Abstract
Asparaginases are a cornerstone of treatment protocols for acute lymphoblastic leukemia (ALL) and are used for remission induction and intensification treatment in all pediatric regimens and in the majority of adult protocols. Extensive clinical data have shown that intensive asparaginase treatment improves clinical outcomes in childhood ALL. Three asparaginase preparations are available; the native asparaginase derived from Escherichia coli (E. coli-asparaginase), a pegylated form of this enzyme (PEG-asparaginase) and a product isolated from Erwinia chrysanthemi, i.e. Erwinia asparaginase. Clinical hypersensitivity reactions and silent inactivation due to antibodies against E.coli-asparaginase, lead to inactivation of E-Coli asparaginase in up to 60% of cases. Current treatment protocols include E. coli-asparaginase or PEG-asparaginase for first-line treatment of ALL. Typically, patients exhibiting sensitivity to one formulation of asparaginase are switched to another product to ensure they receive the most efficacious treatment regimen possible. Erwinia asparaginase is used as a second- or third-line treatment in European and US protocols. Despite the universal inclusion of asparaginase in such treatment protocols, there is much debate regarding the optimal formulation and dosage of these agents. This manuscript provides an overview of available evidence to make recommendations for optimal use of Erwinia asparaginase in the treatment of ALL.
Keywords: acute lymphoblastic leukemia, asparagine depletion, asparaginase, Erwinia asparaginase, Erwinase, ALL
Introduction
The long-term outcome of acute lymphoblastic leukemia (ALL) has improved dramatically over the last few decades through the development of effective treatments and well-designed treatment protocols. Long-term event-free survival rates in children are currently around 80%1-6 and overall survival rates are close to or exceeding 90%.4 Although overall survival rates in adults have improved in recent years, only 38–50% achieve long-term survival.7;8 The superior outcome achieved in childhood ALL has been attributed to a higher proportion of favorable genetic subtypes, more effective treatment options and better compliance with treatment by patients, parents and doctors when compared with adult ALL patients, alongside a poorer tolerance of adults to some chemotherapy regimens.9-11 While the majority of recent regimens for adult ALL are based on pediatric schedules, there is room for further treatment refinement in these patients.9;10;12;12-17
Among the drugs used in the treatment of ALL are bacterial-derived enzymes, referred to as asparaginases.6;18 Three main types of asparaginase have been used to date: native asparaginase derived from Escherichia coli (E. coli-asparaginase: Kidrolase®, EUSA Pharma; Elspar®, Ovation Pharmaceuticals; Crasnitin™, Bayer; Leunase®, sanofi-aventis; Asparaginase medac™, Kyowa Hakko); a pegylated form of the native E. coli-asparaginase (polyethylene glycol [PEG]-asparaginase: Oncaspar™, Enzon Pharmaceuticals Inc);19 and an enzyme isolated from Erwinia chrysanthemi, referred to as Erwinia asparaginase (Erwinase®, EUSA Pharma).18 It is important to note that some of these preparations are no longer available in all countries. A fourth, new, recombinant E. coli-asparaginase preparation is currently undergoing clinical evaluation; it is engineered to have an amino acid sequence identical to that of Asparaginase medac™, with initial data showing a comparable efficacy and toxicity profile to those of the other E. coli-asparaginases.20 An asparaginase encapsulated into homologous red blood cells has recently been proposed as a new approach to maintain enzyme activity, while reducing formation of anti-asparaginase antibodies.21 In addition, a pegylated form of recombinant Erwinia asparaginase is under preclinical study.22
The parenteral administration of asparaginase results in rapid and complete deamination of the amino acid asparagine and, to a lesser extent glutamine,23-26 leading to depletion of asparagine, especially in the plasma23;27-31 and, in part, the cerebrospinal fluid (CSF).32;33 Differences in the biological activity among available E coli-asparaginase preparations have been suggested.32 The tolerated dose has varied among trials,4,25,34 which is also suggestive of differences in the relative potency of the available asparaginase products.
Despite being an essential drug used in all treatment protocols for ALL, there is much debate regarding the optimal formulation and dosage of asparaginase. We provide an overview of available data on the use of asparaginases, and focus on Erwinia asparaginase which has been less well studied compared with other forms.
Asparaginase therapy in ALL
Efficacy data for asparaginases
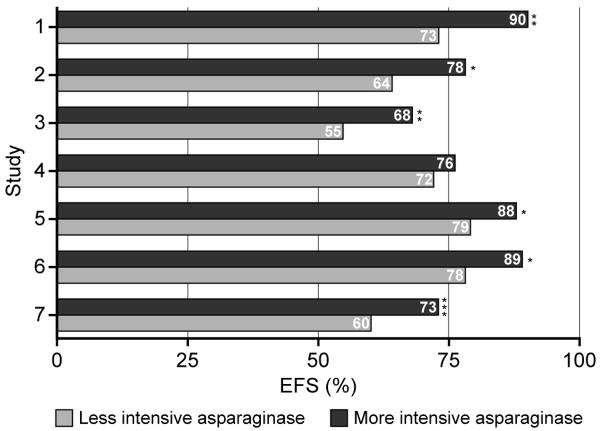
Extensive clinical data support the use of asparaginase therapy in pediatric ALL 2;4;6;35-38 and the benefit of intensive asparaginase treatment compared with less intensive regimens has been demonstrated (Figure 1).2;38-41 In a study conducted by the Dana-Faber Cancer Institute (DFCI) ALL Consortium and designed to improve outcomes and minimize toxicities in pediatric patients with standard-risk or high-risk ALL, 377 children were enrolled to receive a high-dose native E.coli asparaginase (25 000 IU/m2 weekly) or PEG-asparaginase (2 500 IU/m2 every other week) for 30 weeks during intensification therapy. The estimated 5-year event-free survival rate was significantly higher than that of a previously conducted DFCI ALL Consortium study (83%±2% vs 74% ±3%; p<0.01), a finding which was attributed to the prolonged asparaginase intensification.2 In addition, in this study children who tolerated more than 25 weekly doses of asparaginase had a better event-free survival than those who received 25 or fewer doses.2 Furthermore, a randomized study carried out by the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) determined the efficacy of a BFM-type modified chemotherapy regimen with or without prolonged use of high-dose native E.coli asparaginase (25 000 IU/m2 weekly for 20 weeks) during continuation therapy in 355 children with standard-risk ALL.41 Children given asparaginase had a significantly increased 10-year disease-free survival (87.5% vs 78.7%) and overall survival (93.7% vs 88.6%), with a 40% reduction in the relative risk of failure compared with patients who were not treated with asparaginase.41 This finding supports previous data of Amylon et al38 showing that high-dose native E.coli asparaginase (25 000 IU/m2 weekly for 20 weeks) during consolidation significantly improved complete continuous remission in pediatric patients with T-cell ALL and lymphoblastic lymphoma compared with patients treated with lower-dose asparaginase regimen (71.3% vs 57.8%, respectively). The randomized studies conducted by Moghrabi et al40 and Duval et al39 made clear that asparaginase preparations with a shorter half-life result in a poorer event-free survival, albeit less toxicity, compared to the use of asparaginase preparation with a longer half-life given at the same dose and frequency (Figure 1). It is also noteworthy that in a study carried out by Rizzari et al,42 no significant difference in disease-free survival was observed between patients who received standard treatment (10 000 IU/m2 asparaginase for 4 doses during reinduction) and those who received high-dose treatment (25 000 IU/m2 asparaginase weekly for 20 weeks during reinduction and early continuation)
Figure 1.
EFS, event-free survival
Intensification studies
1 Less than or greater than 25 weeks of treatment
2–5 Additional 20 weeks of treatment
6–7 Erwina vs E.Coli-ASP
*p<0.05; **p<0.01; ***p<0.001
As a result of these trials, asparaginases are now a universal component of ALL therapy and are used for remission induction and intensification treatment in every pediatric regimen for ALL. However, much debate remains regarding the optimal formulation and dosage of asparaginase in the treatment of ALL. Therapy aims to achieve serum asparagine depletion but no critical minimum value for efficacy has yet been established.24;25;43 A serum level of asparaginase >100 IU/L corresponds to depletion of asparagine (ie below the level of quantification)27 and therefore is often considered the target trough asparaginase level; complete asparagine depletion is observed less frequently with enzyme concentrations below this level.43;44 However, there is some evidence to suggest that trough asparaginase levels of below 50 IU/L can also result in serum and CSF asparagine depletion.44
Toxicity of asparaginases: hypersensitivity
Asparaginases are associated with a unique set of side effects. Hypersensitivity reactions, due to anti-asparaginase antibody production, have been observed in up to 60% of patients at some time during E. coli asparaginase therapy.45 The development of these antibodies appears to be more commonly observed with native E. coli-asparaginase28;46;47 compared with the pegylated enzyme.28;48 (Table 1) Symptoms of clinical hypersensitivity include anaphylaxis, pain, edema, Quincke's edema, urticaria, erythema, rash and pruritis.46 The route of administration determines the clinical symptoms with a greater incidence of major skin reactions observed with intramuscular (IM) administration compared with intravenous (IV) administration.52 Clinical hypersensitivity occurs almost exclusively in post-induction regimens (ie intensification, re-induction)50;53 when asparaginase has not been given for weeks or months. There are several possible explanations for the rarity of allergic reactions during remission induction. For example, there is a delay in an effective immune response due to the time taken for complement activation and the subsequent production of antibodies18 the symptoms associated with allergy might be masked by intensive corticosteroids treatment that occurs during induction,18 and the frequency of dosing during induction may have a desensitizing effect, since allergic reactions are rarely observed in this phase despite measurable antibody production. Some studies have shown that the incidence of hypersensitivity to asparaginase is similar between age groups,2;54 although others have suggested that infants and younger patients develop antibody and hypersensitivity reactions less frequently than teenagers and adult patients.18
Table 1.
Incidence of specific antibodies induced by the three main asparaginase types
| Asparaginase type | Dose | Concomitant steroid medications | Antibody-positive patients | Citation |
|---|---|---|---|---|
| E. Coli | 10 000 IU/m2 IM three-times weekly for nine doses during induction and nine during re-induction | Prednisolone | 35.5% | Woo et al. 2000 (46) |
| 6000 IU/m2 IM three-times weekly for nine (induction) and six (intensification) doses | Prednisolone/dexamethasone | 26–42% | Avramis et al. 2002 (28) | |
| 6000 IU/m2 SC two-times weekly for 14 doses (induction/intensification) | Prednisolone | 20% | Larson et al. 1998 (47) | |
| PEG | 2500 IU/m2 IM for a total of four doses (induction) and one dose (intensification) | Dexamethasone | 11% | Hawkins et al. 2004 (48) |
| 2500 IU/m2 IM for one dose (induction) and one dose (delayed intensification) | Prednisolone/dexamethasone | 2–11% | Avramis et al. 2002 (28) | |
| Erwinia | 10 000 IU/m2 IM three-times weekly for a total of nine doses (induction/re-induction) | 33% | Wang et al. 2003 (49) | |
| 30 000 IU/m2 IV or IM daily for a total of 10 doses (induction), twice-weekly for a total of four doses (re-induction) | Prednisolone | 21% | Albertsen et al. 2002 (50) | |
| 30 000 IU/m2 IV or IM daily for a total of 10 doses (induction), twice-weekly for a total of four doses (re-induction) | Prednisolone/dexamethasone | 8–10% | Albertsen et al. 2002 (53) |
E. Coli, Escherichia coli; IM, intramuscular; SC, subcutaneous; PEG, polyethylene glycol; IV, intravenous
Antibodies produced in response to asparaginases do not always lead to clinical hypersensitivity, but may instead cause rapid inactivation of the asparaginase, resulting in suboptimal asparagine depletion. This is commonly referred to as ‘silent hypersensitivity’ or ‘silent inactivation’45;55;56 and may occur in approximately 30% of the patients.45 Development of anti-asparaginase antibodies can thus confer resistance to asparaginase therapy and is associated with higher plasma levels of asparagine57 and reduced therapeutic efficacy in some,55;58 but not all, studies.46;47 This inconsistency of anti-asparaginase antibodies as a prognostic indicator may be explained by the efficacy of the overall treatment regimens and the use of alternative asparaginase preparations following allergic reactions, which may mitigate the adverse effects of silent hypersensitivity.
Typically, patients exhibiting clinical allergy symptoms to one formulation of asparaginase are switched to another product to ensure they receive the most efficacious treatment regimen possible.45;56 However, since patients with silent hypersensitivity lack clinical symptoms and routine antibody monitoring is often not implemented, asparaginase switching does not usually occur in this setting.45 PEG-asparaginase has a relatively lower immunogenicity due to the covalent conjugation to monomethoxy polyethylene glycol59 and often replaces E. coli-asparaginase in patients who develop allergic reaction. This switch may not be optimal because antibodies against E. coli-asparaginase can cross-react with PEG-asparaginase.49;60 Moreover, PEG-asparaginase may also induce silent inactivation56 with antibodies resulting in a fast decline in asparaginase activity.61 Switching from PEG-asparaginase after an allergic reaction to E. coli-asparaginase is not considered a viable treatment option.62
Other toxicity associated with asparaginases
Pancreatitis occurs in 4–18% of pediatric patients, depending upon the definition used in the study, and can cause significant morbidity.54;63;64 Adolescents appear to be at higher risk for developing this condition than younger children.54 Pancreatitis tends to occur after the first few weeks of asparaginase, suggesting a predisposition to this complication rather than a cumulative drug effect.64 Re-treatment with asparaginase after an episode of pancreatitis is associated with a high risk of recurrence64 and so further doses of asparaginase are often omitted, which may negatively impact event-free survival.2 Other asparaginase-related toxicities include abnormalities of hemostasis (including central nervous system [CNS] thromboses and hemorrhage, and peripheral deep venous thromboses in 2–4% of patients), hyperglycemia and abnormalities of lipid metabolism.59;65 As with pancreatitis, thrombotic complications are more common in adolescents and adults than in younger children.54 In adult patients, liver toxicity with elevated liver enzymes or increased bilirubin is a frequent clinical problem.8
Erwinia asparaginase in ALL
Currently, there are no widely accepted guidelines for the use of asparaginases, especially Erwinia asparaginase. A number of comparative studies have been conducted with Erwinia asparaginase and native E. coli preparations; the dose and schedules of asparaginase in these studies have been inconsistent and outcomes have been variable.39;40;66;67 However, the efficacy of Erwinia asparaginase following hypersensitivity to E. coli-asparaginase preparations has been demonstrated.45;68 The differences in these results highlight the need for recommendations to provide guidance for the optimal use of Erwinia asparaginase in the treatment of ALL.
Efficacy data for Erwinia asparaginase
Eden et al66 carried out a non-randomized study (UKALL VIII) comparing the toxicity of IM administration of Erwinia asparaginase with E. coli-asparaginase (6000 IU/m2 three-times weekly for 3 weeks) in 758 unselected children with ALL. No apparent difference in event-free survival was observed after 4.5 years of follow-up, but the incidence of neurotoxicity, pancreatitis and life-threatening sepsis was significantly lower in children treated with Erwinia asparaginase compared with those who received E. coli-asparaginase (neurotoxicity: 2% vs 4%; pancreatitis: 0% vs 2%; sepsis: 18% vs 20%).66 Results from this early trial led to the first randomized study (European Organisation for Research and Treatment of Cancer-Children's Leukemia Group [EORTC-CLG] 58881) comparing Erwinia asparaginase with E. coli-asparaginase39 and included 700 children (aged <18 years) with ALL (93%) or lymphoblastic non-Hodgkin's lymphoma (7%). Patients were randomized to receive the same dosage of either asparaginase (10 000 IU/m2 IV twice-weekly for a total of eight doses in the induction phase and four doses in the re-induction phase). Significantly more patients administered Erwinia asparaginase failed to achieve complete remission compared with those who received E. coli-asparaginase (4.9% vs 2%) and the relapse rate was higher, resulting in reduced event-free survival. Overall 6-year survival was also significantly superior but coagulopathy was more common in patients administered E. coli-asparaginase compared with the Erwinia asparaginase group (83.9% vs 75.1%).39
A subsequent randomized study, DFCI-ALL-95-01, compared administration of Erwinia asparaginase (25 000 IU/m2 once in induction followed by once-weekly doses for 20 weeks during intensification) with the same doses of E. coli-asparaginase in 286 ALL patients (aged 0–18 years).40 The 139 children given Erwinia asparaginase had significantly reduced toxicity (10% vs 24%, p<0.01) and fewer allergic reactions (6% vs 14%, p=0.03) compared with 147 E. coli-asparaginase-treated patients, but had significantly lower 5-year event-free survival (78% ±4% vs 89% ±3%).40 There were also significantly more relapses involving the CNS in children receiving Erwinia asparaginase compared with E. coli-asparaginase-treated patients (6% vs 1%).40
Another study by Kwok et al67 compared the efficacy of Erwinia asparaginase and E. coli-asparaginase in 116 children with ALL. Erwinia asparaginase was administered at a dose of 10 000 IU/m2 IM and E. coli-asparaginase at 7500 to 10 000 IU/m2 IM twice-weekly for eight doses during remission induction. Patients treated with Erwinia asparaginase were 6.7 times more likely to have residual leukemia levels ≥ 10−2 in bone marrow compared with E. coli-asparaginase-treated patients.67
Due to the shorter half-life of Erwinia asparaginase compared with the E. coli-derived preparations,55 a higher dose and increased frequency of treatment is required to ensure adequate serum enzyme activity and complete serum asparagine depletion. It is therefore possible that the inferior outcome of patients treated with Erwinia asparaginase in these trials (with parallel decreases in adverse reactions) is a result of insufficient dose and frequency of this preparation.11;40;62;69 Indeed, Boos et al25 reported that only 26% of samples from ALL patients had complete depletion of asparagine (ie ≤0.1 μmol/L) 3 days after administration of Erwinia asparaginase (10 000 IU/m2 at 3-day intervals). In addition, physiological asparagine levels recovered faster after Erwinia asparaginase than E. coli preparations.25
In one study, Erwinia asparaginase (30 000 IU/m2 IV or IM) was given daily during induction therapy and twice a week for 2 weeks during re-induction phase. The trough levels (measured immediately before the next administration) were below 100 IU/L in approximately two-thirds of samples during reinduction.43 Consequently, the majority of patients failed to achieve complete depletion of asparagine during re-induction. Similarly, in a DFCI ALL Consortium trial, in which patients switched to Erwinia asparaginase (25 000 IU/m2 twice-weekly) after allergy to E. coli-asparaginase, 83% of patients had serum enzyme activity levels at or above 100 IU/L 3 days after administration, but only 42% of patients maintained that level 4 days post-dosing.68 These data highlight that even with relatively high Erwinia asparaginase doses (25 000–30 000 IU/m2), a twice-weekly regimen was still associated with inadequate enzyme levels in most patients.43;68 Despite these findings, treatment with twice-weekly Erwinia asparaginase after E. coli-asparaginase allergy did not adversely impact rates of event-free survival in the DFCI ALL Consortium trial.68
Evidence from Viera Pinheiro et al26 suggests increased dosing frequency enhances Erwinia asparaginase activity. In this study, patients with ALL and non-Hodgkin's lymphoma were administered Erwinia asparaginase (20 000 IU/m2 three-times weekly) and trough asparagine levels and asparaginase activity were assessed 2 and 3 days following therapy.26 Mean serum asparaginase trough levels were above the target level of 100 IU/L 2 days after administration of Erwinia asparaginase (mean asparaginase level: 156 IU/L), although the activity fell after 3 days (mean asparaginase activity: 50 IU/L). Finally, Erwinia asparaginase administered at 10 000 U/m2 IV every second day resulted in a median trough activity of 115 U/L 2 days after administration but asparaginase activities were below 100 U/L in 45% of samples.70 Taken together, these data show that even a regimen of three-times weekly dosing (with a 2-day interval at weekends) yields inadequate asparaginase trough activity for at least part of the treatment schedule (typically at the weekend). In this regard, all the comparative studies in which Erwinia asparaginase yielded ‘inferior’ outcomes included less frequent and/or lower absolute doses than those used by Viera Pinheiro et al26 and therefore serum asparagine levels may not have been sufficiently depleted.
Second-line treatment with Erwinia asparaginase
Despite the apparently inferior outcomes of comparative studies of Erwinia asparaginase with E. coli-derived preparations, a study of 1001 high-risk pediatric ALL patients treated with nine doses of native E. coli-asparaginase during induction (6000 IU/m2 three-times weekly for 3 weeks) demonstrated the efficacy of switching products after clinical hypersensitivity.45 Results from an interim analysis of 280 patients, who were evaluated for at least 30 months after induction, showed that 41% developed clinical allergic reactions with positive antibody formation and were switched to Erwinia asparaginase. The antibody-positive patients with allergic symptoms were switched to Erwinia asparaginase, resulting in a reduction in their hazard ratio for treatment failure from 3.2 to 0.6. In contrast, 29% of patients had silent hypersensitivity and continued to receive E coli-asparaginase; these children had poorer outcomes.45 This demonstrates that awareness of the presence of asparaginase antibodies (in the absence of allergy) and subsequent switching to Erwinia asparaginase might mitigate the adverse effects of silent hypersensitivity.
Studies have shown cross-reactivity between patients' antibodies against E. coli-asparaginase and PEG-asparaginase, but not between those against E. coli-asparaginase and Erwinia asparaginase.49;60 Moreover, asparagine concentrations were less depleted by PEG-asparaginase than by Erwinia asparaginase in a small study of patients with antibodies against E. coli-asparaginase.57 Interestingly, one study showed that patients may also develop antibodies to the non-protein PEG moiety of PEG-asparaginase.71 This was associated with rapid clearance of PEG-asparaginase in a subgroup of pediatric patients who otherwise did not present a clinical manifestation of hypersensitivity or allergy. Furthermore, a population pharmacokinetic model demonstrated a fast decline in asparaginase activity in a group of patients, most likely related to the development of antibodies against PEG-asparaginase.61 It has therefore been suggested that anti-PEG level monitoring/screening or asparaginase activity measurements could allow for modification in PEG-asparaginase dosing or the use of an alternative asparaginase.61;71 So far, the presence of anti-PEG antibodies has not been confirmed by others. Routine antibody assessment or measurement of asparaginase levels has been proposed to predict future allergic reaction or to alert physicians to the possibility of silent hypersensitivity.18;26;46
As yet, there are no data from large well-designed studies to demonstrate a preference for Erwinia asparaginase over PEG-asparaginase in patients developing hypersensitivity to E.coli-asparaginase and there is no consensus opinion on this. After allergic reactions to E.coli preparations, substitution with an alternative asparaginase should be based on drug monitoring.25 Erwinia asparaginase appears to be well tolerated in children with previous allergy to E.coli-asparaginase.68 Allergic reactions to Erwinia asparaginase have also been reported in up to 33% of patients switching to Erwinia asparaginase after clinical hypersensitivity to native E. coli-asparaginase.68;72
Current status of and recommendations for the use of Erwinia asparaginase
Both E. coli-asparaginase and PEG-asparaginase can be used as first-line treatment in pediatric ALL protocols, depending upon country. Before a temporary interruption in 2002 that resulted from manufacturing issues related to vial stoppers, Erwinia asparaginase was considered the best alternative in cases of clinical hypersensitivity to these enzymes.62 Erwinia asparaginase production was reinstated in 2006 and previous European licenses are planned for reinstatement, together with a process of mutual recognition in other European countries and full approval in the USA.
Which patients should receive Erwinia asparaginase?
Patients developing allergic reactions to one asparaginase should be switched to an alternative product, to ensure maximum clinical benefit in terms of survival. Second-line asparaginase therapy should be dictated by protocols or regulatory/availability factors and the type of asparaginase used in front-line therapy; some protocols advise Erwinia asparaginase as a preferable preparation after allergic reaction to native E. coli-asparaginase, whilst others prescribe PEG-asparaginase as replacement for native E. coli-asparaginase and Erwinia asparaginase as third-line drug.
Several clinical trial groups in Europe and the United States allow the use of Erwinia asparaginase as a second-line agent (eg Nordic Society of Pediatric Hematology and Oncology [NOPHO], German Multicenter Acute Lymphoblastic Leukemia Study Group [GMALL], EORTC-58951, French Acute Lymphoblastic Leukemia group [FRALLE], Children's Oncology Group [COG]), DFCI ALL Consortium and, for others, as a third-line treatment (AIEOP, ALL-BFM-2000, Dutch Childhood Oncology Group [DCOG-ALL-10], Czech Republic protocols). Selection of the individual asparaginase is determined by availability, treatment protocol and treatment status of the patients (ie asparaginase-naïve or relapsed) (Table 2) and various Erwinia asparaginase dosing regimens are in use (Table 3).
Table 2.
Current regional use of asparaginases (source: EUSA Pharma)
| North America, UK, Australia and New Zealand | Europe (BFM zone) | Rest of World | ||
|---|---|---|---|---|
| Children | Naïve patients | First-line: PEG-asparaginase | First-line: E. coli-asparaginase | First-line: E. coli-asparaginase |
| Second-line: Erwinia asparaginase | Second-line: Erwinia asparaginase or PEG-asparaginase | Second-line: Erwinia asparaginase or PEG-asparaginase | ||
| Relapsed | First-line: PEG-asparaginase | First-line: PEG-asparaginase | First-line: E. coli-asparaginase | |
| Second-line: Erwinia asparaginase | Second-line: Erwinia asparaginase | Second-line: Erwinia asparaginase or PEG-asparaginase | ||
| Adults | Naïve patients | First-line: E. coli-asparaginase or PEG-asparaginase | First-line: E. coli-asparaginase or PEG-asparaginase | First-line: E. coli-asparaginase |
| Second-line: Erwinia asparaginase or PEG-asparaginase | Second-line: Erwinia asparaginase | Second-line: Erwinia asparaginase or PEG-asparaginase |
BFM, Berlin-Frankfurt-Münster; PEG, polyethylene glycol; E. Coli, Escherichia coli
Table 3.
ALL protocols currently used for Erwinia asparaginase (second- or third-line treatment)
| Protocol | Second-line treatment |
|---|---|
| NOPHO | Erwinia asparaginase 20 000 IU/m2 two–three-times/week (×6) |
| AIEOP | NB: Erwinia asparaginase as third-line |
| Erwinia asparaginase 20 000 IU/m2 every other day | |
| GMALL 07/2003 and 01/2003 | Erwinia asparaginase 20 000 IU/m2 three-times weekly (×5); IV (10 000 IU/m2 in patients older than 55 years) |
| COG | Erwinia asparaginase 25 000 IU/m2 three-times weekly (×6); IM |
| COALL-07-03 | Erwinia asparaginase 45 000 IU/m2 (×2) |
| Czech Republic | NB: Erwinia asparaginase as third-line |
| Induction and late intensification | |
| • Erwinia asparaginase 10 000 IU/m2 twice-weekly | |
| HR blocks | |
| • Erwinia asparaginase 10 000 IU/m2 twice-weekly | |
| First relapse | |
| • Erwinia asparaginase 10 000 IU/m2 twice-weekly | |
| DCOG ALL-10 | NB: Erwinia asparaginase as third-line |
| Induction | |
| • Erwinia asparaginase 10 000 IU/m2 two–three times/week | |
| Intensification (standard or medium risk) | |
| • Erwinia asparaginase 10 000 IU/m2 two–three times/week | |
| HR blocks | |
| Erwinia asparaginase 10 000 IU/m2, two–three times/week | |
| BFM-2000 | Protocol 1 |
| • Erwinia asparaginase 10 000 IU/m2 every 2 days IM/IV | |
| Protocol II | |
| • Erwinia asparaginase 10 000 IU/m2 every 2 days IM/IV | |
| Block HR-1 | |
| Erwinia asparaginase 10 000 IU/m2 every 2 days IM/IV | |
| EORTC-58951 | Erwinia asparaginase 20 000 IU/m2 two–three times/week; IM |
| FRALLE- 2000 | Induction, delayed intensification(s): Erwinia asparaginase 12 000 IU/m2 three times/week; IM, ie double dose compared to E.coli asparaginase |
| St Jude | Induction: |
| Erwinia asparaginase 20 000 IU/m2 three times/week (×6) IM; | |
| Post-remission: 30 000 or 42 000 IU/m2 twice-weekly IM for 30 weeks (standard-/high-risk patients), twice-weekly for 4 weeks during first and second reinduction (low-risk patients) | |
| DFCI ALL Consortium | Post-induction consolidation: |
| Erwinia Asparaginase 25 000 IU/m2 twice-weekly IM for 30 weeks |
NOPHO, Nordic Society of Pediatric Hematology and Oncology; AIEOP, Associazione Italiana Ematologia Oncologia Pediatrica; GMALL, German Multicenter Acute Lymphoblastic Leukemia Study Group; COG, Children's Oncology Group; COALL, Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia; DCOG, Dutch Childhood Oncology Group; BFM, Berlin-Frankfurt-Münster; EORTC, European Organization for Research and Treatment of Cancer; FRALLE, French Acute Lymphobalstic Leukemia group; DFCI, Dana-Faber Cancer Institute; IV, intravenous; IM, intramuscular; HR, high risk
Recommendations
Erwinia asparaginase should be used for the second- or third-line treatment of ALL, depending upon regulatory requirements, in patients developing hypersensitivity to E. coli asparaginase preparations
Erwinia asparaginase should be prescribed if switching from PEG-asparaginase is required (ie second-line use of native E. coli-asparaginase is not justified)
Erwinia asparaginase dosing and schedule
Due to the short half-life of Erwinia asparaginase,55 a higher dose and increased dosing frequency is required to ensure optimal asparagine depletion. Current evidence suggests that Erwinia asparaginase should be administered at dosages of at least 20 000 IU/m2 three-times weekly, ie every second day with a 3-day interval at the weekend.26 Twice-weekly dosing at higher doses (25 000–30 000 IU/m2) has been associated with suboptimal trough serum enzyme activity, but not consistently with inferior event-free survival43;68 and, as a result, this dosing regimen is still utilized by some groups.
As Erwinia asparaginase requires frequent dosing to maintain asparagine depletion, therapeutic drug monitoring data (specifically for serum asparaginase levels) could assist in determining whether increasing the interval between doses is possible and could therefore help to minimize inconvenience to both patients and physicians. Furthermore, the development of pegylated Erwinia asparaginase with a longer half-life would make the dosing schedule more convenient for patients.
Recommendations
Erwinia asparaginase should be administered at dosages of at least 20 000 IU/m2 multiple times per week (eg three-times weekly)
Duration of treatment
The optimal duration of Erwinia asparaginase treatment has yet to be established, although it has been suggested that prolonged intensification results in improved survival. This was demonstrated in a study by Silverman et al2, where the 5-year event-free survival rate of patients who received at least 26 weeks of asparaginase therapy was significantly better than those who tolerated 25 weeks or fewer of therapy (90% vs 73%). This study, together with the studies presented above and summarized in Figure 1, suggest that prolonged and intensified therapy with asparaginase improves outcome of children with ALL.
If Erwinia asparaginase is used as second-line treatment to replace native E.coli-asparaginase or PEG-asparaginase the duration of treatment depends on the protocol and the yielded duration of asparagine depletion. Also, the duration of asparaginase treatment will depend on the backbone of combination chemotherapy that is given.
Recommendations
Prolonged intensification with asparaginase to optimize survival benefits
Route of administration of Erwinia asparaginase
Intravenous administration results in higher peak plasma concentrations, while IM administration results in a concurrent slower increase of asparaginase activity due to the depot effect. Accordingly, administration of 10 000 U/m2 Erwinia asparaginase applied every second day results in median trough activities of 115 U/L (determined from 58 samples of 15 patients) when applied intravenously, and of 151 U/L (determined from 39 samples of 14 patients) when applied intramuscularly. After IM administration, only 15% of analyzed samples showed asparaginase activities below the desired activity of 100 U/L, while 45% of samples were below 100 U/L, when Erwinia asparaginase was administered intravenously.70
However, Rizzari et al44 found no significant differences in mean enzyme activity or frequency of samples showing complete asparagine depletion after IV or IM administration of Erwinia asparaginase 10 000 IU/m2 every 3 days (eight doses) administered in the induction phase.44 Similarly, Albertsen et al43 found comparable complete asparagine depletion in patients given a more intense regimen of IV or IM administration at 30 000 IU/m2 daily for 10 days in the induction phase.43 In this study, however, Erwinia asparaginase administered via the IM route produced trough asparaginase plasma levels significantly lower (by approximately 28%) than IV administration. During the subsequent re-induction phase (30 000 IU/m2 twice-weekly for 2 weeks), no differences were observed between the two routes in terms of trough asparaginase activities or in the proportion of patients who failed to achieve complete asparagine depletion.43 Finally, no significant differences have been observed between two routes of administration of Erwinia asparaginase 30 000 IU/m2 twice-weekly for 2 weeks as a re-induction regimen in terms of neutralizing asparaginase antibody formation.50
The results of studies investigating the optimal route for the administration of asparaginase are inconsistent and therefore further studies are required to determine whether IV or IM administration of Erwinia asparaginase is associated with any meaningful clinical differences.24
Recommendations
No recommendations are made for the route of administration as more data are required to define the optimal route. However, most groups in Europe currently use IV asparaginases, while North American groups more often administer this agent intramuscularly
Monitoring of asparaginase trough levels and/or depletion of asparagine
Initially, the US Food and Drug Administration required that asparagine levels were used as the primary outcome measure in clinical trials. Asparaginase therapy aims to achieve serum asparagine depletion, but no critical minimum value for efficacy has yet been established24;25;43 and asparagine levels are difficult to measure accurately when asparaginase is present in blood because the enzyme can continue to breakdown asparagine ex vivo if the sample is not immediately processed and stored on ice. Therefore, monitoring of asparaginase levels is more reliable than measurement of asparagine itself. A serum level of asparaginase >100 IU/L and possibly >50 IU/L corresponds to depletion of asparagine (ie below the level of quantification)44;73; complete asparagine depletion is observed less frequently with enzyme concentrations below this level.43;44 However, clinical testing to measure asparaginase levels or asparagine depletion is not routinely carried out, although therapeutic drug monitoring is offered in Europe to guide therapeutic decisions (Boos et al. personal communication).
Recommendations
Due to technical difficulties in measuring serum asparagine levels, monitoring asparaginase levels is more reliable and therefore recommended for adaptation of asparaginase dosing in individual cases and for trials in which regulatory authorities ask for pharmacokinetic and pharmacodynamic endpoints
Monitoring of Erwinia asparaginase antibody levels
Although it has been advocated previously to determine anti-asparaginase antibody levels to determine whether alterations in dosing regimen should be employed to overcome the risk of silent hypersensitivity, monitoring of asparaginase levels should be sufficient to identify silent hypersensitivity, since not all antibodies lead to asparaginase inactivation.
Recommendations
No recommendations are made for monitoring antibody status
Conclusions
Advances in therapies for ALL have led to improved long-term survival rates for pediatric and adult patients. Asparaginases form a cornerstone of ALL treatment protocols with three main preparations for use in treatment protocols: the native E. coli-asparaginase, a pegylated form (PEG-asparaginase) and an alternative enzyme isolated from Erwinia chrysanthemi, referred to as Erwinia asparaginase. Despite the availability of these agents, much debate remains regarding the optimal formulation and dose for the treatment of pediatric and adult ALL patients. This manuscript aims to provide recommendations, based on data available in the literature, to ensure optimal use of Erwinia asparaginase. Patients who receive an asparaginase as first-line treatment for ALL and develop anti-asparaginase antibodies should be switched to another asparaginase preparation to ensure maximum survival benefit. Monitoring of asparaginase levels is preferable to assess the extent of serum asparagine depletion and to identify cases of silent inactivation. Erwinia asparaginase is a valid second- or third-line therapy, depending upon protocols, regulatory factors and availability. Evidence from published studies suggests that Erwinia asparaginase should be administered at a dose of at least 20 000 IU/m2 three-times weekly, via either the IV or IM route. Further clinical and pharmacokinetic studies of Erwinia asparaginase will help to optimize the use of this agent.
Acknowledgments
This work was supported in part by grant CA-21765 from the US National Institutes of Health, by the American Lebanese Syrian Associated Charities, and by EUSA Pharma. The authors would also like to acknowledge Marion James, PhD, ScopeMedical Ltd. for writing and editorial assistance.
Footnotes
Conflict of interest:
Pieters R is involved in scientific collaborations with different companies producing and developing asparaginases.
Hunger S is the Ergen Family Chair in Pediatric Cancer.
Boos J: Served personally as consultant and participated in advisory boards for different asparaginase-selling companies, including EUSA Pharma and former license holders. In addition, J Boos is also involved in scientific collaborations with different companies producing and developing asparaginase
Rizzari C. is involved in scientific researches supported by different companies producing and/or marketing asparaginase products.
Silverman L served on advisory board for EUSA Pharma and as a consultant for Enzon Pharmaceuticals.
Baruchel A had received honorarium for lecture from OPI
Gökbuget N is involved in scientific collaborations with different companies producing and developing asparaginases
Schrappe M is involved in scientific collaborations with different companies producing and developing asparaginases.
Pui CH had received honorarium for lecture from EUSA Pharma.
Reference List
- 1.Silverman LB, Declerck L, Gelber RD, Dalton VK, Asselin BL, Barr RD, et al. Results of Dana-Farber Cancer Institute Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1981-1995) Leukemia. 2000;14:2247–2256. doi: 10.1038/sj.leu.2401980. [DOI] [PubMed] [Google Scholar]
- 2.Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dordelmann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477–4489. doi: 10.1182/blood-2007-09-112920. [DOI] [PubMed] [Google Scholar]
- 6.Pieters R, Carroll WL. Biology and treatment of acute lymphoblastic leukemia. Pediatr Clin North Am. 2008;55:1–20. doi: 10.1016/j.pcl.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, Thomas D, O'Brien S, Cortes J, Giles F, Jeda S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 8.Goekbuget N, Baumann A, Beck J, Boos J, Brueggemann M, Diedrich H, et al. PEG-asparaginase in adult acute lymphoblastic leukemia (ALL): efficacy and feasibility analysis with increasing dose levels. Blood. 2008;112 abstract [302] [Google Scholar]
- 9.de Bont JM, Holt B, Dekker AW, van der Does-van den Berg, Sonneveld P, Pieters R. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18:2032–2035. doi: 10.1038/sj.leu.2403538. [DOI] [PubMed] [Google Scholar]
- 10.Schiffer CA. Differences in outcome in adolescents with acute lymphoblastic leukemia: a consequence of better regimens? Better doctors? Both? J Clin Oncol. 2003;21:760–761. doi: 10.1200/JCO.2003.11.116. [DOI] [PubMed] [Google Scholar]
- 11.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 12.Boissel N, Auclerc MF, Lheritier V, Perel Y, Thomas X, Leblanc T, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27:2269–2277. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]
- 14.Huguet F, Raffoux E, Thomas X, Leguay T, Chevallier P, Escoffre M, et al. Towards a pediatric approach in adults with acute lymphoblastic leukemia (ALL): the GRAALL-2003 Study. Blood. 2006;108 abstract [147] [Google Scholar]
- 15.Storring JM, Brandwein J, Gupta V, Schuh AC, Schimmer A, Yee K, et al. Treatment of adult acute lymphoblastic leukemia (ALL) with a modified DFCI pediatric regimen - The Princess Margaret Experience. Blood. 2006;108 abstract [1875] [Google Scholar]
- 16.Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A, et al. Adolescents with acute lymphoblastic leukaemia: Outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2006;48:254–261. doi: 10.1002/pbc.20749. [DOI] [PubMed] [Google Scholar]
- 17.Linker C, Damon L, Ries C, Navarro W. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:2464–2471. doi: 10.1200/JCO.2002.07.116. [DOI] [PubMed] [Google Scholar]
- 18.Avramis VI, Tiwari PN. Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int J Nanomedicine. 2006;1:241–254. [PMC free article] [PubMed] [Google Scholar]
- 19.Oncospar™. Summary of Product Characteristics. Enzon Pharmaceuticals; 2006. [Google Scholar]
- 20.Pieters R, Appel I, Kuehnel HJ, Tetzlaff-Fohr I, Pichlmeier U, van dV I, et al. Pharmacokinetics, pharmacodynamics, efficacy, and safety of a new recombinant asparaginase preparation in children with previously untreated acute lymphoblastic leukemia: a randomized phase 2 clinical trial. Blood. 2008;112:4832–4838. doi: 10.1182/blood-2008-04-149443. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand Y, Thomas X, Baruchel A, Mazingue F, Auvrignon A, Corm S, et al. GRASPALL 2005.01 Clinical StudyL L-asparaginase loaded into red blood cells is effective at depleting serum asparagine in children and adults with relapsed acute lymphoblastic leukaemia (ALL) Blood. 2008;112 abstract [306] [Google Scholar]
- 22.Allas S, Sahakian P, Fichtner I, Abribat T. Pharmacokinetics and pharmacodynamics in mice of a pegylated recombinant Erwinia Chrysanthemi-derived l-asparaginase. Blood. 2009;114 abstract [2033] [Google Scholar]
- 23.Miller HK, Salser JS, Balis ME. Amino acid levels following L-asparagine amidohydrolase (EC.3.5.1.1) therapy. Cancer Res. 1969;29:183–187. [PubMed] [Google Scholar]
- 24.Avramis VI, Martin-Aragon S, Avramis EV, Asselin BL. Pharmacoanalytical assays of Erwinia asparaginase (erwinase) and pharmacokinetic results in high-risk acute lymphoblastic leukemia (HR ALL) patients: simulations of erwinase population PK-PD models. Anticancer Res. 2007;27:2561–2572. [PubMed] [Google Scholar]
- 25.Boos J, Werber G, Ahlke E, Schulze-Westhoff P, Nowak-Gottl U, Wurthwein G, et al. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur J Cancer. 1996;32A:1544–1550. doi: 10.1016/0959-8049(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 26.Vieira Pinheiro JP, Ahlke E, Nowak-Gottl U, Hempel G, Muller HJ, Lumkemann K, et al. Pharmacokinetic dose adjustment of Erwinia asparaginase in protocol II of the paediatric ALL/NHL-BFM treatment protocols. Br J Haematol. 1999;104:313–320. doi: 10.1046/j.1365-2141.1999.01192.x. [DOI] [PubMed] [Google Scholar]
- 27.Appel IM, Kazemier KM, Boos J, Lanvers C, Huijmans J, Veerman AJ, et al. Pharmacokinetic, pharmacodynamic and intracellular effects of PEG-asparaginase in newly diagnosed childhood acute lymphoblastic leukemia: results from a single agent window study. Leukemia. 2008;22:1665–1679. doi: 10.1038/leu.2008.165. [DOI] [PubMed] [Google Scholar]
- 28.Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2002;99:1986–1994. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 29.Ollenschlager G, Roth E, Linkesch W, Jansen S, Simmel A, Modder B. Asparaginase-induced derangements of glutamine metabolism: the pathogenetic basis for some drug-related side-effects. Eur J Clin Invest. 1988;18:512–516. doi: 10.1111/j.1365-2362.1988.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 30.Broome JD. L-Asparaginase: discovery and development as a tumor-inhibitory agent. Cancer Treat Rep. 1981;65(Suppl 4):111–114. [PubMed] [Google Scholar]
- 31.Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL) Oncologist. 2007;12:991–998. doi: 10.1634/theoncologist.12-8-991. [DOI] [PubMed] [Google Scholar]
- 32.Ahlke E, Nowak-Gottl U, Schulze-Westhoff P, Werber G, Borste H, Wurthwein G, et al. Dose reduction of asparaginase under pharmacokinetic and pharmacodynamic control during induction therapy in children with acute lymphoblastic leukaemia. Br J Haematol. 1997;96:675–681. doi: 10.1046/j.1365-2141.1997.d01-2089.x. [DOI] [PubMed] [Google Scholar]
- 33.Vieira Pinheiro JP, Wenner K, Escherich G, Lanvers-Kaminsky C, Wurthwein G, Janka-Schaub G, et al. Serum asparaginase activities and asparagine concentrations in the cerebrospinal fluid after a single infusion of 2,500 IU/m(2) PEG asparaginase in children with ALL treated according to protocol COALL-06-97. Pediatr Blood Cancer. 2006;46:18–25. doi: 10.1002/pbc.20406. [DOI] [PubMed] [Google Scholar]
- 34.Liang DC, Hung IJ, Yang CP, Lin KH, Chen JS, Hsiao TC, et al. Unexpected mortality from the use of E. coli L-asparaginase during remission induction therapy for childhood acute lymphoblastic leukemia: a report from the Taiwan Pediatric Oncology Group. Leukemia. 1999;13:155–160. doi: 10.1038/sj.leu.2401260. [DOI] [PubMed] [Google Scholar]
- 35.Ertel IJ, Nesbit ME, Hammond D, Weiner J, Sather H. Effective dose of L-asparaginase for induction of remission in previously treated children with acute lymphocytic leukemia: a report from Childrens Cancer Study Group. Cancer Res. 1979;39:3893–3896. [PubMed] [Google Scholar]
- 36.Clavell LA, Gelber RD, Cohen HJ, Hitchcock-Bryan S, Cassady JR, Tarbell NJ, et al. Four-agent induction and intensive asparaginase therapy for treatment of childhood acute lymphoblastic leukemia. N Engl J Med. 1986;315:657–663. doi: 10.1056/NEJM198609113151101. [DOI] [PubMed] [Google Scholar]
- 37.Sallan SE, Hitchcock-Bryan S, Gelber R, Cassady JR, Frei E, III, Nathan DG. Influence of intensive asparaginase in the treatment of childhood non-T-cell acute lymphoblastic leukemia. Cancer Res. 1983;43:5601–5607. [PubMed] [Google Scholar]
- 38.Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–342. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 39.Duval M, Suciu S, Ferster A, Rialland X, Nelken B, Lutz P, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children's Leukemia Group phase 3 trial. Blood. 2002;99:2734–2739. doi: 10.1182/blood.v99.8.2734. [DOI] [PubMed] [Google Scholar]
- 40.Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pession A, Valsecchi MG, Masera G, Kamps WA, Magyarosy E, Rizzari C, et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:7161–7167. doi: 10.1200/JCO.2005.11.411. [DOI] [PubMed] [Google Scholar]
- 42.Rizzari C, Valsecchi MG, Arico M, Conter V, Testi A, Barisone E, et al. Effect of protracted high-dose L-asparaginase given as a second exposure in a Berlin-Frankfurt-Munster-based treatment: results of the randomized 9102 intermediate-risk childhood acute lymphoblastic leukemia study--a report from the Associazione Italiana Ematologia Oncologia Pediatrica. J Clin Oncol. 2001;19:1297–1303. doi: 10.1200/JCO.2001.19.5.1297. [DOI] [PubMed] [Google Scholar]
- 43.Albertsen BK, Schroder H, Jakobsen P, Muller HJ, Carlsen NT, Schmiegelow K. Monitoring of Erwinia asparaginase therapy in childhood ALL in the Nordic countries. Br J Clin Pharmacol. 2001;52:433–437. doi: 10.1046/j.0306-5251.2001.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizzari C, Zucchetti M, Conter V, Diomede L, Bruno A, Gavazzi L, et al. L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E. coli L-asparaginase as first exposure. Ann Oncol. 2000;11:189–193. doi: 10.1023/a:1008368916800. [DOI] [PubMed] [Google Scholar]
- 45.Panosyan EH, Seibel NL, Martin-Aragon S, Gaynon PS, Avramis IA, Sather H, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children's Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26:217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Woo MH, Hak LJ, Storm MC, Sandlund JT, Ribeiro RC, Rivera GK, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18:1525–1532. doi: 10.1200/JCO.2000.18.7.1525. [DOI] [PubMed] [Google Scholar]
- 47.Larson RA, Fretzin MH, Dodge RK, Schiffer CA. Hypersensitivity reactions to L-asparaginase do not impact on the remission duration of adults with acute lymphoblastic leukemia. Leukemia. 1998;12:660–665. doi: 10.1038/sj.leu.2401007. [DOI] [PubMed] [Google Scholar]
- 48.Hawkins DS, Park JR, Thomson BG, Felgenhauer JL, Holcenberg JS, Panosyan EH, et al. Asparaginase pharmacokinetics after intensive polyethylene glycol-conjugated L-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clin Cancer Res. 2004;10:5335–5341. doi: 10.1158/1078-0432.CCR-04-0222. [DOI] [PubMed] [Google Scholar]
- 49.Wang B, Relling MV, Storm MC, Woo MH, Ribeiro R, Pui CH, et al. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia. 2003;17:1583–1588. doi: 10.1038/sj.leu.2403011. [DOI] [PubMed] [Google Scholar]
- 50.Albertsen BK, Schroder H, Jakobsen P, Avramis VI, Muller HJ, Schmiegelow K, et al. Antibody formation during intravenous and intramuscular therapy with Erwinia asparaginase. Med Pediatr Oncol. 2002;38:310–316. doi: 10.1002/mpo.10096. [DOI] [PubMed] [Google Scholar]
- 51.Albertsen BK, Schmiegelow K, Schroder H, Carlsen NT, Rosthoj S, Avramis VI, et al. Anti-Erwinia asparaginase antibodies during treatment of childhood acute lymphoblastic leukemia and their relationship to outcome: a case-control study. Cancer Chemother Pharmacol. 2002;50:117–120. doi: 10.1007/s00280-002-0466-y. [DOI] [PubMed] [Google Scholar]
- 52.Nesbit M, Chard R, Evans A, Karon M, Hammond GD. Evaluation of intramuscular versus intravenous administration of L-asparaginase in childhood leukemia. Am J Pediatr Hematol Oncol. 1979;1:9–13. [PubMed] [Google Scholar]
- 53.Albertsen BK, Schroder H, Ingerslev J, Jakobsen P, Avramis VI, Muller HJ, et al. Comparison of intramuscular therapy with Erwinia asparaginase and asparaginase Medac: pharmacokinetics, pharmacodynamics, formation of antibodies and influence on the coagulation system. Br J Haematol. 2001;115:983–990. doi: 10.1046/j.1365-2141.2001.03148.x. [DOI] [PubMed] [Google Scholar]
- 54.Barry E, DeAngelo DJ, Neuberg D, Stevenson K, Loh ML, Asselin BL, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25:813–819. doi: 10.1200/JCO.2006.08.6397. [DOI] [PubMed] [Google Scholar]
- 55.Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol. 1999;457:621–629. [PubMed] [Google Scholar]
- 56.Wenner KA, Vieira Pinheiro JP, Escherich G, Wessalowski R, Jorch N, Wolff J, et al. Asparagine concentration in plasma after 2,500 IU/m(2) PEG-asparaginase i.v. in children with acute lymphoblastic leukemia. Klin Padiatr. 2005;217:321–326. doi: 10.1055/s-2005-872516. [DOI] [PubMed] [Google Scholar]
- 57.Hak LJ, Relling MV, Cheng C, Pei D, Wang B, Sandlund JT, et al. Asparaginase pharmacodynamics differ by formulation among children with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2004;18:1072–1077. doi: 10.1038/sj.leu.2403351. [DOI] [PubMed] [Google Scholar]
- 58.Zalewska-Szewczyk B, Andrzejewski W, Mlynarski W, Jedrychowska-Danska K, Witas H, Bodalski J. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48:931–936. doi: 10.1080/10428190701292049. [DOI] [PubMed] [Google Scholar]
- 59.Fu CH, Sakamoto KM. PEG-asparaginase. Expert Opin Pharmacother. 2007;8:1977–1984. doi: 10.1517/14656566.8.12.1977. [DOI] [PubMed] [Google Scholar]
- 60.Zalewska-Szewczyk B, Gach A, Wyka K, Bodalski J, Mlynarski W. The cross-reactivity of anti-asparaginase antibodies against different L-asparaginase preparations. Clin Exp Med. 2009;9:113–116. doi: 10.1007/s10238-008-0026-9. [DOI] [PubMed] [Google Scholar]
- 61.Hempel G, Muller HJ, Lanvers-Kaminsky C, Wurthwein G, Hoppe A, Boos J. A population pharmacokinetic model for pegylated-asparaginase in children. Br J Haematol. 2009 doi: 10.1111/j.1365-2141.2009.07923.x. epub ahead of print Oct 11. [DOI] [PubMed] [Google Scholar]
- 62.Gadner H, Masera G, Schrappe M, Eden T, Benoit Y, Harrison C, et al. The Eighth International Childhood Acute Lymphoblastic Leukemia Workshop (‘Ponte di legno meeting’) report: Vienna, Austria, April 27-28, 2005. Leukemia. 2006;20:9–17. doi: 10.1038/sj.leu.2404016. [DOI] [PubMed] [Google Scholar]
- 63.Alvarez OA, Zimmerman G. Pegaspargase-induced pancreatitis. Med Pediatr Oncol. 2000 Mar;34(3):200–5. doi: 10.1002/(sici)1096-911x(200003)34:3<200::aid-mpo7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 64.Kearney SL, Dahlberg SE, Levy DE, Voss SD, Sallan SE, Silverman LB. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2009;53:162–167. doi: 10.1002/pbc.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parsons SK, Skapek SX, Neufeld EJ, Kuhlman C, Young ML, Donnelly M, et al. Asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Blood. 1997;89:1886–1895. [PubMed] [Google Scholar]
- 66.Eden OB, Shaw MP, Lilleyman JS, Richards S. Non-randomised study comparing toxicity of Escherichia coli and Erwinia asparaginase in children with leukaemia. Med Pediatr Oncol. 1990;18:497–502. doi: 10.1002/mpo.2950180612. [DOI] [PubMed] [Google Scholar]
- 67.Kwok CS, Kham SK, Ariffin H, Lin HP, Quah TC, Yeoh AE. Minimal residual disease (MRD) measurement as a tool to compare the efficacy of chemotherapeutic drug regimens using Escherichia Coli-asparaginase or Erwinia-asparaginase in childhood acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2005;47:299–304. doi: 10.1002/pbc.20684. [DOI] [PubMed] [Google Scholar]
- 68.Vrooman LM, Supko JG, Neuberg DS, Asselin BL, Athale UH, Clavell L, et al. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54:199–205. doi: 10.1002/pbc.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogawa C, Ohara A, Manabe A, Hanada R, Takahashi H, Koh K, et al. Treatment outcome of discontinuted l-asparaginase in children with standard-risk acute lymphoblastic leukemia: Tokyo Children's Cancer Study Group (TCCSG) Study L99-15. Blood. 2005;106 abstract [878] [Google Scholar]
- 70.Schrey D, Borghorst S, Lanvers-Kaminsky C, Hempel G, Gerss J, Möricke A, et al. Therapeutic drug monitoring of asparaginase in the ALL-BFM 2000 Protocol between 2000 and 2007. Pediatr Blood Cancer. doi: 10.1002/pbc.22417. In press. [DOI] [PubMed] [Google Scholar]
- 71.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–11. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 72.Billett AL, Carls A, Gelber RD, Sallan SE. Allergic reactions to Erwinia asparaginase in children with acute lymphoblastic leukemia who had previous allergic reactions to Escherichia coli asparaginase. Cancer. 1992;70:201–206. doi: 10.1002/1097-0142(19920701)70:1<201::aid-cncr2820700131>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 73.Rizzari C, Citterio M, Zucchetti M, Conter V, Chiesa R, Colombini A, et al. A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica. 2006;91:24–31. [PubMed] [Google Scholar]