Abstract
Endothelial cells (ECs) express a Nox2 enzyme, which, by generating reactive oxygen species (ROS), contributes to EC redox signaling and angiotensin II (AngII)-induced endothelial dysfunction. ECs also express abundantly an adenosine A2A receptor (A2AR), but its role in EC ROS production remains unknown. In this study, we investigated the role of A2AR in the regulation of Nox2 activity and signaling in ECs with or without acute AngII stimulation. In cultured ECs (SVEC4–10), AngII (100 nm, 30 min) significantly increased Nox2 membrane translocation and association with A2AR. These were accompanied by p47phox, ERK1/2, p38 MAPK, and Akt phosphorylation and an increased ROS production (169 ± 0.04%). These AngII effects were inhibited back to the control levels by a specific A2AR antagonist (SCH58261), or adenosine deaminase, or by knockdown of A2AR or Nox2 using specific siRNAs. Knockdown of A2AR, as determined by Western blotting, decreased Nox2 and p47phox expression. In wild-type mouse aorta, SCH58261 significantly reduced acute AngII-induced ROS production and preserved endothelium-dependent vessel relaxation to acetylcholine. These results were further confirmed by using aortas from A2AR knock-out mice. In conclusion, A2AR is involved in the regulation of EC ROS production by Nox2. Inhibition or blockade of A2AR protects ECs from acute AngII-induced oxidative stress, MAPK activation, and endothelium dysfunction.
Keywords: Cell Surface Receptor, Endothelium, Gene Knockout, MAP Kinases (MAPKs), Reactive Oxygen Species (ROS), Angiotensin II, Nox2
Introduction
Endothelial cell (EC)2 metabolism produces abundant adenosine, which signals through its four subtypes of G protein-coupled cell surface receptors (A1R, A2AR, A2BR, and A3R) and is involved in the regulation of vascular function (1, 2). Among these adenosine receptors, A2AR has been found to be extensively expressed in vascular ECs and had been widely reported to play a major role in mediating adenosine-induced endothelium-dependent vessel relaxation (3). Knock-out of A2AR reduced aortic relaxation and endothelial function in mice (4). However, this notion has been challenged by studies showing that A2AR was not involved in the relaxation of the isolated mouse aorta to adenosine and its analogues (5) and was not involved in mediating adenosine-induced Ca2+ influx in ECs, which is crucial for endothelium-dependent vessel relaxation (6).
ECs also express constitutively an NADPH oxidase (Nox), which, by generating reactive oxygen species (ROS) as second messengers, contributes to the regulation of EC function (7). The Nox enzyme comprises a cytochrome b, which can be further divided into one catalytic subunit (a member of the Nox family) and one p22phox. To date, five members of the Nox family have been identified (Nox1–5) (8), and Nox2 and Nox4 are the major Nox isoforms expressed in ECs and represent important enzymatic sources of EC ROS production (7, 9, 10). Nox2 is a highly glycosylated protein and requires the presence of regulatory subunits, i.e. p40phox, p47phox, p67phox, and rac1, for its activation (11). In response to pathophysiological stimulations such as shear stress, angiotensin II (AngII), or inflammatory cytokines (TNFα), the activity of Nox2 (but not Nox4) is up-regulated, and excessive ROS production from Nox2 outstrips endogenous antioxidant defense and causes EC dysfunction (7, 12).
Several studies have reported a role of A2AR in the regulation of ROS production by Nox2 enzyme, although the outcomes differed from one organ to another. For example, in neutrophils A2AR deficiency caused Nox2 activation, and increased O2˙̄ production exacerbated inflammatory responses and caused oxidative damage to tissues (13). In the lung, knock-out of A2AR increased tracheal ROS production from Nox2, which compromised tracheal relaxation in allergic mice (14). However, in the context of neurodegerative diseases, blockade of A2AR appeared to be beneficial in reducing oxidative damage (15). Similar results were found in the heart where genetic knock-out or pharmacological blockade of the A2AR decreases cardiac ROS production from Nox2 enzyme (16). Many patients suffering from neurodegenerative diseases have endothelial dysfunction characterized by excessive ROS production from Nox2 enzyme (17), and it is possible that A2AR blockade might be beneficial in these endothelial dysfunction-related neurodegenerative and cardiovascular diseases. This hypothesis has been supported by a recent study showing that knock-out of A2AR protects ApoE knock-out mice from atherosclerosis (18).
AngII has pleiotropic acute and chronic effects on many cell types and plays an important role in the pathophysiology of cardiovascular diseases, including hypertension, atherosclerosis, and heart failure (19). AngII is also a potent activator of Nox2, and increased ROS production contributes to AngII-induced EC dysfunction and vessel constriction (19). In the present study, we investigated in detail the effects of A2AR blockade or knockdown on basal and acute AngII-induced endothelial ROS production by Nox2 and on redox-signaling in cultured ECs and in mouse aortas isolated from wild-type (WT) and A2AR knock-out (KO) mice.
EXPERIMENTAL PROCEDURES
Materials and Reagents
Affinity-purified rabbit polyclonal antibodies to p40phox and Nox2 were kindly provided by Dr. F. Wientjes (University College London, UK). Polyclonal antibodies against p22phox, Nox2, Nox4, p40phox, p47phox, p67phox, rac1, and A2AR were from Santa Cruz Biotechnology. Antibodies to phospho-ERK1/2, phospho-p38 MAPK, phospho-JNK, and phospho-Akt were from Cell Signaling Technology. DHE (dihydroethidium) was purchased from Invitrogen. SCH58261 (7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazole-[4,3-e]-1,2,4-triazolo[1,5-c] pyrimidine), CGS21680, (2-[4-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine), and other reagents and chemicals were from Sigma unless stated otherwise.
Animals
WT and A2AR KO male CD1 mice at 10–12 weeks of age were used for aorta isolation. All studies were performed in accordance with protocols approved by the Home Office under the Animals (Scientific Procedures) Act 1986 UK.
Cell Culture and Cell Stimulation
The mouse lymph node microvascular endothelial cell line (SVEC4–10) was obtained from the American Type Culture Collection and grown in Dulbecco's modified Eagle's medium (DMEM), containing 10% (v/v) heat-inactivated fetal calf serum, 100 IU of penicillin, and 100 mg/ml streptomycin. Cells were seeded on the day before the experiment to achieve ∼90% confluence and preincubated with vehicle (5% FCS/DMEM or 5%FCS/DMEM/0.5% dimethyl dulfoxide) as control or with a selective A2AR antagonist (SCH58261, 100 nm dissolved in 0.5% dimethyl sulfoxide) or adenosine deaminase (2 units/ml in 5% FCS/DMEM) or a selective A2AR agonist CGS21680 (100 nm in 5% FCS/DMEM/0.5% dimethyl sulfoxide) for 30 min. 100 nm AngII was added after that and incubated for 30 min in the presence of SCH58261 or adenosine deaminase or CGS21680. Cells were washed three times with PBS and scraped into ice-cold Hanks' balanced salt solution supplemented with 0.8 mm MgCl2 and 1.8 mm CaCl2. Cells were disrupted by rapid freezing in liquid nitrogen followed by homogenization and sonication. Cell homogenates were used for measuring ROS production or for immunoblotting.
In Vitro Knockdown of A2AR and Nox2 Using siRNAs
These experiments were performed as described previously (9). The control siRNA and A2AR siRNA were purchased from Santa Cruz Biotechnology. The nucleotide sequences of Nox2 siRNA and a random negative control siRNA were exactly as described previously (20) and synthesized by VWR International, LLC. Lipofectamine 2000 plusTM (Invitrogen) was used as a transfection reagent in serum-free DMEM as described previously (9). Forty-eight hours after the transfection, cells were used for further experiments.
ROS Production
O2˙̄ production by homogenates of cultured cells was measured using lucigenin (5 μm)-enhanced chemiluminescence (BMG Lumistar, Germany) as described previously (16). O2˙̄ production was expressed as arbitrary mean light units/min measured over 20 min. The specificity of O2˙̄ thus measured was confirmed by adding 10 mm tiron, a nonenzymatic scavenger of O2˙̄, to quench the O2˙̄-dependent chemiluminescence. Other enzymatic sources of O2˙̄ production were also identified by preincubation of homogenates with inhibitors such as N-ω-nitro-l-arginine methyl ester (100 μm), rotenone (50 μm), oxypurinol (100 μm), and diphenyleneiodonium (20 μm).
As alternative approaches, ROS generation in adherent cells or in aorta sections with or without AngII (30 min) stimulation were also measured by 2.7-dichlorofluorescein (200 nm) or DHE (2 μm) fluorescence, respectively (9). Images were captured under confocal microscopy, and the fluorescence intensity was quantified from at least three random fields (1,024 × 1,024 pixels) per slide, from three slides per experimental condition. Experiments were repeated at least three times for cell cultures or using aortic sections from at least six animals.
Immunoblotting
This was performed exactly as described previously (9). Equal amounts of protein from different samples were loaded, and α-tubulin in the same sample was used as a loading control. The blots were then developed using ECL reagent (Amersham Biosciences), and images were captured using an imaging system (UVP BioImager) and quantified. For the quantification of AngII-induced MAPK and Akt phosphorylation, the levels of phosphorylated specific bands were normalized to the levels of the same total protein detected in the same sample.
Immunofluorescence Confocal Microscopy
Experiments were performed as described previously (16). Briefly, cells were cultured onto chamber slides and fixed, and slides were coated. Primary antibodies were used at 1:250–500 dilution in PBS with 0.1% BSA for 30 min at room temperature. Biotin-conjugated anti-rabbit or anti-goat (1:1,000 dilution) were used as secondary antibodies. Specific binding was detected by extravidin-FITC or streptavidin-Cy3. Images were acquired on a Zeiss LS510 confocal microscopy system.
Aorta Organ Bath Experiments
Thoracic aortas (n = 9 mice) were carefully dissected free from surrounding fat tissue and cut into 3–4-mm-long rings. The aorta rings were incubated at 37 °C with 200 nm AngII in serum-free DMEM in the presence or absence of either 100 nm SCH58261 or 20 mm tiron for 45 min. Aortic rings were then suspended in an organ bath (37 °C) containing Krebs-Henseleit solution (119 mm NaCl, 4.7 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 2.5 mm CaCl2, 25 mm NaHCO3, 11.1 mm glucose, pH 7.4) gassed with 95% O2/5% CO2, and connected to isometric force displacement transducers. Endothelium-dependent relaxation to acetylcholine (0.001–10 μm added cumulatively) and endothelium-independent vessel relaxation to sodium nitroprusside (0.0001–1 μm added cumulatively) were tested in rings preconstricted to 70% of their maximal phenylephrine (0.001–10 μm added cumulatively)-induced tension.
Statistics
Data were presented as means ± S.D. from at least three experimental results taken from three independent cell cultures for each condition. In the case of the organ bath studies, nine mice were used, and the data presented are the mean ± S.D. from these results. Comparisons were made by an unpaired t test, with Bonferonni correction for multiple testing. p < 0.05 was considered statistically significant.
RESULTS
Effects of A2AR Blockade on EC ROS Production and Signaling
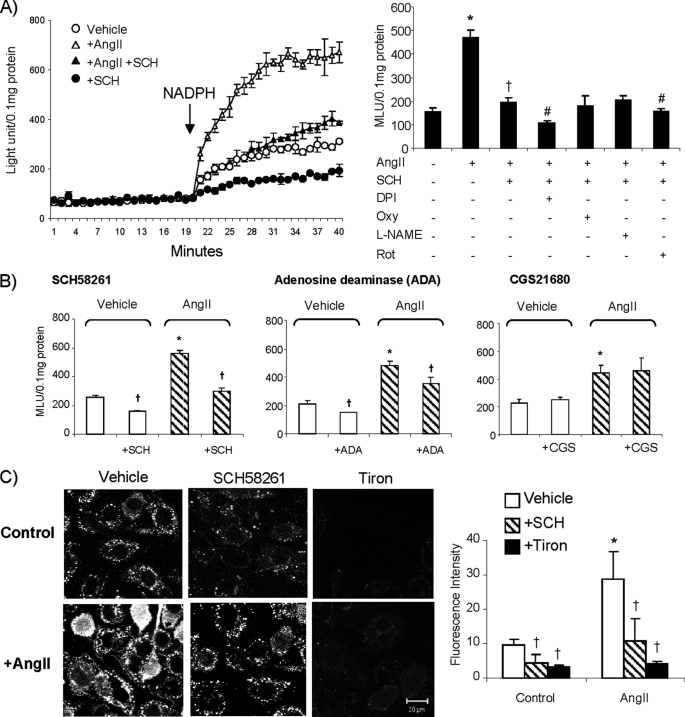
To assess the effects of A2AR blockade on EC ROS production, we treated cells with a selective A2AR antagonist SCH58261 (100 nm for 60 min) and examined the NADPH-dependent O2˙̄ production by lucigenin chemiluminescence (Fig. 1, A and B). There was a basal level of O2˙̄ production by cells maintained in culture medium only. Treatment of cells with SCH58261 slightly but significantly reduced basal (without AngII) NADPH-dependent O2˙̄ production. AngII (100 nm for 30 min) stimulation significantly increased O2˙̄ production, and this was significantly inhibited by diphenyleneiodonium (82 ± 1.9%, a flavoprotein inhibitor) and tiron (61 ± 1.5%, an O2˙̄ scavenger) but not by oxypurinol (a xanthine oxidase inhibitor), or N-ω-nitro-l-arginine methyl ester (a nitric oxide synthase inhibitor). There was a slight but significant reduction in ROS levels by rotenone (21 ± 9%, an inhibitor of mitochondria complex-1 enzymes) suggestive of some low level involvement of mitochondria (data not shown).
FIGURE 1.
EC ROS production. A, O2˙̄ production detected by lucigenin chemiluminescence. Left panel, kinetic measurement of O2˙̄. NADPH was added after 20 min of measurement. Right panel, effect of different enzyme inhibitors on NADPH-dependent O2˙̄ production in the presence of SCH58261. *, p < 0.05 for the indicated values versus the values without AngII. †, p < 0.05 for the indicated values versus AngII values. #, p < 0.05 for the indicated values versus values of AngII + SCH58261. B, effects of SCH58261, adenosine deaminase (ADA), and CGS21680 on NADPH-dependent O2˙̄ production measured by lucigenin chemiluminescence. MLU, mean light unit. C, O2˙̄ production by intact adherent cells detected by DCF fluorescence. *, p < 0.05 for the indicated AngII values versus vehicle control values. †, p < 0.05 for indicated values versus the values without SCH58261 or adenosine deaminase or CGS21680 or tiron in the same group. Error bars, S.D.
AngII-induced ROS production was inhibited back to the control levels in the presence of SCH58261. When cells were treated with AngII plus SCH58261, the inhibitory effect of diphenyleneiodonium on ROS production was reduced to 49 ± 8%, but the inhibition by rotenone remained the same (24 ± 9%) (Fig. 1A, right). These data suggested that the inhibitory effect of SCH58261 was on flavoproteins but not on mitochondrial enzymes. We examined also the effect of adenosine depletion by pretreatment of ECs with 2 units/ml adenosine deaminase. Similar to the SCH58261 effect, depletion of adenosine significantly reduced both basal and the AngII-induced EC O2˙̄ production (Fig. 1B, center). Preincubation of ECs with a selective A2AR agonist CGS21680 (100 nm) had no significant effect on the basal or the acute AngII-induced ROS production (Fig. 1B, right).
As an alterative method, we detected ROS production (without adding NADPH) by in situ DCF fluorescence on adherent ECs (Fig. 1C). Similar to the results from lucigenin chemiluminescence, treatment of cells with SCH58261 significantly reduced the basal DCF fluorescence. AngII stimulation significantly increased the DCF fluorescence, and this increase was significantly inhibited back to control levels in ECs treated with SCH58261. The detection of O2˙̄ was confirmed by using tiron, a cell membrane-permeable O2˙̄ scavenger, which almost abolished the DCF fluorescence.
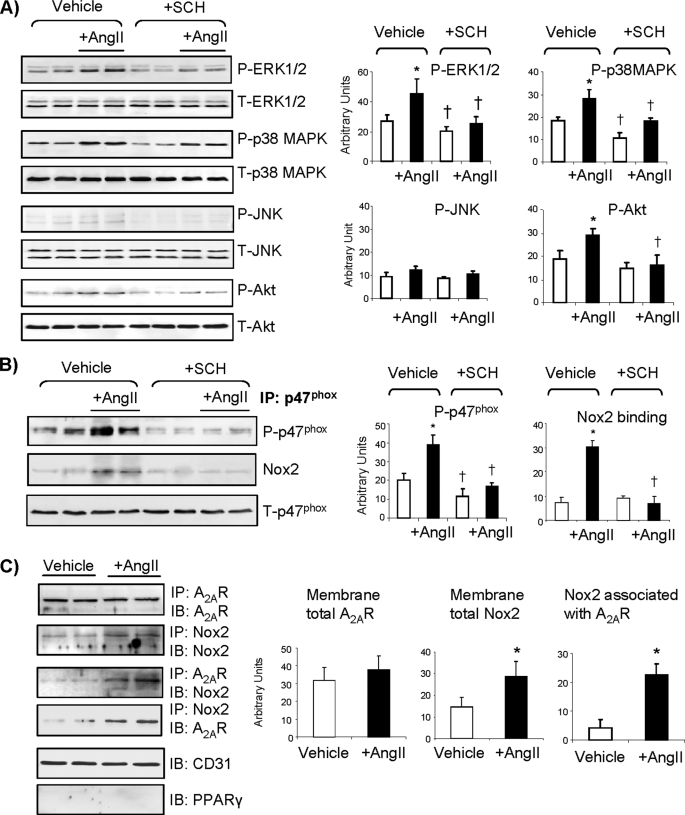
The effects of the A2AR antagonist SCH58261 on AngII-induced redox-sensitive MAPK and Akt activation were investigated using specific monoclonal antibodies against phospho-ERK1/2, phospho-p38 MAPK, phospho-JNK and phospho-Akt (Fig. 2). There was some level of phosphorylation of ERK1/2 and p38 MAPK detected in the control cells, and the levels of their phosphorylation were significantly less in cells treated with SCH58261. AngII stimulation (30 min) increased ERK1/2 and p38 MAPK phosphorylation significantly, and these AngII effects were absent in cells pretreated with SCH58261 (Fig. 2A). Similarly, AngII stimulation caused significant Akt phosphorylation, and this was completely inhibited by SCH58261. The levels of phosphorylated JNK were very low, and it was hard to see any changes.
FIGURE 2.
Effects of SCH58261 on protein phosphorylation and expression. A, the levels of phospho-protein bands were quantified and normalized to the levels of the total proteins detected in the same samples. B, p47phox was immunoprecipitated down and detected for serine phosphorylation and binding to Nox2. C, two-way immunoprecipitation for the detection of AngII-induced Nox2 association with A2AR is shown. *, p < 0.05 for AngII values versus vehicle controls. †, p < 0.05 for SCH58261values versus values without SCH58261 in the same treatment group. Error bars, S.D.
p47phox phosphorylation and binding to Nox2 have been found to be a prerequisite of Nox2 enzyme activation (19). To explore the link between A2AR blockade to ERK1/2 and p38 MAPK inactivation, and the inhibition of Nox2-derived ROS production, we immunoprecipitated down the p47phox and examined the effects of A2AR blockade on AngII-induced p47phox serine phosphorylation and the complex formation with Nox2. We found that SCH58261 significantly reduced the levels of both basal (without AngII) and AngII-induced p47phox phosphorylation, and this resulted in a significant reduction of Nox2 co-immunoprecipitated down with p47phox (Fig. 2B). These results indicated that reduced ROS production by A2AR blockade was due to the inhibition of p47phox phosphorylation and Nox2 activation.
AngII-induced Nox2 Membrane Translocation and Association with A2AR
Nox2 has been reported to translocate to the EC membrane in response to AngII stimulation (7, 12); therefore, we investigated the potential link between Nox2 and A2AR in response to AngII stimulation by two-way co-immunoprecipitation. We immunoprecipitated down A2AR and detected the presence of Nox2 and then confirmed this by immunoprecipitating down Nox2 and detecting the presence of A2AR. We found no changes in the level of membrane A2AR expression in response to acute AngII stimulation. However, there were significant increases in the levels of membrane Nox2 expression and association with the A2AR (Fig. 2C). The purity of the membrane preparation was confirmed by the positive expression of CD31, which is an EC surface marker, and the negative expression of peroxisome proliferator-activated receptor γ, which is a nuclear receptor.
Effects of A2AR Knockdown on Nox2 Expression, ROS Production, and AngII Signaling
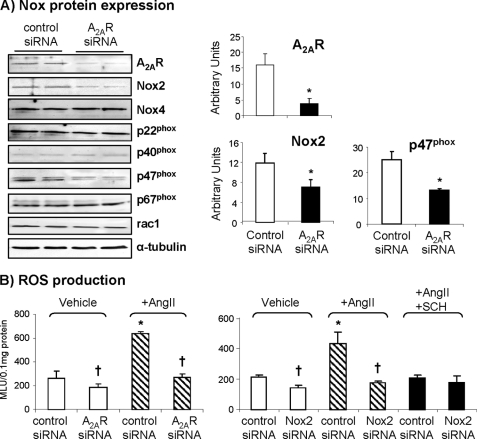
To define further the role of A2AR in acute AngII-induced ROS production by Nox2 enzyme and endothelial dysfunction, we performed in vitro transient knockdown of A2AR using siRNA and examined the protein levels of A2AR, Nox2, Nox4, and p22phox and the regulatory subunits of Nox2, i.e. p40phox, p47phox, p67phox, and rac1 by immunoblotting (Fig. 3A). A2AR protein was detected in cells transfected with a scrambled control siRNA, and the level of expression was significantly reduced to just detectable in cells transfected with A2AR siRNA. Knockdown of A2AR significantly reduced the protein levels of Nox2 and p47phox, which is a major regulatory subunit of the Nox2 enzyme. However, the levels of Nox4, p22phox, p40phox, p67phox, and rac1 were not significantly affected by A2AR knockdown (Fig. 3A). We then looked at the NADPH-dependent ROS production and found that knockdown of A2AR significantly reduced both the basal and acute AngII-induced ROS production in these cells (Fig. 3B, left). Parallel experiments with Nox2 siRNA further confirmed that AngII-induced ROS production was from Nox2 enzyme. Thus, in vitro knockdown of Nox2 significantly reduced the basal ROS production and completely abolished the AngII-induced ROS production. Adding SCH58261 significantly reduced AngII-induced ROS production in cells transfected with control siRNA and had no effect on cells tranfected with Nox2 siRNA (Fig. 3B, right).
FIGURE 3.
Effects of A2AR siRNA on Nox2 expression and activity. A, protein bands were quantified and normalized to the levels of α-tubulin detected in the same samples. B, NADPH-dependent O2˙̄ production detected by lucigenin chemilunescence. MLU, mean light unit. *, p < 0.05 for indicated values versus vehicle control siRNA values. †, p < 0.05 for indicated values versus control siRNA values in the same treatment group. Error bars, S.D.
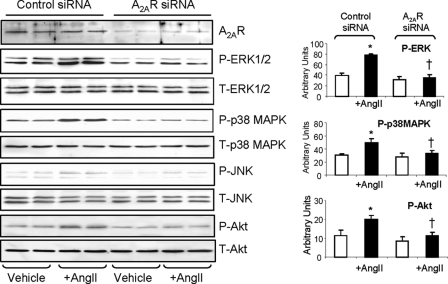
The effects of A2AR knockdown on acute AngII-induced phosphorylation of MAPKs and Akt was also investigated (Fig. 4). Supportive of the results with the A2AR antagonist, SCH58261, knockdown of A2AR completely abolished AngII-induced phosphorylation of ERK1/2, p38 MAPK, and Akt compared with cells transfected with control siRNA. The level of phosphorylated JNK was again almost undetectable. Put together, our data strongly suggested that A2AR is involved in the regulation of endothelial ROS production by Nox2, and this requires the ERK1/2, p38 MAPK and Akt signaling. Blockade or knockdown of A2AR inhibited MAPK activation and thereafter inhibited AngII effects on EC ROS production.
FIGURE 4.
Effects of A2AR siRNA on MAPK and Akt phosphorylation. The phospho-protein bands were quantified and normalized to the total levels of the same proteins detected in the same samples. *, p < 0.05 for AngII values versus values without AngII in the same group. †, p < 0.05 for indicated values versus AngII values in control siRNA group. Error bars, S.D.
Effects of SCH57261 or Genetic knock-out of A2AR on Mouse Aorta ROS Production and Relaxation
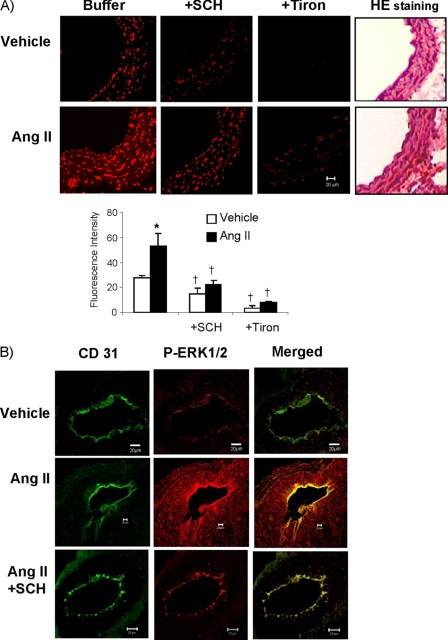
To clarify the in vivo relevance of A2AR blockade by SCH58261 on vascular function, we freshly isolated mouse aortas, incubated the aortic rings with or without 200 nm AngII (for 45 min) and then examined the ROS production by DHE fluorescence on vessel sections. Tiron was used to confirm the detection of O2˙̄ (Fig. 5A). Similar to our cell culture experimental results, acute AngII stimulation significantly increased DHE fluorescence throughout the vessel wall, and SCH58261 treatment significantly reduced both the basal and the AngII-induced DHE fluorescence. Vessel structure was shown by hematoxylin and eosin staining on parallel sections. Because ERK1/2 phosphorylation was a prominent response to acute AngII stimulation in cell experiments, we looked at AngII-induced ERK1/2 phosphorylation in the sections of aortas pretreated with or without SCH58261 (Fig. 5B). To help to visualize the endothelium, the section was labeled with CD31 which is an EC marker, and ERK1/2 phosphorylation was detected using a specific monoclonal antibody. Compared with control vessels treated with vehicle only, ERK1/2 phosphorylation was seen throughout the vessel wall and strongly in the endothelium in AngII-stimulated aortas, and this was largely inhibited in vessels pretreated with A2AR antagonist SCH58261.
FIGURE 5.
ROS production and ERK1/2 phosphorylation in mouse aorta sections. A, ROS production detected by DHE fluorescence. Tiron (a specific O2˙̄ scavenger) was used to confirm the detection of O2˙̄. *, p < 0.05 for AngII values versus vehicle values. †, p < 0.05 for indicated values versus values in the same treatment group without SCH58261 or tiron. n = 6 animals. B, confocal images of ERK1/2 phosphorylation in aortic sections. CD31 (an endothelial marker) was labeled in green (FITC), and phospho-ERK1/2 was labeled in red (Cy3). The yellow color in merged images indicates ERK1/2 phosphorylation detected in the endothelium. Error bars, S.D.
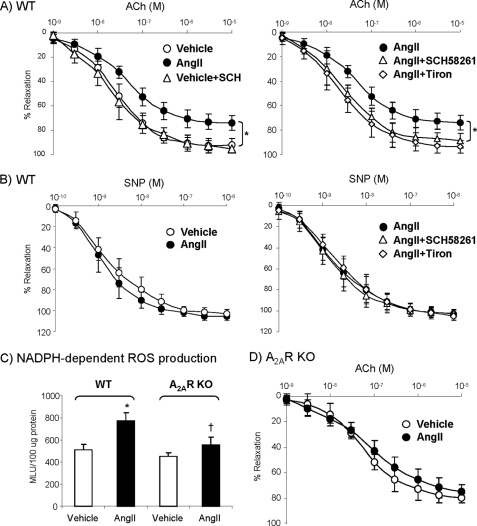
We then looked at the effects of SCH58261 on endothelium-dependent vessel relaxation to acetylcholine using WT aortic rings (Fig. 6A). Compared with control aortas, treatment with 200 nm AngII (for 45 min) severely compromised the endothelium-dependent vessel relaxation to acetylcholine. Addition of 100 nm SCH58261 or 20 mm tiron during AngII stimulation preserved endothelium-dependent vessel relaxation to acetylcholine back to the control levels. There was no significant difference in vessel relaxation to sodium nitroprusside, an endothelium-independent vasodilator, under the same treatments confirming that the SCH58261 effects were on the endothelium not the vascular smooth muscle. (Fig. 6B). To determine further the role of A2AR on promoting AngII-induced ROS, we used aortas isolated from A2AR KO mice and examined the ROS production by aorta homogenates. Knock-out of A2AR significantly reduced AngII-induced ROS production (Fig. 6C) and inhibited AngII impairment of endothelium-dependent vessel relaxation to acetylcholine (Fig. 6D) compared with WT vessel (Fig. 6A).
FIGURE 6.
Effect of SCH58261 or A2AR KO on vessel relaxation. A, endothelium-dependent relaxation to acetylcholine of WT aortic rings. *, p < 0.05 for AngII values versus vehicle controls (left panel) or versus the values in the presence of SCH58261 or tiron (right panel). B, endothelium-independent relaxation to sodium nitroprusside (SNP)of WT aortic rings. C, NADPH-dependent ROS production detected by lucigenin chemiluminescence. MLU, mean light unit. *, p < 0.05 for AngII versus vehicle controls in WT. †, p < 0.05 for A2AR KO versus WT treated with AngII. D, endothelium-dependent relaxation to acetylcholine (ACh) of A2AR KO aortic rings. Error bars, S.D.
DISCUSSION
Endothelial dysfunction characterized by excessive ROS production from Nox2 activation has been found to play an important role in the pathogenesis of many cardiovascular diseases such as hypertension and atherosclerosis. Therapies that inhibit Nox2 activation are urgently required to protect the endothelium from ROS damage. The present study provides the first evidence that inactivation of A2AR through pharmacological blockade or in vitro knockdown or genetic knock-out of A2AR effectively inhibits endothelial ROS production from Nox2 and attenuates AngII-induced oxidative stress, MAPK activation, and endothelial dysfunction.
A2AR are highly expressed on ECs (21). Classically, A2AR activation in ECs was believed to mediate endothelium-dependent vessel relaxation, and genetic A2AR deficiency has been shown to result in loss of endothelium-mediated relaxation (3, 4). However, A2AR inactivation has been shown to protect ApoE knock-out mice from atherosclerosis, which may provide the potential for treating atherosclerosis (18). Although the role of endothelial A2AR and the involvement of Nox2-derived ROS production were not examined in that particular study, a study from another group by cross-breeding p47phox knock-out mice and ApoE knock-out mice demonstrated a similar result of reduced atherosclerosis and clearly pointed out a crucial role of Nox2-derived ROS in the pathogenesis of atherosclerosis (22). Our study extends and supports this suggestion and provides a novel possible mechanism for this effect. In the current study we carried out a detailed investigation on the effects of A2AR blockade on ROS production by ECs. We found that treatment of cells with a specific A2AR antagonist, SCH58261, significantly inhibited ROS production from Nox2 and thereafter abolished acute AngII-induced MAPK and Akt activation. We have also shown that A2AR blockade inhibited AngII-induced p47phox phosphorylation and complex formation with Nox2, which are prerequisites for AngII-induced Nox2 activation and ROS production (12). The removal of adenosine by adenosine deaminase also reduced ROS production, which added further evidence for the requirement of A2AR signaling for ROS production. The lack of effect of the selective A2AR agonist CGS21680 may be because (i) enough adenosine is already present in the culture medium to activate the A2AR at its maximum level, and this is supported by the experiment using adenosine deaminase such that eliminating adenosine mimics the effect of SCH58261 or (ii) an increase in A2AR activity alone cannot promote further ROS production under culture conditions.
The inhibitory effects of A2AR blockade on Nox2 activity were further confirmed by showing that transient knockdown of A2AR using siRNA reduced significantly the ROS production and the protein expression of Nox2 and p47phox, but not Nox4. Moreover, knockdown of Nox2 using siRNA completely abolished AngII-induced ROS production by EC. Similar results were obtained in vessel sections where SCH58261 inhibited AngII-induced ROS production as detected by DHE fluorescence, and preincubation of vessels with SCH58261 attenuated AngII effects on impairing vessel relaxation and preserved endothelial function. In line with these studies, we found also that genetic knock-out of A2AR inhibited ROS production in the aortas and preserved endothelial function.
AngII is the dominant effector of the renin-angiotensin system and is implicated in the pathogenesis of disorders such as hypertension, where one of the major mechanisms of its effects is through oxidative damage to the endothelium due to Nox2 activation (12). The present study is the first report to demonstrate the potential of an A2AR antagonist to attenuate acute AngII-induced Nox2 activation. Thus, in cultured EC or in aortic vessels pretreated with SCH58261, AngII-induced ROS production and MAPK and Akt activation were abrogated in particular in the endothelium, and SCH58261 or knock-out of A2AR preserved endothelium-dependent vessel relaxation to acetylcholine. Although vascular smooth muscle cells are the predominant cellular component in the vessel wall, Nox2 expression is very low or undetectable in vascular smooth muscle cells.
Both the MAPK family and Akt are the downstream signaling pathways of A2AR, via Gs/cAMP-dependent (1) or -independent pathways (23), and ERK1/2 and p38MAPK have been found to phosphorylate p47phox. Therefore, the potential mechanistic link from A2AR blockade to the reduction of both basal and AngII-induced Nox2 activation is that the A2AR blockade inhibited MAPK activation and thereafter reduced the levels of p47phox phosphorylation and binding to Nox2. Long term A2AR inactivation (in the case of A2AR knockdown) results in reduced Nox2 expression. Our data clearly demonstrated that A2AR signaling is necessary to promote AngII-induced MAPK and p47phox phosphorylation. Another interesting observation from the current study is that AngII induced Nox2 plasma membrane translocation and association with A2AR, and this was confirmed by the two-way co-immunoprecipitation of Nox2 or A2AR. A2AR has been found to bind to several signaling molecules, including ERK1/2 and p38 MAPK (24). The significance of AngII-induced Nox2 association with A2AR requires further investigation.
In summary, we have reported for the first time that blockade of A2AR with an antagonist, SCH58261, removal of adenosine by adenosine deaminase, knockdown of A2AR using siRNA, or genetical knock-out of A2AR effectively inhibited basal and acute AngII-induced ROS production by Nox2 in ECs. This in turn significantly protected endothelium function from AngII-induced oxidative damage. Antagonists to A2AR may therefore have therapeutic potential to inhibit Nox2 activation and to treat diseases related to endothelial oxidative stress.
This work was supported by the British Heart Foundation Grant PG/06/073/21118 and the Wellcome Trust Grant 078637/Z/05/Z.
- EC
- endothelial cell
- A2AR
- adenosine A2A receptor
- AngII
- angiotensin II
- DHE
- dihydroethidium
- Nox
- NADPH oxidase
- ROS
- reactive oxygen species
- DCF
- 2,7-dichlorafluorescein.
REFERENCES
- 1.Fredholm B. B., Ijzerman A. P., Jacobson K. A., Klotz K. N., Linden J. (2001) Pharmacol. Rev. 53, 527–552 [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson K. A., Gao Z. G. (2006) Nat. Rev. Drug Discov. 5, 247–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabrizchi R., Bedi S. (2001) Pharmacol. Ther. 91, 133–147 [DOI] [PubMed] [Google Scholar]
- 4.Ponnoth D. S., Sanjani M. S., Ledent C., Roush K., Krahn T., Mustafa S. J. (2009) Am. J. Physiol. Heart Circ. Physiol. 297, H1655–H1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentice D., Boon K., Hourani S. (2001) Eur. J. Pharmacol. 415, 251–255 [DOI] [PubMed] [Google Scholar]
- 6.Cheng K. T., Leung Y. K., Shen B., Kwok Y. C., Wong C. O., Kwan H. Y., Man Y. B., Ma X., Huang Y., Yao X. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 913–918 [DOI] [PubMed] [Google Scholar]
- 7.Li J. M., Shah A. M. (2004) Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1014–R1030 [DOI] [PubMed] [Google Scholar]
- 8.Bengtsson S. H., Gulluyan L. M., Dusting G. J., Drummond G. R. (2003) Clin. Exp. Pharmacol. Physiol. 30, 849–854 [DOI] [PubMed] [Google Scholar]
- 9.Fan L. M., Teng L., Li J. M. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1651–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J. M., Fan L. M., George V. T., Brooks G. (2007) Free Radic. Biol. Med. 43, 976–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumimoto H., Miyano K., Takeya R. (2005) Biochem. Biophys. Res. Commun. 338, 677–686 [DOI] [PubMed] [Google Scholar]
- 12.Li J. M., Wheatcroft S., Fan L. M., Kearney M. T., Shah A. M. (2004) Circulation 109, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 13.Ernens I., Rouy D., Velot E., Devaux Y., Wagner D. R. (2006) Circ. Res. 99, 590–597 [DOI] [PubMed] [Google Scholar]
- 14.Nadeem A., Ponnoth D. S., Ansari H. R., Batchelor T. P., Dey R. D., Ledent C., Mustafa S. J. (2009) J. Pharmacol. Exp. Ther. 330, 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melani A., Gianfriddo M., Vannucchi M. G., Cipriani S., Baraldi P. G., Giovannini M. G., Pedata F. (2006) Brain Res. 1073–1074, 470–480 [DOI] [PubMed] [Google Scholar]
- 16.Ribé D., Sawbridge D., Thakur S., Hussey M., Ledent C., Kitchen I., Hourani S., Li J. M. (2008) Free Radic. Biol. Med 44, 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park L., Anrather J., Zhou P., Frys K., Pitstick R., Younkin S., Carlson G. A., Iadecola C. (2005) J. Neurosci. 25, 1769–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Zhang W., Zhu C., Bucher C., Blazar B. R., Zhang C., Chen J. F., Linden J., Wu C., Huo Y. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1046–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landmesser U., Cai H., Dikalov S., McCann L., Hwang J., Jo H., Holland S. M., Harrison D. G. (2002) Hypertension 40, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hingtgen S. D., Tian X., Yang J., Dunlay S. M., Peek A. S., Wu Y., Sharma R. V., Engelhardt J. F., Davisson R. L. (2006) Physiol. Genomics 26, 180–191 [DOI] [PubMed] [Google Scholar]
- 21.Sands W. A., Palmer T. M. (2005) Immunol. Lett. 101, 1–11 [DOI] [PubMed] [Google Scholar]
- 22.Barry-Lane P. A., Patterson C., van der Merwe M., Hu Z., Holland S. M., Yeh E. T. H., Runge M. S. (2001) J. Clin. Invest. 108, 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte G., Fredholm B. B. (2003) Cell. Signal. 15, 813–827 [DOI] [PubMed] [Google Scholar]
- 24.Zezula J., Freissmuth M. (2008) Br. J. Pharmacology 153, S184–S190 [DOI] [PMC free article] [PubMed] [Google Scholar]