Abstract
Brain and liver mitochondria isolated by a discontinuous Percoll gradient show an oxidized redox environment, which is reflected by low GSH levels and high GSSG levels and significant glutathionylation of mitochondrial proteins as well as by low NAD(P)H/NAD(P) values. The redox potential of brain mitochondria isolated by a discontinuous Percoll gradient method was calculated to be −171 mV based on GSH and GSSG concentrations. Immunoblotting and LC/MS/MS analysis revealed that succinyl-CoA transferase and ATP synthase (F1 complex, α-subunit) were extensively glutathionylated; S-glutathionylation of these proteins resulted in a substantial decrease of activity. Supplementation of mitochondria with complex I or complex II respiratory substrates (malate/glutamate or succinate, respectively) increased NADH and NADPH levels, resulting in the restoration of GSH levels through reduction of GSSG and deglutathionylation of mitochondrial proteins. Under these conditions, the redox potential of brain mitochondria was calculated to be −291 mV. Supplementation of mitochondria with respiratory substrates prevented GSSG formation and, consequently, ATP synthase glutathionylation in response to H2O2 challenges. ATP synthase appears to be the major mitochondrial protein that becomes glutathionylated under oxidative stress conditions. Glutathionylation of mitochondrial proteins is a major consequence of oxidative stress, and respiratory substrates are key regulators of mitochondrial redox status (as reflected by thiol/disulfide exchange) by maintaining mitochondrial NADPH levels.
Keywords: Glutathione, Mitochondria, Oxidation Reduction, Oxidative Stress, Thiol, NADPH, Glutathionylation, Mitochondrial Substrates, Redox
Introduction
GSH is the major nonprotein thiol and redox regulator in mitochondria as well as cells (1, 2). Mitochondria contain a distinct GSH pool important in maintaining a reduced matrix environment and detoxifying H2O2 produced in the matrix (3–5). However, mitochondria are devoid of GSH synthesis machinery and rely strictly on GSH import from the cytosol (6, 7). Despite this, the redox status of the mitochondrial GSH pool can function independently of the cytoplasmic redox status. Maintenance of the mitochondrial redox status is largely regulated by the concerted actions of NAPDH-dependent enzymes including: GSSG reductase, thioredoxin, peroxiredoxin III, and glutaredoxin that directly or indirectly utilize NADPH to keep the mitochondrial matrix reduced (4, 6, 8). Consequently, mitochondrial GSH is predominant in the reduced form with the GSH:GSSG ratio being greater then 100:1 and mitochondrial proteins being predominantly in the reduced thiol state (9, 10). In oxidative stress conditions, increased GSSG formation may lead to glutathionylation of redox-sensitive proteins through thiol-disulfide exchange (11) as follows.
A number of mitochondrial proteins have been identified as being glutathionylated, including subunits of complex I (12) and complex IV (13, 14), aconitase (15, 16), and pyruvate dehydrogenase (17); glutathionylation of these proteins is usually associated with a decrease of their activities. Because of the disruption in protein function that GSSG can cause, cells generally pump GSSG out of the cytoplasm into the extracellular matrix. Export of GSSG has been shown to occur in liver and other tissues during times of oxidative stress (1, 18). Mitochondria, however, do not appear to export GSSG (19), and GSSG transporters have not been observed on the organelle membranes. This suggests that in oxidative stress conditions, GSSG is sequestered in mitochondria, and glutathionylation of mitochondrial proteins may ensue, leading to a decrease in their activity.
NADPH is central for the maintenance of the mitochondrial redox status, because of the direct dependence of GSSG reductase and thioredoxin on NADPH as an electron donor for the reduction of GSSG and disulfide bonds, respectively (10). Glutaredoxin 2, the major protein in mitochondria involved in deglutathionylation of mitochondrial proteins, requires GSH to deglutathionylate proteins and therefore is indirectly dependent on NADPH (20). The majority of NADPH in mitochondria is believed to be mainly generated from nicotinamide nucleotide transhydrogenase, which utilizes NADH to generate NADPH from NADP+ (21). Mitochondria also contain malic enzymes and NADP+-dependent isocitrate dehydrogenase, which are believed to be important sources of NADPH (10). It is therefore likely that mitochondrial GSH redox status is dependent on NADPH production from various respiratory substrates (i.e. pyruvate, glutamate, and malate). However, the direct regulation of mitochondrial GSH levels by respiratory substrates has not been directly demonstrated. Although the key proteins in maintaining mitochondrial redox status have been identified, the dynamic regulation of mitochondrial GSH redox status in the face of an oxidative challenge has not been clearly demonstrated. This study examines the factors that modulate mitochondrial thiol redox status during the isolation procedure and in energized mitochondria as well as the influence of the latter in mitochondrial protein S-glutathionylation.
EXPERIMENTAL PROCEDURES
Chemicals
DTT, Percoll, metaphosphoric acid, CHAPS,2 rotenone, KCN, and antimycin A were from Sigma.
Mitochondria Isolation
Mitochondria from rat brain or liver was isolated by using either differential centrifugation (22, 23) or discontinuous Percoll gradient (24, 25). The isolation buffer for both methods consisted of 250 mm sucrose, 20 mm HEPES, 1 mm EDTA, 1 mm EGTA, 1.0% (w/v) BSA, and 25 μl/100 ml protease inhibitor mixture, pH 7.4.
Differential Centrifugation
Livers from rats were excised, washed, and homogenized in isolation buffer using a loose Teflon pestle. The homogenate was centrifuged at 1000 × g for 10 min at 4 °C, the pellet was removed, and the centrifugation process was repeated. The resulting supernatant was centrifuged at 9,000 × g for 15 min to generate the mitochondria pellet, which was washed with isolation buffer, and the high speed centrifugation was repeated. The mitochondria pellet was resuspended in isolation buffer (without BSA) before Western blot analysis and other assays.
Discontinuous Percoll Gradient
Brain and liver were excised, washed, and homogenized in isolation buffer using a loose Teflon pestle. The homogenate was centrifuged at 1000 × g for 10 min at 4 °C, the pellet was removed, and the centrifugation process was repeated. The resulting supernatant was centrifuged at 9,000 × g for 15 min to generate the mitochondria pellet. The mitochondria pellet was dissolved in isolation buffer containing 15% (v/v) Percoll and centrifuged at 10,000 × g for 10 min. The mitochondria pellet was gently removed from the Percoll solution and layered on top of three discontinuous Percoll gradient tubes (15, 23, and 40% (v/v)). The Percoll gradient was spun at 30,700 × g for 5 min at 4 °C. The mitochondria layer, which resides in the interface between 40 and 23% (v/v) Percoll, was carefully removed using a pipette and suspended in isolation buffer. To remove the Percoll, mitochondria were spun at 10,000 × g for 10 min and washed, and the process was repeated twice. The mitochondria were suspended in isolation buffer (without BSA) before HPLC and other measurements.
Submitochondrial Particles
Mitochondria were sonicated by Branson Sonifier 150 internal sonicator six times with 10–20% power output each time for 10 s with a 1-min interval. Intact mitochondria were spun down at 8250 × g for 10 min. Supernatant containing the submitochondrial particles was transferred and diluted with mitochondrial isolation buffer and spun down at 80,000 × g for 40 min using a Type 60 Ti rotor. Mitochondria were kept on ice throughout the procedure.
Broken Mitochondria
Broken mitochondria were prepared by three series of freezing and thawing in a 2% (w/v) CHAPS solution. Intact mitochondria were spun down at 8250 × g for 10 min.
Western Blotting
Mitochondrial samples were solubilized in a nonreducing SDS sample buffer, separated by Laemmli SDS/PAGE, and transferred onto PVDF membranes. Using appropriate antibodies, the immunoreactive bands were visualized with an enhanced chemiluminescence reagent.
LC/MS/MS
In-gel Tryptic Digest
Protein bands from SDS-PAGE were excised from the gels using biopsy punches (Acuderm, Lauderdale, FL). In-gel tryptic digest was carried out using trypsin that was reductively methylated to reduce autolysis (Promega, Madison, WI). The digestion reaction was carried out overnight at 37 °C. Digestion products were extracted from the gel with a 5% formic acid, 50% (v/v) acetonitrile solution (2×), and one acetonitrile extraction followed by evaporation using an APD SpeedVac (ThermoSavant, Milford, MA).
Analysis of Tryptic Peptide Sequence Tags by Tandem Mass Spectrometry
The dried tryptic digest samples were cleaned with ZipTip and resuspended in 10 μl of 60% (v/v) formic acid. Chromatographic separation of the tryptic peptides was achieved using a ThermoFinnigan Surveyor MS-Pump in conjunction with a BioBasic-18 100 × 0.18-mm reverse phase capillary column (ThermoFinnigan, San Jose, CA). Mass analysis was done using a ThermoFinnigan LCQ Deca XP Plus ion trap mass spectrometer equipped with a nanospray ion source (ThermoFinnigan) employing a 4.5-cm-long metal needle (Hamilton, 950-00954) using data-dependent acquisition mode. Electrical contact and voltage application to the probe tip took place via the nanoprobe assembly. Spray voltage of the mass spectrometer was set to 2.9 kV and heated capillary temperature at 190 °C. The column was equilibrated for 5 min at 1.5 μl/min with 95% solution A, 5% solution B (A, 0.1% formic acid in water; B, 0.1% formic acid in acetonitrile) prior to sample injection. A linear gradient was initiated 5 min after sample injection ramping to 35% A, 65% B after 50 min, and 20% A, 80% B after 60 min. Mass spectra were acquired in the m/z 400–1800 range.
Protein Identification
Protein identification was carried out with the MS/MS search software Mascot 1.9 (Matrix Science) with confirmatory or complementary analyses with TurboSequest as implemented in the Bioworks Browser 3.2, build 41 (ThermoFinnigan). NCBI Rattus norvegicus protein sequences were used as the primary search data base, and searches were complemented with the NCBI nonredundant protein data base.
Measurement of Mitochondrial Thiol Content, GSH, GSSG, and Pyridine Nucleotides
Thiol levels in mitochondria were measured using 5,5′-dithiobis(nitrobenzoic acid) as described previously (26). GSH and GSSG concentrations were analyzed using HPLC electrochemical detection as described previously (27). NAD+, NADH, NADP+, and NADPH levels were measured by HPLC as described previously (28). Briefly, brain homogenate and isolated mitochondria were homogenized in buffer (0.06 m KOH, 0.2 m KCN, and 1 mm bathophenanthroline disulfonic acid) followed by chloroform extraction. Chloroform extraction was carried out by centrifugation at 14,000 rpm in a microcentrifuge at 4 °C; the resulting aqueous supernatant with soluble pyridine nucleotides was collected and extracted thrice to remove lipids and proteins. Finally, it was filtered with a 0.45-μm positively charged filter (Pall Life Sciences) to remove RNA and DNA in microcentrifuge at 4 °C. The mobile phase consisted of 0.2 m ammonium acetate (buffer A) at pH 5.5 and HPLC grade methanol (buffer B). A gradient program with initial conditions as 100% buffer A and 0% buffer B was set. From 0 to 4 min, we used 0 to 3% B, and from 4 to 23 min, we used 3–6.8% B, followed by washing the column with 50% A and 50% B, and re-equilibrated to initial conditions for next run. Quantitation of pyridine nucleotides was performed by integrating the peaks and adding the cyanide adducts as detected by the fluorescence spectrophotometer (λexc = 330 nm; λem = 460 nm).
Mitochondrial Redox Status
The redox potential of the glutathione redox couple was quantified by the Nernst equation (Ehc = E0 + 30 log ([GSSG]/[GSH]2), wherein Eo is taken as −264 mV at pH 7.4 (29). The Nernst potential was calculated from the experimentally determined molar concentration of GSH and GSSG in mitochondria at the value of ∼1 μl/mg of protein. The Nernst potential takes into account not only the GSH/GSSG ratio but also the absolute concentration of GSH, where any changes in the latter will change the redox potential even without significant changes in the GSH/GSSG ratio (9).
Enzyme Activity
Succinyl-CoA transferase activity was assayed in broken mitochondria as described previously (30); activity was expressed in nmol of acetyl-CoA produced per min/mg of protein. ATP synthase activity was assayed in submitochondrial particles by coupling its activity to pyruvate kinase and lactate dehydrogenase and monitoring consumption of NADH (−d[NADH]/dt) at 340 nm (31).
Statistical Analysis
Statistical analysis was performed using Student's t test for unpaired data or analysis of variance (*, p < 0.05; **, p < 0.01). The results are expressed as the means ± S.E.
RESULTS
Influence of Isolation Methods on Mitochondrial GSH and GSSG Levels
Differential centrifugation and discontinuous Percoll gradient are currently the most commonly used methods for isolation of mitochondria from several tissues. The use of Percoll increases sample purity, but prolonged exposure to Percoll itself may have negative effects on mitochondria, and therefore Percoll must be removed by a series of buffer washes. Protocols utilizing Percoll often incorporate reducing agents, such as DTT or mercaptoethanol, in the isolation buffer (24). Table 1 lists the mitochondrial GSH and GSSG values following isolation by either differential centrifugation or discontinuous Percoll gradient: in liver mitochondria, the former isolation method afforded higher GSH levels and lower GSSG levels than those observed with the latter isolation method. The loss of mitochondrial GSH observed when the organelles were isolated by a discontinuous Percoll gradient cannot be accounted for in terms of GSH oxidation to GSSG (i.e. an amount of GSSG greater than 6 nmol/mg of protein would be expected from oxidation of GSH). This suggests that mechanisms other than GSH oxidation were involved in GSH depletion when mitochondria were isolated by a discontinuous Percoll gradient. (Differential centrifugation was not used to isolate brain mitochondria because of high lipid content in the brain; efficient removal of lipid content is achieved through utilization of a Percoll gradient). In both brain and liver mitochondria, the inclusion of DTT in the isolation buffer increased GSH levels and decreased GSSG levels. In isolated liver mitochondria, DTT increased GSH levels from 5.23 ± 0.95 to 8.41 ± 0.17 nmol/mg of protein, while simultaneously reducing GSSG levels from 0.91 ± 0.21 to 0.02 ± 0.07 nmol/mg of protein (Table 1).
TABLE 1.
Mitochondrial GSH concentrations and mitochondrial isolation methods
Mitochondria were isolated as described under “Experimental Procedures.” Isolated mitochondria were pelleted and resuspended in 5% (w/v) metaphosphoric acid to precipitate proteins. Supernatant was analyzed for GSH and GSSG content by HPLC.
| Method | [GSH] | [GSSG] |
|---|---|---|
| nmol/mg of protein | nmol/mg of protein | |
| Differential centrifugation | ||
| Liver | 13.91 ± 0.64 | 0.37 ± 0.09 |
| Discontinuous Percoll gradient | ||
| Liver | 5.23 ± 0.95 | 0.91 ± 0.21 |
| Brain | 0.93 ± 0.08 | 1.06 ± 0.03 |
| Discontinuous Percoll gradient + DTTa | ||
| Liver | 8.41 ± 0.21 | 0.02 ± 0.07 |
| Brain | 5.54 ± 0.17 | 0.09 ± 0.01 |
a DTT was present in the mitochondria isolation buffer at a concentration of 1 mm.
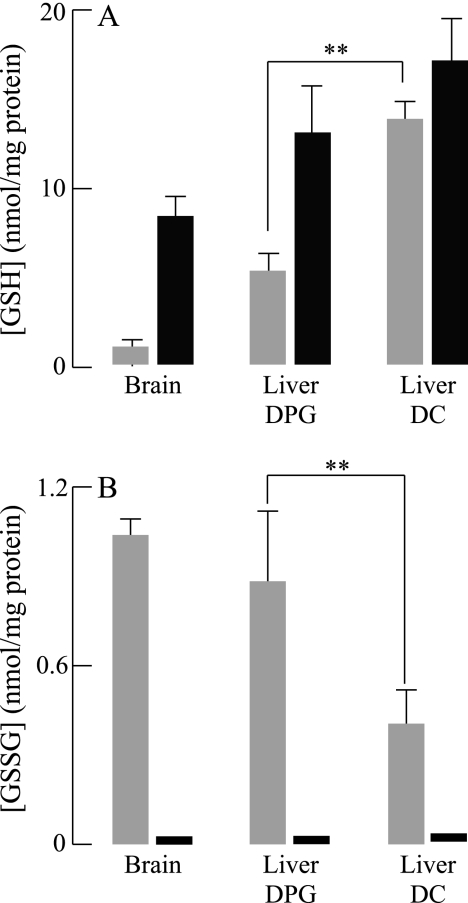
Liver mitochondria GSH levels obtained using a discontinuous Percoll gradient were still significantly lower than those obtained using differential centrifugation, even if DTT was present in the isolation buffer. The effects of DTT were more striking in isolated brain mitochondria, where DTT increased mitochondrial GSH levels from 0.93 ± 0.08 to 5.23 ± 0.95 nmol/mg of protein (Table 1). GSSG levels also fell from 1.06 ± 0.03 to 0.09 ± 0.01 nmol/mg in the presence of DTT in the isolation buffer. These values suggest that the reduction of GSSG by DTT cannot account for the substantial increase in GSH (reduction of 1 mol GSSG yields stoichiometrically 2 mol GSH). The brain mitochondria redox status was calculated as −171 mV, a value obtained by the Nernst equation and based on the GSH and GSSG experimental values in Fig. 1.
FIGURE 1.
Effect of DTT on GSH and GSSG levels in isolated brain and liver mitochondria. Liver mitochondria were isolated using differential centrifugation (DC) or discontinuous Percoll gradient (DPG) as described under “Experimental Procedures.” Brain mitochondria were isolated using discontinuous Percoll gradient. Following isolation, mitochondria were incubated at 37 °C for 5 min with or without DTT (1 mm). Mitochondria were subsequently pelleted, resuspended in 5% metaphosphoric acid, and centrifuged once more. GSH (A) and GSSG (B) levels were measured in the supernatant using HPLC with electrochemical detection. Shaded bar, no DTT added; black bar, plus 1 mm DTT.
GSH Oxidation and Mixed Disulfide Formation
The loss of mitochondrial GSH in mitochondria isolated by a discontinuous Percoll gradient may be accounted for by mechanisms such as increased export of GSH and protein mixed disulfide formation (i.e. GSH oxidation to GSSG followed by Equation 1). Treatment of mitochondria with DTT after isolation allows distinguishing these two pathways (Fig. 1): treatment of liver mitochondria with DTT (after isolation with discontinuous Percoll gradient) resulted in recovery of GSH to levels similar to those observed in liver mitochondria isolated by differential centrifugation (Fig. 1A). Although DTT treatment decreased GSSG to undetectable levels (Fig. 1B), the amount of GSH formed upon GSSG reduction represents a small part of the increase in GSH following DTT treatment. It may be suggested that part of the increase in GSH levels can be attributed to the reduction of protein-mixed disulfides (S-glutathionylated proteins) by DTT. A slight increase in GSH levels and a complete loss of GSSG was observed with DTT treatment in isolated liver mitochondria using differential centrifugation, thus implying that with this mitochondrial isolation method, some GSH was oxidized to GSSG, and the latter supported S-glutathionylation of mitochondrial proteins.
GSH Levels in Energized Mitochondria
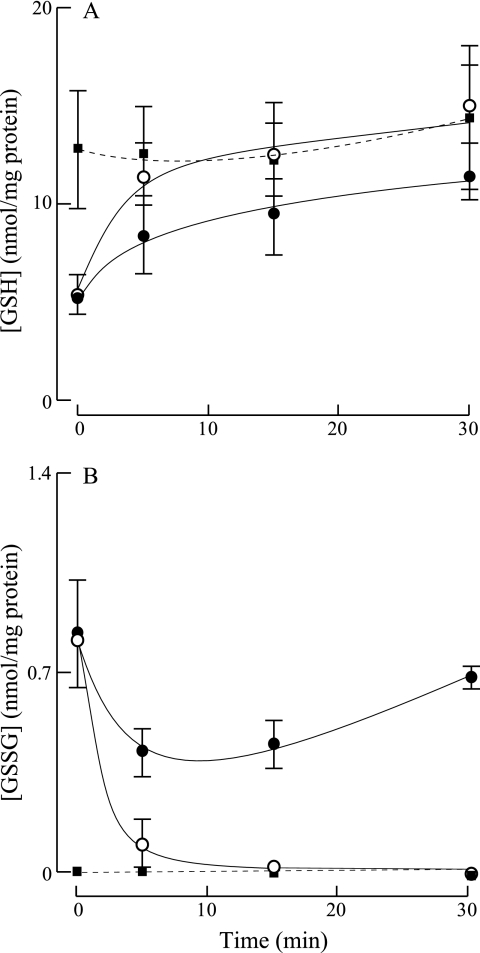
Brain (Fig. 2, top panel) and liver (Fig. 3A) mitochondria, isolated by discontinuous Percoll gradient and energized with complex I substrates (glutamate/malate) and ADP (state 3 respiration), showed a rapid increase in GSH levels along with a drop in GSSG to almost undetectable levels (Figs. 2, bottom panel, and 3B). Similar effects were observed when DTT was added to the mitochondrial suspension. A slight increase in GSH levels was observed as a function of time in the absence of added respiratory substrates (Fig. 2, top panel); this may be partly due to the occurrence of endogenous substrates that may support the reduction of protein mixed disulfides. Liver mitochondria, isolated by differential centrifugation, in state 3 respiration (complex I respiratory substrates and ADP) showed a slight increase in GSH levels (t0 = 14.3 ± 0.21; t30 = 15.2) (Fig. 4, top panel) and a decrease in GSSG levels (Fig. 4, bottom panel) over time.
FIGURE 2.
Effect of respiratory substrates on GSH and GSSG levels in brain mitochondria. Brain mitochondria were isolated using a discontinuous Percoll gradient. Following isolation, mitochondria were incubated with either glutamate/malate/ADP (○) or DTT (■). ●, no additions. At the indicated times, mitochondrial GSH and GSSG levels were measured using HPLC with electrochemical detection as described under “Experimental Procedures.” The assay conditions were: glutamate/malate, 2.4 mm; ADP, 0.07 mm; and DTT, 1 mm.
FIGURE 3.
Effect of respiratory substrates on GSH and GSSG levels in liver mitochondria. Liver mitochondria were isolated using a discontinuous Percoll gradient. Following isolation, mitochondria were incubated with either glutamate/malate/ADP (○) or DTT (■). ●, no additions. At the indicated times, mitochondrial GSH and GSSG levels were measured using HPLC with electrochemical detection. The assay conditions were as described in the legend to Fig. 2.
FIGURE 4.
Effect of respiratory substrates on GSH and GSSG levels in liver mitochondria isolated by differential centrifugation. Liver mitochondria were isolated using differential centrifugation. Following isolation, mitochondria were incubated with either glutamate/malate/ADP (●) or DTT (■). ○, no additions. At the indicated times, mitochondrial GSH and GSSG levels were measured using HPLC with electrochemical detection. The concentrations of reactants are as described in the legend to Fig. 2.
Brain GSH levels were calculated to be ∼10 nmol/mg of protein when reduced by DTT or respiratory substrates. 5,5′-Dithiobis(nitrobenzoic acid) measurement in energized mitochondria (glutamate/malate plus ADP) was estimated to be ∼18.8 nmol/mg of protein. This suggests that the total protein thiol content in mitochondria was well in excess of mitochondrial GSH levels, thus supporting the notion that mitochondrial proteins contain enough free thiols that are sensitive to glutathionylation.
These findings suggest that (a) mitochondrial respiratory substrates support a process that rapidly reduces mitochondrial GSSG and S-glutathionylated proteins (to release GSH) and (b) the formation of GSSG and mixed disulfides that occurs during isolation is partly due to a lack of respiratory substrates.
The redox potential of energized brain mitochondria was calculated as −291 mV, a calculation based on GSH and GSSG values at 30 min of incubation with malate/glutamate (Fig. 2). It may be surmised that energized mitochondria are in a more reduced state (from −171 to −291 mV).
Mitochondrial S-Glutathionylated Proteins
Immunoblotting of mitochondria isolated by a discontinuous Percoll gradient using an anti-GSH antibody revealed three major glutathionylated protein bands at ∼ 40, ∼60, and 70 kDa (Fig. 5A). Treatment with respiratory substrates (glutamate/malate) and ADP decreased the ∼60- and 70-kDa band intensities significantly; DTT treatment was more effective than that by respiratory substrates, especially on the 40-kDa band. Immunoprecipitation followed by LC/MS/MS analyses of brain mitochondria (Fig. 5B) allowed identification of the 70-kDa band as succinyl-CoA transferase (gi 34854196, 68.260 kDa), a mitochondrial matrix enzyme involved in ketone body metabolism. The 60-kDa band was identified as ATP synthase (H+ transporting, mitochondrial F1 complex, α subunit, and isoform 1; gi 40538742, 59.831 kDa) (Table 2). The 40-kDa protein could not be immunoprecipitated and identified by LC/MS/MS. However, a 40-kDa band identified as NADP+-dependent isocitrate dehydrogenase was shown to be sensitive to S-glutathionylation under oxidative stress conditions (32).
FIGURE 5.
Identification of glutathionylated mitochondrial proteins: regulation by respiratory substrates. A, immunoblotting of glutathionylated proteins in mitochondria isolated using a discontinuous Percoll gradient. Following isolation, brain mitochondria were incubated at 37 °C for 10 min in the presence or absence of either glutamate/malate (7.5 mm) plus ADP (50 μm) or DTT (1 mm). Mitochondria were lysed in a 2% CHAPS/nonreducing and reducing buffer and underwent a series (three times) of freezing and thawing to ensure maximal protein release. Then mitochondria were separated via SDS-PAGE, transferred to a nitrocellulose membrane, and probed using a Virogen GSH antibody (1:500). B, immunoprecipitation (IP) of glutathionylated mitochondrial proteins following mitochondria isolation using discontinuous Percoll gradient and identification using LC/MS/MS. Following isolation, brain mitochondria were treated with 2% CHAPS/nonreducing buffer and immunoprecipitated using GSH antibody (1:200). Immunoprecipitation proteins were then separated via SDS-PAGE. Protein bands stained by SYPRO® Ruby were excised and identified by LC/MS/MS.
TABLE 2.
LC/MS/MS analyses of F1α-ATPase and succinyl-CoA transferase
36 total F1α-ATPase peptides (18 unique) were identified by LC/MS/MS (total mascot scores ranged from 409 to 955). Two total SCOT peptides (one unique) were identified by LC/MS/MS (total mascot score was 80). Xc and Δcn values were calculated using TurboSequest software. Expect scores were calculated by Mascot software; expect scores below 0.05 are considered significant.
| Xc | Δcn | Expect scores | |
|---|---|---|---|
| F1α-ATPase | |||
| FESAFLSHVVSQHQSLLGNIR | 5.97 | 0.67 | 7.2 × 10−7 |
| ILGADTSVDLEETGR | 4.38 | 0.60 | 5.7 × 10−8 |
| VVDALGNAIDGKGPVGSK | 4.23 | 0.50 | 2.1 × 10−5 |
| Succinyl-CoA transferase | |||
| AAGTTVVEVEEIVDIGSFAPEDIHIPK | 5.39 | 0.00 | 4.1 × 10−7 |
Post-translational redox protein modifications, such as S-glutathionylation, are frequently associated with changes in activity (10). The activities of ATP synthase and succinyl-CoA transferase in brain mitochondria isolated with a discontinuous Percoll gradient (in the absence of DTT to yield higher levels of S-glutathionylated proteins) were 582 ± 48 and 294 ± 84 nmol/min/mg protein, respectively (Table 3); supplementation with glutamate/malate and ADP increased these values to 1866 ± 12 and 666 ± 18 nmol/min/mg protein, respectively. These data demonstrate that a major consequence of glutathionylation is a decline in mitochondrial bioenergetic capacity. The decrease in intensity in the ∼60- and 70-kDa bands in Fig. 5A tallies with the increase in activity of ATPase and succinyl-CoA transferase (Table 3) upon energizing mitochondria with complex I substrates and ADP.
TABLE 3.
Mitochondrial glutathionylated proteins and enzyme activities: effect of substrate supplementation
Freshly isolated mitochondria with or without supplementation with substrates (2.4 mm glutamate, 2.4 mm malate, and 0.7 mm ADP) were subjected to either freeze/thaw (broken mitochondria) or sonication (submitochondrial particles), and the ATP synthase and succinyl-CoA transferase activities were measured as described under “Experimental Procedures.”
| Activity | |
|---|---|
| nmol/min/mg of protein | |
| ATP synthase (−d[NADH]/dt) | |
| Glutathionylated mitochondria | 582 ± 48 |
| Substrate-supplemented mitochondria | 1870 ± 12 |
| Succinyl-CoA transferase (+d[Acetyl-CoA]/dt) | |
| Glutathionylated mitochondria | 294 ± 84 |
| Substrate-supplemented mitochondria | 666 ± 18 |
Complex I and Complex II Substrates Increase Mitochondrial GSH and Pyridine Nucleotide Levels
GSH concentration in mitochondria isolated by a discontinuous Percoll gradient is 1.73 ± 0.39 nmol/mg of protein (Table 4). Electron donors to complex I (glutamate/malate) and complex II (succinate) (state 4 respiration) increased GSH levels ∼3-fold. In the presence of ADP (state 3 respiration), GSH levels increase 5.0–6.3-fold. This may suggest that state 3 respiration provides optimal conditions to increase mitochondrial GSH levels probably from deglutathionylation of S-glutathionylated proteins. ADP treatment alone (in the absence of respiratory substrates) did not increase mitochondrial GSH (data not shown). Inhibition of electron flow (in presence of glutamate/malate and ADP) with either rotenone (complex I inhibitor) or antimycin A (blocking electron transfer at the bc1 segment) did not completely abrogate the increase in GSH levels (or protein deglutathionylation) but decreased it by 30–40% (compared with values during state 3 respiration). It may be surmised that a maximal increase in GSH values occurs during state 3 respiration. These conditions resulted in low GSSG levels (near detection limit) whether or not inhibitors of electron transport were present.
TABLE 4.
Mitochondrial respiratory state and GSH content
Brain mitochondria were isolated as described under “Experimental Procedures.” Isolated mitochondria were incubated in respiration buffer for 10 min with their respective substrates at room temperature. All of the inhibitors were preincubated with mitochondria for 5 min prior to substrate supplementation. Mitochondria were then pelleted and resuspended in 5% (w/v) metaphosphoric acid to precipitate proteins. Supernatant was analyzed for GSH and GSSG content by HPLC. The assay conditions were: glutamate/malate, 2.4 mm; ADP, 0.7 mm; rotenone, 5 μm; antimycin A, 1 μg/mg protein.
| [GSH] | [GSSG] | [GSH]/[GSSG] | |
|---|---|---|---|
| nmol/mg of protein | nmol/mg of protein | ||
| No substrates | 1.03 ± 0.19 | 1.63 ± 0.30 | 0.63 ± 0.00 |
| With succinate | 5.35 ± 0.18 | 0.10 ± 0.02 | 52.26 ± 8.44 |
| With succinate and ADP | 8.71 ± 0.55 | 0.13 ± 0.03 | 68.36 ± 12.17 |
| With glutamate/malate | 4.61 ± 0.26 | 0.12 ± 0.04 | 43.33 ± 6.50 |
| With glutamate/malate and ADP | 10.93 ± 0.78 | 0.21 ± 0.04 | 56.95 ± 5.22 |
| With glutamate/malate and ADP and rotenone | 6.07 ± 0.22 | 0.15 ± 0.02 | 42.33 ± 2.67 |
| With glutamate/malate and ADP and antimycin A | 7.39 ± 0.16 | 0.19 ± 0.02 | 38.95 ± 2.70 |
Metabolism of glutamate/malate in mitochondria results in the generation of NADH, which funnels the electrons through the respiratory chain. Supplementation of brain mitochondria (isolated by a continuous Percoll gradient) with glutamate/malate (state 4 respiration) led to an increase of NADH (Fig. 6, top panel); the pyridine nucleotide levels were further increased during state 3 respiration (respiratory substrates plus ADP) and, expectedly, in the presence of rotenone (upon inhibition of NADH utilization by complex I). A similar increase in NADH and NADPH was observed with succinate treatment (data not shown). Mitochondrial NADH can be subsequently converted to NADPH by nicotinamide nucleotide transhydrogenase: the [NADPH]/[NADP] values were increased upon supplementation of mitochondria with glutamate/malate (state 4 respiration), glutamate/malate plus ADP (state 3 respiration), and glutamate/malate plus rotenone (Fig. 6, bottom panel). These findings support the notion that mitochondrial reduced pyridine nucleotides are required to reverse S-glutathionylation of mitochondrial proteins.
FIGURE 6.
Effect of mitochondrial respiratory substrates and inhibitors on NADH/NAD+ and NADPH/NADP+ values. Brain mitochondria were isolated using a discontinuous Percoll gradient. Following isolation, mitochondria were incubated with mitochondrial respiratory substrates and/or inhibitors for 10 min at 37 °C. Pyridine nucleotides were measured by HPLC with fluorescence detection as described under “Experimental Procedures.” G/M, glutamate/malate. The assay conditions were: glutamate/malate, 2.4 mm; ADP, 0.7 mm; and rotenone, 5 μm.
Respiratory Substrates Prevent H2O2-induced Mitochondrial GSH Oxidation and Protein S-Glutathionylation
Treatment of the brain mitochondria with H2O2 resulted in a rapid drop in GSH levels and increase GSSG levels (Fig. 7, A and B). H2O2 had no effect on GSH and GSSG levels (remained near detection limit levels) of respiratory substrate-supplemented mitochondria. H2O2 treatment resulted primarily in the glutathionylation of ATP synthase (Fig. 7C), which was substantially prevented by respiratory substrates plus ADP treatment. These findings confirm the importance of respiratory substrates in regulating GSH redox status in the presence of physiologic oxidants such as H2O2. ATP synthase appears to be one of the major mitochondrial proteins glutathionylated during times of oxidative stress. Although succinyl-CoA transferase, ATP synthase, and other unidentified protein(s) (∼40-kDa band) were the major proteins observed to be glutathionylated (Fig. 5), it is possible that other key mitochondrial proteins were also glutathionylated during oxidative stress but were not recognized because of limited sensitivity of the antibody (33). H2O2 treatment, on the other hand, appeared to preferentially cause the glutathionylation of ATP synthase, which may be related to GSSG levels.
FIGURE 7.
Regulation of brain mitochondria GSH and GSSG by respiratory substrates following H2O2 treatment. Brain mitochondria were isolated by a discontinuous Percoll gradient with a buffer containing DTT to conserve mitochondrial GSH. Isolated mitochondria were resuspended in a respiration buffer and incubated with various concentrations of H2O2 for 10 min at 37 °C. When added, glutamate/malate concentration was 2.4 mm. A, brain mitochondria GSH content. B, brain mitochondria GSSG content. C, immunoblotting of glutathionylated ATP synthase.
DISCUSSION
Mitochondrial GSH plays a key role in mitochondria functionality and a more significant role than cytosolic GSH in determining cellular function and viability (1–3, 7). This study shows that mitochondrial GSH levels and redox status are readily modified by the isolation procedure: although a discontinuous Percoll gradient yields mitochondria of higher purity, it promotes GSH oxidation and S-glutathionylation of mitochondrial proteins. The effects of this isolation procedure were more pronounced in brain mitochondria than liver mitochondria (even liver mitochondria isolated by this procedure showed a 60% decrease in GSH levels).
Cells generally export GSSG under oxidative stress conditions to prevent protein S-glutathionylation (1, 18). However, mitochondria have never been shown to release GSSG even when treated with oxidants such as tert-butyl hydroperoxide (19). Although mitochondria contain a GSH transport system, GSSG transporters have not been identified in mitochondrial membranes. This study further supports the notion that GSSG does not leave the mitochondrion and that the disulfide is involved in mitochondrial protein S-glutathionylation. This effect was particularly pronounced in brain mitochondria with a large pool of glutathionylated proteins.
ATP synthase (α subunit) and succinyl-CoA transferase were the two major proteins that were preferentially glutathionylated in brain mitochondria isolated by a discontinuous Percoll gradient. Interestingly, these two enzymes were also the two major proteins identified to be nitrated with age in brain (31), thus suggesting that ATPase and succinyl-CoA transferase can undergo several types of post-translational modifications under oxidative and nitrosative stress. Glutathionylation of these enzymes resulted in decreased activities, which may impair energy production in mitochondria. However, regulation of mitochondrial protein function/activity by S-glutathionylation has some advantages, for the process is readily reversible and sensitive to the mitochondria redox status: in conditions of severe oxidative stress, protein S-glutathionylation may protect protein thiols from oxidation to sulfinic and sulfonic derivatives, which are more stable irreversible type modifications (2, 11).
Mitochondria energized with glutamate/malate and ADP showed a dramatic increase in GSH levels, whereas GSSG and S-glutathionylated proteins became nearly undetectable. Regulation of thiol/disulfide redox status by mitochondrial respiratory substrates is likely to involve generation of NADPH, which is utilized by GSSG reductase (catalyzing the conversion of GSSG to GSH) and which also supports the recovery of S-glutathionylated proteins by the glutaredoxin-2 system (20). Several pathways have been identified as sources of NADPH in mitochondria: NADP+-dependent isocitrate dehydrogenase, malic enzyme, and nicotinamide nucleotide transhydrogenase (10). The extensive protein glutathionylation and GSSG formation in mitochondria isolated by a discontinuous Percoll gradient suggest that the NADPH-dependent reducing enzymes (i.e. GSSG reductase, glutaredoxin, and thioredoxin) that normally reduce disulfide bonds (i.e. GSSG and glutathionylation) formed in the matrix were not functioning optimally, possibly because of a lack of NADPH. Both glutamate/malate and succinate treatment can generate NADH and malate, which can be used to generate NADPH by nicotinamide nucleotide transhydrogenase and malic enzymes, respectively. One interesting observation was that recovery of S-glutathionylated proteins was greater during state 3 respiration (in the presence of ADP): because ATP synthase was a major protein glutathionylated upon H2O2 exposure, it may be that ADP treatment causes a conformational change that allowed easier access to glutaredoxin-2.
The regulation of GSH redox status by respiratory substrates was observed to occur following an H2O2 challenge: in the absence of mitochondrial substrates, H2O2 induced significant GSSG formation; however, energized mitochondria can handle high levels of exogenous H2O2 (with little GSSG and S-glutathionylated proteins being formed). The maintenance of GSH in substrate-treated mitochondria following a H2O2 challenge was likely due to a steady rate of NADPH formation, which supported GSSG reductase activity to reduce GSSG back to GSH. In the absence of substrates, the steady-state levels of NAD(P)H are low and cannot support the reduction of GSSG. This is similar to observations made with antimycin and rotenone, mitochondrial inhibitors that greatly increase steady-state levels of H2O2 (5, 22). GSH levels remained elevated in mitochondria treated with antimycin and rotenone when respiratory substrates were present. Taken together, these findings underscore the significance of a steady flux of respiratory substrates to maintain GSH redox status in mitochondria during oxidative stress.
This work was supported, in whole or in part, by National Institutes of Health Grants AA016911 (to D. H.), AG016718 (to E. C.), and DK067215 (to N. K.). This work was also supported by Tobacco-Related Disease Research Program Grant 16RT-0186 (to E. C.).
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
REFERENCES
- 1.Kaplowitz N., Aw T. Y., Ookhtens M. (1985) Annu. Rev. Pharmacol. Toxicol. 25, 715–744 [DOI] [PubMed] [Google Scholar]
- 2.Han D., Hanawa N., Saberi B., Kaplowitz N. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 291, G1–G7 [DOI] [PubMed] [Google Scholar]
- 3.Meister A. (1995) Biochim. Biophys. Acta. 1271, 35–42 [DOI] [PubMed] [Google Scholar]
- 4.Reed D. J. (1990) Annu. Rev. Pharmacol. Toxicol. 30, 603–631 [DOI] [PubMed] [Google Scholar]
- 5.Han D., Canali R., Rettori D., Kaplowitz N. (2003) Mol. Pharmacol. 64, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 6.Griffith O. W., Meister A. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 4668–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Checa J. C., Kaplowitz N., García-Ruiz C., Colell A. (1998) Semin. Liver Dis. 18, 389–401 [DOI] [PubMed] [Google Scholar]
- 8.Chang T. S., Cho C. S., Park S., Yu S., Kang S. W., Rhee S. G. (2004) J. Biol. Chem. 279, 41975–41984 [DOI] [PubMed] [Google Scholar]
- 9.Schafer F. Q., Buettner G. R. (2001) Free Radic. Biol. Med. 30, 1191–1212 [DOI] [PubMed] [Google Scholar]
- 10.Yap L. P., Garcia J. V., Han D., Cadenas E. (2009) Adv. Drug Deliv. Rev. 61, 1283–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurd T. R., Costa N. J., Dahm C. C., Beer S. M., Brown S. E., Filipovska A., Murphy M. P. (2005) Antioxid. Redox Signal. 7, 999–1010 [DOI] [PubMed] [Google Scholar]
- 12.Taylor E. R., Hurrell F., Shannon R. J., Lin T. K., Hirst J., Murphy M. P. (2003) J. Biol. Chem. 278, 19603–19610 [DOI] [PubMed] [Google Scholar]
- 13.Fratelli M., Demol H., Puype M., Casagrande S., Eberini I., Salmona M., Bonetto V., Mengozzi M., Duffieux F., Miclet E., Bachi A., Vandekerckhove J., Gianazza E., Ghezzi P. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3505–3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratelli M., Demol H., Puype M., Casagrande S., Villa P., Eberini I., Vandekerckhove J., Gianazza E., Ghezzi P. (2003) Proteomics 3, 1154–1161 [DOI] [PubMed] [Google Scholar]
- 15.Han D., Canali R., Garcia J., Aguilera R., Gallaher T. K., Cadenas E. (2005) Biochemistry 44, 11986–11996 [DOI] [PubMed] [Google Scholar]
- 16.Eaton P., Byers H. L., Leeds N., Ward M. A., Shattock M. J. (2002) J. Biol. Chem. 277, 9806–9811 [DOI] [PubMed] [Google Scholar]
- 17.Odin J. A., Huebert R. C., Casciola-Rosen L., LaRusso N. F., Rosen A. (2001) J. Clin. Invest. 108, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sies H., Cadenas E. (1985) Philos. Trans. R. Soc. Lond. B Biol. Sci. 311, 617–631 [DOI] [PubMed] [Google Scholar]
- 19.Olafsdottir K., Reed D. J. (1988) Biochim. Biophys. Acta 964, 377–382 [DOI] [PubMed] [Google Scholar]
- 20.Holmgren A., Johansson C., Berndt C., Lönn M. E., Hudemann C., Lillig C. H. (2005) Biochem. Soc. Trans. 33, 1375–1377 [DOI] [PubMed] [Google Scholar]
- 21.Rydström J. (2006) Biochim. Biophys. Acta 1757, 721–726 [DOI] [PubMed] [Google Scholar]
- 22.Han D., Williams E., Cadenas E. (2001) Biochem. J. 353, 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han D., Antunes F., Daneri F., Cadenas E. (2002) Methods Enzymol. 349, 271–280 [DOI] [PubMed] [Google Scholar]
- 24.Schroeter H., Boyd C. S., Ahmed R., Spencer J. P., Duncan R. F., Rice-Evans C., Cadenas E. (2003) Biochem. J. 372, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson M. F., Sims N. R. (2000) Brain Res. Protoc. 5, 95–101 [DOI] [PubMed] [Google Scholar]
- 26.Ellman G. L. (1959) Arch. Biochem. BIophys. 82, 70–77 [DOI] [PubMed] [Google Scholar]
- 27.Yap L. P., Sancheti H., Ybanez M. D., Garcia J. V., Cadenas E., Han D. (2010) Methods Enzymol. 473, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaidman L. K., Leung A. C., Adams J. D., Jr. (1995) Anal. Biochem. 228, 312–317 [DOI] [PubMed] [Google Scholar]
- 29.Jones D. P. (2002) Methods Enzymol. 348, 93–112 [DOI] [PubMed] [Google Scholar]
- 30.Williamson D. H., Bates M. W., Page M. A., Krebs H. A. (1971) Biochem. J. 121, 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam P. Y., Yin F., Hamilton R. T., Boveris A., Cadenas E. (2009) Free Radic. Res. 43, 431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kil I. S., Park J. W. (2005) J. Biol. Chem. 280, 10846–10854 [DOI] [PubMed] [Google Scholar]
- 33.Yap L. P., Garcia J. V., Han D. S., Cadenas E. (2010) Biochem. J. 428, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]