Abstract
Background
Pregnancy in older women is of great relevance, particularly in developed countries where many women experience pregnancy late in the childbearing age.
Methods
A hospital-based data analysis of 9506 delivery records from 1998 to 2003 at the Liverpool Women's Hospital was undertaken to assess pregnancy outcomes in older women of reproductive age.
Results
Overall, 2.4 % of mothers were >40 years of age (advanced), 5.6% were <20 years (adolescents), and 92% were between 20 and 40 years. The prevalence of low birthweight (LBW), preterm birth, and small for gestational age by maternal age category followed a U-shaped curve with nadirs in the middle age classes. The gestational age of older mothers was 1 week shorter than that for women aged 26–30 years (p = 0.005). Prim-iparaes >40 years were at higher risk for delivering a LBW (9.4% vs. 5.3%, p = 0.005) or a very preterm baby (8.9% vs. 4.4%, p = 0.001) than were multiparous mothers of the same age. There was an association between maternal advanced age and LBW (adjusted OR [AOR], 1.7, 95% CI 1.4-2.5, p = 0.001), preterm birth (AOR 1.4, 95% CI 1.1-2.4, p = 0.04), or very preterm birth (AOR 1.6, 95% CI 1.2-3.5, p = 0.002) after controlling for prenatal alcohol and smoking exposure, household deprivation, maternal anemia, obesity, parity, and single parenthood.
Conclusions
Pregnancy in older women is associated with adverse birth outcomes, particularly in primigravidas. Increased health promotion is required to highlight the risk of adverse birth outcomes in women who become pregnant for the first time in the late childbearing years.
Introduction
Having a baby late in life is now an accepted norm in industrial societies.1 Advanced maternal reproductive age is often considered to occur in women ≥40 years, although inequalities in birth outcomes are reported in women >35 years. Developments in contraception and obstetric care as well as greater equality in the workplace have shifted the age distribution of the female population of reproductive age and influenced the timing of childbearing in these commu-nities.2 In the United States, fetal anomalies, delivery prior to 34 weeks, and stillbirth have been reported to be twice as common in advanced age women as in young or mature adults3; >13% of all births are to women ≥35 years, and 22% of these births are to primigravidas.4 A large study of 1,282,172 live singleton births between 1980 and 2000 in Scotland reported that inequalities in adverse birth outcomes, including low birthweight (LBW), were almost 4 times higher in mothers aged > 35 years compared with younger mothers.5
Although older mothers are expected to be well informed and have greater knowledge about labor and its complica-tions,2 delayed childbearing may be harmful for the mother or fetus, especially when associated with other behavioral factors. These include psychosocial issues,6 smoking practices,7–9 alcohol exposure,10, stress,11 and late primiparity or grand multiparity.12 These factors may be compounded by employment conflicts or low educational standards in areas of social deprivation. The contributions of cardiovascular disease, diabetes, and obesity to late-life pregnancies have been reported.13–15 Adverse pregnancy outcomes in older mothers could in part relate to increased morbidity and obstetric problems during pregnancy and delivery,16 although the exact mechanisms underlying these greater risks for adverse birth outcomes among older mothers remain poorly understood.
Although the association between maternal age and pregnancy outcome has been partially explored through community surveys,17–19 the role of parity has been little studied. This study examined the correlation of maternal age as an independent factor for adverse birth outcomes in a U.K. population from Liverpool. Many previous studies of this aspect of reproductive health have used U.S. data.3,12,13,20 The present study examines this association for a U.K. population with a high prevalence of maternal smoking in pregnancy and substantial social deprivation. It was hypothesized that older primigravidas would be at increased risk for adverse birth outcomes.
Materials and Methods
This was a hospital-based retrospective analysis of 9506 birth registry records in the Liverpool Women's Hospital between 1998 and 2003, as birth registry data were only available from 1998 onward. The first 500 live singleton birth records for the first 3 months of each year were analyzed as a representative sample of the birth registry data for the period of collection. Those with multiple pregnancy, diabetes, eclampsia, and preeclampsia were excluded.
Data were available on maternal body weight and hemoglobin concentration at first antenatal visit, as well as maternal age, parity, ethnicity, fetal sex, gestational age, and birthweight. The information on prenatal smoking and alcohol exposure were obtained from the hospital booking form. Maternal age was grouped by 5-year class intervals into adolescents (<20 years), adults (20–25 years, 26–30 years, 31–35 years, 36–40 years), and women of advanced age (>40 years).
A smoker was defined as self-reported smoking of at least one cigarette per day during pregnancy and alcohol exposure as self-reported consumption of at least one unit of alcohol per week during pregnancy. Gestational age was taken as the number of completed weeks of gestation based on the estimated delivery date as determined by the date of the last normal menstrual period (LMP) and confirmed by ultrasound examination. LBW was classified as <2500 g, very LBW as <1500 g, preterm birth as <259 days (37 weeks) gestation, and very preterm birth as <35 weeks gestation. Small for gestational age (SGA) was defined as birthweight < 10th percentile of the birthweight-for-gestational ages sex-specific curve.21 Maternal anemia was defined as a hemoglobin level at booking of <11 g/dL, grand multiparity as maternal parity ≥ 5, overweight as a body mass index (BMI) between 25 and 29, and obesity as a BMI ≥ 30 kg/m.2,22 Townsend deprivation score was used to assess household deprivation, and values >+6 were considered as deprived.23,24
Continuous variables were summarized using means and standard deviations (SD). Probability values were two-tailed, with a significance level of ≤0.05. Chi-square, Fisher's exact, ANOVA, and Bonferroni tests were used as appropriate to compare birth outcomes by maternal age classes. Backward stepwise logistic regression was used to determine the association of maternal age, LBW, and preterm birth, controlling for prenatal alcohol and smoking exposure, household deprivation, maternal anemia, obesity, parity, and single parenthood, which showed a significant association in univariate analysis.
The study was approved by the Liverpool Women's Hospital and the Liverpool School of Tropical Medicine Research Ethics Committee.
Results
A total of 9506 births were available. Mean age ± SD was 29 years ± 6. Overall, 32% of mothers smoked during pregnancy, and 33.1% reported prenatal alcohol exposure. For all ages, mean birthweight (± SD) was 3344 g (± 640), gestational age was 276 days (± 21) (39.4 weeks), and hemoglobin at booking was 11.4 g/dL (± 1.3).
Table 1 summarizes maternal characteristics by age class. The proportion of mothers > 40 years of age who reported pregnancy smoking was lower than the proportion of mothers < 25 years of age. The prevalence of maternal anemia significantly decreased from 44.3% in adolescents to 36.0% in advanced age mothers (p < 0.001), with the lowest prevalence among mothers aged 26–30 years (27.6%). More adolescents than mothers aged >40 lived in low socioeconomic households (14.0% vs. 6.1%, p < 0.001), and more adolescents were unemployed or single mothers.
Table 1.
Maternal Characteristics
| |
Maternal age classes |
||||||
|---|---|---|---|---|---|---|---|
| |
<20 |
20–25 |
26–30 |
31–35 |
36–40 |
>40 |
|
| Characteristics, % | n = 534 | n = 2299 | n = 2557 | n = 2596 | n = 1216 | n = 229 | p valuea |
| Overweightb | 18.0 | 10.7 | 22.2 | 34.9 | 31.1 | 23.1 | 0.01 |
| Obesec | 10.7 | 2.6 | 7.8 | 26.1 | 15.4 | 19.9 | 0.01 |
| Anemia | 44.3 | 33.3 | 27.6* | 28.9 | 32.1 | 36.0 | 0.01 |
| Primiparous | 99.6 | 89.4 | 78.1 | 71.9 | 57.8 | 42.8 | <0.001 |
| Multiparousd | 0.4 | 10.6 | 21.7 | 27.2 | 41.0 | 50.0 | <0.001 |
| Grand multiparouse | 0.0 | 0.0 | 0.2 | 0.9 | 1.2 | 7.2 | <0.001 |
| Pregnancy smoking | 46.2* | 33.4* | 26.6 | 25.3 | 24.4 | 24.0 | <0.001 |
| Prenatal alcohol use | 26.6* | 29.8 | 32.0 | 34.0 | 33.5 | 33.6 | 0.005 |
| Smoking and alcohol | 17.9 | 17.0 | 13.3 | 10.6 | 9.9 | 12.5 | <0.001 |
| Nonwhite ethnicity | 4.9 | 4.3 | 3.9 | 2.5 | 1.6 | 4.5 | 0.09 |
| Household deprivationf | 14.0* | 12.5* | 17.4* | 9.7 | 18.5 | 6.1 | 0.12 |
| Unemployment | 36.5 | 29.2 | 19.1 | 22.0 | 17.3 | 21.0 | 0.07 |
| Single parent | 29.5 | 19.2 | 17.5 | 16.2 | 9.7 | 11.2 | 0.04 |
p < 0.001, linear association.
BMI 25–29 kg/m2.
BMI ≥ 30 kg/m2.
Parity ≥ 2.
Parity ≥ 5.
Townsend score ≥ +6.
p < 0.005, differences with <40 years (chi-square).
Birth outcomes by maternal age class are summarized in Table 2. Mothers of advanced age delivered lighter babies compared with those 26–40 years of age. The mean birth-weight differences were 162 ± 94 g compared with those 36–40 years, 191 ± 55 g compared with those 31–35 years, and 140 ± 42 g compared with those 26–30 years (all differences, p < 0.05). Mean gestational age was shorter in advanced age (273 ± 23 days) (39.0 weeks) compared with adults aged 26–30 years (279 ± 19 days) (39.8 weeks) (p = 0.05), although it was similar to that for adolescents (274 days) (39.1 weeks). The prevalence of SGA was higher in mothers >40 years compared with those aged 26–30 or 31–35 years (both p < 0.005).
Table 2.
Birth Outcomes by Maternal Age Category
| |
Maternal age classes |
|||||
|---|---|---|---|---|---|---|
| |
<20 |
20–25 |
26–30 |
31–35 |
36–40 |
>40 |
| Birth outcome | n = 534 | n = 2299 | n = 2557 | n = 2596 | n = 1216 | n = 229 |
| Mean BWa ± SDb | 3216 ± 689 | 3310 ± 607 | 3344 ± 629* | 3395 ± 642* | 3366 ± 681* | 3204 ± 587 |
| Mean GAc ± SD | 274 ± 26 | 277 ± 21 | 279 ± 19* | 274 ± 20 | 274 ± 24 | 273 ± 23 |
| Mean GA, weeks | 39.1 | 39.6 | 39.9 | 39.1 | 39.1 | 39.0 |
| LBW, % | 10.5 | 6.7 | 6.0** | 6.8 | 7.9 | 9.8 |
| Very LBWd, % | 5.2 | 2.9** | 3.1** | 2.8** | 3.7 | 4.9 |
| Preterm, % | 12.5 | 8.1 | 8.0** | 9.2 | 10.9 | 12.3 |
| Very preterme, % | 7.4 | 6.4 | 4.3** | 4.4** | 6.3 | 5.7 |
| SGAf, % | 2.7 | 2.4 | 1.9** | 1.7** | 1.6** | 4.0 |
| M/Fg sex ratio | 0.97 | 1.1 | 1.1 | 0.98 | 0.96 | 0.94 |
Birthweight (g).
SD, standard deviation.
Gestational age (days).
Birthweight <2000 g.
<35 weeks gestation.
Small for gestational age.
Male/female.
p < 0.005, differences with <40 years (Bonferroni); **p < 0.005, differences with <40 years (chi-square).
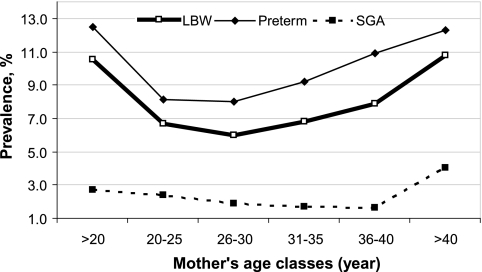
The prevalence of LBW and preterm birth and SGA by maternal age category follows a time-dependent, U-shaped curve with nadirs in the middle-age classes. The lowest point for LBW or preterm birth was observed for mothers aged 26–30 years (Fig. 1).
FIG. 1.
Prevalence of adverse birth outcomes by maternal age category.
Among 229 women of advanced age, 74 were primiparas, and their babies were 233 g (± 92) lighter than babies of multipara of the same age (p = 0.01). The prevalence of LBW was significantly higher for primiparous women of this age compared with multiparas (9.4% vs. 5.3%, p = 0.005). There were no differences between these parity groups for gestational age or prevalence of preterm birth. Compared to multiparas aged ≥40 years, primiparas in the same age group were less likely to be unemployed (14.2% vs. 30.1%, p = 0.001) and less likely to be of lower socioeconomic status (5.5% vs. 12.7%, p = 0.02). They were also more likely to deliver a very preterm baby (8.9% vs. 4.4%, p < 0.001) (Table 3).
Table 3.
Birth Outcomes and Maternal Characteristics by Parity in Mothers Aged >40 Years
| Birth outcomes | Primiparas n = 74 | Multiparas n = 153 | p valuea |
|---|---|---|---|
| Mean birthweight (g) | 3169 ± 607 | 3402 ± 699 | 0.01 |
| Mean gestational age (days) | 272 ± 22 | 273 ± 22 | 0.8 |
| LBW, % | 9.4 | 5.3 | 0.005 |
| Very LBWb, % | 7.3 | 2.8 | <0.001 |
| Preterm birth, % | 13.2 | 11.4 | 0.1 |
| Very preterm birthc, % | 8.9 | 4.4 | <0.001 |
| SGA, % | 1.7 | 2.0 | 0.6 |
| Single parenthood | 7.4 | 4.8 | 0.12 |
| Unemployment | 14.2 | 30.1 | 0.001 |
| Low socioeconomic statusd | 5.5 | 12.7 | 0.02 |
Independent sample t test or chi-square.
Birthweight <2000 g.
Delivery before 35 weeks gestation.
Townsend score ≥ +6.
A backward stepwise logistic regression analysis showed an association between maternal advanced age and LBW (adjusted OR [AOR], 1.7, 95% CI 1.4-2.5, p = 0.001), preterm birth (AOR 1.4, 95% CI 1.1-2.4, p = 0.04), or very preterm birth (AOR 1.6, 95% CI 1.2-3.5, p = 0.002), controlling for prenatal alcohol and smoking exposure, household deprivation, maternal anemia, obesity, parity, and single parenthood (Table 4).
Table 4.
Odds Ratio for Adverse Birth Outcomes in Mothers Aged >40 Yearsa
| Outcome | Crude | Adjustedb |
|---|---|---|
| Low birthweight | 2.6 (1.7–3.0), p = 0.001 | 1.7 (1.4–2.5), p = 0.001 |
| Preterm birth | 1.9 (1.3–3.0), p = 0.01 | 1.4 (1.1–2.4), p = 0.04 |
| Very preterm birth | 2.2 (1.5–4.2), p < 0.001 | 1.6 (1.2–3.5), p = 0.002 |
| Small for gestational age | 1.6 (1.1–2.5), p = 0.05 | 1.4 (0.6–3.5), p = 0.4 |
Women aged 20–40 are the reference group.
Controlling for prenatal alcohol and smoking exposure, anemia, household deprivation, obesity, parity, and single parenthood.
Discussion
This was a hospital-based retrospective analysis undertaken in Liverpool using a large sample of birth registries. The sample was representative, as the Liverpool Women's Hospital is the main delivery facility in this area covering almost all deliveries in this catchment population. As data collection was limited to the first 3 months of each year, seasonal factors may have influenced pregnancy outcomes. We have previously reported an increasing prevalence of adolescent pregnancies during this period and an increased prevalence of pregnancy smoking in adolescents.25 The present study addressed risk characteristics of advanced maternal age as an independent factor associated with adverse pregnancy outcomes, with emphasis on parity-specific associations. The number of women >40 years of age was relatively small compared to other age categories, and this resulted in larger confidence intervals for birthweight and gestational age. Nevertheless, pregnancy in advanced age was associated with LBW and preterm delivery independent of parity.
We found that maternal advanced age was an independent factor associated with LBW and preterm birth outcomes. Primiparas >40 years of age were at higher risk for delivering a LBW baby than multiparous women of the same age. After controlling for prenatal alcohol and smoking exposure, household deprivation, maternal anemia, obesity, parity, and single parenthood, advanced maternal age remained an independent factor associated with these pregnancy outcomes. These findings are consistent with those of other studies. A large Italian study (n = 3,616,622) has shown that compared with 20–29-year-old mothers, those aged 30-35 years were at increased risk of preterm birth (OR 1.3, 95% CI 1.2-1.4), and this risk estimate increased to 1.9 (1.82.1) for women >35 years.16 A retrospective analysis of 22,985 births in the United States between 1995 and 2003 reported that delivery prior to 34 weeks was twice as common in women ≥35 years of age compared with younger mothers, after excluding women with other indications for antepartum testing or fetal anomalies.3 The LBW risk estimates associated with maternal age >35 years were 5.3% (95% CI, 4.7-6.0) among African Americans, 4.3% (95% CI 1.7-6.9) among Puerto Ricans, and 3.7% (95% CI 2.8-4.5) in Mexican Americans, compared with 2.6% (95% CI 2.4-2.7) in non-Hispanic whites.26 In the present sample, only a small proportion of births were nonwhite, although these increased slightly among women >40 years of age (p = 0.09) (Table 1). The prevalence estimates for LBW (9.8%) and preterm birth (12.3%) in women >40 years in the present study were higher than for previously published reports from the United States. The level of risk in this population and the clinical significance are considerable in terms of perinatal health and morbidity. The U.S. studies are typically much larger, with more diverse populations.27 The Liverpool sample represents many women from lower socioeconomic areas with chronic smoking histories, and although the statistical analysis adjusted for social deprivation and prenatal smoking, this may not adequately control for chronic health changes that result from past smoking and environmental exposures. The generalizability of the study may be limited for these reasons, and analyses of comparative U.K. birth registries are required to further assess these results.
The contribution of advanced paternal age on fetal birth outcomes has also been considered by others. The increased risk of multiple birth with increased paternal age independent of parity has been reported, although paternal age was not a risk factor for LBW or preterm birth.28 Data abstracted from birth records compiled in North Dakota among Native American and Caucasian infants from 1978 to 1992 showed that maternal and paternal age both had a U-shaped effect on preterm birth. However, although the risk for preterm birth was increased for both teenage mothers and fathers, the risk was also significantly increased only for mothers between the ages of 36 and 40 and older than 40. There was also a U-shaped relationship between LBW and parental age, with a statistically significant difference between age groups.29 The age-dependent U-shaped association, which frequently is encountered for morbidity and mortality stud-ies,30 was observed for adverse birth outcomes in this study. Recognition of the lowest risk point in the U-shaped association could be emphasized as the preferred maternal age category for optimizing maternal and fetal health.
The biological mechanisms causing increased LBW and preterm birth in older women are unclear. A recent study on triplets born to older mothers showed an increased likelihood for stillbirths in women aged ≥ 40 years compared with younger mothers (20–29 years), although the prevalence of neonatal, perinatal, and infant mortality was lower in the older mothers.31 A population-based study of 15,795 singleton live births between 1995 and 1997 in the United States showed that older smokers had significantly higher fetal morbidity, suggesting that advanced maternal age increased smoking-associated fetal morbidity independent of pregnancy smoking.32 Mothers of advanced age are more likely to take medication and have a medical disorder, such as arthritis, chronic hypertension, depression, cancer, or myocardial infarction, which are independent risk factors for fetal growth restriction.33
Increased alcohol consumption during pregnancy has been associated with low socioeconomic status and de-pression,11 which could influence pregnancy outcome, but in this sample, women over 40 years reported a similar drinking pattern to those aged 26–40 years. In this study, primiparous women were significantly more likely to be employed and to be of higher socioeconomic status than multiparas. These mothers may have delayed their first pregnancy to accommodate occupation and social lifestyle. Heavy and binge drinking during pregnancy is more common in mothers from disadvantaged backgrounds, and light drinking is more common in those of higher socioeconomic status.23,25,34 Socioeconomic status and parity did not account for the higher frequency of adverse birth outcomes among older women, however, and other biological mechanisms may be involved.35 Furthermore, this study showed a peak prevalence of anemia (44.3%) among adolescents (<20 years), followed by mothers aged >40 years, which could influence perinatal outcomes in these age groups. The prevalence of preterm delivery and LBW, respectively, is reported as 4 times and 1.9 times increased among anemic women.36
These results are limited because of the lack of information on medical interventions, including fertility treatments and quality of antenatal care, and by misclassification due to maternal self-reporting of alcohol and smoking exposure during pregnancy in the absence of reliable laboratory biomarkers.37 Underreporting may be a consequence of the social stigma of these exposures in pregnancy.
In conclusion, the prevalence of pregnancy late in life was associated with a high prevalence of adverse birth outcomes, particularly in primigravidas. Increased health promotion is required to highlight the risk of adverse birth outcomes in women who become pregnant for the first time in the late childbearing years.
Acknowledgments
We are grateful for the assistance of M. Delpisheh, F. Yavandi, and S. Drammond.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ventura SJ. Martin JA. Curtin SC. Menacker F. Hamilton BE. Births: Final data for 1999. Natl Vital Stat Rep. 2001;49:1–100. [PubMed] [Google Scholar]
- 2.Freeman-Wang T. Beski S. The older obstetric patient. Curr Obstet Gynaecol. 2002;12:41–46. [Google Scholar]
- 3.Miller DA. Is advanced maternal age an independent risk factor for uteroplacental insufficiency? Am J Obstet Gynecol. 2005;192:1974–1980. doi: 10.1016/j.ajog.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 4.Martin JA. Hamilton BE. Ventura SJ. Births: Preliminary data for 2000. Natl Vital Stat Rep. 2001;49:1–20. [PubMed] [Google Scholar]
- 5.Fairley L. Leyland AH. Social class inequalities in perinatal outcomes: Scotland 1980–2000. J Epidemiol Community Health. 2006;60:31–36. doi: 10.1136/jech.2005.038380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray RF. Indurkhya A. McCormick MC. Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics. 2004;114:736–743. doi: 10.1542/peds.2003-1150-L. [DOI] [PubMed] [Google Scholar]
- 7.Delpisheh A. Brabin L. Brabin BJ. Pregnancy, smoking and birth outcomes. Womens Health. 2006;2:389–404. doi: 10.2217/17455057.2.3.389. [DOI] [PubMed] [Google Scholar]
- 8.Delpisheh A. Kelly Y. Brabin BJ. Passive cigarette smoke exposure in primary school children in Liverpool. Public Health. 2006;120:65–69. doi: 10.1016/j.puhe.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Bailey BA. Byrom AR. Factors predicting birth weight in a low-risk sample: The role of modifiable pregnancy health behaviors. Matern Child Health J. 2007;11:173–179. doi: 10.1007/s10995-006-0150-7. [DOI] [PubMed] [Google Scholar]
- 10.Bada HS. Das A. Bauer CR, et al. Low birth weight and preterm births: Etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25:631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor MJ. Whaley SE. Health care provider advice and risk factors associated with alcohol consumption following pregnancy recognition. J Stud Alcohol. 2006;67:22–31. doi: 10.15288/jsa.2006.67.22. [DOI] [PubMed] [Google Scholar]
- 12.Babinszki A. Kerenyi T. Torok O. Grazi V. Lapinski RH. Berkowitz RL. Perinatal outcome in grand and great-grand multiparity: Effects of parity on obstetric risk factors. Am J Obstet Gynecol. 1999;181:669–674. doi: 10.1016/s0002-9378(99)70511-9. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsson B. Ladfors L. Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104:727–733. doi: 10.1097/01.AOG.0000140682.63746.be. [DOI] [PubMed] [Google Scholar]
- 14.Canterino JC. Ananth CV. Smulian J. Harrigan JT. Vintzileos AM. Maternal age and risk of fetal death in singleton gestations: USA, 1995–2000. J Matern Fetal Neonatal Med. 2004;15:193–197. doi: 10.1080/14767050410001668301. [DOI] [PubMed] [Google Scholar]
- 15.Raymond EG. Cnattingius S. Kiely JL. Effects of maternal age, parity, and smoking on the risk of stillbirth. Br J Obstet Gynaecol. 1994;101:301–306. doi: 10.1111/j.1471-0528.1994.tb13614.x. [DOI] [PubMed] [Google Scholar]
- 16.Astolfi P. De Pasquale A. Zonta L. Late childbearing and its impact on adverse pregnancy outcome: Stillbirth, preterm delivery and low birth weight. Rev Epidemiol Sante Publique. 2005;53(Spec No 2):2S97–105. [PubMed] [Google Scholar]
- 17.Smith GC. Shah I. White IR. Pell JP. Dobbie R. Previous preeclampsia, preterm delivery, and delivery of a small for gestational age infant and the risk of unexplained stillbirth in the second pregnancy: A retrospective cohort study, Scotland, 1992–2001. Am J Epidemiol. 2007;165:194–202. doi: 10.1093/aje/kwj354. [DOI] [PubMed] [Google Scholar]
- 18.Lawlor DA. Smith GD. O'Callaghan M, et al. Epidemiologic evidence for the fetal overnutrition hypothesis: Findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165:418–24. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 19.Newburn-Cook CV. Onyskiw JE. Is older maternal age a risk factor for preterm birth and fetal growth restriction? A systematic review. Health Care Women Int. 2005;26:852–875. doi: 10.1080/07399330500230912. [DOI] [PubMed] [Google Scholar]
- 20.Cleary-Goldman J. Malone FD. Vidaver J, et al. Impact of maternal age on obstetric outome. Obstet Gynecol. 2005;105:983–990. doi: 10.1097/01.AOG.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- 21.Williams RL. Creasy RK. Cunningham GC. Hawes WE. Norris FD. Tashiro M. Fetal growth and perinated viability in California. Obstet Gynecol. 1982;59:624–632. [PubMed] [Google Scholar]
- 22.World Health Organization. Report of a WHO Consultation on Obesity. Geneva: World Health Organization; Jun 3–5, 1997. 1998. Obesity: Preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 23.Delpisheh A. Kelly Y. Rizwan S. Brabin BJ. Socio-economic status, smoking during pregnancy and birth outcomes: An analysis of cross-sectional community studies in Liverpool (1993–2001) J Child Health Care. 2006;10:140–148. doi: 10.1177/1367493506062553. [DOI] [PubMed] [Google Scholar]
- 24.Townsend P. Phillimore P. Saracci R. Health and deprivation: Inequality and the north. London: Croom Helm; 1988. [Google Scholar]
- 25.Delpisheh A. Attia E. Drammond S. Brabin BJ. Adolescent smoking in pregnancy and birth outcomes. Eur J Public Health. 2006;16:168–172. doi: 10.1093/eurpub/cki219. [DOI] [PubMed] [Google Scholar]
- 26.Khoshnood B. Wall S. Lee KS. Risk of low birth weight associated with advanced maternal age among four ethnic groups in the United States. Matern Child Health J. 2005;9:3–9. doi: 10.1007/s10995-005-2446-4. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman MC. Jeffers S. Carter J. Duthely L. Cotter A. Gonzalez-Quintero VH. Pregnancy at or beyond age 40 years is associated with an increased risk of fetal death and other adverse outcomes. Am J Obstet Gynecol. 2007;196:e11–e13. doi: 10.1016/j.ajog.2006.10.862. [DOI] [PubMed] [Google Scholar]
- 28.Tough SC. Faber AJ. Svenson LW. Johnston DW. Is paternal age associated with an increased risk of low birthweight, preterm delivery, and multiple birth? Can J Public Health. 2003;94:88–92. doi: 10.1007/BF03404578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel EL. Kruger M. Burd L. Effects of maternal and paternal age on Caucasian and Native American preterm births and birth weights. Am J Perinatol. 2002;19:49–54. doi: 10.1055/s-2002-20173. [DOI] [PubMed] [Google Scholar]
- 30.Martinussen T. Sorensen TI. Age-dependent U-shaped risk functions and Aalen's additive risk model. Biometrics. 1998;54:989–1001. [PubMed] [Google Scholar]
- 31.Salihu HM. Aliyu MH. Akintobi TH. Pierre-Louis BJ. Kirby RS. Alexander GR. The impact of advanced maternal age (≥ 40 years) on birth outcomes among triplets: A population study. Arch Gynecol Obstet. 2005;271:132–137. doi: 10.1007/s00404-003-0573-y. [DOI] [PubMed] [Google Scholar]
- 32.Salihu HM. Shumpert MN. Aliyu MH. Kirby RS. Alexander GR. Smoking-associated fetal morbidity among older gravidas: A population study. Acta Obstet Gynecol Scand. 2005;84:329–334. doi: 10.1111/j.0001-6349.2005.00552.x. [DOI] [PubMed] [Google Scholar]
- 33.Bewley S. Davies M. Braude P. Which career first? BMJ. 2005;331:588–589. doi: 10.1136/bmj.331.7517.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delpisheh A. Topping J. Reyad M. Tang A. Brabin BJ. Prenatal alcohol consumption, maternal CYP17 gene polymorphisms and pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2008;138:49–53. doi: 10.1016/j.ejogrb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Astone NM. Misra D. Lynch C. The effect of maternal socioeconomic status throughout the life span on infant birthweight. Paediatr Perinat Epidemiol. 2007;21:310–318. doi: 10.1111/j.1365-3016.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- 36.Lone FW. Qureshi RN. Emanuel F. Maternal anaemia and its impact on perinatal outcome. Trop Med Int Health. 2004;9:486–490. doi: 10.1111/j.1365-3156.2004.01222.x. [DOI] [PubMed] [Google Scholar]
- 37.Delpisheh A. Topping J. Reyad M. Tang A. Brabin BJ. Smoking exposure in pregnancy: Use of salivary cotinine in monitoring. Br J Midwifery. 2007;15:216–220. [Google Scholar]