker-Hopfe H, Brakemeier EL, Schlattmann P, et al. Metabolic alterations in the dorsolateral prefrontal cortex after treatment with high-frequency repetitive transcranial magnetic stimulation in patients with unipolar major depression. J Psychiatr Res. 2006 doi: 10.1016/j.jpsychires.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117:455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Maher JW, Johlin FC, Heitshusen D. Long-term follow-up of thoracoscopic splanchnicectomy for chronic pancreatitis pain. Surg Endosc. 2001;15:706–709. doi: 10.1007/s004640080093. [DOI] [PubMed] [Google Scholar]
- Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991;4:47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187:433–437. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- Moisset X, Bouhassira D, Denis D, Dominique G, Benoit C, Sabate JM. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain. 2010;14:142–148. doi: 10.1016/j.ejpain.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Patrizi F, Freedman SD, Pascual-Leone A, Fregni F. Novel therapeutic approaches to the treatment of chronic abdominal visceral pain. ScientificWorldJournal. 2006;6:472–490. doi: 10.1100/tsw.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattany PM, Yezierski RP, Widerstrom-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, et al. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–905. [PMC free article] [PubMed] [Google Scholar]
- Rattner DW, Fernandez-del Castillo C, Warshaw AL. Pitfalls of distal pancreatectomy for relief of pain in chronic pancreatitis. Am J Surg. 1996;171:142–145. doi: 10.1016/s0002-9610(99)80089-0. discussion 145–146. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Cohen BM. Functional brain imaging in the elderly. J Nucl Med. 1993;34:1101–1102. [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Volkmann J, Enck P, Frieling T, Witte OW, Freund HJ. Different cortical organization of visceral and somatic sensation in humans. Eur J Neurosci. 1999;11:305–315. doi: 10.1046/j.1460-9568.1999.00429.x. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081–1088. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Cattaneo Z, Battelli L, Pascual-Leone A. Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J Neurophysiol. 2008;99:2725–2730. doi: 10.1152/jn.01392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126:79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007;176:603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res. 2005;163:1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- Wu X, Gao J, Yan J, Fan J, Owyang C, Li Y. Role for NMDA receptors in visceral nociceptive transmission in the anterior cingulate cortex of viscerally hypersensitive rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G918–927. doi: 10.1152/ajpgi.00452.2007. [DOI] [PubMed] [Google Scholar]
- Yan H, Zuo XN, Wang D, Wang J, Zhu C, Milham MP, et al. Hemispheric asymmetry in cognitive division of anterior cingulate cortex: a resting-state functional connectivity study. Neuroimage. 2009;47:1579–1589. doi: 10.1016/j.neuroimage.2009.05.080. [DOI] [PubMed] [Google Scholar]
- Zorn BH, Watson LR, Steers WD. Nerves from pelvic plexus contribute to chronic orchidalgia. Lancet. 1994;343:1161. doi: 10.1016/s0140-6736(94)90266-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.