Abstract
Background/Aims
The underlying mechanisms of steatosis, the first stage of non-alcoholic fatty liver disease (NAFLD) that is characterized by the accumulation of lipids in hepatocytes, remain unclear. Our study aimed to investigate the hypothesis that cigarette smoke is known to change circulating lipid profiles and thus may also contribute to the accumulation of lipids in the liver.
Methods
Mice and cultured hepatocytes were exposed to sidestream whole smoke (SSW), a major component of “second-hand” smoke and a variety of cellular and molecular approaches were used to study the effects of cigarette smoke on lipid metabolism.
Results
SSW increases lipid accumulation in hepatocytes by modulating the activity of 5′-AMP-activated protein kinase (AMPK) and sterol response element binding protein-1 (SREBP-1), two critical molecules involved in lipid synthesis. SSW causes dephosphorylation/ inactivation of AMPK, which contributes to increased activation of SREBP-1. These changes of activity lead to accumulation of triglycerides in hepatocytes.
Conclusion
These novel findings are important because they point to another risk factor of smoking, i.e., that of contributing to NAFLD. In addition, our results showing that both AMPK and SREBP are critically involved in these effects of smoke point to the potential use of these molecules as targets for treatment of cigarette smoke-induced metabolic diseases.
Keywords: Kinases, Fatty liver, Non-alcoholic fatty liver diseases, Transcription factors, Sidestream whole smoke
1. Introduction
Cigarette smoke contains more than 47,000 toxic substances which significantly harm almost every organ of the body, leading to a variety of diseases and syndromes [1]. Cigarette smoke is composed of MSW (mainstream whole, “first-hand”) and SSW (side stream whole, major component of “second-hand” smoke) smokes. Smoking has been identified as one of the major risk factors for the development of atherosclerosis, the major component of cardiovascular disease [2–4] that manifests itself, among other things, by high lipid levels in the blood [5,6]. Furthermore, increasing evidence suggests that risk for cardiovascular disease incidence is associated with non-alcoholic fatty liver diseases (NAFLD) independently of the classical risk factors and features of this metabolic syndrome [7–10]. In the various stages of NAFLD, hepatic steatosis (the accumulation of lipid in the liver tissue) has become a significant public health concern because it tends to develop into more harmful hepatitis and cirrhosis. Because lipids in steatosis are stored as triglycerides in hepatocytes, understanding what causes this accumulation and how it occurs may contribute to elucidation of NAFLD [11].
Sterol regulatory element-binding proteins (SREBPs) are a family of transcription factors that control the expression of genes required for the biosynthesis of cholesterol, fatty acids, triglycerides, and phospholipids. The three isoforms of SREBP precursors located on the endoplasmic reticulum membrane, designated SREBP-1a, SREBP-1c, and SREBP-2 [12], have different functions and abundance in various animal tissues. The SREBP precursors are activated by a two-step cleavage process that releases the active form that then translocates to the nucleus of the cell to stimulate gene expression [13]. SREBP-1c preferentially controls the expression of genes involved in triglyceride synthesis and accumulation, such as fatty acid synthase (FAS) and acetyl coenzyme-A carboxylase (ACC), whereas SREBP-2 activity has been more closely linked to regulation of genes involved in cholesterol synthesis and uptake, such as low-density lipoprotein receptor (LDLR) and 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR) [14–18]. In the liver tissue, the predominant form of SREBPs is SREBP-1c [19].
Another important modulator of lipid metabolism is 5′-AMP-activated protein kinase (AMPK). AMPK was first identified as a kinase that phosphorylates and inactivates ACC, the rate-limiting enzyme in fatty acid biosynthesis [20]. AMP binds and activates AMPK primarily by causing conformational changes that allows Thr172 phosphorylation to occur by upstream kinases. Activation of AMPK in the liver, skeletal muscle, and adipose tissue improves the status of type 2 diabetes by preventing ATP depletion, increasing fatty acid oxidation, decreasing blood glucose, etc. It has also been found that AMPK activity is inhibited in alcohol-induced fatty liver disease [21].
Although both AMPK and SREBP are related to the metabolism of the cell, the relationship between the two is not clear. We hypothesize that components of tobacco smoke cause lipid accumulation in the liver tissue of mice exposed to “second-hand” smoke by modulating the activities of AMPK and that this enzyme is important in the activation of SREBP-1, the central modulator for triglyceride synthesis. The elucidation of the mechanisms of lipid accumulation in hepatocytes caused by cigarette smoke may help understand processes involved in atherogenesis and in initiation of NAFLD, and suggest possible ways of treating both metabolic diseases.
2. Materials and methods
2.1. Smoke solution preparation
Sidestream whole (SSW) smoke solution was prepared from 1R3F research grade cigarettes (University of Kentucky, Louisville, KY). SSW smoke was bubbled into 10 mL serum free media for the duration of 30 puffs as previously described [22] using a puffer box built by the University of Kentucky. SSW smoke was collected from the burning end of the cigarette. The pH of the smoke solutions was adjusted to 7.4. The solution is aliquoted and kept at −20 °C (stable for up to 6 weeks).
2.2. Exposure of the animals to smoke
Six- to eight-week-old male apoB100 transgenic mice on 57BL/6SJL background [23] were fed a high-fat diet and were exposed to smoke for 19 weeks as described previously [24]. All animal experiments were conducted in accordance with US Public Health Service/US Department of Agriculture guidelines. Experimental protocols were approved by the University of California Riverside Institutional Animal Care and Use Committee.
2.3. Lipid content analysis
Lipid extraction and analysis were performed as described previously [24].
2.4. Oil Red O staining
Cells or cryo-sections of liver tissue were washed with cold PBS, fixed with 4% paraformaldehyde in PBS for 15 min, and stained for 20 min in freshly diluted Oil Red O solution (0.3% Oil Red O in isopropanol: H2O = 3:2), and washed twice with water.
2.5. Immunoblotting
Immunoblot analysis was performed as previously described by us [25].
2.6. Infection of the hepatocytes to detect AMPK activity
AML12 cells or HEP3B cells were infected with null virus (Adnull), an adenovirus expressing the constitutively active form of AMPK (Ad-AMPK-CA) [26], or the dominant negative form of AMPK (Ad-AMPK-DN), at 50 multiplicities of infection. AMPK phosphorylation was analyzed by immunoblotting using a phosphor-specific antibody, anti-phospho-AMPK Thr-172.
Cells were incubated with compound C for 30 min followed by infection with Ad-AMPK-CA or Ad-AMPK-DN and cultured for 24 h for immunoblotting or for 48 h for lipid measurement. When used with SSW and/or SREBP inhibitor 25HC, the cells were incubated with 25-HC for 6 h first, followed by infection with Ad-AMPK-CA or Ad-AMPK-DN, addition of SSW solution to final concentration of 1:40 and cultured for 48 h for lipid measurement.
2.7. RNA isolation, one-step RT-PCR and quantitative real-time PCR
Total hepatic RNA was isolated and purified from 5 × 106 cells or 30 mg of fresh liver tissues using the RNeasy Mini Kit from QIAGEN following manufacturer’s instructions. Sequences of the primer sets used: SREBP-1c, 5′-GGAGCCATGGATTGCACATT-3′ and 5′-CCTGTCTCACCCCCAGCATA-3′; FAS, 5′- GTCCACCCCAAGCAGGCACACA-3′ and 5′-CTGGCAGCCCACCATGCTGTA-3′; ACC1, 5′- GGACAGACTGATCGCAGAGAAAG-3′ and 5′-TGGAGAGCCCCACACACA-3′; LDLR, 5′- GATGGACCAGGCCCCTAACT-3′ and 5′-GGTGTCAGCCACAGATACGCT-3′; HMGCR, 5′-ATGCATGGCCTCTTTGTGGCC-3′ and 5′- CTGCCAAATTGGACGA CCCTC-3′; ADRP, 5′-CTTGTGTCCTCCGCTTATGTCAGT-3′ and 5′-CTGCTCCTTTGGTCTTATCCACCA-3′, GAPDH (glyceraldehyde-3-phosphate dehydrogenase): 5′-GCCCATCACCATCTTCCA G-3′ and 5′-ACGCCACAGCTTTCCAGAG-3′. The PCR optimal cycle number and annealing temperature for each gene was determined to obtain detectable but non-saturating PCR product. Quantitative real-time PCR was performed with an Biorad iQ5 real-time PCR detection system and iQ SYBR Green Supermix (BioRad laboratories) according to the manufacturer’s instructions.
2.8. Statistical analysis
Animal experiments were performed with 5–6 mice per group with values presented as means ± SD. All the experiments were repeated at least 3 times. The significance of variability was determined by Student’s t-test or ANOVA and the Bonferroni Multiple Comparisons Test. A P value < 0.05 was considered significant.
3. Results
3.1. SSW causes lipid accumulation in the liver
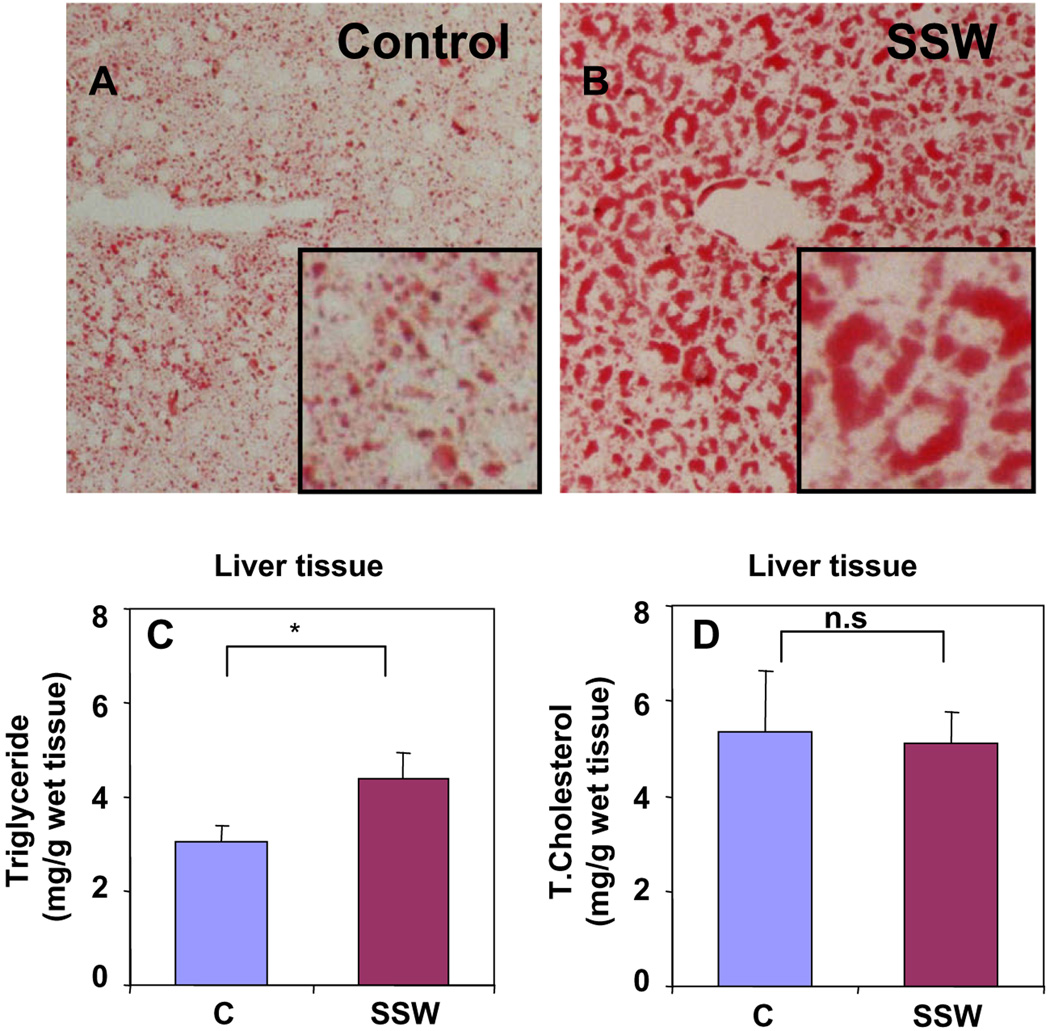
To test our hypothesis that SSW stimulates lipid synthesis and accumulation in hepatocytes, we used ApoB100 mice on a high-fat diet and exposed them to SSW as described in Section 2. These mice were chosen because under such feeding conditions they produce levels of lipid that lead to development of atherosclerosis [27–29], which in turn has been associated with NAFLD [30–32]. To detect the effects of SSW on lipid accumulation in the hepatocytes, mice were exposed to smoke for 19 weeks, the cellular lipid content was evaluated in liver tissue sections stained with Oil Red O, and the levels of lipid in extracts of liver tissue which were quantified using commercially available kits. We observed more lipid accumulation in the hepatocytes of mice exposed to SSW (Fig. 1A and B). The levels of triglycerides in the extracts of the liver tissue are significantly increased from 3.08 ± 0.55 mg/g wet weight for control group to 4.39 ± 1.39 mg/g wet weight for SSW group (Fig. 1C). However, there is no significant change in the levels of total cholesterol (Fig. 1D).
Fig. 1.
SSW causes lipid accumulation in liver tissue of the mice exposed to cigarette smoke. (A–B) Sections of liver tissue from mice exposed to fresh air (A) or SSW (B) for 19 weeks. Tissues were fixed and sections were stained with Oil Red O to observe the accumulation of neutral lipids. Pictures are shown at 200× magnification. Inserts show enlargements of a small area of the larger pictures. (C–D) Lipid levels in the liver tissue of mice exposure to SSW for 19 weeks. Lipids were extracted and measured using commercial kit for the level of triglyceride (C) and total cholesterol (D). *p < 0.05 relative to control.
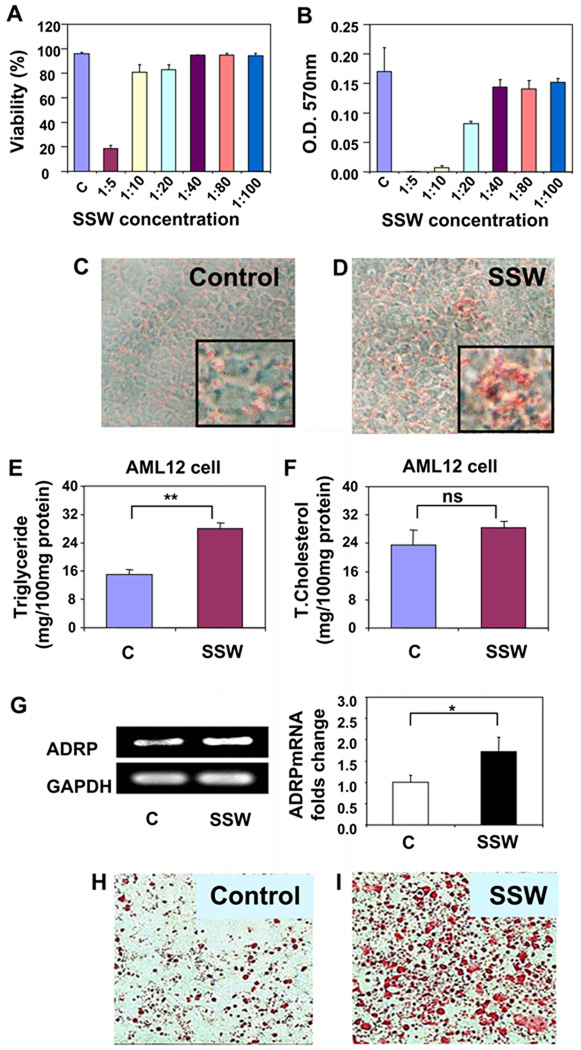
Primary hepatocytes are difficult to culture and do not survive well [33–35]. Therefore, we used AML12 cells, a cell line that has been used extensively for studies of hepatocyte function [36–38]. The appropriate concentration of cigarette smoke solution for the treatment of the cultured hepatocytes was determined by two methods: the trypan blue viability assay and the MTT assay. Although in the trypan blue assay, more than 80% of the cells survived in SSW solution at 1:20 dilution (Fig. 2A), when the MTT assay was used, the cells showed a significant decrease in viability at this concentration (Fig. 2B). Because the MTT assay measures biochemical changes in the mitochondria and is more sensitive in measuring viability, we chose to use the SSW solution at 1:40, the dilution that showed no significant difference in viability between the smoke-treated and control cells. In addition, we had previously determined that at this concentration, the nicotine level is ~0.5 µg/ml [22], which is within the concentration ranges of nicotine in the tissues of passive smokers (range 0.195–3.12 µg/ml) [39].
Fig. 2.
Lipid accumulation in hepatocytes treated with SSW solution. (A–B) Cytotoxicity of SSW at different concentrations. AML-12 hepatocytes were grown to confluence. Two days post-confluence, cells were treated with or without increasing dilutions of SSW in culture media. The cytotoxicity of the SSW solution to AML12 was assessed by: (A) viability measured by cell counting using trypan blue in 24 well plate; (B) viability measured by the MTT assay in 96-well plates. (C–D) AML12 cells were treated with media alone (C) or 1:40 SSW solution (D), fixed and stained for neutral lipids with Oil Red O. Pictures are shown at 200× magnification. Inserts show enlargements of the pictures. (E–F) Lipid concentration of cell extracts of AML12 that were exposed to fresh media or 1:40 diluted SSW solution. Triglyceride (E) and total cholesterol (F) content were measured using commercial kits. OD, optical density. *p < 0.05 relative to controls. (G) RT-PCR shows the result of expression of ADRP, a membrane protein of lipid droplets, in AML12 cells treated with fresh media or 1:40 diluted SSW solution. (H–I) HEP3B cells were treated with media alone (H) or 1:40 SSW solution (I), and prepared and analyzed like in (C) and (D).
Our results show that there is more lipid accumulation in the AML12 hepatocytes when they are cultured in medium containing 1:40 SSW, as observed by Oil Red O staining (Fig. 2C–D). The triglyceride levels in the cell extracts of SSW treated cells were significantly increased from 15.04 ± 1.44 mg/100 mg protein for control group to 28.10 ± 1.41 mg/100 mg protein for SSW treated cells (Fig. 2E). In addition, we observed that there was no significant change in the total cholesterol levels (Fig. 2F), much as was observed in vivo. Furthermore, RT-PCR showed higher levels of the RNA encoding adipose differentiation-related protein (ADRP), a protein primarily present in the membrane of the lipid droplets in liver cells (Fig. 2G). Lipid accumulation was also observed in HEP3B hepatocyte (Fig. 2H and I).
3.2. SSW stimulates activation of SREBP-1
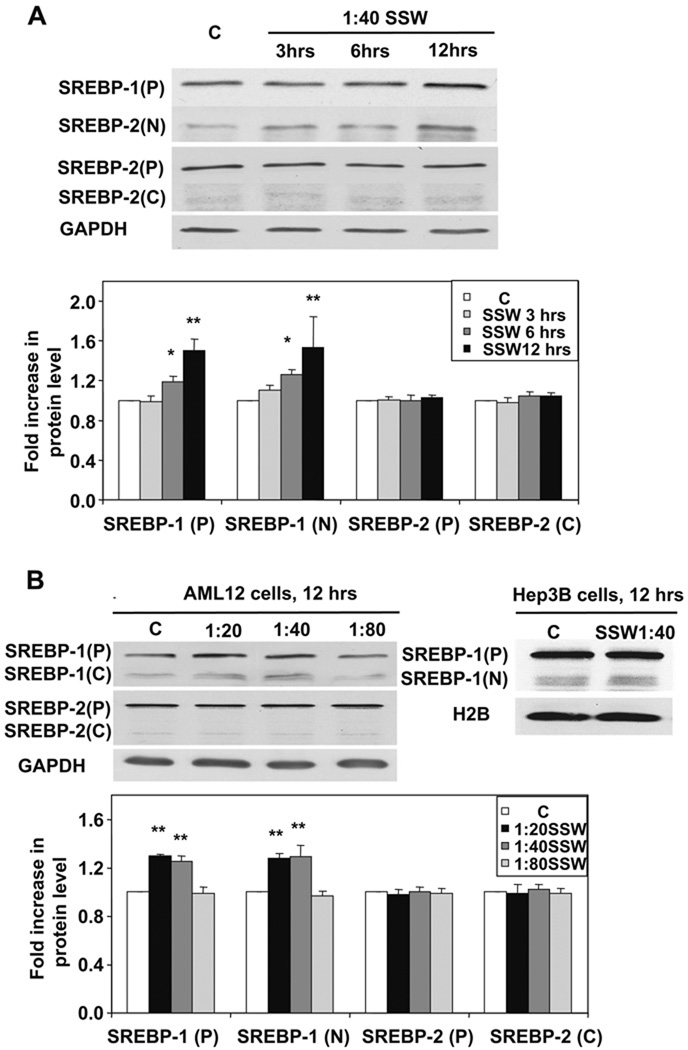
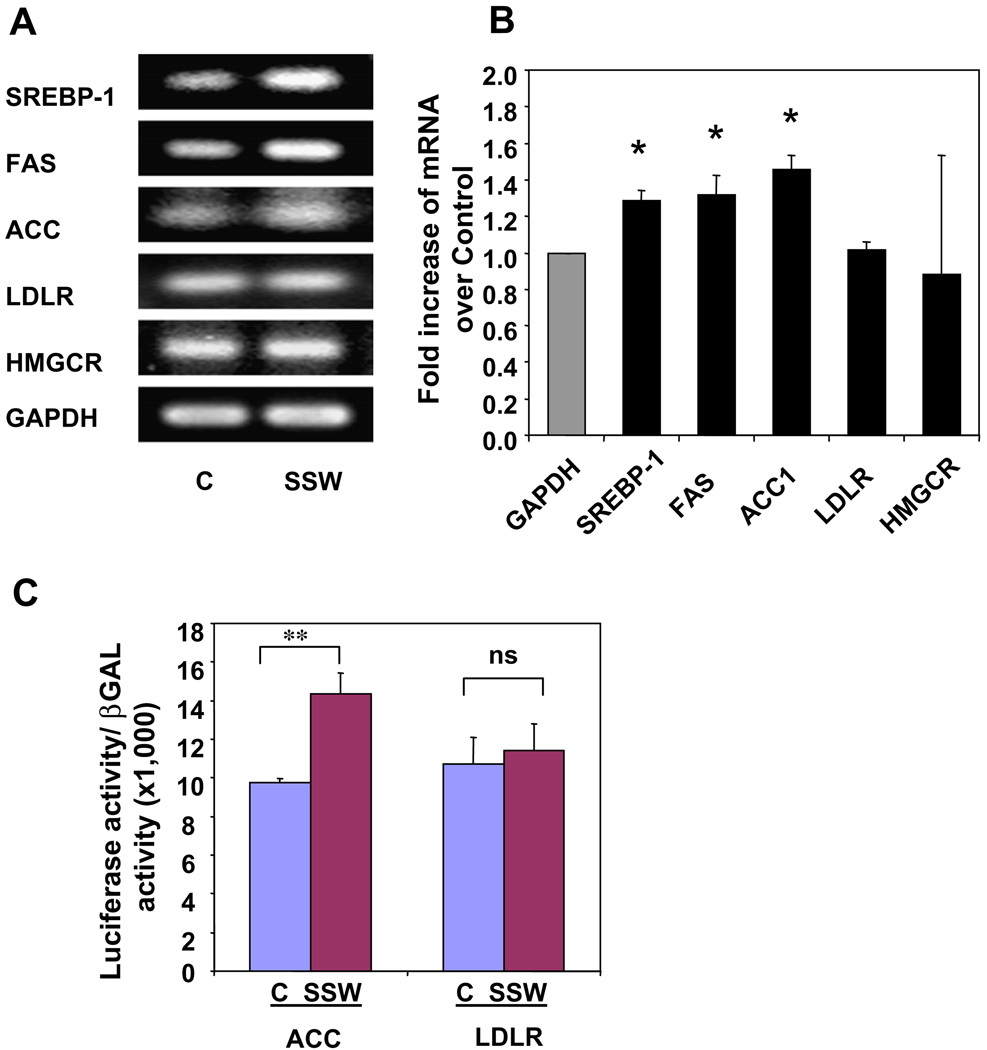
One major reason for lipid accumulation in hepatocytes is increase in lipid synthesis. In lipid metabolism, SREBPs play essential roles in hepatic triglyceride and cholesterol syntheses. When the cells were treated with smoke, SREBP-1 was activated in a time-dependent manner as shown by the presence of increased levels of the mature/active form, the N-terminus of the molecule (Fig. 3A). However, activation of SREBP-2, represented by a cleavage product created during protein activation, the C-terminus, was not apparent (Fig. 3A). Furthermore, there was also a dose-dependent expression and activation of SREBP-1, but not SREBP-2 (Fig. 3B). The expression of SREBP-1N was at about the same level at 1:80 compared to the control, because 1:80 dilution of SSW does not affect the cells. To confirm that SSW affects SREBP-1 function but not that of SREBP-2, we performed RT-PCR on cells exposed to SSW and examined the expression of genes that are stimulated by the binding of the active forms of these SREBPs to their elements in the DNA, such as FAS and ACC for SREBP-1 and LDLR and HMGCR for SREBP-2. Increase in expression of FAS and ACC were detected, whereas those of LDLR and HMGCR were not, suggesting the activation of SREBP-1 but not SREBP-2 (Fig. 4A). The RT-PCR results were further supported by quantitative real-time PCR (Fig. 4B). We further tested the effects of SSW on SREBP-1 using a luciferase assay. The cells were co-transfected with plasmids containing pCMV2 β-Gal and pGL2-luc-ACC or pGL2-Luc-LDLR, in which the luciferase expression is under the control of the ACC or LDLR promoters. The non-sterol-regulated cytomegalovirus promoter β-galactosidase expression construct was included as an internal control for normalization. SSW stimulated the expression of ACC whereas the expression of LDLR was not increased, showing that SREBP-1 is indeed activated by SSW (Fig. 4C).
Fig. 3.
SSW causes SREBP-1 activation. (A) Time-dependence of SREBP expression and activation. AML 12 cells were treated with 1:40 SSW for 0, 3, 6, and 12 h; the cell lysates were harvested at indicated time-points and analyzed by immunoblot for SREBP1 or SREBP2. P, precursor forms of the SREBPs; N, N-terminus of SREBP1 (active form); C, C-terminus of SREBP2 (indicates release of the active N-terminus). (B) Dose-dependent effects of SSW on SREBP activation and expression. The AML12 cells were treated with SSW at different concentrations (1:20, 1:40, and 1:80 dilutions) for 12 h. The HEP3B cells treated with 1:40 SSW for 12 h also showed SREBP-1 activation. GAPDH was detected to show equal loading.
Fig. 4.
SSW-induced SREBP-1 activation, and expression of down stream genes. (A–B) Gene expression of molecules down-stream of SREBPs. The AML 12 cells were treated with 1:40 dilution of SSW or fresh media for 8 h, and total RNA extracted. Gene expression for SREBP-1, FAS, ACC, LDLR, HMGCR, and GAPDH was analyzed by RT-PCR (A) and by quantitative real-time PCR (B). (C) AML12 cells cultured in 12-well plates were transiently transfected with LDLR-Luc (1.0 µg/well) or ACC-Luc (1.0 µg) plus β-gal (0.1 µg). Thirty-six hours after transfection, the cells were treated with 1:40 dilution SSW or fresh media for 12 h. Luciferase activity was measured and normalized to that of β-gal. All of the values presented here represent a ratio of luciferase to β-Gal to correct for nonspecific variations in the transfection and assay procedures. The data represent the ratio of luciferase activity (relative light units) for the indicated test plasmid to the β-galactosidase activity (OD 420 nM/h) expressed from the control cytomegalovirus promoter. The average of at least five independent experiments for each plasmid performed in duplicate is presented.
3.3. SSW stimulates AMPK dephosphorylation/inactivation
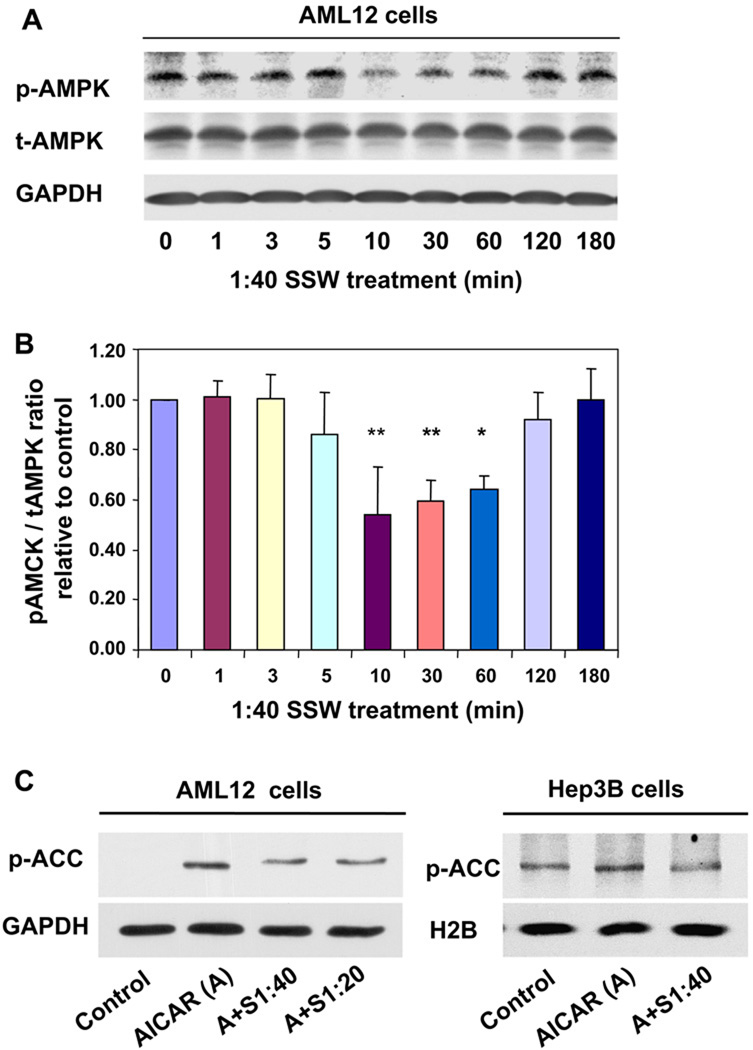
AMPK is another molecule that has been shown to be important in lipid metabolism. Our immunoblot analysis shows that phosphorylation of AMPK decreases after exposure of cells to SSW solution for 10 min, and then gradually returns to normal phosphorylation levels after 2 h (Fig. 5A–B). In order to show that SSW-induced dephosphorylation of AMPK corresponds to a decrease in activity/function of this kinase, we used its substrate ACC as a marker. It is known that when AMPK is active (i.e., phosphorylated) it phosphorylates ACC, rendering this enzyme inactive and, as a consequence, keeping lipid synthesis at low levels [40,41]. Because the levels of endogenous AMPK are low and they are difficult to detect under normal conditions, we used 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR), a stimulator of AMPK activity, to increase the levels of AMPK phosphorylation/activation and determine the effects of SSW on this phosphorylation/ activation. The results show that the level of ACC phosphorylation significantly increased after the cells were treated with AICAR, and this increase was blocked when the cells were also treated with SSW solution, in both AML12 and HEP3B cell lines (Fig. 5C). Because AMPK phosphorylates ACC and the phosphorylated form of this enzyme is the inactive molecule, we conclude that SSW decreases not only the phosphorylation of AMPK but also its activity. This leads to the decreased phosphorylation and thus increased activity of ACC.
Fig. 5.
SSW induces AMPK dephosphorylation/inactivation. (A) AMPK phosphorylation in SSW treated AML12 cells. The cells were treated with 1:40 SSW solution. Cell lysates were harvested at a series of time points (0–3 h), and equal amounts of total protein were prepared, separated (100 µg) by SDS-PAGE and analyzed by immunoblotting for phosphorylation of AMPK. (B) Bar graph shows the ratio of band density of the pAMPK: total AMPK. (C) AMPK activity assay. AML12 cells or HEP3B cells were treated with AICAR, an AMPK activator at 0.5 mM for 1 h, and then incubate with or without 1:20 or 1:40 diluted SSW for 30 min. Equal amount of protein from cell lysates were separated by SDS–PAGE, and the level of phosphorylated ACC was shown by immunoblot analysis. GAPDH was used for equal loading.
3.4. AMPK phosphorylation/activation inhibits SREBP-1 activation
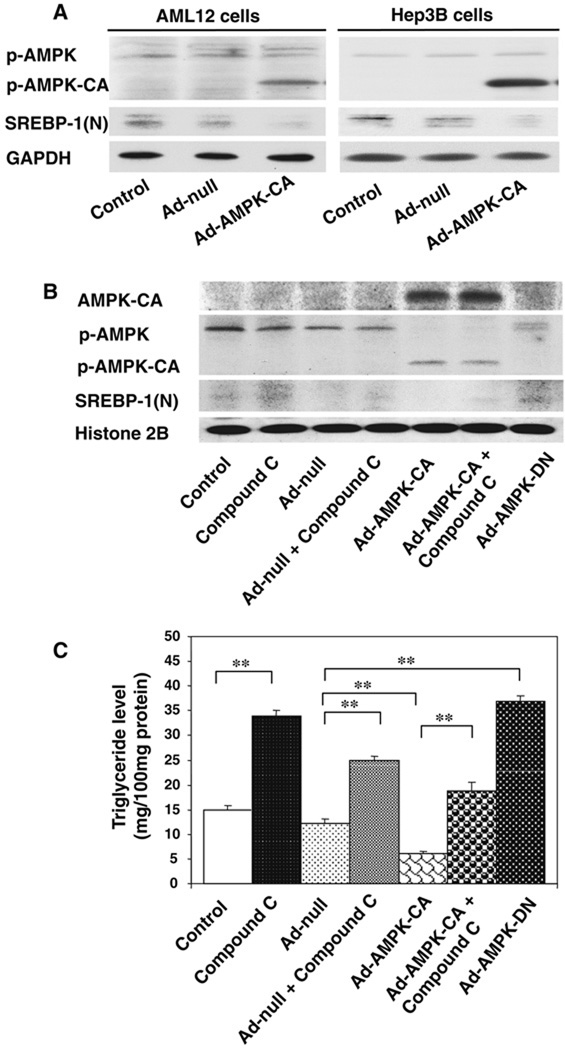
To determine whether AMPK affects SREBP-1 activation, cells were transiently transfected with an adenovirus containing the gene for constitutively active AMPK (Ad-AMPK-CA) and then examined for the activation status of SREBP-1 (Fig. 6). In both AML12 and HEP3B cell lines, expression of Ad-AMPK-CA significantly increased phosphorylation/activation of this protein. Under the same conditions, SREBP-1 activation was decreased (Fig. 6A). To make the link between AMPK inactivation and SREBP-1 activation, the AML12 cells were treated with compound C, an inhibitor of AMPK, as well as with constitutively activated or dominant negative forms of this enzyme (Fig. 6B). Ad-AMPK-CA inhibited SREBP-1 activation that could be at least in part reversed by compound C. Conversely, the cells transfected with Ad-AMPK-DN inhibited the phosphorylation/activation of this enzyme resulting in SREBP-1 activation. This was also seen when compound C alone was used (Fig. 6B). These results, taken together, show that inactivation of AMPK leads to an increase in SREBP-1 activity. To show that the inactivation of AMPK/activation of SREBP leads to an increase in lipid accumulation in hepatocytes, we used the same conditions as above to treat the cells and measured the triglyceride levels in the cell extracts (Fig. 6C). Increased levels of triglycerides correlate well with AMPK inactivation using compound C or the dominant negative form of this enzyme. Conversely, decreased levels of trigycerides occurred when the cells were treated with the constitutively activated form of AMPK.
Fig. 6.
AMPK phosphorylation/activation leads to inhibition of SREBP-1 activation. (A) AMPK activation decreases SREBP activity in AML12 cells. Both AML12 cells and HEP3B cells were infected with control null virus (Ad-null, 50 multiplicities of infection), or an adenovirus expressing the constitutively active form of AMPK (Ad-AMPK-CA) for 24 h. The phosphorylation of AMPK and ACC were analyzed by immunoblotting. (B) Inhibition of AMPK activation can reverse the decrease of SREBP activity caused by AMPK activation. AML12 cells were treated with or without compound C (20 µM), as well as with constitutively activated (Ad-AMPK-CA) or dominant negative (Ad-AMPK-DN) forms of AMPK for 24 h. (C) AMPK activation leading to decreased level of triglyceride. The treatment was the same as in (B), but for 48 h, and triglycerides were measured using commercial kit. **p < 0.01.
3.5. Activity of AMPK and SREBP-1 in liver tissue
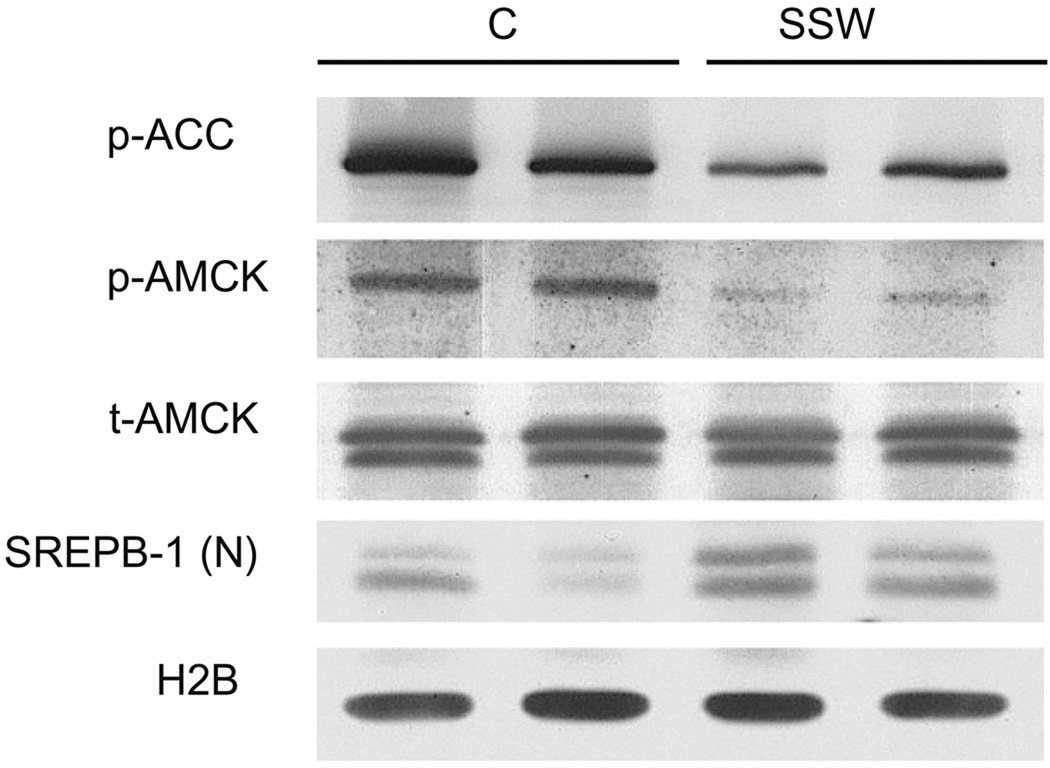
To further demonstrate that SSW causes lipid accumulation in hepatocytes, we measured the levels of SREBP-1 level and of the phosporylation of ACC and AMPK in liver tissue of the mice that were exposed to smoke. The results show decreased phosphorylation of ACC and AMPK, and increased levels of SREBP-1(N), the activated form of this protein (Fig. 7). Also, these findings correlate well with those obtained when we used AML 12 cells.
Fig. 7.
Activity of AMPK and SREBP-1 in the liver tissue from mice exposed to SSW. Mice were exposed to fresh air or SSW for 19 weeks, liver tissues collected and extracted using lysing buffer. Equal amount of extracted proteins were loaded on SDS–PAGE and analyzed for the levels of phospho-AMPK, phospho-ACC, and SREBP-1 (N) using Western blot.
3.6. SSW-induced AMPK inactivation/SREBP-1 activation leads to lipid accumulation in hepatocytes
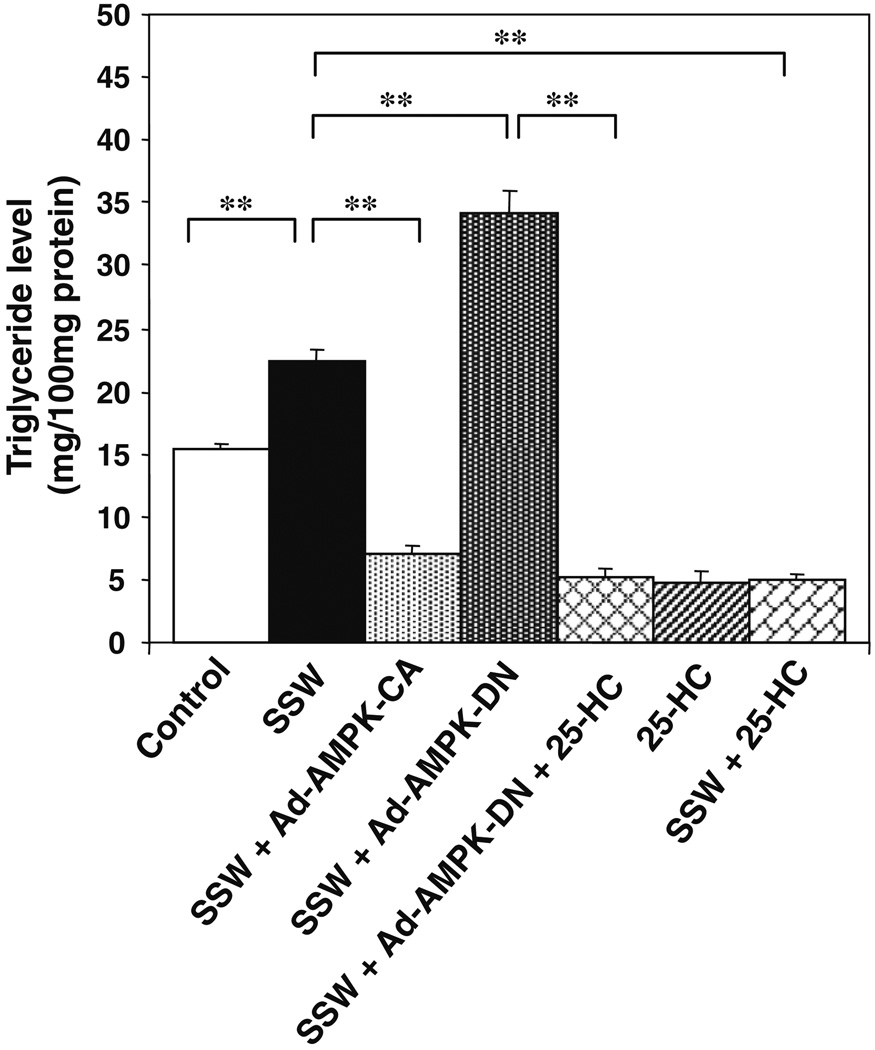
To establish the link between SSW and AMPK inactivation/SREBP-1 activation with lipid accumulations in hepatocytes, we treated the cells with SSW in the presence of constitutively activated or the dominant negative forms of AMPK and/or in the presence of the SREBP-1 inhibitor 25-hydroxycholesterol, which decrease SREBP activation in this cell type. (Fig. 8). When cells were exposed to SSW, triglyceride levels were increased, and this increase could be blocked when the constitutively activated form of AMPK was present, whereas the dominant negative form of AMPK greatly increased the already elevated triglyceride level in the cells stimulated by SSW. Furthermore, 25-HC, an inhibitor of SREBP-1, when in the presence of the dominant negative form of AMPK, inhibited triglyceride accumulation stimulated by SSW.
Fig. 8.
SSW-induced AMPK innactivation/SREBP-1 activation leads to lipid accumulation in hepatocytes. AML12 cells cultured in 35 mm plates were incubated with or without 25-HC (10 µg/ml) for 6 h, then transfected with or without Ad-AMPK-CA or Ad-AMPK-DN (50 multiplicities of infection) for 5 h followed by incubation with fresh media or media containing 1:40 diluted SSW and/or 25-HC for 48 h. Triglycerides were measured using commercial kits. **p < 0.01.
3.7. Adiponectin reverses the effects of SSW on AMPK and SERBP-1
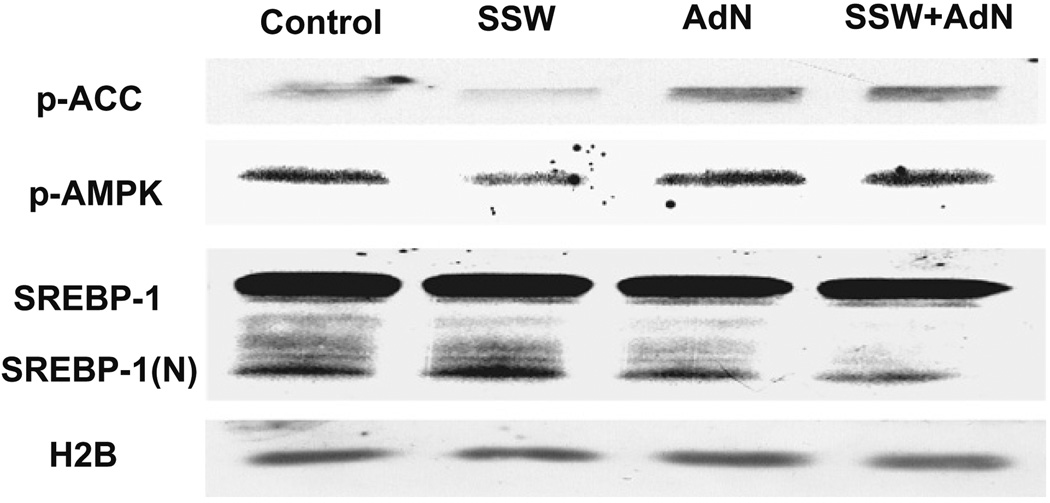
To determine whether adiponectin inhibits the effects of SSW on AMPK inactivation/SREBP-1 activation leading to lipid accumulations in hepatocytes, we treated the cells with recombinant adiponectin followed by exposure to SSW. The results show that adiponectin was able to reverse the effects of SSW resulting in increased levels of activated/phosphorylated ACC and AMPK, and in decreased levels of activated SREBP-1 (Fig. 9).
Fig. 9.
Adiponectin affects regulation of AMPK/SERBP-1 by SSW. AML 12 cells were incubated with or without adiponectin (10 ng/ml) for 12 h, then treated with 1:40 SSW solution for 30 min. Proteins extracts were then applied to SDS–PAGE and the level of phosphorylated ACC, phosphorylated AMPK and the activated form of SREBP-1(N) was shown by immunoblot analysis. HEP3B was used for equal loading.
4. Discussion
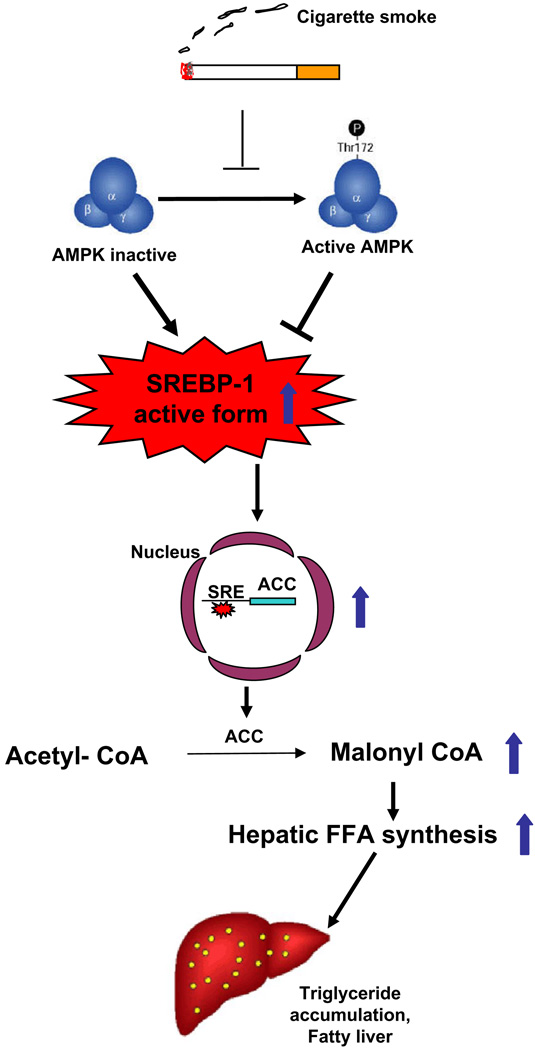
Cigarette smoke has long been recognized as one of the most preventable non-hereditary factors contributing to cardiovascular disease [2–4]. Evidence is mounting that there are relationships between cardiovascular disease and metabolic diseases such as NAFLD and diabetes [7–10]. These diseases all exhibit high lipid levels. Therefore, it is logical to speculate that changes in liver function, the organ where lipid synthesis takes place, affect the initiation and development of these diseases. However, in most studies, patients who smoke are not included or smoking habits are not counted as factors, hence, nothing is known about the relationship between cigarette smoke and fatty liver, or about the smoke-induced mechanisms of lipid accumulation in liver. Because second-hand smoke is a major toxicant that affects children and the elderly living in the household of adults who smoke, we used the major components of the second-hand smoke, SSW, for our studies. Here we show that SSW: (1) stimulates lipid accumulation in hepatocytes, both in liver tissue and in cultured cells and (2) results in inactivation of AMPK which, in turn, contributes to increased activation of SREBP-1 which leads to accumulation of triglycerides in hepatocytes. These results advance the knowledge in the field, by deciphering the mechanisms by which SSW stimulates lipid accumulation in hepatocytes (Fig. 10).
Fig. 10.
Mechanism of cigarette smoke-induced lipid accumulation in hepatocytes.
AMPK, one of the energy metabolism regulators, acts as a key “master switch” by phosphorylating target enzymes. However, AMPK can also be activated in situations without detectable changes in the AMP/ATP ratio, such as in response to sheer stress [42,43], increased osmotic pressure [44] anti-diabetic drugs metformin [45] and thiazolidinediones [46,47], and in the regulation of whole body glucose homeostasis [48,49]. Our results show that SSW smoke inhibits AMPK phosphorylation, and hence its function, within 10 min of exposure (Fig. 5A and B). Consequently, AMPK cannot phosphorylate/innactivate ACC. ACC is involved in lipid synthesis in the liver and, because it is a direct substrate for AMPK, ACC phosphorylation or lack of it, reflects the activity of AMPK. Our observation that ACC phosphorylation decreases after SSW exposure confirms the SSW-mediated inhibition of AMPK.
Because both SREBP and AMPK are involved in lipid metabolism, we hypothesized the existence of a relationship between these two important molecules. Our studies extend existing knowledge by showing that cigarette smoke increases lipid accumulation in the hepathocytes through neosynthesis of triglycerides via inactivation of AMPK and activation of SREBP-1. The SREBP inhibitor 25-HC blocks the increase of triglycerides in AML12 cells, and this occurs not only in the presence of SSW or of the expression of a dominant negative form of AMPK, but also in the presence of both SSW and of the dominant negative form of AMPK (Fig. 8). Moreover, constitutive activation of AMPK also reduced increase of triglycerides caused by SSW in hepatocytes. The AMPK inactivation is transient after exposure to SSW, however, repeated exposure to SSW may have a cumulative effect on the lipid accumulation in hepatocytes shown in Fig. 1. Changes in AMPK activity can be transient as reported previously in IEC-6 cells and in primary cultures of rat hepatocytes exposed to adenosine [50] or in alveolar epithelial cells exposed to elevated CO2 [51] or in prostate cancer cells exposed to adiponectin [52]. AMPK is also transiently inactivated by exercise in mononuclear cells [53]. Taken together, our results show that SSW increases lipid accumulation in hepatocytes by increasing SREBP-1 activity via the inactivation of AMPK.
With sedentary life style, unhealthy food habits, and stress, the percentage of people suffering from metabolic diseases, such as diabetes and obesity, has reached an alarming level. Because metabolic diseases have disturbed lipid metabolism, elucidating the link between AMPK innactivation and SREBP activation may be helpful in treatment of these diseases. For example, the level of adiponectin, a cytokine secreted by adipocytes, was decreased in obese people and those with diabetes [54–56]. Interestingly, adiponectin stimulates the activation of AMPK [57,58]. Thus, it is not surprising to see that metformin [45] and thiazolidinediones [46,47], the anti-diabetic drugs that can stimulate adiponectin expression in adipocytes, relieve symptoms of obesity, diabetes, and atherosclerosis. Overexpression of adiponectin in vivo inhibits SREBP-1 expression and offsets the development of diet-induced obesity in rats [59], and anti-diabetic drugs thiazolidinediones were also found to block the expression of SREBP-1c [60]. Therefore, it is very possible that anti-diabetes drugs stimulate adiponectin production, by stimulating AMPK activation and consequently causing SREBP inactivation, thus decreasing lipid synthesis and improving the metabolic condition of these patients. We have previously shown that the level of adiponectin in circulation was decreased in the animals exposed to cigarette smoke [24], in a manner similar to that of smokers [61,62]. These smoke-mediated decreases in adiponectin may cause decreased AMPK activation and increase SREBP activation, leading to lipid accumulation. This may provide an explanation for the observation that second- hand smoke contributes to metabolic diseases.
In conclusion, we show that second-hand cigarette smoke stimulates lipid accumulation in the liver and that this effect is mediated by AMPK and SREBP-1. Furthermore, we show for the first time that inactivation of AMPK stimulates activation of SREBP and leads to an increase in lipid accumulation. These findings point to new molecular targets for therapy that can reverse the effects of second-hand smoke on atherosclerosis and development of NAFLD.
Acknowledgements
We thank M. Petreaca for discussion and critical reading of the paper, F. Sladek for the CMV-β-galactosidase plasmid, and K. Chellapa for help with the Luciferase assay.
Abbreviations
- ACC
acetyl-CoA carboxylase
- ADRP
adipose differentiation-related protein
- AICAR
5-aminoimidazole-4-carboxamide ribonucleoside
- AMPK
AMP-activated protein kinase
- ApoB
apolipoprotein B
- FAS
fatty acid synthase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HMGCR
3-hydroxy-3-methylglutaryl CoA reductase
- MSW
mainstream whole
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide, a tetrazole
- SREBPs
sterol-regulated element-binding proteins
- SSW
sidestream whole
- LDLR
low density lipopotein receptor
- NAFLD
non-alcoholic fatty liver diseases
- TPM
total particulate matter
- TG
triglyceride
- TC
total cholesterol
Footnotes
The underlying research reported in the study was funded by NIH (HL77448 and HL89940) and in part by the Tobacco-Related Disease Research Program TRDRP (11DT-0244). The authors who have taken part in this study declared that they do not have anything to disclose regarding funding from industry or conflict of interest with respect to this manuscript.
References
- 1.Smith CJ, Fischer TH. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158:257–267. doi: 10.1016/s0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, Hiratzka LF, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation. 1998;97:1876–1887. doi: 10.1161/01.cir.97.18.1876. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 5.Brown MS, Goldstein JL. How Ldl receptors influence cholesterol and atherosclerosis. Sci Am. 1984;251:58–66. doi: 10.1038/scientificamerican1184-58. [DOI] [PubMed] [Google Scholar]
- 6.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22:437–443. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- 8.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 11.Bradbury MW. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194–G198. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- 12.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JL, Rawson RB, Brown MS. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch Biochem Biophys. 2002;397:139–148. doi: 10.1006/abbi.2001.2615. [DOI] [PubMed] [Google Scholar]
- 14.Pai JT, Guryev O, Brown MS, Goldstein JL. Differential stimulation of cholesterol and unsaturated fatty acid biosynthesis in cells expressing individual nuclear sterol regulatory element-binding proteins. J Biol Chem. 1998;273:26138–26148. doi: 10.1074/jbc.273.40.26138. [DOI] [PubMed] [Google Scholar]
- 15.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nohturfft A, Brown MS, Goldstein JL. Topology of SREBP cleavage-activating protein, a polytopic membrane protein with a sterol-sensing domain. J Biol Chem. 1998;273:17243–17250. doi: 10.1074/jbc.273.27.17243. [DOI] [PubMed] [Google Scholar]
- 18.Foufelle F, Ferre P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stapleton D, Gao G, Michell B, Widmer J, Mitchelhill K, Teh T, et al. Mammalian 5′-AMP-activated protein kinase non-catalytic subunits are homologs of proteins that interact with yeast Snf1 protein kinase. J Biol Chem. 1994;269:29343–29346. [PubMed] [Google Scholar]
- 21.García-Villafranca J, Guillén A, Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie. 2008;90:460–466. doi: 10.1016/j.biochi.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Wong LS, Green HM, Feugate JE, Yadav M, Nothnagel EA, Martins-Green M. Effects of “second-hand“ smoke on structure and function of fibroblasts, cells that are critical for tissue repair and remodeling. BMC Cell Biol. 2004;5:13. doi: 10.1186/1471-2121-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boren J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity TL. Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective apo-B100. J Clin Invest. 1998;101:1084–1093. doi: 10.1172/JCI1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan H, Wong LS, Bhattacharya M, Ma C, Zafarani M, Yao M, et al. The effects of second-hand smoke on biological processes important in atherogenesis. BMC Cardiovasc Disord. 2007;7:1. doi: 10.1186/1471-2261-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petreaca ML, Yao M, Liu Y, DeFea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007;18:5014–5023. doi: 10.1091/mbc.E07-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 27.Linton MF, Farese RV, Jr, Chiesa G, Grass DS, Chin P, Hammer RE, et al. Transgenic mice expressing high plasma concentrations of human apolipoprotein B100 and lipoprotein(a) J Clin Invest. 1993;92:3029–3037. doi: 10.1172/JCI116927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veniant MM, Sullivan MA, Kim SK, Ambroziak P, Chu A, Wilson MD, et al. Defining the atherogenicity of large and small lipoproteins containing apolipoprotein B100. J Clin Invest. 2000;106:1501–1510. doi: 10.1172/JCI10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell-Huynh DA, Farese RV, Jr, Johnson DF, Flynn LM, Pierotti V, Newland DL, et al. Transgenic mice expressing high levels of human apolipoprotein B develop severe atherosclerotic lesions in response to a high-fat diet. J Clin Invest. 1995;95:2246–2257. doi: 10.1172/JCI117915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 31.Loria P, Lonardo A, Bellentani S, Day CP, Marchesini G, Carulli N. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: an open question. Nutr Metab Cardiovasc Dis. 2007;17:684–698. doi: 10.1016/j.numecd.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24:1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 33.Mitry RR, Hughes RD, Dhawan A. Progress in human hepatocytes: isolation, culture & cryopreservation. Semin Cell Dev Biol. 2002;13:463–467. doi: 10.1016/s1084952102001350. [DOI] [PubMed] [Google Scholar]
- 34.Donkin SS, Armentano LE. Preparation of extended in vitro cultures of bovine hepatocytes that are hormonally responsive. J Anim Sci. 1993;71:2218–2227. doi: 10.2527/1993.7182218x. [DOI] [PubMed] [Google Scholar]
- 35.Gregory PG, Connolly CK, Toner M, Sullivan SJ. In vitro characterization of porcine hepatocyte function. Cell Transplant. 2000;9:1–10. doi: 10.1177/096368970000900101. [DOI] [PubMed] [Google Scholar]
- 36.Wu JC, Merlino G, Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci USA. 1994;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada W, Maeshima A, Zhang Y-Q, Hasegawa Y, Kuwano H, Kojima I. Assessment of the function of the {beta}C-subunit of activin in cultured hepatocytes. Am J Physiol Endocrinol Metab. 2004;287:E247–E254. doi: 10.1152/ajpendo.00390.2003. [DOI] [PubMed] [Google Scholar]
- 38.Masson S, Scotte M, Francois A, Coeffier M, Provot F, Hiron M, et al. Changes in growth factor and cytokine mRNA levels after hepatectomy in rat with CCl4-induced cirrhosis. Am J Physiol Gastrointest Liver Physiol. 1999;277:G838–G846. doi: 10.1152/ajpgi.1999.277.4.G838. [DOI] [PubMed] [Google Scholar]
- 39.Russell MAH, Feyerabend C. Blood and urinary nicotine in nonsmokers. The Lancet. 1975;305:179–181. doi: 10.1016/s0140-6736(75)91355-0. [DOI] [PubMed] [Google Scholar]
- 40.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 41.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Lee T-S, Kolb EM, Sun K, Lu X, Sladek FM, et al. AMP-activated protein kinase is involved in endothelial no synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 43.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 44.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 45.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konrad D, Rudich A, Bilan PJ, Patel N, Richardson C, Witters LA, et al. Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia. 2005;48:954–966. doi: 10.1007/s00125-005-1713-7. [DOI] [PubMed] [Google Scholar]
- 47.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 48.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnes BR, Zierath JR. Role of AMP-activated protein kinase in the control of glucose homeostasis. Curr Mol Med. 2005;5:341–348. doi: 10.2174/1566524053766103. [DOI] [PubMed] [Google Scholar]
- 50.Aymerich I, Foufelle F, Ferre P, Casado FJ, Pastor-Anglada M. Extracellular adenosine activates AMP-dependent protein kinase (AMPK) J Cell Sci. 2006;119:1612–1621. doi: 10.1242/jcs.02865. [DOI] [PubMed] [Google Scholar]
- 51.Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na, K-ATPase endocytosis. J Clin Invest. 2008;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barb D, Neuwirth A, Mantzoros CS, Balk SP. Adiponectin signals in prostate cancer cells through Akt to activate the mammalian target of rapamycin pathway. Endocr Relat Cancer. 2007;14:995–1005. doi: 10.1677/ERC-06-0091. [DOI] [PubMed] [Google Scholar]
- 53.Moir H, Butcher L, Jones KP, Hughes MG, Neale H, Jia H, et al. AMPK inactivation in mononuclear cells: a potential intracellular mechanism for exercise-induced immunosuppression. Appl Physiol Nutr Metab. 2008;33:75–85. doi: 10.1139/H07-135. [DOI] [PubMed] [Google Scholar]
- 54.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 55.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 56.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J-i, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 57.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 58.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 59.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA. 2003;100:14217–14222. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kakuma T, Lee Y, Higa M, Wang Z-w, Pan W, Shimomura I, et al. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci USA. 2000;97:8536–8541. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyazaki T, Shimada K, Mokuno H, Daida H. Adipocyte derived plasma protein, adiponectin, is associated with smoking status in patients with coronary artery disease. Heart. 2003;89:663–664. doi: 10.1136/heart.89.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thamer C, Stefan N, Stumvoll M, Häring H, Fritsche A. Reduced adiponectin serum levels in smokers. Atherosclerosis. 2005;179:421–422. doi: 10.1016/j.atherosclerosis.2004.12.039. [DOI] [PubMed] [Google Scholar]