Abstract
Whether intrauterine exposures to alcohol, tobacco, marijuana, or cocaine predispose offspring to substance use in adolescence has not been established. We followed a sample of 149 primarily African American/African Caribbean, urban adolescents recruited at term birth until age 16 to investigate intrauterine cocaine exposure (IUCE). We found that in Kaplan-Meier analyses higher levels of IUCE were associated with a greater likelihood of initiation of any substance (licit or illicit), as well as marijuana and alcohol specifically. Adolescent initiation of other illicit drugs and cigarettes were analyzed only in the “any” summary variable since they were used too infrequently to analyze as individual outcomes. In Cox proportional hazard models controlling for intrauterine exposure to alcohol, tobacco, and marijuana and demographic and postnatal covariates, those who experienced heavier IUCE had a greater likelihood of initiation of any substance, and those with lighter intrauterine marijuana exposure had a greater likelihood of initiation of any substance as well as of marijuana specifically. Time-dependent higher levels of exposure to violence between ages of 8 and 16 were also robustly associated with initiation of any licit or illicit substance, and of marijuana, and alcohol particularly.
Keywords: Prenatal drug exposure, Adolescent substance use, Violence
1. Introduction
By definition, women who continue to use licit or illicit psychoactive substances in pregnancy, even at low levels, may be considered to have problem substance use since they are unable to discontinue use in spite of potential adverse consequences to themselves and their children. Children whose parents have a history of problem licit or illicit substance use may themselves be at elevated risk for becoming substance users and abusers in adolescence and adulthood [37], a risk ascribed both to environmental and genetic factors[55]. However, after acknowledging familial risk including risks of living with substance using parents or parental figures in school age or adolescence, [5,20] surprisingly little is known about whether there is an association of intrauterine exposures to psychoactive substances, licit or illicit, with the offspring’s own age of initiation of substance use.
Substance use and pubertal maturation are linked[53]. The maturing of the brain in adolescence ushers in an epoch when previously undetectable effects of intrauterine insults may manifest and a time of unusual vulnerability to negative effects of the adolescent’s own substance use, particularly because of the immaturity of the frontal cortex and subcortical monoaminergic systems [33]. The earlier the initiation of psychoactive substances, the greater the risk of neuropsychological perturbations and of eventual problem substance use [18,32,45,58–60,63,65], and other negative psychosocial outcomes such as incarceration [62].
The animal data on intrauterine substance exposure and later substance use are inconclusive, conflicting, and focus on adult rather than adolescent animals. Animal studies of intrauterine cocaine exposure (IUCE) provide an instructive example. One study [56] suggested that in male mice intrauterine exposure to higher dosages of cocaine increased the probability of the mouse acquiring cocaine self-administration in adulthood, but a second study [35] suggested that the reinforcing efficacy of cocaine was reduced in adult male rats with IUCE. Mice with IUCE, compared to unexposed, also showed reduced cocaine conditioned place-preference for high doses of cocaine, though the preference for low and medium doses was not altered [24].
Published findings on whether intrauterine exposures affect human offsprings future licit and illicit substance use are also contradictory and vary with the demographic characteristics of the sample, and document substance use or problem use rather than age at first use. Two studies in a predominantly European American, middle class United States sample [10,11] do not report age of alcohol initiation, but suggest that intrauterine alcohol exposure confers incremental risks for offspring to develop alcohol “problems,” but a similar study from Australia [5] found that maternal alcohol use during the youngster’s early adolescence, but not the use in pregnancy, predicted adolescent alcohol use. Data from the National Collaborative Perinatal Project (NCPP) [17] suggested that intrauterine tobacco exposure increases the offspring’s risk of developing nicotine addiction, a finding replicated in Australia [49]. Gender specific effects were found in a retrospective study of treatment-seeking smokers suggesting intrauterine tobacco exposure was significantly associated with earlier age of tobacco initiation in males and accelerated daily use in females [50]. Intrauterine marijuana exposure has been found to predict offspring’s marijuana use at age 14 in a sample which was 50% African American [20]. In the same sample, in contrast to the studies of European American children, it was found that intrauterine tobacco exposure did not predict offspring use once current maternal use was considered. A single study to date in a predominantly African American low income cohort suggests that boys, but not girls, with IUCE were more likely than unexposed peers at age 11 years to engage in high-risk behaviors, including tobacco use [15]. These findings were not replicated in a multi-site multiethnic sample by other researchers [42] where risk behaviors correlated with the child’s post-natal violence exposure, but not with history of intrauterine exposures.
Additionally, there is not always a homology between the intrauterine exposure and the substance that is used by the offspring. For example, a longitudinal study following European-Canadian middle-class children reported that intrauterine marijuana exposure appears to predict the offspring’s tobacco use [54], and a retrospective study of adopted adults reported that intrauterine alcohol exposure, independent of home environment, may increase the risk of dependence not only on alcohol but on tobacco and other drugs [70].
Previous studies suggest it is important to perform analyses that evaluate multiple intrauterine exposures in the context of postnatal environmental factors which have also been linked in epidemiologic studies to increased risk of early adolescent substance initiation and later substance use disorders. For example, the retrospective Adverse Childhood Exposures (ACE) study identified parental substance use, parental incarceration, household dysfunction and various forms of exposure to violence as factors likely to increase an individual’s own risk of developing a substance use disorder [25].
Conversely, multiple environmental factors in adolescents’ lives have been shown potentially to protect against substance abuse. A longitudinal study of urban African American adolescents identified an inverse relationship between having a higher number of protective factors (such as increased religiosity) and drug abuse [51]. An inverse relationship between religiosity and substance use was also identified in a retrospective study of a nationally representative sample of adolescents as part of the National Comorbidity Study [46]. A longitudinal study of adolescents from their senior year of high school through their first year after high school found parental monitoring to be a protective factor decreasing substance abuse as adolescents enter young adulthood [67].
We hypothesize that adolescents who experienced intrauterine cocaine exposure (IUCE) and intrauterine exposures to other substances are more likely, after confound control, to become themselves early initiators of licit and illicit psychoactive substances, compared to demographically similar adolescents without such exposures. Although we are aware that other child level factors such as lower IQ [26] and childhood externalizing behavior and other psychiatric symptoms [8,13] may be on the causal pathway to substance misuse, we focus on potential familial and environmental confounds rather than these possible mediators, which are addressed only in secondary analyses.
2. METHODS
2.1.1 Sample Recruitment
The IRB of Boston Medical Center (then called Boston City Hospital) approved this study at inception and yearly thereafter. Prior to initiation of the study a Certificate of Confidentiality was obtained from the federal government to protect researchers from being compelled by subpoena to release data regarding study participants. Soon after delivery, all birth mothers gave informed consent to study participation. If the child changed caregivers, similar informed consent was obtained for each new caregiver. Beginning at the 8-year visit children also provided informed assent. The primary goal of the study was to explore the potential impact of intrauterine cocaine exposure (IUCE).
Sample recruitment on the post-partum floor of Boston Medical Center from October 1990 to March 1993 has been published in detail [66]. All mother–infant dyads met the following criteria based on review of mother and infant medical records and confirmed by interviews, biological markers, and infant physical examinations obtained by study personnel: 1) Infant gestational age greater than or equal to 36 weeks; 2) No requirement for neonatal intensive care; 3) No obvious major congenital malformations; 4) No diagnosis of fetal alcohol syndrome in the neonatal record; 5) No history of human immunodeficiency virus seropositivity noted in the mother’s or infant’s medical record; 6) Mother’s ability to communicate fluently in English; 7) No indication by neonatal or maternal urine toxicology screen or history in medical record of mother’s use during pregnancy of illegal opiates, methadone, amphetamines, phencyclidine, barbiturates, or hallucinogens; and 8) Mother aged 18 years or older. These criteria were established to exclude infants with known major risk factors that might confound or obscure the effects, if any, of IUCE.
2.1.2 Method of Intrauterine Cocaine Exposure Classification
Mothers participating in the study were identified as either heavier, lighter, or non-users of cocaine soon after delivery of the index child by interview and by biological markers obtained by clinicians and study personnel. At intake during the maternal post-partum hospitalization, research assistants used the Addiction Severity Index [39], supplemented by study-specific questions, to interview the mothers about pregnancy and lifetime use of cigarettes, alcohol, and illicit drugs.
During the period of study recruitment at Boston Medical Center, urine testing for metabolites of illicit drugs was performed for clinical indications at the discretion of health care personnel, but was not universal. Results of the urine drug Enzyme Multiplied Immunoassay Technique (EMIT) assays, which were obtained for clinical purposes during pre-natal care or labor and delivery from the mother or from the newborn after birth, were recorded for the present study (when available in the medical record). Exposed newborns were targeted for recruitment on the basis of maternal self-report or positive clinical urine assays obtained from either mother or newborn.
However, provisionally unexposed newborns were drawn from the nursery population as a whole, most of whom did not have maternal urine assays performed for drug metabolites for clinical purposes. Therefore, after recruitment and informed consent, additional urine samples were collected from all study mothers for analysis for benzoylecognine, opiates, amphetamines, benzodiazepines, and cannabinoids by radioimmunoassay using commercial kits (Abuscreen RIA, Roche Diagnostics Systems, Inc, Montclair, NJ). Meconium specimens were also sought from all enrolled newborns to be analyzed by radioimmunoassay for benzoylecognine (a cocaine metabolite), opiates, amphetamines, benzodiazepines, and cannabinoids. The radioimmunoassay used was a modification of the method of Ostrea et al. published in detail elsewhere [47,52]. All mother–infant dyads provided at least one biological marker, either urine from mother or infant or meconium that confirmed their exposure or lack of exposure to cocaine during pregnancy. To be classified in the “unexposed” group, mothers had to deny use of cocaine on interview and all available biological markers needed to be negative for cocaine use. In this sample, the mean days of self-reported cocaine use during pregnancy was 20.6 days, with a range from 0 to 264. The mean meconium concentration of benzoylecognine/g was 1143 ng with a range from 0 ng to 17950 ng/g. Before data were analyzed, a composite measure of “heavier” use was a priori defined as the top quartile of meconium concentration for cocaine metabolites (≥3314 ng of benzoylecognine/g meconium) and/or top quartile days of self-reported use ( ≥61 days) during the entire pregnancy. All other use was classified as “lighter” [66]. If benzoylecognine levels in meconium were not in the top quartile, self-report took priority in classifying levels of exposure comparable to the approach of other investigators, where use of cocaine more than twice a week during pregnancy is considered “heavier” use [6,36,61]. Not all infants with IUCE have positive meconium assays [52]. Moreover, meconium samples could not be obtained from 14% of study infants whose exposure status was confirmed by maternal or infant urine assay. Therefore, whichever indicator (self-report or meconium assay) demonstrated higher exposure was used to define exposure category.
2.1.3 Other Intrauterine Drug Exposure Classifications Determined during the Neonatal Period
Identification of prenatal marijuana exposure was based on positive results of urine assay, meconium assay, or maternal self-report. In our previous reports we have analyzed marijuana categorically as exposed or unexposed, since meconium concentration is not entirely valid due to the storage of metabolites in the mother’s body fat [52] and because self-reported use was denied by a third of the marijuana users in this cohort who were identified solely on the basis of meconium or urine assay. However, recently we constructed an a priori index of no marijuana use (negative: self-report, urine, and meconium assays), heavier use (positive: self-report and/or meconium or urine assay and positive urine at delivery or the top quartile of self reported days of use (≥8 days during pregnancy among users), or lighter use (positive: self-report or assay who did not meet criteria for inclusion in the heavier group). This classification index was significantly associated (p< .0001) with level of use of alcohol, cigarettes, and cocaine during the pregnancy and with the infants’ mean birth weight - Marijuana Unexposed 3210 grams (s.d. 477), Lighter 3069 grams (s.d. 464), and Heavier 2943 grams (s.d. 511), p=.02). Therefore, in this analysis, unlike our earlier developmental analyses [28,57], we are able to classify intrauterine marijuana exposure as a three-level ordinal exposure.
At the time the study was initiated there was no established biologic marker for gestational alcohol exposure, and cotinine assays for tobacco metabolites were prohibitively expensive. Therefore, the ascertainment of alcohol and tobacco use in pregnancy only by self-report was state of the art at the time the current sample was recruited. We quantified intrauterine alcohol exposure by mother’s self reported average daily volume of alcohol in drinks per day (in the 30 days prior to delivery, which was highly correlated with use through pregnancy in our sample). During the post-partum interview, mothers reported the average number of cigarettes that they consumed per day while pregnant. For descriptive purposes we categorized alcohol use as none, lighter (<1 drink/day) and heavier (≥1 drinks/day) in the 30 days prior to delivery and cigarettes as none, lighter (<10 cigarettes a day), or heavier (≥10 cigarettes a day) during pregnancy.
2.1.4 Sample Maintenance and Retention
As described in previous publications [12,29–31,57], caregiver/child dyads were assessed post-natally at ages 6 months, 1, 2, 4, 6, 8.5, 9.5, 11, 14 (early adolescence) and 16 (middle adolescence) years. After each study visit, the caregiver and child were given store vouchers and/or age appropriate gifts for completion of the interview and assessment.
One hundred forty-nine of the original 252 study offspring (60%) were assessed during early and mid adolescence waves of follow up. These adolescents did not differ by level of intrauterine exposures to cocaine, tobacco, alcohol, or marijuana, birth weight, gender, or their mother’s age, ethnicity, or education at the time of their birth (p>.05) from the 103 in the birth sample who did not participate in these waves.
2.2 Procedures
2.2.1 Outcome Measures
Adolescents took an audio computer assisted self-interview (ACASI) to assess their substance use. The software was programmed and the computer screen positioned so that the research assistant could not see or access the respondent’s confidential answers. The items on the survey were synthesized from a number of different instruments to address the issues that seemed most relevant to young adolescents from urban environments. In order to assess the participants’ tobacco use, the ACASI included all 10 items of the Hooked on Nicotine Checklist (HONC), developed to assess adolescent nicotine dependence [21]. Additional questions about tobacco, alcohol, and other substance use were taken from several components of the CDC’s 2005 Youth Risk Behavior Surveillance System (YRBSS) [22]. The ACASI also included questions from the Wisconsin YRBS Middle School Questionnaire, the State and Local YRBS, and the Wisconsin YRBS High School Questionnaire. These questions asked about the participants’ past and current use of alcohol and other licit or illicit substances. Using the YRBS format, questions were also added about specific drugs, such as illegally diverted prescription opiates, known to be a problem in our geographic region.
For this analysis of substance use initiation, questions were framed as follows for legal substances which do not require a prescription for adults: “How old were you when you smoked a whole cigarette for the first time?” “How old were you when you had your 1st drink of alcohol other than a few sips?” It was specified that, “a drink of alcohol is equal to having a can of beer (the same size as a soda can), a glass of wine, a wine cooler, or a shot of liquor such as rum, gin, vodka, or whiskey.” For substances that are legal with a prescription but not without (such as amphetamines, steroids, oxycodone and other pain killers, or benzodiazepines) the question was “How old were you when you first tried taking (substance of interest) without a doctor or nurse telling you to take them?” In the case of illicit substances (marijuana, heroin, cocaine, “club drugs”) quantity was not specified, with the question framed “How old were you when you first tried (substance) for the first time?”
Participants selected from a forced choice list of “never” or of age in years beginning at age “8 or younger” for each substance. The same questions were asked at early and middle adolescence. In case of discrepancy between the two ages for a given substance, the middle adolescent answer was chosen for analysis, since answers differed by more than a year primarily for age of alcohol initiation, and there was concern that some early adolescents may not have understood the definition of a “drink.” Adolescent participants also furnished urine to be tested by the United States Drug Testing Laboratories, Inc. using the No-Excuse Urine Panel, a limit of detection panel that screens using EMIT at the lowest validated concentrations that can be achieved with the reagent set and the instrumentation. The GC/MS confirmations used are either at ½ or 1/5 of the SAMHSA screening concentrations depending on the drug class for cannabinoids, opiates, amphetamines and cocaine metabolites and ELISA for cotinine. We classified adolescents as having used a particular substance if either self-report or urine assay was positive. In the 16 instances where the respondent denied initiation of a given substance but the urine assay was positive for that substance, the respondent’s age at the date of the assay was taken as the age of initiation.
2.2.2 Covariates
Potential covariates were ascertained from interviews of caregivers at each research contact since birth, and from the participants themselves by face-to-face interview during school age or by ACASI and face-to-face interview and cognitive assessments during adolescence.
2.2.2.1 Caregiver Measures
Trained research interviewers questioned caregivers at each study contact regarding their own substance use in the last 12 months. For each substance, caregivers were asked “Have you used….?” For each substance for which the caregiver answered affirmatively they were also asked “How many days have you used…during the past 30 days?”, “In the last 12 months, about how many total days were you using…?” and “How many days ago was your most recent use of…?” Caregivers were also asked if child/adolescent participants spent time with household members or with anyone else who used tobacco, alcohol, or any illegal substances; “How many people that your child spends time with use the following drugs?” and the relationship between the adolescent and the substance user. Urine samples were obtained from caregivers and analyzed using the same No Excuse Urine Panel described above for the adolescents.
The participants’ birth mothers or other caregivers were also interviewed to assess familial risk of substance use. The biological father’s substance use was ascertained by birth mother’s report on a study specific question immediately following delivery [28]. Later interviews asked using study specific questions assessed whether the child’s biological father, biological grandmothers, biological grandfathers, biological aunts, biological uncles or biological siblings had problems with alcohol or drugs. The sum of affirmative answers for alcohol and drug problems was the control variable for familial risk.
The identity of the child’s caregiver (birth mother vs. other) and number of changes in caregiver since last contact were ascertained at each study visit and summarized as whether or not the child had lived with the birth mother from birth to mid-adolescence. Whether the child had experienced the incarceration of a parent was also ascertained from the accompanying caregiver at each visit [68]. Caregivers were also asked to complete the Child Behavior Problem Checklist [3,4] at each study contact from age 2 to 11 years. Household food insecurity as a measure of relative material hardship was reported by the caregivers at assessments when the children were ages 8– 16 [19] using the United States Department of Agriculture (USDA) Food Security Scale (FSS) [23], which determines whether or not there was sufficient quantity and quality of food in the preceding twelve months for all households members to lead an active and healthy life.
2.2.2.2 Adolescent Measures
Trained research assistants masked to intrauterine exposure status administered the Wechsler Abbreviated Scale of Intelligence (WASI), [66] to adolescents at age 14. Participants beginning at age 9.5 years were asked at each study contact to indicate their level of pubertal development on a series of schematic drawing derived from the work of Tanner [64]. ACASI questions about peer use were the four questions from Peer Chemical Environment Subscale, taken from the Personal Experience Inventory [70].
Children’s self-report of exposure to violence was ascertained using the Violence Exposure Scale for Children-Revised (VEX-R) [27] designed to assess children’s exposure to violence through self-report using a 21 item, cartoon-based interview. The VEX-R uses a 4-point Likert scale (0=never, 1=once, 2=a few times, 3=lots of times) to determine how many times the child has witnessed different types of violence (e.g. someone being yelled at, being beaten up, or stabbed with a knife) and how many times these violent things have happened to the child him/herself. The VEX-R was administered face to face by trained examiners when subjects visited the testing laboratory at ages 8.5, 9.5 and 11 years.
For the early and middle adolescence research visits we modified the VEX-R to make it more appropriate for adolescent subjects by removing the cartoons and the questions related to spanking, administering the text of other questions administered by ACASI, and making questions more time-specific to determine if the event took place within the past year or at any point in the child’s lifetime.
There is no standard method for scoring the VEX-R [7,16,41,44]. In our previous work with preadolescents [38] the VEX-R was analyzed using a total score, calculated by simply adding up how many times the subject reported experiencing each violent event, without weighting for severity. In this analysis of adolescents without attempting a priori weighting of violent events, to address the non-interval scaling and the skewed nature of the VEX-R total score, we created quartiles by ranking each subject’s VEX-R score at each of the 8.5, 9.5,11, 14 and 16 year old time points, and then subdividing those scores into 4 approximately equal groups. The highest quartile obtained by each subject at each time point was then used to create the time dependent variable used for our analyses, with the 4th quartile representing the highest level of violence exposure for age and the 1st quartile representing the lowest level of exposure. When data were missing at any protocol point, the last value was carried forward. If no such value was available the closest later value was substituted.
Level of supervision by parents or other primary caregivers was ascertained with the measure of Lamborn et al. [43] which evaluates the adolescent’s perception of parenting by summing Likert scoring (1–4) of responses ranging from “never” to “frequently” of questions such as, “My parents know exactly where I am most afternoons after school.” The adolescent’s religiosity was assessed using four questions from the Longitudinal Study of Adolescent Health [48].
2.2.3 Data Analyses
First, we generated descriptive statistics for each variable of interest, with means and standard deviations for continuous variables and counts and percentages for categorical variables, in quartiles (e.g., for VEX-R measured at each protocol age as previously described). Next, in bivariate analyses we compared the three IUCE groups on each of our dependent variables of interest: age at first use of any licit or illicit substance; age at first use of each category of substances --- alcohol, marijuana, tobacco and other drugs. For each, we used Kaplan-Meier analyses together with Cox proportional hazards regression without additional covariates. We generated unadjusted (crude) hazard ratios (HR) and their 95% confidence intervals (CI) from these Cox models as well as from the multivariable models to be described. The IUCE variables in these models were modeled as dummy variables: heavier compared to unexposed; lighter compared to unexposed. We then added a series of potential covariates in a one-at-a-time fashion to the model in order to assess their confounding effects by applying a 10% change-in-estimates criterion for the adjusted hazard ratios compared to the unadjusted. Those variables for which inclusion in the model changed either the hazard ratio for lighter IUCE compared to unexposed or for heavier IUCE compared to unexposed were retained in the full, main effects-only Cox regression model. Variables assessing the intrauterine exposure to marijuana, cigarettes, and alcohol were included in all final models based on a priori theoretical considerations to avoid misattribution of one intrauterine substance exposure to another. We assessed potential collinearity among the independent variables in the final model by examining bivariate correlations and through examination of principal components analysis-based methods as described for linear models by Belsley, Kuh, and Welsch [14]. We found no collinearity in these data among the predictors used in our final statistical models.
We used SAS version 9.1.2 for all of our analyses. Results were deemed statistically significant where two-tailed p < .05.
3. RESULTS
3.1 Sample Characteristics
Table 1 summarizes sample characteristics by the level of IUCE. Compared to those with no IUCE, children with IUCE were more likely to experience higher levels of marijuana, alcohol, and tobacco intrauterine exposure, and to be of lower birth weight, but there were no sex differences by level of exposure. Mothers who used cocaine during pregnancy were more likely to be born in the United States, and to be older at the child’s birth, but the groups did not differ in education level or in the percent who described themselves as African American or African Caribbean (“Black”). The groups did not differ significantly in the number of first or seconddegree relatives who were reported by birth mother or other caregivers as having a drug or alcohol problem.
Table 1.
Sample Characteristics – Age of Drug Use Initiation (N=149)
| Intrauterine Cocaine Exposure Group |
P- value |
|||
|---|---|---|---|---|
| Unexposed (N=69) |
Light (N=53) |
Heavy (N=27) |
||
| Perinatal and Family Covariates | ||||
| Maternal characteristics at birth | ||||
| Mean maternal age at child’s birth (yrs) | 25.2 (5.5) | 28.2 (5.3) | 26.7 (3.8) | 0.008† |
| US born (%) | 78.3 | 92.5 | 96.3 | 0.02* |
| Black-US or other (%) | 92.8 | 84.9 | 88.9 | 0.38* |
| Mean mother’s education (yrs) | 11.6 (1.4) | 11.6 (1.3) | 11.4 (1.2) | 0.84† |
| Marijuana exposure (%) | 10.1 | 34.0 | 33.3 | 0.003* |
| Alcohol (≥ .5 drinks per day) (%) | 0 | 5.7 | 11.1 | 0.03* |
| Cigarettes (≥1/2 pack per day) (%) | 13.0 | 34.0 | 44.4 | 0.002* |
| Child characteristics at birth | ||||
| Male child (%) | 53.6 | 50.9 | 44.4 | 0.72* |
| Mean birthweight (grams) | 3350.0 (513.9) | 3034.1 (373.7) | 2864.6 (348.0) | <.0001† |
| Family substance use at birth | ||||
| Mean number of 1st degree relatives with drug or alcohol problem at intake |
0.6 (0.8) | 0.6 (0.8) | 0.9 (0.9) | 0.2† |
| Mean number of 2nd degree relatives with drug or alcohol problem at intake |
0.4 (0.7) | 0.5 (0.7) | 0.4 (0.8) | 0.88† |
| Postnatal Covariates | ||||
| Mean number of 1st degree relatives with drug or alcohol problem at age14 or 16 |
0.4 (0.6) | 0.5 (0.5) | 0.3 (0.5) | 0.46† |
| Mean number of 2nd degree relatives with drug or alcohol problem at age14 or 16 |
0.8 (1.1) | 1.1 (1.1) | 1.4 (1.3) | 0.09† |
| Strict supervision ever 4th quartile (%) | 36.2 | 40.4 | 22.2 | 0.27* |
| Violence exposure revised (VEX-R) ever 4th quartile (%) |
44.9 | 45.3 | 55.6 | 0.61* |
| Mean religiosity scale | 12.8 (4.5) | 13.6 (4.6) | 14.1 (4.8) | 0.35† |
| Always in birth mother care up to age 16 (%) |
72.5 | 37.7 | 33.3 | <.0001* |
| Mean caregiver changes to age 16 | 0.6 (1.4) | 2.3 (2.3) | 2.5 (2.4) | <.0001† |
| Food insecure ever (%) | 50.7 | 41.5 | 29.6 | 0.16* |
| Incarcerated parent ever (%) | 39.1 | 35.9 | 44.4 | 0.76* |
| Mean peer use score at age 16 | 7.3 (2.8) | 8.0 (3.0) | 9.4 (3.2) | 0.02† |
| Mean CBCL ages 2–10 years | 51.6 (8.2) | 51.8 (9.0) | 52.2 (9.0) | 0.96† |
| Mean WASI IQ | 91.9 (12.4) | 91.4 (13.0) | 94.8 (10.9) | 0.48† |
| Mean maximum lead level (N=117) | 8.7 (4.7) | 8.8 (4.5) | 11.6 (8.6) | 0.12† |
| Mean age at menarche (N=69) | 12.0 (1.1) | 11.8 (1.4) | 12.4 (1.2) | 0.44† |
| Tanner stage-age 9.5 (N=114) | 0.86* | |||
| I/II (%) | 85.7 | 87.2 | 94.7 | |
| III (%) | 8.9 | 5.1 | 5.3 | |
| IV/V (%) | 5.4 | 7.7 | 0 | |
| Tanner stage-age 11 (N=111) | 0.23* | |||
| I/II (%) | 74.6 | 73.7 | 88.9 | |
| III (%) | 14.6 | 21.1 | 0 | |
| IV/V (%) | 10.9 | 5.3 | 11.1 | |
| Tanner stage-age 14 (N=135) | 0.71* | |||
| I/II (%) | 4.8 | 8.3 | 8.0 | |
| III (%) | 30.7 | 27.1 | 40.0 | |
| IV/V (%) | 64.5 | 64.6 | 52.0 | |
| Tanner stage-age 16 (N=139) | 0.03* | |||
| I/II (%) | 0 | 7.7 | 4.2 | |
| III (%) | 11.1 | 7.7 | 25.0 | |
| IV/V (%) | 88.9 | 84.6 | 70.8 | |
| Household/regular contact up to age 6 – cigarettes (%) |
56.9 | 70.0 | 83.3 | 0.05* |
| Ever household/regular contact ages 8–16 cigarettes (%) |
65.2 | 73.6 | 88.9 | 0.06* |
| Household/regular contact up to age 8 –16 alcohol (%) |
1.6 | 16.0 | 16.7 | 0.01* |
| Ever household/regular contact ages 8–16 alcohol (%) |
23.2 | 22.6 | 22.2 | 0.99* |
| Household/regular contact up to age 8 –16 marijuana (%) |
28.1 | 34.0 | 41.7 | 0.46* |
| Ever household/regular contact ages 8–16 marijuana (%) |
42.0 | 41.5 | 44.4 | 0.97* |
| Household/regular contact up to age 8 –16 Cocaine (%) |
9.4 | 22.9 | 8.7 | 0.09* |
| Ever household/regular contact ages 8–16 Cocaine (%) |
11.6 | 20.8 | 14.8 | 0.38* |
| Household/regular contact up to age 8 –16 other drugs (%) |
0 | 8.0 | 8.3 | 0.03* |
| Ever household/regular contact ages 8–16 other drugs (%) |
0 | 9.4 | 7.4 | 0.02* |
1st degree relatives include father and siblings. 2nd degree relatives include grandparents, aunts and uncles
P-value via chi-square test.
Means (S.D.) P-value via one-way ANOVA.
Adolescents with IUCE were more likely than adolescents without IUCE to have experienced multiple changes in caregivers up to the middle adolescent assessment, and less likely to have always lived with their birth mother. However, we did not find any statistically significant difference in percent who reported the strictest (top quartile) level of parental supervision, or of violence exposure in the top quartile between adolescents with and without IUCE. The three groups of adolescents also did not differ in self-reported religiosity, measured IQ, or average caregiver reported externalizing score on the Child Behavior Problem Checklist between ages 2 and 11, average lead level or age at menarche for girls, although the heavily exposed adolescents were less likely than the other groups to rate themselves as Tanner 1V/V at age 14 and 16. There was a dose related difference in the Mean Peer Use Score at 16 years, with the most heavily IUCE adolescents reporting the highest levels of peer use. In preadolescence, level of IUCE was associated with the percent of children who experienced tobacco, alcohol, cocaine, and other drug use in their household, but no difference in household marijuana use.
Table 1: Sample Characteristics –N=149)
3.2 Bivariate Associations of Intrauterine Cocaine Exposure with Substance Initiation
3.2.1 Initiation of Any Substance
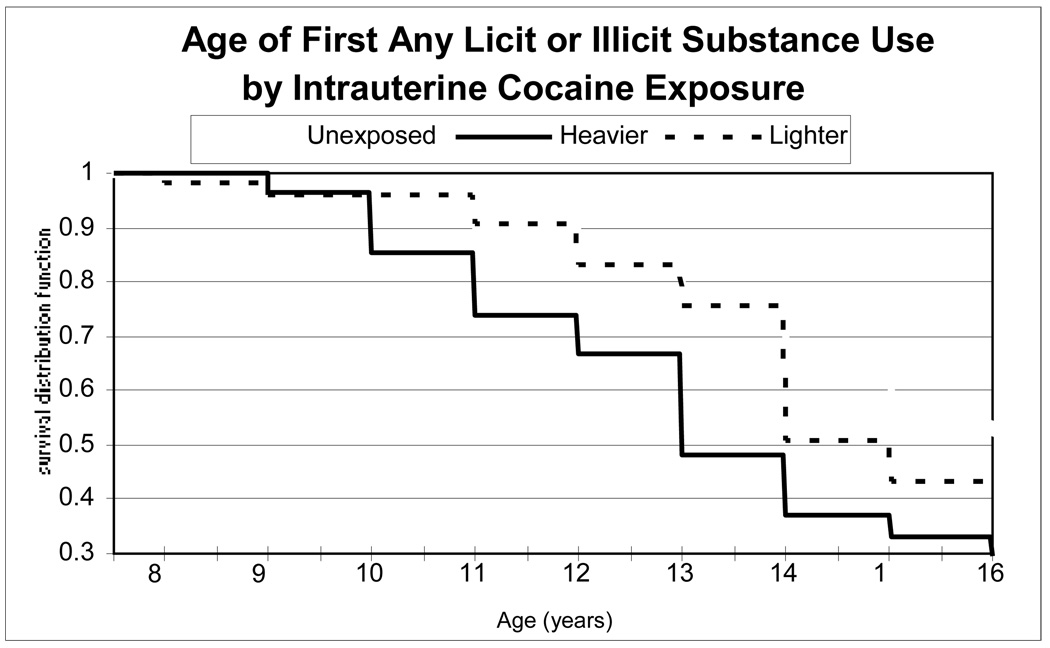
As shown by the bivariate Kaplan-Meier survival curves in Figure 1, earliest age of initiation of any licit or illicit substance varied by IUCE group. In these unadjusted Kaplan-Meier analyses, 74% of the heavier cocaine exposure group had initiated any licit or illicit substance use by age 16, compared to 58% of the lighter cocaine exposure group, and 48% of the unexposed, p=0.02 via a global log rank test (Figure 1). A Cox regression model including only the two IUCE dummy variables showed hazard ratios (HR) of 2.10 for heavier IUCE exposure vs. unexposed (p=.009) and 1.29 for lighter IUCE exposure vs. unexposed (p=.31). The most commonly used substances were alcohol and marijuana, as described in detail in 3.2.2 and 3.2.3. Cigarette use was less common (23% of unexposed, 21% of the lighter IUCE, 30% of the heavier IUCE, p= 0.85 by log rank test). Use of other substances was relatively rare (3.4% cocaine, 3% glue, 1% prescription opiates, 1% amphetamines). No participant acknowledged cocaine use by self report on the ACASI; the urine levels of cocaine metabolites using the No-Excuses panel were all below the level that could rule out passive exposure, so the cocaine use finding is tentative. Although these substances were all considered in the analyses of any initiation of substance use reported above, the number of users of these as individual substances in each group was too small to allow for appropriate multivariable analyses. Excluding the adolescent cocaine urine data did not change the analyses for age of initiation of any substance, since all those with positive urines for cocaine had also initiated other substances.
FIGURE 1.
Age of initiation of any substance use by level of intrauterine cocaine exposure
3.2.2 Initiation of any Marijuana Use
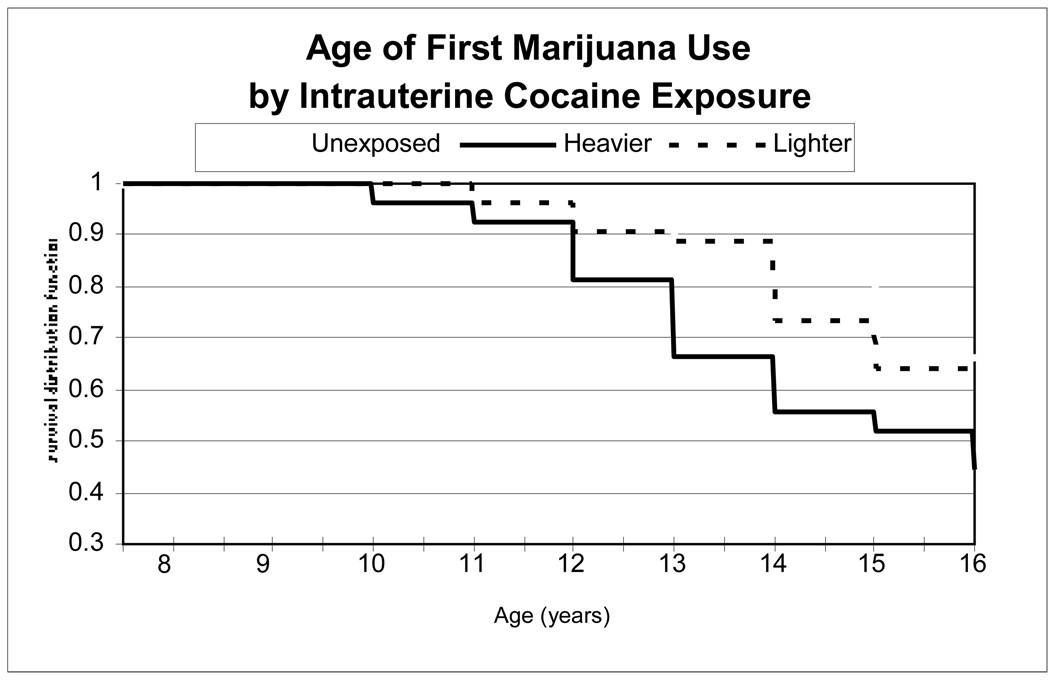
As shown in Figure 2, in Kaplan-Meier analysis 56% of the heavier, 38% of the lighter, and 35% of the unexposed IUCE groups had used marijuana up to age 16 (global log rank test p=.06; Figure 1b). The unadjusted hazard ratio for the heavier vs. unexposed contrast was 2.07 (p=.03) and for lighter vs. unexposed was 1.17 (p=.61).
Figure 2.
Age of initiation of marijuana use by level of intrauterine cocaine exposure
3.2.3 Initiation of any Alcohol Use
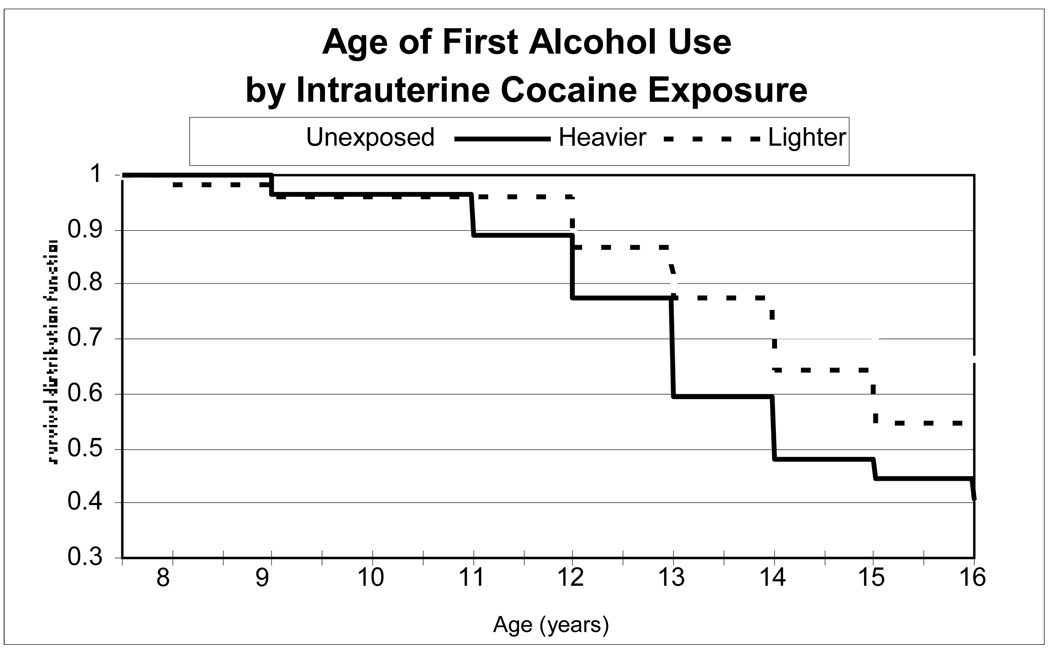
As seen in Figure 3, in Kaplan-Meier analysis, 59% of the heavier, 45% of the lighter, and 33% of the unexposed IUCE groups had used alcohol up to age 16 (global log rank test p=.05), with a hazard ratio of 2.10 for the heavier IUCE group compared to unexposed (p=.02) and 1.38 for the lighter IUCE group compared to unexposed (p=.27).
FIGURE 3.
Age of initiation of alcohol use by level of intrauterine cocaine exposure
FIGURE 3 Age of initiation of any alcohol use by level of intrauterine cocaine exposure
3.3 Multivariable Analyses
Because of the relatively low prevalence of cigarette and other drug use, we restricted the multivariable analyses which follow to age at first use of any licit or illicit substance as a composite outcome and to marijuana, and alcohol as individual outcomes.
To arrive at the final models, we tested and excluded the following variables (because they did not change the relationship of IUCE with outcome by more than 10%): 1st degree relative (father, sister, brother) with history of drug or alcohol problem, 2nd degree relative (grandmother, grandfather, aunt, uncle) with history of drug or alcohol problem, history of having an incarcerated parent, birth mother’s education, and whether she was born in the United States and the child’s birth weight, child’s gender, adolescent religiosity, experience of strict supervision by caregivers, time dependent household food security from age 8.5 to 16 years, time dependent ascertainment of household members' use of alcohol, cigarettes, and cocaine from the participant’s age 8.5–16 years, number of caretaker changes from birth through middle adolescence, and peer substance use. Of all the variables retained in the multivariable analyses which follow, only VEX-R scores in the 3rd and 4th quartiles had hazard ratios of comparable or greater magnitude than those found for intrauterine exposure to cocaine or marijuana and so only these are discussed in detail.
3.3.1 Initiation of Any Licit or Illicit Substance Use
As seen in Table 2, in an adjusted model that included the level of intrauterine exposures to alcohol, marijuana, and tobacco, VEX-R quartiles at the time of substance initiation or censoring (i.e the last available observation up to which time there was no substance use), African American/Caribbean maternal ethnicity, maternal age at delivery, and having had the birth mother as the sole caretaker from birth, we found a moderate-to-strong positive adjusted association between IUCE and earlier age-at-initiation, with hazard ratios of 2.19 for heavier IUCE exposure vs. unexposed (95% C.I.: 1.10, 4.36, p=.03) and 1.69 for lighter IUCE exposure vs. unexposed (95% C.I.: 0.95, 3.03, p=.07). There was also a statistically significant association of younger maternal age at delivery with an increased likelihood of substance initiation by age 16 (HR=0.94 per year of mother’s age; p=.02) as well as of having had VEX-R scores in the third or fourth quartiles compared to the first fourth quartile: HR=3.44 (95% C.I.: 1.70, 6.94, p=.0006); third quartile: HR=2.29 (95% C.I. 1.10, 4.75 p=.03). Levels of prenatal alcohol or cigarette exposure were not statistically significant predictors of age at initiation of any licit or illicit substance use. There was suggestive evidence, however, of a positive association with lighter but not heavier intrauterine marijuana exposure with substance initiation (HR of 2.14, 95% C.I.: 1.07, 4.28; p=.03).
Table 2.
Cox proportional hazards regression models of initiation of any substance, marijuana, and alcohol N=149
| Any substance use1 |
Any marijuana use2 |
Any alcohol use3 | ||||
|---|---|---|---|---|---|---|
| HR (95% C.I.)* | p | HR (95% C.I.)* | p | HR (95% C.I.)* | p | |
| Intrauterine cocaine exposure4 |
||||||
| Heavier | 2.19 (1.10, 4.36) | 0.03 | 1.40 (0.62, 3.15) | 0.42 | 1.73 (0.79, 3.82) | 0.17 |
| Lighter | 1.69 (0.95, 3.03) | 0.07 | 1.29 (0.64, 2.60) | 0.47 | 1.86 (0.96, 3.63) | 0.07 |
| Intrauterine marijuana exposure4 |
||||||
| Heavier | 0.84 (0.39, 1.83) | 0.66 | 1.36 (0.56, 3.28) | 0.50 | 0.69 (0.26, 1.84) | 0.46 |
| Lighter | 2.14 (1.07, 4.28) | 0.03 | 2.86 (1.20, 6.77) | 0.02 | 2.12 (0.94, 4.74) | 0.07 |
| Nat. log, av. daily vol. alcohol, 30 days pre-delivery |
1.16 (0.63, 2.14) | 0.64 | 1.42 (0.74, 2.75) | 0.29 | 1.54 (0.76, 3.11) | 0.23 |
| Nat. log, prenatal daily cigarettes |
0.97 (0.79, 1.19) | 0.77 | 1.15 (0.89, 1.48) | 0.29 | 1.07 (0.85, 1.36) | 0.56 |
| Birth mother African-American/ Caribbean5 |
0.66 (0.29, 1.48) | 0.31 | 0.89 (0.34, 2.31) | 0.80 | 0.38 (0.12, 1.27) | 0.12 |
| Birth mother continuous caretaker6 |
0.83 (0.51, 1.34) | 0.44 | 0.76 (0.42, 1.38) | 0.37 | 0.88 (0.49, 1.56) | 0.65 |
| Birth mother age at delivery (years) |
0.94 (0.90, 0.99) | 0.02 | 0.94 (0.88, 0.99) | 0.03 | 0.95 (0.90, 1.00) | 0.06 |
| Violence Exposure Revised (VEX-R)7 |
||||||
| 4th quartile | 3.44 (1.70, 6.94) | 0.0006 | 5.05 (1.95, 13.07) | 0.0008 | 3.96 (1.74, 8.99) | 0.001 |
| 3rd quartile | 2.29 (1.10, 4.75) | 0.03 | 3.60 (1.40, 9.29) | 0.008 | 2.82 (1.20, 6.62) | 0.02 |
| 2nd quartile | 1.81 (0.89, 3.68) | 0.10 | 1.40 (0.51, 3.82) | 0.52 | 1.09 (0.44, 2.69) | 0.85 |
| Marijuana user in household8 |
2.02 (0.92, 4.41) | 0.08 |
HR: hazard ratio; CI: confidence interval
n=84 events, n=65 censored; model p=0.001
n=59 events, 90 censored; model p=0.0014
n=63 events, n=86 censored; p=0.0002
vs. unexposed
vs. non African-American/Caribbean
vs. not in continuous care of birth mother
vs. 1st quartile
vs. not
3.3.2 Initiation of Marijuana Use
As shown in Table 2, after adjustment for the same covariates as in the Cox model for any substance use by age 16 plus an additional covariate of having a member of the adolescent’s household who used marijuana (which changed the relationship between predictor and outcome more than 10% as described above), the heavier IUCE group had only 1.40 times the hazard for marijuana use compared to those unexposed to IUCE (95% C.I.: 0.62, 3.15, p=.42). Likewise, for those in the lighter IUCE group compared to the unexposed, there was no significant association with an increased likelihood of initiation of marijuana use by age 16 (HR=1.29, 95% C.I.: 0.64, 2.60, p=.47). There was significant evidence of a positive association with lighter marijuana exposure (HR =2.86, 95% C.I.: 1.20, 6.77; p=.02), as well as for and having had a VEX-R score in the fourth quartile versus the first quartile (HR=5.05 95% C.I.: 1.95, 13.07, p=.0008) and for VEX-R scores in the third quartile compared to the first (HR=3.60, 95% C.I.: 1.40, 9.29, p=.008).
3.3.3. Initiation of alcohol use
In a Cox regression analysis adjusting for the same covariates as in the Cox model for any substance use by age 16, since no other variables met our criteria for inclusion in this analysis, (Table 2), we found similar, elevated, but non-significant levels of association with initiation of alcohol use by age among the heavier IUCE (HR=1.86, 95% C.I: 0.96, 3.63, p=.17) and lighter IUCE (HR=1.73, 95%C.I.: 0.79, 3.82, p=.07) groups versus the unexposed. As in the Cox analyses for any substance use and marijuana use by age 16, we found that those who had VEX-R scores in the fourth quartile had a markedly and statistically significantly increased likelihood of alcohol use by age 16 compared to those in the first quartile (HR=3.96, 95% C.I.: 1.74, 8.99, p=.001), as did those in the third quartile versus the first quartile (HR=2.82, 95% C.I.: 1.20, 6.62, p=.02).
3.3.4 Interaction terms and secondary analyses
From the preceding models, we examined potential effect modification of IUCE and VEX-R effects by including interaction terms of level of prenatal marijuana, alcohol and cigarette exposures in the model and assessing their statistical significance at the 0.05 level (those not meeting this criterion were not included in subsequent models). Likewise, we verified that the proportional hazards assumption was met by including interaction terms of the IUCE and VEX-R variables with age-at-first use and found them not to be statistically significant at the 0.05 level. In secondary analyses results were not substantially changed after including adolescent IQ and average externalizing CBCL score through age 11 years as fixed covariates, and the participant’s self reported Tanner pubertal stage as a time dependent covariate beginning at age 9.5 years. (Analyses available from the authors on request)
4.1 Discussion
This is one of only a few published studies in humans which evaluates a possible association between prospectively identified intrauterine exposure to cocaine or marijuana with initiation of adolescent substance use, after considering intrauterine exposure to tobacco and alcohol, and potentially relevant covariates. Consistent with neonatal [66] and adolescent neuropsychological findings [57] in this cohort, there appears to be an ordered relationship after confound control between the heavier IUCE and the initiation of any licit or illicit psychoactive substance, and for alcohol but not marijuana initiation. The relationship between intrauterine marijuana exposure and the adolescent’s own use of any licit or illicit substance or of marijuana is not as clearly ordered as that for IUCE. We do not have a definitive explanation for this. One criterion for “heavier” marijuana use in our sample required the mother or infant to have positive urine toxic screen at delivery indicating exposure in the last month of pregnancy. It may be that exposure to marijuana earlier rather than later in gestation confers greater risk for adolescent substance initiation or there may be unmeasured covariates which explain these findings.
While our findings are intriguing from a neuroteratologic perspective, and seem to indicate that heavier IUCE increases the risk for early substance use initiation, it is crucial not to over-interpret them from a clinical or public policy perspective. It is important to stress that this analysis does not evaluate problem or heavy substance use, but only age at first use which, while concerning, by no means indicates that an adolescent will inevitably develop a substance use disorder. The substances most commonly used in our cohort were those which are widely available and commonly used by other adolescents nationally and in our region: alcohol and marijuana. Alcohol is currently used by approximately 45% of adolescents age 12–17 in Massachusetts and 46% nationally while approximately 41% of adolescents in Massachusetts and 38% of adolescents nationally have ever used marijuana.
In contrast, use is less prevalent of “hard drugs” such as cocaine (9% in Massachusetts and 7% nationally) or illegal amphetamines (4% both in Massachusetts and nationally) [1]. Questions about whether early initiation of alcohol and marijuana for these adolescents presages future “hard drug” use will only be answered through further follow up study. We have no evidence to suggest whether children with intrauterine cocaine or marijuana exposure will or will not go on to become adult addicts. Additionally, it is important to remember that even at age 16 years, even at the highest levels of intrauterine cocaine exposure, 26% of our sample were complete abstainers from any licit or illicit psychoactive substance use while nearly half of the intrauterine unexposed adolescents had initiated substance use.
As far as we know, the strong prospective relationship we identified between the highest time dependent levels of childhood and adolescent exposure to violence and licit or illicit substance initiation, has been noted only in one other prospective study which extended just to age 11 [42] years, although this relationship has been reported in a large retrospective study [25]. High levels of exposure to violence as reported through childhood and adolescence by the adolescents were associated with the initiation of any licit or illicit substance, of marijuana, and of alcohol with hazard ratios as high, or higher, than those associated with any prenatal exposure, including cocaine (see Table 2) Notably high levels of violence exposure were robustly associated with initiation of alcohol and marijuana use even when heavier intrauterine exposures to cocaine or marijuana were not.
4.2 Limitations
The study has a number of limitations including sample size. In a larger sample certain effects that are statistically marginal in our analyses (for example, the association of heavier IUCE with early alcohol initiation) might be more robust and multivariable analysis of initiation of tobacco and illicit substances other than marijuana would be possible. Our sample is also by design quite homogeneous, so findings should not be generalized to adolescents born prematurely, from non Black ethnic groups, or different levels of socioeconomic privilege. Undoubtedly some of the 17 cocaine using women for whom neonatal meconium samples were not available minimized self reported use, and therefore some heavier users may have been misclassified as lighter. Misclassifying heavier users as lighter would decrease the likelihood of finding ordinal dose effect, so that any found in this study are robust. Our characterization of familial risk is quite crude without confirmation by detailed genogram or genetic markers. Therefore, we cannot rule out that the apparent impact of intrauterine exposures on offspring’s substance initiation is a marker for unmeasured genetic factors, rather than a neuroteratologic mechanism. While we attempted to control for numerous psychosocial variables, we do not know the impact of unmeasured or unanalyzed covariates, such as post-natal lead levels which did not differ between exposure groups but for which we do not have a complete data set.
4.3 Conclusions
Comprehensive treatment of pregnant women who are struggling with use of any psychoactive substance, whether licit or illicit, should be a clinical and public health priority. Effective prevention of early substance use initiation also requires focus on the post-natal quality of life for impoverished children and adolescents, particularly protection from high levels of exposure to violence, which correlate in this cohort with as great or greater odds of early substance use initiation than intrauterine exposures to cocaine or marijuana.
In the United States during pregnancy approximately 1% [2,34] of women use cocaine and between 3% [34] and 6% [9] use marijuana. However, 17% of children age 12–17 witness violence and 39% are the victims of violence [40]. The role that violence plays on the initiation of licit or illicit substance use even after controlling for multiple intrauterine exposures and other factors thus has far broader implications for public health than intrauterine exposure to illicit drugs, but receives less attention in the public discourse.
Acknowledgments
This research was supported by the National Institutes on Drug Abuse (NIDA) grant number DA06532 to Dr. Frank and by grant MO1 RR00533 and RR025771 from the National Institutes of Health/National Center for Research Resources, a component of the National Institutes of Health (NIH). This work would not have been completed without the wise guidance and unfailing support of Dr. Vincent Smeriglio to us and all the other longitudinal projects. We are everlastingly grateful to him. We would also like to thank Shayna Soenksen, Heather Baldwin, Laura Anatale, and Mattia Chason for their diligence in performing study assessments and to the participants and their caregivers who have worked with us for more than 19 years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no financial, personal, or other relationships with people or organizations that may inappropriately influence the authors' submitted work.
References
- 1.Comparison Between Massachusetts Students and U.S. Students 2007 YRBS. Centers for Disease Control and Prevention. 2007 [Google Scholar]
- 2.National Pregnancy and Health Survey: Drug Use Among Women Delivering Livebirths: 1992. Rockville, MD: National Institute on Drug Abuse; 1996
- 3.Achenbach TM. Manual for Child Behavior Checklist 4–18, 1991 Profile. Burlington, VT: Department of Psychiatry University of Vermont; 1991. [Google Scholar]
- 4.Achenbach TM. Department of Psychiatry. Burlington, VT: University of Vermont; 1992. Manual for the child behavior checklist/2–3 and 1992 profile. [Google Scholar]
- 5.Alati R, Najman JM, Kinner SA, Mamun AA, Williams GM, O'Callaghan M, Bor W. Early predictors of adult drinking: a birth cohort study. Am J Epidemiol. 2005;162:1098–1107. doi: 10.1093/aje/kwi320. [DOI] [PubMed] [Google Scholar]
- 6.Alessandri SM, Bendersky M, Lewis M. Cognitive functioning in 8- to 18-month-old drug-exposed infants. Dev Psychol. 1998;34:565–573. doi: 10.1037//0012-1649.34.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardila-Rey A, Killen M, Brenick A. Moral Reasoning in Violent Contexts: Displaced and Non-Displaced Colombian Children's Evaluations of Moral TransgressionsRetaliations and Reconciliation. Social Development. 2008;18:182–209. doi: 10.1111/j.1467-9507.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong TD, Costello EJ. Community Studies on Adolescent Substance Use, Abuse, or Dependence and Psychiatric Comorbidity. Journal of Consulting and Clinical Psychology. 2002;70:1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- 9.Arria A, Derauf C, Lagasse L, Grant P, Shah R, Smith L, Haning W, Huestis M, Strauss A, Grotta SD, et al. Methamphetamine and Other Substance Use During Pregnancy: Preliminary Estimates From the Infant Development, Environment, and Lifestyle (IDEAL) Study. Maternal and Child Health Journal. 2006;10:293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 10.Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- 11.Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-Year Longitudinal Analysis of the Effects of Prenatal Alcohol Exposure on Young Adult Drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- 12.Beeghly M, Martin B, Rose-Jacobs R, Cabral H, Heeren T, Augustyn M, Bellinger D, Frank DA. Prenatal cocaine exposure and children's language functioning at 6 and 9.5 years: moderating effects of child age, birthweight, and gender. Journal of Pediatric Psychology. 2006;31:98–115. doi: 10.1093/jpepsy/jsj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belcher HME, Shinitzky HE. Substance Abuse in Children: Prediction, Protection, and Prevention. Archives of Pediatric and Adolescent Medicine. 1998;152:952–960. doi: 10.1001/archpedi.152.10.952. [DOI] [PubMed] [Google Scholar]
- 14.Belsley DA, Kuh E, Welsch RE. Regression diagnostics: identifying influential data and sources of collinearity. New York: Wiley; 1980. [Google Scholar]
- 15.Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28:467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- 16.Brandt R, Ward C, Dawes A, Flisher A. Epidemiological Measurement of Children’s and Adolescents’ Exposure to Community Violence: Working with the Current State of the Science. Clinical Child and Family Psychology Review. 2005;8 doi: 10.1007/s10567-005-8811-4. [DOI] [PubMed] [Google Scholar]
- 17.Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- 18.Carlin A, O'Malley S. Neuropsychological consequences of drug abuse. In: Grant I, Adams K, editors. Neuropsychological assessment of neuropsychiatric disorders. New York: Oxford University Press; 1996. pp. 486–503. [Google Scholar]
- 19.Cook JT, Frank DA. Food security, poverty, and human development in the United States. Ann N Y Acad Sci. 2008;1136:193–209. doi: 10.1196/annals.1425.001. [DOI] [PubMed] [Google Scholar]
- 20.Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313–1322. doi: 10.1111/j.1360-0443.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- 21.DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- 22.Eaton DK, Kann L, Kinchen S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Shanklin S others. Youth risk behavior surveillance--United States, 2005. MMWR Surveill Summ. 2006;55:1–108. and others. [PubMed] [Google Scholar]
- 23.U. S. Economic Research Service. U.S. household food security module: Three-stage design, with screeners. 2008 [Google Scholar]
- 24.Estelles J, Rodriguez-Arias M, Maldonado C, Aguilar MA, Minarro J. Gestational exposure to cocaine alters cocaine reward. Behav Pharmacol. 2006;17:509–515. doi: 10.1097/00008877-200609000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventative Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 26.Fergusson D, Horwood L, Ridder E. Show me the child at seven II: Childhood intelligence and later outcomes in adolescence and young adulthood. Child Psychology and Psychiatry. 2005;46:850–858. doi: 10.1111/j.1469-7610.2005.01472.x. [DOI] [PubMed] [Google Scholar]
- 27.Fox NA, Leavitt LA. VEX-R: The Violence Exposure Scale for Children - Revised. 1995 [Google Scholar]
- 28.Frank DA, Brown J, Johnson S, Cabral H. Forgotten fathers: an exploratory study of mothers' report of drug and alcohol problems among fathers of urban newborns. Neurotoxicol Teratol. 2002;24:339–347. doi: 10.1016/s0892-0362(02)00196-4. [DOI] [PubMed] [Google Scholar]
- 29.Frank DA, Rose-Jacobs R, Beeghly M, Augustyn M, Bellinger D, Cabral H, Heeren T. Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics. 2002;110:1143–1152. doi: 10.1542/peds.110.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank DA, McCarten KM, Robson CD, Mirochnick M, Cabral H, Park H, Zuckerman B. Level of in utero cocaine exposure and neonatal ultrasound findings. Pediatrics. 1999;104:1101–1105. doi: 10.1542/peds.104.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank DA, Rose-Jacobs R, Beeghly M, Wilbur MB, Bellinger D, Cabral H. Level of prenatal cocaine exposure and 48-month IQ: importance of preschool enrichment. Neurotoxicol Teratol. 2005;27:15–28. doi: 10.1016/j.ntt.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Fried PA WB, Gray R. Neurocognitive consequences of cigarette smoking in young adults--a comparison with pre-drug performance. Neurotoxicol Teratol. 2006;28:517–525. doi: 10.1016/j.ntt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Havens J, Simmons LA, Shannon LM, Hansen WF. Factors associated with substance use during pregnancy: Results from a national sample. Drug and Alcohol Dependence. 2008;99:89–95. doi: 10.1016/j.drugalcdep.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Hecht GS, Spear NE, Spear LP. Alterations in the reinforcing efficacy of cocaine in adult rats following prenatal exposure to cocaine. Behav Neurosci. 1998;112:410–418. doi: 10.1037//0735-7044.112.2.410. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence for neurobehavioral effects of in utero cocaine exposure. The Journal of pediatrics. 1996;129:581–590. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JL, Leff M. Children of Substance Abusers: Overview of Research Findings. Pediatrics. 1999;103:1085–1099. [PubMed] [Google Scholar]
- 38.Joseph N, Augustyn M, Frank DA, Cabral H. Preadolescents’ report of exposure to violence: Association with friends’ and own substance use. Journal of Adolescent Health. 2006;38:669–674. doi: 10.1016/j.jadohealth.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaminer Y, Bukstein O, Tarter RE. The Teen-Addiction Severity Index: rationale and reliability. Int J Addict. 1991;26:219–226. doi: 10.3109/10826089109053184. [DOI] [PubMed] [Google Scholar]
- 40.Kilpatrick DG, Saunders BE, Smith DW. Youth Victimization: Prevalence and Implications. National Institute of Justice. 2003 [Google Scholar]
- 41.Kolko DJ, Hurlburt MS, Zhang J, Barth RP, Leslie LK, Burns BJ. Posttraumatic Stress Symptoms in Children and Adolescents Referred for Child Welfare Investigation: A National Sample of In-Home and Out-of-Home Care. Child Maltreatreatment. 2009 doi: 10.1177/1077559509337892. [DOI] [PubMed] [Google Scholar]
- 42.Lagasse L, Hammond J, Jing LIU, Lester B, Shankaran S, Bada H, Bauer CR, Higgins R, Abhik DAS. Violence and Delinquency, Early Onset Drug Use, and Psychopathology in Drug-Exposed Youth at 11 Years. Annals of the New York Academy of Sciences. 2006;1094:313–318. doi: 10.1196/annals.1376.041. [DOI] [PubMed] [Google Scholar]
- 43.Lamborn S, Mounts N, Steinberg L, Dornbusch S. Patterns of Competence and Adjustment among Adolescents from Authoritative, Authoritarian, Indulgent, and Neglectful Families. Child Development. 1991;62:1049–1065. doi: 10.1111/j.1467-8624.1991.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 44.Marie-Alsana W, Haj-Yahia M. Violence Among Arab Elementary School Pupils in Israel. Journal of Interpersonal Violence. 2006;21:58–88. doi: 10.1177/0886260505281604. [DOI] [PubMed] [Google Scholar]
- 45.Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of International Neuropsychological Society. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller L, Davies M, Greenwald S. Religiosity and substance use and abuse among adolescents in the National Comorbidity Survey. Journal of the American Academy of Child and Adolescent Psychology. 2000;39:1190–1197. doi: 10.1097/00004583-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 47.Mirochnick M, Frank DA, Cabral H, Turner A, Zuckerman B. Relation between meconium concentration of the cocaine metabolite benzoylecgonine and fetal growth. The Journal of pediatrics. 1995;126:636–638. doi: 10.1016/s0022-3476(95)70367-5. [DOI] [PubMed] [Google Scholar]
- 48.Nonnemaker JM, McNeely CA, Blum RW. Public and private domains of religiosity and adolescent health risk behaviors: evidence from the National Longitudinal Study of Adolescent Health. Social Science and Medicine. 2003;57:2049–2054. doi: 10.1016/s0277-9536(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 49.O'Callaghan FV, O'Callaghan M, Najman JM, Williams GM, Bor W, Alati R. Prediction of adolescent smoking from family and social risk factors at 5 years, and maternal smoking in pregnancy and at 5 and 14 years. Addiction. 2006;101:282–290. doi: 10.1111/j.1360-0443.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 50.Oncken C, McKee S, Krishnan-Sarin S, O'Malley S, Mazure C. Gender effects of reported in utero tobacco exposure on smoking initiation, progression and nicotine dependence in adult offspring. Nicotine Tob Res. 2004;6:829–833. doi: 10.1080/1462220042000282555. [DOI] [PubMed] [Google Scholar]
- 51.Ostaszewski K, Zimmerman MA. The Effects of Cumulative Risks and Promotive Factors on Urban Adolescent Alcohol and Other Drug Use: A Longitudinal Study of Resiliency. American Journal of Community Psychology. 2006;38:237–249. doi: 10.1007/s10464-006-9076-x. [DOI] [PubMed] [Google Scholar]
- 52.Ostrea EM, Jr, Brady M, Gause S, Raymundo AL, Stevens M. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992;89:107–113. [PubMed] [Google Scholar]
- 53.Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the Onset of Substance Use and Abuse. Pediatrics. 2004;114:e300–e306. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porath AJ, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol. 2005;27:267–277. doi: 10.1016/j.ntt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Prescott CA, Madden PAF, Stallings MC. Challenges in Genetic Studies of the Etiology of Substance Use and Substance Use Disorders: Introduction to the Special Issue. Behavior Genetics. 2006;36:473–482. doi: 10.1007/s10519-006-9072-9. [DOI] [PubMed] [Google Scholar]
- 56.Rocha BA, Mead AN, Kosofsky BE. Increased vulnerability to self-administer cocaine in mice prenatally exposed to cocaine. Psychopharmacology. 2002;163:221–229. doi: 10.1007/s00213-002-1140-0. [DOI] [PubMed] [Google Scholar]
- 57.Rose-Jacobs R, Waber D, Beeghly M, Cabral H, Appugleise D, Heeren T, Marani J, Frank DA. Intrauterine cocaine exposure and executive functioning in middle childhood. Neurotoxicol Teratol. 2009;31:159–168. doi: 10.1016/j.ntt.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roselli M, Ardila A, Lubomski M, Murray S, King K. Personality profile and neuropsychological test performance in chronic cocaine-abusers. International Journal of Neuroscience. 2001;110:55–72. doi: 10.3109/00207450108994221. [DOI] [PubMed] [Google Scholar]
- 59.Rourke SB, Loberg T. Neurobehavioral correlates of alcoholism. In: Grant I, Adams K, editors. Neuropsychological assessment of neuropsychiatric disorders. New York: Oxford University Press; 1996. pp. 423–485. [Google Scholar]
- 60.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Review. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singer LT, Arendt R, Minnes S, Salvator A, Siegel AC, Lewis BA. Developing language skills of cocaine-exposed infants. Pediatrics. 2001;107:1057–1064. doi: 10.1542/peds.107.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slade E, Stuart E, Salkever D, Karakus M, Green K, Ialongo N. Impacts of age of onset of substance use disorders on risk of adult incarceration among disadvantaged urban youth: A propensity score matching approach. Drug and Alcohol Dependence. 2008;95:1–13. doi: 10.1016/j.drugalcdep.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solowij N. Cannabis and cognitive functioning. New York: Cambridge University Press; 1998. [Google Scholar]
- 64.Tanner JM. Growth and endocrinology of the adolescent. In: Gardner L, editor. Growth and endocrinology of the adolescent. 2nd Edition. Philadelphia, PA: W.B. Saunders; 1975. pp. 14–64. [Google Scholar]
- 65.Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. Journal of International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- 66.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 67.White HR, McMorris BJ, Catalano R, Fleming CB, Haggerty KP, Abbott RD. Increases in alcohol and marijuana use during the transition out of high school Into emerging adulthood: The effects of leaving home, going to college, and high school protective factors. Journal on the Study of Alcohol. 2006;67:810–822. doi: 10.15288/jsa.2006.67.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilbur MB, Marani JE, Appugliese D, Woods R, Siegel JA, Cabral HJ, Frank DA. Socioemotional effects of fathers' incarceration on low-income, urban, school-aged children. Pediatrics. 2007;120:e678–e685. doi: 10.1542/peds.2006-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winters KC, Henly GA. Western Psychological Services, Personal experience inventory (PEI) : manual. Los Angeles, Calif.: Western Psychological Services; 1989. [Google Scholar]
- 70.Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–920. [PubMed] [Google Scholar]