Abstract
Although human skin fibroblast (HSF) elastase has been characterized as a membrane-bound metalloproteinase, little is known about its structure, amino acid sequence, and encoding gene. As there are similarities in the molecular weights and inhibitory profiles of HSF elastase and neprilysin (neutral endopeptidase 24.11 (NEP)), in this study we tested the hypothesis that they are identical using immunoprecipitation and transfection methods. An immunoprecipitation study demonstrated that HSF elastase activity co-immunoprecipitated with anti-NEP in lysates of cultured HSF. Transfection of an NEP cDNA expression vector into COS-1 cells elicited the expression of HSF elastase and NEP activities in the transfected cells. These findings strongly suggest that HSF elastase is identical to NEP, which functions mainly in neuron-associated cells to degrade neuropeptides. Analysis of the expression pattern of NEP revealed that its expression was remarkably up-regulated at the gene, protein, and enzymatic activity levels during the replicative senescence of cultured HSF. Further, the activity of NEP was markedly enhanced in a pattern similar to elastase activity during the intrinsic aging of mouse skin, in UVA-exposed HSF as well as in HSF treated with conditioned medium from UVB-exposed human keratinocytes. Analysis of the cytokine profile for the stimulation of NEP and HSF elastase activities in HSF demonstrated that among the 11 cytokines tested, IL-1α, IL-1β, IL-6, IL-8, and GM-CSF had the potential to significantly stimulate both activities similarly, again supporting the identity of HSF elastase and NEP.
Keywords: Aging, Elastin, Enzymes, Fibroblast, Gene Expression, Neutral Endopeptidase, Ultraviolet Light, Elastase, Photo-aging
Introduction
Cumulative exposure to sunlight elicits the formation of wrinkles, which are associated with marked decreases in skin elasticity (1–3). We demonstrated previously that skin elasticity is remarkably diminished in an early phase of long term UV irradiation at a dose of less than 1 minimal erythemal dose (MED), which is accompanied by degeneration of the elastic fiber network (consisting of premature oxytalan, elaunin fibers, and mature elastic fibers) (1, 2). We also reported that elastase activity is gradually and markedly increased in the wrinkling process prior to the onset of wrinkle formation in UVB-exposed hairless mouse skin (4), which suggests the deep involvement of elastases in the damage of the elastic fiber network. The evidence that the exposure of animal skin to UVB at less than a suberythemal dose can cause wrinkles, despite the lack of inflammatory cell infiltration (5), led us to speculate that skin fibroblast elastase is mainly responsible for the degradation of elastic fibers. While characterizing the elastase-like enzyme derived from skin fibroblasts, we found that fibroblast elastase, which exhibits the characteristics of a metalloproteinase, is remarkably inhibited by metalloproteinase inhibitors but not by other inhibitors, which indicates that the fibroblast elastase belongs to the metalloproteinase family. To determine the contribution of fibroblast elastase activity to the deterioration of elastins associated with wrinkling, we used a synthetic metalloproteinase inhibitor that does not inhibit type I or type IV collagenases. There was a preventive effect of a topical application of the inhibitor on wrinkle formation, which suggested that skin fibroblast elastase plays a critical role in wrinkle formation through its degradation of elastic fibers.
There is some evidence that skin fibroblasts synthesize the “elastase,” but its specific enzyme species is still unknown. It is a 94-kDa membrane-bound type metalloproteinase, and its optimum pH is in the neutral region. This elastase mainly hydrolyzes premature oxytalan and elaunin fibers, but it has only a limited activity toward mature dermal elastic fibers (6). It has also been reported to have increased activity in cutis laxa and actinic elastosis (7) and in UV-irradiated skin (4) and to have no elastolytic activity on ligamentum nuchae elastin (7).
There are several candidate enzymes for skin fibroblast elastase, including 92- and 72-kDa type IV collagenase (gelatinase) (8, 9), neutrophil elastase (10), metalloelastase (11), cathepsin G (12), and proteinase 3 (13). Among those candidates, only the 92-/72-kDa type IV collagenase matches the properties of a metalloproteinase, but it is not consistent with the characteristics of skin fibroblast elastase in terms of molecular weight or membrane binding property. Because we noticed similarities between fibroblast elastase and neprilysin (neutral endopeptidase 24.11 (NEP))2 (EC 3.4.24.11) in terms of their sizes (97 kDa), their membrane-bound metalloproteinase nature, and their inhibitory profiles in this study, we have characterised these two enzymes by immunoprecipitation and transfection methods. We now report the co-identity of these two enzymes.
EXPERIMENTAL PROCEDURES
Materials
The synthetic substrate for elastase, N-succinyl-tri-alanyl-p-nitroaniline (STANA), was purchased from the Peptide Institute Inc. (Osaka, Japan). Phosphoramidone was obtained from Roche Applied Science. Two mouse monoclonal anti-human CD10 antibodies, IMO113 and IMO112, were purchased from Immuntech (Marseille, France). Another clone of anti-human CD10 antibody, H-321, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse IgG (PP54) was purchased from Chemicon (Tokyo). Rabbit anti-human MT-MMP-1 (MMP-14) antibody, RP1-MTM1, was purchased from Triple Point Biologics (Forest Grove, OR). Horseradish peroxidase-conjugated goat polyclonal anti-mouse IgG was obtained from Transduction Laboratories (Lexington, KY), diaminobenzidene substrate from Funakoshi (Tokyo), and protein A-agarose from Santa Cruz Biotechnology. Human elastin labeled with the chromophore Remazol Brilliant Blue (elastin-RBB) was purchased from the Elastin Products Co. (Owensville, MO). TRIzol reagent, Lipofectamine reagent, and culture media were obtained from Invitrogen. Avian myeloblastosis virus (AMV) reverse transcriptase was obtained from PerkinElmer and oligo d(T)18 from New England Biolabs (Ipswich, MA). The gelatin zymography kit was obtained from Yagai Corp. (Yamagata, Japan). Recombinant human cytokines (IL-1α, IL-1β, IL-6, IL-7, IL-8, IL-10, IL-12, IL-15, TNF-α, GM-CSF, and CSF-1) were purchased from R&D Systems (Minneapolis, MN). ELISA kits for IL-1α, IL-1β, IL-6, IL-8, and GM-CSF were obtained from Beckman Coulter Inc. (Brea, CA). Other chemicals, of reagent grade, were purchased from Sigma.
Cell Cultures
Human skin fibroblasts (HSF) (Dainippon Sumitomo Pharmaceutical Co., Ltd., Osaka, Japan) and COS-1 cells (ATCC, Manassas, VA) were cultivated in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum, 100 μg/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B at 37 °C in a 95% air, 5% CO2 atmosphere. Human epidermal keratinocytes derived from newborn male Caucasians (Kurabo, Osaka, Japan) were cultivated in EpiLife KG2 medium (Kurabo) with supplements as detailed above.
Animals
Female albino hairless ICR/HR mice were used in this study. This strain was established from a cross between a hairless strain, HR/HR (originally from Nisseiken Corp, Osaka, Japan), and a normal haired strain, HaM/ICR. These mice were maintained in our laboratory by hairless brother/normal haired sister mating for several years. The animals were fed ad libitum and housed under conventional conditions at a controlled temperature (23 ± 2 °C), humidity (55 ± 10%), and light (12 h light/12 h dark) without any UV emission. Animals were cared for in accordance with our institutional guidelines. After they reached a certain age, mice were sacrificed with CO2 gas. The dorsal skins of the hairless mice were biopsied and, after removal of the subcutaneous tissue, homogenized and solubilized in 0.1% Triton-X 100, 0.2 m Tris-HCl (pH 8.0) buffer followed by ultrasonication and then centrifugation (3000 rpm for 20 min) to obtain supernatants for enzyme assays (elastase and NEP activities).
Extraction of Enzyme
Fibroblasts were cultured to confluence and then were washed three times with phosphate-buffered saline (PBS), scraped into PBS, and centrifuged at 4 °C, 1000 rpm for 5 min. The cell pellets were lysed with 0.1% Triton-X 100, 0.2 m Tris-HCl (pH 8.0) buffer followed by ultrasonication for 5 min on ice. Clear supernatants obtained after centrifuging the cell residues (2000 rpm, 10 min) were used as the fibroblast enzyme solution.
Immunostaining
HSF were cultured on glass chamber slides (Nunc), which were then treated with cold acetone for 10 min followed by drying in air. Blocking was performed using PBS with 3% BSA for 2 h at room temperature. The slides were then washed three times with PBS for 5 min each. They were then incubated overnight at 4 °C in PBS with 8 μg/ml primary antibody (anti-human CD10 antibody; IMO113) or with control mouse IgG. They were then washed three times for 5 min each and incubated with the secondary antibody for 30 min at 37 °C. After washing three more times for 5 min each, staining was performed using the diaminobenzidene substrate.
Immunoprecipitation
One ml of each human skin fibroblast elastase solution (200 μg protein/ml) was added to 5 μg of mouse monoclonal anti-human CD10 antibody (IMO113 or IMO112), rabbit anti-human MT-MMP-1 (MMP-14) antibody (RP1-MTM1), or control mouse IgG and incubated for 1 h at 4 °C. One hundred μl of protein A-agarose was added and incubated for 30 min at 4 °C followed by centrifugation (15,000 rpm, 2 min). The supernatants (0.9 ml each) were transferred to new tubes, and these steps were repeated two more times to obtain the immunoprecipitated enzyme solutions. Immunoprecipitates and the supernatants at each step were taken and used for enzyme activity assays or Western blotting.
Measurement of Elastase Activity
Elastase activity was measured using the synthetic substrate STANA as described previously by Nakagawa et al. (14). In brief, 100 μl of each enzyme solution was dispensed into 96-well plates, which were preincubated for 15 min at 37 °C. After the addition of 2 μl of 62.5 mm STANA, a further incubation was performed for 1 h at 37 °C. The release of p-nitroaniline was measured by absorbance at 405 nm and enzymatic activities expressed as unit/mg of protein, 1 unit representing the activity that releases 1 nmol of nitroaniline/h.
Elastase activity using insoluble elastin was performed in 500-μl tubes. Insoluble human elastin-RBB (130 μg) was added to 400 μl of human skin fibroblast elastase solution and incubated for 20 h at 37 °C. Each tube was centrifuged for 5 min at 15,000 rpm, and the supernatants were measured for absorbance at 605 nm as elastase activity.
Measurement of NEP Activity
NEP activity was measured as described previously by Casey et al. (15). Fibroblast enzyme solutions were diluted 10 times with MES buffer (300 mm NaCl, 100 mm 2-[N-morpholino]ethanesulfonic acid (pH 6.5)), or immunoprecipitates were dispersed with 500 μl of MES buffer and then dispensed in 100-μl aliquots into 96-well microplates. Two μl of substrate solution (10 mm glutaryl-Ala-Ala-Phe-4-methoxy-2-naphthylamine/dimethylformamide) was added to each well and incubated for 1 h at 37 °C to form Phe-4-methoxy-2-naphthylamine. The enzyme reaction was stopped by adding 1 μl of 400 μm phosphoramidone and then 20 mU of aminopeptidase M and was incubated for 15 min at 37 °C to form 4-methoxy-2-naphthylamine. The amounts of 4-methoxy-2-naphthylamine formed were measured using a spectrophotofluorometer at an excitation wavelength of 340 nm and an emission wavelength of 425 nm.
Western Blotting
Immunoprecipitates and supernatants were resolved on 10% SDS-PAGE and then blotted to PVDF membranes (Bio-Rad). After blocking with 3% bovine serum albumin containing Tris-HCl (pH 7.5) and 100 mm NaCl, the membranes were treated with mouse monoclonal anti-human NEP antibody (IMO 113) at room temperature for 1 h. They were then treated with anti-mouse IgG conjugated to horseradish peroxidase and incubated with Amersham Biosciences ECL reagents and exposed to x-ray film for specified times to detect bands.
Cloning of Human NEP cDNA
Cloning of the full-length human NEP cDNA was performed using RT-PCR. Total RNA was extracted from cultured HSF using TRIzol reagent. Two μg of total RNA was treated with AMV reverse transcriptase and oligo d(T)18 to obtain single-stranded cDNA. To amplify the NEP cDNA, we used the following primers: sense, 5′-ATGGATATAACTGATATCAACACT-3′ (1–24 bp); and antisense, 5′-AGAAGTGCCGGGTTTGGTGATC-3′ (2211–2233 bp).
The reaction conditions for PCR were 94 °C for 2 min for 1 cycle, 94 °C for 30 s, 55 °C for 30 s, 72 °C for 3 min for 35 cycles, and 72 °C for 10 min for 1 cycle. Products from the PCR reactions were inserted into the pCR3.1 mammalian cell expression vector using T4 DNA ligase. Eight clones were selected and inserted in the correct sense by mapping with restriction enzymes.
Transfection of the NEP cDNA Expression Vector into Cells
Eight μl of Lipofectamine reagent and 1 μg of DNA were added to 200 μl of Opti-MEM I medium, incubated at room temperature for 45 min, and then added to 800 μl of Opti-MEM I medium. COS-1 cells or CHO-K1 cells were washed with Opti-MEM I medium and added to the medium mixture above followed by incubation for 5–12 h at 37 °C, 5% CO2. After that, 1 ml of DMEM containing 10% FCS was added, and the cells were cultured for 24 h. After the medium was exchanged, a further 48–72 h of culture provided the NEP cDNA-transfected cells.
Analysis of Human NEP Gene Expression by RT-PCR
Total RNAs were extracted from cultured HSF using the TRIzol reagent. One μg of total RNA was treated with AMV reverse transcriptase and oligo d(T)18 to obtain single-stranded cDNA. To amplify the NEP cDNA, we used the two primer sets reported by Cohen et al. (16): NEP1 sense, 5′-GTCCTGCTCCTCACCATCATAGC-3′ (238–260 bp), and NEP1 antisense, 5′-CGATCTTCAGGTTTAGCCGTAGC-3′ (991–1013 bp); and NEP3 sense, 5′-GCTACGGCTAAACCTGAAGATCG-3′ (991–1013 bp), and NEP3 antisense, 5′TTCGTGTCCTATGACCATGCC-3′ (1870–1890 bp). PCR reaction conditions were 94 °C for 2 min for 1 cycle, 94 °C for 30 s, 67 °C for 1 min, 72 °C for 1 min for 25 cycles, and 72 °C for 10 min for 1 cycle.
UV Irradiation
Irradiation with UVA and UVB was performed using a FL20SBL lamp (Panasonic Co., Osaka, Japan), and a GL20SE lamp (Sankyo Denki Co., Ltd., Kanagawa, Japan), respectively.
Statistics
Data for statistical analysis are presented as means ± S.D. of at least three separate experiments. Comparisons between groups were analyzed by Student's t test or ANOVA, and p values of 0.05 or less were considered statistically significant.
RESULTS
Immunoprecipitation Studies
Because metalloproteinases with enzymatic properties similar to HSF-derived elastase were predicted to be NEP or MMP-14, we performed immunoprecipitation analysis with antibodies to anti-NEP and to anti-MMP-14 to clarify their identities. Immunoprecipitation followed by measurement of elastase activity demonstrated that elastase activity was reduced in the supernatant following immunoprecipitation with anti-NEP (IM0112 and/or IM0113) but not with anti-MMP-14 antibodies (Fig. 1).
FIGURE 1.
Elastase activity in supernatants after immunoprecipitation with anti-NEP and anti-MMP-14 antibodies. Supernatants before and after immunoprecipitation of HSF fibroblasts with anti-NEP or anti-MMP-14 antibodies or control mouse IgG were measured using the synthetic substrate STANA for elastase activity. Values represent means ± S.D. from three independent experiments. **, p < 0.01 (ANOVA, Dunnett's test).
A similar immunoprecipitation study demonstrated that elastase activity was also depleted in the supernatants following immunoprecipitation with the anti-NEP antibody (Fig. 2A), which indicates that HSF have detectable elastase activity that corresponds to an anti-NEP-recognizable protein. Inhibition with thiorphan revealed that it almost completely suppressed the elastase activity in the supernatants following immunoprecipitation with the control IgG. A similar suppression of elastase activity in the supernatant following immunoprecipitation with the control IgG was seen with the typical metalloproteinase inhibitor phosphoramidone (Fig. 2A). On the other hand, the precipitates from the same assay had a distinct activity of elastase following immunoprecipitation with the anti-NEP antibody (IM0113) (Fig. 2B), which further indicates that HSF have detectable elastase activities that correspond to an anti-NEP-recognizable protein. Inhibition with thiorphan revealed that it almost completely suppressed the elastase activity present in the precipitate following immunoprecipitation with the anti-NEP antibody (IM0113).
FIGURE 2.
Elastase activity in supernatants (A) and in immunoprecipitates (B) following immunoprecipitation with the anti-NEP antibody and the inhibitory effects of thiorphan or phosphoramidone. Extracts of HSF were subjected to immunoprecipitation with the anti-NEP antibody or control IgG. A, supernatants of immunoprecipitation with or without thiorphan (1 μm) or the metalloproteinase inhibitor phosphoramidone (1 μm) were assayed for elastase activity. B, immunoprecipitates with the anti-NEP antibody or control IgG with or without thiorphan (1 μm) were assayed for elastase activity. Values represent means ± S.D. from three independent experiments. **, p < 0.01 (ANOVA, Dunnett's test).
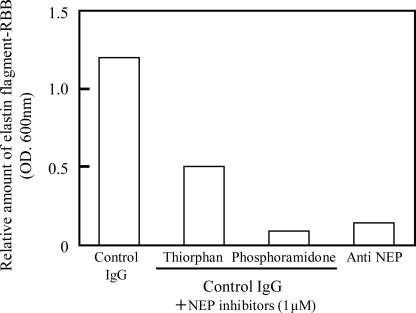
An enzyme assay using human lung elastin-RBB as another substrate for elastase demonstrated that (consistent with the results in Fig. 2) whereas supernatants following immunoprecipitation with the control IgG have significant elastase activity, treatment with the anti-NEP-antibody markedly reduced the elastin-degrading activity, indicating that HSF have distinct levels of elastin-degrading activity that correspond to an anti-NEP-recognizable protein (Fig. 3). Inhibition using thiorphan or phosphoramidone revealed that both inhibitors markedly suppressed the elastase activity in the supernatants following immunoprecipitation with the control IgG, although the inhibitory effect of thiorphan was weaker than that of phosphoramidone, probably because of its constitutive instability during the long incubation (∼20 h) required for the enzyme assay.
FIGURE 3.
Elastin-degrading activity in supernatants following immunoprecipitation with the anti-NEP antibody and the inhibitory effect of thiorphan or phosphoramidone. Extracts of HSF were subjected to immunoprecipitation with the anti-NEP antibody or a control IgG. Supernatants immunoprecipitated with or without thiorphan (1 μm) or phosphoramidone (1 μm) were measured for elastin-degrading activity using human elastin-RBB as a substrate. Values represent the means of two independent experiments.
Immunostaining of NEP in HSF
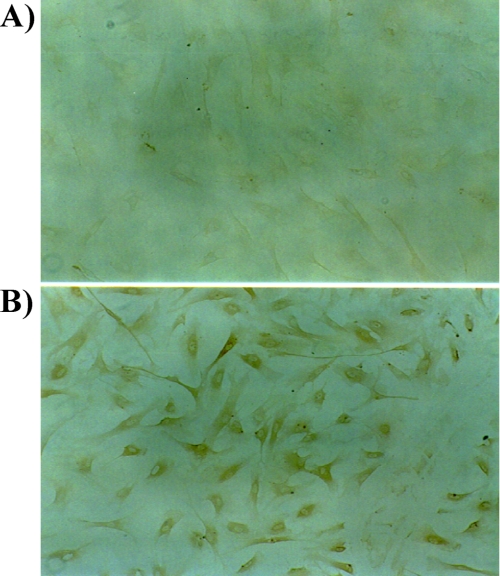
Immunohistochemistry of HSF with the anti-NEP antibody showed positive staining on the plasma membrane and cytoplasm but not within the nuclei (Fig. 4). This indicates the presence of NEP in HSF, which is consistent with results reported by Lorkowski et al. (17).
FIGURE 4.
Localization of NEP in HSF. Immunostaining of HSF with control IgG (A) and with an anti-NEP antibody (B) is shown. Positive and negative staining was observed in plasma membranes and in nuclei, respectively. There was no specific staining with the control IgG.
Transfection of a Human NEP Expression Vector
Expression vectors with an inserted human NEP cDNA were transfected into COS-1 cells. Total RNAs were then extracted from the COS-1 cells and analyzed by RT-PCR with the NEP primer set. NEP cDNA-transfected COS-1 cells expressed NEP mRNA to an extent similar to HSF (data not shown). Although extracts of non-transfected COS-1 cells had extremely low levels of NEP activity, transfection of the human NEP cDNA expression vector elicited a marked up-regulation of NEP activity (Fig. 5B). A similar transfection of COS-1 cells induced a marked increase in elastase activity, although non-transfected COS-1 cells had detectable levels of elastase activity (Fig. 5A).
FIGURE 5.
Elastase and NEP activities of NEP cDNA-transfected COS-1 cells. Elastase activity (A) was measured using STANA as a substrate. NEP activity (B) was measured using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphthylamine as a substrate. Values represent means ± S.D. from three independent experiments. **, p < 0.01 (t test).
NEP Is Not Able to Convert 72-kDa Gelatinase to 68-kDa Gelatinase
As Beranger et al. (18) had reported that fibroblast elastase has an ability to convert 72-kDa progelatinase A to active 66-kDa gelatinase, we next determined whether NEP was able to convert 72-kDa to 66-kDa gelatinase. Analysis by gelatin zymography revealed that there was no detectable level of the converting activity in lysates of HSF or NEP cDNA-transfected COS-1 cells (data not shown). Thus, it is unlikely that NEP elicits elastase activity via the activation of gelatinase.
Changes in Elastase/NEP Activities during Replicative Senescence of HSF
The results reported above demonstrate that the elastase activity of HSF can be ascribed to NEP. Thus, it is of considerable interest to know the chronological and photo-aging effects on their enzymatic activities. The elastase and NEP activities during replicative senescence of HSF demonstrated that an increasing passage number of HSF in culture is associated with the enhanced activities of both elastase and NEP (Fig. 6, A and B). This senescence-dependent increase in their activities was accompanied by the increased expression of NEP protein as revealed by Western blotting (Fig. 6C) but not by increased expression of NEP mRNA transcripts as revealed by RT-PCR (Fig. 6D).
FIGURE 6.
Activities of elastase and NEP in HSF during passage in culture and their gene and protein expression levels. Elastase activity (A) and NEP activity (B) were measured using the corresponding synthetic substrates. Values represent means ± S.D. from three independent experiments. **, p < 0.01 (ANOVA, Tukey test). Western blotting (C) and RT-PCR (D) for NEP were performed on the same samples used for assays of enzyme activities.
Changes in Elastase/NEP Activities during Chronological Aging of Mouse Skin
To determine changes in elastase/NEP activities during the chronological aging of skin, we used the dorsal skin of hairless mice at ages 2, 4, 9, 16, and 21 months. The results indicate that the activities of both elastase and NEP were increased in an age-dependent manner (Fig. 7). This indicates that the activities of elastase/NEP correlate with the chronological aging of hairless mouse skin.
FIGURE 7.
Elastase and NEP activities in the dorsal skin of mice at different ages. Elastase activity (A) and NEP activity (B) were measured as per unit area of skin biopsy. Values represent means ± S.D. from five independent experiments. **, p < 0.01; *, p < 0.05 versus 2 months (ANOVA, Dunnett's test).
Effects of UV Irradiation on Elastase/NEP Activities of HSF
We had already reported that elastase activity in mouse skin is elevated by UV irradiation (4). Thus, it was of interest to determine changes in elastase/NEP activities following UV exposure of HSF. UVA irradiation at doses of 1 or 5 J/cm2 elicited significant increases in elastase activity in a dose-dependent manner at 72 h post-irradiation (Fig. 8, A and B). Although UVB irradiation at a dose of 1 mJ/cm2 had no effect on elastase or NEP activities of HSF at 72 h post-irradiation, UVB irradiation at a dose of 5 mJ/cm2 significantly down-regulated both activities at 72 h post-irradiation (Fig. 8, A and B). There was no visible damage in cultured HSF at 72 h post-irradiation at doses of less than 5 J/cm2 for UVA and 5 mJ/cm2 for UVB (data not shown).
FIGURE 8.
Elastase and NEP activities in HSF exposed to UVA or UVB or treated with the conditioned medium from UVB-exposed human keratinocytes. Elastase activity (A) and NEP activity (B) were measured in HSF at 72 h post-irradiation with UVA (1 or 5 J/cm2) or UVB (1 or 5 mJ/cm2). Elastase activity (C) and NEP activity (D) were measured in HSF cultured for 24 h with conditioned medium prepared 72 h after UVB exposure of cultured human keratinocytes. Values represent means ± S.D. from five independent experiments (C, D). Values represent means ± S.D. from three independent experiments (A, B). **, p < 0.01; *, p < 0.05 (ANOVA, Dunnett's test (A and B); t test (C and D).
Effects of Medium Conditioned by UVB-exposed Human Keratinocytes on Elastase/NEP Activity of HSF
We had already reported that cumulative UVB exposure of hairless mouse skin elicits a gradual increase in the elastase activity of the whole skin (excluding the subcutaneous tissue) (4). As UVB is not able to penetrate the dermis, it is conceivable that UVB-exposed keratinocyte-derived soluble factors penetrate the dermis to stimulate dermal fibroblasts, resulting in the increased elastase activity. Therefore, we determined whether medium conditioned by UVB-exposed human keratinocytes has a potential to stimulate elastase/NEP activities in dermal fibroblasts. Analysis of enzyme activities revealed that medium conditioned for 72 h post-irradiation by UVB (20 mJ/cm2)-exposed human keratinocytes caused cultured human fibroblasts to significantly increase elastase/NEP activities after 24 h of incubation (Fig. 8, C and D).
Effects of Various Cytokines on Elastase/NEP Activities of HSF
We next studied the effects of a variety of cyotokines or chemokines known to be released by UVB-exposed keratinocytes on elastase/NEP activities in HSF. Analysis of enzyme activities revealed that elastase and NEP activities were both significantly stimulated by IL-1α, IL-1β, IL-6, IL-8, and GM-CSF after 24 h of treatment (Fig. 9).
FIGURE 9.
Elastase and NEP activities in HSF treated with various cytokines. Elastase activity (A) and NEP activity (B) were measured in HSF treated with various cytokines for 48 h. Values represent means ± S.D. from three independent experiments. **, p < 0.01; *, p < 0.05 (ANOVA, Dunnett's test).
Cytokine Secretion by UVB-exposed Human Keratinocytes
Because five cytokines were identified that were capable of stimulating elastase/NEP activities in HSF, we determined whether the levels of those cytokines are increased in UVB-exposed human keratinocytes. ELISAs revealed that among the cytokines tested, UVB irradiation at a dose of 20 mJ/cm2 elicited a significant increase in IL-8 and GM-CSF, but not in IL-1α, IL-1β, or IL-6, at 72 h post-irradiation (Fig. 10A).
FIGURE 10.
Release of cytokines into medium conditioned by UVB-exposed human keratinocytes (A) and by UVA-exposed HSF (B). Cytokine concentrations in medium conditioned by human keratinocytes at 72 h post-irradiation with UVB (20 mJ/cm2 ■ or 0 mJ/cm2  (A)) and by HSF at 72 h post-irradiation with UVA (1 J/cm2
(A)) and by HSF at 72 h post-irradiation with UVA (1 J/cm2  , 5 J/cm2 ■, or 0 J/cm2 □ (B)) were measured using ELISA kits. Values represent means ± S.D. from three independent experiments. N.D., not detectable; **, p < 0.01; *, p < 0.05 (t test, Bonferroni).
, 5 J/cm2 ■, or 0 J/cm2 □ (B)) were measured using ELISA kits. Values represent means ± S.D. from three independent experiments. N.D., not detectable; **, p < 0.01; *, p < 0.05 (t test, Bonferroni).
Effects of UVA Irradiation on Cytokine Secretion by HSF
To elucidate the involvement of autocrine mechanisms for the secreted cytokines in the UVA stimulation of elastase/NEP activities, we measured the levels of the five elastase/NEP-stimulating cytokines secreted by UVA-exposed human fibroblasts. ELISAs revealed that UVA irradiation at doses of 1.0 or 5.0 J/cm2 did not elicit any significant increase in any of the cytokines tested at 72 h post-irradiation (Fig. 10B).
DISCUSSION
NEP was first reported in 1974 as an enzyme that cleaves insulin B chain from the rabbit renal pelvic membrane (19). Its encoding gene is on chromosome 3, and it has a molecular weight of 98,000, is characterized by a neutral optimum pH, and is a plasma membrane-bound metalloproteinase (17). Many peptides have been found to be substrates for NEP, for example, enkephalin (20, 21), substance P (22), bombesin-like peptide (16, 23), endothelin (24, 25), bradykinin (22) and others (26). The other name for NEP, enkephalinase, reflects the fact that NEP can degrade enkephalin in association with nerves (21, 27, 28). In relation to the lymph system (30, 31), NEP has recently been identified by gene homology as CD10, which is a differentiation marker of B cells and neutrophils (32). However, there has been no report linking NEP with HSF elastase.
Our immunoprecipitation study with an anti-NEP antibody revealed that elastase activity in extracts of HSF co-precipitates with the anti-NEP antibody. Those precipitates exhibited elastase activity for both a synthetic substrate and insoluble elastin and also contained NEP activity. Furthermore, thiorphan, a specific inhibitor of NEP (26, 33), and phosphoramidone, a metalloproteinase inhibitor (34, 35), almost completely suppress the elastase activity in cell extracts and in the immunoprecipitates. The sum of these findings strongly suggests that HSF elastase is identical to NEP.
It was hypothesized that the Suc(Ala)3NA-degrading activity of fibroblast elastase could be attributed to a two-step reaction sequence of endopeptidase and aminopeptidase (36) in which Suc(Ala)3NA is initially cleaved into Suc(Ala)2 and Ala-NA by endopeptidase after which Ala-NA is degraded into alanine and paranitroaniline by aminopeptidase. In this study, immunoprecipitation with the anti-NEP antibody showed a definite elastase activity when measured using Suc(Ala)3NA as a substrate. Additionally, NEP gene transfection into COS-1 cells, which have no endogenous NEP, elicited a marked expression of elastase activity, although consistent with a recent report (37), the aminopeptidase activity of mock-transfected COS-1 cells occurred at a negligible level when measured using Ala-NA and Leu-NA as substrates (data not shown). Thus, it is likely that the HSF elastase activity can be attributed mainly to the enzymic activity of NEP.
As for the effect of senescence on the activities of fibroblast elastase (measured using Suc(Ala)3NA as the substrate) and NEP (using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphthylamine), we found that both activities are up-regulated during passage of cultured HSF, which is accompanied by a marked increase (∼2.5–11.5-fold at passages 10–17 compared with passage 4) in NEP protein, despite the fact that there is only a 1.2–1.3-fold increase in NEP mRNA. The up-regulated protein expression without a corresponding increase in mRNA has been reported previously in other studies of cancer and innate immunity and explained in terms of enhanced translation speed due to the up-regulation of translation control factors such as initiation factor and elongation factor (38). Further, we found that both activities (elastase and NEP) are significantly up-regulated in the skin of mice as they age chronologically, which also supports the identity of HSF elastase to NEP. This result showing aging dependence is consistent with reports (39) for fibroblast elastase activity, as well as for NEP activity, as an aging biomarker in several tissues (40, 41). Such an age-dependent augmentation of HSF elastase activity may be involved in spontaneous skin aging phenomena such as sagging (42).
Recently we demonstrated that fibroblast elastase activity is significantly elevated when wrinkling of the skin is induced by repetitive UVB exposure with doses of less than 1 MED on the dorsal skin of hairless mice (4). Wrinkle formation can be significantly suppressed by a topical application of a specific inhibitor for skin fibroblast elastase (4). Therefore, it is conceivable that among the elastin-degrading enzymes described above, HSF elastase/NEP plays a central role in wrinkle formation, which is induced by repetitive non-inflammatory doses of UVB irradiation. In this study, we found that NEP activity is significantly elevated by exposure of HSF to UVA but not to UVB irradiation. The conditioned medium from UVB-exposed human keratinocytes elevated NEP and elastase activities in HSF, which suggests that basement membrane-permeable cytokines (for instance, IL-8 and GM-CSF) produced by the UVB-exposed epidermis are possible stimulatory factors for up-regulating the expression of NEP in the dermis of UVB-exposed skin (43–45). Thus, the fact that both types of UV lights directly or indirectly elevate both NEP and elastase activities also supports their identity and suggests that the elastase activity stimulated by sunlight plays an important role in the loss of skin elasticity, which leads to wrinkle formation.
In the connection between photoaging-inducible HSF elastase and neuropeptide-degradable NEP as the same enzyme, it is of considerable interest to determine whether there is a mechanistic link between skin photoaging and neuropeptides. Thus, NEP is well known to play an essential role in the regulation of neurotransmission (17) and in the neuroimmunocutaneous system (46, 47). In UV-exposed skin, it seems likely that the up-regulated expression of NEP is associated with the degradation of a variety of neuropeptides, leading to the down-regulation of neuropeptide function as regulatory factors of inflammation (29) in concert with the elastin fiber degeneration that results in photoaging.
Footnotes
- NEP
- neutral endopeptidase
- STANA
- N-succinyl-tri-alanyl-p-nitroaniline
- AMV
- avian myeloblastosis virus
- HSF
- human skin fibroblast(s)
- ANOVA
- analysis of variance
- RBB
- Remazol Brilliant Blue.
REFERENCES
- 1.Takema Y., Yorimoto Y., Kawai M., Imokawa G. (1994) Br. J. Dermatol. 131, 641–648 [DOI] [PubMed] [Google Scholar]
- 2.Takema Y., Imokawa G. (1998) Dermatology 196, 397–400 [DOI] [PubMed] [Google Scholar]
- 3.Habig J., Vocks E., Kautzky F., Dahm M., Borelli S. (1996) Hautarzt 47, 515–520 [DOI] [PubMed] [Google Scholar]
- 4.Tsuji N., Moriwaki S., Suzuki Y., Takema Y., Imokawa G. (2001) Photochem. Photobiol. 74, 283–290 [DOI] [PubMed] [Google Scholar]
- 5.Learn D. B., Moloney S. J. (1991) Photodermatol. Photoimmunol. Photomed. 8, 195–199 [PubMed] [Google Scholar]
- 6.Godeau G., Hornebeck W. (1988) Pathol. Biol. 36, 1133–1138 [PubMed] [Google Scholar]
- 7.Schwartz E., Cruickshank F. A., Lebwohl M. G. (1988) Clin. Chim. Acta 176, 219–224 [DOI] [PubMed] [Google Scholar]
- 8.Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. (1991) J. Biol. Chem. 266, 7870–7875 [PubMed] [Google Scholar]
- 9.Shipley J. M., Doyle G. A., Fliszar C. J., Ye Q. Z., Johnson L. L., Shapiro S. D., Welgus H. G., Senior R. M. (1996) J. Biol. Chem. 271, 4335–4341 [DOI] [PubMed] [Google Scholar]
- 10.Campbell E. J., Campbell M. A. (1988) J. Cell Biol. 106, 667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro S. D., Griffin G. L., Gilbert D. J., Jenkins N. A., Copeland N. G., Welgus H. G., Senior R. M., Ley T. J. (1992) J. Biol. Chem. 267, 4664–4671 [PubMed] [Google Scholar]
- 12.Boudier C., Godeau G., Hornebeck W., Robert L., Bieth J. G. (1991) Am. J. Respir. Cell Mol. Biol. 4, 497–503 [DOI] [PubMed] [Google Scholar]
- 13.Kao R. C., Wehner N. G., Skubitz K. M., Gray B. H., Hoidal J. R. (1988) J. Clin. Invest. 82, 1963–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa K., Tsuji T., Kadoya A., Hamada T. (1987) Skin Res. 29, 793–797 [Google Scholar]
- 15.Casey M. L., Smith J. W., Nagai K., Hersh L. B., MacDonald P. C. (1991) J. Biol. Chem. 266, 23041–23047 [PubMed] [Google Scholar]
- 16.Cohen A. J., Bunn P. A., Franklin W., Magill-Solc C., Hartmann C., Helfrich B., Gilman L., Folkvord J., Helm K., Miller Y. E. (1996) Cancer Res. 56, 831–839 [PubMed] [Google Scholar]
- 17.Lorkowski G., Zijderhand-Bleekemolen J. E., Erdös E., G., von Figura K., Hasilik A. (1987) Biochem. J. 248, 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beranger J. Y., Godeau G., Frances C., Robert L., Hornebeck W. (1994) Cell Biol. Int. 18, 715–722 [DOI] [PubMed] [Google Scholar]
- 19.Kerr M. A., Kenny A. J. (1974) Biochem. J. 137, 477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oka T., Hiranuma T., Liu XF., Ohgiya N., Iwao K., Matsumiya T. (1993) Nippon Yakurigaku Zasshi 101, 197–207 [DOI] [PubMed] [Google Scholar]
- 21.Saria A., Hauser K. F., Traurig H. H., Turbek C. S., Hersh L., Gerard C. (1997) Neurosci. Lett. 234, 27–30 [DOI] [PubMed] [Google Scholar]
- 22.Solan N. J., Ward P. E., Sanders S. P., Towns M. C., Bathon J. M. (1998) Inflammation 22, 107–121 [DOI] [PubMed] [Google Scholar]
- 23.Aguayo S. M., Schuyler W. E., Murtagh J. J., Jr., Roman J. (1994) Am. J. Respir. Cell Mol. Biol. 10, 635–642 [DOI] [PubMed] [Google Scholar]
- 24.Vijayaraghavan J., Scicli A. G., Carretero O. A., Slaughter C., Moomaw C., Hersh L. B. (1990) J. Biol. Chem. 265, 14150–14155 [PubMed] [Google Scholar]
- 25.Graf K., Schäper C., Gräfe M., Fleck E., Kunkel G. (1998) Basic Res. Cardiol. 93, 11–17 [DOI] [PubMed] [Google Scholar]
- 26.Roques B. P., Noble F., Daugé V., Fournié-Zaluski M. C., Beaumont A. (1993) Pharmacol. Rev. 45, 87–146 [PubMed] [Google Scholar]
- 27.Krämer H. H., Schmidt K., Leis S., Schmelz M., Sommer C., Birklein F. (2005) Exp. Neurol. 195, 179–184 [DOI] [PubMed] [Google Scholar]
- 28.Roques B. P., Fournié-Zaluski M. C., Soroca E., Lecomte J. M., Malfroy B., Llorens C., Schwartz J. C. (1980) Nature 288, 286–288 [DOI] [PubMed] [Google Scholar]
- 29.Toyoda M., Nakamura M., Nakada K., Nakagawa H., Morohashi M. (2005) Br. J. Dermatol. 153, Suppl. 2, 13–22 [DOI] [PubMed] [Google Scholar]
- 30.Mari B., Guerin S., Maulon L., Belhacene N., Farahi Far D., Imbert V., Rossi B., Peyron J. F., Auberger P. (1997) FASEB J. 11, 869–879 [DOI] [PubMed] [Google Scholar]
- 31.Stefano G. B., Parmen L. R., Hughes T. K., Jr. (1992) J. Neurochem. 41, 9–14 [DOI] [PubMed] [Google Scholar]
- 32.Letarte M., Vera S., Tran R., Addis J. B., Onizuka R. J., Quack E. J. (1988) J. Exp. Med. 198, 1247–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Lombaert S., Erion M. D., Tan J., Blanchard L., el Chehabi L., Ghai R. D., Sakane Y., Berry C., Trapani A. J. (1994) J. Med. Chem. 37, 498–511 [DOI] [PubMed] [Google Scholar]
- 34.Suda H., Aoyagi T., Takeuchi T., Umezawa H. (1973) J. Antibiot. 26, 621–623 [DOI] [PubMed] [Google Scholar]
- 35.Oefner C., D'Arcy A., Hennig M., Winkler F. K., Dale G. E. (2000) J. Mol. Biol. 296, 341–349 [DOI] [PubMed] [Google Scholar]
- 36.Homsy R., Pelletier-Lebon P., Tixier J. M., Godeau G., Robert L., Hornebeck W. (1988) J. Invest. Dermatol. 91, 472–477 [DOI] [PubMed] [Google Scholar]
- 37.Constam D. B., Togbler A. R., Rensing Ehl A., Kenler I., Hersh L. B., Fontana A. (1995) J. Biol. Chem. 270, 26930–26939 [DOI] [PubMed] [Google Scholar]
- 38.Sonenberg N. (2008) Biochem. Cell Biol. 86, 178–183 [DOI] [PubMed] [Google Scholar]
- 39.Labat-Robert J., Kern P., Robert L. (1992) Ann. N.Y. Acad. Sci. 673, 16–22 [DOI] [PubMed] [Google Scholar]
- 40.Kletsas D., Caselgrandi E., Barbieri D., Stathakos D., Franceschi C., Ottaviani E. (1998) Mech. Ageing Dev. 102, 15–23 [DOI] [PubMed] [Google Scholar]
- 41.Solmi R., Tietz C., Zucchini C., Gualandi G., Pugnaloni A., Talassi O., Castaldini C., Simonelli L., Biagini G. (1996) Mech. Ageing Dev. 92, 31–41 [DOI] [PubMed] [Google Scholar]
- 42.Bolognia J. L. (1995) Am. J. Med. 98, 99S-103S [DOI] [PubMed] [Google Scholar]
- 43.Mckenzie R. C., Park E. S., Brown W. R., Shivji G. S., Sauder D. N. (1994) Photodermatol. Photoimmunol. Photomed. 10, 74–79 [PubMed] [Google Scholar]
- 44.Nozaki S., Abrams J. S., Pearce M. K., Sauder D. N. (1991) J. Invest. Dermatol. 97, 10–14 [DOI] [PubMed] [Google Scholar]
- 45.Kondepudi A., Johnson A. (1993) Am. J. Respir. Cell Mol. Biol. 8, 43–49 [DOI] [PubMed] [Google Scholar]
- 46.Nadel J. A. (1991) Eur. Respir. J. 4, 745–754 [PubMed] [Google Scholar]
- 47.Misery L. (1996) Pathol. Biol. 44, 867–874 [PubMed] [Google Scholar]