Abstract
Deposition of amyloid β (Aβ) in the brain is closely associated with Alzheimer disease (AD). Aβ is generated from amyloid precursor protein (APP) by the actions of β- and γ-secretases. In addition to Aβ deposition in the brain parenchyma, deposition of Aβ in cerebral vessel walls, termed cerebral amyloid angiopathy, is observed in more than 80% of AD individuals. The mechanism for how Aβ accumulates in blood vessels remains largely unknown. In the present study, we show that brain endothelial cells expressed APP770, a differently spliced APP mRNA isoform from neuronal APP695, and produced Aβ40 and Aβ42. Furthermore, we found that the endothelial APP770 had sialylated core 1 type O-glycans. Interestingly, Ο-glycosylated APP770 was preferentially processed by both α- and β-cleavage and secreted into the media, suggesting that O-glycosylation and APP processing involved related pathways. By immunostaining human brain sections with an anti-APP770 antibody, we found that APP770 was expressed in vascular endothelial cells. Because we were able to detect O-glycosylated sAPP770β in human cerebrospinal fluid, this unique soluble APP770β has the potential to serve as a marker for cortical dementias such as AD and vascular dementia.
Keywords: Alzheimer Disease, Amyloid, Endothelium, Glycoprotein Secretion, Secretion
Introduction
Alzheimer disease (AD)3 is characterized by intracellular accumulation of neurofibrillary tangles and extracellular deposits of amyloid β (Aβ) peptides in the brain (1, 2). Aβ is generated from amyloid precursor protein (APP) by the sequential actions of β-secretase, BACE1 (β-site APP-cleaving enzyme 1) (3–6), and γ-secretase (7). Because most early-onset familial AD patients have gene mutations that influence the processing or aggregation of Aβ, and the neurites associated with Aβ plaques are often damaged (2), the process of Aβ deposition in the brain seems to be strongly associated with AD pathogenesis. Even though APP has been proposed to have a receptor-like function and binds to multiple extracellular matrix proteins such as heparin and collagen (8, 9), understanding the biological functions of APP remains an important scientific and intellectual challenge (10). Two paralogs of APP are known in mammals and are designated APP-like proteins 1 and 2. Interestingly, triple knock-out mice lacking all three APP family members die shortly after birth (11), suggesting redundant functions of the APP family proteins.
APP has three alternatively spliced isoforms: APP695, APP751, and APP770 (12, 13). Compared with APP695, APP751 contains an additional Kunitz-type protease inhibitor (KPI) domain, whereas APP770 contains a KPI domain plus an OX2 domain. APP695 is most abundantly expressed in neurons, whereas APP751 and APP770 show more ubiquitous expression patterns (14). The secreted form of APP containing a KPI domain, also known as the protease nexin 2, potentially inhibits certain serine proteases, most notably several prothrombotic enzymes (15); very limited information is available concerning the functions of the OX2 domain. In the present study, we show that brain microvascular endothelial cells (BMECs) express significant levels of APP770, from which Aβ40 and Aβ42 are produced, and that the endothelial APP770 has multiple Ο-glycosylation chains, which potentially play important roles in APP processing.
EXPERIMENTAL PROCEDURES
Materials
The sources of the materials used in this study were as follows: tissue culture media and reagents including DMEM from Invitrogen; recombinant peptide N-glycosidase (PNGase) from New England BioLabs; O-glycosidase from Roche Applied Science; Arthrobacter ureafaciens sialidase from Nacalai Tesque; protein A-Sepharose Fast Flow from GE Healthcare; protein molecular weight standards from Bio-Rad; peanut agglutinin (PNA; biotinylated Arachis hypogaea) lectin and a series of lectin-conjugated agaroses from Seikagaku Corp.; Aβ40 from Peptide Institute; BCA protein assay reagents and sulfo-NHS-LC-biotin from Thermo Fisher Scientific; all other chemicals were from Sigma or Wako Chemicals. An anti-APP (C15) rabbit polyclonal antibody against endogenous membrane-bound (intact) APP was a generous gift from Dr. Kei Maruyama (Saitama Medical University, Saitama, Japan). The commercially available antibodies used were as follows: mouse monoclonal anti-APP 22C11 (Chemicon); anti-human sAPPα (6E10; Signet Laboratories); anti-GAPDH (Chemicon); anti-KPI (Chemicon); anti-OX2 (Chemicon); anti-human sAPPβ (IBL Co.); goat polyclonal anti-platelet endothelial cell adhesion molecule (Santa Cruz Biotechnology). The clinical study was approved by the Ethical Committee of RIKEN and Fukushima Medical University (No. 613). All animal experiments were performed in compliance with the institutional guidelines for animal experiments of RIKEN.
Expression Plasmids and Cell Culture
Human APP770 FLAG-pcDNA was constructed by inserting a human APP770 sequence generated by PCR (primers 1 and 2) at the SalI and HindIII sites of pcDNA, and a FLAG domain fragment was generated by annealing primers 3 and 4 at the HindIII and XbaI sites of pcDNA. A ViraPower Adenoviral Expression system (Invitrogen) was used to produce recombinant adenoviruses carrying human APP-FLAG, according to the manufacturer's protocol. A series of APP770-pcDNA3.1 mutants was generated using a QuikChange site-directed mutagenesis kit (Stratagene). To generate the APPOX2All mutant, in which all of the possible O-glycosylation sites in the OX2 domain were deleted, APPS346A,S348A was constructed first, followed by creation of the APPOX2All mutant using APPS346A,S348A as a template and the primers APPSOX2A forward and reverse. To generate the APPall mutant, in which all the reported O-glycosylation sites (16) in the whole APP770 region were deleted, we used the APPOX2all mutant as a template and the primers APPT366A,T367A and APPT651A to change Thr366, Thr367, and Thr651 to Ala. The primer information is shown in Table 1, A and B. Human BMECs (Applied Cell Biology Research Institute) were cultured in CS-C complete medium with or without FBS and used within four passages. Human umbilical vein endothelial cells (TaKaRa) were cultured in EMB-2 (TaKaRa) containing 2% FBS and EGM-2TM SingleQuots (TaKaRa) and used within four passages. Primary liver sinusoidal endothelial cells were prepared from mouse livers using CD146 MicroBeads (Miltenyi Biotech) according to the manufacturer's protocol (17). COS-7 cells were cultured in DMEM containing 10% FBS. Cortical neurons were isolated from C57BL/6CrSlc mice as described previously (18).
TABLE 1.
Primers used in this study
Patients and Samples
A patient (60 years of age) who died from a nonketotic hyperosmolar coma was recruited for this study. Cerebrospinal fluid (CSF) samples were collected from patients with AD.
PCR Analysis of APP Transcripts
Total RNA was isolated from cells using the Sepasol reagent (Nacalai Tesque, Inc.), and 5 μg of the isolated RNA was reverse-transcribed with random hexamers using a SuperScript III RT kit (Invitrogen) according to the manufacturer's protocol. The obtained cDNA samples were subjected to PCR analyses with primers A and D for APP695, APP751, and APP770, primers B and D for APP751 and APP770, and primers C and D for APP770. PCR was performed for 28 cycles (95 °C for 40 s, 56 °C for 40 s, and 72 °C for 90 s). The primer information is shown in Table 1C.
Immunohistochemistry
Brain tissue was fixed in phosphate-buffered 15% formalin solution and embedded in paraffin. A pair of serial sections (5-μm thick) was stained with hematoxylin and eosin or incubated with an anti-OX2 antibody (1:100) overnight at 37 °C followed by biotinylated anti-rabbit IgG (1:200). Bound antigens were visualized with the avidin and biotin-peroxidase complex method (ABC kit, Vector Laboratories).
APP Detection
Cell lysates were solubilized in T-PER buffer (Thermo Fischer Scientific, Inc.) containing a protease inhibitor mixture (Roche Applied Science). For lectin precipitation, BMEC lysates (0.1 mg of protein) were incubated with Sambucus sieboldiana agglutinin-, Maackia amurensis agglutinin-, PNA-, phytohemagglutinin-E4-, Ricinus communis agglutinin 120-, concanavalin A-, or jacalin-agarose (20 μl each) for 16 h. For APP immunoprecipitation, BMEC lysates (0.25 mg of protein) were incubated with the anti-APP C15 antibody (5 μg) for 16 h. The precipitated samples were washed three times with T-PER buffer prior to SDS-PAGE analysis or sialidase treatment. Human CSF (0.2–0.5 ml) or media from cultured cells (0.5–1 ml) were incubated with heparin-Sepharose (Thermo Fisher Scientific). The precipitates were washed three times with PBS. The samples were then subjected to SDS-PAGE (5–20% gradient gel) and transferred to nitrocellulose membranes. For Western blot analyses, the membranes were incubated with anti-APP 22C11 (1:1000 dilution), anti-APP C15 (1:1000 dilution), anti-sAPPβ (1:500 dilution), anti-sAPPα (1:1000 dilution), anti-KPI (1:250 dilution), or anti-OX2 (1:250 dilution) antibodies. Appropriate horseradish peroxidase-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories), anti-mouse, and anti-rabbit IgG (GE Healthcare) antibodies were used as the secondary antibodies (1:1,000 dilution). For lectin blot analyses, the membranes were incubated with PNA-biotin (1:100 dilution) and horseradish peroxidase-streptavidin (1:1000 dilution; GE Healthcare). A chemiluminescent substrate (Thermo Fisher Scientific) was used for detection of the bound antibodies. As a loading control, we detected GAPDH using the same membranes by incubation with an anti-GAPDH antibody (1:250 dilution; Chemicon). The detected signals were quantified with a luminoimage analyzer LAS-1000 PLUS (Fujifilm).
Aβ Quantification
BMECs were infected with adenovirus preparations for APP770-FLAG overexpression and cultured in Opti-MEM for 8 h. The levels of Aβ40 and Aβ42 in the media were determined using a human amyloid β(1–40) or (1–42) assay kit (IBL Co.) for BMECs and a human/rat β-amyloid(40) or (42) ELISA Kit (Wako Chemicals) for mouse neurons.
Glycosidase Digestion
Cell lysates, APP precipitates from cell lysates, culture media, and heparin-precipitated samples from culture media were incubated in the presence or absence of Arthrobacter ureafaciens sialidase (4 milliunits) and/or O-glycosidase (2 milliunits) for 18 h.
Cell Surface Biotinylation
BMECs grown in 10-cm culture plates were labeled with Sulfo-NHS-LC-Biotin for 30 min at 4 °C. After washing the plates three times with 0.1 m glycine in PBS (pH 8.0) and once with PBS, cell lysates were prepared. NHS-SS-biotin was used for internalization assays. After two washes with ice-cold PBS, the cells were cultured in prewarmed CS-C media for 0 or 1 h at 37 °C. After washing once with ice-cold CS-C media and twice with PBS/10% FBS, the remaining cell surface biotin was removed by incubating the cells in buffer containing the nonpermeant reducing agent GSH (50 mm GSH, 75 mm NaCl, 0.3% NaOH, and 10% FBS) twice for 20 min each at 4 °C (19). After the reaction was quenched by 5 mg/ml of iodoacetamide in PBS containing 1% BSA, cell lysates were prepared. Biotinylated cell surface proteins were pulled down from the lysates with streptavidin-Sepharose (GE Healthcare) for further analysis.
Benzyl N-Acetyl Galactosamine (GalNAc) Treatment
Subconfluent BMECs grown on 10-cm tissue culture plates were incubated in the presence of benzyl GalNAc (2 mm) for 18 h, before cell lysates and culture media samples were prepared for further analysis.
RESULTS
Brain Endothelial Cells Express APP770
The human brain expresses three alternatively spliced APP mRNA isoforms: APP695, APP751, and APP770 (12, 13) (Fig. 1A), whereas neurons exhibit restricted expression of APP695 (14, 20, 21). This suggests that cell type-specific APP splicing events occur in the brain. In the present study, we focused on brain endothelial cells and established primary human BMECs to characterize the brain endothelial APP. Western blot analysis using an anti-APP C-terminal antibody (C15) showed that BMECs expressed equivalent or rather higher levels of APP compared with primary neurons (Fig. 1B). Interestingly, the endothelial APP exhibited two discrete bands, based on gel mobility after SDS-PAGE. Because the anti-KPI and anti-OX2 antibodies also detected these signals, we concluded that endothelial cells expressed APP770. Neither the anti-KPI nor the anti-OX2 antibodies detected neuronal APP, thereby confirming previous reports that neurons solely express APP695 (14, 20). When we analyzed BMEC lysates with the anti-KPI or anti-OX2 antibodies, we observed an additional band between the two APP bands. This additional band is most likely the processed form of APP, sAPP, which lacks the C-terminal sequence of APP. Using the OX2 antibody, we could detect APP770 in endothelial cells prepared from mouse brains (supplemental Fig. S1). Furthermore, human umbilical vein endothelial cells also expressed the two forms of APP, although their expression levels were significantly lower than those in BMECs (Fig. 1B), indicating that APP770 is broadly expressed in endothelial cells.
FIGURE 1.
Characterization of APP in brain endothelial cells. A, schematic diagrams of the three alternatively spliced APP isoforms: APP695, APP751, and APP770. Compared with APP695, APP751 has an additional KPI domain, whereas APP770 has a KPI domain plus an OX2 domain. The recognition sites of a series of anti-APP antibodies and primers A, B, C, and D used to analyze the APP transcripts are shown. Two N-glycosylation sites are also shown. B, Western blot analysis of APP in cell lysates (20 μg of protein) from BMECs, mouse primary neurons and human umbilical vein endothelial cells using anti-APP C15, anti-KPI, anti-OX2, anti-platelet endothelial cell adhesion molecule (PECAM), and anti-GAPDH antibodies. C, total RNA was extracted from BMECs, neurons, human umbilical vein endothelial cells (HUVEC), and liver sinusoidal endothelial cells (LSEC), reverse-transcribed, and then subjected to PCR to detect the respective APP transcripts. APP695, APP751, and APP770 plasmids were used as standards.
To analyze the APP transcripts, we isolated RNA from endothelial cells and primary neurons. Reverse-transcribed cDNA samples were then analyzed by PCR using a series of oligonucleotide primers to detect APP695, APP751, and APP770. The results confirmed the cell type-specific APP expression, as neurons expressed APP695, whereas endothelial cells expressed APP770 and not APP695 (Fig. 1C).
Endothelial APP770 Has Sialylated Core 1 Type O-Glycans
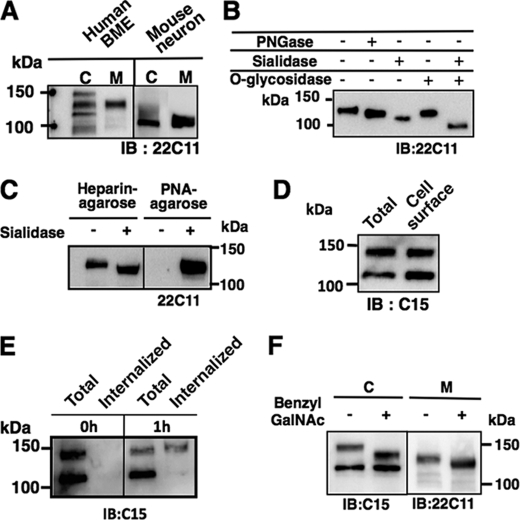
Because APP potentially has two N- and multiple O-glycans (16, 22, 23), we expected that the high molecular weight APP770 (APP-H) would be heavily glycosylated. We first performed glycosidase treatment, in which sialidase-treated and -untreated APP were incubated with PNGase. We found that the low molecular weight APP770 (APP-L) was resistant to sialidase treatment, indicating that APP-L contains no sialic acid (Fig. 2A). After PNGase treatment, both APP-H and APP-L moved slightly faster on SDS-PAGE gels, indicating that both forms contain N-glycan chains, although their molecular weight difference cannot be explained by N-glycans alone. Furthermore, the mobility difference between APP-H and de-N-glycosylated APP-H on SDS-PAGE gels was similar to that after sialidase treatment. This suggests that few sialic acids, if any, were present in the N-glycans of APP-H. Indeed, it seems that both APP-H and APP-L were sensitive to endoglycosidase H, which cleaves high mannose and some hybrid types of N-glycans (supplemental Fig. S2). After sialidase treatment, the mobility of APP-H shifted markedly and became closer to that of APP-L (Fig. 2, A and B). Similarly, after treatment with O-glycosidase, which only cleaves unsubstituted core 1 and 3 type O-glycans, the mobility of APP-H perfectly matched that of APP-L (Fig. 2B). We also found that sialidase-treated APP-H reacted with PNA lectin that specifically detects core 1 type O-glycans (Fig. 2C). Furthermore, a series of lectin pulldown assays showed that APP-H and APP-L have affinities for Sambucus sieboldiana agglutinin- and concanavalin A-lectin, respectively (Fig. 2D). In addition to the above results, judging from the fact that Sambucus sieboldiana agglutinin lectin recognizes Siaα2,6Gal/GalNAc, we concluded that APP-H has α2,6-sialylated core 1 type O-glycans.
FIGURE 2.

Glycan analysis of brain endothelial APP. A, BMEC lysates (20 μg of protein) were incubated in the presence or absence of PNGase and with or without sialidase. The digested samples were analyzed using the anti-APP C15 antibody. B, BMEC lysates (6 μg of protein) were incubated in the presence or absence of sialidase O-glycosidase and then immunostained with the anti-APP C15 antibody. C, APP precipitated from BMEC lysates (0.25 mg of protein) using the anti-APP C15 antibody (5 μg) was incubated in the presence or absence of sialidase (4 milliunits). The digested samples were subjected to Western blot analysis with the anti-APP 22C11 antibody or PNA lectin blot analysis. D, BMEC lysates (100 μg of protein) were incubated with Sambucus sieboldiana agglutinin (SSA)-, Maackia amurensis agglutinin (MAA)-, E4-phytohemagglutinin (PHA-E4)-, concanavalin A (ConA)-, Ricinus communis agglutinin (RCA)120-, or jacalin-agarose for 16 h at 4 °C. The lectin-precipitated samples were washed and analyzed by Western blotting with the anti-APP C15 antibody. IB, immunoblot.
When we overexpressed APP695, APP751, or APP770 in COS cells, mobility differences between the two APP forms were always clearly observed in the case of APP770 (Fig. 3A). These preliminary data suggested the presence of O-glycans in the OX2 domain. Therefore, we generated a series of APP770 mutants in which each Ser/Thr residue within the OX2 domain was individually changed to Ala (Fig. 3B). We then overexpressed wild-type APP770 and its mutants in COS cells. Even though the O-glycosylation machinery is not well developed in COS cells, we detected a lower level of APP-H (Fig. 3C). Mutations at Ser346, Ser348, and Thr352 had no effect on the mobility of the APP mutants on SDS-PAGE gels, whereas the APPT353A mutant, in which Thr353 was changed to Ala, migrated faster than wild-type APP770 (Fig. 3, B and C). These results suggest that an O-glycan is attached to Thr353. The findings that both APPT353A and APPOX2all, in which all of the Ser/Thr residues within the OX2 domain were mutated to Ala, still exhibited two discrete bands suggested the presence of additional O-glycosylation sites in domains other than the OX2 domain in APP770. Perdivara et al. (16) recently showed that APP695 is modified with O-glycans at Thr291, Thr292, and Thr576 (Thr366, Thr367, and Thr651 for APP770). Therefore, we next generated the APP770 mutant APPall, in which Thr366, Thr367, Thr651, and Thr652 were additionally changed to Ala from APPOX2all. APPall mostly showed a single band (Fig. 3C), indicating that we had mutated all of the O-glycosylation sites of APP770 in the APPall mutant.
FIGURE 3.
Analysis of a series of APP770 mutants. A, human APP695, APP751, and APP770 were individually overexpressed in COS cells. The obtained cell lysates (1 μg of protein) together with lysates of BMECs (5 μg of protein) and neurons (10 μg of protein) were analyzed by Western blotting with the anti-APP C15 antibody. B, schematic diagrams of the APP770 mutants used in this study. C, wild-type APP770 or its mutants were expressed in COS cells and subjected to Western blot analysis with the anti-APP C15 antibody. The black and gray arrowheads show APP-H and APP-L, respectively. cont, control; IB, immunoblot.
O-Glycosylated APP770 Is Preferentially Secreted to Media
Next, we analyzed the APP metabolites in endothelial cells. We performed Western blot analyses of BMEC lysates and culture media with the anti-APP 22C11 antibody, which recognizes the N-terminal APP region and therefore detects sAPP. We observed three bands in the cell lysates using the anti-22C11 antibody. In the media, we detected a single sAPP band that migrated with the middle band observed in the cell lysates (Fig. 4A), suggesting that sAPP is solely derived from APP-H. Indeed, sAPP was sensitive to both sialidase and O-glycosidase treatments (Fig. 4B). Furthermore, after sialidase treatment, most of the sAPP was precipitated with PNA lectin (Fig. 4C), indicating that sAPP mostly contains sialylated core 1 type O-glycan chains. It should be noted that we failed to detect sAPP without O-glycans. Considering that APP cleavage at the α-site seems to occur at the cell surface, whereas cleavage at the β-site occurs during the endocytotic pathway (24), it is possible that APP without O-glycosylation is unable to move to the cell surface to encounter either α- or β-secretase. However, cell surface biotinylation experiments showed that both APP-H and APP-L reached the cell surface (Fig. 4D). We next studied how cell surface APP is metabolized in the cell. To analyze APP internalization, we next labeled the surface of BMECs with a disulfide cleavable biotinylation reagent (19). Internalization was then induced at 37 °C for 1 h. Interestingly, we found that only APP-H was endocytosed (Fig. 4E). This suggests that cell surface APP-H uses a specific intracellular trafficking pathway to encounter both α- and β-secretase. Furthermore, benzyl GalNAc, an inhibitor of O-glycan chain elongation, failed to inhibit sAPP secretion into the media (Fig. 4F).
FIGURE 4.
Characterization of sAPP secreted from BMECs. A, intact APP in cell lysates (6 μg) and soluble secreted sAPP pulled-down with heparin-agarose from the media of BMECs and mouse primary neurons were analyzed by Western blotting with the anti-APP 22C11 antibody. C, cell lysates; M, media. B, sAPP pulled down with heparin-agarose from the BMEC media was incubated in the presence or absence of sialidase and O-glycosidase and then analyzed by immunoblotting (IB) with the anti-APP 22C11 antibody. C, media from BMEC cultures (0.5 ml) were incubated in the presence or absence of Arthrobacter ureafaciens sialidase (4 milliunits) for 18 h. The digested samples were incubated with heparin-agarose or PNA agarose (20 μl each). The precipitated samples were analyzed by Western blotting with the anti-APP 22C11 antibody. D, following cell-surface biotinylation of BMECs, biotinylated cell surface proteins were precipitated with streptavidin-Sepharose and then analyzed by Western blotting with the anti-APP C15 antibody. E, after cell-surface labeling of BMECs using NHS-SS-biotin, the cells were incubated for 0 or 1 h to allow internalization of the biotinylated proteins. Cell surface biotin was then stripped by reductive treatment. Total proteins, or internalized biotinylated proteins that were precipitated with streptavidin-Sepharose, were analyzed by Western blotting with the anti-APP C15 antibody. F, BMECs were cultured in the presence of benzyl GalNAc, and then intact APP and sAPP were analyzed by Western blotting with the anti-APP C15 and 22C11 antibodies, respectively.
Aβ is Produced from Brain Endothelial Cells
Since there is limited information regarding the expression levels and activities of the endothelial α-secretase and β-secretase enzymes for the processing of APP770, we characterized the soluble sAPP770 secreted from BMECs. Using specific antibodies against sAPPα and sAPPβ, we successfully detected both sAPP770α and sAPP770β (Fig. 5A). Since the amyloidogenic β-secretase pathway is present in endothelial cells, it is reasonable to consider that these cells also have γ-secretase activity to produce Aβ peptides. Even though endogenous Aβ was not detectable, we detected both Aβ40 and Aβ42 in the culture media of BMECs overexpressing APP770, and the ratio of endothelial Aβ42/Aβ40 was similar to that in neurons (Fig. 5B).
FIGURE 5.
Analysis of APP770 in the human brain. A, sAPP770 secreted from BMECs was analyzed by Western blotting with anti-APP 22C1, anti-OX2, anti-sAPPα, and anti-sAPPβ antibodies. B, culture media from BMECs cultured in Opti-MEM were analyzed for their levels of Aβ40 and Aβ42 (n = 3; upper panel). The ratios of Aβ42/Aβ40 secreted from BMECs and neurons are shown as the means ± S.E. (n = 4) (lower panel). C, paraffin-embedded human cerebral sections were analyzed by hematoxylin-eosin staining (upper panel) and immunostaining with the anti-OX2 antibody (lower panel). The gray and black arrowheads show the nuclei of endothelial cells and smooth muscle cells, respectively. The arrow shows the OX2-immunoreactive endothelium. Scale bar, 20 μm. D, sAPP in human CSF samples (0.5 ml each) was pulled down with heparin-agarose (30 μl) and then analyzed by Western blotting with anti-APP 22C11, anti-sAPPβ, and anti-OX2 antibodies.
APP770 is Expressed in Cerebral Vessels and sAPP770β Is Secreted into the CSF
To clarify whether APP770 is indeed expressed in cerebral vessels, we first analyzed cerebral cortex sections with an anti-OX2 antibody to determine the localization of APP770. The luminal regions of the venous and venular endothelial cells, but not smooth muscle cells, were stained with the anti-OX2 antibody (Fig. 5C). No immunohistochemical signals were observed in the vessels of the arachnoid. Next, we investigated whether the CSF contains sAPP770β, the N-terminal β-secretase cleavage product of APP770. sAPP was pulled-down from CSF using heparin-agarose and then immunostained with anti-APP 22C11, anti-OX2 and anti-sAPPβ antibodies. We detected two bands with the anti-APP 22C11 antibody, of which only the upper band was detected with the anti-OX2 antibody (Fig. 5D), indicating that the upper band is derived from APP770. Since both the upper and lower bands were detected with the anti-sAPPβ antibody, both forms contain β-secretase cleavage products.
DISCUSSION
The dementia of AD is closely associated with accumulation of Aβ in the brain parenchyma and in the walls of blood vessels in the brain (25–27). Aβ deposition in the cerebral vasculature contributes to cerebral amyloid angiopathy, which shows a prevalence of >80% among AD individuals and 10–40% in elderly people without AD (28). Microbleeds in the brain occur in most cerebral amyloid angiopathy cases (25). Although important roles for cerebral vascular smooth muscle cells in vascular Aβ clearance were recently highlighted (29), the regions where vascular Aβ is produced for deposition remain to be determined.
We have shown for the first time that brain endothelial cells express APP770. First, we unambiguously detected APP770 expression in human BMECs. Using an anti-APP770 antibody that recognizes the OX2 domain for immunohistochemical staining of the cerebral cortex, we found that APP770 was expressed in venous and venular endothelial cells. We also showed that Aβ40 and Aβ42 were produced in BMECs. Therefore, our study points out the possibility that endothelial Aβ peptides could be the source of the Aβ deposits in cerebral vessel walls. Even though our immunohistochemical study is somewhat different from the previous observations in which Aβ deposits are frequently found in cortical arteries, the major distribution of APP770 in the brain might be different from where Aβ is mainly produced from APP770 to cause vascular Aβ deposits.
Perdivara et al. (16) recently identified the core 1 type O-glycans attached to residues Thr291, Thr292 and Thr576 of APP695. Here, we have shown that APP770 has an additional O-glycan chain at residue Thr353 within the OX2 domain. Furthermore, a series of site-directed mutations within the KPI domain had no effect on the mobility of APP770 (Fig. S3), indicating the absence of O-glycans in the KPI domain. A large ectodomain of APP consists of several subdomains, such as the E1 (30), E2 (31), and KPI domains (32). The web application POODLE-S (Prediction of Order and Disorder by Machine Learning) predicts that the other regions, in which all of the O-glycans are located, are intrinsically unstructured (33) (supplemental Fig. S4). Therefore, it is unlikely that the addition of any O-glycans to APP770 would result in conformational changes that might affect the α- or β-secretase cleavage. Because we found that O-glycosylated APP is selectively internalized, how O-glycosylation of APP would modulate its intracellular trafficking to the same intracellular compartment as α- and β-secretase remains an interesting and unanswered question. Because APP695 was also shown to contain O-glycans, whether our finding applies to neuronal APP also remains to be resolved. We found that BMECs expressed higher amounts of O-glycosylated APP770 than did COS cells, suggesting that endothelial cells possess a highly developed O-glycosylation machinery. Interestingly, endothelial O-glycan deficiency causes blood/lymphatic misconnections (34), indicating a fundamental role for O-glycans in vascular development.
In this study, we were able to discriminate CSF sAPP770β from other types of sAPPβ. As BACE1 seems to be a stress-response protein (35), one of our ongoing projects is elucidation of the critical factors required to increase sAPP770β secretion. We are now establishing a sandwich ELISA system to quantify sAPP770β in CSF or plasma. Because the CSF sAPP770β appears to be mainly derived from brain endothelial cells, sAPP770β would be a potential biomarker for diagnosing cerebrovascular dementia or AD.
Supplementary Material
Acknowledgments
We thank Dr. Shoichi Ishiura (The University of Tokyo) for the human APP751 and APP770 pcDNAs and Dr. Kei Maruyama (Saitama Medical School) for the anti-APP C15 antibody. We also thank Dr. Makoto Higuchi (National Institute of Radiological Sciences) for valuable discussions.
This work was supported in part by grants from the Systems Glycobiology research project (to N. T.) and Grant 21570154 from the Ministry of Education, Science, Sports, and Culture of Japan (to S. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- AD
- Alzheimer disease
- Aβ
- amyloid β-peptide
- APP
- amyloid precursor protein
- BACE
- β-site APP-cleaving enzyme
- BMEC
- brain microvascular endothelial cell
- CSF
- cerebrospinal fluid
- KPI
- Kunitz-type protease inhibitor
- sAPP
- soluble APP
- PNA
- peanut agglutinin (Arachis hypogaea lectin)
- PNGase
- peptide-N glycosidase
- APP-H
- high molecular weight APP770
- APP-L
- low molecular weight APP770.
REFERENCES
- 1.Selkoe D. J. (2001) Physiol. Rev. 81, 741–766 [DOI] [PubMed] [Google Scholar]
- 2.Tanzi R. E., Bertram L. (2005) Cell 120, 545–555 [DOI] [PubMed] [Google Scholar]
- 3.Yan R., Bienkowski M. J., Shuck M. E., Miao H., Tory M. C., Pauley A. M., Brashier J. R., Stratman N. C., Mathews W. R., Buhl A. E., Carter D. B., Tomasselli A. G., Parodi L. A., Heinrikson R. L., Gurney M. E. (1999) Nature 402, 533–537 [DOI] [PubMed] [Google Scholar]
- 4.Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) Science 286, 735–741 [DOI] [PubMed] [Google Scholar]
- 5.Sinha S., Anderson J. P., Barbour R., Basi G. S., Caccavello R., Davis D., Doan M., Dovey H. F., Frigon N., Hong J., Jacobson-Croak K., Jewett N., Keim P., Knops J., Lieberburg I., Power M., Tan H., Tatsuno G., Tung J., Schenk D., Seubert P., Suomensaari S. M., Wang S., Walker D., Zhao J., McConlogue L., John V. (1999) Nature 402, 537–540 [DOI] [PubMed] [Google Scholar]
- 6.Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Nature 398, 513–517 [DOI] [PubMed] [Google Scholar]
- 7.De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J. S., Schroeter E. H., Schrijvers V., Wolfe M. S., Ray W. J., Goate A., Kopan R. (1999) Nature 398, 518–522 [DOI] [PubMed] [Google Scholar]
- 8.Beher D., Hesse L., Masters C. L., Multhaup G. (1996) J. Biol. Chem. 271, 1613–1620 [DOI] [PubMed] [Google Scholar]
- 9.Small D. H., Nurcombe V., Moir R., Michaelson S., Monard D., Beyreuther K., Masters C. L. (1992) J Neurosci. 12, 4143–4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinhard C., Hébert S. S., De Strooper B. (2005) EMBO J. 24, 3996–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herms J., Anliker B., Heber S., Ring S., Fuhrmann M., Kretzschmar H., Sisodia S., Müller U. (2004) EMBO J. 23, 4106–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. (1988) Nature 331, 525–527 [DOI] [PubMed] [Google Scholar]
- 13.Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. (1988) Nature 331, 528–530 [DOI] [PubMed] [Google Scholar]
- 14.Wertkin A. M., Turner R. S., Pleasure S. J., Golde T. E., Younkin S. G., Trojanowski J. Q., Lee V. M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 9513–9517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu F., Davis J., Miao J., Previti M. L., Romanov G., Ziegler K., Van Nostrand W. E. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18135–18140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perdivara I., Petrovich R., Allinquant B., Deterding L. J., Tomer K. B., Przybylski M. (2009) J. Proteome Res. 8, 631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitazume S., Imamaki R., Ogawa K., Komi Y., Futakawa S., Kojima S., Hashimoto Y., Marth J. D., Paulson J. C., Taniguchi N. (2010) J. Biol. Chem. 285, 6515–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hama E., Shirotani K., Masumoto H., Sekine-Aizawa Y., Aizawa H., Saido T. C. (2001) J. Biochem. 130, 721–726 [DOI] [PubMed] [Google Scholar]
- 19.Aroeti B., Mostov K. E. (1994) EMBO J. 13, 2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 6338–6342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato Y., Liu C., Wojczyk B. S., Kobata A., Spitalnik S. L., Endo T. (1999) Biochim Biophys Acta. 1472, 344–358 [DOI] [PubMed] [Google Scholar]
- 23.Tomita S., Kirino Y., Suzuki T. (1998) J. Biol. Chem. 273, 6277–6284 [DOI] [PubMed] [Google Scholar]
- 24.Ehehalt R., Keller P., Haass C., Thiele C., Simons K. (2003) J. Cell. Biol. 160, 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vonsattel J. P., Myers R. H., Hedley-Whyte E. T., Ropper A. H., Bird E. D., Richardson E. P., Jr. (1991) Ann. Neurol. 30, 637–649 [DOI] [PubMed] [Google Scholar]
- 26.Van Broeckhoven C., Haan J., Bakker E., Hardy J. A., Van Hul W., Wehnert A., Vegter-Van der Vlis M., Roos R. A. (1990) Science 248, 1120–1122 [DOI] [PubMed] [Google Scholar]
- 27.Rovelet-Lecrux A., Hannequin D., Raux G., Le Meur N., Laquerrière A., Vital A., Dumanchin C., Feuillette S., Brice A., Vercelletto M., Dubas F., Frebourg T., Campion D. (2006) Nat. Genet. 38, 24–26 [DOI] [PubMed] [Google Scholar]
- 28.Greenberg S. M., Gurol M. E., Rosand J., Smith E. E. (2004) Stroke 35, 2616–2619 [DOI] [PubMed] [Google Scholar]
- 29.Bell R. D., Deane R., Chow N., Long X., Sagare A., Singh I., Streb J. W., Guo H., Rubio A., Van Nostrand W., Miano J. M., Zlokovic B. V. (2009) Nat. Cell. Biol. 11, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahms S. O., Hoefgen S., Roeser D., Schlott B., Gührs K. H., Than M. E. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 5381–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Ha Y. (2004) Mol. Cell. 15, 343–353 [DOI] [PubMed] [Google Scholar]
- 32.Hynes T. R., Randal M., Kennedy L. A., Eigenbrot C., Kossiakoff A. A. (1990) Biochemistry 29, 10018–10022 [DOI] [PubMed] [Google Scholar]
- 33.Shimizu K., Hirose S., Noguchi T. (2007) Bioinformatics 23, 2337–2338 [DOI] [PubMed] [Google Scholar]
- 34.Fu J., Gerhardt H., McDaniel J. M., Xia B., Liu X., Ivanciu L., Ny A., Hermans K., Silasi-Mansat R., McGee S., Nye E., Ju T., Ramirez M. I., Carmeliet P., Cummings R. D., Lupu F., Xia L. (2008) J. Clin. Invest. 118, 3725–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassar R., Kovacs D. M., Yan R., Wong P. C. (2009) J. Neurosci. 29, 12787–12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.