Abstract
Background
Case management (CM) is a systematic approach to supplement physician-centered cardiovascular disease (CVD) prevention efforts. Research is limited on its implementation and efficacy in low-income, ethnic minority populations.
Methods
We conducted a randomized clinical trial to evaluate a nurse and dietitian-led CM program for reducing major cardiovascular risk factors in low-income, primarily ethnic-minority patients in a county healthcare system, 63% of whom had Type II diabetes mellitus. The primary outcome was the Framingham risk score (FRS).
Results
A total of 419 patients at elevated risk of CVD events were randomized and followed for a mean of 16 months (81% retention). Mean FRS was significantly lower for the CM vs. UC group at follow-up (7.80 [95% CI, 7.21 to 8.38] vs. 8.93 [95% CI, 8.36 to 9.49]; P=.001) after adjusting for baseline FRS. This is equivalent to 5 fewer heart disease events per 1000 individuals per year attributable to the intervention or 200 individuals receiving the intervention to prevent one event per year. The pattern of group differences in the FRS was similar in subgroups defined a priori by sex and ethnicity. The main driver of these differences was lowering mean (SD) systolic (−4.2 [18.5] mm Hg vs. 2.6 [22.7] mm Hg; P=.003) and diastolic (−6.0 [11.6] mm Hg vs. 3.0 [11.7] mm Hg; P=.02) blood pressure for the CM vs. UC group.
Conclusions
Nurse and dietitian case management targeting multifactor risk reduction can lead to modest improvements in CVD risk factors among high-risk patients in low-income, minority populations receiving care in county health clinics.
Keywords: cardiovascular diseases, prevention, risk factors, trials
Cardiovascular disease (CVD) affects 80.7 million Americans with estimated national costs of $448.5 billion in 2008.1 Age, sex, high blood pressure (BP), smoking, dyslipidemia, obesity, and diabetes are widely recognized as major risk factors, frequently clustering and interacting multiplicatively in predicting risk for coronary and other atherosclerotic vascular diseases.2 While CVD and its major risk factors affect every racial/ethnic group and social class, they disproportionately burden ethnic minorities and low income communities.1, 3 These population subgroups also are more likely to receive inadequate cardiac care compared with Whites and higher income individuals.4
Innovative approaches are needed to supplement traditional care models that emphasize episodic, acute delivery of physician services.5 Case management (CM) is a comprehensive, longitudinal approach involving multidisciplinary teams of physicians and other clinicians, who cooperate to identify, manage and coordinate care of patients with costly, high-risk conditions. Evidence supports the efficacy of intensive CM, particularly in diabetes6 and prevention of subsequent events for patients with existing CVD.7 Experience with CM among low-income, ethnic minority patients is limited, although some studies show favorable results.8-11 Translation of proven interventions into community practice is a strategic imperative for eliminating health disparities.12
Chronic disease management in low-income, ethnic minorities is a unique challenge for local health care systems, particularly county systems that serve the most disadvantaged. Such systems are often under-resourced to cope with a complex clinical load and use a primary care delivery model ill-suited to provide comprehensive disease management with continued follow-up support. The Stanford and San Mateo Heart to Heart (HTH) project was a two-arm RCT to evaluate the feasibility and clinical utility of a CM model of multifactor CVD risk reduction for low-income, ethnically diverse patients served by the county healthcare system in San Mateo County, California. We hypothesized that compared with usual care, CM participants would experience greater improvements in Framingham risk scores13 and in individual modifiable risk factors.
METHODS
A complete description of the research design and methods of HTH is available.14 We therefore provide a condensed description.
Recruitment
A branch of San Mateo County, California government, the San Mateo Medical Center (SMMC) serves the County’s sizable low-income population, most of whom have Medicaid or a County-sponsored indigent care plan. Patients were recruited between October 2003 and April 2005 from four SMMC outpatient clinics. These clinics were chosen for their accommodating clinic environment, patient volume, patient demographics, and established adult primary care services. All data acquisition and CM visits took place within the clinics where the patients received primary care services.
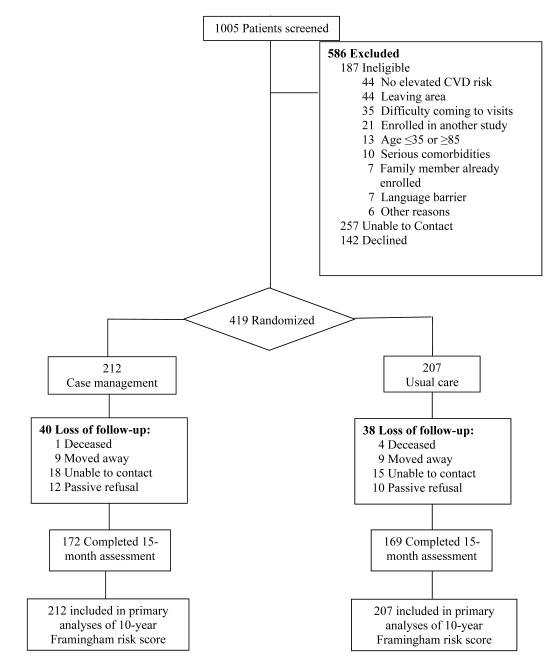
Physicians at study clinics were asked to refer patients based on simplified, partial eligibility criteria. Referred patients were then formally screened by study staff for interest in HTH and eligibility based on self-completed questionnaires and clinical measurements. Of 1005 patients referred, 267 were unreachable and 142 declined participation (Figure 1). Through screenings by phone or at baseline visits, 187 additional patients were excluded for medical, psychosocial or personal reasons that would prevent them from providing informed consent or complying with study protocols. A total of 419 (41%) patients were eligible and provided informed consent to participate. Participants were men and women between the ages of 35 and 85 years who had moderately to severely elevated levels of major modifiable CVD risk factors with or without prior history of atherosclerotic CVD or diabetes mellitus.14 The study was approved by Institutional Review Boards at Stanford University and SMMC.
Figure 1.
Patient flowchart.
Randomization
Participants were equally randomized to the CM group or the usual care (UC) group, using the permuted block method (block size=6) stratified by gender and ethnicity (Hispanic vs. Non-Hispanic) within each clinic. Concealment of treatment allocation was achieved by having study staff not involved in recruitment, intervention or assessment generate the sequence of treatment allocations and prepare randomization letters. The letters were sealed in sequentially numbered opaque envelopes and opened first-hand by patients upon randomization following completion of baseline assessment.
Intervention
Participants in both UC and CM groups were instructed to continue routine medical care with their PCP. In addition, CM participants received a one-on-one nurse- and dietitian-led case management intervention previously demonstrated to reduce multiple major risk factors in patients with or at risk for CVD, including medically underserved patients.7, 9 As in our prior studies, case managers emphasized behavior change and medical management strategies. HTH differed by focusing on high-risk patients served by public health primary care clinics. Unlike prior interventions, all patients had primary care physicians who integrated their care with the case managers’ semi-autonomous, protocol-based approach to risk factor management.
Nurse and dietitian case managers were trained and supervised by a senior nurse practitioner (KB) and the principal investigator (RSS). Principal CM strategies included: 1) intensive, individualized care, 2) continuity of care and coordination with primary and specialty care, 3) self-management support, 4) implementation of evidence-based treatment guidelines for primary and secondary CVD prevention,15, 16 and 5) behavioral counseling to improve physical activity, nutrition, weight management, stress reduction, and medication adherence. The theoretical underpinning of behavior change protocols was derived from Social Cognitive Theory17 and the Transtheoretical Model of Behavior Change.18 Guided by intervention protocols, intensity of case management and treatment goals were individualized based on the patient’s clinical and risk factor status, personal preferences and available resources (home, work, community, and health care access). Case management was delivered in Spanish or English during face-to-face clinic visits supplemented by telephone consultations, as needed. For non-English, non-Spanish speaking patients, CM visits were translated by an accompanying adult family member or friend. Protocol-designated visits had scheduled durations of 30-60 minutes and occurred at 4- to 6-week intervals during the initial 6 months and every 2 to 3 months thereafter, with a per-patient target of 8 to 10 visits over 15 months. Each visit began with a brief physical examination and a review of the patient’s risk reduction plan, progress and problems. Counseling was then provided and referrals made as needed.
Baseline and Follow-up Assessments
Participants completed assessments at baseline and at 15 months. Follow-up assessments were completed by research staff other than the case managers who had been directly responsible for the patient’s care. Additionally, charts with baseline visit data were not available to staff at the follow-up visit. A fasting blood sample was obtained by fingerstick19 for analysis of blood glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and calculation of low-density lipoprotein cholesterol (LDL-C) (LDX Analyzer, Cholestech Corporation, Hayward, CA). Plasma hemoglobin A1c (HbA1c) was also measured (Cholestech GDX Analyzer). Height (baseline only), weight, and waist circumference were measured, and body mass index (BMI) calculated. Resting BP was measured in the seated position in both upper arms using well-maintained equipment and properly sized cuffs, and the average of the two readings was used.
Primary Outcome
The pre-specified primary outcome variable was the global cardiovascular risk score, according to sex-specific Framingham point score algorithms of Wilson et al. in 1998.13 Although those algorithms were developed for prediction of absolute coronary risk among patients without clinical manifestations of coronary heart disease (CHD), they combine the major risk factors recognized for coronary and other atherosclerotic CVD: age, sex, high BP, smoking, dyslipidemia, and diabetes status.2 We used risk scores as a composite measure of change in modifiable major risk factors, rather than as a predictor of risk, for both patients with and without known CVD. This approach has been previously used in evaluating effects of multifactor risk reduction interventions.20, 21 As an outcome measure, advantages of FRS outweighed known calibration issues in minority populations 22 and possible lack of specificity for patients with diabetes. Baseline age was applied when calculating FRS at 15 months. All participants older than 74 years (n=13) were coded as age 74 years.
Statistical Analysis
To assess group comparability on baseline demographic and clinical characteristics, Student’s t tests for continuous variables and Pearson χ2 tests for categorical variables were performed. Following our a priori primary analysis plan, intervention effect on the primary outcome, FRS, at 15 months was examined on an intention-to-treat basis using a mixed-effects regression model adjusted for baseline FRS. The model also took into account random effects associated with physicians and clinics in a hierarchical structure. The magnitudes and patterns of missing data were examined by randomized group and no significant differences were detected. Missing outcomes at the 15-month follow-up were imputed using the baseline-observation-carried-forward method. The same mixed-effects model was used for protocol-specific subgroup analyses defined by sex and ethnicity. Sensitivity analyses were performed to assess the robustness of analytical results. First, we evaluated intervention effects using alternate risk models. We repeated the primary analyses with alternative global cardiovascular risk scores and stroke risk scores available from Framingham and others.23 24, 25 Although limited by sample size, we also analyzed subgroups using coronary risk functions specific to patients with Type 2 diabetes (UKPDS)26 (63% of study sample) and those with existing CVD27, 28 (19%). Second, we evaluated the sensitivity of study results to missing outcomes by replacing missing data with values from a multiple regression imputation model.29 Third, we performed a complete-case analysis on the subset of patients who attended a 15-month follow-up visit to estimate efficacy. Results from these sensitivity analyses were consistent with those from primary analyses; we report the latter only. In addition to between-group differences, mean (95% confidence interval [CI]) changes from baseline to 15 months by group are reported. All analyses of individual risk factors were performed on the subset of patients with complete follow-up and were compared using Student’s t tests. All reported P values and 95% CIs are 2-sided. Statistical significance was set at P<.05. All analyses were performed using SAS 4.1 (SAS Institute, Cary, NC).
This trial was designed to enroll 400 patients equally to the CM or UC group. This sample size was powered to detect a group difference in the FRS at 15 months of 1/2 of a standard deviation (SD) at an α level of 0.01 and power of 87% after accounting for a 25% loss to follow-up.
RESULTS
Baseline Characteristics
We exceeded our target sample size and enrolled 419 low-SES, ethnically diverse patients to CM (n=212) and UC (n=207). Baseline demographic and clinic characteristics were similar between the CM and UC groups (Table 1). CM patients, however, were less likely to have completed 8th grade (p=0.02). The rate of retention was 81% for a mean follow-up of 16 months (range: 7-25 months) and did not vary by randomized group (P=0.89).
Table 1.
Baseline characteristics of participants
| Characteristic | Overall (n=419) |
CM Group (n=212) |
UC Group (n=207) |
P value |
|---|---|---|---|---|
| Demographic and Social | ||||
| Age, year | 55.1 ± 9.6 | 54.4 ± 9.5 | 55.8 ± 9.7 | 0.17 |
| Female, % | 65.6 | 64.6 | 66.7 | 0.66 |
| Hispanic, % | 63.0 | 63.2 | 62.8 | 0.93 |
| African American, % | 9.6 | 9.9 | 9.2 | 0.80 |
| Asian/Pacific Islander, % | 11.9 | 11.3 | 12.6 | 0.70 |
| Less than 8th grade, % | 44.9 | 50.7 | 39.0 | 0.02 |
| Unemployed, disabled, retired, % |
60.5 | 63.2 | 57.7 | 0.26 |
| Unable to speak, read or understand English, % |
49.1% | 50.5 | 48.1 | 0.62 |
| Health Status | ||||
| 10-year FRS | ||||
| men | 7.2 ± 2.9 | 7.1± 3.1 | 7.5 ± 2.7 | 0.42 |
| women | 9.8 ± 4.3 | 9.6 ± 4.4 | 10.0 ± 4.3 | 0.51 |
| TC, mg/dL | 190.2 ± 41 | 187.7 ± 39.7 | 192.7 ± 42.4 | 0.22 |
| LDL-c, mg/dL | 104.2 ± 32.7 | 104.2 ± 33.6 | 104.2 ± 31.8 | 0.99 |
| HDL-c, mg/dL | 45.7 ± 12.1 | 45.0 ± 12.2 | 46.3 ± 12.1 | 0.27 |
| TC: HDL-c | 4.5 ± 1.7 | 4.4 ± 1.4 | 4.5 ± 1.9 | 0.72 |
| TG, mg/dL | 201.0 ± 105.7 | 196.4 ± 101.1 | 205.5 ± 110.1 | 0.38 |
| SBP, mmHg | 133.9 ± 19.8 | 132.7 ± 19.4 | 135.1 ± 20.2 | 0.20 |
| DBP, mmHg | 79.6 ± 10.4 | 79.6 ± 10.6 | 79.6 ± 10.1 | 0.94 |
| CVD, % | 18.9% | 17.9% | 19.8% | 0.62 |
| Diabetes, % | 63.0% | 64.2% | 61.8% | 0.62 |
| A1C, %† | 7.6 ± 1.7 | 7.6 ± 1.7 | 7.7 ± 1.7 | 0.87 |
| FBG, mg/dL† | 159.8 ± 58.3 | 161.2 ± 62.2 | 158.2 ± 54.2 | 0.68 |
| Metabolic syndrome, % | 59.2% | 59.0% | 59.4% | 0.92 |
| Cigarette Smoking, % | 16.2% | 16.0% | 16.4% | 0.96 |
| Family history of CAD or stroke, % |
45.4% | 44.3% | 46.4% | 0.68 |
| Body mass index, kg/m2 | ||||
| men | 33 ± 7.6 | 33.1 ± 7.1 | 32.9 ± 8.2 | 0.87 |
| women | 35.4 ± 8.6 | 35.2 ± 7.2 | 35.5 ± 9.9 | 0.76 |
| Waist circumference, in | ||||
| men | 41.0 ± 7.2 | 41.2 ± 6.5 | 40.9 ± 8.0 | 0.83 |
| women | 42.2 ± 6.1 | 42.4 ± 6.1 | 42.1 ± 6.2 | 0.68 |
Plus-minus values are mean ± SD. To convert values for cholesterol to millimoles per liter, multiply by 0.026. To convert values for triglycerides to millimoles per liter, multiply by 0.011. To convert values for glucose to millimoles per liter, multiply by 0.056. To convert values for waist circumference to cm, multiply by 2.54.
Values are reported only for patients with diagnosed diabetes.
Primary Outcome: Framingham Risk Score
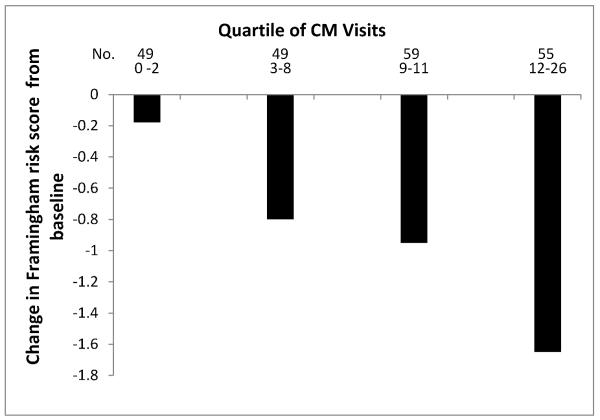
Compared with baseline, mean FRS decreased in the CM group (−0.92; 95% CI, −1.28 to −0.57) whereas it remained unchanged in the UC group (−0.19; −0.56 to 0.18) (Table 2). Among patients randomly assigned to receive CM, the amount of decline in FRS was associated with the number of face-to-face visits (r =0.22, P=.001; Figure 2). The mean (SD) number of CM visits was 8.0 (5.3), equivalent to 11.2 (6.8) hours of face-to-face contact time.
Table 2.
Mean Framingham risk score at 15 months and change from baseline for all patients and by sex and ethnicity
| Analysis | CM Group 95% CI |
UC Group 95% CI |
Difference between groups |
P value |
|---|---|---|---|---|
| All Mean |
7.80 (7.21 to 8.38) | 8.93 (8.36 to 9.49) | −1.13 (−1.94 to −0.32) | 0.001 |
| Change from baseline |
−0.92 (−1.28 to −0.57) | −0.19 (−0.56 to 0.18) | −0.73 (−1.24 to −0.22) | 0.001 |
| Men Mean |
6.05 (5.32 to 6.79) | 7.51 (6.83 to 8.19) | −1.45 (−2.45 to −0.45) | 0.002 |
| Change from baseline |
−0.99 (−1.53 to −0.44) | 0.06 (−0.52 to 0.64) | −1.05 (−1.83 to −0.26) | 0.002 |
| Women Mean |
8.75 (7.99 to 9.52) | 9.64 (8.88 to 10.39) | −0.89 (−1.96 to 0.18) | 0.06 |
| Change from baseline |
−0.88 (−1.35 to −0.42) | −0.31 (−0.79 to 0.17) | −0.57 (−1.24 to 0.10) | 0.06 |
| Hispanics Mean |
7.87 (7.17 to 8.58) | 8.69 (7.94 to 9.44) | −0.82 (−1.84 to 0.20) | 0.07 |
| Change from baseline |
−0.78 (−1.23 to −0.34) | −0.26 (−0.77 to 0.24) | −0.52 (−1.19 to 0.15) | 0.07 |
| Non-Hispanics Mean |
7.67 (6.62 to 8.71) | 9.32 (8.47 to 10.18) | −1.66 (−3.00 to −0.31) | 0.004 |
| Change frombaseline | −1.15 (−1.75 to −0.56) | −0.07 (−0.61 to 0.48) | −1.09 (−1.89 to −0.29) | 0.004 |
Figure 2.
Mean change in Framingham risk score from baseline by quartile of face-to-face visits completed by patients randomly assigned to receive case management.
Compared with the UC group, the FRS of the CM group was significantly lower at 15 months (difference between groups, −1.13; 95% CI, −1.94 to −0.32; P=.001) after adjusting for baseline FRS and the effects of clinic and physician (Table 2). This is equivalent to 5 less heart disease events per 1000 individuals per year attributable to the intervention or 200 individuals receiving the intervention to prevent one event per year. Variations in the intervention effect did not differ significantly among physicians or clinics. In addition, mean FRS at 15 months was consistently lower for CM vs. UC across tertiles of baseline FRS (data not shown).
The pattern of results was similar in subgroups defined by sex and ethnicity, although results were not quite significant for women and Hispanics. Compared with the UC group, the CM group decreased the FRS by a mean of 1.45 points (P=.002) in men (equivalent to 5 fewer heart disease events per 1000 individuals per year), 0.89 points (P=.06) in women (4 fewer events), 0.82 points (P=.07) in Hispanics (3 fewer events), and 1.66 points (P=.004) in non-Hispanics (6 fewer events, Table 2).
Secondary Outcomes
Table 3 displays changes from baseline in selected clinical and metabolic risk factors by randomized group. Mean (SD) change from baseline in systolic BP was −4.2 (18.5) mm Hg in the CM group and 2.6 (22.7) mm Hg in the UC group (P=.003). Diastolic BP declined in both groups but magnitude of reduction was significantly greater for the CM group (P=.02). For diabetics, those in the CM group demonstrated a significantly greater decrement in fasting blood glucose than UC patients (−21.0 vs. −1.4 mg/dL; P=.01). For the CM vs. UC group, mean changes in LDL-C, HDL-C, BMI, and for diabetic patients, HbA1c were favorable for CM, but not statistically significant. Unfavorable, but non-significant mean changes in triglycerides and waist circumference in women were noted for CM.
Table 3.
Changes from baseline in individual risk factors for patients with complete follow-up
| Risk Factor* | n | CM Group mean ± SD |
n | UC Group mean ± SD |
P value |
|---|---|---|---|---|---|
| TC, mg/dL | 166 | −14.7 ± 38.0 | 167 | −15.4 ± 38.6 | 0.87 |
| LDL-c, mg/dL | 145 | −14.9 ± 35.2 | 147 | −10.6 ± 33.0 | 0.29 |
| HDL-c, mg/dL | 164 | −0.9 ± 10.8 | 163 | −2.5 ± 11.9 | 0.22 |
| TG, mg/dL | 163 | −2.7 ± 89.9 | 165 | −9.4 ± 97.7 | 0.52 |
| SBP, mmHg | 170 | −4.2 ± 18.5 | 170 | 2.6 ± 22.7 | 0.003 |
| DBP, mmHg | 170 | −6.0 ± 11.6 | 170 | −3.0 ± 11.7 | 0.02 |
| A1C, % † | 107 | −0.02 ± 1.6 | 99 | 0.3 ± 1.5 | 0.15 |
| FBG, mg/dL † | 107 | −21.0 ± 56.5 | 102 | −1.4 ± 52.5 | 0.01 |
| BMI, kg/m2 | |||||
| men | 55 | −0.3 ± 1.8 | 53 | 0.1 ± 2.5 | 0.29 |
| women | 109 | −0.2 ± 2.7 | 107 | −0.02 ± 2.1 | 0.63 |
| Waist circumference, in | |||||
| men | 47 | −0.2 ± 2.8 | 48 | 0.3 ± 2.7 | 0.32 |
| women | 101 | 0.2 ± 4.0 | 103 | −0.3 ± 4.2 | 0.47 |
To convert values for cholesterol to millimoles per liter, multiply by 0.026. To convert values for glucose to millimoles per liter, multiply by 0.056. To convert values for waist circumference to cm, multiply by 2.54.
Values are reported only for patients with diagnosed diabetes.
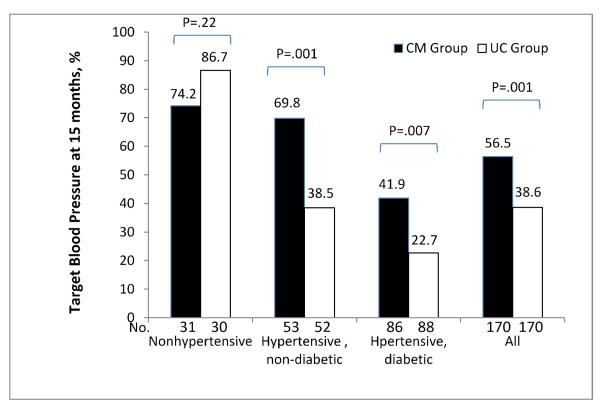
Patients were divided into three categories according to baseline clinical status: nonhypertensive, hypertensive and non-diabetic, and hypertensive and diabetic. Blood pressure control was defined as systolic BP <140 mm Hg and diastolic BP <90 mm Hg for non-diabetics and systolic BP <130 mm Hg and diastolic BP <80 mm Hg for diabetics (no participant had chronic kidney disease). Target BP was achieved at 15 months in 56% of CM patients and 38% of UC patients overall (P=.001, Figure 3). Blood pressure control rates were also higher in the CM group, compared with the UC group, for hypertensive patients with (42% vs. 23%; P=.007) or without diabetes (70% vs. 38%, P=.001). BP control rate among patients who were non-hypertensive at baseline did not differ significantly by randomized group. Our search for mediators of the intervention’s effect on systolic BP identified reduction in dietary saturated fats as a mediating variable (P=0.03), while number of antihypertensive medications was not.
Figure 3.
Percentage of participants with target blood pressure at 15 months by randomized group according to hypertension and diabetes status. Target blood pressure is <140/90 mmHg for the nonhypertensive and hypertensive, non-diabetic subgroups and is <130/80 mmHg for the hypertensive, diabetic group.
Use of alternative prediction models indicated substantial agreement with the FRS model. Models that relied heavily on blood pressure (e.g., Framingham stroke score) suggested more dramatic improvements associated with CM than we report, while those relying more heavily on other parameters (e.g., UKPDS) show favorable, but non-significant net changes.
The 15-month intervention cost $896 per person in 2008 dollars including labor, supplies, and office space. In routine practice we expect that the intervention would be slightly less intensive but would extend beyond 15 months. If a RN delivers all care then we estimate a cost of $371 for year one and $337 annually thereafter (2008 dollars). If instead an internist delivers all care then the estimated cost rises to $686 in year one and $647 annually thereafter.
Serious Adverse Events
Five patients died during the study; four died in the UC group and one died in the CM group (P=.21). Emergency department (ED) visits were equally likely among the CM participants (28%) compared to those in UC (25%, P=.46). The SMMC system uses their emergency room for all after-hours urgent and emergency care for patients seen in the primary care clinics. Among intervention participants, two episodes of diabetic hypoglycemia occurred resulting in ED visits, likely related to the increased intensity of glucose control strategies in CM. The rates of hospitalizations were similar between the two groups (18 hospitalizations per 100 participants in CM vs. 16 in UC). Hospitalizations for cardiac diagnoses (including chest pain) were more frequent in CM (8.0 hospitalization per 100 participants) than in UC (3.4, P=.04). The Data and Safety Monitoring Board determined that none of the deaths, cardiovascular events, or other hospitalizations were causally related to study participation. CM participants, however, likely had more opportunities to receive advice to have cardiac symptoms evaluated.
DISCUSSION
We tested a CM intervention targeting multifactor cardiovascular risk reduction for persons at elevated risk of CVD events in a low-income, predominantly ethnic minority, largely diabetic population in a county healthcare system. The intervention significantly lowered global cardiovascular risk score, compared with usual care. The intervention effect on global risk score was similar for all subgroups by sex and ethnicity. While not always statistically significant, CM yielded favorable outcomes for individual cardiovascular risk factors with reduced BP the leading driver of reduced aggregate cardiovascular risk score.
The intervention provided high intensity contact time with highly trained nurse and dietitian care managers, with mean face-to-face contact time of 11.2 hours per participant over an average 16 months follow-up (about 45 minutes per month). Due to gaps in SMMC administrative records we could not determine how the intervention affected time spent with primary care or specialty providers. Given the population’s social marginalization, substantial mobility between housing locations, travel in and out of the United States, and need to prioritize survival issues over preventive health care, participant retention was an acknowledged challenge. Nonetheless, participant retention was 81% over a mean follow-up of 16 months. A high rate of retention (91% over 12 months) also was observed in our previous study using the same CM model in a similarly low-income, multiethnic population of patients.9 This smaller (n=148), predecessor intervention focused on indigent patients without primary care providers seen in free clinics. The current study extended the care model of our previous study, including integrating CM and physician activities, and allowing CMs to initiate and titrate some medications. Our previous study reported significant improvements in BP, lipids, and blood glucose for CM, relative to usual care. Similarly, other studies evaluating multifactor cardiovascular risk reduction approaches also have demonstrated success in recruiting and retaining medically underserved, ethnic minority patients and achieving clinically meaningful changes in biologic risk factors.8-11
CM can enhance chronic disease care by facilitating guideline-concordant, patient-centered interventions that improve outcomes through intensive, individualized, longitudinal care.6, 7 Evidence supporting the utility of CM for multifactor cardiovascular risk reduction, however, is derived primarily from studies in patient populations with good access to health care. Our study adds to a growing body of evidence8-11 demonstrating the feasibility and efficacy of multifactor cardiovascular risk CM in medically underserved populations. Our success in modifying cardiovascular risk factors in HTH was less than expected based on recent clinical trials of multifactor risk reduction interventions among patients at varying levels of CVD risk.7-9 Levels of LDL-C and TC at baseline were normal or borderline in a majority of our participants, which likely reduced our ability to effect more pronounced improvements in FRS. Also, the high prevalence of diabetes (63%) presented special challenges. Furthermore, several factors specific to a multiethnic, low-income population may have led to less favorable results. These include cultural/language barriers, increased emotional stress due to low SES, financial barriers to medications, focus on survival issues without a long-term perspective, and limited resources to facilitate lifestyle changes. However, such populations might particularly benefit from multifactor cardiovascular risk management: most current care focuses on acute care needs, so that baseline prevention services may be particularly lacking. Further, gaps between guideline and actual risk factor parameters are wider in these populations despite their adverse cardiovascular risk factor profiles4 – the so-called “inverse care law” whereby medical care is most lacking for patients in greatest need.30
Research also shows that, to maximize benefits of multifactor cardiovascular risk management for low-income, ethnic minority patients, strategies that address known social, cultural, and financial barriers to optimal health care for disadvantaged populations are needed.8 Clinical prevention services, including clinical CM, will fall short of their promise if provided in isolation from a patient’s living environment.31 It may be unrealistic to expect patients to implement advice given in medical settings without a complimentary strategy focused on their home and neighborhood environments.32 In HTH, case managers did coordinate access to community resources (e.g., smoking cessation programs and pharmacy support programs), but all direct CM services were provided within health centers. Previous research has shown that outreach by community health workers can improve CVD prevention.8
Our recruitment process yielded participants who were a high-risk subset of the SMMC population. As a population requiring more intensive outpatient services, they form an important group in which to assess the effectiveness of CM. While use of point-of-care laboratory testing provided immediate feedback, this strategy introduced additional measurement variation and may have hampered detection of outcome differences. The Framingham risk functions13 integrate the risk factors that account for most of CVD burden.2 Consistent findings when other risk functions (including those specific to patient with diabetes and existing CVD)23, 26-28 were applied suggests out result’s robustness. We acknowledge that these models were not developed for persons with established CVD,13, 23 who accounted for 19% of our sample. In addition, application of any risk function developed in a single cohort to populations with differing background risk can be associated with misclassifications and might ideally require recalibration or consideration of other risk factors to improve prediction.22 These concerns are mitigated by using the FRS as a composite measure of change in modifiable risk factors, not as a predictor of risk.
Our CM approach to multifactor cardiovascular risk reduction was efficacious. These findings suggest that a multifactor risk reduction approach can foster improved cardiovascular care and outcomes for high-risk patients in low-income, ethnic minority populations.
Acknowledgments
Funding sources: This research was primarily funded by a research award from the National Heart, Lung, and Blood Institute (R01 HL070781). It was also supported with resources and the use of facilities at the Veteran Affairs Palo Alto Health Care System, Menlo Park, CA. Neither of these organizations played a role in the design, implementation or reporting of the study. Additional resources were provided by the San Mateo Medical Center. This organization provided guidance on the design, implementation and reporting of the project.
Footnotes
Disclosures: The authors declare no conflict of interests related to the contents of this report. RSS has consulted for Bayer Corporation and has had grants/contracts with Procter and Gamble, GlaxoSmithKline, Toyo Shinyaku, and Wako. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Drs. Stafford, Ma, and Xiao had access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Jan 29;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003 Aug 20;290(7):891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Benjamin EJ, Fabunmi RP, Bonow RO. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: executive summary. Circulation. 2005 Mar 15;111(10):1339–1349. doi: 10.1161/01.CIR.0000157740.93598.51. [DOI] [PubMed] [Google Scholar]
- 4.Lillie-Blanton M, Rushing OE, Ruiz S, Mayberry R, Boone L. [Last accessed August 4, 2009];Racial/Ethnic Differences in Cardiac Care: The Weight of the Evidence. http://www.kff.org/uninsured/loader.cfm?url=/commonspot/security/getfile.cfm&PageID =14167.
- 5.Ostbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005 May-Jun;3(3):209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight K, Badamgarav E, Henning JM, et al. A systematic review of diabetes disease management programs. Am J Manag Care. 2005 Apr;11(4):242–250. [PubMed] [Google Scholar]
- 7.Haskell WL, Alderman EL, Fair JM, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP) Circulation. 1994 Mar;89(3):975–990. doi: 10.1161/01.cir.89.3.975. [DOI] [PubMed] [Google Scholar]
- 8.Becker DM, Yanek LR, Johnson WR, Jr., et al. Impact of a community-based multiple risk factor intervention on cardiovascular risk in black families with a history of premature coronary disease. Circulation. 2005 Mar 15;111(10):1298–1304. doi: 10.1161/01.CIR.0000157734.97351.B2. [DOI] [PubMed] [Google Scholar]
- 9.Haskell WL, Berra K, Arias E, et al. Multifactor cardiovascular disease risk reduction in medically underserved, high-risk patients. Am J Cardiol. 2006 Dec 1;98(11):1472–1479. doi: 10.1016/j.amjcard.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Dennison CR, Post WS, Kim MT, et al. Underserved urban african american men: hypertension trial outcomes and mortality during 5 years. Am J Hypertens. 2007 Feb;20(2):164–171. doi: 10.1016/j.amjhyper.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Sisk JE, Hebert PL, Horowitz CR, McLaughlin MA, Wang JJ, Chassin MR. Effects of nurse management on the quality of heart failure care in minority communities: a randomized trial. Ann Intern Med. 2006 Aug 15;145(4):273–283. doi: 10.7326/0003-4819-145-4-200608150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005 Mar 15;111(10):1332–1336. doi: 10.1161/01.CIR.0000158134.24860.91. [DOI] [PubMed] [Google Scholar]
- 13.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May 12;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Lee KV, Berra K, Stafford RS. Implementation of case management to reduce cardiovascular disease risk in the Stanford and San Mateo Heart to Heart randomized controlled trial: study protocol and baseline characteristics. Implement Sci. 2006;1:21. doi: 10.1186/1748-5908-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002 Jul 16;106(3):388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 16.Smith SC, Jr., Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006 May 16;113(19):2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 17.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice Hall; Englewood Cliffs, N.J.: 1986. [Google Scholar]
- 18.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983 Jun;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 19.Carey M, Markham C, Gaffney P, Boran C, Maher V. Validation of a point of care lipid analyser using a hospital based reference laboratory. Ir J Med Sci. 2006 Oct-Dec;175(4):30–35. doi: 10.1007/BF03167964. [DOI] [PubMed] [Google Scholar]
- 20.Lear SA, Ignaszewski A, Linden W, et al. The Extensive Lifestyle Management Intervention (ELMI) following cardiac rehabilitation trial. Eur Heart J. 2003 Nov;24(21):1920–1927. doi: 10.1016/j.ehj.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Wister A, Loewen N, Kennedy-Symonds H, McGowan B, McCoy B, Singer J. One-year follow-up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. CMAJ. 2007 Oct 9;177(8):859–865. doi: 10.1503/cmaj.061059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Agostino RB, Sr., Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. Jama. 2001 Jul 11;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB, Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008 Feb 12;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007 Feb 14;297(6):611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994 Jan;25(1):40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 26.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci (Lond) 2001 Dec;101(6):671–679. [PubMed] [Google Scholar]
- 27.Califf RM, Armstrong PW, Carver JR, D’Agostino RB, Strauss WE. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for coronary disease events. Task Force 5. Stratification of patients into high, medium and low risk subgroups for purposes of risk factor management. J Am Coll Cardiol. 1996 Apr;27(5):1007–1019. doi: 10.1016/0735-1097(96)87733-3. [DOI] [PubMed] [Google Scholar]
- 28.D’Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000 Feb;139:272–281. doi: 10.1067/mhj.2000.96469. [DOI] [PubMed] [Google Scholar]
- 29.Little RJA, Rubin DB. Statistical Analysis with Missing Data. John Wiley & Sons, Inc.; New York: 1987. [Google Scholar]
- 30.Hart JT. The inverse care law. Lancet. 1971 Feb 27;1(7696):405–412. doi: 10.1016/s0140-6736(71)92410-x. [DOI] [PubMed] [Google Scholar]
- 31.Frank LD, Andresen MA, Schmid TL. Obesity relationships with community design, physical activity, and time spent in cars. Am J Prev Med. 2004 Aug;27(2):87–96. doi: 10.1016/j.amepre.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Berra K, Ma J, Klieman L, et al. Implementing cardiac risk-factor case management: lessons learned in a county health system. Crit Pathw Cardiol. 2007 Dec;6(4):173–179. doi: 10.1097/HPC.0b013e31815b5609. [DOI] [PubMed] [Google Scholar]