Abstract
Objective
The pro-inflammatory cytokine interleukin (IL)-6 has been linked with health morbidity, particularly risk for cardiovascular disease. The purpose of this study was to investigate the potential protective role of coping self-efficacy on the relationship between caregiving stress and circulating concentrations of IL-6.
Methods
A total of 62 elderly Alzheimer’s caregivers (mean age = 74 years) were assessed for plasma concentrations of IL-6, caregiving-related overload, and coping self-efficacy. Multiple regression was used to examined the main effects of stress and self-efficacy, as well as the interaction between stress and self-efficacy, in predicting plasma IL-6 after controlling for age, gender, resting blood pressure, and obesity.
Results
There was a significant interaction between stress and self-efficacy in predicting IL-6. Post-hoc examination indicated that when self-efficacy was low, stress was significantly related to IL-6 (β = .43). However, when self-efficacy was high, stress was not significantly related to IL-6 (β = -.10).
Conclusion
Caregiving stress in combination with low coping self-efficacy is significantly related to IL-6, a known risk marker for health morbidity, particularly CVD. However, stress was not associated with IL-6 with high self-efficacy. While limited and preliminary, these results point to a potential protective effect of self-efficacy on caregiver health that can be tested in longitudinal studies.
Keywords: Coping, Personal Control, Cardiovascular Disease, Inflammation, Elderly
OBJECTIVE
An extensive focus in research has been on the relationship between caregiver stress and health outcomes. The pro-inflammatory cytokine interleukin 6 (IL-6) has been found to be a biological indicator of physiological changes. Chronic elevation of IL-6 has been implicated in downstream health consequences including physical disability (1), hypertension (2), subclinical atherosclerosis (3), and risk for coronary heart disease (CHD) (4, 5). IL-6 is secreted during acute stress from different sources (6, 7) and produces increased stimulation of the hypothalamic-pituitary-adrenal (HPA) axis in humans (8). Stress has also been found to have deleterious cardiovascular health effects. Indeed, Danesh and colleagues (5) concluded that long-term elevation of IL-6 levels present an increased CHD risk equivalent to established risk factors such as elevated total cholesterol and systolic blood pressure. Similarly, others have found that overproduction of IL-6 has been linked with morbidity and mortality in older adults (9). It is stress-related elevations of IL-6 that will be examined in this study.
An overproduction in IL-6 has been found to be associated with increased age (1). Within these age-related changes, elderly spousal Alzheimer’s caregivers are a population at significant risk for elevated concentrations of IL-6 due to the constant daily stresses associated with caregiving. For example, a cross-sectional study of spousal Alzheimer’s caregivers and noncaregiver controls found that overall, age accounted for a significant portion of the relationship with IL-6 (10). However, post-hoc analyses revealed that increasing age was significantly associated with elevated plasma concentrations of IL-6 in Alzheimer’s caregivers but not in controls (10). Because age was associated with IL-6 only in caregivers, these findings suggest that age-associated increases in IL-6 may occur as a function of stress. In a separate longitudinal study, spousal Alzheimer’s caregivers demonstrated significant longitudinal increases in IL-6 over a 6-year time-period (11). This study also found no such relationship in elderly non-caregivers, suggesting a potential age-related dysregulation of IL-6 among the chronically stressed compared to their non-caregiving peers. Coincidently, other studies confirm that caregivers are at increased risk of hypertension (12), cardiovascular disease (CVD) (13), and mortality (14), findings that, interestingly enough, concur with the previously-described links between elevated IL-6 and these outcomes.
Although caregivers experience stress from multiple sources, recent evidence suggests that not all caregivers experience negative health outcomes. Psychological factors, including personal mastery, self-efficacy, and coping self-efficacy, have been found to play an important role in the subjective and objective health experiences of caregivers. While coping self-efficacy is of interest for this study, all three constructs will be briefly discussed to clarify how they differentiate from each other and how they are related to caregiver health.“Personal mastery” was defined by Pearlin (21) as a general sense of control over one’s life and circumstances, and has been found to be associated with physical and psychological health outcomes. Individuals with a high sense of mastery are more likely to view stressors as under their control and more of a challenge than a problem. For example, Mausbach and colleagues (15) reported that increases in caregiver stress were associated with psychiatric morbidity only in individuals who felt a diminished sense of personal mastery over their lives. Similar results have been found regarding the protective role of personal mastery on the vascular biomarker plasminogen activator inhibitor (PAI)-1 (16) and overall health symptoms (17) in caregivers. A sense of control also appears to be directly associated with reduced sympathetic arousal (18) and immune cell functioning (19, 20) in caregivers. Therefore, mounting evidence suggests the importance of defining the circumstances under which caregiving-related stress may actually be harmful.
“Self-efficacy” is a similar yet separate construct that can be differentiated from personal mastery. Bandura indicated that the more specific construct of “self-efficacy” pertains to the belief that one has the capacity to successfully perform specific behaviors or to influence specific life domains (21, 22). In regard to caregivers, higher self-efficacy has been associated with reductions in depressive symptoms (23, 24), reduced health risk behavior patterns (25), and improved physical health (26, 27). Similar results have been observed in non-caregivers, including a negative relationship between self-efficacy and cardiac function and heart failure hospitalizations (28) and between self-efficacy and reduced heart rate increases under stress (29).
“Coping self-efficacy” is a form of self-efficacy particularly relevant to caregivers as it relates to the degree to which one believes he/she could carry out actions necessary to respond to (i.e., cope with) a challenge (30, 31). While all forms of self-efficacy pertain to one’s belief for performing task-specific behaviors (e.g., lifting heavy weights, running a particular speed), self-efficacy for coping relates specifically to one’s belief that he/she can perform specific coping behaviors (e.g., stop unpleasant thoughts when under stress). Bandura demonstrated that high levels of coping self-efficacy attenuated cardiac acceleration (i.e., degree of heart rate increase) and blood pressure (29) and later found similar results pertaining to catecholamine secretion under stress (32).
While coping self-efficacy appears to attenuate catecholamine secretion and heart rate increase, little is known about the relationship between coping self-efficacy in IL-6 concentrations. However, catecholamine secretion appears to stimulate secretion of IL-6, suggesting that coping self-efficacy might also be helpful in reducing the effects of stress on IL-6 production. For example, since Bandura’s findings, research has demonstrated that catecholamine secretion (33, 34) is associated with IL-6 secretion in both animals and humans (6, 35). Similarly, a positive association has been found between plasma norepinephrine and IL-6 concentrations in individuals with posttraumatic stress disorder (36). Moreover, while circulating levels of IL-6 in the context of chronic stress may stem from different cell sources such as from immune-competent blood mononuclear cells, the endothelium, the liver, and adipose tissue (4, 37), it was shown that human adipocytes secrete IL-6 after incubation with catecholamines (38). Stressed individuals exhibit decreased vagal tone, which, in turn, might underlie enhanced production of proinflammatory cytokines by tissue macrophages, including IL-6, giving rise to low-grade systemic inflammation (39). Therefore, if self-efficacy improves autonomic dysregulation, it is possible that self-efficacy may also have similar attenuating effects on IL-6 levels. However, no previous study has examined the moderating role of self-efficacy on the relations between stress and IL-6 concentrations.
The purpose of this study was to examine whether coping self-efficacy plays a protective role against elevations in IL-6. Given it has already been established that coping self-efficacy has been associated with reduced sympathetic arousal (29, 32), and that sympathetic arousal is positively related to IL-6 (6, 35, 36), we hypothesized that coping self-efficacy would be a significant moderator of the relations between stress (i.e., feeling overloaded by the caregiving role) and circulating concentrations of plasma IL-6.
METHODS
Participants
A total of 62 Alzheimer’s caregivers were the participants in this study. All were enrolled in a study examining the psychobiological consequences of chronic stress. To be eligible for the study all participants were required to be at least 55 years of age and caring for a spouse with a physician diagnosis of Alzheimer’s disease (AD). In addition, all caregivers were required to be free of serious health conditions (e.g., cancer) and must have been providing in-home care for their spouses (i.e., care recipients could not be placed in a long-term care facility or residing with another family member). Participants were primarily recruited from local caregiver support groups, referral from the University of California San Diego (UCSD) Alzheimer’s Disease Research Center (ADRC), and referral from other participants. The study protocol was approved by the UCSD Institutional Review Board and all participants provided informed consent prior to enrolling in the study.
Measures
Interleukin-6 (IL-6) Levels
A research nurse obtained venous blood samples from all participants between 8:00-11:00 a.m. using a 22-gauge forearm catheter. Blood was dispensed in EDTA tubes and spun at 3000g for 10 minutes at 4–8° C. Plasma IL-6 was determined by use of a commercially available ELISA kit (R&D Systems, Minneapolis, MN). Each sample was measured in duplicate. The precision and sensitivity performance values of these assays are excellent. Specifically the intra-assay CV (%) was 2.2, the inter-assay CV (%) was 3.9, and the assay sensitivity was <0.71pg/ml. Our lab has ample experience in performing this assay (10).
Caregiver Stress
The Role Overload scale (40) was used to summarize overall caregiver stress. This scale consists of 4 statements: “You are exhausted when you go to bed at night”; “You have more things to do than you can handle”; “You don’t have time just for yourself”, and “You work hard but never seem to make any progress”. Using a 4-point Likert-type scale, participants rated the extent to which each statement described them from 0 = “not at all” to 3 = “Completely”. The 4 items were summed to create an overall Overload score ranging from 0-12, with higher scores indicating greater levels of stress. High correlations have been demonstrated between the Role Overload scale and other measure of caregiver stress (41, 42). For the present sample, Cronbach’s alpha was .76.
Coping Self-Efficacy
All participants completed the Coping Self-Efficacy Scale (31), which is a 13-item scale that assesses self-efficacy in 3 domains: a) using Problem-Focused Coping (6 items) (e.g., Think of only one part of the problem), b) Stop unpleasant emotions and thoughts (4 items) (e.g., take your mind off unpleasant thoughts), and c) Get support from friends and family (3 items) (e.g., get friends to help you with the things you need). Participants were asked how confident or certain they were that they could perform the specific actions presented in each domain, with response options ranging from 0-10 with 0 = “cannot do at all”, 5 = “moderately certain can do”, and 10 = “certain can do”. Previous research shows that reliability and validity of this questionnaire are excellent (31). Because the focus of the current study was how caregivers managed caregiving-related stresses, we utilized the Self-efficacy for Problem-Focused Coping scale in our analysis. The 6 items of this scale were summed to create an overall self-efficacy score (possible range = 0-60) and Cronbach’s alpha for the current study was .87.
Demographic and Health Characteristics
In addition to the above psychometric constructs, the following data was collected: a) caregiver and care recipient age, b) caregiver sex, c) caregiver years of caregiving, d) caregiver years of education, and e) caregiver weight in kilograms and height in meters. The latter information was used to calculate body mass index (BMI), which was equal to the participant’s weight divided by height squared. During an in-home visit with each caregiver, a research nurse collected three resting blood pressure readings which were averaged to obtain an overall blood pressure value.
Each care recipient’s dementia severity was determined using the Clinical Dementia Rating (CDR) scale (43). For this scale, caregivers responded to a series of questions regarding their spouse’s functioning in six domains: a) memory, b) orientation, c) judgment and problem-solving, d) community affairs, e) home and hobbies, and f) personal care. Functioning in these domains was used to determine an overall dementia rating score ranging from 0 (lowest) to 3 (highest) with higher scores indicating greater dementia severity.
Data Analysis
Prior to analyzing our data, each continuous variable was examined for normality using the formula described by Tabachnik and Fidel (44), with a conventional alpha level set at p < .01. Using this criterion, IL-6 was significantly skewed and required log10 transformation. Our primary hypotheses were tested using a multiple regression analysis with (log10) IL-6 as our dependent variable. As recommended by Kraemer and Blasey (45), linear independent variables were centered at their means and binary variables were centered as +0.5 and -0.5. As described by Kraemer and Blasey, non-centered variables may produce regression coefficients that are irrelevant and misleading, whereas centered variables help to diminish problems associated with multicollinearity. The following variables were included as independent variables in the analysis: a) caregiver age in years, b) caregiver sex, c) BMI, d) systolic blood pressure, e) role overload, and f) self-efficacy for using problem-focused coping.
To test whether the relationship between role overload and IL-6 was moderated by self-efficacy, an overload-by-self efficacy interaction term was included in the regression model. If the interaction term was significant at p < .05, post-hoc analyses were conducted to determine the nature of the interaction (46). Prior to these analyses, we created a high self-efficacy (i.e., centered self-efficacy – 1 SD) and low self-efficacy variable (i.e., centered self-efficacy + 1 SD). Each of these variables was then multiplied by the (centered) role overload variable to create interaction terms. With these variables, we conducted two regression analyses, each of which included the main effect for role overload, one of the conditional self-efficacy variables (high self-efficacy or low self-efficacy), and the interaction of the overload and self-efficacy variable (i.e., overload-by-low self efficacy and overload-by-high self efficacy). The primary effect examined in each of these post-hoc analyses was the association between role overload and IL-6, as this association helped us determine the slope between overload and IL-6 for low and high levels of self-efficacy. For more details on this approach, see Holmbeck et al. (46).
RESULTS
Participants
Overall, our sample was primarily female (71%) and elderly (mean age ± SD = 74.2 ± 7.8). The average length of time participants had been providing care was approximately 5 years, and most (54%) were caring for a spouse with moderate dementia. More details on the demographic and health characteristics of our sample are presented in Table 1.
Table 1.
Caregiver and Care Recipient Demographic and Health Characteristics
| Characteristic | |
|---|---|
| Age, M (SD) | 74.2 (7.8) |
| Female, n (%) | 44 (71.0) |
| Education, n (%) | |
| < High School | 1 (1.6) |
| High School or Equivalent | 15 (24.2) |
| Some College | 17 (27.4) |
| College graduate or above | 29 (46.7) |
| Years Caregiving, M (SD) | 4.7 (3.8) |
| Care Recipient CDR Score, n (%) | |
| 1 | 23 (37.1) |
| 2 | 34 (54.8) |
| 3 | 5 (8.1) |
| Body Mass Index, M (SD) | 26.1 (4.2) |
| Systolic Blood Pressure, M (SD) | 134.6 (16.4) |
| Interleukin-6 (pg/ml), M (SD) | 1.5 (2.6) |
| Role Overload, M (SD) | 5.2 (3.1) |
| SE for using Problem-Focused Coping, M (SD) | 44.5 (10.0) |
Note. SE = Self-efficacy; CDR = Clinical Dementia Rating.
Self-efficacy Moderator Analysis
Our overall regression model was significant (F=2.54, df = 8,53; p = .020) and explained 27.7% of the total (log10) IL-6 variance (adjusted R2 = 16.8%). Results of our regression are presented in Table 2. Specifically, we found a significant interaction between role overload and self-efficacy (t = -2.017, df = 53, p = .049), suggesting a potential moderating effect. No other variables were significantly related to IL-6 values (all p-values > .094).
Table 2.
Regression model predicting (log10) IL-6
| Variable | B (SE) | t-value | p-value |
|---|---|---|---|
| Age | 0.008 (0.005) | 1.702 | .095 |
| Sex (female) | 0.094 (0.088) | 1.069 | .290 |
| BMI | 0.014 (0.009) | 1.629 | .109 |
| Systolic Blood Pressure | -0.002 (0.002) | -0.784 | .436 |
| CDR | 0.032 (0.062) | 0.518 | .606 |
| Role Overload | 0.016 (0.013) | 1.171 | .247 |
| Self-efficacy | -0.002 (0.004) | -0.369 | .714 |
| Overload-by-Self efficacy | -0.003 (0.001) | -2.017 | .049 |
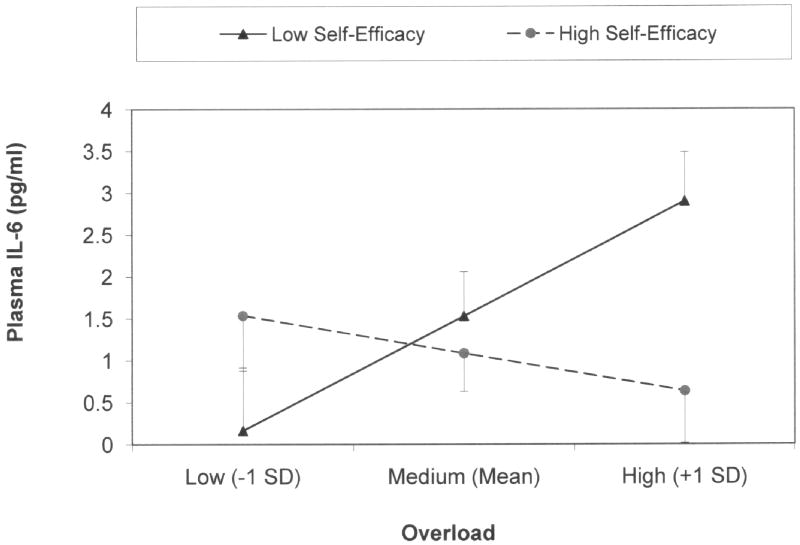
The significant overload-by-self efficacy interaction was follow-up with two post-hoc tests. Results of these tests indicated that role overload was significantly related to (log10) IL-6 when self-efficacy was low (t = 2.60, df = 53, p = .012). In contrast, when self-efficacy was high, there was not a significant relationship between overload and IL-6 (t = -0.47, df = 53, p = .638). A graphic depiction of this interaction effect is presented in Figure 1.
Figure 1.
Relationship between caregiver stress (i.e., overload) and raw Plasma IL-6 (pg/ml) for low and high self-efficacy. When self-efficacy was low, overload was significantly related to IL-6 (β = .53). When self-efficacy was high, overload was not significantly related to IL-6 (β = -.17). Values represent M±SE.
DISCUSSION
The purpose of the current study was to determine if increased levels of coping self-efficacy, namely self-efficacy for using problem-focused coping, significantly altered the relations between stress and plasma concentrations of IL-6 in spousal Alzheimer’s caregivers. Results confirmed our hypothesis such that stress, conceptualized as role overload, was significantly related to IL-6 when self-efficacy was low but not when self-efficacy was high.
These results are complimentary to those found by Bandura, who found that high levels of coping self-efficacy helped reduce the impact of acute stress on blood pressure and heart rate (29) and catecholamine secretion (32). The current study contributes to Bandura’s findings in that overstimulation of the sympathetic nervous system acts to stimulate secretion of IL-6 (6, 35). Therefore, to the extent that self-efficacy attenuates the sympathetic response to stress the expected result would be reduced concentrations of IL-6. Indeed, we have previously shown that greater perceived sense of control is associated with reduced sympathetic arousal (i.e., norepinephrine reactivity to acute stress) in elderly Alzheimer’s caregivers (18). It is therefore our speculation that caregivers with low levels of coping self-efficacy and who experience daily and chronic exposure to stressors (e.g., care receivers engaging in potentially dangerous behaviors) experience repeated activation of the sympathoadrenalmedulary (SAM) system, eventually leading to downstream consequences including dysregulation of IL-6 and potentially cardiovascular morbidity (12, 13, 47, 48) and mortality (14). However, caregivers with greater overall coping self-efficacy may be protected from these consequences of stress.
One positive implication of these findings is that self-efficacy is a malleable construct that is often the focus of psychotherapy treatments. For example, Steffen (49) and Coon et al. (23) have conducted randomized trials of psychological interventions for increasing self-efficacy. Both of these trials reported that caregivers in the active intervention conditions showed significant increases in self-efficacy, which in turn mediated the efficacy of the interventions for improving psychological outcomes. While the results of a study like ours, with limitations of its cross sectional design, require caution in extrapolation, they do raise the intriguing possibility that if psychosocial interventions can successfully improve self-efficacy in caregivers in the midst of ongoing stressors, then perhaps they might also have long-term beneficial implications for the health of caregivers, most notably cardiovascular health (1-5).
With regard to self-efficacy and cardiovascular health, other studies of non-caregiving populations have emphasized self-efficacy as a treatment target. These interventions conceptualize that improving self-efficacy may have direct or residual effects on health, particularly CVD risk. In a study examining illness perceptions in young adults with insulin dependent diabetes mellitus, Griva, Myers, & Newman (50) found that higher self-efficacy predicted both physiological and behavioral outcomes in the self-management of diabetes. Other interventions that improved self-efficacy have shown simultaneous improvements in physical activity (51, 52) and improved physical functioning after CABG surgery in those with ischemic heart failure (53). Again, the message from these studies is that self-efficacy is an important treatment target that is believed to produce behavior change and ultimately positive health outcomes.
In interpreting our findings, we acknowledge several study limitations. First, our study was cross-sectional and we do not know if self-efficacy has any protective effect against rising IL-6 over time. Indeed, Kiecolt-Glaser and colleagues (11) demonstrated that caregivers are susceptible to accelerated increases in IL-6 over time. How self-efficacy moderates this slope can be tested in future research. A second related limitation is that we do not know if increasing caregivers’ self-efficacy causes decreases in circulating IL-6. If our findings are confirmed, then future intervention studies that actively target self-efficacy and IL-6 to determine if change in self-efficacy mediates any change in IL-6 may be warranted.
Elevations in IL-6 have been linked to cardiovascular illness in multiple studies (2-5), and research suggests that chronically stressed caregivers are at increased risk for both elevated IL-6 (10, 11) and CVD (13, 47). While results of our study suggest that increased self-efficacy may provide downstream benefits such as reduced risk for CVD, it is premature to conclude that increasing self-efficacy in caregivers has any protective effects on risk for actual development of CVD. Therefore, future studies should examine under what circumstances (e.g., low self-efficacy) caregivers develop CVD.
In sum, we found that in elderly Alzheimer’s caregivers increased stress was significantly related to increased IL-6 when coping self-efficacy was low but not when self-efficacy was high. If confirmed, these results suggest that psychosocial interventions for caregivers may be considered to target coping self-efficacy so that caregivers may respond more confidently to the stresses of caregiving. More adaptive coping may then result in not only reduced IL-6 but may potentially reduce caregivers’ risk for development of CVD.
Acknowledgments
Financial support for this study was provided by the National Institute on Aging, grant AG 015301 to Igor Grant. Additional support was provided through grant AG031090 to Brent T Mausbach.
Footnotes
Disclosure of Competing Interest: No disclosures to report.
References
- 1.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. Journal of the American Geriatrics Society. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 2.Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Valkonen VP, Fuentes R, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44:859–865. doi: 10.1161/01.HYP.0000146691.51307.84. [DOI] [PubMed] [Google Scholar]
- 3.Amar J, Fauvel J, Drouet L, Ruidavets JB, Perret B, Chamontin B, et al. Interleukin 6 is associated with subclinical atherosclerosis: a link with soluble intercellular adhesion molecule 1. J Hypertens. 2006;24:1083–1088. doi: 10.1097/01.hjh.0000226198.44181.0c. [DOI] [PubMed] [Google Scholar]
- 4.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 5.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. The Journal of clinical endocrinology and metabolism. 1993;77:1690–1694. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- 9.Jenny NS, Tracy RP, Ogg MS, Luong le A, Kuller LH, Arnold AM, et al. In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–2071. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 10.von Känel R, Dimasdale JE, Mills PJ, Ancoli-Israel S, Patterson TL, Mausbach BT, et al. Effect of Alzheimer caregiving stress and age on frailty markers Interleukin-6, C-reactive protein, and d-Dimer. J Gerontol A Biol Sci Med Sci. 2006;61A:963–969. doi: 10.1093/gerona/61.9.963. [DOI] [PubMed] [Google Scholar]
- 11.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. PNAS. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw WS, Patterson TL, Ziegler MG, Dimsdale JE, Semple SJ, Grant I. Accelerated risk of hypertensive blood pressure recordings among Alzheimer caregivers. Journal of Psychosomatic Research. 1999;46:215–227. doi: 10.1016/s0022-3999(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 13.Mausbach BT, Patterson TL, Rabinowitz Y, Grant I, Schulz R. Depression and Distress Predict time to Cardiovascular Disease in Dementia Caregivers. Health Psychol. 2007;26:539–544. doi: 10.1037/0278-6133.26.5.539. [DOI] [PubMed] [Google Scholar]
- 14.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 15.Mausbach BT, Patterson TL, von Kanel R, Mills PJ, Ancoli-Israel S, Dimsdale JE, et al. Personal Mastery Attenuates the Effect of Caregiving Stress on Psychiatric Morbidity. J Nerv Ment Dis. 2006;194:132–134. doi: 10.1097/01.nmd.0000198198.21928.e7. [DOI] [PubMed] [Google Scholar]
- 16.Mausbach BT, von Känel R, Depp C, Patterson TL, Aschbacher K, Mills PJ, et al. The moderating effect of personal mastery on the relations between stress and Plasminogen Activator Inhibitor-1 (PAI-1) antigen. Health Psychol. 2008;27:S172–S179. doi: 10.1037/0278-6133.27.2(Suppl.).S172. [DOI] [PubMed] [Google Scholar]
- 17.Mausbach BT, Patterson TL, Von Känel R, Mills PJ, Dimsdale JE, Ancoli-Israel S, et al. The attenuating effect of personal mastery on the relations between stress and Alzheimer caregiver health: A five-year longitudinal analysis. Aging and Mental Health. 2007;11:637–644. doi: 10.1080/13607860701787043. [DOI] [PubMed] [Google Scholar]
- 18.Roepke SK, Mausbach BT, Aschbacher K, Ziegler MG, Dimsdale JE, Mills PJ, et al. Personal mastery is associated with reduced sympathetic arousal in stressed Alzheimer caregivers. American Journal of Geriatric Psychiatry. 2008;16:310–317. doi: 10.1097/JGP.0b013e3181662a80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mausbach BT, Aschbacher K, Mills PJ, Roepke SK, Von Känel R, Patterson TL, et al. A 5-year longitudinal study of the relationships between stress, coping, and immune cell ß2-adrenergic receptor sensitivity. Psychiatry Research. 2008;160:247–255. doi: 10.1016/j.psychres.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mausbach BT, Mills PJ, Patterson TL, Aschbacher K, Dimsdale JE, Ancoli-Israel S, et al. Stress-related reductions in personal mastery are related to reduced immune cell beta2-adrenergic receptor sensitivity. International Psychogeriatrics. 2007;19:935–946. doi: 10.1017/S1041610206004364. [DOI] [PubMed] [Google Scholar]
- 21.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 22.Bandura A. Self-efficacy: The exercise of control. New York: W H Freeman; 1997. [Google Scholar]
- 23.Coon DW, Thompson L, Steffen A, Sorocco K, Gallagher-Thompson D. Anger and depression management: Psychoeducational skill training interventions for women caregivers of a relative with dementia. The Gerontologist. 2003;43:678–689. doi: 10.1093/geront/43.5.678. [DOI] [PubMed] [Google Scholar]
- 24.Zeiss A, Gallagher-Thompson D, Lovett S, Rose J, McKibbin C. Self-efficacy as a mediator of caregiver coping: Development and testing of an assessment model. Journal of Clinical Geropsychology. 1999;5:221–230. [Google Scholar]
- 25.Rabinowitz YG, Mausbach BT, Thompson LW, Gallagher-Thompson D. The relationship between self-efficacy and cumulative health risk associated with health behavior patterns in female caregivers of elderly relatives with Alzheimer’s dementia. Journal of Aging and Health. 2007;19:946–964. doi: 10.1177/0898264307308559. [DOI] [PubMed] [Google Scholar]
- 26.Fortinsky RH, Kercher K, Burant CJ. Measurement and correlates of family caregiver self-efficacy for managing dementia. Aging & mental health. 2002;6:153–160. doi: 10.1080/13607860220126763. [DOI] [PubMed] [Google Scholar]
- 27.Mowat J, Laschinger HK. Self-efficacy in caregivers of cognitively impaired elderly people: a concept analysis. J Adv Nurs. 1994;19:1105–1113. doi: 10.1111/j.1365-2648.1994.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar U, Ali S, Whooley MA. Self-efficacy as a marker of cardiac function and predictor of heart failure hospitalization and mortality in patients with stable coronary heart disease: Findings from the Heart and Soul Study. Health Psychology. 2009;28:166–173. doi: 10.1037/a0013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandura A, Reese L, Adams NE. Microanalysis of action and fear arousal as a function of differential levels of perceived self-efficacy. J Pers Soc Psychol. 1982;43:5–21. doi: 10.1037//0022-3514.43.1.5. [DOI] [PubMed] [Google Scholar]
- 30.Bandura A. Perceived self-efficacy. In: Mays V, Albee G, Schneider S, editors. Prevention of AIDS: Psychological Approaches. Newbury Park, CA: Sage; 1989. pp. 128–141. [Google Scholar]
- 31.Chesney MA, Neilands TB, Chambers DB, Taylor JM, Folkman S. A validity and reliability study of the coping self-efficacy scale. Br J Health Psychol. 2006;11:421–437. doi: 10.1348/135910705X53155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandura A, Taylor CB, Williams SL, Mefford IN, Barchas JD. Catecholamine secretion as a function of perceived coping self-efficacy. J Consult Clin Psychol. 1985;53:406–414. doi: 10.1037//0022-006x.53.3.406. [DOI] [PubMed] [Google Scholar]
- 33.Kotchen TA. Chapter 241. Hypertensive Vascular Disease. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison’s Principles of Internal Medicine. 17 [Google Scholar]
- 34.Widmaier EP, Raff H, Strang KT. Vander’s Human Physiology: The Mechanisms of Body Function. 10. New York, NY: McGraw-Hill; 2006. [Google Scholar]
- 35.Papanicolaou DA, Petrides JS, Tsigos C, Bina S, Kalogeras KT, Wilder R, et al. Exercise stimulates interleukin-6 secretion: inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol. 1996;271:E601–605. doi: 10.1152/ajpendo.1996.271.3.E601. [DOI] [PubMed] [Google Scholar]
- 36.Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- 37.Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 38.Vicennati V, Vottero A, Friedman C, Papanicolaou DA. Hormonal regulation of interleukin-6 production in human adipocytes. Int J Obes Relat Metab Disord. 2002;26:905–911. doi: 10.1038/sj.ijo.0802035. [DOI] [PubMed] [Google Scholar]
- 39.Thayer JF. Vagal tone and the inflammatory reflex. Cleve Clin J Med. 2009;76(Suppl 2):S23–26. doi: 10.3949/ccjm.76.s2.05. [DOI] [PubMed] [Google Scholar]
- 40.Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 41.Li LW. From Caregiving to Bereavement: Trajectories of Depressive Symptoms Among Wife and Daughter Caregivers. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2005;60B:P190–P198. doi: 10.1093/geronb/60.4.p190. [DOI] [PubMed] [Google Scholar]
- 42.Campbell P, Wright J, Oyebode J, Job D, Crome P, Bentham P, et al. Determinants of burden in those who care for someone with dementia. Int J Geriatr Psychiatry. 2008;23:1078–1085. doi: 10.1002/gps.2071. [DOI] [PubMed] [Google Scholar]
- 43.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 44.Tabachnick BG, Fidel LS. Using Multivariate Statistics. 4. Needham Heights, MA: Allyn & Bacon; 2001. [Google Scholar]
- 45.Kraemer HC, Blasey CM. Centring in regression analyses: A strategy to prevent errors in statistical inference. International Journal of Methods in Psychiatric Research. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S women A prospective study. Am J Prev Med. 2003;24:113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 48.Shaw WS, Patterson TL, Semple SJ, Ho S, Irwin MR, Hauger RL, et al. Longitudinal analysis of multiple indicators of health decline among spousal caregivers. Annals of Behavioral Medicine. 1997;19:101–109. doi: 10.1007/BF02883326. [DOI] [PubMed] [Google Scholar]
- 49.Steffen AM. Anger management for dementia caregivers: A preliminary study using video and telephone interventions. Behavior Therapy. 2000;31:281–299. [Google Scholar]
- 50.Griva K, Myers LB, Newman S. Illness perceptions and self efficacy beliefs in adolescents and young adults with insulin dependent diabetes mellitus. Psychology & Health. 2000;15:733–750. [Google Scholar]
- 51.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, 3, Blair SN. Reduction in cardiovascular disease risk factors: 6-month results from Project Active. Preventive medicine. 1997;26:883–892. doi: 10.1006/pmed.1997.0218. [DOI] [PubMed] [Google Scholar]
- 52.Witmer JM, Hensel MR, Holck PS, Ammerman AS, Will JC. Heart Disease Prevention for Alaska Native Women: A Review of Pilot Study Findings. Journal of Women’s Health. 2004;13:569–578. doi: 10.1089/1540999041280981. [DOI] [PubMed] [Google Scholar]
- 53.Barnason S, Zimmerman L, Nieveen J, Schmaderer M, Carranza B, Reilly S. Impact of a home communication intervention for coronary artery bypass graft patients with ischemic heart failure on self-efficacy, coronary disease risk factor modification, and functioning. Heart Lung. 2003;32:147–158. doi: 10.1016/s0147-9563(03)00036-0. [DOI] [PubMed] [Google Scholar]