Abstract
The oxygenation of polyunsaturated fatty acids by lipoxygenases (LOX) is associated with a lag phase during which the resting ferrous enzyme is converted to the active ferric form by reaction with fatty acid hydroperoxide. Epidermal lipoxygenase-3 (eLOX3) is atypical in displaying hydroperoxide isomerase activity with fatty acid hydroperoxides through cycling of the ferrous enzyme. Yet eLOX3 is capable of dioxygenase activity, albeit with a long lag phase and need for high concentrations of hydroperoxide activator. Here, we show that higher O2 concentration shortens the lag phase in eLOX3, although it reduces the rate of hydroperoxide consumption, effects also associated with an A451G mutation known to affect the disposition of molecular oxygen in the LOX active site. These observations are consistent with a role of O2 in interrupting hydroperoxide isomerase cycling. Activation of eLOX3, A451G eLOX3, and soybean LOX-1 with 13-hydroperoxy-linoleic acid forms oxygenated end products, which we identified as 9R- and 9S-hydroperoxy-12S,13S-trans-epoxyoctadec-10E-enoic acids. We deduce that activation partly depends on reaction of O2 with the intermediate of hydroperoxide cleavage, the epoxyallylic radical, giving an epoxyallylic peroxyl radical that does not further react with Fe(III)-OH; instead, it dissociates and leaves the enzyme in the activated free ferric state. eLOX3 differs from soybean LOX-1 in more tightly binding the epoxyallylic radical and having limited access to O2 within the active site, leading to a deficiency in activation and a dominant hydroperoxide isomerase activity.
Keywords: Arachidonic Acid, Eicosanoid-specific Enzymes, Enzyme Catalysis, Lipoxygenase Pathway, Oxygen Radicals
Introduction
Lipoxygenases (LOX)2 are a class of non-heme iron dioxygenases that incorporate molecular oxygen into polyunsaturated fatty acids to give fatty acid hydroperoxides (1). Widely expressed in animals and plants, and also in some bacteria and fungi, LOX enzymes participate in diverse physiological and pathological processes. Mammalian 12R-LOX and epidermal lipoxygenase-3 (eLOX3), for example, play an indispensable role in skin barrier formation (2, 3), whereas plant LOX enzymes are involved in stress responses and development (4). A distinctive characteristic of LOX enzymes in general is that the resting enzyme requires activation before catalytic reactions with fatty acids and molecular oxygen can begin (5–7).
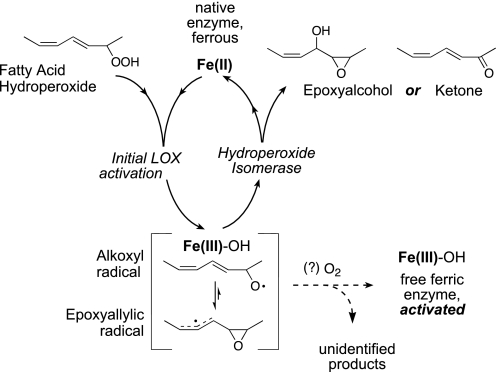
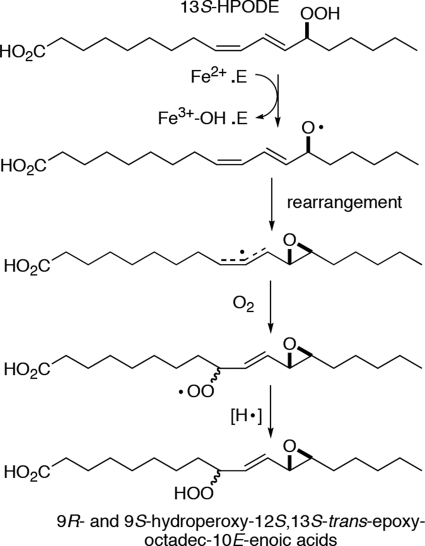
Mechanistically, soybean LOX-1 is the most extensively studied and is generally considered as the prototypical LOX enzyme. The non-heme iron of soybean LOX-1 exists in two oxidation states, ferric and ferrous. The ferric enzyme is responsible for the catalytic activity (8) and preferentially oxygenates polyunsaturated fatty acids with an ω6 and ω9 pentadiene unit at the “S” face of the ω6 carbon, e.g. to convert linoleic acid to 13S-hydroperoxyoctadecadienoic acid (13S-HPODE). The ferrous enzyme is inactive with fatty acids but can react with fatty acid hydroperoxides such as 13S-HPODE to become the ferric enzyme (9, 10). Because the newly isolated enzyme is usually in the ferrous state, such a reaction with fatty acid hydroperoxides is required for the enzyme to enter the catalytic cycle, hence being known as the LOX activation step. In this activation process, as the proportion of ferric enzyme gradually increases, the rate of fatty acid oxygenation will also increase, manifesting itself in the progress curve of fatty acid oxygenation as a lag phase (11). A distinctive feature of LOX activation is that it is a single turnover event; once the enzyme is oxidized to the ferric form, it will no longer react with fatty acid hydroperoxides (12). Because of this single-turnover nature of the reaction, the products from LOX activation are formed in minute amounts and have not been identified. Although substantial evidence suggests that an alkoxyl radical is produced in LOX activation (13), the fate of this alkoxyl radical is poorly understood. Scheme 1 illustrates transformation of a fatty acid hydroperoxide to an alkoxyl radical as well as subsequent reactions and rearrangement discussed throughout the paper.
SCHEME 1.
eLOX3, on the other hand, is an atypical LOX with a prominent hydroperoxide isomerase activity (14). Using this hydroperoxide isomerase activity, ferrous eLOX3 converts fatty acid hydroperoxides via an alkoxyl radical intermediate to epoxyalcohols and ketones (Scheme 1). Unlike the single-turnover LOX activation step, this hydroperoxide isomerase activity fulfills a catalytic cycle so that the free ferrous enzyme will be regenerated upon product release. Although previously unknown, the dioxygenase activity of eLOX3 has recently been reexamined and uncovered in this laboratory (15). Using this dioxygenase activity, ferric eLOX3 oxygenates the synthetic fatty acid, 9E,11Z,14Z-20:3ω6, to the specific 9S-hydroperoxide and oxygenates arachidonic acid to a mixture of HPETEs including the bis-allylic 7-HPETE. However, we have found this dioxygenase activity unusual in exhibiting a pronounced lag phase, which points to a deficiency in eLOX3 activation by fatty acid hydroperoxides. We have also found that this long lag phase is partially alleviated by introduction of an Ala-to-Gly mutation at a conserved residue (Ala-451) that has been implicated in the control of O2 access in the LOX active site (16–21).
An alkoxyl radical is postulated as a common intermediate in both the hydroperoxide isomerase cycling pathway and the single turnover LOX activation step (Scheme 1). O2 is not involved in hydroperoxide isomerase cycling, but we questioned whether O2 is directly involved in LOX activation. Oxygen uptake has been observed in aerobic incubations of soybean LOX-1 with fatty acid hydroperoxides (22). The participation of O2 in LOX activation has also been suggested in a kinetic study of rabbit reticulocyte 15-LOX (23). If O2 is involved in activation, we postulated further that a lack of or limited O2 access in the enzyme active site could be the explanation for both the prominent hydroperoxide isomerase activity and the inefficient enzyme activation observed in eLOX3. To explore these ideas by using kinetic and analytical chemical approaches, we compared and contrasted the rates and the products of reactions of soybean LOX-1, the A451G eLOX3, and wild-type eLOX3 with fatty acid hydroperoxides at various oxygen concentrations.
EXPERIMENTAL PROCEDURES
Materials
Arachidonic and linoleic acids and methyl esters were purchased from NuChek Prep Inc. (Elysian, MN). 15S-HPETE or 13S-HPODE was synthesized by reacting soybean LOX-1 with arachidonic acid or linoleic acid at pH 9 followed by SP-HPLC purification (24). [18O]13S-HPODE was synthesized under 18O2 atmosphere (25). Nordihydroguaiaretic acid (NDGA) was purchased from Cayman Chemical Co. (Ann Arbor, MI). Triphenylphosphine (TPP) was purchased from Sigma. Soybean LOX-1 was purchased from Sigma and Cayman Chemical and labeled as type V and P1-purified, respectively. The enzyme from both sources gave identical products, although in our hands the enzyme from Sigma was about 10 times as active as the purified enzyme from Cayman Chemical Co. (26). The concentration label in this study refers to the Sigma enzyme assuming 80% purity of the preparation. H218O (∼95%) was purchased 25 years ago from the Monsanto Research Corp. (Miamiburg, OH).
Expression and Purification of Human eLOX3
The cDNA of human eLOX3 with an N-terminal His6 tag was subcloned into the pCW vector, and the protein was expressed in Escherichia coli and purified according to a previously published protocol (27). The A451G mutagenesis in eLOX3 was performed using QuikChangeTM site-directed mutagenesis kit. The mutation was verified by DNA sequencing. Then the A451G eLOX3 was expressed and purified as the wild-type eLOX3.
Reaction Incubations and Extraction
Initial incubations were conducted on an analytical scale in 100 mm sodium phosphate buffer, pH 7.5, at various O2 concentrations at room temperature and were monitored using a spectrophotometer from PerkinElmer Life Sciences. The typical reaction volume was 1 ml. O2-saturated buffer was made by bubbling ∼40 ml of buffer in a 50-ml conical tube with O2 for 30 min. The O2-saturated buffer was used for the incubations after the buffer temperature reverted to room temperature. For the anaerobic incubations, the buffer was rendered anaerobic using an oxygen-consuming system consisting of glucose, glucose oxidase, and catalase according to a previously published protocol (26). To verify the results, each anaerobic reaction was also repeated in N2-saturated phosphate buffer inside a N2-inflated glovebag in the absence of the glucose-glucose oxidase-catalase system (26), and identical results were found. If methyl arachidonate is one of the substrates, sodium deoxycholate (final concentration 1–2 mm) was used to help solubilize the methyl ester. The incubation mixture was acidified to pH 5 and then extracted using an Oasis HLB cartridge (Waters Corp.).
For structural identification of the products of LOX activation by 13S-HPODE, large scale incubations of unpurified soybean LOX-1 from Cayman Chemical (250 μl of the commercial preparation, catalogue no. 60712) with 13S-HPODE (66 μm) in the presence of NDGA (80 μm) were performed in 60 ml of air-saturated 50 mm sodium borate, pH 10 buffer, for 15 min. In addition, 20 μl of bovine liver catalase (the commercial preparation, catalogue no. C3155, Sigma) was added to the incubation to eliminate hydrogen peroxide generated from NDGA autoxidation (28). Although borate would form a complex with NDGA and thus reduce the reaction efficiency (28), we found the reaction was largely complete under the used conditions. (Other buffers can be used to eliminate this problem.) The reaction mixture was acidified to about pH 4 and extracted by methylene chloride.
HPLC-Diode Array Analysis
RP-HPLC analysis typically used a Waters Symmetry C18 column (0.46 × 25 cm), a flow rate of 1 ml/min, and a solvent system of methanol/water/acetic acid (80:20:0.01, by volume). For better resolution of the epoxyalcohol or epoxyallylic hydroperoxide products, we performed SP-HPLC analysis using a Beckman Silica Ultrasphere column (0.46 × 25 cm), a flow rate of 1 ml/min, and a solvent system of hexane/isopropyl alcohol/acetic acid (100:2:0.02). The monomeric and dimeric products from the anaerobic reaction of soybean LOX-1 or A451G eLOX3 can be conveniently separated using a Waters Symmetry C18 column (0.46 cm × 25 cm), a flow rate of 1 ml/min, and a solvent system of methanol/water/acetic acid (95:5:0.01, by volume).
Derivatization and GC-MS Analysis
Free fatty acid products were dissolved in a small volume (20–50 μl) of methanol and then converted to the methyl ester by adding ethereal diazomethane. TMS ether derivatives were prepared by treatment with bis(trimethylsilyl)-trifluoroacetamide (10 μl) and pyridine (2 μl) at room temperature for 2 h. Subsequently, the reagents were evaporated under a stream of nitrogen, and the samples were dissolved in hexane for GC-MS.
Analysis of the methyl ester trimethylsilyl ether derivatives of the products was carried out in the positive ion electron impact mode (70 eV) using a Thermo Finnigan Trace DSQ ion trap GC-MS with the Xcalibur data system. Samples were injected at 150 °C, and after 1 min the temperature was programmed to 300 °C at 20 °C/min. The spectrum shown for each compound was averaged from ∼10 spectra collected during elution of the GC peak.
ESI-LC-MS
ESI-LC-MS was performed using a Thermo Finnigan LC Quantum or ion trap instrument. For analysis of the aerobic reactions of soybean LOX-1 or A451G eLOX3 with 13S-HPODE or 15S-HPETE in the presence of NDGA, a Waters Symmetry C18 column (0.2 × 15 cm) was eluted with acetonitrile/water/ammonium acetate (50:50:10 mm) at 0.2 ml/min. Mass spectra were acquired over the mass range m/z 200–500 at 2 s per scan under the negative ion mode. For analysis of monomer products from the anaerobic reactions of soybean LOX-1 or A451G eLOX3 with 13S-HPODE and arachidonic acid, we used a Phenomenex C18 column (2.6 μm, 0.3 × 10 cm), methanol/water/ammonium acetate (80:20:10 mm) and a flow rate of 0.3 ml/min. These conditions were held for 5 min, and a gradient program (from the weak solvent to pure methanol) was then used to elute the remaining arachidonic acid substrate and dimer products. Mass spectra were acquired over the mass range m/z 200–700 at 2 s per scan under the negative ion mode. For further analysis of the dimer products, we used a Phenomenex C18 column (2.6 μm, 0.3 × 10 cm), a flow rate of 0.3 ml/min, and a solvent system of methanol/water/ammonium acetate (90:10:10 mm), with MS detecting negative ions in the range from m/z 500 to 650. HPLC-diode array analysis was also performed for each reaction under identical LC conditions.
NMR
1H and 1H,1H-COSY NMR spectra were recorded on a Bruker DXR 600 MHz spectrometer at 298 K. The ppm values were reported relative to residual nondeuterated solvent (δ = 7.16 ppm for C6H6).
RESULTS
Effect of O2 Concentration or the A451G Mutation on the Lag Phase of eLOX3
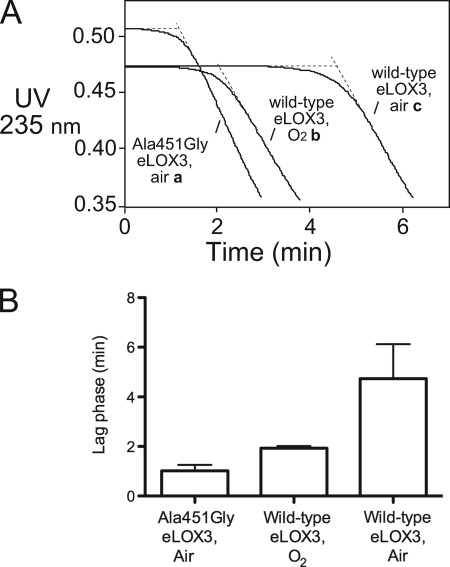
Our previous study showed that eLOX3 is capable of oxygenating the synthetic fatty acid 9E,11Z,14Z-20:3ω6 to a specific hydroperoxide and that the reaction exhibits a pronounced lag phase during which the enzyme is slowly activated by trace amounts of fatty acid hydroperoxides (15). As known with other LOX enzymes (5–7), this lag phase can be eliminated using exogenous fatty acid hydroperoxide activators such as 13S-HPODE (15). To elucidate the role of O2 in eLOX3 activation, first we compared the reactions of eLOX3 with 9E,11Z,14Z-20:3ω6 in air- and O2-saturated buffer ([O2] ∼240 μm and ∼1.2 mm, respectively). We found a consistently shorter lag phase in O2-saturated buffer than in air-saturated buffer (Fig. 1, curves b and c). Only the duration of the lag phase, not the maximal oxygenation rate, was affected. Thus, O2 appeared to promote eLOX3 activation. We also found that the eLOX3 mutant, A451G eLOX3, shows a shorter lag phase than wild-type eLOX3 (Fig. 1, curves a and c). Mutation of Ala to Gly in this position is implicated in oxygenation control in many other LOX enzymes (16–21), and the result in Fig. 1 is consistent with this mutation in eLOX3 improving the access of O2 within the enzyme active site.
FIGURE 1.
Increased O2 concentration or the A451G mutation shortened the lag phase in the reaction of eLOX3 with 9E,11Z,14Z-20:3ω6. Note that this synthetic fatty acid contains a conjugated diene with strong 235 nm absorbance, whereas the oxygenation product has a conjugated triene absorbing at 270 nm (15); consequently, reaction is associated with an absorbance decrease at 235 nm. A, reaction progress curves monitored at 235 nm. Reaction was conducted in sodium phosphate buffer, pH 7.5, at room temperature and was started by addition of enzyme. Wild-type eLOX3 concentration is 0.10 μm. A451G eLOX3 concentration is 0.13 μm. 9E,11Z,14Z-20:3 ω6 concentration is 18–20 μm. B, statistical bar representation of the lag phase duration of the reactions in A. The lag phase duration is defined as the time axis intercept of a straight line through the portion of the reaction progress curve where the rate is maximal (10). The data are presented as the mean ± S.D. For each of the reactions of wild-type eLOX3 in air- and O2-saturated buffer, n = 4. For the reaction of A451G eLOX3 in air-saturated buffer, n = 3. p < 0.01 for every pair of reactions.
Effect of O2 Concentration or the A451G Mutation on the Rate of Reaction of eLOX3 with 13S-HPODE
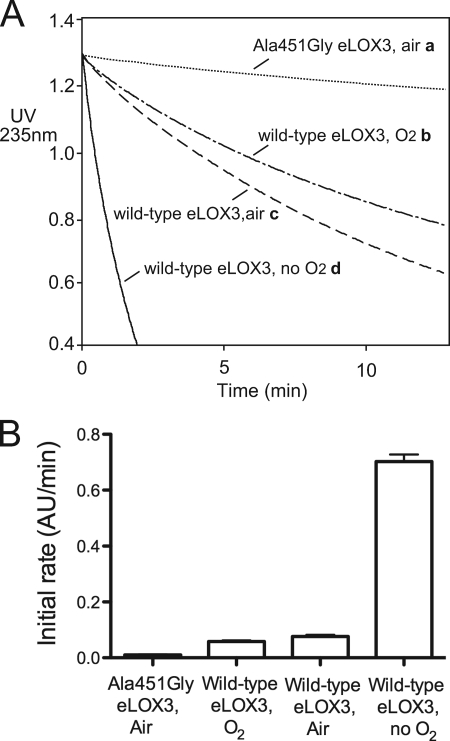
Fatty acid hydroperoxide activators of LOX-catalyzed oxygenation of polyunsaturated substrates are consumed in the activation process (29). This also occurs in 13S-HPODE-activated eLOX3-catalyzed oxygenations, as we demonstrated previously (15). Here, we studied the influence of O2 concentration or the A451G mutation on the consumption of 13S-HPODE when incubated by itself with the enzyme. We observed an increased rate of 13S-HPODE disappearance on shifting from O2-saturated buffer to normoxic buffer, and a further dramatic ∼9-fold increase in rate under anaerobic conditions (Fig. 2, curves b–d). Thus, the initial rate of reaction with 13S-HPODE was inversely related to the oxygen concentration. We also found that compared with wild-type eLOX3 in air-saturated buffer, A451G eLOX3 reacted very slowly with 13S-HPODE (Fig. 2, curves a and c), a result compatible with improved access to O2 within the A451G mutant active site.
FIGURE 2.
Increased O2 concentration or the A451G mutation decreased the rate of the reaction of eLOX3 with 13S-HPODE. A, reaction progress curves monitored at 235 nm. Reaction was conducted in sodium phosphate buffer, pH 7.5, at room temperature and was started by addition of enzyme. Wild-type eLOX3 concentration is 0.25 μm. A451G eLOX3 concentration is 0.32 μm. Note that the oxygenation rate of wild-type eLOX3 at 0.25 μm is similar to the oxygenation rate of A451G eLOX3 at 0.32 μm (Fig. 1). 13S-HPODE concentration is 52–55 μm. B, statistical bar representation of the initial rate of the reactions in A. The data are presented as the mean ± S.D. For each of the reactions of wild-type eLOX3 in air- and O2-saturated buffer, n = 4. For the reaction of wild-type eLOX3 under anaerobic conditions, n = 3. For the reaction of A451G eLOX3 in air-saturated buffer, n = 3. p < 0.01 for every pair of reactions.
Although counterintuitive, this inhibitory effect of O2 or the A451G mutation on the rate of 13S-HPODE consumption (Fig. 2) is in fact consistent with its stimulatory effect on eLOX3 activation (Fig. 1). Because O2 or the A451G mutation promotes eLOX3 activation, i.e. the single turnover reaction of ferrous eLOX3 with 13S-HPODE to give ferric eLOX3, it will necessarily impede the ferrous enzyme cycling pathway, i.e. the far more productive pathway of 13S-HPODE consumption (cf. Scheme 1).
Identification of the Products in LOX Activation by 13S-HPODE
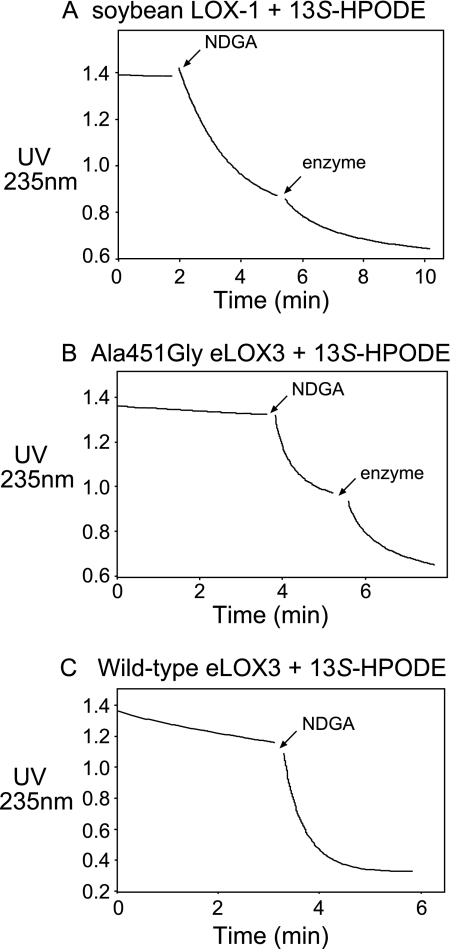
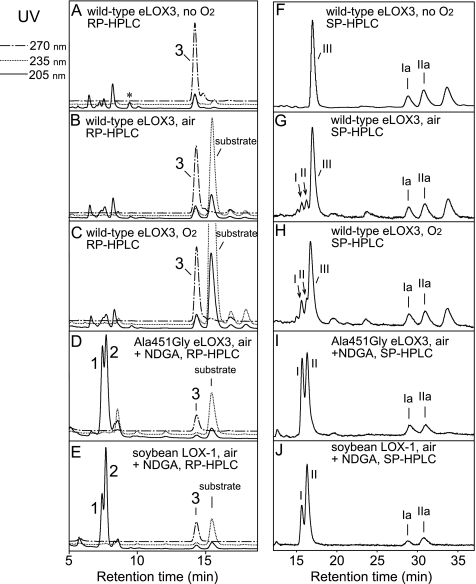
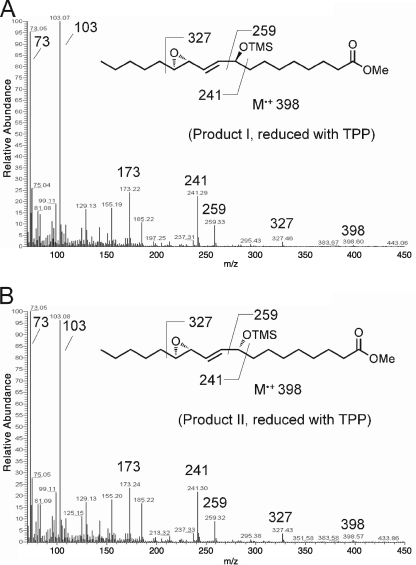
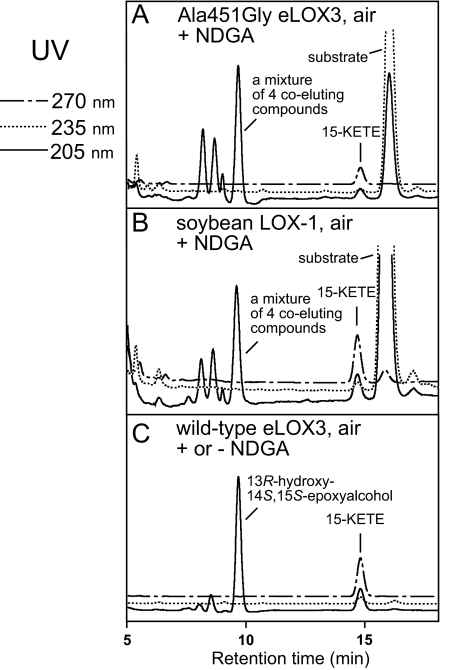
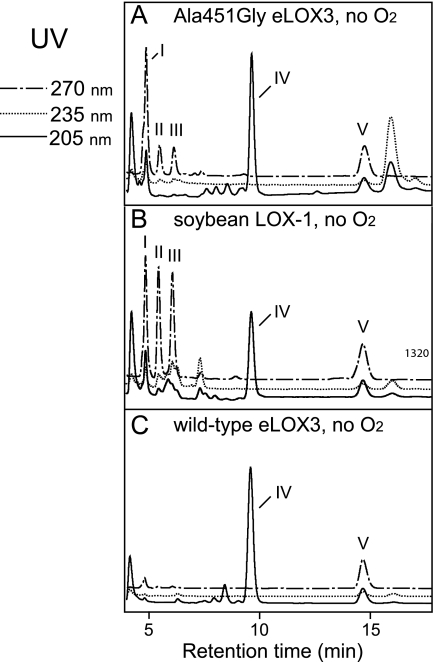
As noted in the Introduction, for LOX enzymes such as soybean LOX-1 that are efficiently activated to the ferric state, the activation process is a single turnover event that produces only traces of hydroperoxide-derived products. NDGA affords an opportunity to obtain the products in LOX activation in large amounts, because it helps regenerate the ferrous enzyme and thus accelerates transformation of the activating hydroperoxide (28), in our experiments of 13S-HPODE (Fig. 5A). Hence, we incubated soybean LOX-1 with 13S-HPODE in the presence of NDGA and identified the products by RP- and SP-HPLC, LC- and GC-MS, and NMR. The keto derivative 13-KODE (product 3 in Fig. 3E) was only a minor product. The major products (1 and 2 in Fig. 3E) showed end absorbance at 205 nm and similar retentions times on RP-HPLC to C18 epoxyalcohol standards. On SP-HPLC, products 1 and 2 were further resolved into four products (products I and II, 23 and 58%, respectively (a ratio of 28:72), and products Ia and IIa, 6 and 13%, respectively, Fig. 3J). Upon TPP reduction, products I and II were converted to products Ia and IIa, respectively. LC-MS showed that products Ia and IIa both have the relative molecular mass of 312, consistent with the structure of a linoleic acid-derived epoxyalcohol, and that products I and II both have the relative molecular mass of 328, which, together with the TPP reduction experiment, suggests that products I and II are hydroperoxide counterparts of products Ia and IIa, i.e. epoxyallylic hydroperoxides. GC-MS analysis indicated that both products Ia and IIa are stereoisomers of 9-hydroxy-12,13S-epoxyoctadecenoic acid (Fig. 4, A and B). 1H and 1H,1H-COSY NMR analysis confirmed such a structure and further revealed that both products Ia and IIa contain a trans-epoxide between C-12 and C-13 (J = 2.0 Hz) and a trans double bond between C-10 and C-11 (J = 15.5 Hz) (supplemental Fig. S1 and S2). Finally, the stereochemistry of the 9-hydroxyl in products Ia and IIa was assigned as 9S and 9R, respectively, based on their order of elution on silica SP-HPLC (30). Thus, the major products in the activation of soybean LOX-1 by 13S-HPODE, products I and II, are 9S- and 9R-hydroperoxy-12S,13S-trans-epoxyoctadec-10E-enoic acid, respectively (Scheme 2).
FIGURE 5.
Effect of NDGA on the rates of reaction with 13S-HPODE. NDGA (20 μm) stimulated the reaction of soybean LOX-1 (0.06 μm A), A451G eLOX3 (0.32 μm, B), or wild-type eLOX3 (0.25 μm, C) with 13S-HPODE (55 μm). The incubations were performed in sodium phosphate buffer, pH 7.5, and initiated by addition of enzyme. NDGA was added at the indicated point. For the reaction of A451G eLOX3 or soybean LOX-1, a second equal aliquot of enzyme was added at the indicated point.
FIGURE 3.
RP- and SP-HPLC analysis of the products from the reactions with 13S-HPODE. Analysis of wild-type eLOX3 (A–C and F–H), A451G eLOX3 (D and I), and soybean LOX-1 following reaction with 13S-HPODE (E and J) is shown. For wild-type eLOX3, O2 concentration was varied in the incubation, from 0 (A and F) to 240 μm (B and G) to 1.2 mm (C and H). For A451G eLOX3 and soybean LOX-1, NDGA was included in the incubation. RP-HPLC analysis used a Waters Symmetry C18 column (0.46 × 25 cm), a flow rate of 1 ml/min, and a solvent system of methanol/water/acetic acid (80:20:0.01, by volume). SP-HPLC analysis used a Beckman Silica Ultrasphere column (0.46 × 25 cm), a flow rate of 1 ml/min and a solvent system of hexane/isopropyl alcohol/acetic acid (100:2:0.02). *, control experiments indicated that this peak was not an enzymatic product.
FIGURE 4.
GC-MS analysis of products I and II. Mass spectra of the TMS ether methyl ester derivative of the TPP-reduced major products (product I and II in Fig. 3) from the reaction of soybean LOX-1 or A451G eLOX3 with 13S-HPODE in the presence of NDGA are shown. A, product I; B, product II.
SCHEME 2.
Similarly to soybean LOX-1, A451G eLOX3 barely reacted with 13S-HPODE alone and NDGA stimulated this reaction by ∼90-fold (Fig. 5B). As the reaction proceeded, the enzyme was quickly and irreversibly inactivated (Fig. 5B). This type of LOX inactivation has been documented for soybean LOX-1 (and is also evident in Fig. 5A) and is partly ascribed to hydrogen peroxide generated from reaction of NDGA-derived radical intermediates with O2 (28). RP-HPLC, SP-HPLC, LC-MS analyses, and also TPP reduction of the products from the reaction of A451G eLOX3 in the presence of NDGA revealed that 13S-HPODE was converted to the same major products I and II as in the reaction of soybean LOX-1, albeit with a different ratio of 42:58 (Fig. 3I and Scheme 2). On prolonged incubations with an excess of enzyme, the epoxyallylic hydroperoxide products I and II were further converted by A451G eLOX3 to an epoxyketone compound (9-keto-12S,13S-trans-epoxyoctadec-10E-enoic acid), which was identified directly by 1H and 1H,1H-COSY NMR and, following NaBH4 treatment, by chromatographic comparison with epoxyalcohol standards (data not shown).
For the reaction of wild-type eLOX3 with 13S-HPODE, a considerable stimulation (∼23-fold) was also observed in the presence of NDGA (Fig. 5C). However, whether NDGA was present or not, 13S-HPODE was converted to a different set of products, among which the two most prominent were 13-KODE (product 3 in Fig. 3) and one stereoisomer of 11-hydroxy-12,13S-epoxyoctadec-9Z-enoic acid (product III in Fig. 3, identified by GC-MS in supplemental Fig. S3). Despite this, careful examination revealed that the epoxyallylic hydroperoxides I and II were also present as minor products. On TPP reduction, these two products were converted to the epoxyalcohols Ia and IIa. Importantly, the production of I and II by wild-type eLOX3 was O2-dependent; under anaerobic conditions, I and II were absent and only 13-KODE and epoxyalcohols were produced (Fig. 3F); in air-saturated buffer, I and II constituted 7% of total products (Fig. 3G); in O2-saturated buffer, the yield of I and II was increased to 11% (Fig. 3H).
Identification of the Products in LOX Activation by 15S-HPETE
Similarly, soybean LOX-1 or A451G eLOX3 reacted with 15S-HPETE in air-saturated buffer in the presence of NDGA to give a nonspecific mixture consisted of mainly epoxyallylic hydroperoxides (Fig. 6, A and B, and supplemental Fig. S4). Wild-type eLOX3, on the other hand, gave a specific epoxyalcohol, threo-13R-hydroxy-14S,15S-trans-epoxyeicosa-5Z,8Z,11Z-trienoic acid followed by 15-KETE (Fig. 6C) (14).
FIGURE 6.
RP-HPLC analysis of the products from the reactions with 15S-HPETE. Products from reactions of 15S-HPETE with A451G eLOX3 (A), soybean LOX-1 (B), and wild-type eLOX3 (C) are shown. RP-HPLC analysis used a Waters Symmetry C18 column (0.46 × 25 cm), a flow rate of 1 ml/min, and a solvent system of methanol/water/acetic acid (80:20:0.01, by volume).
Reaction of Soybean LOX-1 or A451G eLOX3 with 13S-HPODE under Anaerobic Conditions
As already demonstrated, the conversion of 13S-HPODE by wild-type eLOX3 was dramatically accelerated under anaerobic conditions giving exclusively epoxyalcohols and 13-KODE (Figs. 2 and 3, A and F). We questioned whether the same applies to soybean LOX-1 and A451G eLOX3. The anaerobic reaction of soybean LOX-1 with 13S-HPODE has been extensively investigated (31–34), and these findings were confirmed in this study. The anaerobic conditions afforded only a mild stimulation on the reaction of soybean LOX-1 with 13S-HPODE alone (22, 34); at submicromolar enzyme concentrations, the rate of consumption of 13S-HPODE by soybean LOX-1 was negligible even under anaerobic conditions compared with wild-type eLOX3. When further stimulated by linoleic acid or arachidonic acid (which serves as a LOX-reducing agent under anaerobic conditions), the reaction yielded not only epoxyalcohols and 13-KODE but also significant amounts of C13 aldehydes and fatty acid dimers (supplemental Figs. S5 and S6).
Although considered under “Discussion,” it might be helpful to note at this stage that both the anaerobic and aerobic behavior of soybean LOX-1 and A451G eLOX3 is consistent with the concept that their reaction with hydroperoxides is often followed by dissociation of the fatty acid radicals produced, leaving the enzyme in the activated ferric state and thus able to oxygenate polyunsaturated substrates, while unable to further react with hydroperoxides.
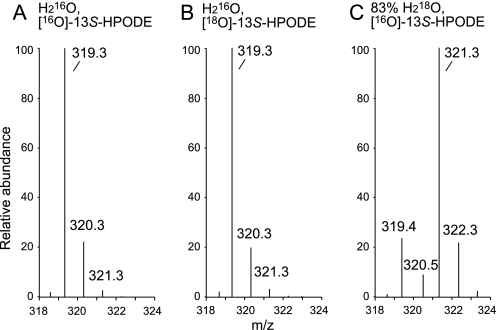
Consistent with this, we found that A451G eLOX3 also required a LOX-reducing agent to react with 13S-HPODE under anaerobic conditions (Fig. 7) and gave products qualitatively similar to those from soybean LOX-1, yet with one notable exception. In addition to epoxyalcohols, 13-KODE, C13 aldehydes, and fatty acid dimers, the reaction of A451G eLOX3 with 13S-HPODE and arachidonic acid or methyl arachidonate unexpectedly gave considerable amounts of (methyl) HETEs (Figs. 8 and 9 and supplemental Figs. S7 and S8A). These were hydroxy products, not hydroperoxides. The relative amounts of these HETE products (5-HETE + 9-HETE > 15-HETE + 11-HETE > 8-HETE + 12-HETE) were consistent with the preference of this enzyme in hydrogen abstraction under aerobic conditions (H-7 > H-13 > H-10) (15), pointing to a similar hydrogen abstraction step during this anaerobic HETE synthesis. Incubations with 13S- [18O]HPODE or in H218O followed by LC-MS analysis led to the unexpected finding that the hydroxyl of these HETE products was derived exclusively from water (Fig. 10). The mechanism of formation of these HETE products is considered under “Discussion.” As to the leading question, we concluded that for soybean LOX-1 or A451G eLOX3, epoxyalcohols and ketone are formed from 13S-HPODE in better yields anaerobically than aerobically, yet the synthesis of these compounds is prevented by other factors from being the only mechanism in operation. Their formation may be hindered by spontaneous dissociation of the radical intermediates from the enzyme, see under “Discussion.”
FIGURE 7.
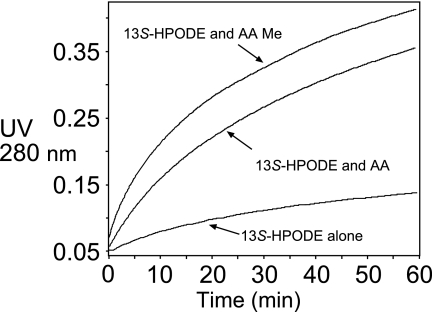
Progress curves of the anaerobic reactions of A451G eLOX3 (0.23 μm) with 13S-HPODE (90 μm). Reactions were conducted in the absence or presence of fatty acids (arachidonic acid or methyl arachidonate, 70 μm) in sodium phosphate buffer, pH 7.5, started by addition of enzyme, and monitored at 280 nm. The anaerobic conditions are described under “Experimental Procedures.”
FIGURE 8.
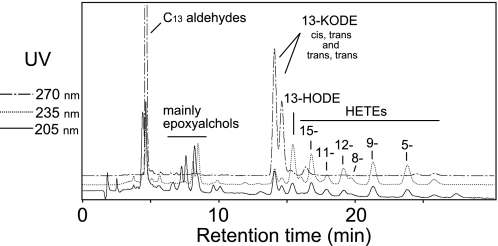
RP-HPLC analysis of the monomeric products from the anaerobic reaction of A451G eLOX3 with 13S-HPODE and arachidonic acid. RP-HPLC analysis used a Waters Symmetry C18 column (0.46 × 25 cm), a flow rate of 1 ml/min, and a solvent system of methanol/water/acetic acid (80:20:0.01, by volume).
FIGURE 9.
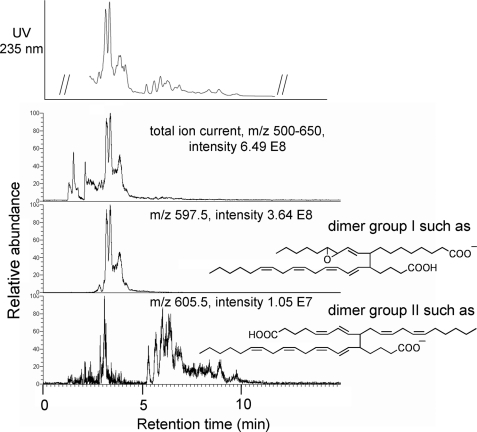
RP-HPLC-UV and LC-ESI-MS analysis of the dimer products from the anaerobic reaction of A451G eLOX3 with 13S-HPODE and arachidonic acid. RP-HPLC analysis used a Phenomenex C18 column (2.6 μm, 0.3 × 10 cm), a flow rate of 0.3 ml/min, and a solvent system of methanol/water (90:10 by volume) containing 10 mm ammonium acetate. MS analysis detected negative ions in the range from m/z 500 to 650. Under these conditions, arachidonic acid eluted at 3.0 min (data not shown).
FIGURE 10.
Mass spectra of 15-HETE formed in the reaction of A451G eLOX3 with 13S-HPODE and arachidonic acid. A, reaction with [16O]13S-HPODE in H216O. B, reaction with [18O]13S-HPODE in H216O. C, reaction with [16O]13S-HPODE in 83% H218O. The mass spectra were obtained in LC-ESI-MS analysis using a Phenomenex C18 column (2.6 μm, 0.3 × 10 cm), a flow rate of 0.3 ml/min, and a solvent system of methanol/water (80:20, by volume), containing 10 mm ammonium acetate. MS analysis detected negative ions in the range from m/z 200 to 700.
As a control, incubation of wild-type eLOX3 with both 13S-HPODE and methyl arachidonate under identical anaerobic conditions showed that 13S-HPODE was converted to the same set of epoxyalcohols and 13-KODE as in the absence of methyl arachidonate, whereas methyl arachidonate largely remained unconverted (supplemental Fig. S8B). This can be explained if wild-type eLOX3 reacts with 13S-HPODE strictly through the cycling pathway under anaerobic conditions and thus remains in the ferrous state when in the free enzyme form, which is inactive toward methyl arachidonate. Similarly, A451G eLOX3 failed to metabolize methyl arachidonate when 12R-HPETE instead of 13S-HPODE was co-incubated under anaerobic conditions (supplemental Fig. S8C). Unlike 13S-HPODE, 12R-HPETE was converted by A451G eLOX3 to epoxyalcohol and ketone products (8R-hydroxy-11R,12R-epoxyeicosa-5Z,9E,14Z-trienoic acid and 12-KETE) whether O2 is present or not, implying that with 12R-HPETE the hydroperoxide isomerase activity of A451G eLOX3 is dominant. Taken together, these results emphasized the importance of the enzyme shuttling between the two oxidation states during the synergistic conversion of (methyl) arachidonate and 13S-HPODE by A451G eLOX3 or soybean LOX-1.
Reaction of Soybean LOX-1 or A451G eLOX3 with 15S-HPETE under Anaerobic Conditions
When 15S-HPETE alone was used as substrate, significant conversion by either soybean LOX-1 or A451G eLOX3 did occur under anaerobic conditions (for comparison of rates under aerobic and anaerobic conditions, see supplemental Fig. S9). Part of the reason could be that 15S-HPETE itself serves as a reducing agent to stimulate the reaction, due to the presence of the C5–C9 and C8–C12 pentadiene systems on the molecule. Strikingly, for both soybean LOX-1 and A451G eLOX3, the major product (>50% yield) is the single epoxyalcohol threo-13R-hydroxy-14S,15S-trans-epoxyeicosa-5Z,8Z,11Z-trienoic acid (product IV, Fig. 11, A and B), the same molecule as found in the reaction of wild-type eLOX3 with 15S-HPETE either aerobically or anaerobically (Figs. 6C and 11C). (The identification of product IV has been described in detail previously (26).). Yet unlike in wild-type eLOX3 reaction, besides the epoxyalcohol and 15-KETE (product V), other products were also present, including C15 aldehydes (product I), leukotriene-type 8,15-diols (product II and III), and for soybean LOX-1, two unusual 5,15-diols (26). Thus, for soybean LOX-1 and A451G eLOX3, anaerobic conditions promoted specific epoxyalcohol synthesis from 15S-HPETE, yet the “perfected” epoxyalcohol synthesis as catalyzed by wild-type eLOX3 was still not achieved.
FIGURE 11.
RP-HPLC analysis of the products from anaerobic reactions with 15S-HPETE. A, A451G eLOX3. B, soybean LOX-1. C, wild-type eLOX3. RP-HPLC analysis used a Waters Symmetry C18 column (0.46 × 25 cm), a flow rate of 1 ml/min, and a solvent system of methanol/water/acetic acid (80:20:0.01, by volume).
DISCUSSION
In this study, we have demonstrated that O2 is involved in LOX activation, a central piece of evidence being that O2 itself is incorporated into the products from LOX activation. Our study also demonstrates that for eLOX3, the enzyme activation and the hydroperoxide isomerase activity are in competition and are reciprocally regulated by O2. To explain the preponderance of the hydroperoxide isomerase activity over the enzyme activation in eLOX3 under normoxic conditions, we propose a model of limited O2 access in its enzyme active site. For soybean LOX-1, although complexity arises to suggest that the enzyme activation may occur to some extent even under anaerobic conditions, O2 if present will penetrate into the enzyme active site and help maximize the efficiency of enzyme activation. In a context of enzyme evolution, we suggest that restricting O2 access to the enzyme active site is one crucial mechanism that enables eLOX3 to develop the unique hydroperoxide isomerase activity that distinguishes itself from soybean LOX-1 and other typical LOX enzymes.
Chemical Model of LOX Activation
It has long been recognized that ferrous ion in aqueous solutions readily induces homolytic cleavage of the peroxide bond in an alkyl hydroperoxide to give an alkoxyl radical and a hydroxide, with itself is being converted to the ferric ion (35, 36). A polyunsaturated fatty acid-derived alkoxyl radical usually exists in equilibrium with an epoxyallylic radical, and the fate of such an alkoxyl/epoxyallylic radical is usually one of the following: 1) combination of the epoxyallylic radical with other radicals, favored in the presence of an efficient radical trap; 2) loss of the β-hydrogen from the alkoxyl radical to become a ketone, favored in the presence of a strong oxidant that helps abstract the β-hydrogen; 3) carbon-carbon chain cleavage resulting in an aldehyde and an alkyl radical, favored if a γ double bond exists to help stabilize the alkyl radical fragment (37, 38). Despite these complexities, the pioneering work led by Gardner and co-workers (35, 39–41) suggests that in a simple system such as aqueous ferrous ion, cysteine, and 13S-HPODE, the major route of the alkoxyl/epoxyallylic radical under aerobic conditions is the combination of the epoxyallylic radical with O2, an efficient radical trap, followed by hydrogen abstraction to give epoxyallylic hydroperoxides, which, in the cysteine/Fe(II) system, will be further converted to epoxyketones.
Mechanism of LOX Activation
Based on the above chemical precedence, we suggest for LOX activation a similar mechanism from an alkoxyl radical to the epoxyallylic hydroperoxide products, with O2 playing the central role as a radical trap. Yet it merits attention that the ratio of the two epoxyallylic hydroperoxide products from soybean LOX-1 epimeric at C-9 (28:72, Fig. 3J) is clearly deviated from the ratio of 50:50 as predicted in the chemical system. We surmise that in LOX activation the combination of the epoxyallylic radical with O2 occurs largely under the enzymatic control, i.e. within the enzyme active site where oxygen supplies to the two faces of the molecule are often unequal. Once produced, these epoxyallylic peroxyl radicals will then be released into the solution, leaving the enzyme in the activated ferric state and free to react with polyunsaturated fatty acid substrates.
Up to this point, the enzyme activation is complete, yet the fate of the epoxyallylic peroxyl radical in the solution is uncertain. It is often suggested in the literature that two such epoxyallylic peroxyl radicals in solution would combine by the Russell mechanism to give epoxyketone, epoxyalcohol, and singlet oxygen (42) or that the epoxyallylic peroxyl radical would transfer an oxygen atom to certain susceptible compounds (e.g. to epoxidize a double bond) with itself being converted to an epoxyalcohol (13, 43). Although we cannot exclude these possibilities, here we have demonstrated, in agreement with the chemical model, that the major route for an epoxyallylic peroxyl radical in solution is to abstract a hydrogen atom from a certain source to become an epoxyallylic hydroperoxide. It might be argued that this could be an artifact brought by NDGA we used in the study to regenerate the enzyme and thus to amplify the reaction, because NDGA is also a free radical scavenger. However, we consider this rather unlikely, because we found in an analogous reaction of Anabaena LOX that when a large quantity of purified enzyme instead of NDGA was used, the product pattern of the Anabaena LOX activation by 9R-HPODE remained largely unchanged (although in this case, the epoxyallylic hydroperoxides were further dehydrated to epoxyketones as in the chemical model).3 The chemical transformations involved in LOX activation are summarized in Scheme 2.
Is O2 Required for LOX Activation?
It is natural to ask whether the observed incorporation of O2 into the products is necessary for LOX activation/oxidation. An alternative scenario is that the alkoxyl radical is produced in the enzyme active site and then simply released into the solution resulting in LOX activation. This study unexpectedly provides two different answers to this question for the two enzymes, eLOX3 and soybean LOX-1, suggesting variability within the LOX family.
For eLOX3, O2 is required for the enzyme activation, as suggested by three lines of evidence. First, increasing O2 concentration promotes the enzyme activation in the oxygenation of 9E,11Z,14Z-20:3ω6 (Fig. 1). Second, the anaerobic incubation of eLOX3 with 13S-HPODE and methyl arachidonate results in the production of exclusively epoxyalcohols and 13-KODE from 13S-HPODE with no conversion of methyl arachidonate, the latter suggesting that the enzyme is never activated to the ferric form under anaerobic conditions. From a different perspective, for eLOX3, the hydroperoxide isomerase activity with 13S-HPODE operates at full speed only when the competing enzyme activation pathway is fully suppressed under the anaerobic conditions (Fig. 2). Third, in sharp contrast to the second point, in the presence of O2, eLOX3 will be activated by 13S-HPODE and will then convert (methyl) arachidonate into a mixture of (methyl) HPETEs (15). Given this stringent O2 requirement, we further deduce that the relative low efficiency of eLOX3 activation is a consequence of limited oxygen access in the enzyme active site.
For soybean LOX-1, however, O2 seems only to help maximize the efficiency of enzyme activation. Although the hydroperoxide isomerase activity as indicated by epoxyalcohol and ketone synthesis is indeed promoted by the anaerobic conditions, it is not the only mechanism in operation. That the enzyme activation/oxidation occurs to some extent even in the absence of O2 is suggested by two observed transformations, both ascribed to the ferric enzyme (26, 45, 46). Under anaerobic conditions and in the presence of 13S-HPODE, linoleic or arachidonic acid is converted via the corresponding fatty acid radical to fatty acid dimers (13, 45). And 15S-HPETE is converted via either C-7 or C-10 centered radicals to allylic epoxides such as 14,15-LTA4 (Fig. 11B) (25). This O2-independent enzyme activation is best explained if a fraction of the alkoxyl/epoxyallylic radical intermediate is released into the solution, probably because of a low affinity of the enzyme for this radical intermediate, leaving the enzyme in the activated ferric state. Indeed, some of the released alkoxyl/epoxyallylic radicals are subsequently trapped in fatty acid dimers, which have been shown to form in the solution as opposed to in the enzyme active site (47). Nevertheless, it is beyond doubt that the efficiency of enzyme activation will be maximized under aerobic conditions, when the fatty acid hydroperoxide is efficiently utilized for enzyme activation with minimal conversion to epoxyalcohols and ketones.
Effect of the A451G Mutation on eLOX3 Activation
Strikingly, the single amino acid substitution A451G causes eLOX3 to switch from the hydroperoxide isomerase cycling pathway to the single turnover enzyme activation pathway in the reaction with 13S-HPODE or 15S-HPETE. The close resemblance of A451G eLOX3 to soybean LOX-1 in the chemistry of enzyme activation by 13S-HPODE or 15S-HPETE is evident either under aerobic conditions or under anaerobic conditions. To explain this effect of the A451G mutation, we have considered two possibilities not mutually exclusive. In the first possibility, this mutation opens up space either directly (18) or indirectly (48), thereby allowing O2 to react with the epoxyallylic radical intermediate in the enzyme active site. Consistent with this possibility is the finding by Cristea and Oliw (49) that the opposite mutation in the fungal manganese-LOX, G316A, produces the opposite effect, i.e. a switch from dioxygenase to hydroperoxide isomerase activity. Presumably, O2 is barred from reacting with the epoxyallylic radical intermediate when Gly-316 is replaced with Ala in manganese-LOX.
In the second possibility, the A451G mutation results in a lower affinity of the enzyme for the alkoxyl/epoxyallylic radical intermediate, which once released will also lead to enzyme activation. This will not be unexpected because this residue lies within the active site and may be directly involved in the substrate/intermediate/product binding (18, 48). Consistent with this possibility, the two epoxyallylic hydroperoxide products from 13S-HPODE are in a ratio of 42:58 (cf. 28:72 in soybean LOX-1) and thus may derive comparatively more from combination of the epoxyallylic radical with O2 in the solution. Also, as already explained for soybean LOX-1, the formation of dimers containing an epoxyallylic structure in the anaerobic reaction of A451G eLOX3 with 13S-HPODE and arachidonic acid (Fig. 9) is strong evidence for the occurrence of the alkoxyl/epoxyallylic radical leakage.
It should be emphasized, however, that this switch to the enzyme activation pathway does not apply to every fatty acid hydroperoxide. 12R-HPETE, for example, is still predominantly converted to an epoxyalcohol and 12-KETE by A451G eLOX3. This probably explains why this mutation only partially alleviates the pronounced lag phase exhibited by wild-type eLOX3 in fatty acid oxygenation (Fig. 1) (15).
In summary, the A451G mutation is associated with diminished hydroperoxide cycling and more efficient activation. Possible explanations are an enhanced availability of O2 in the active site, allowing O2 to more efficiently intercept the initial product of hydroperoxide cleavage (the epoxyallylic radical), forming the epoxyallylic peroxyl radical, which tends to dissociate from the active site, leaving the enzyme in the activated ferric state and/or the epoxyallylic radical binds less tightly in the A451G mutant, and more readily leaves the active site. Either way, the consequence is a shorter lag phase in the A451G mutant and less consumption of hydroperoxide through hydroperoxide isomerase cycling.
Novel Mechanism of HETE Synthesis
In this study, the anaerobic reaction of A451G eLOX3 with 13S-HPODE and (methyl) arachidonate unexpectedly gives significant amounts of HETEs. LOX enzymes are known to operate through free radical mechanisms. The free radical nature of this HETE synthesis is also suggested by the product pattern (5-HETE + 9-HETE > 11-HETE + 15-HETE > 8-HETE + 12-HETE) that is consistent with the preference of this enzyme in hydrogen atom abstraction (H-7 > H-13 > H-10) (15). Thus, it seems paradoxical that the HETEs generated in a free radical reaction derive the hydroxyl group from water, which is often considered as evidence for an ionic mechanism.
We suspect that what is actually incorporated into the HETE products is the hydroxide ligand of the non-heme ferric iron in the enzyme active site rather than water in the bulk solvent. The hydroxide ligand of the non-heme iron can be conceived as a trapped hydroxyl radical, and its combination with an arachidonate carbon radical to give HETEs seems feasible on chemical grounds. (In fact it mimics the rebound hydroxylation step in the hydroperoxide isomerase cycle producing epoxyalcohols (14).) Notably, for HETE synthesis this mechanism necessitates two molecules of the ferric enzyme to generate one molecule of HETE. The first molecule of ferric enzyme abstracts a hydrogen from arachidonic acid to generate the corresponding carbon radical and then releases it into the solution; this released arachidonic acid radical then fetches the trapped hydroxyl radical from a second molecule of ferric enzyme and becomes the final HETE product. In addition, this mechanism assumes that the hydroxide ligand of the non-heme iron readily exchanges with water in the bulk solvent (44, 50). It would be of interest to further investigate the mechanism of this unusual HETE synthesis. Also, it remains to be ascertained whether this type of HETE synthesis is unique to A451G eLOX3 or is general among LOX enzymes. In the anaerobic reaction of soybean LOX-1 with arachidonic acid and 13S-HPODE, 15-HETE, and 11-HETE were also observed among the minor products, but the origin of the hydroxyl group in these HETE products was not investigated here due to their minute amounts.
Conclusions
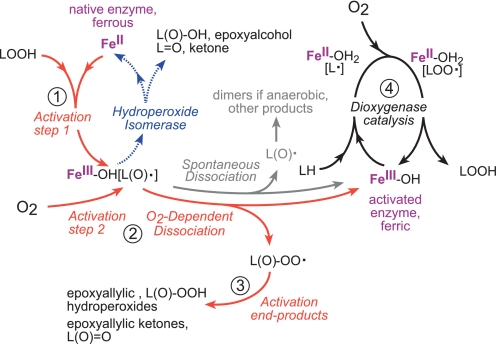
In this study, we have established the role of O2 in LOX activation and elucidated the chemistry involved (Fig. 12). The key event leading to LOX activation is dissociation of a ferric LOX-radical intermediate complex. O2 facilitates this dissociation and arrests the competing hydroperoxide isomerase cycling pathway by reacting with the epoxyallylic radical intermediate to give an epoxyallylic peroxyl radical, which, due to its limited reactivity within the enzyme active site, will leave the enzyme resulting in enzyme activation.
FIGURE 12.
Activation of LOX from ferrous to the active ferric species by reaction with fatty acid hydroperoxide (LOOH). The initial fatty acid alkoxyl radical intermediate rearranges to an enzyme-associated epoxyallylic radical intermediate, Fe(III)-OH[L(O)•]. This enzyme-intermediate complex can cycle back to the free ferrous LOX giving epoxyalcohol product via the hydroperoxide isomerase “oxygen rebound” pathway or dissociate, resulting in the free ferric LOX available for dioxygenase catalysis. Dissociation is facilitated by reaction with O2, giving an epoxyallylic peroxy radical L(O)-OO• that cannot react with Fe(III)-OH, will thus leave the enzyme, and will be reduced in the solution to the epoxyallylic hydroperoxide products we identified (products I and II in Fig. 3). Spontaneous dissociation, almost absent with eLOX3, but more prominent with soybean LOX-1 or A451G eLOX3, under anaerobic conditions can also lead to enzyme oxidation, with the dissociated epoxyallylic radical undergoing nonenzymatic transformations in the solution to various products, including fatty acid dimers.
Supplementary Material
Acknowledgment
We thank William E. Boeglin for help with eLOX3 expression and purification.
This work was supported, in whole or in part, by National Institutes of Health Grant AR-051968 (to A. R. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S9 and an additional reference.
Y. Zheng and A. R. Brash, unpublished observations.
- LOX
- lipoxygenase
- HPODE
- hydroperoxyoctadecadienoic acid
- HPETE
- hydroperoxyeicosatetraenoic acid
- HETE
- hydroxyeicosatetraenoic acid
- KODE
- ketooctadecadienoic acid
- KETE
- ketoeicosatetraenoic acid
- RP-HPLC
- reversed phase-HPLC
- SP-HPLC
- straight phase-HPLC
- NDGA
- nordihydroguaiaretic acid
- TPP
- triphenylphosphine
- eLOX3
- epidermal lipoxygenase-3
- TMS
- trimethylsilyl.
REFERENCES
- 1.Schneider C., Pratt D. A., Porter N. A., Brash A. R. (2007) Chem. Biol. 14, 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brash A. R., Yu Z., Boeglin W. E., Schneider C. (2007) FEBS J. 274, 3494–3502 [DOI] [PubMed] [Google Scholar]
- 3.Fürstenberger G., Epp N., Eckl K. M., Hennies H. C., Jørgensen C., Hallenborg P., Kristiansen K., Krieg P. (2007) Prostaglandins Other Lipid Mediat. 82, 128–134 [DOI] [PubMed] [Google Scholar]
- 4.Feussner I., Wasternack C. (2002) Annu. Rev. Plant Biol. 53, 275–297 [DOI] [PubMed] [Google Scholar]
- 5.Ford-Hutchinson A. W., Gresser M., Young R. N. (1994) Annu. Rev. Biochem. 63, 383–417 [DOI] [PubMed] [Google Scholar]
- 6.Schewe T., Rapoport S. M., Kühn H. (1986) Adv. Enzymol. 58, 191–272 [DOI] [PubMed] [Google Scholar]
- 7.Vliegenthart J. F. G., Veldink G. A. (1982) Free Radic. Biol. 5, 29–64 [Google Scholar]
- 8.Schilstra M. J., Veldink G. A., Vliegenthart J. F. G. (1994) Biochemistry 33, 3974–3979 [DOI] [PubMed] [Google Scholar]
- 9.Schilstra M. J., Veldink G. A., Verhagen J., Vliegenthart J. F. G. (1992) Biochemistry 31, 7692–7699 [DOI] [PubMed] [Google Scholar]
- 10.Schilstra M. J., Veldink G. A., Vliegenthart J. F. G. (1993) Biochemistry 32, 7686–7691 [DOI] [PubMed] [Google Scholar]
- 11.Haining J. L., Axelrod B. (1958) J. Biol. Chem. 232, 193–202 [PubMed] [Google Scholar]
- 12.de Groot J. J., Garssen G. J., Veldink G. A., Vliegenthart J. F. G., Boldingh J. (1975) FEBS Lett. 56, 50–54 [DOI] [PubMed] [Google Scholar]
- 13.Qian S. Y., Yue G. H., Tomer K. B., Mason R. P. (2003) Free Radic. Biol. Med. 34, 1017–1028 [DOI] [PubMed] [Google Scholar]
- 14.Yu Z., Schneider C., Boeglin W. E., Marnett L. J., Brash A. R. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9162–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y., Brash A. R. (2010) J. Biol. Chem. 285, 39866–39875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreou A. Z., Vanko M., Bezakova L., Feussner I. (2008) Phytochemistry 69, 1832–1837 [DOI] [PubMed] [Google Scholar]
- 17.Boeglin W. E., Itoh A., Zheng Y., Coffa G., Howe G. A., Brash A. R. (2008) Lipids 43, 979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffa G., Brash A. R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15579–15584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffa G., Imber A. N., Maguire B. C., Laxmikanthan G., Schneider C., Gaffney B. J., Brash A. R. (2005) J. Biol. Chem. 280, 38756–38766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meruvu S., Walther M., Ivanov I., Hammarström S., Fürstenberger G., Krieg P., Reddanna P., Kuhn H. (2005) J. Biol. Chem. 280, 36633–36641 [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y., Boeglin W. E., Schneider C., Brash A. R. (2008) J. Biol. Chem. 283, 5138–5147 [DOI] [PubMed] [Google Scholar]
- 22.Verhagen J., Veldink G. A., Vliegenthart J. F. G., Boldingh J. (1979) in Advances in the Biochemistry and Physiology of Plant Lipids (Appelqvist L., Liljenberg C. eds) pp. 231–236, Elsevier-North-Holland Biomedical Press, Amsterdam [Google Scholar]
- 23.Ivanov I., Saam J., Kuhn H., Holzhütter H. G. (2005) FEBS J. 272, 2523–2535 [DOI] [PubMed] [Google Scholar]
- 24.Brash A. R., Song W. (1996) Methods Enzymol. 272, 250–259 [DOI] [PubMed] [Google Scholar]
- 25.Gao B., Boeglin W. E., Zheng Y., Schneider C., Brash A. R. (2009) J. Biol. Chem. 284, 22087–22098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y., Brash A. R. (2010) J. Biol. Chem. 285, 13427–13436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boutaud O., Brash A. R. (1999) J. Biol. Chem. 274, 33764–33770 [DOI] [PubMed] [Google Scholar]
- 28.Kemal C., Louis-Flamberg P., Krupinski-Olsen R., Shorter A. L. (1987) Biochemistry 26, 7064–7072 [DOI] [PubMed] [Google Scholar]
- 29.Kühn H., Wiesner R., Stender H., Schewe T., Lankin V. Z., Nekrasov A., Rapoport S. M. (1986) FEBS Lett. 203, 247–252 [DOI] [PubMed] [Google Scholar]
- 30.Van Os C. P., Vliegenthart J. F. G., Crawford C. G., Gardner H. W. (1982) Biochim. Biophys. Acta 713, 173–176 [Google Scholar]
- 31.Garssen G. J., Vliegenthart J. F. G., Boldingh J. (1971) Biochem. J. 122, 327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garssen G. J., Vliegenthart J. F. G., Boldingh J. (1972) Biochem. J. 130, 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salzmann U., Kühn H., Schewe T., Rapoport S. M. (1984) Biochim. Biophys. Acta 795, 535–542 [DOI] [PubMed] [Google Scholar]
- 34.Verhagen J., Bouman A. A., Vliegenthart J. F. G., Boldingh J. (1976) Biochim. Biophys. Acta 486, 114–120 [DOI] [PubMed] [Google Scholar]
- 35.Gardner H. W., Weisleder D., Kleiman R. (1978) Lipids 13, 246–252 [Google Scholar]
- 36.O'Brien P. J. (1969) Can. J. Biochem. 47, 485–492 [DOI] [PubMed] [Google Scholar]
- 37.Gardner H. W. (1989) Free Radic. Biol. Med. 7, 65–86 [DOI] [PubMed] [Google Scholar]
- 38.Marnett L. J., Wilcox A. L. (1995) Biochem. Soc. Symp. 61, 65–72 [DOI] [PubMed] [Google Scholar]
- 39.Gardner H. W., Crawford C. G. (1981) Biochim. Biophys. Acta 665, 126–133 [DOI] [PubMed] [Google Scholar]
- 40.Gardner H. W., Jursinic P. A. (1981) Biochim. Biophys. Acta 665, 100–112 [DOI] [PubMed] [Google Scholar]
- 41.Gardner H. W., Kleiman R. (1981) Biochim. Biophys. Acta 665, 113–124 [DOI] [PubMed] [Google Scholar]
- 42.Reeder B. J., Wilson M. T. (1998) Biochem. J. 330, 1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dix T. A., Fontana R., Panthani A., Marnett L. J. (1985) J. Biol. Chem. 260, 5358–5365 [PubMed] [Google Scholar]
- 44.Nelson M. J. (1988) J. Am. Chem. Soc. 110, 2985–2986 [Google Scholar]
- 45.de Groot J. J., Garssen G. J., Vliegenthart J. F. G., Boldingh J. (1973) Biochim. Biophys. Acta 326, 279–284 [DOI] [PubMed] [Google Scholar]
- 46.de Groot J. J., Veldink G. A., Vliegenthart J. F. G., Boldingh J., Wever R., van Gelder B. F. (1975) Biochim. Biophys. Acta 377, 71–79 [DOI] [PubMed] [Google Scholar]
- 47.van der Heijdt L. M., van der Lecq F., Lachmansingh A., Versluis K., van der Kerk-van Hoof A., Veldink G. A., Vliegenthart J. F. G. (1993) Lipids 28, 779–782 [Google Scholar]
- 48.Neau D. B., Gilbert N. C., Bartlett S. G., Boeglin W., Brash A. R., Newcomer M. E. (2009) Biochemistry 48, 7906–7915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cristea M., Oliw E. H. (2006) J. Biol. Chem. 281, 17612–17623 [DOI] [PubMed] [Google Scholar]
- 50.Knapp M. J., Klinman J. P. (2003) Biochemistry 42, 11466–11475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.