Abstract
Hemorrhagic shock due to trauma (HS/T) induces an inflammatory response that can contribute to end-organ injury. The pathways involved in the initiation and propagation of HS/T-induced inflammation are incompletely understood. Here, we hypothesized that the DNA sensor TLR9 would have a role in inflammatory signaling after HS/T. Using mice expressing a nonfunctional, mutant form of TLR9, we identified a role of TLR9 in driving the initial cytokine response and liver damage in a model of hemorrhagic shock and bilateral femur fracture. Circulating DNA levels were found to correlate with the degree of tissue damage. Experiments using chimeric mice show that TLR9 on both bone marrow-derived cells and parenchymal cells are important for the TLR9-mediated liver and tissue damage, as well as systemic inflammation following HS/T. These data suggest that release of DNA may be a driver of the inflammatory response to severe injury as well as a marker of the extent of tissue damage. One of the sensors of DNA in the setting of HS/T appears to be TLR9.
Keywords: TLR9, trauma, damage-associated molecular pattern (DAMP), hemorrhagic shock, femur fracture, chimera
INTRODUCTION
Trauma is the leading cause of death and morbidity in the United States in people under the age of 45. Morbidity and mortality following trauma results, in part, from the excessive activation of inflammatory signaling pathways and the subsequent development of the systemic inflammatory response syndrome (SIRS). SIRS can lead to multiple organ dysfunction syndrome (MODS) and death (1–3). The most severely injured patients typically experience a combination of global hypoperfusion, in combination with extensive tissue injury and this combination is typically required for the development of early MODS (4, 5). Significant improvements in trauma patient management have decreased the severity and mortality of MODS, but the syndrome still remains a significant contributor to not only early MODS but also late morbidity resulting from nosocomial infections (1–3). SIRS induced by polytrauma is characterized by elevated levels of inflammatory mediators such as IL6 and IL10. However, the exact mechanisms by which trauma activates inflammation have not been fully clarified. Recently, much interest has developed in the prognostic and diagnostic utility of circulating cell-free DNA in trauma and critically ill patients (6–9). More recently, Zhang, et al. (10) demonstrated circulating mitochondrial DNA released from cellular disruption by trauma is a key link between trauma, inflammation and SIRS.
It is known that tissue injury in the absence of infection, hemorrhage, or ischemia/reperfusion (I/R) can trigger the release of endogenous damage-associated molecular patterns (DAMPs). These DAMPs can be recognized by pattern recognition receptors and serve as an early trigger to initiate inflammation after injury (11). The best characterized pattern-recognition receptors are the Toll-like receptors (TLRs). Studies in murine trauma and I/R models have strongly implicated TLR4 (12–14) and to a lesser extent TLR2 (15, 16) in the injury response. Other TLRs capable of recognizing endogenous molecules, such as TLR9 have not been as well studied. TLR9 is a sensor for bacterial DNA rich in unmethylated CpG motifs and can also be activated by DNA from mammalian cells (17–20). TLR9 has been found in immune cells, such as macrophages and dendritic cells (21), as well as non-immune cells including endothelial cells (22) and hepatocytes (23).
Although TLR9 has been intensely studied in the innate immune response to infection, its role in sterile challenges including drug-induced liver injury (24, 25), and I/R liver injury (26, 27), has only recently emerged. The importance of TLR9 in initiating the systemic inflammatory response and driving end-organ injury after systemic insults is unknown. Using mice expressing a nonfunctional, mutant form of TLR9 (28), we identified a role for TLR9 in driving the initial cytokine response and liver damage in a model of hemorrhagic shock and bilateral femur fracture (HS/T). Circulating DNA levels were found to correlate with the degree of tissue damage. Experiments using chimeric mice show that TLR9 on both bone marrow and non-bone marrow derived cells are important for the TLR9-mediated liver and tissue damage, as well as inflammatory responses following HS/T.
MATERIALS AND METHODS
Mice
Mice used in the experimental protocols were housed in accordance with University of Pittsburgh (Pittsburgh, PA, USA) and National Institutes of Health (NIH; Bethesda, MD, USA) animal care guidelines in specific pathogen-free conditions. Male C57BL/6 (WT) mice obtained from Charles Rivers, and TLR9 mutant mice (CpG1) provided by Dr. B. Beutler (The Scripps Research Institute, La Jolla, CA, USA), 8 to 12 weeks old and weighing 20–30 g, were used in chimera generation. The animals were maintained in the University of Pittsburgh Animal Research Center with a 12-h light-dark cycle and free access to standard laboratory feed and water. All animals were fasted for ~12 h prior to experimental manipulation and were acclimatized for 7 days prior to being used.
Chimeric animals
Chimeric mice were generated by adoptive transfer of donor BM cells into irradiated recipient animals as described previously using combinations of WT (C57BL/6) and TLR9 Mu (TLR9 mutant) mice (28, 29). The following recipient/donor combinations were produced: WT/WT, WT/Mu, Mu/Mu, Mu/WT. Recipient mice were exposed to an otherwise lethal 1000 cGy from a 137Cesium source (Nordion International Inc., Ontario, Canada) 6 h before receiving 5 million bone marrow cells by tail vein injection. The bone marrow cells were prepared in a sterile manner from the tibia and femur bones of the donor mice. Control mice had C57BL/6 donor and recipient as the positive control, and TLR9 mutant donor and recipient as the negative control. Crossed groups had C57BL/6 donors with TLR9 mutant recipients, or TLR9 mutant donors with C57BL/6 recipients. All animals were monitored two to three times weekly for the first 2 wk to ensure successful bone marrow engraftment. Chimeric animals were maintained under the same conditions as described above and underwent the experimental injury protocol or sham procedure more than 8 weeks after the adoptive transfer to ensure stable engraftment.
Chloroquine treatments
For chloroquine treatment experiment, chloroquine (Sigma, St. Louis, MO, USA) 60 mg/kg in 200 μl or an equal volume of PBS was administrated intraperitoneally at 14 h and 1 h prior to induction of femur fracture/hemorrhagic shock.
Femur fracture/hemorrhagic shock model
As described previously (30, 31), animals were anesthetized with i.p. sodium pentobarbital (70 mg/kg) and inhaled isofluorane (Abbott Labs, Chicago, IL, USA). A closed, mid-shaft, fracture of the femoral diaphysis was then induced via a three-bending technique with controlled local soft tissue damage related to the fracture. Then, utilizing the sterile technique, unilateral groin dissections were performed, and femoral arteries were cannulated with tapered PE-10 tubing, flushed with heparin sulfate (Pharmacia, Uppsala, Sweden, and Upjohn, Kalamazoo, MI, USA), for a total of ~2 U heparin per animal. The groin catheter was connected to a blood pressure transducer (Micro-Med) for continuous MAP readings. Mice were allowed to recover from the inhalational anesthesia for 10 min before initiation of hemorrhage. After baseline blood-pressure readings, repeated three times, mice were hemorrhaged to a MAP of 25 mmHg over 5 min. Total withdrawn blood was recorded every 10 min, and mice were maintained at a MAP of 25 mmHg for 150 min. The mice were then resuscitated over 10 min with two times the maximal shed blood amount in Lactated Ringer's solution through the arterial catheter. After post-resuscitation blood pressure readings, catheters were removed, vessels were ligated, and groin incisions were closed with 4-0 nylon sutures, and buprenorphine was injected for analgesia. At corresponding times after the end of hemorrhage, the animals were killed under inhalational anesthesia. Plasma from postmortem blood samples was obtained for biomarkers and blood chemistry analysis. Organs were snap-frozen in liquid nitrogen for biochemical analysis.
Plasma cytokines assay
Plasma IL-6 and IL-10 levels were used as a means of evaluating systemic inflammation [31, 32], and were quantified with commercial ELISA kits (R&D Systems Inc., Minneapolis, MN, USA).
Circulating DNA measurement
In some experiments, plasma double-stranded (ds) DNA and single stranded (ss) DNA were analyzed for analysis of cell-free DNA levels by fluorimetric assay. For this purpose, plasma dsDNA and ssDNA quantification was performed using PicoGreen dsDNA kit and OliGreen ssDNA kit (Invitrogen/Molecular Probes, Eugene, Oregon, USA) respectively, according to the manufacturer's instructions. Briefly, PicoGreen or OliGreen reagent was diluted 1:200 with TE (pH=7). Each reaction contained 50 μl of a dye solution plus a sample DNA made up to 50 μl in TE. Each sample DNA was analyzed in two duplicated dilution series. Standard curves were constructed by serial dilution of lambda DNA stock provided by the manufacturer. Black microtiter plates (Nunc, Denmark) were read in FLx800 fluorometer (Bio Tek Instruments, Winooski, Vermont, USA) at an excitation wavelength of ~480 nm and emission of ~520 nm. Blank values were subtracted and replicates averaged for each sample.
Liver damage assessment
To assess hepatic function and cellular injury following femur fracture/hemorrhagic shock, Plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using the Dri-Chem 7000 Chemistry Analyzer (Heska Co., Loveland, Colo, USA).
Apoptosis assay
Apoptosis with terminal deoxynucleotidyl-transferase dUTP nick end-labeling (TUNEL) assay was performed to confirm cell death using a kit from Promega (Madison, WI, USA). Following cryopreservation as described above in the HS/T methods, sections of 5 μm were prepared and processed for the TUNEL assay according to the manufacturer's protocol and counterstained with Hoeschst (bisbenzimidie) nuclear stain. TUNEL positive cells were quantitated using a Metamorph image acquisition and analysis system (Chester, PA, USA) using a Nikon microscope. TUNEL-positive cells were then counted blindly and expressed as a percentage of the total cell number.
Histopathology
Ethanol fixed samples were processed in an automated Excelsior processor in which tissue was taken through a series of alcohols from 70% to 100%, cleared using Xylenes and infiltrated with paraffin on a vacuum system. 4 μm thick sections were cut after embedding in a paraffin block, and baked on slides for 1 hour at 58°C. After cooling, the slides were stained with standard hematoxylin-eosin staining, and then assessed for necrosis and inflammatory infiltrate.
Statistical analysis
The data are expressed as mean ± standard error of the mean. Comparisons between groups were performed using one-way analysis of variances (ANOVA) and a post-hoc Tukey test. Probability values less than 0.05 were considered statistically significant. When individual studies are demonstrated, these are representative of at least three independent studies.
RESULTS
A comparison of circulating DNA levels, ALT, and AST levels and IL-6 and IL-10 levels following hemorrhagic shock
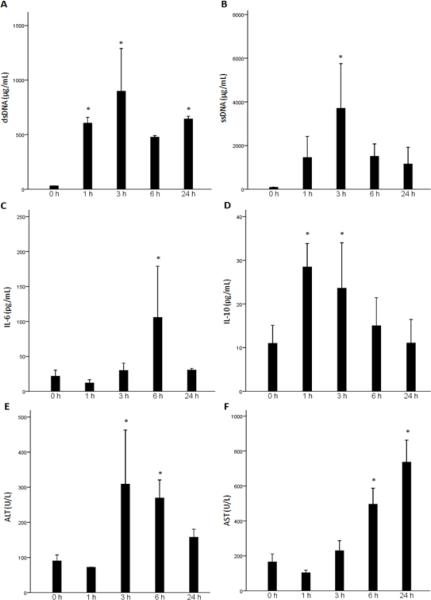
Levels of circulating DNA are known to increase after tissue trauma (6–8). Recent evidence suggests that hypomethylated DNA, such as that found in mitochondria can act as a trigger of inflammation following injury (10). Therefore, release of cell-free DNA can serve as both a marker of tissue damage and possibly an immune activator. Here, we compared the timing and extent of DNA accumulation in the circulation following resuscitated HS with the appearance of other typical markers of tissue damage, the transaminases ALT and AST. We also assessed the onset and magnitude of the systemic inflammatory response by measuring circulating IL-6 and IL-10 levels. As shown in Fig. 1, levels of both dsDNA and ssDNA were increased over baseline by 1 hour following resuscitation from HS. The greatest increases were measured at 3 hrs with persistent elevations evident at 24 hrs, the latest time point assessed. Levels of ssDNA were nearly 4-fold higher that measured for dsDNA and reached nearly 4ug/ml. Interestingly, increases in levels of ALT and AST over baseline were not seen until 3 hrs following resuscitation with the highest elevation detected at 6hrs for ALT and 24 hrs for AST.
Fig. 1.
Measurements of systemic cell-free DNA, inflammation, and liver damage following HS. C57BL/6 mice underwent hemorrhagic shock (HS), or sham procedure. Plasma double-stranded (ds) DNA (A) and single-stranded (ss) DNA (B) levels were measured for analysis of cell-free plasma DNA release. Plasma IL-6 (C) and IL-10 (D) levels were measured for analysis of systemic inflammation. Plasma alanine aminotransferase (ALT, E) and aspartate aminotransferase (AST, F) levels were analyzed for analysis of liver damage. (*, P<0.05 vs. baseline) Data represent means ± SEM; n = 3 mice for each time point.
Of the cytokine markers of inflammation following trauma, IL-6 and IL-10 are upregulated the earliest and correlate best with the extent of tissue damage (32, 33). We found that IL-10 levels were already significantly increased at the 1 hr time point followed by a gradual decline. Increases in IL-6 levels followed and were highest at the 6 hr time point. Taken together, this data shows that the increases in circulating cell-free DNA occurs early following injury and precede even more classical markers of tissue damage. The increases also parallel the upregulation of inflammatory markers.
TLR9 is involved in the systemic inflammatory response following injury
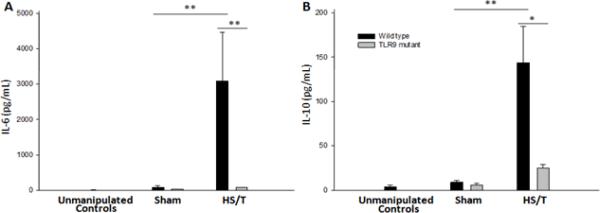
To determine if one of the key innate immune sensors of DNA is involved in the systemic inflammatory response following injury, we subjected TLR9 mutant mice and their wild type counterparts to a combination of HS and bilateral femur fracture (HS/T) as described in methods. Measurements of IL-6 and IL-10 at 6hrs demonstrated the expected significant increase in both cytokines (Fig. 2). In contrast, TLR9 mutant mice exhibited minimal elevation in either IL-6 or IL-10. Both strains had low to undetectable levels at baseline. These data demonstrate that TLR9 signaling is required for the typical systemic cytokine response observed following severe injury.
Fig. 2.
Circulating IL-6 and IL-10 levels are lower in TLR9 mutant mice after HS/T. WT and TLR9 mice were subjected to bilateral femur fracture and hemorrhagic shock (HS/T), or sham procedure. Plasma IL-6 (A) and IL-10 (B) levels were measured to assess the systemic inflammatory response. TLR9 mutant mice subjected to HS/T exhibited lower IL-6 and IL-10 levels as compared with WT mice subjected to HS/T (*, P<0.05; **, P<0.01). Data represent means ± SEM; n = 6–8 mice group.
TLR9 signaling mediates liver damage and increases in circulating DNA levels following injury
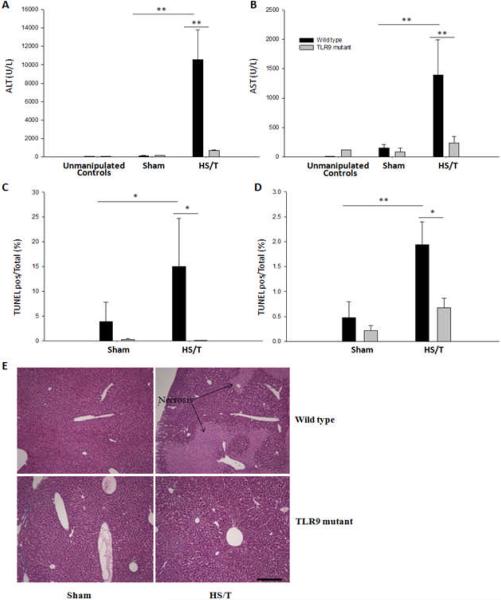
The delayed end-organ and tissue damage that follows resuscitation from HS is, in part, due to the activation of inflammatory pathways (34). To determine if TLR9 also contributed to end-organ damage in our model, we assessed liver injury at 6hrs following HS/T. Circulating ALT and AST, as well as histology were used to establish the extent of liver damage. As shown in Fig. 3, no liver damage was seen at baseline in either strain. Marked liver damage was seen in the wild type mice subjected to HS/T. However, TLR9 mutant mice displayed minimal liver damage. This included a near absence of liver necrosis, as well as TUNEL positive cells. To seek additional evidence for the importance of TLR9 signaling in HS/T-induced liver damage, we pre-treated wild type mice with chloroquine. Chloroquine inhibits signaling through endosomal receptors like TLR9 (35). As shown in Fig. 4, chloroquine pretreatment suppressed liver damage following HS/T as measured by circulating ALT levels.
Fig. 3.
TLR9 mutant mice exhibit less liver damage and apoptosis after HS/T. WT and TLR9 mice were subjected to bilateral femur fracture and hemorrhagic shock (HS/T), or sham procedure. Plasma ALT (A) and AST (B) levels were analyzed for analysis of liver damage. TLR9 mutant mice demonstrated significantly lower ALT and AST levels as compared with WT mice after HS/T. The number of In situ TdT-mediated dUTP nick-end labelling (TUNEL) positive nuclei in liver (C) of TLR9 mutant mice, expressed as a percent of the total number of nuclei, was reduced compared with WT mice after HS/T (*, P<0.05; **, P<0.01). Data represent means ± SEM; n = 6–8 mice group. H&E (D, magnification × 400) liver tissue histology of TLR9 mutant mice showed significantly less necrosis (→) compared with WT following fracture. Data shown represent 1 of 3 independent experiments yielding similar results.
Fig. 4.
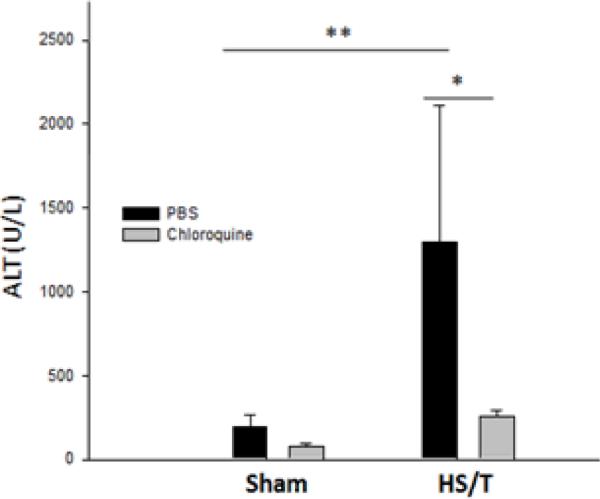
Chloroquine reduces liver damage following HS/T. Serum ALT levels were analyzed for liver damage after HS/T. Chloroquine (30mg/kg i.p.) 14 h and 1 h before trauma significantly prevented HS/T induced increases in plasma ALT, which was shown in the phosphate buffered saline (PBS) controlled mice (*, P<0.05; **, P<0.01). Data represent means ± SEM; n = 4–6 mice group.
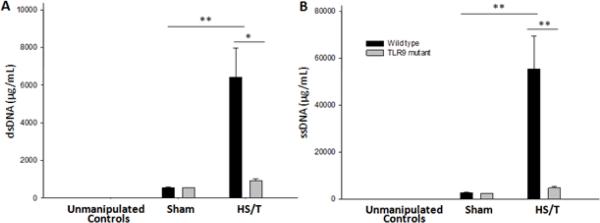
As described above, circulating DNA could serve as both a mediator and a marker of tissue damage. To determine if the increases in circulating DNA observed following HS required TLR9, we assessed circulating dsDNA and ssDNA levels in wild type and TLR9 mutant mice at 6 hrs following HS/T. Sham treated animals exhibited a minor increase over baseline in both strains for dsDNA and ssDNA (Fig. 5). However, only the wild type mice exhibited the expected increase in circulating DNA levels following HS/T. These data indicate that signaling through TLR9 is involved in liver damage and is also required for increases in circulating DNA levels following injury. This does not rule out a role for the DNA in driving the inflammatory response but instead indicates that TLR9 signaling leads to tissue damage manifested by DNA release.
Fig. 5.
Circulating DNA levels are lower in TLR9 mutant mice following HS/T. WT and TLR9 mutant mice were subjected to bilateral femur fracture and hemorrhagic shock (HS/T), or sham procedure. Plasma double-stranded (ds) DNA (A) and single-stranded (ss) DNA (B) levels were measured for analysis of DNA release following injury. TLR9 mutant mice subjected to HS/T showed significantly lower cell-free plasma dsDNA and ssDNA levels as compared with WT (*, P<0.05; **, P<0.01). Data represent means ± SEM; n = 6–8 mice group.
TLR9 on both bone marrow and non-bone marrow derived cells is involved in HS/T-induced tissue damage
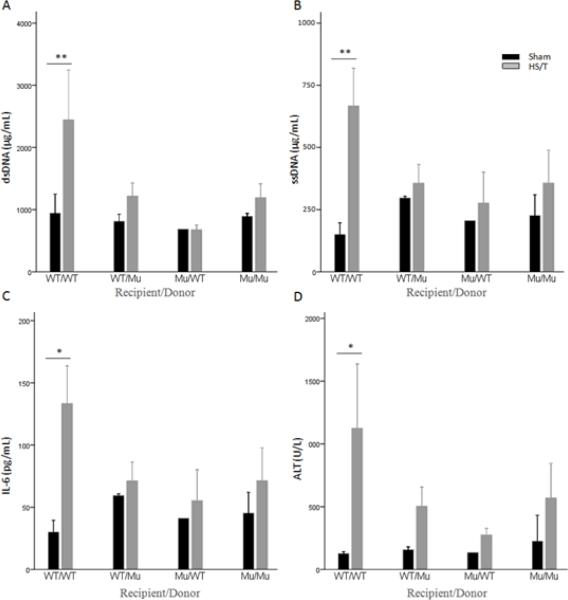
TLR9 is expressed on a number of cell types including leukocytes (21), endothelial cells (22), and hepatocytes (23). To assess the importance of TLR9 on cells of bone marrow origin, such as leukocytes and non-bone marrow derived cells, we generated bone marrow chimeric mice. Following lethal irradiation either wild type or TLR9 mutant mice were reconstituted with bone marrow from wild type or mutant donors. We (31) and others (36) have previously shown that this approach leads to a near complete conversion of liver macrophages to donor phenotype by 10 weeks. Mice from the four groups were subjected to either sham manipulation or HS/T and liver damage assessed at 6hrs. As shown in Fig. 6, the control group combinations exhibited the expected results with WT/WT (recipient/donor) subjected to HS/T exhibiting significant increases in circulating ALT, AST, IL-6 and DNA levels and the MU/MU mice protected from liver injury and exhibited lower IL-6 and DNA levels. The test group combinations where either only the bone marrow derived cells or only the non-bone marrow derived cells expressed the mutant form of the TLR9 were also protected from liver and tissue damage and had lower circulating IL-6 levels than the WT/WT combination. These findings suggest that TLR9 on multiple cell types participates in the injury response in this model.
Fig. 6.
Cell-free DNA release and liver injury following HS/T require functional TLR9 on bone marrow (BM)-derived cells and non-BM-derived cells. Chimeric mice underwent bilateral femur fracture and hemorrhagic shock (HS/T), or sham procedure. Plasma dsDNA (A) and ssDNA (B) levels were analyzed to assess cell-free DNA release after trauma. Plasma IL-6 (C) levels were analyzed for inflammatory response and ALT (D) for liver injury. WT/WT mice (recipient/donor) demonstrated significant increases in plasma ssDNA and dsDNA, Il-6, and ALT after HS/T, which was not seen in WT/Mu, Mu/WT, or Mu/Mu mice (A and B, **, P<0.01). (C and D, *, P<0.05). Data represent means ± SEM; n = 4–6 mice group. ssDNA, single stranded DNA; dsDNA, double stranded DNA; WT, wild type; Mu, TLR9 mutant.
DISCUSSION
There is increasing evidence that the systemic inflammatory response following trauma is initiated and driven by the stimulation of pattern recognition receptors of the innate immune system (10, 33, 37). Furthermore, there is some evidence that this receptor activation is driven by danger associated molecular pattern (DAMP) molecules released by damaged or stressed tissues (24, 38). Recent findings implicate mitochondrial DNA as a DAMP which triggers PMN activation via TLR9 following trauma (10) Here, we show that signaling through TLR9 is also involved in the initial systemic inflammatory response in a model of HS and trauma (HS/T). Signaling through TLR9 also contributes to liver injury in this model. Studies using chimeric mice indicate that TLR9 on both bone marrow-derived cells and parenchymal cells is part of this response. HS/T induces the release of DNA into the circulation; however much of this is dependent on TLR9 signaling confirming that increases in circulating DNA levels results from tissue damage. Thus, TLR9 is another pattern recognition receptor important not only to the host response to infection, but also the response to injury.
It is now well established that molecules of both microbial and host origin can activate signaling through a common set of innate immune receptors (39, 40). We have suggested that this could be one explanation for the similarity of the systemic inflammatory response seen in severely injured and septic patients (33). Best studied in this regard are the TLRs, where TLR2, TLR3, TLR4, and TLR9 have been shown to participate in the aspects of the inflammatory in a range of tissue injury models (41). Of these, TLR4 has been most extensively studied in systemic injury models. Mice deficient in TLR4 signaling have been shown to exhibit less end-organ damage and a diminished inflammatory response following HS (42) or bilateral femur fracture (31, 34). As seen here with TLR9, it appears that TLR4 on both bone-marrow and non-bone marrow derived cells are critical to these responses (44). Although the triggers of TLR4 signaling following trauma are uncertain, one potential candidate is high mobility group box 1 (HMGB1) (43). Neutralizing anti-HMGB1 antibodies mimic the TLR4 deficient state (13). Studies using TLR2 knockout mice indicate that TLR2 also contributes to trauma-induced responses in pulmonary contusion (44) and burns (45). Our current study adds TLR9 to the growing list of pattern recognition receptors involved in the HS and tissue trauma- induced systemic inflammatory response. How the various TLRs interact or coordinate to the response to injury is uncertain. However, it is possible that there is both some redundancy in the response, as well as cell and tissue-specific aspects to TLR function.
The only known ligand for TLR9 is hypomethylated DNA also known as DNA rich in CpG motifs. CpG DNA is especially abundant in bacteria and mitochondrial DNA. Zhang, et al. (10) recently reported the detection of mitochondrial DNA in the circulation of trauma patients and demonstrated a TLR9-mediated activation of PMN. Lo, et al (46) have reported increased levels of circulating DNA in humans even after minor trauma while other work has shown that DNA release correlates with tissue damage in trauma and sepsis (9, 47, 48). Tissue damage leads to release of cellular components such as DNA, HMGB1, and heat shock proteins, all of which can serve as DAMPs (27, 49, 50). HMGB1 has been shown to be an early mediator of trauma and injury, and partly mediates mitochondrial DNA-mediated inflammatory response in a variety of innate immune cells (10, 21, 51). The observation that HMGB1 can interact with DNA to facilitate TLR9 signaling (21) suggests that certain DAMPs may act in concert to trigger TLR signaling. Although mechanisms by which DNA is released into the circulation are unclear, most authors believe this is secondary to cell death (52, 53). In our experimental model, we found significant increases in circulating cell-free DNA by 1 hr following resuscitation. Levels of ssDNA were the highest and peaked at 3 hrs following resuscitation in a model of HS alone. The elevation of DNA levels occurred in parallel with the peak in IL-10 and prior to the peak in IL-6 suggests that circulating DNA may participate in the stimulation of the early cytokines response to trauma. However, the increase in circulating DNA measured at the 6 hrs time point in our HS/T was dependent, in part, on intact TLR9 signaling. Thus, much of the rise in circulating DNA is likely to reflect the extent of tissue damage. Whether the increases in circulating DNA resulting from TLR9 signaling then continue to propagate the immno-inflammatory response to trauma is unknown. What does appear to be clear from our study and the work of others is that DNA is an important mediator of the immunologic response of mammals to injury.
In addition to driving the inflammatory response after HS/T, TLR9 signaling also contributed to liver injury. It is likely that the liver damage was at least partially the result of the activation of inflammatory pathways. Recent reports have shown that liver damage, as well as inflammatory responses in the liver in models of I/R (26, 27), acetaminophen-induced toxicity (25), and steatohepatitis (54) are driven by TLR9. Therefore, TLR9 dependent responses may be especially prominent in the liver. This is supported by our observation that the gut is not protected in HS/T in TLR9 mutant mice [Chhindler, et al. Manuscript Submitted]. Gribar, et al (29) have shown that TLR9 signaling counteracts the pro-inflammatory TLR4 signaling in enterocytes. Thus, the functions of TLR9 may be organ specific. Our results using chimeric mice support the idea that TLR9 on bone marrow-derived cells and parenchymal cells contribute to the response. Imeada, et al (25) showed that TLR9 expression is prominent on liver sinusoidal endothelial cells and that DNA from hepatocytes can trigger endothelial cell activation. The specific TLR9 positive cell types involved in either the systemic cytokine response of the liver damage were not identified as part of our study.
In summary, our results provide clear evidence that signaling through TLR9 on multiple cell types participates in the immuno-inflammatory response to severe injury. Liver damage is one consequence of TLR9-mediated signaling in this setting. Taken in the context of previous studies demonstrating a role for other TLRs in similar models, a paradigm emerges where several TLRs driven by diverse DAMPs initiate the host immune response to injury.
ACKNOWLEDGMENTS
This work was supported by NIH grant GM-53789-09 (to T. R. B.) and in part by NSFC grants 30700786 and 2007-ZDi-13 (to X. R.). R. G. is a recipient of an American College of Surgeons Resident Scholarship. The authors acknowledge valuable discussion with Debra Williams, and the technical assistance of Meihua Bo, Lauryn Kohut, and Alicia Frank.
Nonstandard abbreviations used
- TLRs
Toll-like receptors
- HS
hemorrhagic shock
- HS/T
hemorrhagic shock + bilateral femur fracture
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ssDNA
single stranded DNA
- dsDNA
double stranded DNA
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRENCES
- 1.Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40:912–918. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–1345. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Faist E, Baue AE, Dittmer H, Heberer G. Multiple organ failure in polytrauma patients. J Trauma. 1983;23:775–787. doi: 10.1097/00005373-198309000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Strecker W, Gebhard F, Rager J, Bruckner UB, Steinbach G, Kinzl L. Early biochemical characterization of soft-tissue trauma and fracture trauma. J Trauma. 1999;47:358–364. doi: 10.1097/00005373-199908000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Moore EE. Synergy of bone fractures, soft tissue disruption, and hemorrhagic shock in the genesis of postinjury immunochaos: the pathway to multiple organ failure. Crit Care Med. 1998;26:1305–1306. doi: 10.1097/00003246-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Gahan PB, Swaminathan R. Circulating nucleic acids in plasma and serum. Recent developments. Ann N Y Acad Sci. 2008;1137:1–6. doi: 10.1196/annals.1448.050. [DOI] [PubMed] [Google Scholar]
- 7.Lam NY, Rainer TH, Chan LY, Joynt GM, Lo YM. Time course of early and late changes in plasma DNA in trauma patients. Clin Chem. 2003;49:1286–1291. doi: 10.1373/49.8.1286. [DOI] [PubMed] [Google Scholar]
- 8.Rainer TH, Lo YM, Chan LY, Lam NY, Lit LC, Cocks RA. Derivation of a prediction rule for posttraumatic organ failure using plasma DNA and other variables. Ann N Y Acad Sci. 2001;945:211–220. doi: 10.1111/j.1749-6632.2001.tb03888.x. [DOI] [PubMed] [Google Scholar]
- 9.Rainer TH. Plasma DNA, prediction and post-traumatic complications. Clin Chim Acta. 2001;313:81–85. doi: 10.1016/s0009-8981(01)00653-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar TR. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Birch SE, He R, Tawadros P, Szaszi K, Kapus A, Rotstein OD. Remote ischemic preconditioning by hindlimb occlusion prevents liver ischemic/reperfusion injury: the role of High Mobility Group-Box 1. Ann Surg. 2010;251:292–299. doi: 10.1097/SLA.0b013e3181bfda8c. [DOI] [PubMed] [Google Scholar]
- 13.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Xiang M, Yuan Y, Xiao G, Zhang J, Jiang Y, Vodovotz Y, Billiar TR, Wilson MA, Fan J. Hemorrhagic shock augments lung endothelial cell activation: role of temporal alterations of TLR4 and TLR2. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1670–1680. doi: 10.1152/ajpregu.00445.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer J. TLR9 in health and disease. Int Rev Immunol. 2005;25:155–181. doi: 10.1080/08830180600743107. [DOI] [PubMed] [Google Scholar]
- 18.Lamphier MS, Sirois CM, Verma A, Golenbock DT, Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann N Y Acad Sci. 2006;1082:31–43. doi: 10.1196/annals.1348.005. [DOI] [PubMed] [Google Scholar]
- 19.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 20.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 21.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Armas M, Simon-Santamaria J, Pettersen I, Moens U, Smedsrod B, Sveinbjornsson B. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J Hepatol. 2006;44:939–946. doi: 10.1016/j.jhep.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. Role of Toll-Like Receptors in Changes in Gene Expression and NF-{kappa}B Activation in Mouse Hepatocytes Stimulated with Lipopolysaccharide. Infect. Immun. 2002;70:3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maher JJ. DAMPs ramp up drug toxicity. J Clin Invest. 2009;119:246–249. doi: 10.1172/JCI38178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi X-h, Shah S, Ozolek JA, Hackam DJ. Reciprocal Expression and Signaling of TLR4 and TLR9 in the Pathogenesis and Treatment of Necrotizing Enterocolitis. J Immunol. 2009;182:636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan X, Shi H, Xia G, Xiao Y, Dong J, Ming F, Wang S. Encapsulated Bifidobacteria reduced bacterial translocation in rats following hemorrhagic shock and resuscitation. Nutrition. 2007;23:754–761. doi: 10.1016/j.nut.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Mollen KP, Levy RM, Prince JM, Hoffman RA, Scott MJ, Kaczorowski DJ, Vallabhaneni R, Vodovotz Y, Billiar TR. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83:80–88. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- 32.Sherry RM, Cue JI, Goddard JK, Parramore JB, DiPiro JT. Interleukin-10 is associated with the development of sepsis in trauma patients. J Trauma. 1996;40:613–6. doi: 10.1097/00005373-199604000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 34.Levy RM, Prince JM, Yang R, Mollen KP, Liao H, Watson GA, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. Am J Physiol Regul Integr Comp Physiol. 2006;291:R970–R976. doi: 10.1152/ajpregu.00793.2005. [DOI] [PubMed] [Google Scholar]
- 35.Ertel W, Morrison MH, Ayala A, Chaudry IH. Chloroquine attenuates hemorrhagic shock-induced immunosuppression and decreases susceptibility to sepsis. Arch Surg. 1992;127:70–75. doi: 10.1001/archsurg.1992.01420010084012. [DOI] [PubMed] [Google Scholar]
- 36.Makui H, Soares RJ, Jiang W, Constante M, Santos MM. Contribution of Hfe expression in macrophages to the regulation of hepatic hepcidin levels and iron loading. Blood. 2005;106:2189–2195. doi: 10.1182/blood-2005-02-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Yuan Y, Li Y, Zhang J, Xiao G, Vodovotz Y, Billiar TR, Wilson MA, Fan J. Interacting neuroendocrine and innate and acquired immune pathways regulate neutrophil mobilization from bone marrow following hemorrhagic shock. J Immunol. 2009;182:572–580. doi: 10.4049/jimmunol.182.1.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 40.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 41.Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR. Early events in the recognition of danger signals after tissue injury. J Leukoc Biol. 2008;83:546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 42.Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, Billiar TR. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202:407–417. doi: 10.1016/j.jamcollsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538–R1544. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 44.Hoth JJ, Hudson WP, Brownlee NA, Yoza BK, Hiltbold EM, Meredith JW, McCall CE. Toll-like receptor 2 participates in the response to lung injury in a murine model of pulmonary contusion. Shock. 2007;28:447–452. doi: 10.1097/shk.0b013e318048801a. [DOI] [PubMed] [Google Scholar]
- 45.Cairns BA, Barnes CM, Mlot S, Meyer AA, Maile R. Toll-like receptor 2 and 4 ligation results in complex altered cytokine profiles early and late after burn injury. J Trauma. 2008;64:1069–1077. doi: 10.1097/TA.0b013e318166b7d9. [DOI] [PubMed] [Google Scholar]
- 46.Lo YM, Chiu RW. The biology and diagnostic applications of plasma RNA. Ann N Y Acad Sci. 2004;1022:135–139. doi: 10.1196/annals.1318.022. [DOI] [PubMed] [Google Scholar]
- 47.Saukkonen K, Lakkisto P, Varpula M, Varpula T, Voipio-Pulkki LM, Pettila V, Pulkki K. Association of cell-free plasma DNA with hospital mortality and organ dysfunction in intensive care unit patients. Intensive Care Med. 2007;33:1624–1627. doi: 10.1007/s00134-007-0686-z. [DOI] [PubMed] [Google Scholar]
- 48.Wijeratne S, Butt A, Burns S, Sherwood K, Boyd O, Swaminathan R. Cell-free plasma DNA as a prognostic marker in intensive treatment unit patients. Ann N Y Acad Sci. 2004;1022:232–238. doi: 10.1196/annals.1318.036. [DOI] [PubMed] [Google Scholar]
- 49.Kaczorowski DJ, Tsung A, Billiar TR. Innate immune mechanisms in ischemia/reperfusion. Front Biosci (Elite Ed) 2009;1:91–98. doi: 10.2741/E10. [DOI] [PubMed] [Google Scholar]
- 50.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margraf S, Logters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;30:352–358. doi: 10.1097/SHK.0b013e31816a6bb1. [DOI] [PubMed] [Google Scholar]
- 53.Butt AN, Swaminathan R. Overview of circulating nucleic acids in plasma/serum. Ann N Y Acad Sci. 2008;1137:236–342. doi: 10.1196/annals.1448.002. [DOI] [PubMed] [Google Scholar]
- 54.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-Like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1[beta] in Mice. Gastroenterology. doi: 10.1053/j.gastro.2010.03.052. In Press, Uncorrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]