Abstract
The prostaglandin E2 (PGE2) G protein-coupled receptor (GPCR), EP2, plays important roles in mouse skin tumor development (Chun, K. S., Lao, H. C., Trempus, C. S., Okada, M., and Langenbach, R. (2009) Carcinogenesis 30, 1620–1627). Because keratinocyte proliferation is essential for skin tumor development, EP2-mediated signaling pathways that contribute to keratinocyte proliferation were investigated. A single topical application of the EP2 agonist, butaprost, dose-dependently increased keratinocyte replication via activation of epidermal growth factor receptor (EGFR) and PKA signaling. Because GPCR-mediated activation of EGFR can involve the formation of a GPCR-β-arrestin-Src signaling complex, the possibility of a β-arrestin1-Src complex contributing to EP2-mediated signaling in keratinocytes was investigated. Butaprost induced β-arrestin1-Src complex formation and increased both Src and EGFR activation. A role for β-arrestin1 in EP2-mediated Src and EGFR activation was demonstrated by the observation that β-arrestin1 deficiency significantly reduced Src and EGFR activation. In agreement with a β-arrestin1-Src complex contributing to EGFR activation, Src and EGFR inhibition (PP2 and AG1478, respectively) indicated that Src was upstream of EGFR. Butaprost also induced the activation of Akt, ERK1/2, and STAT3, and both β-arrestin1 deficiency and EGFR inhibition (AG1478 or gefitinib) decreased their activation. In addition to β-arrestin1-dependent EGFR activation, butaprost increased PKA activation, as measured by phospho-GSK3β (p-GSK3β) and p-cAMP-response element-binding protein formation. PKA inhibition (H89 or RP-adenosine-3′,5′-cyclic monophosphorothioate (RP-cAMPS)) decreased butaprost-induced cAMP-response element-binding protein and ERK activation but did not affect EGFR activation, whereas β-arrestin1 deficiency decreased EGFR activation but did not affect butaprost-induced PKA activation, thus indicating that they were independent EP2-mediated pathways. Therefore, the results indicate that EP2 contributed to mouse keratinocyte proliferation by G protein-independent, β-arrestin1-dependent activation of EGFR and G protein-dependent activation of PKA.
Keywords: G protein-coupled Receptors (GPCR), Prostaglandins, Receptors, Signal Transduction, Skin, EP2, GPCR Signaling Pathways, Beta-Arrestin1, Butaprost, Mouse Skin Keratinocyte
Introduction
The prostaglandins (PGs)2 generated via the cyclooxygenases (COX-1 and COX-2) are known to have roles both in the maintenance of normal physiology and in various pathological conditions (1). In mouse skin, for example, PGE2 is the major PG produced and contributes to keratinocyte replication and skin tumor formation (2–4). However, the mechanisms by which PGs contribute to these processes are only beginning to be resolved. The biological activities of PGE2 are mediated through the interaction with four G protein-coupled receptors (GPCRs): EP1, EP2, EP3, and EP4 (5, 6). These receptors differ in their PGE2 binding affinities and the downstream signaling pathways activated. Early studies indicated that EP1 and EP3 couple to Gαq to activate Ca2+ signaling and Gαi to inhibit adenylyl cyclase, respectively, whereas EP2 and EP4 couple to Gαs to stimulate adenylyl cyclase (6, 7). Furthermore, data derived from EP knock-out and EP-overexpressing mice as well as receptor-specific agonists and antagonists indicated important roles for EP2 in the formation and growth of several tumor types, including skin tumors (8–13).
Classically, GPCRs were thought to activate only G protein-mediated pathways, and it was thought that G protein-dependent signaling was regulated by receptor internalization/desensitization following the formation of a complex with β-arrestin1 or -2 (14, 15). However, more recent studies have indicated that the GPCR-β-arrestin complex can activate G protein-independent signaling pathways (16, 17). GPCR-β-arrestin complexes function as scaffolds for the binding/activation of signaling effectors that include PI3K, Ras, and ERK1/2 (17–19). Furthermore, subsequent studies demonstrated that Src was a component of the GPCR-β-arrestin complex and that the binding/activation of Src facilitated GPCR-β-arrestin-mediated signaling, including the transactivation of epidermal growth factor receptor (EGFR) (16, 17).
For the PGE2 receptors, EP2 and EP4, most studies have focused on their ability to activate G protein-dependent signaling pathways. Early studies by Regan and colleagues (6, 7) demonstrated that PGE2 stimulation of EP2 and EP4 activated adenylate cyclase with the subsequent activation of PKA and downstream effectors such as cAMP-response element-binding protein (CREB) and glycogen synthase kinase 3β (GSK3β), although a PI3K/Akt pathway appeared to be a major pathway for EP4. Donnini et al. (20) reported that the EP2 agonist, butaprost, stimulated PKA and Src activation in cultured cells and that both activated PKA and Src contributed to EGFR activation, although a pathway for Src activation was not described. In genetically modified mice, EP2 overexpression increased (12) and EP2 deficiency decreased (11, 13) PKA activation and mouse skin tumor formation.
Only a limited number of studies have investigated the abilities of EP2 and EP4 to complex with and/or signal via β-arrestin-dependent mechanisms. Furthermore, initial studies investigating the interactions of EP2 and EP4 with the β-arrestins concluded that EP4, but not EP2, could be desensitized/internalized following PGE2 stimulation (21, 22), suggesting that the EP2 was unable to form a functional complex with the β-arrestins. To date, the only PGE2 receptor reported to form a signaling complex with a β-arrestin is EP4, and these studies indicated that the EP4-β-arrestin1 complex resulted in Src (23) and Src-EGFR (24) activation. Although early studies indicated that EP2 was not internalized by PGE2 stimulation (21, 22), we recently reported that EP2 could form a complex with β-arrestin1 and Src in mouse skin and papillomas following ligand stimulation of EP2. However, our data did not allow us to determine whether the EP2-β-arrestin1-Src complex contributed to signaling or was only a mechanism for EP2 desensitization (13).
In the present study, we provide evidence that ligand (butaprost) stimulation of EP2 in mouse skin leads to the formation of a β-arrestin1-Src complex that contributes to the transactivation of EGFR. The contribution of β-arrestin1 to EP2-mediated signaling was obtained by comparing the abilities of butaprost-treated WT and β-arrestin1-deficient mice (25) with activated Src-EGFR and the downstream effectors: ERK, Akt, and STAT3. In addition, the data indicate that butaprost stimulation of EP2 also leads to G protein-dependent activation of PKA and the downstream effectors: CREB and GSK3β. Furthermore, by using EGFR and PKA pathway-selective inhibitors, we demonstrate that both β-arrestin1-dependent EGFR activation and G protein-dependent PKA activation contribute to keratinocyte replication in vivo. Thus, EP2-mediated signaling induces keratinocyte replication via both G protein-dependent activation of PKA and G protein-independent, β-arrestin1-dependent activation of EGFR.
EXPERIMENTAL PROCEDURES
Materials
PGE2 and butaprost, a highly selective agonist for EP2 (5), were obtained from Cayman Chemical (Ann Arbor, MI). Bromodeoxyuridine (BrdU) was purchased from GE Healthcare. PP2 was purchased from Alexis Biochemicals (San Diego, CA). AG1478 and H89 were obtained from Calbiochem and Santa Cruz Biotechnology (Santa Cruz, CA). RP-cAMPS and gefitinib were purchased from Tocris Bioscience (Ellisville, MO).
Animals
Female CD-1 mice were obtained from Charles River Laboratories (Wilmington, MA) and were used at 6 weeks of age. β-Arrestin1−/− mice (25) were obtained from Dr. Lefkowitz (Duke University Medical Center, Durham, NC) and maintained on a C57Bl6 background. Six-week-old CD-1 or β-arrestin1 WT and deficient mice were shaved 2 days before a single treatment with butaprost or PGE2 in 200 μl of acetone. The inhibitors, AG1478, gefitinib, H89, RP-cAMPS, and PP2, were applied as described in the legends for Figs. 1–3 and 5 for the individual experiments. Mice were housed in the animal husbandry facilities of the NIEHS/National Institutes of Health according to the Association for the Assessment and Accreditation of Laboratory Animal Care guidelines. All studies were approved by the NIEHS Animal Care and Use Committee. Food and water were provided ad libitum.
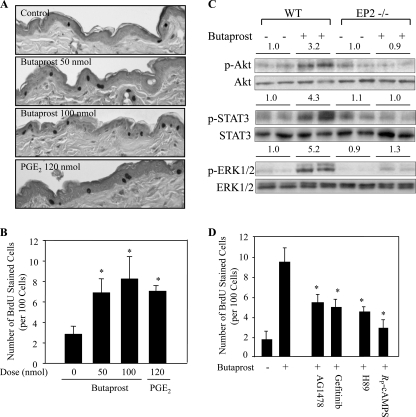
FIGURE 1.
EP2 activation increased epidermal proliferation. A, BrdU incorporation into epidermal keratinocytes. Six-week-old female CD-1 mice were treated with acetone, butaprost (50 or 100 nmol), or PGE2 (120 nmol) for 24 h, and BrdU was injected i.p. 1 h before sacrifice. The skin sections were immunostained with an antibody against BrdU. Skin sections are shown at a magnification of 200×. B, number of BrdU-stained cells in the skin of butaprost- and PGE2-treated mice. *, p < 0.05 versus vehicle-treated mice. C, butaprost treatment of EP2−/− mice did not significantly increase the levels of p-Akt, p-STAT3, and p-ERK1/2. WT and EP2−/− mice were pretreated topically with acetone or 100 nmol of butaprost and sacrificed 2 h following butaprost treatment. The phosphorylation status of ERK1/2, Akt, and STAT3 was determined by Western blot analysis using antibodies for p-ERK1/2 (Thr202/Tyr204), p-Akt (Ser473), or p-STAT3 (Tyr705). Total EGFR, ERK1/2, Akt, and STAT3 served as controls for protein loading and membrane transfer. Each lane represents an individual mouse. The intensities of the bands were determined by densitometry, and the ratios of the activated to total protein are shown above each lane. The number above each lane shows the mean -fold intensity from two mice. D, PKA and EGFR inhibitors suppressed butaprost-induced keratinocyte proliferation. Mice were pretreated topically with AG1478 (100 nmol), gefitinib (67 nmol), H89 (500 nmol), or RP-cAMPS (12 nmol) 30 min prior to treatment with 100 nmol of butaprost and sacrificed 24 h following butaprost treatment. *, p < 0.05 versus mice treated with butaprost alone. In B and D, BrdU-stained cells were counted in five skin sections from each of five mice. Data are expressed as the mean ± S.D. (n = 5) of the number of stained cells per 100 basal cells in each group.
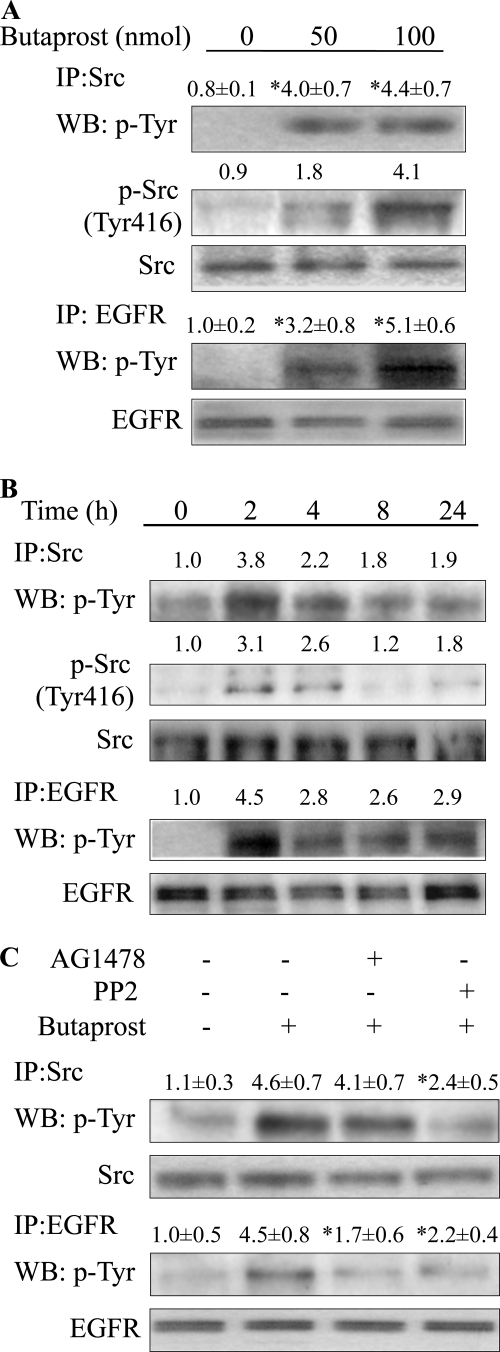
FIGURE 2.
Butaprost induced Src and EGFR activation. A, butaprost dose-dependently induced phosphorylation of Src and EGFR. Mice were treated with butaprost (50 or 100 nmol) and sacrificed at 2 h after treatment. *, p < 0.05 versus vehicle-treated mice. IP, immunoprecipitation; WB, Western blot. B, butaprost (100 nmol) induced phosphorylation of Src and EGFR in a time-dependent manner. C, AG1478 and PP2 decreased EGFR phosphorylation. The dorsal skin was treated with PP2 (100 nmol) or AG1478 (100 nmol) for 30 min before butaprost (100 nmol) treatment, and skin sections were isolated 2 h later. *, p < 0.05 versus butaprost-treated mice. In A–C, Src and EGFR were immunoprecipitated from skin lysates (200 μg) by an anti-Src or an anti-EGFR antibody. p-Src and p-EGFR were detected by Western blot analysis by using a monoclonal anti-p-Tyr antibody. The data are representative of two independent experiments. In A and B, skin lysates (50 μg) were immunoblotted for p-Src (Tyr416). In A–C, the intensities of the bands were determined by densitometry as described in the legend for Fig. 1. In A and C, the relative intensities are presented as mean value ± S.D. (n = 3) from two independent experiments.
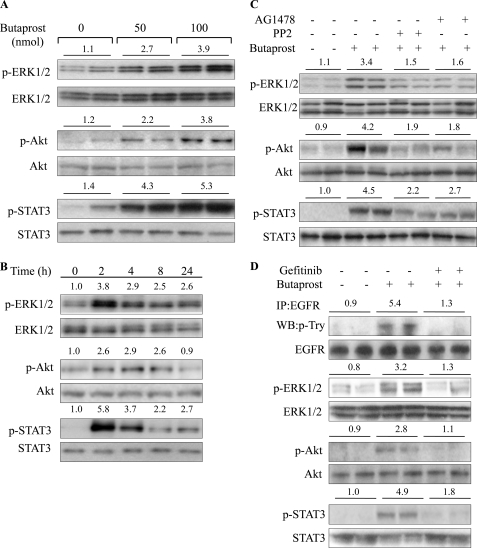
FIGURE 3.
Butaprost induced ERK1/2, Akt, and STAT3 phosphorylation. A, butaprost induced dose-dependent phosphorylation of ERK1/2, Akt, and STAT3. Mice were treated with butaprost (50 or 100 nmol) and sacrificed 2 h after treatment. B, butaprost (100 nmol) induced phosphorylation of ERK1/2, Akt, and STAT3 in a time-dependent manner. C, the Src and EGFR inhibitors suppressed butaprost-induced phosphorylation of ERK1/2, Akt, and STAT3. Mice were pretreated with PP2 (100 nmol) or AG1478 (100 nmol) for 30 min and then treated with 100 nmol of butaprost for 2 h. D, the EGFR inhibitor, gefitinib, suppressed butaprost-induced phosphorylation of EGFR, ERK1/2, Akt, and STAT3. Mice were pretreated with gefitinib (67 nmol) for 30 min and then treated with 100 nmol of butaprost for 2 h. EGFR was immunoprecipitated (IP) from skin lysates, and p-EGFR was determined by Western blot (WB) analysis by using a monoclonal anti-p-Tyr antibody. In A–D, the phosphorylation status was determined by Western blot analysis using antibodies against indicated proteins. The densitometry data are representative of two independent experiments. In A, C, and D, each lane represents an individual mouse. The number above each lane shows the mean -fold intensity from two mice.
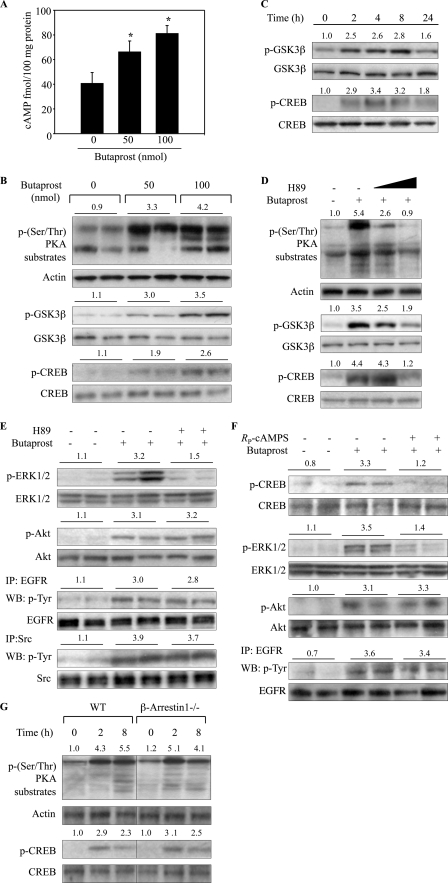
FIGURE 5.
Butaprost activated the cAMP/PKA pathway. A, butaprost induced cAMP production. Mice were treated with butaprost (50 or 100 nmol) for 2 h, and tissue lysates (100 μg protein) were assayed for cAMP levels as described under “Experimental Procedures.” Values are means ± S.D. (n = 5). *, p < 0.05 versus vehicle-treated mice. B, butaprost induced dose-dependent phosphorylation of PKA substrates, GSK3β, and CREB. Mice were sacrificed at 2 h after 50 or 100 nmol of butaprost treatment. C, butaprost (100 nmol) induced time-dependent phosphorylation of GSK3β and CREB. D, H89 dose-dependently decreased PKA activity. Mice were treated with H89 (100 or 500 nmol) for 30 min before butaprost (100 nmol) treatment, and skin sections were isolated 2 h later. E, H89 suppressed butaprost-induced phosphorylation of ERK1/2 but not Akt, EGFR, or Src. Mice were pretreated with H89 (500 nmol) for 30 min and then treated with 100 nmol of butaprost for 2 h. Skin lysates were immunoprecipitated (IP) with an anti-EGFR or an anti-Src antibody and immunoblotted (WB) with an anti-p-Tyr antibody. Total EGFR and Src served as controls for protein loading and membrane transfer. F, the PKA inhibitor, RP-cAMPS, suppressed butaprost-induced phosphorylation of CREB and ERK1/2 but not Akt or EGFR. Mice were pretreated with RP-cAMPS (12 nmol) for 30 min and then treated with 100 nmol of butaprost for 2 h. Skin lysates were immunoprecipitated with an anti-EGFR antibody and immunoblotted with an anti-p-Tyr antibody. G, butaprost induced phosphorylation of PKA substrates and CREB in both WT and β-arrestin1−/− mice. WT and β-arrestin1−/− mice were treated for the indicated times with 100 nmol of butaprost. In B–G, skin lysates (80 μg) were immunoblotted with the indicated antibodies. The number above each lane shows the relative intensities of the bands as described in the legend for Fig. 1. The data are representative of two independent experiments. In B, E, and F, each lane represents an individual mouse. The number above each lane shows the mean -fold intensities for two mice.
BrdU Incorporation
The mice were euthanized 24 h after butaprost or PGE2 treatment, and BrdU (200 μl of 10 mm BrdU solution per mouse) was injected i.p. 1 h before sacrifice. The dorsal skin was removed, fixed in formalin, and processed for paraffin embedding and immunohistochemical staining with a monoclonal rat anti-BrdU antibody (BD Biosciences). BrdU-positive and BrdU-negative basal cells were counted in five randomly selected areas of each skin section, and the mean percentage of BrdU-positive cells for each treatment group was determined.
cAMP Immunoassay
cAMP measurement was carried out as described previously (26) using a kit from BioVision (Mountain View, CA). Samples (100 μg of protein) were added to the protein A-coated 96-well plates, and incubations were carried out with a cAMP antibody for 1 h at room temperature. cAMP conjugated with horseradish peroxidase was added to the sample mixture, and the immunoassay was conducted per the supplier's instructions. The reactions were terminated by the addition of 1.0 n HCl, and the A450 nm was determined. Calculations were based on a standard curve for each experiment.
Western Blot Analysis
For isolation of protein from mouse skin, the dorsal skin was excised and immediately placed in liquid nitrogen and pulverized with a mortar and pestle. The pulverized skin was homogenized at 4 °C with a Polytron tissue homogenizer in 0.5 ml of 1× cell lysis buffer (Cell Signaling Technology, Danvers, MA) containing 1 mm phenylmethylsulfonyl fluoride. Lysates were centrifuged at 12,000 × g for 15 min, and supernatants containing 50–80 μg of protein were boiled in sample loading buffer for 5 min and loaded on CriterionTM 4–20% Tris-HCl precast gels (Bio-Rad Laboratories). After electrophoresis for 2 h, the proteins were transferred to PVDF membranes and blocked with 5% nonfat dry milk-PBST buffer (phosphate-buffered saline containing 0.1% Tween 20) for 1 h at room temperature. The membranes were incubated overnight at 4 °C with 1:500–1,000 dilution of the following antibodies: p-Akt (Ser473), Akt, p-ERK1/2 (Thr202/Tyr204), ERK1/2, p-STAT3 (Tyr705), STAT3, p-GSK3β (Ser9), GSK3β, p-CREB (Ser133), and CREB and p-(Ser/Thr) PKA substrate polyclonal antibodies (Cell Signaling Technology); EGFR and Src (Santa Cruz Biotechnology); β-arrestin1 (Abcam, Cambridge, MA); and phosphotyrosine (Upstate Biotech Millipore, Lake Placid, NY). The blots were incubated for 1 h with 1:5,000 dilution of the horseradish peroxidase-conjugated secondary anti-rabbit (Sigma-Aldrich) or anti-mouse (Cell Signaling Technology, Beverly, MA) antibody. The transferred proteins were visualized with an enhanced chemiluminescence detection kit (GE Healthcare UK Ltd., Buckinghamshire, UK).
Immunoprecipitations
To preclear tissue lysates, supernatants were incubated for 1 h with gentle rocking at 4 °C by adding 1.0 μg of the appropriate control IgG (corresponding to the host species of the primary antibody) and 20 μl of the appropriate suspended protein A/G-agarose (Santa Cruz Biotechnology). Following centrifugation at 1,000 × g for 30 s at 4 °C, the supernatants containing 200 μg protein were transferred to a microcentrifuge tube, and 1.0 μg primary antibody was added followed by incubation for 2 h at 4 °C. Twenty μl of protein A/G-agarose was then added and incubated at 4 °C overnight with rotation. The immunoprecipitates were collected by centrifugation at 1,000 × g for 30 s at 4 °C, and the pellet was gently washed four times with 1.0 ml of cell lysis buffer. After the final wash, the supernatants were discarded, and the pellets were resuspended in electrophoresis sample buffer for loading.
Statistical Analysis
Data are expressed as means ± S.D. Statistical significance was determined by the Student's t test, and p values <0.05 were considered statistically significant.
RESULTS
PGE2 and the EP2 Agonist, Butaprost, Increased Epidermal Cell Proliferation
The data in Fig. 1, A and B, show that a single topical application of butaprost dose-dependently increased epidermal keratinocyte proliferation in the mouse epidermis by 2.1- and 2.6-fold when BrdU incorporation was used as a measure of cell replication. When PGE2 was used as a positive control, a single topical treatment increased BrdU incorporation by about 2.1-fold (Fig. 1, A and B). Fig. 1A also shows that BrdU incorporation following PGE2 or butaprost treatment was primarily located in the basal cells of the epidermis. The selectivity of butaprost for EP2 was shown by the negligible increase of p-ERK1/2, p-Akt, and p-STAT3 levels in butaprost-treated EP2−/− mice when compared with EP2+/+ mice (Fig. 1C). Based on the observation that butaprost stimulated epidermal keratinocyte proliferation, EP2-activated signaling pathways that contributed to keratinocyte proliferation were identified using pathway-specific inhibitors. The data in Fig. 1D indicate that butaprost-induced BrdU incorporation was reduced by about 50% by the EGFR inhibitor, AG1478, and about 60% by the PKA inhibitor, H89. Because both AG1478 and H89 can inhibit kinases in addition to EGFR and PKA (27, 28), the effects of additional inhibitors of EGFR and PKA on keratinocyte replication were determined. In agreement with the effects observed with AG1478 and H89, the EGFR inhibitor, gefitinib, and the PKA inhibitor, RP-cAMPS, inhibited keratinocyte replication by about 50 and 68%, respectively. Thus, the inhibitor data indicate important roles for EGFR and PKA signaling in EP2-stimulated epidermal cell replication.
Butaprost Induced the Activation of Src and EGFR
Because the EGFR inhibitors decreased epidermal cell proliferation (Fig. 1D) and recent studies have shown that PGE2 activation of Src could mediate the activation of EGFR (29–31), the ability of butaprost to activate Src and EGFR was investigated. The data in Fig. 2, A and B, indicate that a single topical application of butaprost dose- and time-dependently increased Src phosphorylation, including phosphorylation at the activation site, Tyr416 (32). Butaprost also dose- and time-dependently increased EGFR phosphorylation (Fig. 2, A and B). The time course data indicate that Src and EGFR were maximally activated at about 2 h after treatment and that elevated activation was still evident at 24 h after treatment (Fig. 2B). The levels of total Src and EGFR were not altered by butaprost treatment. The data in Fig. 2C further indicate that the Src kinase inhibitor, PP2, decreased Src and EGFR phosphorylation, whereas the EGFR inhibitor, AG1478, inhibited EGFR phosphorylation but did not inhibit Src phosphorylation. These data indicate that activated Src is upstream of EGFR.
Butaprost Induced the Activation of ERK1/2, Akt, and STAT3
Activated ERK1/2, Akt, and STAT3 are EGFR effectors known to contribute to cell replication and skin tumor formation (33–36); therefore, the effect of butaprost on their activation was investigated. The data in Fig. 3, A and B, indicate that butaprost increased the activation of ERK1/2, Akt, and STAT3 in a dose- and time-dependent manner. Furthermore, because EGFR had been reported to contribute to the activation of these effectors, the effects of Src and EGFR inhibition on the activation of ERK, Akt, and STAT3 were determined. The data in Fig. 3C indicate that the EGFR inhibitor, AG1478, and the Src inhibitor, PP2, significantly reduced butaprost-induced phosphorylation of ERK1/2, Akt, and STAT3. To confirm the contribution of EGFR to the activation of ERK1/2, Akt, and STAT3, the effect of the EGFR inhibitor, gefitinib, on their activation was also determined. The data in Fig. 3D indicate that gefitinib also significantly reduced butaprost-induced phosphorylation of EGFR, ERK1/2, Akt, and STAT3. Thus, these data indicate that EP2-mediated activation of Src and EGFR contributes to the activation of ERK1/2, Akt, and STAT3.
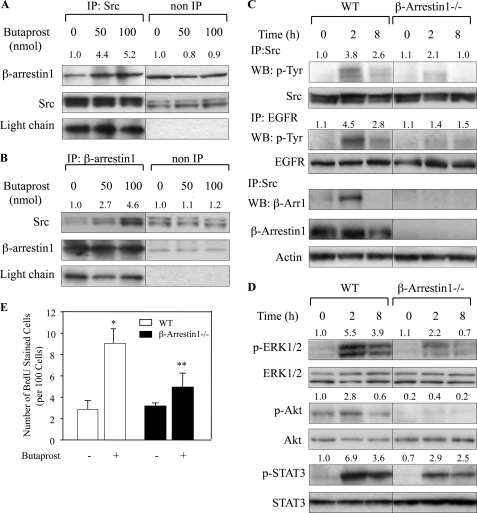
Butaprost Induced β-Arrestin1-Src Complex Formation in Mouse Skin, and β-Arrestin1 Deficiency Decreased the Activation of Src and EGFR
Because the data in Fig. 2, A–C, indicated that ligand stimulation of EP2 led to the activation of Src, which then contributed to the activation of EGFR, possible mechanisms for the activation of Src by EP2 were investigated. Recent studies have indicated that ligand stimulation of a GPCR can induce the formation of a GPCR-β-arrestin-Src complex and Src activation (17, 24, 37), and we recently reported the formation of an EP2-β-arrestin1-Src complex in TPA-EP2 agonist-induced mouse skin papillomas (13). The Western blot data in Fig. 4, A and B, show that a single treatment of butaprost alone dose-dependently increased the levels of a β-arrestin1-Src complex as indicated by immunoprecipitation with antibodies to Src and β-arrestin1, respectively. The data also indicate that butaprost treatment did not increase the levels of total Src or β-arrestin1. Thus, the data indicate that butaprost stimulation of EP2 leads to the increased formation of a β-arrestin1-Src complex in mouse skin.
FIGURE 4.
Butaprost induced β-arrestin1-Src complex formation. A, butaprost increased β-arrestin1-Src complex formation. Mice were sacrificed at 2 h after 50 or 100 nmol of butaprost treatment. Skin lysates (200 μg) were immunoprecipitated (IP; non-IP, not immunoprecipitated) with a monoclonal anti-Src antibody, and β-arrestin1 and Src were detected by Western blotting. B, butaprost increased the formation of a β-arrestin1-Src complex. Skin lysates (200 μg) were immunoprecipitated with a polyclonal anti-β-arrestin1 antibody and subjected to Western blot analysis using a monoclonal Src antibody. In A and B, nonspecific IgG light chain served as a control for protein loading and membrane transfer. The number above each lane shows the relative intensities of the bands to IgG light chain. C, butaprost induced phosphorylation of Src and EGFR and increased the β-arrestin1-Src complex in WT but not in β-arrestin1−/− mice. WT and β-arrestin1−/− mice were treated for the indicated times with 100 nmol of butaprost. Src and EGFR were immunoprecipitated from protein lysates (200 μg) and subjected to Western blot (WB) analysis using an anti-p-Tyr or an anti-β-arrestin1 antibody. D, butaprost induced phosphorylation of ERK1/2, Akt, and STAT3 in WT but not in β-arrestin1−/− mice. WT and β-arrestin1−/− mice were treated for the indicated times with 100 nmol of butaprost. Skin lysates were immunoblotted with the indicated antibodies. In C and D, the number above each lane shows the mean-fold intensity as determined by densitometry. In A–D, the data presented are representative of two independent experiments. E, β-arrestin1 deficiency suppressed butaprost-induced keratinocyte proliferation. WT and β-arrestin1−/− mice were pretreated topically with acetone or 100 nmol of butaprost and sacrificed 24 h following butaprost treatment. *, p < 0.05 versus mice treated with acetone alone. **, p < 0.05 versus butaprost-treated WT mice. BrdU-stained cells were counted in five skin sections from each of five mice. Data are expressed as the mean ± S.D. (n = 5) of the number of stained cells per 100 basal cells in each group.
To determine whether the β-arrestin1-Src complex contributed to Src activation in butaprost-treated mouse skin, butaprost-induced activation of Src in WT and β-arrestin1-deficient mice was compared. The data in Fig. 4C show that a single butaprost treatment induced time-dependent activation of Src in WT mouse skin but that Src activation was significantly decreased in β-arrestin1−/− mice. Furthermore, the data in Fig. 4C indicate that p-EGFR, which is activated by Src (Fig. 2C), was also decreased in butaprost-treated β-arrestin1−/− mouse skin. The data, as expected, also show that β-arrestin1 could be immunoprecipitated by an antibody to Src from WT but not from β-arrestin1−/− mouse skin. Butaprost-induced activation of ERK1/2, Akt, and STAT3 was also significantly decreased in β-arrestin1−/− mice when compared with WT mice (Fig. 4D). Furthermore, butaprost-induced keratinocyte replication as measured by BrdU incorporation was decreased by about 40% in β-arrestin1−/− mouse skin when compared with WT mouse skin (Fig. 4E), indicating that β-arrestin1-mediated signaling contributes to keratinocyte replication. Thus, the data indicate that a β-arrestin1-Src complex contributes to keratinocyte replication and that Src-EGFR-activated signaling pathways are involved.
Butaprost Increased PKA Activation via a β-Arrestin1-independent Pathway
Because our previous study indicated that EP2 stimulation caused increases in cAMP levels and PKA activation in TPA-EP2 agonist-treated mouse papillomas (13), studies were conducted to determine whether butaprost treatment alone could increase cAMP levels and PKA activation in mouse skin. The data in Fig. 5A show that cAMP levels were dose-dependently increased 1.6- and 2.2-fold at 2 h after butaprost treatment. To determine whether PKA activation was also increased by a single treatment with butaprost, PKA activity was measured using antibodies that recognize PKA-phosphorylated proteins that contained the threonine and serine motifs, RXXT and RRXS (26, 38), and the PKA-phosphorylated effectors, p-GSK3β and p-CREB. The data in Fig. 5B show that butaprost induced dose-dependent increases in the levels of PKA-phosphorylated proteins and the effectors, p-GSK3β and p-CREB. Fig. 5C shows that butaprost induced time-dependent increases in p-GSK3β and p-CREB formation. To confirm that the formation of the phosphorylated PKA substrates, p-GSK3β and p-CREB, was due to PKA, the PKA inhibitor, H89, was shown to decrease their phosphorylation (Fig. 5D). The data in Fig. 5E also show that H89 significantly inhibited butaprost-induced phosphorylation of ERK1/2, but not Akt, whereas EGFR inhibition reduced the activation of both ERK1/2 and Akt (Fig. 3C).
To determine whether PKA was involved in Src or EGFR activation, the effects of H89 and another PKA inhibitor, RP-cAMPS, on Src-EGFR activation were determined. The data in Fig. 5E show that H89 did not affect butaprost-induced phosphorylation of Src or EGFR, indicating that their activation was not dependent on PKA. The data in Fig. 5F also indicate that RP-cAMPS, like H89 (Fig. 5D), significantly reduced butaprost-induced phosphorylation of CREB and ERK1/2 but did not affect butaprost-induced phosphorylation of EGFR and the downstream effector Akt. Thus, the data indicate that PKA activation was not a major pathway contributing to the activation of EGFR.
Conversely, to determine whether the β-arrestin1/Src pathway contributed to PKA activation, butaprost-induced activation of PKA, as measured by the levels of PKA-phosphorylated proteins and p-CREB formation, was compared in WT and β-arrestin1−/− mice. The data in Fig. 5G show that the levels of PKA-phosphorylated proteins and p-CREB were similar in both butaprost-treated WT and butaprost-treated β-arrestin1−/− mouse skin, indicating that the β-arrestin1 pathway does not contribute to the activation of PKA in mouse skin. However, although PKA signaling does not contribute to the activation of β-arrestin1-Src-EGFR and the β-arrestin1 signaling does not contribute to the activation of PKA in butaprost-treated mouse skin, there are downstream effectors, such as ERK1/2, which are activated via both pathways.
DISCUSSION
In the present study, we report that butaprost-induced stimulation of the PGE2 receptor, EP2, in mouse skin activates β-arrestin1-dependent signaling pathways. We previously reported that TPA-EP2 ligand treatment of mouse skin papillomas caused the formation of an EP2-β-arrestin1-Src complex, but because of the approach used, we were unable to show that the complex activated signaling pathways (13). In the present study, we demonstrate that butaprost alone induced β-arrestin1-Src complex formation, and by comparing WT and β-arrestin1−/− mice, we show that β-arrestin1 contributed to Src activation and the subsequent transactivation of EGFR. EGFR then activated the downstream effectors, ERK1/2, Akt, and STAT3. We also demonstrate that butaprost stimulation of EP2 led to the activation of PKA and the downstream effectors, GSK3β and CREB. Furthermore, our results indicate that the butaprost-induced β-arrestin1 and PKA pathways are independent, although some downstream effectors such as ERK1/2 are activated in both pathways. In addition, the data obtained using inhibitors of EGFR and PKA indicate that both β-arrestin1-dependent activation of EGFR and G protein-dependent activation of PKA play key roles in mouse skin keratinocyte replication.
The present findings in combination with our previous findings (13) indicate that agonist-induced stimulation of EP2 resulted in the formation of a complex involving EP2, β-arrestin1, and Src. Furthermore, the decreased activation of Src and EGFR in butaprost-treated β-arrestin1−/− mice (Fig. 4C) indicated that the EP2-β-arrestin1 complex contributed to their activation, and Src and EGFR inhibitors indicated that activated Src contributed to the transactivation of EGFR (Fig. 2C). Although several GPCRs have been shown to signal via β-arrestin-dependent pathways (16, 17, 39, 40), EP4 is the only PGE2 receptor that has been reported to form a complex with β-arrestin that leads to the activation of signaling pathways. PGE2-stimulated EP4 has been reported to form a complex with β-arrestin1 that leads to Src activation (23, 24) and the transactivation of EGFR (24). However, EP2-β-arrestin-mediated signaling has not been previously reported. In fact, PGE2 stimulation of EP2 was reported not to cause β-arrestin-mediated internalization of EP2, whereas PGE2-stimulated EP4 was rapidly internalized (21, 22). In contrast, we recently reported that ligand-stimulated EP2 can form an EP2-β-arrestin1-Src complex in mouse skin (13), and the results from that study combined with the data reported herein demonstrate that the EP2-β-arrestin1-Src complex leads to G protein-independent, β-arrestin1-dependent signaling and enhanced keratinocyte proliferation.
Early reports concluded that the formation of a GPCR-β-arrestin complex was a mechanism for uncoupling GPCR-mediated G protein signaling and down-regulating the GPCR (14, 15); however, more recent studies have indicated that the GPCR-β-arrestin complex actually contributes to GPCR-mediated signaling (17, 18). This G protein-independent signaling is due to the ability of the β-arrestins to function as scaffolds for the binding and activation of signaling effectors such as: Src, Ras, ERK1/2, and other members of the MAPK family (18). Moreover, the ability of β-arrestins to bind and activate Src thereby provides the GPCRs with mechanisms to cross-talk with effectors/receptors that are activated by tyrosine phosphorylation. In addition, although GPCR-mediated G protein signaling is transient, GPCR-β-arrestin-mediated signaling can be protracted (41). Indeed, the levels of p-EGFR, p-Src, and p-ERK1/2 were elevated in WT mice when compared with β-arrestin1−/− mice and remained elevated for 8 h following a single butaprost treatment (Fig. 4C). The elevated levels of the EP2-β-arrestin1-Src complex observed in skin tumors combined with protracted β-arrestin-dependent signaling could give tumor cells a growth advantage when compared with surrounding normal cells.
The activation of Src, a non-receptor tyrosine kinase, following ligand-stimulated GPCR interaction with β-arrestin has been reported by several investigators (17, 37). Activated Src itself is known to modulate a variety of cellular functions including proliferation, survival, adhesion, and migration, and Src overexpression has been observed in a number of tumor types, including skin tumors (42). In the present study, we observed that Src was activated following butaprost stimulation of EP2 via a β-arrestin1-mediated mechanism. The mechanisms by which the GPCR-β-arrestin complex led to Src activation are unclear, but we observed that Src was phosphorylated at a site required for its activation, Tyr416 (32), in skin (Fig. 2, A and B) and in papillomas (13).
Previous studies demonstrated that activated Src can transactivate EGFR (43–45). In the present study, we demonstrated that EP2 activated EGFR by a Src-dependent mechanism and previously reported that the Src-specific activation site on EGFR (Tyr845) was phosphorylated (13). However, in addition to the direct activation of EGFR by Src, recent studies have demonstrated that Src can activate matrix metalloproteinases, causing the release of EGFR ligands such as heparin binding EGF (39, 46). Heparin binding EGF then binds to EGFR, causing EGFR dimerization and autophosphorylation (46). Although our data indicated that direct activation of EGFR by activated Src occurred (13), the data did not rule out the possibility that ligand activation of EGFR also occurred. EGFR is a receptor tyrosine kinase that is known to have a role in keratinocyte proliferation as well as in skin tumor formation (13, 47). Indeed, we reported previously (13) and herein that inhibition of EGFR reduced mouse skin papilloma formation and keratinocyte replication, respectively.
EGFR is a multifunctional tyrosine kinase receptor with the ability to active multiple effectors and downstream signaling pathways (48, 49). In the current study, we observed that EP2 agonist-mediated transactivation of EGFR resulted in the activation of the downstream signaling effectors: ERK1/2, Akt, and STAT3. ERK1/2 and Akt are known to be activated in cultured cells by PGE2 (20, 48, 50) and to play important roles in cell proliferation and skin tumor formation (33, 36). Several reports indicated that PGE2-stimulated ERK1/2 and Akt activation were mediated by both activated EGFR and activated PKA (20, 48, 51) and that both effectors contributed to keratinocyte replication and survival (33, 35). However, in the present studies, Akt activation was suppressed by EGFR inhibition but not by inhibition of PKA (Figs. 3, C and D, and 5, E and F). Thus, Akt was activated primarily via the EGFR pathway. On the other hand, butaprost-induced ERK1/2 activation was decreased by inhibition of both EGFR and PKA, indicating that ERK1/2 activation occurred via both the PKA and the EGFR pathways.
STAT3 is a transcription factor that modulates various physiological functions (34), and recent studies have shown that STAT3 plays critical roles in keratinocyte proliferation and mouse skin tumor formation (52, 53). The primary mechanisms for the activation of STAT3 in keratinocytes are via activated EGFR (53) or Src (54). In agreement with previous studies, we found that butaprost induced STAT3 phosphorylation and that STAT3 activation was diminished by inhibition of EGFR or Src (Fig. 3C). These results provide evidence that the EP2-mediated activation of Src-EGFR contributes to the activation of STAT3 in the mouse epidermis.
In addition to the β-arrestin-mediated pathways described above, various in vitro studies had shown that EP2 stimulation caused increases in intracellular cAMP formation and activation of PKA (6, 7, 20). Activated PKA has been shown to phosphorylate GSK3β, thereby preventing GSK3β from phosphorylating β-catenin and allowing β-catenin-Tcf-dependent transcription to occur (7). PKA also stimulates the phosphorylation of CREB, which is known to play an important role in cell proliferation (6, 7). In our studies, we observed that EP2-mediated PKA activation led to increases in the levels of p-GSK3β, p-ERK1/2, and p-CREB and that their activation was decreased by PKA inhibition. However, PKA inhibition did not affect EGFR phosphorylation (Fig. 5, E and F), suggesting that PKA was not involved in EP2-mediated activation of EGFR. Thus, the combined data suggest that both the PKA and the EGFR pathways contribute to epidermal cell replication but that the pathways are independent when activated via EP2.
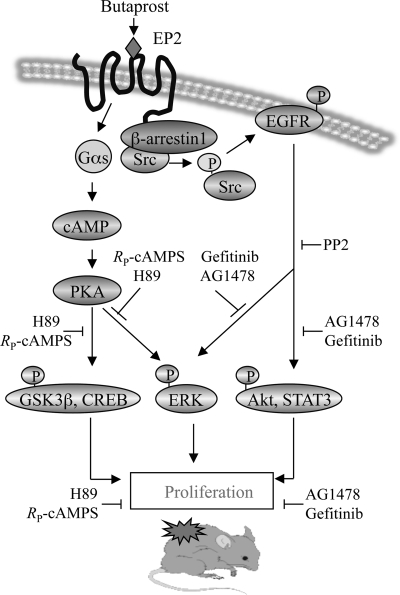
In summary, the results show that the PGE2 receptor, EP2, activates both β-arrestin1-dependent and G protein-dependent pathways that contribute to keratinocyte proliferation and papilloma formation in mouse skin. Fig. 6 illustrates a proposed scheme of the signaling pathways activated by EP2 in mouse skin. Stimulation of EP2 receptor activates PKA and its downstream effectors, GSK3β, CREB, and ERK1/2, via a G protein-dependent mechanism. In addition, EP2 also activates EGFR and its downstream effectors, ERK1/2, Akt, and STAT3, via a G protein-independent mechanism that requires β-arrestin1. Thus, the data indicate that EP2 induced keratinocyte proliferation via signaling pathways involving β-arrestin1-dependent activation of EGFR and the classical G protein-dependent activation of PKA.
FIGURE 6.
Proposed EP2-mediated signaling pathways that contributed to keratinocyte replication and mouse skin papilloma formation. EP2 stimulation by butaprost activated PKA and its downstream effectors, p-GSK3β, p-CREB, and p-ERK1/2. Butaprost also activated EGFR and its downstream effectors, p-ERK1/2, p-STAT3, and p-Akt. EGFR activation involves Src activation via an EP2-β-arrestin1-Src complex. Activation of the PKA and EGFR pathways induced cell proliferation, thereby contributing to skin tumor formation.
Acknowledgments
We are thankful to Dr. Robert Lefkowitz for supplying the β-arrestin1 mice. We thank Chris Lee for animal husbandry and mouse genotyping. We also thank Dr. Robert Oakley and Dr. John Roberts for critical review of manuscript.
This work was supported, in whole or in part, by the Intramural Research Program of the NIEHS/National Institutes of Health.
- PG
- prostaglandin
- GPCR
- G protein-coupled receptor
- CREB
- cAMP-response element-binding protein
- GSK3β
- glycogen synthase kinase 3β
- EGFR
- epidermal growth factor receptor
- RP-cAMPS
- RP-adenosine-3′,5′-cyclic monophosphorothioate
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- p
- phospho
- EP
- E prostanoid receptor.
REFERENCES
- 1.Smith W. L., DeWitt D. L., Garavito R. M. (2000) Annu. Rev. Biochem. 69, 145–182 [DOI] [PubMed] [Google Scholar]
- 2.Fischer S. M. (2002) J. Environ. Pathol. Toxicol. Oncol. 21, 183–191 [PubMed] [Google Scholar]
- 3.Tiano H. F., Loftin C. D., Akunda J., Lee C. A., Spalding J., Sessoms A., Dunson D. B., Rogan E. G., Morham S. G., Smart R. C., Langenbach R. (2002) Cancer Res. 62, 3395–3401 [PubMed] [Google Scholar]
- 4.Pentland A. P., Needleman P. (1986) J. Clin. Invest. 77, 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breyer R. M., Bagdassarian C. K., Myers S. A., Breyer M. D. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 661–690 [DOI] [PubMed] [Google Scholar]
- 6.Fujino H., Regan J. W. (2003) Trends Pharmacol. Sci. 24, 335–340 [DOI] [PubMed] [Google Scholar]
- 7.Regan J. W. (2003) Life Sci. 74, 143–153 [DOI] [PubMed] [Google Scholar]
- 8.Sonoshita M., Takaku K., Sasaki N., Sugimoto Y., Ushikubi F., Narumiya S., Oshima M., Taketo M. M. (2001) Nat. Med. 7, 1048–1051 [DOI] [PubMed] [Google Scholar]
- 9.Seno H., Oshima M., Ishikawa T. O., Oshima H., Takaku K., Chiba T., Narumiya S., Taketo M. M. (2002) Cancer Res. 62, 506–511 [PubMed] [Google Scholar]
- 10.Chang S. H., Ai Y., Breyer R. M., Lane T. F., Hla T. (2005) Cancer Res. 65, 4496–4499 [DOI] [PubMed] [Google Scholar]
- 11.Sung Y. M., He G., Fischer S. M. (2005) Cancer Res. 65, 9304–9311 [DOI] [PubMed] [Google Scholar]
- 12.Sung Y. M., He G., Hwang D. H., Fischer S. M. (2006) Oncogene 25, 5507–5516 [DOI] [PubMed] [Google Scholar]
- 13.Chun K. S., Lao H. C., Trempus C. S., Okada M., Langenbach R. (2009) Carcinogenesis 30, 1620–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benovic J. L., Kühn H., Weyand I., Codina J., Caron M. G., Lefkowitz R. J. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 8879–8882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hausdorff W. P., Caron M. G., Lefkowitz R. J. (1990) FASEB J. 4, 2881–2889 [PubMed] [Google Scholar]
- 16.Pierce K. L., Tohgo A., Ahn S., Field M. E., Luttrell L. M., Lefkowitz R. J. (2001) J. Biol. Chem. 276, 23155–23160 [DOI] [PubMed] [Google Scholar]
- 17.Luttrell D. K., Luttrell L. M. (2004) Oncogene 23, 7969–7978 [DOI] [PubMed] [Google Scholar]
- 18.Lefkowitz R. J., Shenoy S. K. (2005) Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 19.Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., Lefkowitz R. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnini S., Finetti F., Solito R., Terzuoli E., Sacchetti A., Morbidelli L., Patrignani P., Ziche M. (2007) FASEB J. 21, 2418–2430 [DOI] [PubMed] [Google Scholar]
- 21.Penn R. B., Pascual R. M., Kim Y. M., Mundell S. J., Krymskaya V. P., Panettieri R. A., Jr., Benovic J. L. (2001) J. Biol. Chem. 276, 32648–32656 [DOI] [PubMed] [Google Scholar]
- 22.Desai S., April H., Nwaneshiudu C., Ashby B. (2000) Mol. Pharmacol. 58, 1279–1286 [DOI] [PubMed] [Google Scholar]
- 23.Kim J. I., Lakshmikanthan V., Frilot N., Daaka Y. (2010) Mol. Cancer Res. 8, 569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchanan F. G., Gorden D. L., Matta P., Shi Q., Matrisian L. M., DuBois R. N. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conner D. A., Mathier M. A., Mortensen R. M., Christe M., Vatner S. F., Seidman C. E., Seidman J. G. (1997) Circ. Res. 81, 1021–1026 [DOI] [PubMed] [Google Scholar]
- 26.Chun K. S., Akunda J. K., Langenbach R. (2007) Cancer Res. 67, 2015–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray A. J. (2008) Sci. Signal. 1, re4. [DOI] [PubMed] [Google Scholar]
- 28.Egeblad M., Mortensen O. H., van Kempen L. C., Jäättelä M. (2001) Biochem. Biophys. Res. Commun. 281, 25–31 [DOI] [PubMed] [Google Scholar]
- 29.Tice D. A., Biscardi J. S., Nickles A. L., Parsons S. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 1415–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchanan F. G., Wang D., Bargiacchi F., DuBois R. N. (2003) J. Biol. Chem. 278, 35451–35457 [DOI] [PubMed] [Google Scholar]
- 31.Pai R., Soreghan B., Szabo I. L., Pavelka M., Baatar D., Tarnawski A. S. (2002) Nat. Med. 8, 289–293 [DOI] [PubMed] [Google Scholar]
- 32.Hunter T. (1987) Cell 49, 1–4 [DOI] [PubMed] [Google Scholar]
- 33.Bourcier C., Jacquel A., Hess J., Peyrottes I., Angel P., Hofman P., Auberger P., Pouysségur J., Pagès G. (2006) Cancer Res. 66, 2700–2707 [DOI] [PubMed] [Google Scholar]
- 34.Kim D. J., Chan K. S., Sano S., Digiovanni J. (2007) Mol. Carcinog. 46, 725–731 [DOI] [PubMed] [Google Scholar]
- 35.Skeen J. E., Bhaskar P. T., Chen C. C., Chen W. S., Peng X. D., Nogueira V., Hahn-Windgassen A., Kiyokawa H., Hay N. (2006) Cancer Cell 10, 269–280 [DOI] [PubMed] [Google Scholar]
- 36.Segrelles C., Lu J., Hammann B., Santos M., Moral M., Cascallana J. L., Lara M. F., Rho O., Carbajal S., Traag J., Beltrán L., Martínez-Cruz A. B., García-Escudero R., Lorz C., Ruiz S., Bravo A., Paramio J. M., DiGiovanni J. (2007) Cancer Res. 67, 10879–10888 [DOI] [PubMed] [Google Scholar]
- 37.Luttrell L. M., Daaka Y., Lefkowitz R. J. (1999) Curr. Opin. Cell Biol. 11, 177–183 [DOI] [PubMed] [Google Scholar]
- 38.Bruce J. I., Shuttleworth T. J., Giovannucci D. R., Yule D. I. (2002) J. Biol. Chem. 277, 1340–1348 [DOI] [PubMed] [Google Scholar]
- 39.Noma T., Lemaire A., Naga Prasad S. V., Barki-Harrington L., Tilley D. G., Chen J., Le Corvoisier P., Violin J. D., Wei H., Lefkowitz R. J., Rockman H. A. (2007) J. Clin. Invest. 117, 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luttrell L. M., Gesty-Palmer D. (2010) Pharmacol. Rev. 62, 305–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kendall R. T., Luttrell L. M. (2009) Cell Mol. Life Sci. 66, 2953–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Summy J. M., Gallick G. E. (2003) Cancer Metastasis Rev. 22, 337–358 [DOI] [PubMed] [Google Scholar]
- 43.Normanno N., De Luca A., Bianco C., Strizzi L., Mancino M., Maiello M. R., Carotenuto A., De Feo G., Caponigro F., Salomon D. S. (2006) Gene 366, 2–16 [DOI] [PubMed] [Google Scholar]
- 44.Daub H., Wallasch C., Lankenau A., Herrlich A., Ullrich A. (1997) EMBO J. 16, 7032–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simeonova P. P., Wang S., Hulderman T., Luster M. I. (2002) J. Biol. Chem. 277, 2945–2950 [DOI] [PubMed] [Google Scholar]
- 46.Prenzel N., Zwick E., Daub H., Leserer M., Abraham R., Wallasch C., Ullrich A. (1999) Nature 402, 884–888 [DOI] [PubMed] [Google Scholar]
- 47.El-Abaseri T. B., Fuhrman J., Trempus C., Shendrik I., Tennant R. W., Hansen L. A. (2005) Cancer Res. 65, 3958–3965 [DOI] [PubMed] [Google Scholar]
- 48.Dajani O. F., Meisdalen K., Guren T. K., Aasrum M., Tveteraas I. H., Lilleby P., Thoresen G. H., Sandnes D., Christoffersen T. (2008) J. Cell. Physiol. 214, 371–380 [DOI] [PubMed] [Google Scholar]
- 49.Gschwind A., Zwick E., Prenzel N., Leserer M., Ullrich A. (2001) Oncogene 20, 1594–1600 [DOI] [PubMed] [Google Scholar]
- 50.Ansari K. M., Sung Y. M., He G., Fischer S. M. (2007) Carcinogenesis 28, 2063–2068 [DOI] [PubMed] [Google Scholar]
- 51.Chang S. H., Liu C. H., Wu M. T., Hla T. (2005) Prostaglandins Other Lipid Mediat. 76, 48–58 [DOI] [PubMed] [Google Scholar]
- 52.Chan K. S., Sano S., Kiguchi K., Anders J., Komazawa N., Takeda J., DiGiovanni J. (2004) J. Clin. Invest. 114, 720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan K. S., Carbajal S., Kiguchi K., Clifford J., Sano S., DiGiovanni J. (2004) Cancer Res. 64, 2382–2389 [DOI] [PubMed] [Google Scholar]
- 54.Silva C. M. (2004) Oncogene 23, 8017–8023 [DOI] [PubMed] [Google Scholar]