Abstract
Vangl2 was identified as the gene defective in the Looptail mouse model for neural tube defects (NTDs). This gene forms part of the planar cell polarity pathway, also called the non-canonical Frizzled/Dishevelled pathway, which mediates the morphogenetic process of convergent extension essential for proper gastrulation and neural tube formation in vertebrates. Genetic defects in PCP signaling have strongly been associated with NTDs in mouse models. To assess the role of VANGL2 in the complex etiology of NTDs in humans, we resequenced this gene in a large multi-ethnic cohort of 673 familial and sporadic NTD patients, including 453 open spina bifida and 202 closed spinal NTD cases. Six novel rare missense mutations were identified in 7 patients, five of which were affected with closed spinal NTDs. This suggests that VANGL2 mutations may predispose to NTDs in approximately 2.5% of closed spinal NTDs (5 in 202), at a frequency that is significantly different from that of 0.4% (2 in 453) detected in open spina bifida patients (P=0.027). Our findings strongly implicate VANGL2 in the genetic causation of spinal NTDs in a subset of patients and provide additional evidence for a pathogenic role of PCP signaling in these malformations.
Keywords: VANGL2, neural tube defects, planar cell polarity
Introduction
Neural tube defects (NTDs) represent a group of common congenital malformations of the central nervous system, affecting 1-2 infants per 1000 births (1). They reflect an early failure of the neural tube closure during the first 6 weeks of pregnancy and can occur at any level of the embryonic axis, affecting cranial and/or spinal regions of the developing spinal cord (1). NTDs can be classified as either “open” where the affected nervous tissues are exposed to the environment or “closed” where the defect is covered by skin. The two most common forms of NTDs are “open” and include anencephaly and myelomeningocele (or spina bifida) that result from failure of the neural tube to close at the cranial region and spinal regions respectively (1,2). Anencephaly is characterized by complete absence of the cranial vault and cerebral hemispheres and is invariably lethal (1, 2). In myelomeningocele, the placode protrudes together with the meninges through a bony defect in the midline of the back, therefore being exposed to the environment. The degree of motor deficit is affected by direct injury to the exposed placode during delivery. In addition to spinal anomalies, all patients with myelomeningocele present with Chiari II malformation and hydrocephalus. The subsequent clinical picture of spina bifida includes sensorimotor deficits of the lower extremities, bowel and bladder incontinence, hindbrain dysfunction, and intellectual and psychological disturbances (3).
Closed (skin-covered) NTDs are categorized clinically depending on the presence or absence of a lower back subcutaneous mass. Closed NTDs with a mass are represented by lipomyeloschisis, lipomyelomeningocele, meningocele, and myelocystocele. Closed NTDs without a mass include simple dysraphic states (intradural lipomas, diastematomyelia, teratoma, dermoid, epidermoid, tight filum terminale, persistent terminal ventricle, and dermal sinus) and complex dysraphic states (dorsal enteric fistula, neurenteric cysts, split cord malformations, caudal agenesis, and spinal segmental dysgenesis). The clinical manifestations of closed NTDs are more heterogeneous than those of open NTDs and vary in severity depending on the type of the NTD and the age at presentation (3).
The prevalence of NTDs has declined considerably during the past three decades due mainly to the protective effect of periconceptional administration of folic acid, which reduces their incidence as much as 60-70%, and to termination of affected pregnancies (4). Despite this, thousands of families are affected with NTDs each year urging the need for better understanding the underlying pathogenic mechanisms of these diseases.
NTDs have a complex etiology involving environmental and genetic factors whose identity and number remain largely unknown (1, 5). A genomewide linkage screen in NTDs has identified few candidate regions on chromosomes 2, 7 and 10; however, such studies are complicated by probable genetic heterogeneity and variable penetrance and failed to identify any NTD gene (reviewed in reference 1). Animal models were proven to be instrumental in deciphering the complex causation of NTDs (6). Particularly, these models demonstrated an essential role for the planar cell polarity (PCP) signaling pathway in neurulation. PCP, or tissue polarity, is the process by which cells become polarized within the plane of an epithelium (7). This form of polarity has been well studied in the fly where it can be observed in the distally oriented wing hairs and the highly organized ommatidia (eye units) of the adult eye. Genetic studies in the fly have identified a group of so called “core” PCP genes which include: Frizzled (Fz), Dishevelled (Dsh), Strabismus/ Van Gogh (Stbm/Vang), Flamingo (Fmi), Prickle (Pk) and Diego (Dgo) (7,8). These PCP proteins are highly conserved in vertebrates where they form part of the non canonical Frizzled/Dishevelled pathway that mediates the process of convergent extension (CE) during gastrulation and neural tube formation. CE is a complex morphogenetic process where a group of cell elongates medio-laterally, moves directionally and intercalates with other neighboring cells. This results in convergence at the mediolateral axis and extension at the anteroposterior axis, forming one of the major shaping forces for gastrulation and neural tube formation (9,10).
Looptail (Lp) was the first mouse model to implicate a PCP core gene, Vangl2, in the pathogenesis of NTDs (11,12). Lp mice are characterized by a looped tail appearance in heterozygotes and a severe form of NTDs called craniorachischisis in homozygous embryos where the neural tube fail to form along the entire body axis (13). Using a positional cloning strategy, we and others have identified Vangl2 as the gene mutated in Lp (11,12). Disease-specific missense mutations, p.Asp255Glu and p.Ser464Asn, were identified in Vangl2 in two independent alleles of Lp, Lpm1Jus and Lp, respectively (11,12). Vangl2 encodes a membrane protein whose predicted features include 4 transmembrane (TM) domains and a PDZ-domain binding motif at the carboxy terminus involved in protein-protein interaction (11). In vertebrates, Vangl2 has another homologue called Vangl1 that has similar biochemical functions based on protein similarity and expression data (14). In mice, Vangl1 shows a dynamic pattern of expression in the developing neural tube and genetically interacts with Vangl2 (15). We previously identified 8 novel rare missense mutations in VANGL1 that were associated with NTDs (16,17). Particularly, one de novo mutation, p.Val239Ile, was shown to affect the physical interaction of VANGL1 with the Dishevelled proteins, thereby affecting an important functional aspect of PCP signaling (16). Recently, we demonstrated that p.Val239Ile and another variant p.Met328Thr affect convergent extension in a zebrafish model, further confirming their pathogenic role in NTDs (18).
Initial genetic studies of VANGL2 by our group in a cohort of 137 Italian patients with isolated spinal dysraphisms and 7 fetuses with craniorachischisis, and by others, have failed to identify any potentially pathogenic variant in this gene (16,19). However, given the strong genetic and functional evidence for a role of the VANGL genes in neurulation, and in particular for a role of Vangl2 in NTD development in Lp mice, we sought to assess the role of VANGL2 in human NTDs in a separate and larger cohort of patients. Therefore, in the present study, we screened 673 patients affected with various forms of NTDs and of various ethnic origins for the presence of mutations in VANGL2 by DNA resequencing analysis.
Patients and Methods
Patients and Controls
The cohort consisted of 284 Italian patients recruited at the Spina Bifida Center of the Gaslini Hospital in Genova, Italy and 389 patients recruited at the Children's Memorial Hospital in Chicago, Illinois, United States. Detailed clinical information on both cohorts is as presented previously (17). In summary, all patients included in this study were affected with non-syndromic or isolated NTDs, where 67% of patients were affected with myelomeningocele or open spina bifida and 30% were affected with various forms of closed spinal NTDs. Of all 673 patients, 38% were male and 80% were of Caucasian white non hispanic origin. The two other major ethnic groups present in this cohort consisted of Hispanics (11%) and African Americans (∼5%). The mean age upon clinical presentation is known only for the Italian cohort and is 10.1 years. The folate status is known only for mothers of Italian patients who all lacked periconceptional folic acid supplementation. One hundred twenty patients (18%) had a positive family history documented by clinical records (MRI and X-ray images) obtained from parents of cases.
The control group included in this study comprised 222 healthy random Italian individuals consisting of randomly selected children admitted to the Gaslini Children's Hospital for miscellaneous illnesses and healthy young adults who contributed samples to the blood bank of the Gaslini Institute. All control individuals were unrelated. All samples from patients and controls were collected with the approval of the local ethics committee and written informed consent was obtained from all patients, parents, and control individuals. The control group also included 90 CEU individuals of northern and western European ancestry from the International HapMap project (CEU, the Centre d'Etude du Polymorphisme Humain) (www.hapmap.org) and 1050 individuals from The Human Genome Diversity Project originating from 51 world populations throughout the world (www.cephb.fr/).
Resequencing
The genomic structure of VANGL2 was determined using the NCBI (GeneID: 57216) and Ensembl (transcript ID: ENST00000368061) databases. Primers flanking the exon-intron junctions were developed manually and used to amplify the coding region and the exon-intron junctions of VANGL2 from genomic DNA. Primers' sequences are available upon request. The whole coding region of VANGL2 of 1566 bp along with 123 bp of the 5′ untranslated region (UTR) and 158 bp of the 3′UTR were amplified in a total of 6 amplicons. Each exon was amplified in one amplicon except for the first and second coding exons that were both amplified in one fragment. The portions of the introns that were sequenced ranged from 20 bp to 60 bp with an average of ∼40 bp. PCR was carried out using the Taq DNA polymerase or the Platinum Taq Hi Fidelity polymerase (Invitrogen) as per manufacturer's instructions. Direct dye terminator sequencing of PCR products was carried out using the ABI Prism Big Dye Systems at the Genome Quebec Innovation Center (Montréal, Québec, Canada). Samples were run on ABI 3700 automated sequencer and analyzed using the PhredPhrap software. When possible, variants were confirmed by PCR from a new DNA sample obtained from the patient and were tested in other family members. Genotyping of the large panel of HapMap and HGDP controls was done using the Sequenom iPlex Gold technology (20).
Bioinformatics and statistical tests
Two public databases were queried for the occurrence of the identified mutations in VANGL2: dbSNP ((http://www.ncbi.nlm.nih.gov/snp) and the 1000 genome project (www.1000genomes.org). The potential pathogenic effect of the identified mutations on protein function was predicted using the three software programs: PolyPhen (Polymorphism Phenotyping: http://genetics.bwh.harvard.edu/pph/), SIFT (Sorting Intolerant from Tolerant; http://sift.jcvi.org/) and SNPs3D (http://snps3d.org/). Default conditions were used for the programs. PolyPhen and SIFT predict the possible impact of an amino acid substitution on the structure and function of a human protein using sequence homology and the physical properties of amino acids. SNPs3D identifies the deleterious potential of non-synonymous single base changes by analyzing the effect of the resulting amino acid change on protein stability, utilizing structural information, and by making use of the conservation and type of residues observed at a base change position within a protein family. Multiple alignments of the VANGL proteins were done using the CLUSTAL W program freely available at http://npsa-pbil.ibcp.fr. The Z-Test for Two Proportions was used to compare the frequency of mutations between NTD patients and controls and between closed and open NTDs
Results
Mutation screening of the coding exons and the exon-intron junctions of VANGL2 identified 8 NTD patients with seven rare missense mutations that had not been described previously or reported in the dbSNP or 1000 genome project databases to date (Table 1). All these variants were heterozygous and were absent in all 1362 controls analyzed except for p.Val178Ile that was detected in one HGDP control of Caucasian origin. Initial validation of the potential effect of each of these mutations on protein function was done by analyzing the level of conservation of the amino acid affected. Alignment of seven VANGL2 vertebrate proteins with human VANGL1 and Drosophila Strabismus revealed 32% identity, 18% strong similarity and 9% weak similarity (data not shown).
Table 1.
Rare missense variants (<1%) identified in VANGL2 in neural tube defects patients and controls.
| Amino acid change | Nucleotide change | Freq. in patients | Freq. in controlsa | PolyPhen prediction | SIFT prediction | SNPs3D prediction | |
|---|---|---|---|---|---|---|---|
| Open | Closed | ||||||
| Absolutely conserved | |||||||
| p.Arg105Cys | c.313C>T | 0 | 0 | 1b | Damaging | Intolerant | Deleterious |
| p.Arg135Trp | c.403C>T | 1 | 0 | 0 | Damaging | Intolerant | Deleterious |
| p.Arg177His | c.530G>A | 0 | 1 | 0 | Damaging | Intolerant | Deleterious |
| p.Arg270His | c.809G>A | 0 | 1 | 0 | Damaging | Intolerant | Deleterious |
| Highly conserved | |||||||
| p.Leu242Val | c.724C>G | 1 | 1 | 0 | Benign | Tolerant | Deleterious |
| p.Thr247Met | c.740C>T | 0 | 1 | 0 | Benign | Tolerant | Non-deleterious |
| p.Arg482His | c.1445G>A | 0 | 1 | 0 | Benign | Tolerant | Non-deleterious |
| Poorly conserved | |||||||
| p.Val178Ile | c.532G>A | 0 | 1 | 1 | Benign | Tolerant | Non-deleterious |
| Total | 2 | 6 | 2 | ||||
The control group analyzed for all variants except p.Arg105Cys included 222 Italian controls, 90 CEU individuals (www.hapmap.org) and 1050 HGDP individuals (www.cephb.fr/)
The control group analyzed for this variant included only 222 Italian controls and 65 CEPH individuals.
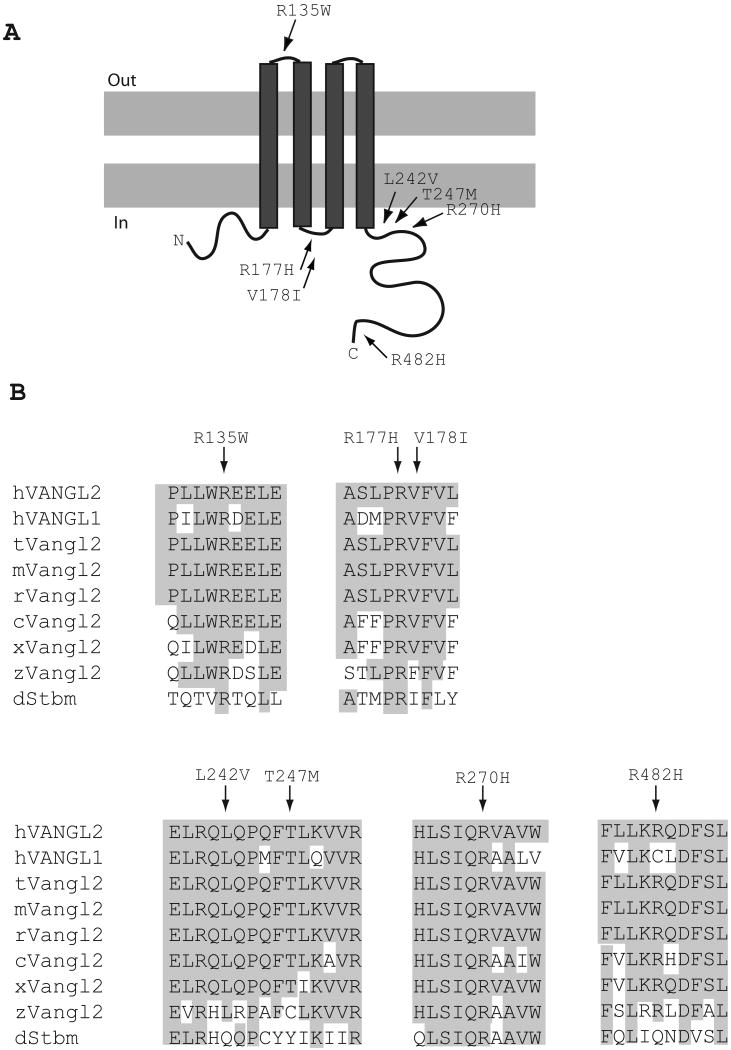
Three VANGL2 variants, p.Arg135Trp, p.Arg177His, p.Arg270His, affect absolutely conserved amino acid residues and were predicted to affect protein function in silico using the PolyPhen, SIFT and SNPs3D programs (Table 1 and Figure 1B). The p.Arg135Trp variant was detected in one patient affected with lumbo-sacral myelomenigocele associated with stabilized hydrocephalus, Chiari II malformation and hydromyelia. This variant was detected in the mother of this patient. The Arg135 residue maps to the predicted extracellular domain of the VANGL2 protein between TM1 and TM2 (Figure 1). The substitution of the basic, positively charged arginine for the neutral, aromatic and hydrophobic tryptophan reduces the average hydrophilicity and surface probability over a window of five and 9 amino acids including the substitution respectively. This may weaken the structural integrity of the VANGL2 protein and may consequently affect its function. The p.Arg177His was detected in one patient affected with diastematomyelia, which represents a closed form of NTDs characterized by a variably elongated separation of the spinal cord in two usually symmetric halves and a hairy tuft lying along the child's back as a clinical marker. The Arg177 is predicted to localize to the intracellular domain between TM2 and TM3 (Figure 1A). The p.Arg270His variant was detected in one patient who presented with right convex dorso-lumbar scoliosis, hydrosyringomyelia and a small fibrolipoma of the filum terminalis. The Arg270 residue is predicted to localize near the cytoplasmic side of TM4 and is part of a motif of 5 residues “LSIQR” that is perfectly conserved across evolution (Figure 1). Although the variants p.Arg177His and p.Arg270His represent an exchange of two basic amino acids that is not considered to be a substantial chemical change, these residues do lie within two absolutely conserved regions of VANGL2 suggesting intolerance for amino acid substitution across evolution.
Figure 1.
VANGL2 mutations in NTD patients. (A) A topological model of VANGL2 protein is shown with the approximate positions of the six novel mutations identified in NTD patients. (B) A partial alignment of human VANGL2 with 8 other Vangl/Stbm sequences. Residues conserved between VANGL2 and other family members are highlighted. The VANGL2 variants found in NTD patients affect conserved residues (indicated by arrows). Accession numbers: human VANGL2 (hVANGL2), NP_065068; human VANGL1 (hVANGL1), NP_620409; taurus Vangl2 (tVangl2), XP_598791; mouse Vangl2 (mVangl2), NP_277044, frog Vangl2 (xVangl2), AAK70879; zebrafishVangl2 (zVangl2), NP_705960 and Drosophila Stbm (dStbm), NP_477177.
Three VANGL2 variants, p.Leu242Val, p.Thr247Met and p.Arg482His, affect highly conserved residues across evolution (Table 1 and Figure 1B). The variant p.Leu242Val was detected in one Italian patient and one American patient, both of Caucasian white origin. The Italian patient was affected with lumbar myeolocystocele and the American patient with myelomeningocele. This variant was detected in the mother of the Italian patient and was absent from his father and unaffected sister. The Leu242 residue is predicted to localize near the cytoplasmic side of TM4 (Figure 1A). A leucine to valine substitution is a conservative chemical change; however, it was predicted to be deleterious using the SNPs3D program and the fact that this variant was identified in two unrelated patients affected with NTDs but no controls suggests that it is most likely pathogenic. The variant p.Thr247Met was detected in a Caucasian white American patient who suffered from lipoma of the filum terminalis, tethered cord and hydrocephalus. This variant affects a highly conserved threonine residue predicted to localize near the cytoplasmic side of TM4, it is replaced by cysteine in zebrafish Vangl2 and tyrosine in the fly Stbm. While both cysteine and tyrosine preserve the hydrophobic nature of the threonine residue, a substitution to methionine is not conservative as it significantly reduces its hydrophilicity. The variant p.Arg482His was detected in one patient affected with caudal agenesis and tethered cord. This variant affects a residue that maps near the carboxyl terminus of the predicted protein (Figure 1A) and that is conserved in all vertebrate orthologues of VANGL2 (Figure 1B).
One VANGL2 variant, p. Val178Ile, affects a poorly conserved residue that is predicted to localize to the intracellular domain between TM2 and TM3 (Figure 1). This variant was detected in one patient affected with lipoma and tethered cord and in his father. The fact that valine at position 178 is replaced by isoleucine in the fly Stbm/Vang argues against a pathogenic role for this variant.
To assess the rate of missense mutations in VANGL2 in the normal population, we sequenced the full ORF and exon-intron junctions of this gene in a cohort of 222 Italian controls and 65 CEPH individuals. In this group of 287 controls, we identified one missense mutation, p.Arg105Cys, in one Italian control, that affects an absolutely conserved arginine residue that is predicted to map to the cytoplasmic side of TM1 (Table 1). A substitution of arginine to cysteine is not conservative as it reduces its hydrophilicity and an introduction of cysteine may result in the formation of intermolecular disulfide bridges leading to abnormal conformational changes of the VANGL2 protein. This variant was predicted to affect protein function in silico using the PolyPhen, SIFT and SNPs3D programs (Table 1). We also detected an excess of missense mutations in the NTD cohort versus controls, 1.2% (8/673) versus 0.7% (2/287) (Table 1); however, the difference was not statistically significant.
In addition, four silent rare mutations, p. Ala111Ala, p. Leu310Leu, p.Tyr414Tyr and p.Leu421Leu, were detected in the coding region of VANGL2 in NTD patients and controls (data not shown). These mutations reside away from the consensus splice junctions, suggesting that they do not affect the gene splicing. Association studies with 3 common silent SNPs, rs12086448:A>G (p.Lys379Lys), rs17380127:A>C (p.Gly445Gly) and rs17380141:G>A (p.Pro467Prol), conducted in the Italian cohort only, failed to show any statistically significant difference in either the allele or genotype frequencies between patients and controls groups (data not shown), consistent with previous studies (17).
Discussion
In this study, we conducted a mutational analysis of the open reading frame and exon-intron junctions of VANGL2 to assess its role in the pathogenesis of NTDs and to evaluate the frequency and type of mutations in this gene in a large cohort of various ethnic origins and affected with various forms of open and closed NTDs. We identified 6 novel heterozygous missense mutations that could be pathogenic based on genetic and initial validation data. Four of these mutations, p.Arg135Trp, p.Arg177His, p. Leu242Val, p.Arg270His, were predicted to be damaging to protein function using bioinformatics' tools, and 2 others, p.Thr247Met and p.Arg482His, affect highly conserved residues across evolution. Further functional studies are necessary to demonstrate the pathogenicity of these 6 rare novel variants.
Three variants, p.Leu242Val, p.Thr247Met and p.Arg270His, map to a highly conserved region near the cytoplasmic side of TM4, indicating a hotspot for NTDs-associated VANGL2 mutations. This “hotspot” region also harbors the mouse Vangl2 mutation in Lp, p.Asp255Glu, that was proven to be pathogenic using various genetic and functional assays (11).
All VANGL2 mutations occurred in sporadic cases of NTDs with no reported family history. They were private except for p.Leu242Val that was identified in two unrelated patients. Genotype-phenotype correlations were attempted in our study even with a small number of mutations. As presented in Table 2, VANGL2 potentially pathogenic mutations were identified in open (2 cases of myelomeningocele) and closed spinal NTDs (5 cases of diastematomyelia, myelocystocele, fibrolipoma or lipoma of the filum terminalis, caudal agenesis and tethered cord). This is consistent with our previous studies demonstrating a role for another PCP gene, VANGL1, in both forms of NTDs, further supporting our hypothesis that a defective PCP could represent a common mechanism contributing to the pathogenesis of both forms of NTDs (16,17). Importantly, the mutation frequency in closed spinal NTDs (2.5% or 5 in 202) was significantly different than that detected in open spina bifida (0.5% or 2 in 453) with a P value of 0.027, suggesting that VANGL2 might have a major effect in closed spinal NTDs, as compared to the open forms of NTDs. This finding needs to be confirmed independently in other NTD cohorts. In mouse models, mutations or knockouts in PCP genes have been strongly implicated in open forms of NTDs including spina bifida and craniorachischisis (6,7). No study has examined the occurrence of closed NTDs in these PCP mutants, and hence the role of a defective PCP signalling in closed forms of NTDs in mouse models has not been investigated yet.
Table 2.
Clinical features of NTD patients carrying novel rare missense mutations in VANGL2
| Sex | Mutation | Spinal dysraphism | Clinical features |
|---|---|---|---|
| M | p.Arg135Trpa | Open | Lumbo-sacral myelomenigocele, stabilized hydrocephalus, Chiari II malformation and hydromyelia, occasional dysphagia and hiccups, diplopia for convergent strabismus and nistagmus on the left eye, anal and urinary incontinence, abnormal bladder, azospermia. |
| F | p.Arg177His | Closed | Diastematomyelia type I with associated dermal sinus, hypopolasia of sacral bone, anomalies of the orientation of the 4th, 5th, 6th, 7th right rib, fusion of the 5th and 6th right ribs, malformed right ear, short neck. |
| F | p.Leu242Vala | Closed | Lumbar myelocystocele, low lying (L3-L4) conus medullaris, hydromyelia at L1 and L2, vesicoureteral reflux. |
| F | p.Leu242Val | Open | Myelomeningoceleb |
| M | p.Thr247Met | Closed | Lipoma of the filum terminalis, tethered cord and hydrocephalus |
| M | p.Arg270His | Closed | Fibrolipoma of the filum terminalis, hydrosyringomyelia cavity extending from D5 to D8, right convex dorso-lumbar scoliosis |
| F | p.Arg482His | Closed | Caudal agenesis and tethered cord, asymmetry of the epiphyseal nucleus of the femoral head, femoral varism, right lumbar convex scoliosis, schisis of the posterior arch at S1, horizontalization of the sacrum, recurrent urinary infections, bilateral dilatation of the kidney pelvis and of the ureters. |
Each of these two variants was detected in the unaffected mother. For all other variants, DNA from parents was not available.
No detailed clinical information was available on this patient.
The cohort included in this study was part of the cohort analyzed for the role of VANGL1 in NTDs (16,17). No overlapping patient carried potentially pathogenic mutations in both VANGL1 and VANGL2. The frequency of VANGL2 mutations in NTDs predicted to be pathogenic in this study (7 in 673 or 1%) is comparable to what we detected in previous studies in its homologue VANGL1 (8 in 817 or 0.9%) (16,17). The type of NTD-associated mutations we detected in both genes is also similar, where they all represent missense heterozygous mutations that were absent in all controls analyzed and affect highly conserved residues (16,17). When DNA from parents was available, one of the unaffected parents carried the VANGL2 mutation indicating incomplete penetrance for this mutation. Similar to our re-sequencing study of VANGL1 in controls, we detected in VANGL2 one missense mutation in one apparently healthy control of a potential pathogenic effect based on our initial validation criteria. These findings confirm our hypothesis that rare missense mutations in VANGL1 and/or VANGL2 represent low penetrance alleles that must interact with other genes and environmental factors to modulate the incidence and severity of NTDs.
Vangl2 has long been proven to be defective in the looptail mouse model for NTDs (11,12). Recently, three novel missense mutations were identified in VANGL2 in two fetuses affected with cranial NTDs and one fetus affected with holoprosencephaly (21). Particularly, two mutations, p.Phe437Ser, and p.Arg353Cys, were shown to affect the interaction of Vangl2 with Dvl suggesting that they are most likely pathogenic (21). We previously demonstrated that mutations in another family member VANGL1 were associated with human spinal NTDs. Here, we present genetic data that demonstrate a potential pathogenic role for VANGL2 in a subset of spinal NTD patients. The evidence is accumulating for an important contribution of PCP genes to the pathogenesis of NTDs in a subset of patients, necessitating a detailed analysis of these genes in large cohorts of human NTDs.
Acknowledgments
We thank all participants who made this study possible. This work was supported by the Canadian Institutes for Health Research (P.G.; MT-13425) (Z.K.), the SickKids Foundation (Z.K.), the Fonds de la Recherche en Santé du Québec (Z.K.), the Gaslini Foundation and Telethon-Italy (Grant no. GGP08051) (V.C.) and the National Institutes of Health (A.G.B., 1R01 NS064159-01A1). We also thank A.S.B.I. (Associazione Spina Bifida Italia).
Footnotes
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Bassuk AG, Kibar A. Genetic basis of neural tube defects. Semin Pediatr Neurol. 2009;16:101–10. doi: 10.1016/j.spen.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Botto LD, Moore CA, Khoury MJ, et al. Neural-tube defects. N Engl J Med. 1999;34:1509–19. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 3.Rossi A, Biancheri R, Cama A, et al. Imaging in spine and spinal cord malformations. Eur J Radiol. 2004;50:177–200. doi: 10.1016/j.ejrad.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 4.MRC. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin StudyResearch Group. Lancet. 1991;338:131–7. [PubMed] [Google Scholar]
- 5.De Marco P, Merello E, Mascelli S, et al. Current perspectives on the genetic causes of neural tube defects. Neurogenetics. 2006;7:201–21. doi: 10.1007/s10048-006-0052-2. [DOI] [PubMed] [Google Scholar]
- 6.Wallingford JB. Neural tube closure and neural tube defects: studies in animal models reveal known knowns and known unknowns. Am J Med Genet C Semin Med Genet. 2005;135:59–68. doi: 10.1002/ajmg.c.30054. [DOI] [PubMed] [Google Scholar]
- 7.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vladar EK, Antic D, Axelrod JD. Planar Cell Polarity Signaling: The Developing Cell's Compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 10.Keller R, Shook D, Skoglund P. The forces that shape embryos: physical aspects of convergent extension by cell intercalation. Phys Biol. 2008;5:15007. doi: 10.1088/1478-3975/5/1/015007. [DOI] [PubMed] [Google Scholar]
- 11.Kibar Z, Vogan KJ, Groulx N, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch JN, Doudney K, Paternotte C, et al. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- 13.Strong LC, Hollander WF. Hereditary Loop-tail in the house mouse. J Hered. 1949;40:329–34. [Google Scholar]
- 14.Torban E, Wang HJ, Groulx N, et al. Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem. 2004;279:52703–713. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- 15.Torban E, Patenaude AM, Leclerc S, et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci USA. 2008;105:3449–54. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kibar Z, Torban E, McDearmid JR, et al. Mutations in Vangl1 are associated with neural tube defects in humans. New Engl J Med. 2007;35:1432–7. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- 17.Kibar Z, Bosoi CM, Kooistra M, et al. Novel mutations in VANGL1 in neural tube defects. Hum Mutat. 2009;30:E706–15. doi: 10.1002/humu.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds A, McDearmid JR, Lachance S, et al. VANGL1 rare variants associated with neural tube defects affect convergent extension in zebrafish. Mech Dev. 2010;127:385–92. doi: 10.1016/j.mod.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doudney K, Ybot-Gonzalez P, Paternotte C, et al. Analysis of the planar cell polarity gene Vangl2 and its co-expressed paralogue Vangl1 in neural tube defect patients. Am J Med Genet A. 2005;136:90–2. doi: 10.1002/ajmg.a.30766. [DOI] [PubMed] [Google Scholar]
- 20.Ehrich M, Böcker S, Van den Boom D. Multiplexed discovery of sequence polymorphisms using base-specific cleavage and MALDI-TOF MS. Nucl Acids Res. 2005;33:e38. doi: 10.1093/nar/gni038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei YP, Zhang T, Li H, et al. VANGL2 mutations in human cranial neural-tube defects. N Engl J Med. 2010;62:2232–5. doi: 10.1056/NEJMc0910820. [DOI] [PubMed] [Google Scholar]