Abstract
The calcium-regulated phosphatase calcineurin intersects with both calcium and cAMP-mediated signaling pathways in the pancreatic β-cell. Pharmacologic calcineurin inhibition, necessary to prevent rejection in the setting of organ transplantation, is associated with post-transplant β-cell failure. We sought to determine the effect of calcineurin inhibition on β-cell replication and survival in rodents and in isolated human islets. Further, we assessed whether the GLP-1 receptor agonist and cAMP stimulus, exendin-4 (Ex-4), could rescue β-cell replication and survival following calcineurin inhibition. Following treatment with the calcineurin inhibitor tacrolimus, human β-cell apoptosis was significantly increased. Although we detected no human β-cell replication, tacrolimus significantly decreased rodent β-cell replication. Ex-4 nearly normalized both human β-cell survival and rodent β-cell replication when co-administered with tacrolimus. We found that tacrolimus decreased Akt phosphorylation, suggesting that calcineurin could regulate replication and survival via the PI3K/Akt pathway. We identify insulin receptor substrate-2 (Irs2), a known cAMP-responsive element-binding protein target and upstream regulator of the PI3K/Akt pathway, as a novel calcineurin target in β-cells. Irs2 mRNA and protein are decreased by calcineurin inhibition in both rodent and human islets. The effect of calcineurin on Irs2 expression is mediated at least in part through the nuclear factor of activated T-cells (NFAT), as NFAT occupied the Irs2 promoter in a calcineurin-sensitive manner. Ex-4 restored Irs2 expression in tacrolimus-treated rodent and human islets nearly to baseline. These findings reveal calcineurin as a regulator of human β-cell survival in part through regulation of Irs2, with implications for the pathogenesis and treatment of diabetes following organ transplantation.
Keywords: Apoptosis, Calcineurin, Diabetes, Insulin, Pancreatic Islet
Introduction
New onset diabetes mellitus is a major complication following solid organ transplantation, often leading to decreased graft survival and increased mortality (1–3). As with other forms of diabetes, hyperglycemia ensues when there is inadequate pancreatic β-cell mass to meet insulin demand (4). Post-transplant diabetes is strongly associated with the use of calcineurin inhibitors, antirejection medications that are widely used in clinical solid organ transplantation (5). This association has prompted long-standing speculation that the calcineurin inhibitors are β-cell toxic and pathogenic in transplant-related β-cell failure.
Calcineurin is a calcium-activated cytosolic phosphatase that is critical for antigen-stimulated T lymphocyte activation (6). Therefore, pharmacologic calcineurin inhibition is highly effective in preventing allograft rejection. However, calcineurin is also expressed in β-cells where it has two well described molecular targets, the nuclear factor of activated T cell (NFAT)2 family of transcription factors (7), and the cAMP-responsive element-binding protein (CREB) transcriptional co-activator, transducer of regulated CREB activity-2 (TORC2) (8). Through dephosphorylation-mediated nuclear localization of these targets, calcineurin integrates calcium and cAMP signals generated by physiologic stimuli, such as hyperglycemia and incretin receptor activation, to alter gene expression (7–9).
A role for calcineurin in β-cell growth and function was recently demonstrated in a murine model of β-cell specific calcineurin deletion. These mice displayed a phenotype of age-dependent diabetes and decreased β-cell proliferation in association with diminished expression of numerous genes known to be critical for β-cell function and proliferation (10). These and other data indicating a role for calcineurin in cell cycle regulation (11) suggested that calcineurin inhibitors might contribute to transplant-related β-cell failure by blunting β-cell proliferation (4). Indeed, Dor and co-workers recently observed that the simultaneous administration of a calcineurin inhibitor and the mammalian target of rapamycin inhibitor, rapamycin, impaired β-cell regeneration during recovery from diphtheria toxin-induced β-cell death (12). Conversely, calcineurin inhibition has also recently been suggested to play a beneficial role in β-cell survival after treatment with cytokines (13) and corticosteroids (14).
The GLP-1 receptor agonist exendin-4 (Ex-4) improves rodent β-cell replication and survival in multiple models (15). GLP-1 receptor activation has been implicated in increasing rodent β-cell replication through multiple trophic pathways, including MAP kinase (16) and downstream of the epidermal growth factor receptor (EGFR) (17), but many of its effects have been associated with the activation of CREB. Ex-4-mediated increases in cAMP lead to activation of protein kinase A followed by phosphorylation and activation of CREB to influence gene expression (18). The ability of Ex-4 to stimulate insulin gene transcription appears to involve the action of calcineurin (9), but the exact mechanism underlying these interactions, as well as the relevance to regulation of β-cell replication and survival, is unknown.
Here, we find that calcineurin inhibition markedly induces apoptosis of human islet β-cells and reduces β-cell replication in rodent islets. Co-administration of Ex-4 partially rescues these defects. Consistent with changes in proliferation and apoptosis, calcineurin inhibition decreases Akt phosphorylation. We identify Irs2 as a novel target of calcineurin whose promoter is directly occupied by NFAT in a calcineurin-sensitive manner. Further, the downstream targets of Akt, the D-cyclins, are reduced in rodent islets following calcineurin inhibition. These findings have significant implications for the pathogenesis and treatment of transplant related β-cell failure.
EXPERIMENTAL PROCEDURES
Human Islets
Human islets were supplied by the University of Pennsylvania Human Islet Isolation Laboratory. Islets were cultured for 4 days in CMRL-1066 Supplemented medium prior to the addition of tacrolimus (Tecoland, Edison, NJ) or vehicle (DMSO) as indicated. Six hours later, islets were washed once with phosphate-buffered saline (PBS) and lysed with TRIzol reagent (Invitrogen) or radioimmune precipitation assay buffer containing protease and phosphatase inhibitors (Calbiochem) for RNA and protein isolation, respectively. For morphometric analysis of replication and survival, human islets were treated with vehicle (DMSO), 10 nm Ex-4 (Bachem Bioscience; King of Prussia, PA), and/or 10 ng/ml tacrolimus for 48 h. For the final 24 h of treatment, BrdU (10 μm; Sigma) was added to the cells prior to harvest for analysis.
Islet Culture
Rat islets were isolated from 300–325-g male Sprague-Dawley rats (Charles River; Wilmington, MA) as described previously (19). Rat islet tissue culture media contained RPMI 1640 medium with l-glutamine, 11 mm glucose, 1% heat-inactivated fetal bovine serum, 10 mm HEPES, 100 units/ml penicillin, 100 mg/ml streptomycin, and 1% anti-mycotic solution (Invitrogen). After overnight culture following isolation (16 h), islets were treated with vehicles (PBS + DMSO), Ex-4 + DMSO, tacrolimus + PBS, or tacrolimus + Ex-4 for 3 and 6 h. At both time points, islets were washed once in PBS and lysed with TRIzol for RNA isolation.
Tissue Culture
INS-1 rat insulinoma cells (clone 832/13) were maintained in growth medium as described (20). Cells were washed once with PBS and placed into defined medium (21) (RPMI 1640 medium without serum, but with 3 mm glucose, 0.1% fatty acid-free bovine serum albumin (Equitech-Bio, Inc., Kerrville, TX), 10 mm HEPES, 1 mm sodium pyruvate, 100 units/ml penicillin, 100 mg/ml streptomycin, and 50 μm β-mercaptoethanol). After overnight incubation, tacrolimus or vehicle (100% ethanol) was added, and 30 min later, glucose, Ex-4, forskolin, or vehicle (PBS) were added. Six hours later, cells were washed once with PBS and lysed with either TRIzol or radioimmune precipitation assay buffer for RNA and protein isolation, respectively.
Animals
Ten- to 12-week-old male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), housed under standard conditions, and allowed free access to food and water unless fasted prior to testing. 0.8 mg/ml BrdU was placed in the drinking water of all mice as indicated under “Results.” Tacrolimus was dissolved in 100% ethanol and administered at the doses indicated in a final solution of 0.9% sodium chloride/5% ethanol by intraperitoneal injection. Ex-4 was dissolved in 0.9% saline/1% BSA solution and administered via an Alzet 2004 subcutaneous miniosmotic pump (Alzet Osmotic Pumps, Cupertino, CA). These studies were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Physiologic Testing
Intraperitoneal glucose tolerance testing, serum insulin measurements, and insulin tolerance testing were performed as described previously (22). Blood urea nitrogen measurement was performed on plasma using a colorimetric assay (Stanbio Laboratory procedure 2050, Boerne, TX). Serum Ex-4 concentration was determined by enzyme immunoassay (Phoenix Pharmaceuticals, Belmont, CA).
Morphologic Analysis
Mouse Models
On the final day of each mouse experiment, deep anesthesia was induced with pentobarbital, and the pancreata were removed, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned for analysis. Antisera were: guinea pig anti-insulin (DakoCytomation; Carpinteria, CA), sheep anti-BrdU (U.S. Biologicals; Swampscott, MA), and species-specific Cy-2- and Cy-3-conjugated secondary antisera. Nuclear labeling was performed with DAPI (Molecular Probes, Eugene, OR) and autofluorescence quenched with Sudan Black (BDH Laboratory Supplies, Poole, England). The β-cell BrdU incorporation rate was determined according to the equation: incorporation rate = [(insulin+/BrdU+)/(total insulin + cells)] × 100. At least 1,000 total β-cell nuclei were counted for each mouse. Section analysis was performed with an Eclipse E600 microscope (Nikon, Japan). Images were captured using a Cool Snap digital camera (Photometrics, Huntington Beach, CA).
Human Islets
The suspension cultures of treated islet cells were fixed for 0.5 h in 4% paraformaldehyde and pelleted in 2% agarose before paraffin embedding and sectioning. Terminal dUTP nick-end labeling (TUNEL) was performed with a commercially available kit (Roche Applied Science) and specific Alexa Fluor 647-dUTP secondary antisera (Invitrogen). TUNEL and BrdU imaging and quantitation of human islet sections were performed utilizing a Zeiss AxioImager M2 microscope (Carl Zeiss MicroImaging, Thornwood, NY) with automated XY stage (Ludl Electronic Products, Hawthorne, NY) and Hamamatsu C4742 digital camera (Hamamatsu Corporation, Bridgewater, NJ). Images were analyzed utilizing Volocity version 5.3.2 software (PerkinElmer Life Sciences).
Chromatin Immunoprecipitation (ChIP)
ChIP assays were performed essentially as described previously in Min6 cells treated for 1 h with vehicle or 30 ng/ml tacrolimus (23). Antibodies used included mouse anti-NFATc1 antiserum (BD Pharmingen) and normal mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Data were analyzed as described previously (22). For detection of the two conserved regions of the mouse Irs2 promoter, primers were designed using Primer3. The two Irs2 NFAT binding sites were located at −1259/−1255 and −1146/−1142 relative to the Irs2 transcriptional start site, respectively. Primers to detect NFAT occupancy of the cyclin D1 and insulin1 promoter were designed using previously identified NFAT binding sites (10). Negative control primers were designed to detect a noncoding intergenic sequence ∼70 kb upstream of the Irs2 transcriptional start site. Primer sequences are listed in supplemental Table 1.
Real Time PCR
Total RNA was DNase-treated (DNA-free; Ambion, Austin, TX) and reverse-transcribed (Superscript II reverse transcriptase; Invitrogen) following the manufacturers' protocols. Primer sequences are indicated in supplemental Table 1. Fluorescence-based real time PCR was performed using the IQ Sybr Green Supermix kit (Bio-Rad) and the IQ-5 Single Color Real Time PCR Detection System (Bio-Rad). Primers were designed using Primer3 as above and tested for linear amplification using serial dilutions of cDNA before use on experimental samples. Expression was determined relative to internal controls (rat actin and human cyclophilin).
Western Blot Analysis
Whole cell lysates were generated by sonication followed by centrifugation to remove insoluble material. Nuclear and cytoplasmic protein fractions were isolated as described (24). Lysates were resolved on 4–12% gradient BisTris gels (Invitrogen) and transferred to nitrocellulose membranes as described (25). Primary antisera were anti-NFATc1 (BD Pharmingen), anti-phosphoserine 133-CREB (Cell Signaling; Danvers, MA), anti-CREB (Cell Signaling), anti-phosphothreonine 308-Akt (Cell Signaling), anti-Akt (Cell Signaling), anti-phospho-Akt substrates (Cell Signaling), anti-Pdx1 (253) (26), anti-tubulin (Cell Signaling), anti-Irs1 (Santa Cruz Biotechnology), anti-Irs2 (Upstate Biotechnology; Lake Placid, NY), anti-phospho-GSK3 (Cell Signaling), anti-GSK3 (Cell Signaling), anti-phospho-S6 (Cell Signaling), anti-S6 (Cell Signaling), anti-actin (Sigma), and anti-cyclophilin B (Affinity Bioreagents; Golden, CO). Secondary antisera incubation and signal detection were performed as described (25). Western blot band intensity analysis was performed using ImageJ version 1.43 software (National Institutes of Health).
Statistical Analysis
Data are presented as mean ± S.E., unless otherwise noted. Statistical comparisons were performed using Student's t test or two-way ANOVA. For 2 × 2 comparisons that reached statistical significance by ANOVA (p < 0.05), a protected Fisher's least significant difference test was utilized for post hoc analysis.
RESULTS
Calcineurin Regulates Human β-Cell Survival
Given the association of tacrolimus with the development of post-transplant diabetes mellitus in humans and the unclear role of calcineurin inhibition as a positive or negative regulator of the β-cell (13, 27), we sought to determine how calcineurin inhibition impacts human β-cell survival and replication. Human islets were treated with tacrolimus (10 ng/ml) or vehicle for 48 h. Tacrolimus increased the rate of human β-cell apoptosis by 3.1-fold in human islets, as detected by TUNEL staining (Fig. 1), with a similar trend found in non-β-cells which was not statistically significant (data not shown). Although tacrolimus induced a similar fold increase in apoptosis in islets from all three donors, we noted a substantial variation in absolute apoptosis rates among the individual donors, consistent with the well appreciated variation among human islet preparations (supplemental Fig. 1). We next sought to determine whether Ex-4 treatment could overcome tacrolimus-induced impairments in β-cell survival. Ex-4 partially reversed the effect of tacrolimus, improving cell survival by greater than 2-fold. Ex-4 alone led to a nearly 40% reduction in β-cell apoptosis, but this effect was not statistically significant (Fig. 1B).
FIGURE 1.
Calcineurin inhibition induces human β-cell apoptosis. A, representative image (magnification, ×40) of vehicle- (upper) and tacrolimus- (lower) treated human islets. Lower right, increased magnification of focal area of β-cell apoptosis in a tacrolimus-treated human islet. Green, insulin; red, TUNEL; blue, DAPI. B, quantitation of human β-cell TUNEL rates after 48-h treatment with vehicle, 10 ng/ml tacrolimus, 10 nm Ex-4, or a combination of tacrolimus and Ex-4 at the same doses. Data are presented as mean ± S.E. (error bars) of three independent human islet preparations and are expressed as fold compared with the vehicle-treated group. Absolute rates of apoptosis are presented in supplemental Fig. 1. 5,000–30,000 human β-cells were counted/treatment group for each preparation. *, p < 0.05.
We also examined BrdU incorporation as a measure of β-cell replication in human islets treated with vehicle, tacrolimus, and/or Ex-4. Following 24 h of BrdU labeling, we found rare BrdU-labeled non-β-cells; however, not a single BrdU-labeled β-cell was identified despite counting nearly 100,000 human β-cells among the three donors (data not shown). These data may reflect the failure of β-cell replication to occur in the ex vivo setting and are consistent with the low levels of β-cell proliferation previously reported in adult humans (28). Taken together, these results demonstrate the deleterious effects of calcineurin inhibition on human β-cell survival.
Tacrolimus Decreases Murine β-Cell Proliferation
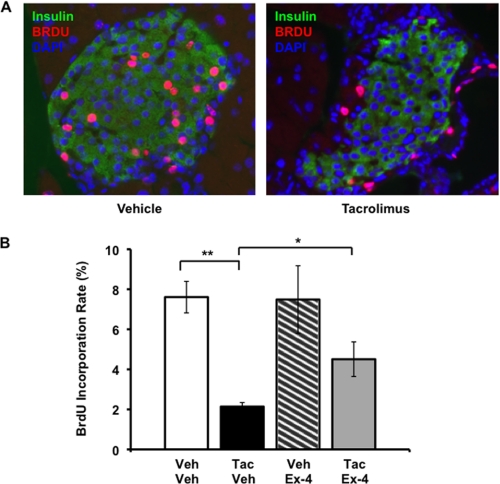
Due to the absence of detectable β-cell replication in cultured human islets, we could not determine whether pharmacologic calcineurin inhibition affects human β-cell proliferation. Therefore, we evaluated the impact of tacrolimus on β-cell proliferation in rodents, which possess higher rates of β-cell replication compared with humans. Ten-week-old male C57BL/6 mice were assigned to treatment with daily tacrolimus (1.0 mg/kg per day intraperitoneally), Ex-4 (20 nmol/kg per day), or vehicle controls for 19 days (4 groups, n = 5–8/group). Ex-4 was delivered continuously by subcutaneous miniosmotic pump. Tacrolimus decreased β-cell proliferation by 72% compared with vehicle-treated controls (Fig. 2), and this effect was consistent across multiple dosages (supplemental Fig. 2). Ex-4 partially reversed this effect, doubling the β-cell BrdU incorporation rate compared with the tacrolimus-only treatment group (Fig. 2B). Mean plasma Ex-4 concentration was ∼2 ng/ml on days 7 and 14 and was not affected by tacrolimus administration (supplemental Table 2). Glucose tolerance testing on day 15 did not reveal a deleterious impact of tacrolimus on glucose tolerance over this time frame, and Ex-4 exerted its well described beneficial effects on glucose tolerance independent of tacrolimus administration (supplemental Fig. 3) (15). No differences in body weight or insulin sensitivity were detected among the four treatment groups (supplemental Fig. 3), suggesting that the effects of tacrolimus or Ex-4 on β-cell proliferation were not due to compensation for alterations in insulin sensitivity. We examined the rate of apoptosis by TUNEL staining, and tacrolimus did not appear to increase the low rate of islet apoptosis in tacrolimus compared with vehicle-treated mice (data not shown). These results demonstrate that, similar to genetic calcineurin deletion (10), pharmacologic calcineurin inhibition markedly inhibits rodent β-cell proliferation.
FIGURE 2.
Ex-4 attenuates the tacrolimus-induced decrease in murine β-cell proliferation. A, representative image of islets from mice treated with vehicle or tacrolimus. Insulin is seen in green, BrdU in red, DAPI in blue. B, quantitation of β-cell BrdU incorporation rates over 14 days of continuous administration in drinking water. All data points are mean ± S.E. (error bars). Bar graphs, open bar, vehicle/vehicle; black bar, 1.0 mg/kg per day tacrolimus/vehicle; hatched bar, vehicle/20 nmol per kg per day Ex-4; gray bar, 1.0 mg/kg per day tacrolimus/20 nmol/kg per day Ex-4. n = 7–8 mice/group except for vehicle/Ex-4 (n = 5). *, p < 0.05; **, p < 0.01.
Calcineurin Regulates Activity of the PI3K/Akt Pathway
Akt, also known as protein kinase B (PKB), is a serine-threonine kinase that acts as a major effector of the Irs/PI3K/Akt pathway to coordinate growth factor signaling (for review, see Refs. 29, 30). Studies in genetic mouse models and human islets support the regulation of β-cell replication, survival, and function by Akt (31–33), and both insulin and insulin-like growth factor-1 are critical activators of this pathway (34–36). To determine whether calcineurin inhibition influences this pathway, we examined the effect of tacrolimus on phosphorylation of Akt. Following tacrolimus treatment in INS-1 cells, phosphorylation of Akt at Thr308, the phosphorylation site necessary for Akt activation, was reduced, whereas total Akt expression was unaffected (Fig. 3, A and B). The reduction in Akt phosphorylation following tacrolimus treatment was associated with reduction in the phosphorylation of Akt substrates in INS-1 cells (Fig. 3C). Similarly, tacrolimus treatment in human islets reduced phosphorylation of Akt substrates (Fig. 3D). Calcineurin inhibition in human islets led to decreased phosphorylation of specific Akt targets GSK3 (Fig. 3E) and ribosomal protein S6 (Fig. 3F), while not affecting total GSK3 and S6 expression. Therefore, calcineurin inhibition directly impacts the activity of the PI3K/Akt pathway in both rodent and human β cells and islets, respectively.
FIGURE 3.
Calcineurin inhibition reduces phosphorylation of Akt and Akt substrates. A, Western blot analysis of INS-1 cells following treatment with vehicle, 30 ng/ml tacrolimus, and/or 10 μm forskolin for 6 h, probed with anti-phospho-Akt (Thr308) (representative of four independent experiments). B, band intensity quantitation of p-Akt/Akt protein ratio in INS-1 cells by Western blotting. Data are presented as fold change from vehicle controls and as the mean ± S.E. (error bars) of four experiments performed in duplicate. C and D, representative Western blot of phospho-Akt substrate protein expression in INS-1 cells (C) and human islets (D) following treatment with vehicle or tacrolimus for 6 h. For human islets, duplicate cultures of islets derived from a single donor are shown (data are representative of experiments performed on islets from three independent donors). E and F, Western blot analysis of phospho- and total GSK3 (E) and phospho- and total S6 (F) expression in human islets following treatment with vehicle or10 ng/ml tacrolimus for 6 h. G, quantitative PCR analysis of Akt isoform mRNA expression in cultured human and rat islets. Isoform expression is presented relative to Akt1 mRNA levels. Data are presented as mean ± S.E. (error bars) of three independent rat islet preparations and four independent human islet preparations (each analyzed in triplicate). *, p < 0.05; **, p < 0.01.
Given the species-specific effects of calcineurin inhibition on cell survival, we examined expression of Akt isoforms in both rodent and human islets. Although all three Akt isoforms are expressed in both rodent and human islets (37, 38), studies in genetic mouse models show distinct effects of each isoform on the regulation of glucose tolerance (39–46). Comparison of the relative mRNA expression of these isoforms between rodent and human islets showed a significant reduction in the relative levels of Akt2 in human islets (Fig. 3G).
Irs2 Is a Novel Transcriptional Target of Calcineurin
Irs2 is a critical adaptor in the insulin receptor/insulin-like growth factor-1 receptor signaling pathway, the activity of which is required for normal murine β-cell replication and survival (29, 47). Further, Irs2 is a CREB target gene (48) and a target of the calcineurin-dependent CREB co-activator TORC2 in hepatocytes (49). Thus, we hypothesized that Irs2 expression might be both sensitive to pharmacologic calcineurin inhibition and a potential mediator of the tacrolimus-induced increase in β-cell apoptosis. Therefore, we examined the impact of tacrolimus on Irs2 expression. Following a 6-h exposure to tacrolimus, Irs2 mRNA expression was consistently reduced by ∼50% in INS-1 cells (Fig. 4A), primary rat islets (Fig. 4D), and primary human islets (Fig. 4E). The reduction in Irs2 mRNA was paralleled by reductions in Irs2 protein levels in INS-1 cells and human islets (Fig. 4, B and F). Thus, calcineurin regulation of Irs2 expression is highly conserved. Tacrolimus also inhibited the previously described glucose stimulation of Irs2 expression (21) (supplemental Fig. 4). Calcineurin appears to regulate Irs2 specifically, as Irs1 expression was not affected by tacrolimus treatment (Fig. 3A).
FIGURE 4.
Tacrolimus decreases Irs2 expression, which is restored by cAMP stimuli. A and B, Irs2 mRNA (A) and protein (B) levels were assessed in INS-1 cells after a 6-h incubation with tacrolimus (30 ng/ml), forskolin (10 μm), or their vehicles as indicated. Each Western blot lane represents a separate culture dish. C and D, quantitative PCR analysis of Irs2 mRNA expression in rat islets following vehicle, tacrolimus, and/or Ex-4 treatment for 3 h (C) or 6 h (D). Data are expressed as mean ± S.E. (error bars) of three experiments performed in triplicate. E, quantitative PCR analysis of Irs2 mRNA expression following treatment with vehicle, 10 ng/ml tacrolimus, and/or 10 nm Ex-4 for 6 h in three independent human islet preparations (studied in triplicate). Data are presented as the mean ± S.E. (error bars) fold mRNA level versus vehicle. F, Irs2 protein expression in representative Western blot of human islets following treatment with vehicle, 10 ng/ml tacrolimus, and/or 10 nm Ex-4 for 6 h. Each Western blot lane represents a separate culture dish from one donor. The experiment was performed with islets from three separate donors in duplicate. *, p < 0.05; **, p < 0.01; †, p < 0.001.
Because activation of cAMP-dependent signaling induces β-cell Irs2 expression and improves β-cell survival (48), we sought to determine whether a cAMP signal could overcome the effect of calcineurin inhibition on Irs2 expression. We examined the effect of forskolin and Ex-4 on the ability of tacrolimus to inhibit Irs2 expression in INS-1 cells, rat islets, and human islets. Forskolin (10 μm) increased Irs2 expression in the presence of tacrolimus in INS-1 cells, restoring expression to the level seen with forskolin alone (Fig. 4, A and B). Similarly, in primary rat islets, tacrolimus reduced Irs2 mRNA expression after 6 h of treatment, whereas Ex-4 stimulated Irs2 expression already beginning at 3 h. After 6 h, Ex-4 normalized the expression of Irs2 in tacrolimus-treated islets, although the level was reduced compared with Ex-4 alone, suggesting a role for calcineurin in Ex-4 stimulation of Irs2 expression, similar to its role in mediating Ex-4 stimulation of insulin gene transcription (Fig. 4, C and D) (7, 9). Ex-4 also normalized the expression of IRS2 in tacrolimus-treated human islets (Fig. 4, E and F).
To determine whether calcineurin could regulate Irs2 via its well known effector NFAT, we scanned the Irs2 promoter and identified two evolutionarily conserved genomic sequences within 1.3 kb upstream of the Irs2 transcriptional start site. Each of the two sequences contains a conserved NFAT consensus binding motif 5′-GGAAA-3′. ChIP assays demonstrated endogenous NFATc1 occupancy of both regions in Min6 cells, which was abrogated in the presence of tacrolimus (Fig. 5). We also observed that the endogenous promoters of insulin and cyclin D1 are occupied by NFATc1 in a manner sensitive to calcineurin inhibition. These genes were previously suggested to be NFAT targets based on occupancy by overexpressed NFATc1 (10).
FIGURE 5.
NFATc1 occupies two regions of the Irs2 promoter in a calcineurin-sensitive manner. Quantitative ChIP analysis in Min6 cells following treatment with vehicle or 30 ng/ml tacrolimus, is expressed as fold enrichment (αNFAT versus normal mouse IgG). Data are displayed as mean ± S.D. (error bars) of a single representative ChIP experiment. Experiment was performed three times. Primers designed to amplify a known NFATc1 binding region of the cyclin D1 and insulin promoter and a cis-control, located in an intergenic region nearly 70 kb upstream of the Irs2 transcriptional start site, were used as positive and negative controls, respectively.
To determine how calcineurin and cAMP signaling might intersect in the pancreatic β-cell, we next assessed whether cAMP signaling impacts tacrolimus-mediated changes in NFAT localization and, conversely, whether calcineurin affects cAMP-stimulated CREB phosphorylation. As assessed by Western blot analysis of nuclear and cytoplasmic extracts in INS-1 cells, tacrolimus resulted in the expected shift in NFATc1 localization from predominantly nuclear to predominantly cytoplasmic as well as a shift in mobility consistent with increased phosphorylation. Neither Ex-4 nor forskolin was able to overcome these effects of calcineurin inhibition (supplemental Fig. 5A and data not shown). Further, the phosphorylation of CREB by forskolin was not impaired by tacrolimus (supplemental Fig. 5B). Thus, the ability of cAMP stimuli to rescue the effect of calcineurin inhibition of Irs2 expression is not mediated via NFAT and is more likely to be mediated by the classical PKA-CREB pathway. Taken altogether, these results indicate that calcineurin inhibition may regulate human β-cell survival as well as function of the PI3K/Akt pathway in part through direct regulation of Irs2 expression.
Tacrolimus Inhibits Rodent D-cyclin Expression, Which Is Improved by Ex-4
D-cyclins are critical regulators of postnatal β-cell proliferation and the G1/S cell cycle transition through their participation in complexes with Cdk4 and Cdk6 to regulate phosphorylation and inactivation of retinoblastoma protein (Rb) (50–52). Activation of Akt leads to integration with the cell cycle machinery by regulating D-cyclin levels (53). In addition, the cyclin D1 promoter has been shown to be occupied by NFATc1 in a calcineurin-sensitive manner (Fig. 4) (10). Therefore, we sought to determine whether calcineurin regulates D-cyclin expression. We assessed cyclin D1 (Ccnd1) and cyclin D2 (Ccnd2) mRNA transcript level in rat islets following treatment with vehicle, tacrolimus, and/or Ex-4. Tacrolimus reduced Ccnd1 and Ccnd2 transcript levels at both 3 and 6 h following treatment (Fig. 6, A and B). Tacrolimus-mediated reductions in Ccnd1, but not Ccnd2, expression were improved by Ex-4 at 6 h following treatment (p < 0.05; Fig. 6A) with a similar trend at 3 h (p = 0.07). Ex-4 alone did not increase Ccnd1 or Ccnd2 transcript levels. Tacrolimus did not cause reductions in Cdk4 expression (Fig. 6C). These data suggest that that calcineurin regulates rodent β-cell replication at least in part through control of D-cyclin expression.
FIGURE 6.
Tacrolimus reduces D-cyclin expression in rodent islets. A–C, quantitative PCR analysis of cyclin D1 (A), cyclin D2 (B), and Cdk4 (C) expression in cultured rat islets following vehicle, tacrolimus, and/or Ex-4 treatment for 3 or 6 h. Data are expressed as mean ± S.E. (error bars) of three experiments performed in triplicate. *, p < 0.05; **, p < 0.01; †, p < 0.001.
DISCUSSION
We demonstrate here that pharmacologic calcineurin inhibition impairs human β-cell survival and murine β-cell proliferation and reduces activity of the PI3K/Akt pathway. We identify Irs2 as a target of calcineurin, likely mediated by direct occupancy by NFAT as well as by the previously described action of TORC2 to co-activate CREB at the Irs2 promoter in hepatocytes. This regulation of Irs2 corresponds to a reduction in the activity of Akt, as reflected by its phosphorylation at Thr308 and reduced phosphorylation of Akt substrates. Finally, we find that the incretin GLP-1 receptor agonist Ex-4 attenuates the survival, proliferative, and gene expression defects induced by calcineurin inhibition, suggesting that clinically available incretin mimetic agents will ameliorate the β-cell toxic effects of calcineurin inhibition.
We identify Irs2 as a novel target of calcineurin inhibition relevant to β-cell growth. Irs2 is an adaptor linking receptor activation to the proximal insulin/ insulin-like growth factor-1 receptor signaling pathway. Irs2 is required for β-cell survival (54) as well as compensatory β-cell mass expansion in response to increased insulin demand (55). Maintenance of Irs2 expression is particularly vital to β-cell survival and resistance to FFA-induced apoptosis (56), suggesting that inhibition of Irs2 expression by tacrolimus could increase β-cell death. Thus, the proapoptotic effect of calcineurin inhibition on human islets that we observe as well as the increase in human islet caspase-3 cleavage and cell damage observed by others in response to calcineurin inhibition (27, 57) suggest that Irs2 likely contributes to human β-cell survival.
Cell cycle progression from G1 to S phase in rodent β-cells is dependent on the activity of D-cyclin/cyclin-dependent kinase complexes, specifically those formed by Ccnd1 and Ccnd2 with Cdk4 (50–52). Although the physiologic integration points between calcineurin and the cell cycle machinery have not been fully elucidated, several lines of evidence suggest that D-cyclin and Cdk4 expression may be calcineurin-dependent and directly regulated by NFAT (10). Tacrolimus-mediated changes in β-cell proliferation may also be TORC2-dependent because the CREB co-activator, TORC2, is expressed in β-cells, activated by calcineurin (8), and is vital for CREB-mediated gene expression (58, 59). Ccnd1 is a CREB target in the β-cell (60–62), and the potential role of TORC2 in Ccnd1 expression remains to be explored. Finally, Akt overexpression positively regulates Ccnd1 and Ccnd2 levels in rodent islets (53). Thus, tacrolimus-induced inhibition of calcineurin-dependent NFAT and TORC2 activation, as well as of PI3K/Akt pathway activity, may decrease expression of critical components of the rodent cell cycle machinery. Future studies will be needed to determine whether these effects are conserved in human islets, as our studies were limited by the observed lack of β-cell replication under baseline conditions.
We observe species-specific effects of calcineurin inhibition on β-cell survival in human and rodent islets. These effects could be due to differences in the response of human and rodent islets to reductions in Irs2 expression. Alternatively, it is possible that calcineurin inhibition leads to Irs2- or Akt-independent effects, or potentially, Akt-dependent effects that differ between humans and rodents. Human and rodent islets, despite similarities in insulin release to secretagogues utilized for the treatment of diabetes, vary in terms of histological composition (63–65), basal levels of replication (66), glucose transporter gene expression (67), and D-cyclin/cyclin-dependent kinase gene expression (68). We report significant differences in relative Akt2 expression between rodent and human islets that could also contribute to the differences we observe in β-cell survival. Akt2 is an important regulator of β-cell survival in mice (44). Irs2 has been recently suggested to have preferential interactions with Akt1 (39). Thus, the higher levels of Akt2 may make rodent islets less susceptible to the effects of calcineurin inhibition on β-cell survival. This is one of several possibilities that merit further investigation. These differences, among others, may underscore the inconsistent translation of novel molecular findings in rodent models to human therapeutic benefit. The species-specific differences of our findings further reinforce the need to translate findings from rodent models to human islets.
An important clinically relevant observation is the demonstration that GLP-1 receptor agonists can correct, at least in part, the deleterious effects of tacrolimus on IRS2 expression and human β-cell survival. Several potential mechanisms could mediate these effects. Activation of cAMP-dependent signaling results in increased intracellular calcium (16) which could potentially override calcineurin inhibition. However, calcineurin-dependent NFAT and TORC activation are highly sensitive to calcineurin inhibition (8, 9), and we demonstrate that cAMP stimuli do not cause NFAT nuclear localization following tacrolimus treatment. Direct CREB phosphorylation by both cAMP-dependent PKA (48) and calcium-activated calmodulin kinase-IV (69) increases CREB-dependent transcriptional activity via recruitment of CBP/p300, providing a calcineurin-independent pathway for the modulation of Irs2 expression. Our demonstration that CREB phosphorylation is maintained following calcineurin inhibition supports this hypothesis.
Overall, our observations indicate that pharmacologic calcineurin inhibition may contribute to post-transplant diabetes by diminishing β-cell survival and replication. This may lead to impaired adaptation to dynamic changes in insulin demand after solid organ transplantation and promote β-cell failure in these settings. Although efforts to move away from calcineurin inhibitors to novel immunosuppressive agents that do not target this pathway or are able to target it in a cell-selective fashion may be beneficial in the future, calcineurin inhibitors are currently an indispensable component of chronic immune suppression for many transplant recipients. Our results specifically support the possibility that pharmacologic approaches to GLP-1 receptor activation may promote β-cell survival and proliferation in the setting of calcineurin inhibitor co-administration.
Supplementary Material
Acknowledgments
We thank Dr. Chris Newgard (Duke University) for the INS-1 832/13 cells, Dr. Morrie Birnbaum for expert advice and helpful discussions in preparation of the manuscript, Daniela Budo for expert technical assistance with immunofluorescence staining of tissue sections, and Dr. Daniella Babu for assistance with human islet experiments and advice regarding Ex-4 optimization.
This work was supported, in whole or in part, by National Institutes of Health Grants DK49210 and DK062965 (to D. A. S.), U42 (RR016600) (to A. N.), and National Institutes of Health Career Development Award K12 (RR017625) (to M. F. C.). This work was also supported by American Diabetes Association Career Development Award 7-01-CD-15 (to D. A. S.), Commonwealth of Pennsylvania Center for Excellence in Regenerative Medicine Grant 4100043362 (to D. A. S., A. N., and J. A. K.), a research grant from The Iacocca Foundation (to D. A. S. and A. N.), Kirschstein NRSA-T32 Award DK007314-27 (to S. A. S.), a grant from the Benjamin and Mary Siddons Measey Foundation (to S. A. S.), and Morphology Core of the University of Pennsylvania Center for Molecular Studies in Digestive and Liver Disease Center Grant P30 DK50306.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1–5.
- NFAT
- nuclear factor of activated T cell
- BisTris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- CREB
- cAMP-responsive element-binding protein
- DMSO
- dimethyl sulfoxide
- Ex-4
- exendin-4
- Irs2
- insulin receptor substrate-2
- TORC2
- transducer of regulated CREB activity-2.
REFERENCES
- 1.Cosio F. G., Kudva Y., van der Velde M., Larson T. S., Textor S. C., Griffin M. D., Stegall M. D. (2005) Kidney Int. 67, 2415–2421 [DOI] [PubMed] [Google Scholar]
- 2.Kasiske B. L., Snyder J. J., Gilbertson D., Matas A. J. (2003) Am. J. Transplant. 3, 178–185 [DOI] [PubMed] [Google Scholar]
- 3.Baid S., Cosimi A. B., Farrell M. L., Schoenfeld D. A., Feng S., Chung R. T., Tolkoff-Rubin N., Pascual M. (2001) Transplantation 72, 1066–1072 [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S. (2000) Endocrinology 141, 1926–1929 [DOI] [PubMed] [Google Scholar]
- 5.Davidson J., Wilkinson A., Dantal J., Dotta F., Haller H., Hernández D., Kasiske B. L., Kiberd B., Krentz A., Legendre C., Marchetti P., Markell M., van der Woude F. J., Wheeler D. C. (2003) Transplantation 75, SS3–S24 [DOI] [PubMed] [Google Scholar]
- 6.Crabtree G. R., Olson E. N. (2002) Cell 109, S67–S79 [DOI] [PubMed] [Google Scholar]
- 7.Lawrence M. C., Bhatt H. S., Watterson J. M., Easom R. A. (2001) Mol. Endocrinol. 15, 1758–1767 [DOI] [PubMed] [Google Scholar]
- 8.Screaton R. A., Conkright M. D., Katoh Y., Best J. L., Canettieri G., Jeffries S., Guzman E., Niessen S., Yates J. R., 3rd, Takemori H., Okamoto M., Montminy M. (2004) Cell 119, 61–74 [DOI] [PubMed] [Google Scholar]
- 9.Lawrence M. C., Bhatt H. S., Easom R. A. (2002) Diabetes 51, 691–698 [DOI] [PubMed] [Google Scholar]
- 10.Heit J. J., Apelqvist A. A., Gu X., Winslow M. M., Neilson J. R., Crabtree G. R., Kim S. K. (2006) Nature 443, 345–349 [DOI] [PubMed] [Google Scholar]
- 11.Kahl C. R., Means A. R. (2004) Mol. Biol. Cell 15, 1833–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nir T., Melton D. A., Dor Y. (2007) J. Clin. Invest. 117, 2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Castillo J. M., García-Martín M. C., Arias-Díaz J., Giné E., Vara E., Cantero J. L. (2009) Cell Transplant. 18, 1237–1246 [DOI] [PubMed] [Google Scholar]
- 14.Ranta F., Avram D., Berchtold S., Düfer M., Drews G., Lang F., Ullrich S. (2006) Diabetes 55, 1380–1390 [DOI] [PubMed] [Google Scholar]
- 15.De León D. D., Crutchlow M. F., Ham J. Y., Stoffers D. A. (2006) Int. J. Biochem. Cell Biol. 38, 845–859 [DOI] [PubMed] [Google Scholar]
- 16.Gomez E., Pritchard C., Herbert T. P. (2002) J. Biol. Chem. 277, 48146–48151 [DOI] [PubMed] [Google Scholar]
- 17.Buteau J., Foisy S., Joly E., Prentki M. (2003) Diabetes 52, 124–132 [DOI] [PubMed] [Google Scholar]
- 18.Drucker D. J. (2006) Cell Metab. 3, 153–165 [DOI] [PubMed] [Google Scholar]
- 19.Scharp D. W., Kemp C. B., Knight M. J., Ballinger W. F., Lacy P. E. (1973) Transplantation 16, 686–689 [DOI] [PubMed] [Google Scholar]
- 20.Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 21.Lingohr M. K., Briaud I., Dickson L. M., McCuaig J. F., Alárcon C., Wicksteed B. L., Rhodes C. J. (2006) J. Biol. Chem. 281, 15884–15892 [DOI] [PubMed] [Google Scholar]
- 22.Sachdeva M. M., Claiborn K. C., Khoo C., Yang J., Groff D. N., Mirmira R. G., Stoffers D. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 19090–19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deramaudt T. B., Sachdeva M. M., Wescott M. P., Chen Y., Stoffers D. A., Rustgi A. K. (2006) J. Biol. Chem. 281, 38385–38395 [DOI] [PubMed] [Google Scholar]
- 24.Schreiber E., Matthias P., Müller M. M., Schaffner W. (1989) Nucleic Acids Res. 17, 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De León D. D., Farzad C., Crutchlow M. F., Brestelli J., Tobias J., Kaestner K. H., Stoffers D. A. (2006) Physiol. Genomics 24, 133–143 [DOI] [PubMed] [Google Scholar]
- 26.Stoffers D. A., Stanojevic V., Habener J. F. (1998) J. Clin. Invest. 102, 232–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson J. D., Ao Z., Ao P., Li H., Dai L. J., He Z., Tee M., Potter K. J., Klimek A. M., Meloche R. M., Thompson D. M., Verchere C. B., Warnock G. L. (2009) Cell Transplant. 18, 833–845 [DOI] [PubMed] [Google Scholar]
- 28.Meier J. J., Butler A. E., Saisho Y., Monchamp T., Galasso R., Bhushan A., Rizza R. A., Butler P. C. (2008) Diabetes 57, 1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elghazi L., Balcazar N., Bernal-Mizrachi E. (2006) Int. J. Biochem. Cell Biol. 38, 157–163 [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni R. N. (2005) Rev. Endocr. Metab. Disord. 6, 199–210 [DOI] [PubMed] [Google Scholar]
- 31.Bernal-Mizrachi E., Wen W., Stahlhut S., Welling C. M., Permutt M. A. (2001) J. Clin. Invest. 108, 1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao P., Roccisana J., Takane K. K., Bottino R., Zhao A., Trucco M., García-Ocaña A. (2005) Diabetes 54, 1664–1675 [DOI] [PubMed] [Google Scholar]
- 33.Tuttle R. L., Gill N. S., Pugh W., Lee J. P., Koeberlein B., Furth E. E., Polonsky K. S., Naji A., Birnbaum M. J. (2001) Nat. Med. 7, 1133–1137 [DOI] [PubMed] [Google Scholar]
- 34.Dickson L. M., Lingohr M. K., McCuaig J., Hugl S. R., Snow L., Kahn B. B., Myers M. G., Jr., Rhodes C. J. (2001) J. Biol. Chem. 276, 21110–21120 [DOI] [PubMed] [Google Scholar]
- 35.Dickson L. M., Rhodes C. J. (2004) Am. J. Physiol. Endocrinol. Metab. 287, E192–E198 [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan S., Bernal-Mizrachi E., Ohsugi M., Permutt M. A. (2002) Am. J. Physiol. Endocrinol. Metab. 283, E784–E793 [DOI] [PubMed] [Google Scholar]
- 37.Holst L. S., Mulder H., Manganiello V., Sundler F., Ahrén B., Holm C., Degerman E. (1998) Biochem. Biophys. Res. Commun. 250, 181–186 [DOI] [PubMed] [Google Scholar]
- 38.Muller D., Huang G. C., Amiel S., Jones P. M., Persaud S. J. (2006) Diabetes 55, 2835–2842 [DOI] [PubMed] [Google Scholar]
- 39.Buzzi F., Xu L., Zuellig R. A., Boller S. B., Spinas G. A., Hynx D., Chang Z., Yang Z., Hemmings B. A., Tschopp O., Niessen M. (2010) Mol. Cell. Biol. 30, 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W. S., Xu P. Z., Gottlob K., Chen M. L., Sokol K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K., Kadowaki T., Hay N. (2001) Genes Dev. 15, 2203–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B., 3rd, Kaestner K. H., Bartolomei M. S., Shulman G. I., Birnbaum M. J. (2001) Science 292, 1728–1731 [DOI] [PubMed] [Google Scholar]
- 42.Cho H., Thorvaldsen J. L., Chu Q., Feng F., Birnbaum M. J. (2001) J. Biol. Chem. 276, 38349–38352 [DOI] [PubMed] [Google Scholar]
- 43.Easton R. M., Cho H., Roovers K., Shineman D. W., Mizrahi M., Forman M. S., Lee V. M., Szabolcs M., de Jong R., Oltersdorf T., Ludwig T., Efstratiadis A., Birnbaum M. J. (2005) Mol. Cell. Biol. 25, 1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garofalo R. S., Orena S. J., Rafidi K., Torchia A. J., Stock J. L., Hildebrandt A. L., Coskran T., Black S. C., Brees D. J., Wicks J. R., McNeish J. D., Coleman K. G. (2003) J. Clin. Invest. 112, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tschopp O., Yang Z. Z., Brodbeck D., Dummler B. A., Hemmings-Mieszczak M., Watanabe T., Michaelis T., Frahm J., Hemmings B. A. (2005) Development 132, 2943–2954 [DOI] [PubMed] [Google Scholar]
- 46.Yang Z. Z., Tschopp O., Hemmings-Mieszczak M., Feng J., Brodbeck D., Perentes E., Hemmings B. A. (2003) J. Biol. Chem. 278, 32124–32131 [DOI] [PubMed] [Google Scholar]
- 47.Crutchlow M. F., Stoffers D. A. (2007) Curr. Opin. Organ Transplant. 12, 55–62 [DOI] [PubMed] [Google Scholar]
- 48.Jhala U. S., Canettieri G., Screaton R. A., Kulkarni R. N., Krajewski S., Reed J., Walker J., Lin X., White M., Montminy M. (2003) Genes Dev. 17, 1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canettieri G., Koo S. H., Berdeaux R., Heredia J., Hedrick S., Zhang X., Montminy M. (2005) Cell Metab. 2, 331–338 [DOI] [PubMed] [Google Scholar]
- 50.Georgia S., Bhushan A. (2004) J. Clin. Invest. 114, 963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kushner J. A., Ciemerych M. A., Sicinska E., Wartschow L. M., Teta M., Long S. Y., Sicinski P., White M. F. (2005) Mol. Cell. Biol. 25, 3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rane S. G., Dubus P., Mettus R. V., Galbreath E. J., Boden G., Reddy E. P., Barbacid M. (1999) Nat. Genet. 22, 44–52 [DOI] [PubMed] [Google Scholar]
- 53.Fatrai S., Elghazi L., Balcazar N., Cras-Méneur C., Krits I., Kiyokawa H., Bernal-Mizrachi E. (2006) Diabetes 55, 318–325 [DOI] [PubMed] [Google Scholar]
- 54.Withers D. J., Burks D. J., Towery H. H., Altamuro S. L., Flint C. L., White M. F. (1999) Nat. Genet. 23, 32–40 [DOI] [PubMed] [Google Scholar]
- 55.Withers D. J., Gutierrez J. S., Towery H., Burks D. J., Ren J. M., Previs S., Zhang Y., Bernal D., Pons S., Shulman G. I., Bonner-Weir S., White M. F. (1998) Nature 391, 900–904 [DOI] [PubMed] [Google Scholar]
- 56.Lingohr M. K., Dickson L. M., Wrede C. E., Briaud I., McCuaig J. F., Myers M. G., Jr., Rhodes C. J. (2003) Mol. Cell. Endocrinol. 209, 17–31 [DOI] [PubMed] [Google Scholar]
- 57.Drachenberg C. B., Klassen D. K., Weir M. R., Wiland A., Fink J. C., Bartlett S. T., Cangro C. B., Blahut S., Papadimitriou J. C. (1999) Transplantation 68, 396–402 [DOI] [PubMed] [Google Scholar]
- 58.Iourgenko V., Zhang W., Mickanin C., Daly I., Jiang C., Hexham J. M., Orth A. P., Miraglia L., Meltzer J., Garza D., Chirn G. W., McWhinnie E., Cohen D., Skelton J., Terry R., Yu Y., Bodian D., Buxton F. P., Zhu J., Song C., Labow M. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12147–12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conkright M. D., Canettieri G., Screaton R., Guzman E., Miraglia L., Hogenesch J. B., Montminy M. (2003) Mol. Cell 12, 413–423 [DOI] [PubMed] [Google Scholar]
- 60.Kang J. H., Kim M. J., Ko S. H., Jeong I. K., Koh K. H., Rhie D. J., Yoon S. H., Hahn S. J., Kim M. S., Jo Y. H. (2006) Diabetologia 49, 969–979 [DOI] [PubMed] [Google Scholar]
- 61.Kim M. J., Kang J. H., Park Y. G., Ryu G. R., Ko S. H., Jeong I. K., Koh K. H., Rhie D. J., Yoon S. H., Hahn S. J., Kim M. S., Jo Y. H. (2006) J. Endocrinol. 188, 623–633 [DOI] [PubMed] [Google Scholar]
- 62.Friedrichsen B. N., Neubauer N., Lee Y. C., Gram V. K., Blume N., Petersen J. S., Nielsen J. H., Møldrup A. (2006) J. Endocrinol. 188, 481–492 [DOI] [PubMed] [Google Scholar]
- 63.Bosco D., Armanet M., Morel P., Niclauss N., Sgroi A., Muller Y. D., Giovannoni L., Parnaud G., Berney T. (2010) Diabetes 59, 1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brissova M., Fowler M. J., Nicholson W. E., Chu A., Hirshberg B., Harlan D. M., Powers A. C. (2005) J. Histochem. Cytochem. 53, 1087–1097 [DOI] [PubMed] [Google Scholar]
- 65.Cabrera O., Berman D. M., Kenyon N. S., Ricordi C., Berggren P. O., Caicedo A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butler P. C., Meier J. J., Butler A. E., Bhushan A. (2007) Nat. Clin. Pract. Endocrinol. Metab. 3, 758–768 [DOI] [PubMed] [Google Scholar]
- 67.De Vos A., Heimberg H., Quartier E., Huypens P., Bouwens L., Pipeleers D., Schuit F. (1995) J. Clin. Invest. 96, 2489–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiaschi-Taesch N., Bigatel T. A., Sicari B., Takane K. K., Salim F., Velazquez-Garcia S., Harb G., Selk K., Cozar-Castellano I., Stewart A. F. (2009) Diabetes 58, 882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mayr B., Montminy M. (2001) Nat. Rev. Mol. Cell Biol. 2, 599–609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.