Abstract
Members of the ATP-binding cassette superfamily couple the energy from ATP hydrolysis to the active transport of substrates across the membrane. The maltose transporter, a well characterized model system, consists of a periplasmic maltose-binding protein (MBP) and a multisubunit membrane transporter, MalFGK2. On the basis of the structure of the MBP-MalFGK2 complex in an outward-facing conformation (Oldham, M. L., Khare, D., Quiocho, F. A., Davidson, A. L., and Chen, J. (2007) Nature 450, 515–521), we identified two mutants in transmembrane domains MalF and MalG that generated futile cycling; although interaction with MBP stimulated the ATPase activity of the transporter, maltose was not transported. Both mutants appeared to disrupt the normal transfer of maltose from MBP to MalFGK2. In the first case, substitution of aspartate for glycine in the maltose-binding site of MalF likely generated a futile cycle by preventing maltose from binding to MalFGK2 during the catalytic cycle. In the second case, a four-residue deletion of a periplasmic loop of MalG limited its reach into the maltose-binding pocket of MBP, allowing maltose to remain associated with MBP during the catalytic cycle. Retention of maltose in the MBP binding site in the deletion mutant, as well as insertion of this loop into the binding site in the wild type, was detected by EPR as a change in mobility of a nitroxide spin label positioned near the maltose-binding pocket of MBP.
Keywords: ABC Transporter, Carbohydrate-binding Protein, Electron Paramagnetic Resonance (EPR), Membrane Proteins, Protein Conformation, Uncoupling
Introduction
The ATP-binding cassette (ABC)2 transporter protein family is one of the largest superfamilies; its members occupy almost 5% of the Escherichia coli genome (1). ABC proteins are implicated in many disease states, including cystic fibrosis (2), hyperinsulinemia (3), macular dystrophy (4), and multidrug resistance in cancer (5). In bacteria, ABC transporters are important virulence factors involved in nutrient uptake and in secretion of toxins and antimicrobial agents. ABC transporters can be divided into exporters and importers based on direction of transport and sequence similarities (6). One basic feature of this family is that they couple the energy from ATP to the transport of substrate across the membrane in bacteria and eukaryotic cells.
The E. coli maltose transporter (MalFGK2) (7, 8) is one of the most studied model systems in the family and has been crystallized in two different conformational states (9, 10). MalFGK2 forms a membrane-associated complex in which the MalF and MalG proteins make up the transmembrane domain, and two MalK proteins form a nucleotide (ATP)-binding domain dimer at the cytoplasmic surface of the membrane. A periplasmic maltose-binding protein (MBP) delivers maltose and maltodextrin substrates to MalFGK2 and stimulates the ATPase activity of the transporter. In a catalytic cycle, the maltose transporter cycles between two conformations (Fig. 1), an inward-facing MalFG conformation, representing the resting state, in which the maltose-binding site in MalF is exposed to the cytoplasm (9), and an outward-facing MalFG conformation, induced by binding of both ATP and MBP, representing the transition state, in which the maltose-binding site in MalF is exposed within an enclosed water-filled cavity that also contains the maltose-binding site of MBP (10). MBP is tightly bound and open in this conformation, and the MalK dimer is closed with two ATPs bound along the dimer interface (11, 12). Coupling of transport to hydrolysis is assumed to occur via this transition state-like intermediate, in which MBP is locked on top of the transporter, delivering maltose as it helps to stabilize the closed, catalytically active conformation of the MalK ATPase on the other face of the membrane (13).
FIGURE 1.
Model for maltose transport (modified from Ref. 12). In the resting state, the MalK dimer is open, and MalFG opens inward. Maltose binding induces closure of MBP, and closed MBP initiates transport and hydrolysis. In the catalytic transition state, the MalK dimer is closed, MalFG opens outward, and MBP is tightly bound in an open conformation. MBP stimulates ATPase activity by stabilizing this catalytic transition state for ATP hydrolysis. The transfer of maltose from MBP to the transmembrane binding site is highlighted by a rectangle, the details of which are shown in Fig. 2. Following ATP hydrolysis, transporter returns to the resting state, maltose is transported, and MBP is released.
MBP consists of two domains joined by a flexible hinge and binds maltose and maltodextrins with high affinity in a cleft between them, triggering a domain rotation that results in closure of the sugar-binding cleft around the substrate (Kd for maltose = 3.5 μm) (14). In the MBP-MalFGK2 system, a second transmembrane-located sugar-binding site was suggested by a study of MBP-independent MalFGK2 mutants that retain specificity for maltose, although they transport the substrate with much lower affinity (Km = 2 mm) (15). The existence of this site is confirmed in the structure of the outward-facing conformation, where maltose is bound to MalF at the base of the enclosed cavity formed by MalF, MalG, and MBP (10). One of the outstanding questions is what drives the substrate out of the high affinity MBP site and into the low affinity transmembrane MalF site. Although the opening of MBP that occurs during the catalytic cycle will likely decrease the affinity of the site in MBP, maltose still binds specifically to open MBP (16) and may not relocate to the membrane with each cycle of ATP hydrolysis. In the crystal structure, the third periplasmic loop of MalG (the P3 loop or “scoop” loop) is positioned in the maltose-binding site of MBP (10), suggesting that it may be a key element in the efficient transfer of maltose from one site to the other (Fig. 2). To test the importance of the scoop loop to the mechanism of translocation, four residues were deleted from this loop to minimize its reach into the binding pocket of MBP. In addition, residue 380 in the MalF binding site (10, 17) was mutated from glycine to aspartate to interfere with the binding of maltose to this site (Fig. 2). Remarkably, each mutation resulted in the uncoupling of maltose transport from MBP-stimulated ATPase activity, suggesting that both are key elements in the mechanism of coupling of transport to ATP hydrolysis. Mutations that lead to uncoupling of ATP hydrolysis and substrate transport have been reported in the yeast multidrug exporter Pdr5 (18, 19). Understanding the basis for uncoupling in these two disparate systems will increase our general knowledge of ABC transporter mechanism.
FIGURE 2.
Structure of the intact outward-facing MBP-MalFGK2 complex (Protein Data Bank code 2R6G) overlaid with the structure of open maltose-bound MBP (code 1JW5). Protein backbones are partially transparent to reveal the location of two maltose-binding sites in the cavity formed by MBP (magenta), MalF (blue), and MalG (yellow) in the outward-facing structure. The MalK dimer in the cytoplasm is not shown. Residues in MalF that bind maltose (orange spheres) are indicated as blue sticks. One of these residues, Gly-380 of MalF (black spheres), was mutated to Asp in an effort to interfere with maltose binding at this site. The position of maltose binding to the isolated open MBP (cyan) is indicated in space fill as black dots. Four residues (Asn-254–Asn-257) in the MalG scoop loop are indicated as yellow sticks. The position of the scoop loop in 2R6G is shown to overlay that of maltose in the 1JW5 structure. Five aromatic residues in MBP involved in maltose binding, which were mutated to Cys and modified with spin label, are shown as magenta sticks. This figure was prepared and the overlay performed in PyMOL (42) with root mean square deviation = 0.871 for the two MBPs.
EXPERIMENTAL PROCEDURES
Mutagenesis
The Stratagene QuikChange II XL site-directed mutagenesis kit was used to generate the following mutations: G380D in MalF; W62C, Y155C, W230C, W340C, and Y341C in MBP; and a deletion of Asn-254–Asn-257 in the scoop loop of MalG. The sequences of the mutant genes were confirmed by DNA sequencing.
Purification of Wild-type and Mutant MalFGK2 Transporters
The strain (HN741) and plasmids for transporter overexpression (pFG23, encoding MalF and MalG, and pKJ, encoding a variant of MalK with a C-terminal 10-amino acid extension (ASASHHHHHH)) have been described (20, 21). The MalFGK2 complex was purified essentially as described (22), except that the NaCl concentration was raised to 300 mm in the column equilibration buffer. The column was washed with equilibration buffer containing 5 mm imidazole, and the protein was eluted with the same buffer containing 150 mm imidazole.
Purification and Spin Labeling of MBP and Measurement of Binding Affinity
The strain BAR1000 (23) was used to overexpress MBP. MBP modified with a C-terminal histidine tag was purified essentially as described (12), except that proteins were mixed with nickel-Sepharose 6 Fast Flow resin (1 ml/liter of cells; GE Healthcare) in the presence of 2 mm dithiothreitol for 45 min. The resin was put in a column, washed with Buffer A (20 mm Tris-HCl (pH 8) and 150 mm NaCl), and eluted with Buffer A containing 100 mm imidazole. For spin labeling of single cysteine mutants, four separate aliquots of a 2-fold molar excess of 1-oxyl-2,2,5,5-tetramethyl-Δ3-pyrroline-3-methyl methanethiosulfonate spin label (Toronto Research Chemicals) were added from a 200 mm stock solution in acetonitrile, once every 15 min, and incubated at room temperature. Excess spin label was removed by dialysis against Buffer A. Protein concentrations were determined by the method of Schaffner and Weissmann (24). Spin labeling efficiency was calculated by comparison of spin label concentration, obtained by double integration of the EPR spectra, and protein concentration. Substrate binding was assessed by quenching of intrinsic fluorescence (25). Briefly, maltose and maltotriose concentrations varying from 0.3 μm to 3 mm were added to 0.25 μm spin-labeled MBP in Buffer A. Measurements were repeated in the presence of 0.3 mm dithiothreitol to remove the spin label from MBP. The Kd values were calculated by fitting data to the equation f = [L]free/(Kd + [L]free) using KaleidaGraph software (Version 3.6).
ATPase Assays
MalFGK2 was reconstituted into proteoliposomes for ATPase assays essentially as described previously (11). Briefly, 50 mg of l-α-phosphatidylcholine (Sigma) was dissolved and sonicated in 1 ml of 20 mm Tris-HCl (pH 8). 200 μl of sonicated lipids were mixed with 20 μl of 15% octyl β-d-glucopyranoside and, finally, 100 μg of transporter (typically 5 μl from a 20-mg/ml stock). Proteoliposomes were formed after a fast dilution of the equivalent of 5 μg of transporter directly into a final volume of 311 μl of the ATPase assay buffer (50 mm HEPES (pH 8), 10 mm MgCl2, 60 μg/ml pyruvate kinase, 32 μg/ml lactate dehydrogenase, 4 mm phosphoenolpyruvate, 0.3 mm NADH, 1.3 mm ATP, 0.3 mm maltose, and variable concentrations of MBP). Hydrolysis of ATP at 23 °C was monitored at 340 nm as the rate of conversion of NADH to NAD+ (26).
In Vitro [3H]Maltose Transport Assays
Proteoliposomes were formed by mixing 200 μl of lipids sonicated in 20 mm MES buffer at pH 6.2, 20 μl of 15% octyl β-d-glucopyranoside, and 100 μg of MalFGK2 and then diluting the mixture into 30 ml of 20 mm MES (pH 6.2) and 5 mm ATP. The proteoliposomes were collected by centrifugation at 100,000 × g for 1 h, resuspended in 4 ml of 20 mm MES (pH 6.2) and 0.25 mm MgCl2, and kept on ice until used. Unless indicated otherwise, 1 ml of proteoliposomes was mixed with MBP at a final concentration of 0.2 μm and [3H]maltose (111 μCi/μmol; American Radiolabeled Chemicals) at a final concentration of 10 μm. 190-μl aliquots were taken after 15, 30, 45, 60, and 75 s; filtered on 25-mm nitrocellulose mixed ester membranes (0.22-μm pore; GE Osmonics Labstore); and washed with 5 ml of 20 mm MES (pH 6.2). The membranes were then dried and counted in CytoScint-ES liquid scintillation fluid (MP Biomedicals). All values were corrected for background counts/min on membranes in the presence of MBP and maltose only (typically equal to 5% of total counts/min of the wild-type transporter).
EPR
X-band EPR spectra were recorded at room temperature on a Bruker EMXplus system fitted with an ER 4119HS cavity. Data were typically collected with a sweep width of 200 G at a microwave frequency of 9.86 GHz and a microwave power of 10 milliwatts and signal-averaged nine times. In each 30-μl sample, MBP at a final concentration of 80 μm was generally mixed with one or more of the following reagents at the indicated final concentrations: 0.1–3 mm maltose, 14 mm ATP, 0.5 mm EDTA, and 80 μm MalFGK2 (in 20 mm Tris-HCl with 10% glycerol, 300 mm NaCl, and 0.01% dodecyl maltoside). Changes in spin label mobility were approximated both empirically by examining shifts in the positions of the m−1 and m+1 peaks and by measuring the central line width (27). Decreases in spin label mobility result in broadening of the EPR line shape (28).
RESULTS
Uncoupling of Maltose Transport from ATPase Activity
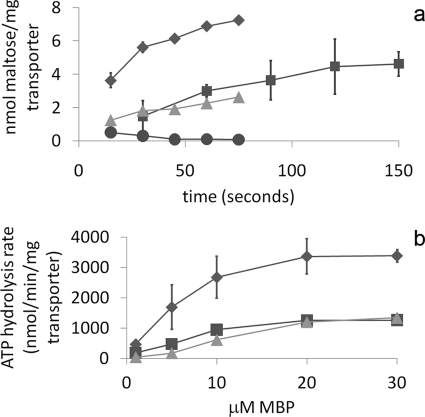
To test the importance of the scoop loop of MalG and the sugar-binding site in MalF to the transport mechanism, both maltose transport and ATPase activities were measured in mutant transporters either lacking Asn-254–Asn-257 in the scoop loop of MalG (MalG-Δscoop) or containing a G380D substitution in the transmembrane binding site (MalF-G380D). The G380D substitution was selected based on prior demonstration of a severe defect in both maltose and maltodextrin transport associated with this substitution that did not affect protein stability (17), as well on its critical location in the maltose-binding pocket of MalF (10). These mutant proteins were overproduced in the presence of the other subunits of the transporter, and the MalFGK2 complexes were purified and reconstituted into proteoliposomes for assay. The yield of purified mutant transporters was comparable with that of the wild-type transporter, typically 3–6 mg of protein/liter of cells. Both the scoop loop deletion and the G380D substitution eliminated maltose transport activity (Fig. 3a). Consistent with a lack of maltose transport activity, both mutants displayed a maltose-minus phenotype on maltose-MacConkey plates.
FIGURE 3.

Uncoupling of transport and ATP hydrolysis in the MalG-Δscoop and MalF-G380D mutants. a, [3H]maltose transport assays using wild-type MBP and proteoliposomes containing purified wild-type or mutant MalFGK2. Each value is the mean of three separate determinations, with S.D. shown as error bars (some smaller than symbols). ♦, wild-type MalFGK2; ■, MalG-Δscoop; ▴, MalF-G380D; ●, wild-type MalFGK2 without MBP. b, stimulation of ATPase activity by wild-type MBP in proteoliposomes containing purified wild-type or mutant MalFGK2. Each data point is the mean of at least four trials, with S.D. shown as error bars. ♦, wild-type MalFGK2; ■, MalG-Δscoop; ▴, MalF-G380D; ●, MalK-E159Q as a negative control.
Purified MalFGK2 reconstituted in proteoliposomes was also used to test the ability of MBP to stimulate the ATPase activity of wild-type and mutant transporters. A transporter containing the MalK-E159Q mutation (10), defective in ATP hydrolysis, was used as a negative control. Like the wild-type transporter, both the MalG-Δscoop and MalF-G380D mutants were able to hydrolyze ATP, and the activities were dependent on the presence of MBP (Fig. 3b). In the presence of 30 μm MBP, both mutants retained ∼50% of the activity compared with the wild type.
The observation that MBP stimulated the ATPase activity of both mutants despite the lack of transport activity argues that the mutant transporters undergo conformational changes similar to those of the wild-type protein, in which the transporter reorients in the presence of MBP and ATP with MBP opening as MalK closes to hydrolyze ATP. To test this hypothesis, we employed a method previously developed in our laboratory to monitor the opening of MBP when in complex with MalFGK2 (12). In this approach, nitroxide spin labels are attached to both lobes of MBP (at positions 41 and 211), and the distance between them is determined from dipolar broadening of the EPR spectra. When MBP is closed with maltose bound, spin labels are 8 Å apart. The addition of MalFGK2, ATP, and EDTA (to prevent ATP hydrolysis) triggers formation of the outward-facing intermediate, MBP opens, and spin-spin interactions are lost, indicative of distances >25 Å between spins (12). Similar transformations occurred using both the MalG-Δscoop and MalF-G380D transporters, suggesting that neither mutation prevents formation of the outward-facing transition state-like conformation (supplemental Fig. S1).
Functional Analysis of Spin-labeled MBP Mutants
In the crystal structure of the outward-facing MBP-MalFGK2 (MalK-E159Q) complex, maltose is present only in the MalF binding site and not in the MBP binding site (Ref. 10; see also Fig. 2). To test whether maltose migrated from the MBP binding pocket to the MalF binding site when the scoop loop was deleted, we attempted to place spin labels on MBP that would experience mobility changes upon maltose binding. In preliminary experiments, five aromatic residues, Trp-62, Tyr-155, Trp-230, Trp-340, and Tyr-341 (Fig. 2), involved in the binding of maltose to MBP (14), were mutated to cysteine, spin-labeled, and tested for function in maltose binding. The rationale for selection of these residues was that the spin label (SL) is roughly the size of a tryptophan residue and might be accommodated in place of an aromatic residue. As shown in Table 1, two of the single cysteine-substituted, spin-labeled proteins retained the ability to bind maltose, the W62C-SL and Y341C-SL mutant MBPs. Trp-62 is normally involved in minor ring stacking and hydrogen bond interactions with the first two glucose moieties at the reducing end of a maltodextrin (14), and removal of the tryptophan appears to explain the reduced affinity of this spin-labeled mutant for maltose (Kd = 141 μm) relative to the wild type (Kd = 3 μm). Maltotriose binds with higher affinity than maltose to this mutant with or without SL present. Tyr-341 interacts with the third glucose moiety from the reducing end (14), and this spin-labeled mutant displayed a binding affinity comparable with that of the wild type for maltose substrates, both with and without SL attached (Table 1). The affinity of the Y341C mutant for maltotriose was reduced by ∼10-fold with or without SL attached.
TABLE 1.
Substrate binding affinity of MBP mutants and efficiency of labeling with SL
| Labeling | Kd for maltose with SL | Kd for maltotriose with SL | Kd for maltose without SL | Kd for maltotriose without SL | |
|---|---|---|---|---|---|
| % | |||||
| Wild-type MBP | NAa | NA | NA | 3 μmb | 0.16 μmc |
| W62C-SL | 123 | 84 μm | 1.6 μm | 141 μm | 1.2 μm |
| Y155C-SL | 87 | >3 mm | >3 mm | >3 mm | 50 μm |
| W230C-SL | 90 | >3 mm | >3 mm | 17 μm | 9 μm |
| W340C-SL | 68 | >3 mm | >3 mm | >3 mm | >3 mm |
| Y341C-SL | 87 | 1.4 μm | 1.0 μm | 1.3 μm | 1.8 μm |
Both W62C-SL and Y341C-SL retained good function in maltose transport and MBP-stimulated ATPase activities (Fig. 4, a and b). In proteoliposomes containing wild-type MalFGK2, both spin-labeled MBPs catalyzed maltose uptake and stimulated ATP hydrolysis at rates of ∼30% of the wild type (Fig. 4, a and b). Because the W62C-SL mutant had a lower affinity for maltose, the concentration of [3H]maltose in the transport assay was raised from 10 to 100 μm, and the time course of the assay was extended compared with that of the normal assay. The efficiency with which the spin-labeled MBPs were able to stimulate ATP hydrolysis was consistent with the idea that ligand binding triggered closure of the two lobes of MBP, as it does in the wild type (29). High rates of MBP-stimulated ATPase are seen in proteoliposomes only in the presence of maltose (8, 30, 31), although low rates of maltose-free MBP-stimulated ATPase have been reported (8, 30), accounting for the ability of both maltose-bound and maltose-free MBPs to stabilize the outward-facing conformation of the transporter in the presence of ATP (11, 12). The failure of these spin-labeled mutants to function at the same rate as the wild type may result from their location at the interface between MBP and MalFGK2. In the outward-facing conformation, Tyr-341 contacts residues 460 and 473 of MalF, whereas Trp-62 contacts residue 255 in the scoop loop of MalG. Modest decreases in MBP-stimulated ATPase, such as seen here, might be caused by a decrease in affinity between MBP and the transporter, a decrease in the rate of a rate-limiting conformational change of the transporter, or a destabilization of the transition state for ATP hydrolysis. Although the ability of 30 μm Y341C-SL MBP to stimulate ATP hydrolysis was unchanged following removal of SL by DTT, the rate of MBP-stimulated ATPase doubled upon removal of SL from the W62C substitution in MBP (supplemental Fig. S2).
FIGURE 4.
In vitro functional assays of spin-labeled MBPs using proteoliposomes containing purified wild-type MalFGK2. The wild-type data sets are the same as in Fig. 3. a, [3H]maltose transport. Each value is the mean of three separate determinations with S.D. shown as error bars. ♦, wild-type MBP; ■, W62C-SL; ▴, Y341C-SL; ●, no MBP. b, MBP-stimulated ATPase activity. Each data point is the mean of at least two trials that varied by <15%. ♦, wild-type MBP; ■, W62C-SL; ▴, Y341C-SL.
Using W62C-SL to Probe the Maltose-binding Pocket of MBP
In Fig. 5, changes in mobility of spin labels attached to MBP were examined as a function of the addition of maltose or maltotriose, MalFGK2, or MalFGK2 plus ATP/EDTA, the latter of which triggers a conformational change to the outward-facing, transition state-like conformation shown in Figs. 1 and 2 in both the presence and absence of maltose (11). These experiments were designed to judge whether maltose was present or absent in the MBP binding site in the transition state conformation of the MalG-Δscoop mutant. In Fig. 5a, the addition of saturating maltose (3 mm) or maltotriose (3 mm) greatly reduced the mobility of the spin label at W62C when MBP alone was present. This decrease in mobility is manifest as an increase in the central peak width (supplemental Table S1) and an increase in the distance between the outer peaks (27, 28). The immobilization of spin label seen in the presence of maltose and maltotriose likely reflects the binding of maltose and/or the closure of the two lobes of MBP that occurs upon ligand binding (29). For reference, the effect of the addition of 40% glycerol to increase viscosity on side chain mobility and/or tumbling of MBP is also shown. W62C-SL remained immobilized upon addition of wild-type or MalG-Δscoop MalFGK2 (Fig. 5b), consistent with our earlier observation that the transporter does not trigger opening of MBP in the absence of nucleotide even though MBP does bind to the inward-facing transporter (12).
FIGURE 5.
EPR spectra using SL at W62C and Y341C in MBP as a maltose or scoop loop sensor. EPR spectra were normalized to the same amount of spin, colored and numbered as indicated. Only the central 100 G of the spectra are shown. a, spectra of W62C-SL MBP alone (trace 1), with maltose (trace 2), with maltotriose (trace 3), and in glycerol (trace 4) are overlaid. b, spectra of W62C-SL MBP plus maltose (trace 2) and with wild-type MalFGK2 (trace 5) and MalG-Δscoop MalFGK2 (trace 6). c, spectra of W62C-SL plus MalG-Δscoop with maltose (trace 6) and with ATP (trace 7) and W62C-SL in glycerol (trace 4). d, spectra of W62C-SL plus wild-type MalFGK2 with maltose (trace 5), with ATP (trace 8), and with both maltose and ATP (trace 9). e, spectra of W62C-SL plus MalG-Δscoop in the absence (trace 7) and presence of maltose or maltotriose (traces 10–12). f, spectra of Y341C-SL MBP alone (trace 13), with maltose (trace 14), with maltotriose (trace 15), and in glycerol (trace 16). g, spectra of Y341C-SL MBP trapped by vanadate (Vi) in complex with wild-type MalFGK2 in the absence and presence of maltotriose (traces 17 and 18). h, spectra of Y341C-SL MBP trapped by vanadate in complex with MalG-Δscoop in the absence and presence of maltotriose (traces 19 and 20).
The addition of ATP and EDTA (to trigger the outward-facing conformation) to the MBP-MalFGK2 system in the absence of maltose (11, 12) increased the mobility of W62C-SL in the MalG-Δscoop system (Fig. 5c) but decreased it in the wild type (Fig. 5d) compared with the spectra with maltose and MalFGK2 present (see also supplemental Table S1). Because both the wild type and Δscoop mutants open MBP under these conditions (supplemental Fig. S1), the decreased mobility seen in the wild type compared with MalG-Δscoop suggested that the insertion of the scoop loop into the maltose-binding pocket of MBP upon reorientation of transmembrane helices and the opening of MBP may be responsible for the immobilization of W62C-SL seen in Fig. 5d. In the MalG-Δscoop mutant, the spectral shape was similar to that seen for unliganded (open) MBP in 40% glycerol (Fig. 5c). Based on the crystal structure of MBP-MalFGK2 (Ref. 10; see Fig. 2), maltose should be absent from the MBP binding site in the wild-type transporter in the outward-facing conformation, and the spectra were essentially unchanged in the presence of ATP/EDTA whether maltose was absent or present (Fig. 5d).
Fig. 5e shows a comparison of the spectra of W62C-SL MBP in the absence and presence of maltose when MBP was in complex with the MalG-Δscoop transporter in the presence of ATP and EDTA. In contrast to the wild-type transporter, there were clear decreases in mobility when maltose was present. These results suggested that, in the absence of the scoop loop, maltose occupied the maltose-binding site in MBP in the outward-facing MBP-MalFGK2 complex. This experiment was performed both at stoichiometric concentrations of maltose (100 versus 80 μm protein) and at higher concentrations (400 μm) with similar results, suggesting that maltose was not transferred to any significant extent from MBP to MalF in the absence of the scoop loop. The use of maltotriose, which saturated spin-labeled W62C MBP at 100 μm (Table 1), gave similar results. If the transporters were locked in the outward-facing conformation through the use of MgATP and vanadate, a transition state analog that substitutes for the γ-phosphate of ATP (13), the spectra were superimposable to those with ATP and EDTA (data not shown).
Using Y341C-SL to Probe the Maltose-binding Pocket of MBP
In contrast to W62C-SL MBP, Y341C-SL MBP bound to both maltose and maltotriose with high affinity (Table 1). However, only maltotriose triggered a mobility change upon binding (Fig. 5f and supplemental Table S1), possibly because Tyr-341 interacts only with the third glucose moiety of a maltodextrin (14). The immobilization due to maltotriose binding was less substantial than that caused by increased viscosity due to the addition of glycerol to MBP (Fig. 5f). When the transporter was locked into the outward-facing conformation through vanadate trapping (13), SL interacting with the wild type was more immobile than SL interacting with the MalG-Δscoop transporter, suggesting that the presence or absence of the scoop loop in the maltose-binding pocket of MBP was sensed by SL at Tyr-341 in the absence of maltose (Fig. 5, compare trace 17 in g and trace 19 in h). The presence of maltotriose in the experiments with the MalG-Δscoop transporter again resulted in a decrease in the mobility of Y341C-SL, suggesting that sugar remained in the MBP binding pocket when the scoop loop was absent (Fig. 5h).
DISCUSSION
Partial deletion of the third periplasmic loop (the P3 loop or scoop loop) of MalG eliminated maltose transport without dramatically altering the ATPase activity of the transporter, creating an uncoupled phenotype. In the wild type, ATP hydrolysis is stimulated by interaction of the MalFGK2 transporter with periplasmic MBP, triggering a set of concerted conformational changes that result in the closure and activation of the nucleotide-binding sites in the MalK dimer. As part of this change, the transmembrane helices reorient from inward-facing to outward-facing to receive maltose from MBP, which has progressed from a closed liganded conformation that is only loosely associated with the transporter (Kd = 10–30 μm) (32) to an open conformation that is tightly associated (12, 13). Crystal structures of isolated MBP reveal that maltose is bound by residues from both lobes in the closed conformation, whereas maltose binds only to the C-terminal lobe in the open conformation, primarily through hydrophobic interactions (16). In the outward-facing structure of the MBP-MalFGK2 complex, the maltose-binding site in MBP is exposed to the maltose-binding site in MalF within an enclosed cavity (10), and residues in the scoop loop partially overlap the position where maltose is bound in the structure of open MBP (Fig. 2) (16). In transporters lacking the scoop loop, the concerted conformational changes needed for stimulation of ATP hydrolysis appeared to be intact; however, they were not sufficient for transport activity. Maltose was retained in the binding pocket of MBP in the MalG-Δscoop mutant, indicating that insertion of this loop into the MBP binding pocket in the wild type played an active role in the transport process.
From the structure of the outward-facing conformation (10), the volume of the cavity formed between MBP, MalF, and MalG is calculated to be ∼6500 Å3, and the effective concentration of one maltose released into this cavity would be ∼260 mm. Hence, it is not surprising that maltose may not be transferred from MBP to MalF without the assistance of the scoop loop even though opening of MBP would reduce the affinity of MBP for maltose in the transition state. With the scoop loop blocking the rebinding of maltose to MBP, the enclosed nature and small size of this cavity virtually ensure that maltose will bind the second site in MalF, which has an estimated affinity of 1 to 2 mm for maltose (15). Comparison of the inward- and outward-facing structures (9) suggests that a periplasmic gate closes just above the maltose-binding site in MalF following ATP hydrolysis as a channel to the cytoplasm opens, ensuring that bound maltose is released inside the cell.
Mutation of glycine 380 in the maltose-binding site of MalF to aspartate (MalF-G380D) also caused an uncoupled phenotype, eliminating maltose transport while causing only a 2-fold reduction in MBP stimulation of ATPase at 30 μm MBP. These results indicated that the transporter was still able to undergo cycles of conformational change and that binding of maltose to the MalF site was not required for ATP hydrolysis to occur. Although it is difficult to predict how a mutation at this site would slow the rate of ATP hydrolysis by 50%, one possibility is that the presence of unbound maltose in the cavity or a charge in the transmembrane region impedes the rate of reorientation of transmembrane helices and closure of the periplasmic gate following ATP hydrolysis. The structure of the maltose transporter in complex with maltose (10) suggested to us that substitution at this position would block binding of maltose to this binding site, although we were not successful in positioning a spin label able to sense the presence or absence of maltose in this site. The data suggested that maltose must bind to this site to be transported, even though one might imagine that insertion of the scoop loop into the MBP binding site might have been sufficient for translocation. The absence of any maltose transport raises the possibility that, in the absence of binding to MalF, a back-reaction, in which maltose returns to MBP following ATP hydrolysis and withdrawal of the scoop loop, is substantially more favorable than one in which it is able to pass through the transporter to the opposite side of the membrane.
A recent study by Gould and Shilton (33) also discusses the importance of the scoop loop in energetic coupling of transport to hydrolysis. On the basis of the observation that mutations in MBP designed to accommodate sucrose in the binding pocket severely interfere with MBP-stimulated ATPase activity, they suggest that the substitutions disrupt interactions between MBP and the scoop loop that are critical for promotion of ATP hydrolysis. In light of our result that the scoop loop deletion retains high levels of MBP-stimulated ATPase (50% of the wild type), we would suggest instead that, although the normal contacts between MBP and the scoop loop may contribute modestly to the stability of the transition state, the mutations of MBP in the cited study may be impeding the normal motion of the scoop loop within the binding pocket, either interfering with the precise formation of the transition state or slowing the rate of conformational changes associated with the formation and/or breakdown of the transition state. The authors comment that the D14L substitution in MBP clashes with the position of Asn-254 in the MalG scoop loop in the outward-facing, transition state-like structure (33). Although the scoop loop is on the opposite side of the membrane from the active site for ATP hydrolysis, achievement of the catalytic transition state for ATP hydrolysis may require the full extension of the scoop loop into the binding pocket to complete the global conformational change involved in MalK closure. Such a mechanism would ensure that maltose leaves the binding site on MBP before ATP hydrolysis can occur.
It is not known whether a structural feature similar to the MalG scoop loop is essential for translocation in other ABC transporters. In the structure of the binding protein-dependent vitamin B12 transporter, periplasmic loops of the transmembrane BtuC protein are inserted into the empty B12-binding cleft of the periplasmic B12-binding protein (34). In ABC proteins involved in efflux, formation of the presumed outward-facing conformation is frequently associated with a loss of high affinity binding for drug substrates (Refs. 35 and 36; see Ref. 37). In the absence of detailed information on substrate binding to efflux pumps, it is not possible to determine whether conformational changes in the transmembrane domain simply reduce affinity, allowing for dissociation by mass action, or actively expel the drugs from the binding site.
In the maltose system, three major steps are involved in the transfer of substrate across the membrane: maltose spontaneously binds to MBP, then transfers from the high affinity site in MBP to the low affinity site in MalF, and finally transfers from the site in MalF into the cell. ATP binding and hydrolysis are associated with the closure of the nucleotide-binding domain MalK and reorientation of transmembrane helices that drive the scoop loop into the maltose-binding cleft of MBP, disrupting the binding site. In contrast, the components of the MalF binding site appear to undergo a rigid-body rotation during reorientation, leaving the binding site essentially unchanged except for its exposure to alternating sides of the membrane (9), so maltose may simply dissociate due to mass action.
The ability to trap the maltose transporter in a transition state-like conformation (13, 22) and the availability of high resolution structures (9, 10) have allowed us to propose a detailed model to explain the uncoupling of transport from ATP hydrolysis in this importer. Mutations that lead to uncoupling of ATP hydrolysis and substrate transport have also been reported in the yeast multidrug exporter Pdr5 (18, 19). Although structures of exporters with substrates bound are so far lacking, making it impossible to understand the molecular basis of uncoupling in the drug efflux pumps, this study offers insights that will ultimately help to unravel the mechanisms of coupling in many different types of ABC systems. In addition, carbohydrate transport and metabolism are vital in some models of microbial pathogenesis (38). The E. coli MBP-MalFGK2 homologs in Streptococcus pneumoniae, group A streptococci, and group B streptococci have been implicated as virulence factors (39–41). The MBP-scoop loop interaction can clearly serve as a unique extracellular drug target in these systems.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants GM49261 and GM070515. This work was also supported by a Purdue Research Foundation research grant.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Table S1.
- ABC
- ATP-binding cassette
- MBP
- maltose-binding protein
- SL
- spin label.
REFERENCES
- 1.Linton K. J., Higgins C. F. (1998) Mol. Microbiol. 28, 5–13 [DOI] [PubMed] [Google Scholar]
- 2.Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. (1990) Nature 347, 358–363 [DOI] [PubMed] [Google Scholar]
- 3.Thomas P. M., Cote G. J., Wohllk N., Haddad B., Mathew P. M., Rabl W., Aguilar-Bryan L., Gagel R. F., Bryan J. (1995) Science 268, 426–429 [DOI] [PubMed] [Google Scholar]
- 4.Allikmets R., Singh N., Sun H., Shroyer N. F., Hutchinson A., Chidambaram A., Gerrard B., Baird L., Stauffer D., Peiffer A., Rattner A., Smallwood P., Li Y., Anderson K. L., Lewis R. A., Nathans J., Leppert M., Dean M., Lupski J. R. (1997) Nat. Genet. 15, 236–246 [DOI] [PubMed] [Google Scholar]
- 5.Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. (1986) Cell 47, 381–389 [DOI] [PubMed] [Google Scholar]
- 6.Davidson A. L., Dassa E., Orelle C., Chen J. (2008) Microbiol. Mol. Biol. Rev. 72, 317–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean D. A., Davidson A. L., Nikaido H. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 9134–9138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson A. L., Shuman H. A., Nikaido H. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 2360–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khare D., Oldham M. L., Orelle C., Davidson A. L., Chen J. (2009) Mol. Cell 33, 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oldham M. L., Khare D., Quiocho F. A., Davidson A. L., Chen J. (2007) Nature 450, 515–521 [DOI] [PubMed] [Google Scholar]
- 11.Orelle C., Ayvaz T., Everly R. M., Klug C. S., Davidson A. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austermuhle M. I., Hall J. A., Klug C. S., Davidson A. L. (2004) J. Biol. Chem. 279, 28243–28250 [DOI] [PubMed] [Google Scholar]
- 13.Chen J., Sharma S., Quiocho F. A., Davidson A. L. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1525–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quiocho F. A., Spurlino J. C., Rodseth L. E. (1997) Structure 5, 997–1015 [DOI] [PubMed] [Google Scholar]
- 15.Treptow N. A., Shuman H. A. (1985) J. Bacteriol. 163, 654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan X., Quiocho F. A. (2002) Biochemistry 41, 706–712 [DOI] [PubMed] [Google Scholar]
- 17.Ehrle R., Pick C., Ulrich R., Hofmann E., Ehrmann M. (1996) J. Bacteriol. 178, 2255–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst R., Kueppers P., Klein C. M., Schwarzmueller T., Kuchler K., Schmitt L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5069–5074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauna Z. E., Bohn S. S., Rutledge R., Dougherty M. P., Cronin S., May L., Xia D., Ambudkar S. V., Golin J. (2008) J. Biol. Chem. 283, 35010–35022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson A. L., Nikaido H. (1990) J. Biol. Chem. 265, 4254–4260 [PubMed] [Google Scholar]
- 21.Chen J., Lu G., Lin J., Davidson A. L., Quiocho F. A. (2003) Mol. Cell 12, 651–661 [DOI] [PubMed] [Google Scholar]
- 22.Sharma S., Davidson A. L. (2000) J. Bacteriol. 182, 6570–6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fikes J. D., Bassford P. J., Jr. (1987) J. Bacteriol. 169, 2352–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaffner W., Weissmann C. (1973) Anal. Biochem. 56, 502–514 [DOI] [PubMed] [Google Scholar]
- 25.Martineau P., Szmelcman S., Spurlino J. C., Quiocho F. A., Hofnung M. (1990) J. Mol. Biol. 214, 337–352 [DOI] [PubMed] [Google Scholar]
- 26.Scharschmidt B. F., Keeffe E. B., Blankenship N. M., Ockner R. K. (1979) J. Lab. Clin. Med. 93, 790–799 [PubMed] [Google Scholar]
- 27.Mchaourab H. S., Lietzow M. A., Hideg K., Hubbell W. L. (1996) Biochemistry 35, 7692–7704 [DOI] [PubMed] [Google Scholar]
- 28.Knowles P. F., Marsh D., Rattle H. W. E. (1972) Magnetic Resonance of Biomolecules: An Introduction to the Theory and Practice of NMR and ESR in Biological Systems, John Wiley & Sons, London [Google Scholar]
- 29.Sharff A. J., Rodseth L. E., Spurlino J. C., Quiocho F. A. (1992) Biochemistry 31, 10657–10663 [DOI] [PubMed] [Google Scholar]
- 30.Gould A. D., Telmer P. G., Shilton B. H. (2009) Biochemistry 48, 8051–8061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall J. A., Ganesan A. K., Chen J., Nikaido H. (1997) J. Biol. Chem. 272, 17615–17622 [DOI] [PubMed] [Google Scholar]
- 32.Jacso T., Grote M., Daus M. L., Schmieder P., Keller S., Schneider E., Reif B. (2009) Biochemistry 48, 2216–2225 [DOI] [PubMed] [Google Scholar]
- 33.Gould A. D., Shilton B. H. (2010) J. Biol. Chem. 285, 11290–11296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hvorup R. N., Goetz B. A., Niederer M., Hollenstein K., Perozo E., Locher K. P. (2007) Science 317, 1387–1390 [DOI] [PubMed] [Google Scholar]
- 35.van Veen H. W., Margolles A., Müller M., Higgins C. F., Konings W. N. (2000) EMBO J. 19, 2503–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramachandra M., Ambudkar S. V., Chen D., Hrycyna C. A., Dey S., Gottesman M. M., Pastan I. (1998) Biochemistry 37, 5010–5019 [DOI] [PubMed] [Google Scholar]
- 37.Qu Q., Chu J. W., Sharom F. J. (2003) Biochemistry 42, 1345–1353 [DOI] [PubMed] [Google Scholar]
- 38.Shelburne S. A., Davenport M. T., Keith D. B., Musser J. M. (2008) Trends Microbiol. 16, 318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones A. L., Knoll K. M., Rubens C. E. (2000) Mol. Microbiol. 37, 1444–1455 [DOI] [PubMed] [Google Scholar]
- 40.Shelburne S. A., 3rd, Sumby P., Sitkiewicz I., Okorafor N., Granville C., Patel P., Voyich J., Hull R., DeLeo F. R., Musser J. M. (2006) Infect. Immun. 74, 4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hava D. L., Camilli A. (2002) Mol. Microbiol. 45, 1389–1406 [PMC free article] [PubMed] [Google Scholar]
- 42.DeLano W. L. (2010) The PyMOL Molecular Graphics System, Version 1.3r1, Schrödinger, LLC [Google Scholar]
- 43.Szmelcman S., Schwartz M., Silhavy T. J., Boos W. (1976) Eur. J. Biochem. 65, 13–19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.