Abstract
Mechanical forces are essential to maintain skeletal integrity, and microgravity exposure leads to bone loss. The underlying molecular mechanisms leading to the changes in osteoblasts and osteoclast differentiation and function remain be to fully elucidated. Due to the infrequency of spaceflights and payload constraints, establishing in vitro and in vivo systems that mimic microgravity conditions becomes necessary. We have established a simulated microgravity (modeled microgravity, MMG) system to study the changes induced in osteoclast precursors. We observed that MMG, on its own was unable to induce osteoclastogenesis of osteoclast precursors, however, 24h of MMG activates osteoclastogenesis-related signaling molecules ERK, p38, PLCγ2, and NFATc1. RANKL (and/or M-CSF) stimulation for 3-4 days in gravity of cells that had been exposed to MMG for 24h, enhanced the formation of very large TRAP positive multinucleated (>30 nuclei) osteoclasts accompanied by an upregulation of osteoclast marker genes- TRAP and cathepsin K. To validate the in vitro system, we established the hindlimb unloading system using BALB/c mice and observed a decrease in BMD of femurs and a loss of 3D microstructure of both cortical and trabecular bone as determined by microCT. There was a marked stimulation of osteoclastogenesis as determined by the total number of TRAP positive multinucleated osteoclasts formed and also an increase in RANKL stimulated osteoclastogenesis from precursors removed from the tibias of mice after 28 days of hindlimb unloading. Contrary to earlier reported findings, we did not observe any histomorphometrical changes in the bone formation parameters. Thus, the above observations indicate that microgravity sensitizes osteoclast precursors for increased differentiation. The in vitro model system described here is potentially a valid system for testing drugs for preventing microgravity induced bone loss by targeting the molecular events occurring in microgravity-induced enhanced osteoclastogenesis.
Keywords: modeled microgravity, osteoblasts, osteoclasts, hindlimb unloading, rotary cell culture system
Introduction
Microgravity is the condition of weightlessness experienced by astronauts during spaceflights that causes severe physiological alterations in the human body, one of the most prominent of which is bone loss [1-3]. Prolonged exposure to a microgravity environment leads to bone loss at an alarming rate of approximately 0.5-2% per month in the load bearing bones [4,5], and the abnormalities reported include osteopenia [6), decreased bone formation, increased bone resorption [7-8] and decreased mineralization [9], which create an increase in fracture risk following return to earth. In particular, bone formation parameters, bone alkaline phosphatase, osteocalcin, and type I procollagen propeptide, were decreased, whereas bone resorption markers, procollagen C-telopeptide, deoxypyridinolines, and pyridinoline were increased in astronauts after 180-day spaceflight [7]. Data collected from crew members aboard the three skylab missions exhibited a 50% increase in urinary collagen cross-link products. [8].
Skeletal abnormalities due to microgravity are not exclusive to humans. Laboratory animals including rats [10-19] and mice [20-21], newts [22] and rhesus monkeys [23] have been used to study microgravity-induced bone loss. Histomorphometric investigations of rats in spaceflight showed an increase in the number of osteoclasts [18-19] and osteoclast-mediated mineral resorption was enhanced and bone formation was decreased in periosteal bones of mice [21].
Due to the infrequency of spaceflights, payload constraints, and small numbers of subjects and experiments, human and animal data related to bone loss during spaceflight is limited. Therefore, several ground based models of microgravity have been developed that simulate microgravity conditions. The –6° head-down tilt model with humans has been shown to simulate the effects of the weightless environment of space. Fourteen weeks of head-down tilt led to a decrease in bone formation markers, and an increase in urinary calcium and bone resorption markers similar to those observed in spaceflight [24]. Several ground–based studies to model microgravity affects on bone were performed using the rat hindlimb unloading model that produces fluid shifts similar to those occurring in space with minimum evidence of stress [25] as indicated by continued weight gain [26], no change in the serum corticosteroid levels and normal circadian rhythms [27]. The mouse hindlimb unloading model was first reported by Simske and co-workers [28] and has been shown to have a reduction in bone mineral density primarily affecting trabecular bone in young growing rats [25] and mice after two weeks of unloading [29].
Although the hindlimb unloading model has enabled us to study the changes occurring in the skeleton as a whole, we also need to understand the effect of the lack of mechanical strain on isolated cells. Thus, some in vitro ground based systems model microgravity (MMG) and permit investigation into the cellular alterations produced in modeled microgravity. We and others have used the Rotary Cell Culture system (RCCS) that was developed at the NASA's Johnson Space Center to model microgravity [30]. It is a double-walled rotating bioreactor wherein cells in medium rotate in the vessels as a solid body and the cells maintain their relative position creating minimal shear stress and mechanical damage of cells. Constant rotation of the biosystem as a solid body subjects cells to changing angular gravitational vector, thus approximating the highly reduced vector found in the actual space environment [31].
Ground-based models of microgravity have focused mainly on osteoblastogenesis [32], and the molecular mechanisms involved in osteoclastogenesis in microgravity have still not been elucidated. In vitro model systems have shown an indirect effect of modeled microgravity on osteoclastogenesis. It was shown that the gene expression of the receptor activator of NFkB ligand (RANKL) and osteoprotegerin is altered in the mouse stromal cell line, ST2 placed in modeled hypogravity conditions [33]. Similarly, Rucci et al reported that modeled microgravity stimulates osteoclastogenesis indirectly by an increase in RANKL/OPG ratio in osteoblasts.[34].
The aim of the present work was to determine whether modeled microgravity exposure per se directly affects the differentiation capacity of osteoclast precursor cells and, and if so, what is the underlying molecular mechanism. Also, there have been conflicting reports in terms of the effect of hindlimb unloading on bone resorption, some reporting that hindlimb unloading increases bone resorption [35] while others suggesting that bone resorption remains unaltered [36,37]. Thus, we have addressed this issue by analyzing the effect of hindlimb unloading on osteoclasts, and the ex vivo analysis of differentiation of osteoclast precursors from hindlimb unloaded mice.
Using the in vitro rotary cell culture system we have demonstrated that modeled microgravity sensitizes osteoclast precursors to differentiation by partial activation of osteoclastogenesis signaling pathways, and using the in vivo hindlimb unloading system we have validated the effect of microgravity on osteoclastogenesis of osteoclast precursors ex vivo. These results suggest a direct effect of microgravity on osteoclast precursors as one mechanism causing microgravity induced bone loss.
MATERIALS AND METHODS
In vitro experimental procedures
Cell Culture
RAW264.7, a murine macrophage cell line was purchased from the American Type Culture Collection (Mannasas, VA). Cells were maintained in Dulbecco's Modified Eagle Medium (Gibco) supplemented with 10% v/v fetal bovine serum (Invitrogen) and antibiotics at 37°C in a 5% CO2 incubator. To isolate mouse bone marrow macrophage precursors, bone marrow was flushed from the cavity of long bones and minced in alpha-modified (MEM-alpha) (Sigma). Cells were cultured in low adhesion plates for 4 days. Supernatant cells were then transferred to fresh plates and cultured in proliferation medium (1:10 parts of Macrophage colony stimulating factor in MEM-alpha).
Rotary Cell Culture System
The Rotary Cell Culture System (RCCS) was purchased from Synthecon and is composed of High Aspect Ratio Vessels (HARVs) which rotate at a set rpm. The HARVs are cylindrical growth chambers that contain an inner cylinder with a capacity of 10ml and an outer cylinder with a gaseous membrane. The rotational motion of the system prevents sedimentation, creating an optimized suspension culture capable of supporting three-dimensional cell growth on microcarrier bead scaffolds. The HARVs, used in the RCCS, have two essential components: Solid body rotation and diffusion-mediated oxygenation. Solid body rotation of the vessel, medium, microcarriers and cells results in minimal shear stress and mechanical damage of cells. Membrane oxygenation allows diffusion of gases to maintain proper growth conditions but prevents turbulence-inducing air space/bubbles [31]. These components are necessary to establish an optimized suspension culture system in microgravity conditions.
Modeled Microgravity (MMG) system for Osteoclastogenesis
2.5X105 RAW264.7 cells or mouse bone marrow macrophage precursors were incubated with 50 mg microcarrier beads (125-212 microns diameter, Solohill Engg., Ann Arbor, MI) at 37°C overnight in each well of low-adhesion 6 well plates. After overnight incubation, cells attach around the surface of beads (Figure 1A). For modeled microgravity (MMG) exposure, beads with cell aggregates in 10ml fresh complete DMEM were placed in the vessels of the rotating bioreactor located in an incubator. The vessel was rotated at 9 rpm (speed previously determined by using fluorescent beads so that they remain in suspension) for 24h. Parallel bead-attached cells were cultured in gravity (G) in low-adhesion plates and in order to have the same density of cells, cells and beads from one well were divided into 3 wells with a total medium of 10ml for all the three wells. All cultures were carried out for 24h and then cells were separated from the beads. Cells were either used to prepare cell lysates or were cultured on normal culture plates with RANKL (10ng/ml) and/or M-CSF.
Fig. 1.

Modeled microgravity system does not alter cell viability. Figure (A) shows the RAW264.7 cells attach around the surface of microcarrier beads in low adhesion culture plates after overnight incubation. The figure shows the cell and beads aggregates at 10X microscopic magnification (Scale bar=100um). Arrows point to the clusters of bead-attached cells. Figure (B) RAW264.7 cells were exposed to modeled microgravity (MMG) and gravity control (G) for 24h and subjected to AnnexinV-PI staining. Values were obtained from three independent experiments and represent mean ± SE. NS=Not Significant.
TRAP staining
After the 24h incubation in G and MMG, cells were separated from the beads, the cells were counted, and 2.5X105 cells were seeded in each well of 6-well plate for stimulation with RANKL (and M-CSF for mouse bone marrow macrophages) and subsequent TRAP staining. After 4 days (for RAW 264.7 cells) or 5-6 days (for mouse bone marrow macrophages, cells were fixed with citrate-acetone-formaldehyde for 2 minutes, and then stained for tartrate-resistant acid phosphatase (TRAP), using a commercially available staining kit (Sigma). Dark red TRAP stained cells containing three or more nuclei were counted as osteoclasts. For further quantification, multinucleated TRAP-positive cells with different number of nuclei were counted in three different categories: 3 to 6 nuclei, 6 to 30 nuclei, and those with greater than 30 nuclei.
Apoptosis Assay
The percentage of apoptotic cells was measured by using an annexin V-FITC apoptosis kit (Biosource). Cells were detached from the beads by vortexing for 5-10 seconds after 24h of MMG or G exposure and Annexin V-FITC was added to the culture medium at a final concentration of 3μg/ml and subsequently incubated for 3 min at room temperature. Cells were collected with the culture supernatant, pelleted by centrifugation and washed twice with culture medium in order to remove excess annexin V-FITC and resuspended in culture medium to a final concentration of 1×106 cells/ml. Prior to flow cytometric analysis, propidium iodide was added to a final concentration of 5μg/ml. A minimum of 10,000 cells per sample were analyzed using flow cytometry. Apoptotic cells were those that were stained for Annexin-V.
Whole cell and nuclear lysate preparation
Whole cell lysates were prepared from cells which were washed with chilled Phosphate Buffered Saline (PBS) and lysed in RIPA buffer [50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitors (PMSF (5mM), pepstatin (1μg/ml), TPCK (70μg/ml), leupeptin (0.5μg/ml), spermidine (1mM) and spermine (1mM) and phosphatase inhibitors (Sigma, MO). Nuclear lysates were prepared from cells as follows: cells were washed with chilled PBS and centrifuged at 800 X g for 5 minutes at 4°C. Nuclear and cytoplasmic lysates were prepared using the NE-PER kit (Pierce) following the manufacturer's instructions. Protein concentration was quantitated using the Pierce BCA protein assay detection system.
Western Blotting
Proteins were resolved by 10% SDS–PAGE and transferred to nitrocellulose membranes. Blots were probed with the primary antibody as indicated for phosphorylated Extracellular Related Kinase (phospho-ERK), total ERK (ERK), phosphorylated c-Jun N-terminal Kinase (phospho-JNK), total JNK (JNK) , phosphorylated p-38 (phospho-p-38), total p-38 (p-38) (Cell Signaling technology); c-fos, and NFATc1 (Santa Cruz Biotechnology). After incubating with the primary antibody overnight at 4°C, membranes were washed and incubated with the appropriate Horse Radish Peroxidase-conjugated secondary antibody for 1 h at room temperature. Membranes were then stripped by incubation with the stripping solution (Pierce) for 15-20 min at room temperature and reprobed as described above to detect other antibodies. Actin antibody (Santa cruz) was used as the loading control. Protein bands were developed by ECL-Advance (Amersham) and visualized in FujiFilm LAS-3000 imager.
RT-PCR and Real Time PCR
Total RNA was extracted using the Trizol procedure. One microgram of RNA was reverse transcribed using M-MLV reverse transcriptase and the equivalent of 0.1μg was used for the PCR reactions. Real-time PCR was performed in MyIQ Biorad single color Real-time PCR detection system using SYBR1 Green dye. The data were evaluated from the MyIQ reports using Microsoft excel. Melting curves and gel analyses were used to verify single products of the appropriate base pair size. RT-PCR was performed with the primers listed in Table 1.
TABLE 1.
PCR PRIMERS
| Primer | Forward | Reverse |
|---|---|---|
| mTRAP | 5′-CGATCACAATCTGCAGTACC-3′ | 5′-ACCCAGTGAGTCTTCAGTCC-3′ |
| mCathepsin K | 5′-CCAGTGGGAGCTATGGAAGA-3′ | 5′-AAGTGGTTCATGGCCAGTTC-3′ |
| m18S | 5′-CGCCGCTAGAGGTGAAATTCT-3′ | 5′-CGAACCTCCGACTTTCGTTCT-3′ |
| mCalcitonin R | 5′-GACAACTGCTGGCTGAGTG-3′ | 5′-GAAGCAGTAGATAGTCGCCA-3′ |
| c-fos | 5′-CTCCCGTGGTCACCTGTACT-3′ | 5′-TTGCCTTCTCTGACTGCTCA-3′ |
| NFATc1 | 5′-CTTCCAGCCTGTCTTCTTGG-3′ | 5′-TGCAAACACAAGCTCTGTCC-3′ |
| RANK | 5′- CAGGACAGGGCTGATGAGAG -3′ | 5′-TGGCTGACATACACCACGATG-3′ |
Animals and experimental procedures
Hindlimb Unloading (HLU) model
6-7 week old male wild-type BALB/c mice were (Harlan Laboratories) were kept under controlled conditions at 24°C on 12:12 light/dark cycles with the light cycle. 12 mice were used and divided into 2 groups (6 mice per group) - control or loaded (CON) and hindlimb unloaded (HLU). All experiments were conducted according to the institutional guidelines for animal welfare. The mice were subjected to hindlimb unloading by tail suspension as described previously with some modifications [29]. Briefly, a steel wire was attached to the entire length of the dorsal surface of the tail with the help of cyanoacrylate glue. A hypoalergic tape was loosely wound around the wire attached to the tail. The other end of the wire was connected to a swivel that would allow 360° rotation of mouse standing at one point. The swivel was attached to a fishing leader that could slide over a horizontal bar at the top of the cage enabling further movement from one end of the cage to another. The height of the bar was adjusted to maintain the body in a 30° tilt with the hindlimbs elevated above the floor of the cage. Mice could easily move around on the cage surface, although they could not lean on walls with their hind limbs. All mice were acclimatized for 1 week before hindlimb unloading and had free access to food pellets. Water was provided in the petri dishes that were changed twice a day. The mice in the unloading group (HLU) were subjected to unloading for 4 weeks. Loaded control mice (CON) were also housed individually under the same conditions except for tail suspension. After 4 weeks of tail suspension, DEXA analysis of femurs was performed following which they were anesthetized with pentobarbital and then sacrificed by cervical dislocation. The femora and tibias were separated from adherent muscles and connective tissues. The femurs were used for histomorphometry and µCT analysis, respectively, after filtration in a 10% neutral buffered formalin solution for 48 h, rinsed with distilled water, and stored in 70% ethanol.
Ex vivo cell analysis
The tibiae were used to flush out bone marrow cells for ex-vivo cell analysis. Mouse bone macrophages were generated from the bone marrow cells and then allowed to differentiate after stimulation with RANKL (20ng/ml) and M-CSF as described earlier in materials and methods. After 4 days of stimulation, half of the cells were used for RNA extraction and cDNA preparation. RT-PCR was performed with the cDNA to determine the levels of osteoclastogenesis markers TRAP, Cathepsin K, Calcitonin Receptor and 18S. After 5 days of stimulation, the other half of cells were TRAP stained and total number of osteoclasts (>3 nuclei) was counted per field using a light microscope.
Measurement of bone mineral density
Bone mineral densitiy of the entire femora were measured by dual-energy X-ray absorptiometry (DXA) using Lunar PIXImus densitometer (GE Lunar Corp, Madison, WI) and expressed as an observed mean (g/cm2) +/- the Standard Deviation within the whole group.
Micro-CT
After euthanizing the animals, femurs were removed and dissected free of soft tissue. The bones were then fixed in 10% v/v formaldehyde and analyzed by μCT using the manufacturer's included 3-D analysis software (μCT 40, Scanco Medical, Basserdorf, Switzerland). The region of interest analyzed was the metaphysis of the proximal femur, for a total of 12 specimens scanned. The trabecular bone just distal to the growth plate in femurs was scanned using a 6-μm slice increment to obtain 209 slices out of which 100 slices were selected in the typical are. For cortical bone, scanning was performed starting at 6.5 mm away from the distal of femur to obtain 25 slices.
Quantitative Histomorphometry
The left femurs of mice were bisected transversely at the midpoint of the shaft. Femora were fixed in 10% v/v buffered formalin, decalcified in EDTA, embedded in paraffin, sectioned and stained for TRAP. Furthermore, tissues were fixed, embedded in methyl methacrylate, sectioned and stained for Von Koss. Quantitative histomorphometric analysis was performed using the area at least 0.5mm below the growth plate, excluding the primary spongiosa and trabecular connected cortical and represented according to the rules described by Parfitt et al [38].
Statistics
Data are expressed as the mean standard error (SE) of at least three independent experiments each performed in triplicate wells. Statistical analysis was performed by the paired Student's t-test in Microsoft Excel. A p value <0.05, was considered statistically significant.
Results
Modeled Microgravity studies
Modeled Microgravity system does not alter medium properties
Medium properties were measured after 24h of MMG or G exposure of RAW264.7 cells and mouse bone marrow macrophages. The pH, pO2 and pCO2 of the medium were collected anaerobically and assayed at 37°C by a Radiometer ABL blood analyzer 700 Series. The pO2 mmHg (G = 176 ± 4.0, MMG = 178.5 ± 14.5); pCO2 mmHg (G = 44.3 ± 2.3, MMG = 49.05 ± 1.6); and pH (G = 7.46 ± 0.05; MMG = 7.42 ± 0.01) of the medium used for culturing RAW264.7 cells, were unaltered in the MMG apparatus. To investigate whether the MMG system alters cell viability, apoptosis was assayed. Exposure in the RCCS for 24h does not stimulate apoptosis in RAW264.7 cells (Figure 1B) or mouse bone marrow macrophages (data not shown). Cells incubated for more than 24h dissociated from the beads; therefore, incubations in MMG greater than 24h were not possible.
MMG exposure activates osteoclastogenesis pathways partially in osteoclast precursors without RANKL treatment
MMG exposure for 24h (with no RANKL treatment) did not lead to stimulation of osteoclastogenesis marker genes. The mRNA levels of osteoclastogenesis marker genes- TRAP (Tartrate Resistant Acid Phosphatase) and Cathepsin K remained undetectable after 24 exposure of MMG, similar to that observed in G (data not shown). Interestingly, there was increased phosphorylation of ERK, p-38 and PLCγ2 after MMG exposure in RAW264.7 cells as compared to G exposed cells. JNK phosphorylation remained unaffected (Figure 2). In addition, we also observed that MMG exposure led to an increase in the mRNA (Figure 3A), protein expression (Figure 3C) and nuclear translocation of NFATc1 (Figure 3E) after MMG. Levels of c-fos, however, remain unaltered (Figure 3A and 3C). There was no significant difference in RANK (RANKL receptor) expression as determined by mRNA (Figure 3B) and protein expression (Figure 3D) after 24h in MMG as compared to that in G.
Fig. 2.

Modeled microgravity activates ERK, p-38 and PLCγ2. RAW264.7 cells were cultured on microcarrier beads exposed to MMG and G for 24h. Whole cell lysates were prepared from cells. Western blots analysis was performed for phosphorylated ERK, total ERK, phosphorylated JNK, total JNK, phosphorylated p-38, phosphorylated PLCγ2, total PLCγ2 and Actin. The figure is representative of 3 independent experiments.
Fig. 3.
Modeled microgravity activates NFATc1 and has no effect on c-fos and RANK expression. RAW264.7 cells were exposed to MMG and G for 24h. RNA was isolated and cDNA was prepared followed by RT-PCR for NFATc1, c-fos, RANK and 18S. Figure (A) shows the RT-PCR for NFATc1, c-fos and 18S. Figure (B) shows the RT-PCR for RANK and 18S. Whole cell lysate was prepared from MMG and G exposed cells and western blot analysis was performed. Figure (C) shows that western blot for NFATc1, c-fos and Actin. Figure (D) shows the western blot for RANK and Actin. Nuclear and Cytoplasmic lysates were prepared from MMG and G exposed cells. Figure (E) shows the western blot for NFATc1, and Actin for cytoplasmic and nuclear lysates. Each figure is a representative of 3 independent experiments.
Osteoclastogenesis after MMG exposure
After 24h MMG exposure of RAW264.7 cells and mouse bone marrow macrophage precursors, the cells were subsequently cultured with RANKL in G for 3 days (RAW264.7 cells) or 4 days (mouse bone marrow macrophages). This treatment resulted in an increase in the mRNA expression of osteoclastogenesis marker genes, TRAP and Cathepsin K (Figure 4). After RANKL stimulation, MMG exposed osteoclast precursors formed significantly higher number of multinucleated osteoclasts especially those with greater than 30 nuclei as compared to the G exposed cells (Figure 5).
Fig. 4.
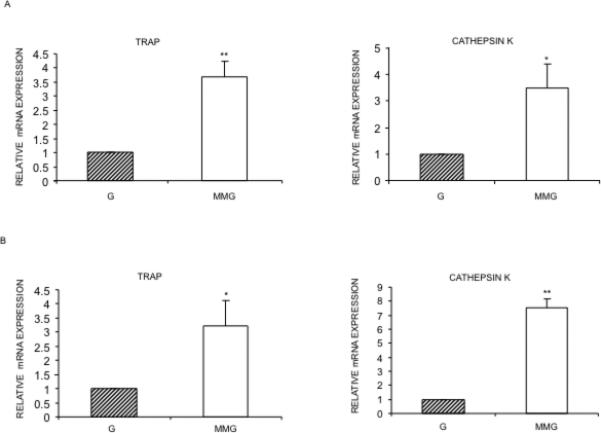
Modeled microgravity mediated TRAP and Cathepsin K expression after RANKL treatment. RAW264.7 cells (A) and mouse bone marrow macrophages (B) exposed to MMG and G for 24h. Cells were separated from the beads and incubated with 10ng/ml RANKL in G for 1 day. RNA was isolated and cDNA prepared followed by quantitative real-time PCR for TRAP, Cathepsin K and 18S. The left panels show the levels of TRAP relative to 18S and right panels shows the level of Cathepsin K relative to 18S, in RAW264.7 cells. Relative gene expression (mean ± SEM) was obtained by normalizing to 18S expression and plotting on a log scale. *p≤ 0.05, **p≤ 0.001.
Fig. 5.

Modeled microgravity stimulates RANKL mediated osteoclastogenesis. RAW264.7 cells (A) and mouse bone marrow macrophages (B) were exposed to MMG and G for 24h. Cells were separated from the beads. RAW264.7 cells (A) were incubated with 10ng/ml RANKL in gravity for 5 days followed by TRAP staining. Mouse bone marrow macrophages (B) were incubated with 10ng/ml RANKL and M-CSF for 5 days. TRAP staining and counting of multinucleated osteoclasts were performed. TRAP positive osteoclasts with 6 to 30 nuclei, and more than 30 nuclei were counted. Values were obtained from three independent experiments and represent mean ± SE. **p≤ 0.005, *p≤ 0.05, NS=Not Significant.
Hindlimb Unloading Studies: Validation of the model
Mean body weight
There was no significant change observed in the body weight of mice during the course of hindlimb unloading. The mean weights of the loaded control group (23.3±3.9) was not found to be statistically different from the hindlimb unloaded group (21.1±1.1g) at the end of 28 days of study.
Hindlimb unloading leads to loss in BMD
To analyze how 28 days of hindlimb unloading affects the bone of BALB/c mice, we first compared the bone mineral density (BMD) of femurs of loaded control and hindlimb unloaded mice by DXA analysis. As expected, hindlimb unloading lead to a significant reduction in the BMD of the femurs as compared to that of the loaded control mice (Figure 6A).
Fig. 6.
DEXA and micro-CT analysis. Figure (A) shows BMD that was measured by DXA in the femurs of loaded control (CON) hindlimb unloaded (HLU) mice after 28 days. Data are the mean ±SEM (n=6). **p<0.001 versus age-matched loaded controls. Micro-CT analysis was performed on trabecular and cortical bones. Figure (B and C) shows representative 3D images by μCT of trabecular bone at the proximal femur of loaded control (CON) and hindlimb unloaded (HLU) male mice. Figures (D and E) show representative 3D images by μCT of cortical bone at the femur of loaded control (CON) and hindlimb unloaded (HLU) male mice. Figure (F) shows comparison of 3D trabecular bone volume fraction (BV/TV) between CON and HLU mice. n=3 **p<0.001. Figure (G) shows comparison of 3D cortical bone volume fraction (BV/TV) between CON and HLU mice. n=3 *p<0.05.
Hindlimb unloading leads to loss of 3D microstructure of femurs
Most reports suggest that hindlimb unloading mainly affects the trabecular bone in growing rats and mice [29,37]. The effect 28 days of hindlimb unloading on 3D microstructure of bone was studied using μCT that showed hindlimb unloading leads to a significant loss in the 3D microstructure at both the trabecular (Figure 6B and 6C) and cortical bones (Figure 6D and 6E). The effect was quantitated in terms of the bone volume per fraction of the total volume at the trabecular and cortical regions (Figure 6F and 6G).
Histomorphometry
Histomorphometry was used to determine whether the decrease in bone was due to an decrease in bone formation, or an increase in bone resorption or both. Histomorphometrical analysis of femurs of loaded control and hindlimb unloaded mice revealed that there was a significant increase in the number of osteoclasts ((N.Ob/BS), loss of number of trabeculae (Tb.No.), decrease in trabecular thickness (Tb.Th.), and an increase in trabecular space (Tb.Sp.). Bone formation parameters (BFR and MAR) and the number of osteoblasts (N.Ob/BS) remained unchanged (Table 2).
TABLE 2.
COMPARISON OF HISTOMORPHOMETRIC PARAMETERS OF 28 DAYS LOADED CONTROL AND HINDLIMB UNLOADED MICE
| Tb.Th(μm) | Tb. N(1/mm) | Tb.Sp (μm) | N.Oc/BS | N.Ob/BS | MAR (μm/d) | BFR(μm/d) | |
|---|---|---|---|---|---|---|---|
| CON (n=6) | 34.1±3.2 | 4.14±0.3 | 218.67±54.4 | 0.49±0.198 | 8.56±3.335 | 2.18±0.419 | 0.40±0.2 |
| HLU (n=6) | 27.41±1.4* | 2.36±0.2* | 406.75±75.2* | 0.80±0.3+ | 9.08±2.7 | 1.97±0.5 | 0.41±0.2 |
| p | 0.005 | 0.0007 | 0.0005 | 0.013 | 0.77 | 0.429 | 0.957 |
Tb.Th, Trabecular thickness; Tb.N, Trabecular number; Tb.Sp, Trabecular Space; N.Oc/BS, Number of osteoclasts per bone surface; N.Ob/BS, Number of osteoblasts per bone surface; MAR, Mineralized apposition rate; BFR, Bone formation rate.
p≤0.005
p ≤0.05 difference between CON and HLU groups.
Ex vivo cell analysis
Bone marrow cells were flushed from the tibiae of loaded control and hindlimb unloaded mice after 28 days (3 mice per group). Mouse bone marrow macrophages were prepared as described earlier [30] and then stimulated with 10ng/ml RANKL and M-CSF for 5 days. Multinucleated osteoclasts formed were TRAP stained and counted. Similar to the in vitro results (Figure 5B), there was an increase in the number of TRAP positive multinucleated osteoclasts (Figure 7A). However, in this case, the effect was on the total number of multinucleated osteoclasts unlike the in vitro observations where the effect was prominently on at larger multinucleated osteoclasts (with >30 nuclei).
Fig. 7.
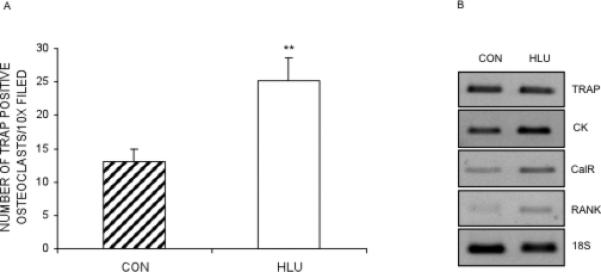
Hindlimb unloading stimulates osteoclastogenesis in ex vivo culture of tibial mouse bone marrow macrophages. Bone marrow macrophages were prepared from the bone marrow cells that were flushed from the tibia of loaded control (CON) and hindlimb unloaded (HLU) mice and cultured with RANKL (20ng/ml) and M-CSF. Figure (A) shows the number of TRAP positive multinucleated cells counted after 5 days of culture. Data is the mean ±SEM (n=5). **p<0.001. Figure (B) shows the RT-PCR performed osteoclastogenesis markers TRAP, CK (Cathepsin K), CalR (Calcitonin Receptor); receptor RANK and 18S after cDNA was prepared from RNA extracted from the cells after 4 days of culture. Data are the representative of 3 independent experiment.
Osteoclastogenesis markers were determined by RT-PCR and after 4 days of ex vivo culture of mouse bone marrow macrophages with RANKL and M-CSF. There was an increase in the expression of Cathepsin K and Calcitonin Receptor. However, contrary to what we observed in vitro (Figure 4A and 4B); there was no change in the level of TRAP mRNA expression (Figure 7B). Interestingly, RANK was also increased.
Discussion
A number of spaceflight studies indicate that bone loss is due to a decrease in osteoblasts and an increase in osteoclasts. Using ground based cell culture systems; the decrease in osteoblasts has been characterized and studied in depth [39-42]. In contrast, very few studies have focused on the direct effect of modeled microgravity on osteoclasts. We have established a ground-based system to study the direct effect of modeled microgravity on osteoclastogenesis and to investigate the molecular mechanism(s) involved. Our results show that short term (24h) modeled microgravity exposure of osteoclast precursors enhances their sensitivity to subsequent treatment with RANKL which stimulates them to form giant multinucleated osteoclasts and an increase in osteoclastogenesis markers TRAP and Cathepsin K. The effect is mediated by a RANKL-independent partial activation of osteoclastogenesis pathways NFATc1, ERK, p-38, and PLCγ2. NFATc1 is regarded as the master regulator of osteoclastogenesis. Once activated, it translocates into the nucleus and acts as a transcription factor for several osteoclast-specific genes. It is activated after RANKL stimulation of osteoclast precursors and when ectopically overexpressed, is sufficient to induce osteoclastogenesis [43]. However, despite NFATc1 activation by MMG, osteoclastogenesis was not enhanced without RANKL treatment. This could be due to the following: First, osteoclast precursors have been exposed to modeled microgravity for only 24h, and not for the normal duration of osteoclastogenesis which is 96h. Thus, the changes that are being induced during the short span of 24h of modeled microgravity conditions might revert back once the cells are plated in the absence of RANKL in normal gravity conditions. Second, it might be due to the fact that c-fos remains unaltered in modeled microgravity and c-fos activation may be necessary to stimulate spontaneous osteoclastogenesis. Cooperative interaction between NFAT and AP-1 (composed of jun and fos) on specific DNA binding sites is required for the transcription of certain osteoclast specific genes. C-fos-/- osteoclast precursors that overexpress NFATc1, do not form osteoclasts in the absence of RANKL [44].
Interestingly, we did not observe any changes in the RANK expression which indicates that there may be involvement of co-stimulatory pathways such as ITAM-motif bearing adaptor molecules DAP12 and its receptor TREM2 that are expressed on osteoclast precursors and, like RANK, have also been shown to activate PLCγ2 [45]. Differentiation of osteoclast precursors has recently been shown to be stimulated during 10 days of spaceflight, however, unlike our study, these experiments have been performed in the presence of M-CSF and RANKL [46]. Monici et al have reported that there is a direct stimulation of human osteoclast-precursor like cells, FLG29.1 in modeled microgravity [47,48]. Contrary to these results, we did not see any direct stimulation of osteoclastogenesis in modeled microgravity. The disparity in the results could be explained by the use of different in vitro systems, different cell-types, and different duration of exposure and/or absence of beads in their system.
Activation of PLCγ2 is known to lead to an increase in intracellular calcium levels by inducing the release of endoplasmic reticulum calcium stores. Thus, intracellular calcium signaling might be a generalized cellular response of cells to microgravity and might explain a number of physiological changes observed in microgravity. Intracellular Ca2+ is known to bind to calmodulin which undergoes a conformational change and induces the activation of several downstream effector proteins including a serine/threonine phosphatase, calcineurin and calmodulin dependent protein kinase II (CaMKII). We have shown previously that CaMKII is involved in osteoclastogenesis [49] and calmodulin is essential for osteoclast activity [50]. Calcineurin is involved in NFATc1 activation and its inhibitors, Cyclosporin A and FK506, have been shown to inhibit osteoclastogenesis in RAW264.7 cells [43], and stimulate osteoblastogenesis in mice [51]. The sensitization of osteoclast precursors cells to RANKL mediated osteoclastogenesis as observed here, and the inhibition of osteoblastogenesis as previously shown by our laboratory in the MMG environment [40-41], could possibly be reversed by calcineurin inhibitors when used at appropriately low concentrations. The effect of calcineurin inhibitors on microgravity induced bone loss should be tested in the established in vitro (rotary cell culture system) and in vivo (mouse hindlimb unloading model) systems.
The mouse hindlimb unloading system has been used by several groups to define the role of bone cells in microgravity induced bone loss. The role of osteocytes as mechanosensors has been emphasized. Aguirre et al [52] found that hindlimb unloading initially leads to osteocyte apoptosis followed by osteoclast recruitment leading to bone loss. Fourteen days of hindlimb unloading of rats resulted in a decrease in the bone mineral density at the tibial metaphysis due to enhancement of bone resorption as determined by an early increase of urinary resorption marker deoxypyridinoline (in seven days) and an increase in the number of osteoclasts as determined by histomorphometry [53].
It has been observed in several unloading studies that bone formation is decreased as detrmined by serum markers of bone formation [54,55]. In one study, bone histomorphometric analysis revealed that unloading leads to a decrease in bone osteoblast surface and bone collagen density indicating a decrease in bone formation parameters [56]. However, we did not observe any significant reduction in bone formation parameters by histomorphometry (Table 2). This descripancy in the results might be due to the fact that, unlike other studies that were performed with mature rodents, we used rapidly growing mice. Li XJ et al [57] have observed by histomorphometry that cancellous bone loss occurred rapidly before 10 weeks of unloading and stabilized at 50% less bone mass after 18 weeks in rats, indicating that bone resorption might be the primary cause of bone loss in the initial periods of unloading and later there is also a decrease in bone formation parameters. Thus, it is possible that we might also have observed a decrease in bone formation parameters if the unloading duration was increased in mice.
Although a number of hindlimb unloading studies have been performed none of them have compared results with an in vitro MMG system. The effect of MMG on osteoclast precursors in our hands is verified in the in vivo hindlimb system. For this purpose, we isolated mouse bone marrow macrophages from the hindlimbs of unloaded mice and cultured them ex vivo and found that they formed more multinucleated TRAP positive osteoclasts than those isolated from the loaded control mice. There was also an increase in the expression of osteoclastogenesis markers cathepsin K and calcitonin receptor but no change in TRAP expression. Although, there is a discrepancy in the effect of HLU and MMG on TRAP expression, this could be because TRAP is an early marker of osteoclastogenesis and although it was measured early on (day 1) after RANKL treatment in MMG exposed cells, TRAP levels were analyzed after 4 days of RANKL treatment in mouse bone macrophages cultured from HLU mice.
Therapies for preventing hindlimb unloading induced bone loss have mainly used bisphosphonates that suppress resorption. Recently, Lloyd et al [58] have showed that the use of a low-dose bisphosphonate, Zolendronic acid in conjunction with Osteoprotegerin prevents bone loss occurring in mice due to 28 days of hindlimb unloading. A study by Bikle et al administered Alendronate alone to suppress unloading-induced bone loss in rats which targets resorption [35]. Very few anabolic studies have been designed that target bone formation pathways for preventing unloading induced bone loss. However, one such study by Turner et al [59] which used intermittent parathyroid hormone at a human therapeutic dose prevented the skeletal changes associated with hindlimb unloading in skeletally mature (6 months old) male rats which was accompanied by an increase of serum markers of bone formation. Although in a gravity environment intermittent administration of parathyroid hormone increases bone mass by increasing formation over resorption whereas continuous administration induces bone loss through large increases in resorption [60,61], Ono et al have observed that constitutively active PTH/PTHrP signaling in osteoblasts was able to suppress unloading-induced bone loss in mice [62]. The mechanism appears to involve osteoblast-mediated regulation of osteoclastic resorption activity. This response of bone in constitutively active PTH receptor suggests a different mechanism of bone loss is involved in unloading. Furthurmore, Baek et al [63] showed that blocking β-adrenergic receptors and replacing Leptin was able to prevent hindlimb unloading unduced bone loss.
A recent study by Lin et al [64] implicates the role of osteocytes in unloading induced bone loss. Here authors present evidence that secretion of sclerostin by osteocytes is increased in unloading. Sclerostin, an inhibitor of Wnt/β-catenin signaling, inhibits osteoblastogenesis. By using the in vitro sytem we have established that both osteoblast and osteoclast precursors show a direct response to reduced gravity. We propose that these direct effects of microgravity occur in addition to the activation of the osteocyte/sclerostin axis. Also, from our studies it would appear that use of our in vitro MMG systems can yield valuable insight into the mechanisms of cellular alterations occurring in microgravity conditions that in turn could lead to identifying novel drug targets. Our in vitro systems (validated by in vivo system), can also be used as drug assessment test system in which drugs that would affect bone formation and resorption differentially can be tested.
Supplementary Material
Supplementary figure: RAW264.7 cells were incubated for overnight in low-adhesion plates. Cells attached around the beads which were then incubated in G and MMG for 24h. Figure shows that the cells remain attached around the beads after incubation(10X magnification).
Acknowledgements
This work was supported by National Institute of Health Grants R01 AR050235 and P30 AR046031 and a NASA grant, NNJ04HB27G (NAG 9-1562).
References
- 1.Blanc S, Normand S, Ritz P, Pachiaudi C, Vico L, Gharib C, Gauquelin-Koch G. Energy and water metabolism, body composition, and hormonal changes induced by 42 days of enforced inactivity and simulated weightlessness. J Clin Endocrinol Metab. 1998;83:4289–4297. doi: 10.1210/jcem.83.12.5340. [DOI] [PubMed] [Google Scholar]
- 2.Fowler JF., Jr Physiological changes during spaceflight. Cutis. 1991;48:291–295. [PubMed] [Google Scholar]
- 3.Vernikos J. Human physiology in space. Bioessays. 1996;18:1029–1037. doi: 10.1002/bies.950181215. [DOI] [PubMed] [Google Scholar]
- 4.Collet P, Uebelhart D, Vico L, Moro L, Hartmann D, Roth M, Alexandre C. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20:547–551. doi: 10.1016/s8756-3282(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 5.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 6.Vico L, Lafage-Proust MH, Alexandre C. Effects of gravitational changes on the bone system in vitro and in vivo. Bone. 1998;22:95S–100S. doi: 10.1016/s8756-3282(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 7.Caillot-Augusseau A, Lafage-Proust MH, Soler C, Pernod J, Dubois F, Alexandre C. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95). Clin Chem. 1998;44:578–585. [PubMed] [Google Scholar]
- 8.Smith SM, Nillen JL, Leblanc A, Lipton A, Demers LM, Lane HW, Leach CS. Collagen cross-link excretion during space flight and bed rest. J Clin Endocrinol Metab. 1998;83:3584–3591. doi: 10.1210/jcem.83.10.5169. [DOI] [PubMed] [Google Scholar]
- 9.Smith SM, Wastney ME, O'Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J Bone Miner Res. 2005;20:208–218. doi: 10.1359/JBMR.041105. [DOI] [PubMed] [Google Scholar]
- 10.Abram AC, Keller TS, Spengler DM. The effects of simulated weightlessness on bone biomechanical and biochemical properties in the maturing rat. J Biomech. 1988;21:755–767. doi: 10.1016/0021-9290(88)90284-9. [DOI] [PubMed] [Google Scholar]
- 11.Vico L, Chappard D, Alexandre C, Palle S, Minaire P, Riffat G, Novikov VE, Bakulin AV. Effects of weightlessness on bone mass and osteoclast number in pregnant rats after a five-day spaceflight (COSMOS 1514). Bone. 1987;8:95–103. doi: 10.1016/8756-3282(87)90077-9. [DOI] [PubMed] [Google Scholar]
- 12.Wronski TJ, Morey ER. Skeletal abnormalities in rats induced by simulated weightlessness. Metab Bone Dis Relat Res. 1982;4:69–75. doi: 10.1016/0221-8747(82)90011-x. [DOI] [PubMed] [Google Scholar]
- 13.Morey ER, Baylink DJ. Inhibition of bone formation during space flight. Science. 1978;201:1138–1141. doi: 10.1126/science.150643. [DOI] [PubMed] [Google Scholar]
- 14.Spengler DM, Morey ER, Carter DR, Turner RT, Baylink DJ. Effects of spaceflight on structural and material strength of growing bone. Proc Soc Exp Biol Med. 1983;174:224–228. doi: 10.3181/00379727-174-41729. [DOI] [PubMed] [Google Scholar]
- 15.Turner RT, Wakley GK, Szukalski BW. Effects of gravitational and muscular loading on bone formation in growing rats. Physiologist. 1985;28:S67–S68. [PubMed] [Google Scholar]
- 16.Wronski TJ, Morey ER. Recovery of the rat skeleton from the adverse effects of simulated weightlessness. Metab Bone Dis Relat Res. 1983;4:347–352. [PubMed] [Google Scholar]
- 17.Davis BA, Sipe B, Gershan LA, Fiacco GJ, Lorenz TC, Jeffrey JJ, Partridge NC. Collagenase and tissue plasminogen activator production in developing rat calvariae: normal progression despite fetal exposure to microgravity. Calcif Tissue Int. 1998;63:416–422. doi: 10.1007/s002239900550. [DOI] [PubMed] [Google Scholar]
- 18.Vico L, Bourrin S, Genty C, Palle S, Alexandre C. Histomorphometric analyses of cancellous bone from COSMOS 2044 rats. J Appl Physiol. 1993;75:2203–2208. doi: 10.1152/jappl.1993.75.5.2203. [DOI] [PubMed] [Google Scholar]
- 19.Kaplansky AS, Durnova GN, Burkovskaya TE, Vorotnikova EV. The effect of microgravity on bone fracture healing in rats flown on Cosmos-2044. Physiologist. 1991;34:S196–S199. [PubMed] [Google Scholar]
- 20.Wronski TJ, Morey ER. Alterations in calcium homeostasis and bone during actual and simulated space flight. Med Sci Sports Exerc. 1983;15:410–414. [PubMed] [Google Scholar]
- 21.Van Loon JJ, Bervoets DJ, Burger EH, Dieudonné SC, Hagen JW, Semeins CM, Doulabi BZ, Veldhuijzen JP. Decreased mineralization and increased calcium release in isolated fetal mouse long bones under near weightlessness. J Bone Miner Res. 1995;10:550–557. doi: 10.1002/jbmr.5650100407. [DOI] [PubMed] [Google Scholar]
- 22.Berezovska OP, Rodionova NV, Grigoryan EN, Mitashov VI. Changes in the numbers of osteoclasts in newts under conditions of microgravity. Adv Space Res. 1998;21:1059–1063. doi: 10.1016/s0273-1177(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 23.Zerath E, Holy X, Andre C, Renault S, Noel B, Delannoy P, Hott M, Marie PJ. Effects of Bion 11 14-day space flight on monkey iliac bone. J Gravit Physiol. 2000;7:S155–S156. [PubMed] [Google Scholar]
- 24.Scheld K, Zittermann A, Heer M, Herzog B, Mika C, Drummer C, Stehle P. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin Chem. 2001;47:1688–1695. [PubMed] [Google Scholar]
- 25.Morey-Holton ER, Globus RK. Hindlimb unloading of growing rats: a model for predicting skeletal changes during space flight. Bone. 1998;22:83S–88S. doi: 10.1016/s8756-3282(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 26.Globus RK, Bikle DD, Morey-Holton E. Effects of simulated weightlessness on bone mineral metabolism. Endocrinology. 1984;114:2264–2270. doi: 10.1210/endo-114-6-2264. [DOI] [PubMed] [Google Scholar]
- 27.Halloran BP, Bikle DD, Cone CM, Morey-Holton E. Glucocorticoids and inhibition of bone formation induced by skeletal unloading. Am J Physiol. 1988;255:E875–E879. doi: 10.1152/ajpendo.1988.255.6.E875. [DOI] [PubMed] [Google Scholar]
- 28.Simske SJ, Guerra KM, Greenberg AR, Luttges MW. The physical and mechanical effects of suspension-induced osteopenia on mouse long bones. J Biomech. 1992;25:489–499. doi: 10.1016/0021-9290(92)90089-j. [DOI] [PubMed] [Google Scholar]
- 29.Sakata T, Sakai A, Tsurukami H, Okimoto N, Okazaki Y, Ikeda S, Norimura T, Nakamura T. Trabecular bone turnover and bone marrow cell development in tail-suspended mice. J Bone Miner Res. 1999;14:1596–1604. doi: 10.1359/jbmr.1999.14.9.1596. [DOI] [PubMed] [Google Scholar]
- 30.Saxena R, Pan G, McDonald JM. Osteoblast and osteoclast differentiation in modeled microgravity. Ann N Y Acad Sci. 2007;1116:494–4988. doi: 10.1196/annals.1402.033. [DOI] [PubMed] [Google Scholar]
- 31.Hammond TG, Hammond JM. Optimized suspension culture: the rotating-wall vessel. Am J Physiol Renal Physiol. 2001;281:F12–F25. doi: 10.1152/ajprenal.2001.281.1.F12. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar D, Nagaya T, Koga K, Kambe F, Nomura Y, Seo H. Rotation in clinostat results in apoptosis of osteoblastic ROS 17/2.8 cells. J Gravit Physiol. 2000;7:P71–P72. [PubMed] [Google Scholar]
- 33.Kanematsu M, Yoshimura K, Takaoki M, Sato A. Vector-averaged gravity regulates gene expression of receptor activator of NF-kappaB (RANK) ligand and osteoprotegerin in bone marrow stromal cells via cyclic AMP/protein kinase A pathway. Bone. 2002;30:553–558. doi: 10.1016/s8756-3282(02)00680-4. [DOI] [PubMed] [Google Scholar]
- 34.Rucci N, Rufo A, Alamanou M, Teti Modeled Microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J Cell Biochem. 2007;100:464–473. doi: 10.1002/jcb.21059. [DOI] [PubMed] [Google Scholar]
- 35.Bikle DD, Morey-Holton ER, Doty SB, Currier PA, Tanner SJ, Halloran BP. Alendronate increases skeletal mass of growing rats during unloading by inhibiting resorption of calcified cartilage. J Bone Miner Res. 1994;9:1777–1787. doi: 10.1002/jbmr.5650091115. [DOI] [PubMed] [Google Scholar]
- 36.Machwate M, Zerath E, Holy X, Hott M, Modrowski D, Malouvier A, Marie PJ. Skeletal unloading in rat decreases proliferation of rat bone and marrow-derived osteoblastic cells. Am J Physiol. 1993;264:E790–E799. doi: 10.1152/ajpendo.1993.264.5.E790. [DOI] [PubMed] [Google Scholar]
- 37.Machwate M, Zerath E, Holy X, Pastoureau P, Marie PJ. Insulin-like growth factor-I increases trabecular bone formation and osteoblastic cell proliferation in unloaded rats. Endocrinology. 1994;134:1031–1038. doi: 10.1210/endo.134.3.8119139. [DOI] [PubMed] [Google Scholar]
- 38.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 39.Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res. 2005;20:1858–1866. doi: 10.1359/JBMR.050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 41.Meyers VE, Zayzafoon M, Gonda SR, Gathings WE, McDonald JM. Modeled microgravity disrupts collagen I/integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. Cell Biochem. 2004;93:697–707. doi: 10.1002/jcb.20229. [DOI] [PubMed] [Google Scholar]
- 42.Zayzafoon M, Meyers VE, McDonald JM. Microgravity: the immune response and bone. Immunol Rev. 2005;208:267–280. doi: 10.1111/j.0105-2896.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 43.Hirotani H, Tuohy NA, Woo JT, Stern PH, Clipstone NA. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J Biol Chem. 2004;279:13984–13992. doi: 10.1074/jbc.M213067200. [DOI] [PubMed] [Google Scholar]
- 44.Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279:26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 45.Feng X. RANKing Intracellular Signaling in Osteoclasts. IUBMB Life. 2005;57:389–395. doi: 10.1080/15216540500137669. [DOI] [PubMed] [Google Scholar]
- 46.Tamma R, Colaianni G, Camerino C, Di Benedetto A, Greco G, Strippoli M, Vergari R, Grano A, Mancini L, Mori G, Colucci S, Grano M, Zallone A. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J. 2009 Aug;23:2549–54. doi: 10.1096/fj.08-127951. [DOI] [PubMed] [Google Scholar]
- 47.Monici M, Fusi F, Paglierani M, Marziliano N, Cogoli A, Pratesi R, Bernabei PA. Modeled gravitational unloading triggers differentiation and apoptosis in preosteoclastic cells. J Cell Biochem. 2006;98:65–80. doi: 10.1002/jcb.20747. [DOI] [PubMed] [Google Scholar]
- 48.Monici M, Agati G, Fusi F, Paglierani M, Cogoli A, Bernabei PA. Gravitational unloading induces osteoclast-like differentiation of FLG 29.1 cells. J Gravit Physiol. 2002;9:261–262. [PubMed] [Google Scholar]
- 49.Seales EC, Micoli KJ, McDonald JM. Calmodulin is a critical regulator of osteoclastic differentiation, function, and survival. J Cell Biochem. 2006;97:45–55. doi: 10.1002/jcb.20659. [DOI] [PubMed] [Google Scholar]
- 50.Zayzafoon M, Fulzele K, McDonald JM. Calmodulin and calmodulin-dependent kinase IIalpha regulate osteoblast differentiation by controlling c-fos expression. J Biol Chem. 2005;280:7049–7059. doi: 10.1074/jbc.M412680200. [DOI] [PubMed] [Google Scholar]
- 51.Yeo H, Beck LH, McDonald JM, Zayzafoon M. Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone. 2007;40:1502–1516. doi: 10.1016/j.bone.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto T, Nakayama K, Kodama Y, Fuse H, Nakamura T, Fukumoto S. Effect of mechanical unloading and reloading on periosteal bone formation and gene expression in tail-suspended rapidly growing rats. Bone. 1998;22:89S–93S. doi: 10.1016/s8756-3282(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 54.Hefferan TE, Evans GL, Lotinun S, Zhang M, Morey-Holton E, Turner RT. Effect of gender on bone turnover in adult rats during simulated weightlessness. J Appl Physiol. 2003;95:1775–1780. doi: 10.1152/japplphysiol.00455.2002. [DOI] [PubMed] [Google Scholar]
- 55.Dehority W, Halloran BP, Bikle DD, Curren T, Kostenuik PJ, Wronski TJ, Shen Y, Rabkin B, Bouraoui A, Morey-Holton E. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am J Physiol 1999. 1999;276:E62–E69. doi: 10.1152/ajpendo.1999.276.1.e62. [DOI] [PubMed] [Google Scholar]
- 56.Huang DW, Wan YM, Shi ZZ, Huang ZM, Li YH, Ma YJ. Effects of hindlimb unloading on bone histomorphometry and bone mass in rats (in Chinese). Space Med Med Eng (Beijing) 2003;16:418–21. [PubMed] [Google Scholar]
- 57.Li XJ, Jee WS, Chow SY, Woodbury DM. Adaptation of cancellous bone to aging and immobilization in the rat: a single photon absorptiometry and histomorphometry study. Anat Rec. 1990 May;227:12–24. doi: 10.1002/ar.1092270103. [DOI] [PubMed] [Google Scholar]
- 58.Lloyd SA, Travis ND, Lu T, Bateman TA. Development of a low-dose anti-resorptive drug regimen reveals synergistic suppression of bone formation when coupled with disuse. J Appl Physiol. 2008;104:729–738. doi: 10.1152/japplphysiol.00632.2007. [DOI] [PubMed] [Google Scholar]
- 59.Turner RT, Evans GL, Lotinun S, Lapke PD, Iwaniec UT, Morey-Holton E. Dose-response effects of intermittent PTH on cancellous bone in hindlimb unloaded rats. J Bone Miner Res. 2007;22:64–71. doi: 10.1359/jbmr.061006. [DOI] [PubMed] [Google Scholar]
- 60.Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem. 2003;89:180–190. doi: 10.1002/jcb.10490. [DOI] [PubMed] [Google Scholar]
- 61.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- 62.Ono N, Nakashima K, Schipani E, Hayata T, Ezura Y, Soma K, Kronenberg HM, Noda M. Constitutively active parathyroid hormone receptor signaling in cells in osteoblastic lineage suppresses mechanical unloading-induced bone resorption. J Biol Chem. 2007;282:25509–25516. doi: 10.1074/jbc.M610782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baek K, Bloomfield SA. Beta-adrenergic blockade and leptin replacement effectively mitigate disuse bone loss. J Bone Miner Res. 2009;24:792–799. doi: 10.1359/jbmr.081241. [DOI] [PubMed] [Google Scholar]
- 64.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin Mediates Bone Response to Mechanical Unloading via Antagonizing Wnt/beta-Catenin Signaling. J Bone Miner Res. 2009 Oct;24:1651–61. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure: RAW264.7 cells were incubated for overnight in low-adhesion plates. Cells attached around the beads which were then incubated in G and MMG for 24h. Figure shows that the cells remain attached around the beads after incubation(10X magnification).




