Abstract
Integrin α1β1 is a collagen receptor that down-regulates collagen and reactive oxygen species (ROS) production, and mice lacking this receptor show increased ROS levels and exacerbated glomerular sclerosis following injury. Caveolin-1 (Cav-1) is a multifunctional protein that is tyrosine-phosphorylated in response to injury and has been implicated in ROS-mediated injury. Cav-1 interacts with integrins, and integrin α1β1 binds/activates T cell protein-tyrosine phosphatase (TCPTP), which is homologous to the tyrosine phosphatase PTP1B known to dephosphorylate Cav-1. In this study, we analyzed whether phosphorylated Cav-1 (pCav-1) is a substrate of TCPTP and if integrin α1β1 is essential for promoting TCPTP-mediated Cav-1 dephosphorylation. We found that Cav-1 phosphorylation is significantly higher in cells lacking integrin α1β1 at base line and following oxidative stress. Overexpression of TCPTP leads to reduced pCav-1 levels only in cells expressing integrin α1β1. Using solid phase binding assays, we demonstrated that 1) purified Cav-1 directly interacts with TCPTP and the integrin α1 subunit, 2) pCav-1 is a substrate of TCPTP, and 3) TCPTP-mediated Cav-1 dephosphorylation is highly increased by the addition of purified integrin α1β1 or an integrin α1 cytoplasmic peptide to which TCPTP has been shown to bind. Thus, our results demonstrate that pCav-1 is a new substrate of TCPTP and that integrin α1β1 acts as a negative regulator of Cav-1 phosphorylation by activating TCPTP. This could explain the protective function of integrin α1β1 in oxidative stress-mediated damage and why integrin α1-null mice are more susceptible to fibrosis following injury.
Keywords: Caveolae, Integrin, Phosphotyrosine, Reactive Oxygen Species (ROS), Protein-tyrosine Phosphatase (Tyrosine Phosphatase)
Introduction
Caveolin-1 (Cav-1) is a key component of caveolae, plasma membrane invaginations enriched in sphingolipids and cholesterol, which function in lipid metabolism, transcytosis, and receptor trafficking (1). Cav-1 interacts with and regulates the localization and function of various transmembrane proteins (1, 2). For instance, Cav-1 negatively regulates TGF-β signaling (3) as well as EGF receptor expression and activation (4). In addition, Cav-1 participates in integrin-mediated signaling, as demonstrated by the finding that Cav-1-mediated integrin β1 endocytosis is critical for regulation of fibronectin turnover (5).
Cav-1 is phosphorylated at tyrosine 14 by the Src family kinases in response to various stimuli, including growth factor-mediated signaling, mechanical stretch, and hyperosmolarity (6–10). Cav-1 is also phosphorylated in response to oxidative stress, since hydrogen peroxide induces Cav-1 phosphorylation in endothelial cells (11–13), fibroblasts (7, 10, 14) and renal cells (15). Finally, Cav-1 phosphorylation increases in vivo in brain injury (16) and in the spinal cord following experimental autoimmune encephalomyelitis (17). However, whether increased pCav-1 contributes to or protects from injury is controversial at present. In this context, increased expression of pCav-1 is associated with cell survival after oxidative stress (15); however, in contrast to these findings, Cav-1 phosphorylation increases paclitaxel-induced apoptosis of breast cancer cells (18) and is required for oxidative stress-induced pulmonary vascular hyperpermeability, a key mediator of acute lung injury (19). Interestingly, Cav-1-null mice are resistant to this type of acute lung injury, and expression of wild type, but not Y14A Cav-1 mutant, in Cav-1-null lung microvessels restores the oxidative stress-induced vascular hyperpermeability (19), suggesting that that tyrosine phosphorylation of Cav-1 plays an important role in the pathogenesis of oxidant-induced pulmonary vascular hyperpermeability. Therefore, decreasing Cav-1 phosphorylation might be beneficial for limiting oxidative stress-induced injury.
Cav-1 can be dephosphorylated by protein-tyrosine phosphatases (PTPs).3 At present, two PTPs, namely low molecular weight PTP (20) and the endoplasmic reticulum-localized PTP1B (21), have been shown to dephosphorylate Cav-1 by directly binding to it. This interaction was shown in vitro using purified proteins. A third tyrosine phosphatase, PTP mu, has also been implicated in Cav-1 dephosphorylation (22); however, it is unclear whether PTP mu directly interacts with Cav-1.
PTP1B is homologous to T cell protein-tyrosine phosphatase (TCPTP), and the two PTPs have overlapping substrate specificity (23). TCPTP is ubiquitously expressed, and it can be found both in the nucleus, as a 45-kDa form, and the endoplasmic reticulum, as a 48-kDa form (24). The nuclear form translocates to the cytoplasm in response to growth factor receptor activation, cellular stress, and oxidative stress (25). TCPTP was also shown to directly interact with the cytoplasmic tail of the integrin α1 subunit using a yeast two-hybrid system (26). This interaction is necessary for the recruitment of TCPTP to the plasma membrane (26) and its activation by the integrin α1 cytoplasmic tail displacing the TCPTP inhibitory C-terminal segment (27).
Integrin α1β1-mediated TCPTP activation leads to increased dephosphorylation of TCPTP substrates, such as the EGF receptor (26), VEGF receptor 2 (28), and PDGF-β receptor (29). This dephosphorylation is required for the negative regulation of the signaling by these receptors. Consistent with this finding, we have shown that integrin α1-null cells and mice have increased basal levels of phosphorylated EGF receptor associated with increased production of profibrotic reactive oxygen species (ROS) (30).
Integrin α1β1 is a major collagen IV receptor that negatively regulates EGF receptor activation (30), collagen IV synthesis, and ROS production (31). Because 1) loss of integrin α1β1 leads to increased ROS production and impaired activation of TCPTP, 2) pCav-1 is associated with oxidative stress-induced injury, and 3) TCPTP is homologous to PTP1B, we hypothesized that loss of integrin α1β1 leads to increased basal levels of pCav-1. We provide evidence that pCav-1 is a new substrate of TCPTP and that TCPTP-mediated Cav-1 dephosphorylation is dependent on integrin α1β1. Thus, the integrin α1β1/TCPTP axis negatively regulates the levels of pCav-1, a key mediator of oxidative stress-mediated injury.
EXPERIMENTAL PROCEDURES
Materials
Chemical reagents were purchased from Sigma unless otherwise indicated. The peptide derived from the non-conserved integrin α1 subunit cytoplasmic domain (N-biotin-RPLKKKMEKRPLKKKMEK) was purchased from GenScript (Piscataway, NJ). TS2/7, a mAb to the extracellular domain of integrin α1β1 (32), was purified on Protein G-agarose (GE Healthcare) from hybridoma supernatants following the manufacturer's instructions. mAb FB12 to the extracellular domain of integrin α1 subunit and polyclonal antibody to the cytoplasmic domain of the integrin α1 subunit were purchased from Chemicon (Temecula, CA). mAb 2297 to Cav-1 and mAb 56 to phosphorylated tyrosine 14 of Cav-1 were purchased from BD Biosciences (San Jose, CA). Polyclonal antibody to the N terminus of Cav-1 was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). mAb 252294 to TCPTP and polyclonal antibody to TCPTP were purchased from R&D Systems (Minneapolis, MN). Phycoerythrin-conjugated anti-mouse IgG antibodies were purchased from Jackson ImmunoResearch (West Grove, PA), horseradish peroxidase (HRP)-conjugated antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and HRP-conjugated avidin was purchased from BD Biosciences (San Jose, CA). Recombinant human TCPTP-37 (amino acids 2–314) and recombinant human PTP1B (amino acids 2–321) were purchased from R&D Systems (Minneapolis, MN). Purified human integrin α1β1 in 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 2 mm MgCl2, containing 0.5% Triton X-100, was purchased from Chemicon (Temecula, CA).
Cell Culture and Transfection
Immortalized or primary mesangial cells (MCs) were isolated from wild type and integrin α1-null mice either crossed (immorto) or not (primary) onto the immorto-mouse background as described previously (31). Immortalized MCs were propagated at 33 °C in the presence of 100 IU/ml IFN-γ. For experiments, cells were cultured at 37 °C without IFN-γ for at least 4 days before use because this is the optimal time for conditionally immortalized MCs to acquire a phenotype similar to that of freshly isolated primary mesangial cells (31). Human embryonic kidney (HEK) 293 cells and CHO cells were maintained in DMEM supplemented with penicillin/streptomycin and 10% FBS. CHO cells expressing integrin α1 subunits (CHO-α1) were generated as described (33). To overexpress TCPTP, 3 × 105 cells in 6-well plates were transfected with 2 μg of human TCPTP either into pCMVSPORT6 for transient transfection or pIRESpuro for stable transfections using Lipofectamine Plus (Invitrogen), and cells were used for experiments 24 h after transfection. Stable clones overexpressing TCPTP were generated using puromycin selection.
Recombinant Protein
To generate full-length recombinant TCPTP (TCPTP-45) carrying an N-terminal His6 tag, TCPTP-45 was amplified using full-length human TCPTP-45 cDNA clone (Invitrogen) as a template, and BamHI and SalI restriction sites were added with the amplification primers and then cloned into pBG100 and transformed in Rosetta (DE3) competent Escherichia coli (Novagen, Madison, WI). Recombinant TCPTP-45 was purified from isopropyl β-d-thiogalactoside-induced bacterial lysates using a TALON metal affinity resin (Clontech, Mountain View, CA) and analyzed by SDS-PAGE and Western blot analysis.
To generate glutathione S-transferase (GST)-Cav-1, full-length human Cav-1 was cloned into pGEX-6p (GE Healthcare) between the BamHI and SalI sites. The plasmid encoding GST-Cav-1 was then transformed in BL21 (Novagen, Madison WI) competent E. coli to generate Cav-1. GST-Cav-1 was purified from isopropyl β-d-thiogalactoside-induced bacterial lysates using glutathione-agarose (Sigma) and analyzed by SDS-PAGE and Western blot analysis. In some experiments, the GST was cleaved from GST-Cav-1 using Pre-Scission protease (GE Healthcare) following the manufacturer's instructions. To generate phosphorylated Cav-1, the plasmid encoding GST-Cav-1 was transformed in TKB1 competent E. coli (Stratagene, La Jolla CA). This strain is a derivative of the BL21 strain with the exception that it harbors a plasmid encoding a tyrosine kinase gene inducible by the addition of indoleacrylic acid (10 μg/ml) (6).
To obtain the His-tagged integrin α1 construct carrying the 10 membrane proximal amino acids of the extracellular domain, the transmembrane domain, and cytoplasmic tail (His-α1TM/Cyt), the sequence encompassing these domains was cloned into a pET21b (Novagen, Madison, WI) between the NdeI and XhoI sites, transformed into BL21(DE3) E. coli cells, and then eluted from Ni2+-NTA resin (Qiagen, Chatsworth, CA) with 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 2 mm MgCl2. Immediately after elution, protein concentration was determined, and its identity was verified by Coomassie staining and immunoblotting. The protein was coated on ELISA plates within 24 h from the elution, which is the time period when this detergent-free preparation is stable.
Solid Phase Ligand Binding Assays
To determine Cav-1 binding, purified recombinant proteins (i.e. His-TCPTP-45, His-TCPTP-37, His-LOV, and His-α1TM/Cyt) were coated at 5 μg/ml in 50 mm sodium bicarbonate buffer, pH 9.5, at 4 °C. After 24 h, the plates were treated with 5 mm iodoacetamide for 30 min at room temperature and then blocked with 0.5% bovine serum albumin (BSA) in 50 mm Tris, pH 7.5, and 150 mm NaCl. Cav-1, at various concentrations in 50 mm Tris, pH 7.5, 150 mm NaCl, 0.1% BSA, and 0.1% Tween 20 was added to the wells and incubated overnight at 4 °C. Bound Cav-1 was detected after extensive washing (50 mm Tris, pH 7.5, 150 mm NaCl, 0.1% Tween) with anti-Cav-1 mAb, followed by HRP-conjugated anti-mouse IgG antibodies. HRP substrate (Bio-Rad) was added to wells, and the absorbance was monitored at 650 nm. BSA-coated wells were used as control for nonspecific binding. To determine nonspecific binding to His-TCPTP, some wells coated with His-TCPTP were incubated with BSA followed by anti-Cav-1 antibodies and HRP-conjugated secondary antibodies.
To determine integrin α1β1 binding to Cav-1, wells coated with increasing amounts of cleaved Cav-1 or GST (as a negative control) were incubated with 10 μg/ml purified human integrin α1β1 in 50 mm Tris, pH 7.5, 150 mm NaCl, 0.1% BSA, and 0.1% Tween 20, and then bound integrin was detected with anti-integrin α1 subunit and HRP-conjugated anti-mouse IgG antibodies.
To determine biotin-RPLKKKMEKRPLKKKMEK binding, His-TCPTP or cleaved Cav-1 was coated at 5 μg/ml as described above, and wells were then incubated with increasing amounts of biotin-RPLKKKMEKRPLKKKMEK in 50 mm Tris, pH 7.5, 150 mm NaCl, 0.1% BSA, and 0.1% Tween 20, followed by HRP-conjugated avidin.
Immunoprecipitation and Western Blotting
HEK 293 cells overexpressing full-length TCPTP were lysed in lysis buffer (1% CHAPS, 50 mm Tris, pH 7.5, 150 mm NaCl, 2 mm MgCl2, 5 mm iodoacetamide, and protease inhibitors (Roche Applied Science)), spun at 16,000 × g for 20 min, and preincubated with BSA-blocked Protein G beads for 1 h at 4 °C. Equal amounts of cell lysates (0.5 mg) were subsequently incubated overnight with either mAb TS2/7 (20 μg), mouse IgG1 isotype control (20 μg), or Protein G beads. The antibody-antigen complexes were captured with BSA-blocked Protein G-agarose beads for 1 h at 4 °C, washed five times with wash buffer (50 mm Tris, pH 7.5, 150 mm NaCl, and 0.1% Tween 20), eluted in sample buffer, resolved by SDS-PAGE in 4–20% gradient gels under reducing conditions, transferred, and then blotted with antibodies to the human integrin α1 subunit, Cav-1, and TCPTP-1.
To analyze the levels of phosphorylated Cav-1, serum-starved cells were treated with either 10% FBS for 30 min or 500 μm H2O2 for 30 min or with the indicated amounts of spermidine for 24 h. Cells were then lysed in sample buffer (62.5 mm Tris, pH 6.8, 2% SDS, 10% glycerol), and equal amounts of cell lysates were resolved on 12% SDS-PAGE, transferred, and probed with anti-Tyr(P)14 or anti-Cav-1 antibodies. Membranes were stripped and reincubated with either anti-FAK or anti-Akt to verify equal loading.
Cav-1 and pCav-1 bands were quantified by densitometry analysis, and the pCav-1 signal was expressed as a pCav-1/Cav-1 ratio. The pCav-1/Cav-1 ratio in control samples was arbitrarily assigned the value of 1, and all of the other pCav-1/Cav-1 ratios were expressed as -fold changes relative to the assigned control value.
In Vitro Caveolin-1 Dephosphorylation Assay
Purified pCav-1 was coated in bicarbonate buffer, pH 9.5, in the presence of protease inhibitors. After 16 h, the plates were blocked with blocking buffer (0.5% BSA in 50 mm Tris, pH 7.5, 150 mm NaCl) and incubated with or without full-length TCPTP (TCPTP-45) or truncated, constitutively active, TCPTP (TCPTP-37) in phosphatase buffer (20 mm Tris, pH 7.2, 0.2 mm EDTA, 0.1 mm EGTA, 2 mm DTT, 0.5 mg/ml BSA, 0.01% Brij, and protease inhibitors) in the presence or absence of purified integrin α1β1 or the α1 cytoplasmic peptide. After 30 min at room temperature, the wells were washed with wash buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 0.1% Tween 20), and the Tyr(P)14-Cav-1 was detected with anti-Tyr(P)14-Cav-1 antibody followed by HRP-conjugated anti-mouse-IgG antibody.
For Western blot analysis of Cav-1 dephosphorylation, phosphorylated Cav-1 was incubated with TCPTP-37 or PTP1B (used as positive control) in the presence or absence of phosphatase inhibitors for 30 min at room temperature and then separated in 12% SDS-PAGE, transferred to nitrocellulose, and probed with anti-Tyr(P)14 or anti-Cav-1 antibodies.
Flow Cytometry
To determine integrin α1β1 expression levels, cells were incubated with anti-integrin α1 antibodies for 45 min, washed, and then incubated with appropriate phycoerythrin-conjugated secondary antibodies and analyzed with a FACScan (BD Biosciences). Data collected in flow cytometry experiments were analyzed using Cell Quest software (BD Biosciences).
Statistical Analysis
Student's t test was used for comparisons between two groups, and analysis of variance using Sigma-Stat software was used to analyze statistical differences between multiple groups. p < 0.05 was considered statistically significant.
RESULTS
Lack of Integrin α1β1 Leads to Increased Levels of Phosphorylated Caveolin-1
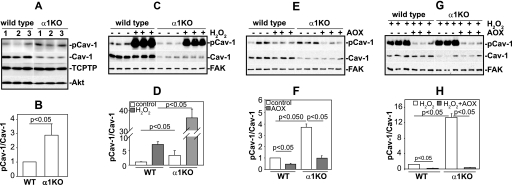
Cav-1 is phosphorylated at tyrosine 14 in response to oxidative stress (6, 8, 34), and increased levels of pCav-1 are associated with tissue injury (16, 17). Because integrin α1β1 negatively regulates ROS production (30, 31) and mice lacking this collagen binding receptor show increased ROS levels and exacerbated oxidative stress-mediated glomerular injury (31), we hypothesized that absence of integrin α1β1 would result in both increased basal and ROS-induced levels of pCav-1. To test this hypothesis, we analyzed the levels of pCav-1 in immortalized wild type and integrin α1-null mesangial cells. As recently reported by our group, integrin α1-null mesangial cells show a significant decrease in the total levels of Cav-1 (35); however, they have a ∼3-fold relative increase in the level of pCav-1 compared with wild type cells (Figs. 1, A and B). The generality of this finding was confirmed when primary cultured mesangial cells from wild type and integrin α1-null mice were analyzed for pCav-1 levels (Fig. 1, C–F).
FIGURE 1.
Levels of phosphorylated caveolin-1 are increased in integrin α1-null mesangial cells. A and B, total cell lysates from serum-starved immortalized mesangial cells (20 μg/lane, n = 3) were analyzed for levels of phosphorylated and total Cav-1 as well as basal levels of endogenous TCPTP. Cav-1 and pCav-1 bands were quantified by densitometry analysis, and the pCav-1 signal is expressed as a pCav-1/Cav-1 ratio. Values are the mean ± S.D. of four experiments and represent -fold changes relative to WT cells (assigned as 1). C and D, total cell lysates from serum-starved primary mesangial cells (20 μg/lane, n = 3) either untreated or treated with H2O2 (500 μm for 30 min) were analyzed for levels of phosphorylated and total Cav-1 as described above. Cav-1 and pCav-1 bands were quantified and expressed as described above (the value of untreated WT cells was assigned as 1). E and F, total cell lysates from serum-starved primary mesangial cells (20 μg/lane, n = 3) either untreated or treated with antioxidants (AOX; 10 μm TEMPOL and 1 μm DPI) for 24 h were analyzed by Western blot for levels of phosphorylated and total Cav-1. Cav-1 and pCav-1 bands were quantified and expressed as described above (the value of untreated WT cells was assigned as 1). G and H, total cell lysates from serum-starved primary mesangial cells (20 μg/lane, n = 3) either untreated or treated with antioxidants for 24 h were treated with H2O2 for 30 min and subsequently analyzed by Western blot for levels of phosphorylated and total Cav-1. Cav-1 and pCav-1 bands were quantified and expressed as described above (the value of H2O2-treated WT cells was assigned as 1).
When we exposed primary mesangial cells to H2O2 and analyzed the levels of pCav-1, we found that the levels of pCav-1 increased in both wild type and integrin α1-null cells; however, the effect was more prominent in α1-null cells (Fig. 1, C and D). To confirm a role for basal and induced ROS in Cav-1 phosphorylation, primary mesangial cells were incubated with antioxidants. Decreased levels of pCav-1 were observed in antioxidant-treated wild type and integrin α1-null cells when compared with untreated cells; however, this effect was more significant in α1-null cells (Fig. 1, E and F). This result supports our previous finding that integrin α1-null mesangial cells produce more basal ROS than their wild type counterparts (31). Furthermore, antioxidant treatment significantly decreased the levels of pCav-1 in cells treated with H2O2 (Fig. 1, G and 1H), clearly demonstrating that induced ROS production is responsible for increased pCav-1 levels.
Overexpression of TCPTP Only Decreases Caveolin-1 Phosphorylation in Integrin α1β1-expressing Cells
Our results suggest that loss of integrin α1β1 leads to increased levels of pCav-1; however, the mechanism is unknown. One possibility is that integrin α1β1 negatively regulates pCav-1 levels via activation of the tyrosine phosphatase TCPTP because 1) integrin α1β1 binds and activates TCPTP (26), and 2) TCPTP is homologous to PTP1B, a protein-tyrosine phosphatase that dephosphorylates Cav-1 (21).
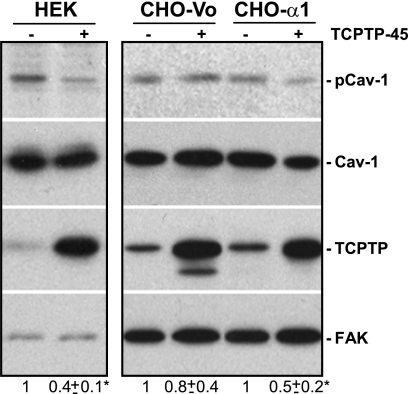
First, we analyzed the levels of basal TCPTP expression in wild type and integrin α1-null mesangial cells and found no differences between the two cell types, despite the increased basal levels of pCav-1 in the α1-null cells (Fig. 1A). To determine whether TCPTP might promote Cav-1 dephosphorylation and whether it requires integrin α1β1 to do so, we overexpressed full-length TCPTP (TCPTP-45; molecular mass 45 kDa) in HEK cells that express endogenous integrin α1β1 (supplemental Fig. 1). Overexpression of TCPTP in HEK cells significantly decreased the levels of pCav-1 compared with cells transfected with empty vector (Fig. 2). To confirm the requirement of integrin α1β1 for Cav-1 dephosphorylation, we overexpressed TCPTP in CHO cells (which do not express endogenous integrin α1β1) transfected with either the human integrin α1 subunit (CHO-α1) or empty vector (CHO-Vo) (supplemental Fig. 1). Overexpression of TCPTP resulted in an ∼50% decrease in pCav-1 levels in CHO-α1 but not CHO-Vo cells (Fig. 2). Thus, TCPTP promotes Cav-1 dephosphorylation in an integrin α1β1-dependent manner.
FIGURE 2.
Expression of integrin α1β1 is required for TCPTP-mediated caveolin-1 dephosphorylation. HEK or CHO cells either expressing (CHO-α1) or not (CHO-Vo) the integrin α1 subunit were transiently transfected with full-length TCPTP (TCPTP-45) or empty vector. 24 h after, the levels of Cav-1 and pCav-1 were analyzed by Western blot (WB) analysis as described under “Experimental Procedures.” Membranes were reincubated with anti-FAK antibodies to verify loading. Cav-1 and pCav-1 bands were quantified by densitometry analysis, and the pCav-1 signal is expressed as a pCav-1/Cav-1 ratio. Values are the mean ± S.D. of three experiments and represent -fold changes relative to cells expressing endogenous TCPTP only. *, significant difference (p < 0.05) between cells transfected with empty vector versus cells transfected with TCPTP.
TCPTP Directly Binds Caveolin-1
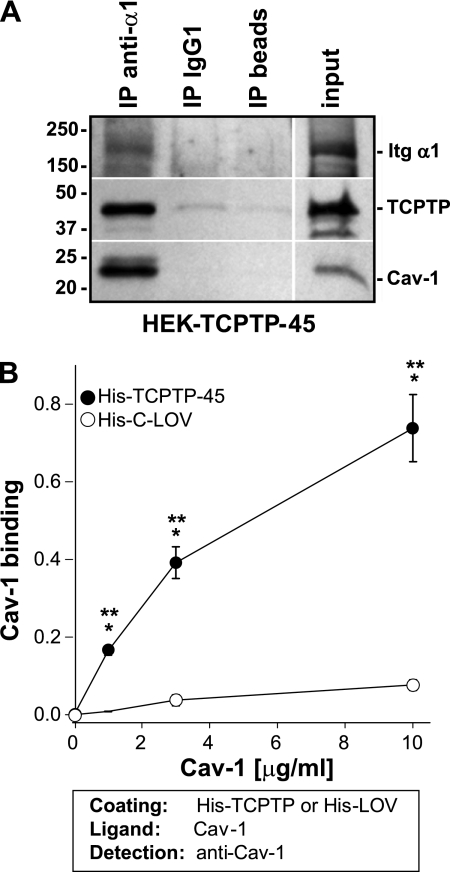
We next determined whether integrin α1β1, TCPTP, and Cav-1 form a functional complex. To do this, we immunoprecipitated lysates from HEK overexpressing full-length TCPTP (HEK-TCPTP-45) with antibodies to the extracellular domain of the human integrin α1 subunit, a mouse isotype control, or protein G beads. Integrin α1, Cav-1, and TCPTP were found in the same complex in samples immunoprecipitated with the anti-integrin α1 antibodies (Fig. 3A), suggesting that TCPTP, in addition to integrin α1β1, could bind directly to Cav-1. To test this possibility, we performed an ELISA on purified full-length His-tagged TCPTP and GST-Cav-1 expressed as recombinant proteins in bacterial cells (supplemental Fig. 2). For some experiments, the GST tag was removed from GST-Cav-1, and this protein is referred to as Cav-1 (supplemental Fig. 2). When increasing amounts of Cav-1 were added to His-TCPTP-45, significant Cav-1 binding was detected; however, this binding was not detected when a control protein, His-tagged C terminus of zebra fish left over gene (His-C-LOV) was used (Fig. 3B). These results indicate that TCPTP directly binds to Cav-1.
FIGURE 3.
Caveolin-1 binds directly to TCPTP. A, equal amount of cell lysates (0.5 mg) derived from HEK cells overexpressing full-length TCPTP were incubated with either anti-integrin α1 antibodies (20 μg), mouse IgG1 isotype control (20 μg), or Protein G beads. The antibody-antigen complexes were resolved by SDS-PAGE in 4–20% gradient gels under reducing conditions, transferred, and then detected with antibodies to the human integrin α1 subunit, TCPTP, and Cav-1. B, immobilized full-length His-TCPTP (His-TCPTP-45) or His-C-LOV (2 μg/ml) was incubated with increasing amounts of purified Cav-1, and bound Cav-1 was detected with anti-Cav-1 antibody. One representative experiment performed in triplicate is shown. Three independent experiments were performed with similar results. Differences between uncoated and coated wells (*) and between His-TCPTP-45 and His-C-LOV (**) were significant with p < 0.05. Error bars, S.D.
The Integrin α1 Subunit Binds Caveolin-1 and TCPTP via Different Domains
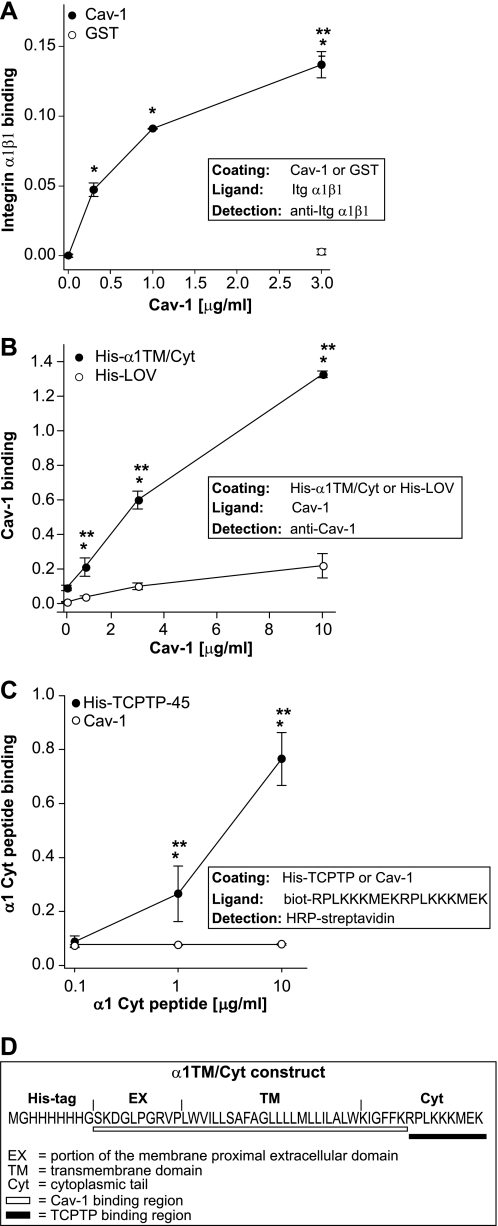
The co-precipitation of Cav-1 with integrin α1β1 and TCPTP (Fig. 3A) might be due to direct binding of Cav-1 to integrin α1β1. This possibility was previously suggested in experiments where Cav-1 was shown to bind the transmembrane domain of the integrin α1 subunit (36); however, these data were obtained by immunoprecipitating wild type and mutated integrin α1 constructs from cell lysates and not with purified proteins. We therefore determined whether the integrin α1 subunit can directly bind Cav-1 and the domain of the integrin α1 subunit involved in this binding by using three different approaches. First, we determined the ability of purified integrin α1β1 to bind immobilized Cav-1 by ELISA. As shown in Fig. 4A, purified integrin α1β1 bound immobilized Cav-1 but not the negative control GST. Second, we analyzed by ELISA the ability of Cav-1 to bind a purified His-tagged integrin α1 construct carrying the first 10 membrane-proximal amino acids of the extracellular domain, the transmembrane domain, and the cytoplasmic tail (supplemental Fig. 3). Cav-1 bound to immobilized integrin α1 construct, but not His-LOV, was used as negative control in a dose-dependent manner (Fig. 4B). Third, we determined whether Cav-1 and TCPTP share the same or different binding sites on the integrin α1 subunit by analyzing the ability of Cav-1 to bind to the integrin α1 tail peptide (RPLKKKMEK) to which TCPTP has been shown to bind (26). Whereas the α1 tail peptide was able to bind immobilized full-length TCPTP, no binding to immobilized Cav-1 was observed (Fig. 4C). Thus, we conclude that Cav-1 directly binds the integrin α1 subunit, and Cav-1 and TCPTP bind to different regions of the integrin α1 subunit (see Fig. 4D for details).
FIGURE 4.
Caveolin-1 binds to the integrin α1 subunit. A, immobilized Cav-1 or GST at the indicated concentrations was incubated with 10 μg/ml purified integrin α1β1, and bound integrin was detected with anti-integrin α1 antibodies. One representative experiment performed in triplicates is shown. Two independent experiments were performed with similar results. B, immobilized His-α1TM/Cyt or His-LOV (5 μg/ml) was incubated with increasing amounts of Cav-1, and bound Cav-1 was detected with anti-Cav-1 antibodies. One representative experiment performed in triplicates is shown. Two independent experiments were performed with similar results. C, immobilized His-TCPTP-45 or Cav-1 (2 μg/ml) was incubated with increasing amounts of biotinylated integrin α1 cytoplasmic peptide. Peptide binding was detected with HRP-conjugated streptavidin. One representative experiment performed in triplicates is shown. Two independent experiments were performed with similar results. D, schematic representation of putative Cav-1 and TCPTP binding regions on the integrin α1 subunit. * and **, as in Fig. 3.
TCPTP Dephosphorylates Caveolin-1
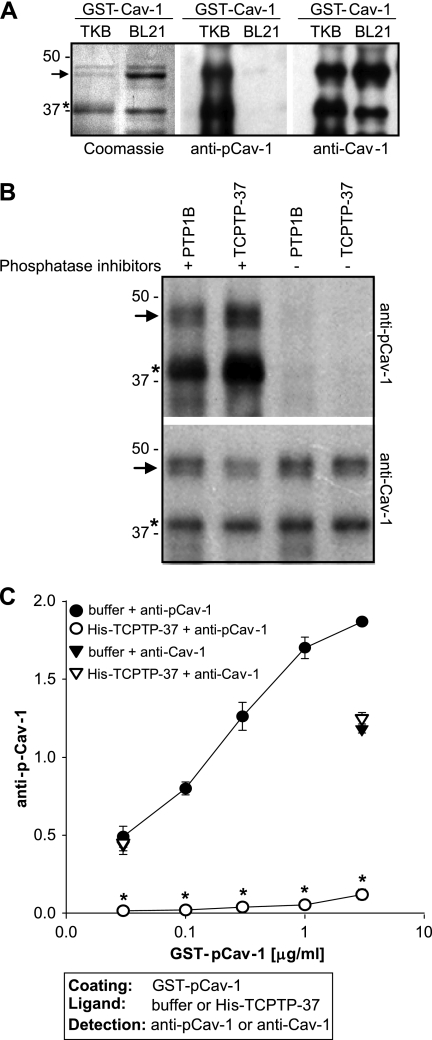
The decreased Cav-1 phosphorylation observed in the CHO-α1 cells overexpressing full-length TCPTP (Fig. 2) could either be due to direct Cav-1 dephosphorylation by TCPTP or to TCPTP inhibiting tyrosine kinases that phosphorylate Cav-1. To determine whether Cav-1 can be directly dephosphorylated by TCPTP, we expressed GST-Cav-1 in TKB1 E. coli to generate pCav-1 (6) (see “Experimental Procedures” for details). As shown in Fig. 5A, GST-Cav-1 expressed in TKB E. coli is phosphorylated, and, therefore, it is recognized by antibodies to both pCav-1 (Tyr(P)14) and total Cav-1. In contrast, GST-Cav-1 expressed in BL21 E. coli is recognized only by antibodies to total Cav-1 (Fig. 5A). To determine whether pCav-1 is a substrate of TCPTP, we initially analyzed the ability of a truncated form of TCPTP to dephosphorylate Cav-1. We used commercially available TCPTP-37 (molecular mass 37 kDa; supplemental Fig. 4A) that lacks the inhibitory C-terminal domain and is constitutively active (27). We incubated GST-pCav-1 with either TCPTP-37 or PTP1B, used as positive control, and analyzed the dephosphorylation rate of GST-pCav-1. Western blot (Fig. 5B) and ELISA (Fig. 5C) showed that TCPTP-37 dephosphorylates Cav-1 efficiently. Importantly, the decrease in Cav-1 dephosphorylation was not due to protein degradation because the levels of total Cav-1 were similar in samples incubated with or without TCPTP-37 (Fig. 5, B and C). These results indicate that pCav-1 is a substrate of constitutively active TCPTP.
FIGURE 5.
Constitutively active TCPTP dephosphorylates caveolin-1. A, Coomassie staining of purified GST-pCav-1 and GST-Cav-1 (2 μg/lane). 20 ng of purified GST-pCav-1 and GST-Cav-1 were separated in 12% SDS-PAGE, transferred to nitrocellulose, and blotted with anti-Tyr(P)14-pCav-1 or anti-Cav-1 antibodies. The arrow indicates full-length constructs, whereas the asterisk indicates a cleaved product still retaining the Cav-1 phosphorylation site (Tyr14). B, GST-pCav-1 (0.4 μg/ml) was incubated with constitutively active TCPTP (TC-37, 0.4 μg/ml) or PTP1B (0.4 μg/ml) with or without tyrosine phosphatase inhibitors. 30 min later, the samples were analyzed by Western blot for levels of phosphorylated and total Cav-1. C, GST-pCav-1, coated at the concentrations indicated, was incubated with either phosphatase buffer or TCPTP-37 followed by incubation with anti-phosphorylated or anti-Cav-1 antibodies. One representative experiment performed in triplicate is shown. Two experiments were performed with similar results. *, indicates significant differences (p < 0.05) between wells coated with pCav-1 incubated with or without His-TCPTP-37. Error bars, S.D.
Integrin α1β1 Increases Full-length TCPTP-mediated Caveolin-1 Dephosphorylation
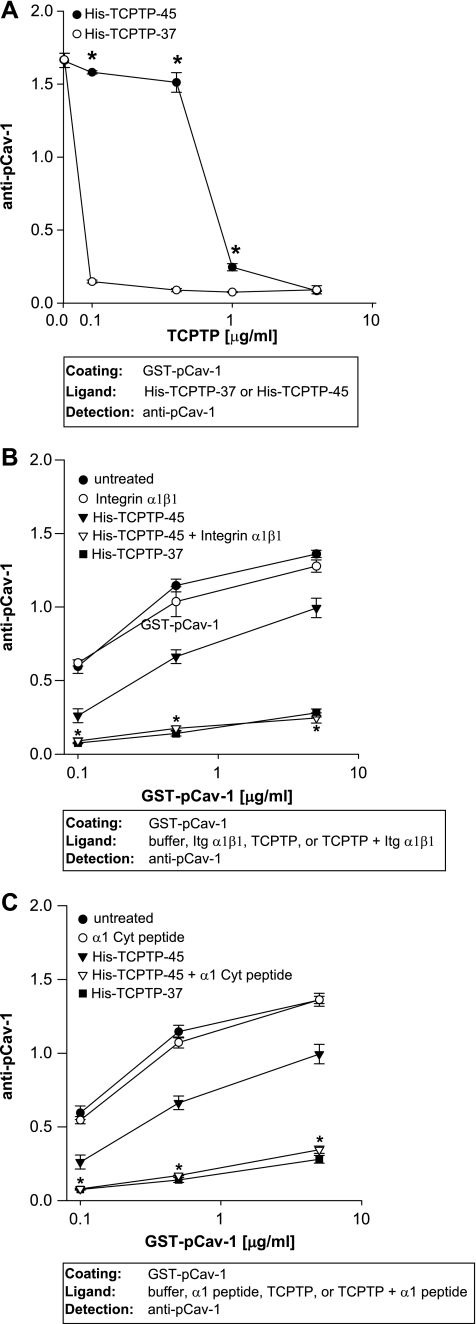
We next investigated whether full-length TCPTP (TCPTP-45) dephosphorylates Cav-1 as efficiently as constitutively active TCPTP (TCPTP-37). To do so, GST-pCav-1 was incubated with TCPTP-37 or TCPTP-45 at concentrations ranging from 0.1 to 4 μg/ml (equivalent to 3–100 nm for both enzymes), and dephosphorylation was analyzed by ELISA. Cav-1 dephosphorylation by TCPTP-45 was significantly lower (∼10-fold lower) than that by TCPTP-37 (Fig. 6A and supplemental Fig. 4B) at low concentrations of enzyme, and this effect was not due to differences in binding of TCPTP-45 or TCPTP-37 to Cav-1, as shown in supplemental Fig. 4C.
FIGURE 6.
Integrin α1β1 enhances full-length TCPTP-mediated caveolin-1 dephosphorylation. A, immobilized pCav-1 (5 μg/ml) was incubated with increasing concentrations of TCPTP-45 or TCPTP-37 followed by incubation with anti-pCav-1 antibodies. One representative experiment performed in triplicate is shown. Two experiments were performed with similar results. Note that full-length TCPTP-45 dephosphorylates Cav-1 less efficiently than constitutively activated TCTPT-37. *, significant differences between wells incubated with TCPTP-45 and those incubated with TCPTP-37. B and C, pCav-1, coated at the concentrations indicated, was incubated with TCPTP-45 (0.4 μg/ml) with or without full-length integrin α1β1 (10 μg/ml) (B) or integrin α1 cytoplasmic peptide (3 μg/ml) (C). The degree of dephosphorylation was subsequently determined by incubation with anti-pCav-1 antibodies. One representative experiment performed in triplicate is shown. Three experiments were performed with similar results. *, significant differences (p < 0.05) between wells coated with pCav-1 incubated with TCPTP-45 and wells incubated with TCPTP-45 plus integrin α1β1 (B) or TCPTP-45 plus α1 Cyt peptide (C). Error bars, S.D.
As the integrin α1 tail has been shown to be required for binding and activation of TCPTP (26), we investigated whether integrin α1β1 could enhance full-length TCPTP-mediated Cav-1 dephosphorylation. Indeed, integrin α1β1 significantly improved full-length TCPTP-mediated Cav-1 dephosphorylation to values similar to those observed with constitutively active TCPTP-37 (Fig. 6B). Similar results were obtained when the integrin α1 cytoplasmic peptide RPLKKKMEK, known to bind TCPTP (26), was used in the assay instead of full-length integrin α1β1 (Fig. 6C). These results suggest that the integrin α1 cytoplasmic domain RPLKKKMEK promotes TCPTP-mediated Cav-1 dephosphorylation.
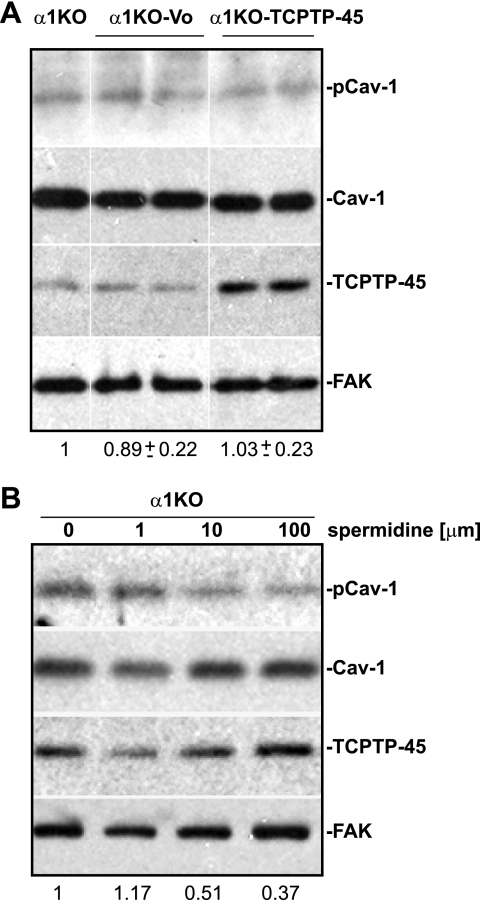
TCPTP Activation Increases Caveolin-1 Dephosphorylation in Integrin α1-null Mesangial Cells
We next determined whether increasing the levels of TCPTP in cells lacking integrin α1β1 expression is sufficient to promote Cav-1 dephosphorylation. To do this, we generated clones of integrin α1-null mesangial cells overexpressing full-length TCPTP (α1KO-TCPTP-45) (Fig. 7A). Interestingly, pCav-1 levels were unchanged in integrin α1KO-TCPTP-45 cells when compared with integrin α1-null cells either untransfected or transfected with empty vector (α1KO-Vo) (Fig. 7A). These results strongly suggest that overexpression of TCPTP is not sufficient to promote Cav-1 dephosphorylation in integrin α1-null mesangial cells.
FIGURE 7.
Activation of TCPTP in integrin α1-null mesangial cells is sufficient to decrease caveolin-1 phosphorylation. A, integrin α1-null mesangial cells either overexpressing (α1KO-TCPTP) or not overexpressing (α1Ko-Vo) full-length TCPTP were analyzed by Western blot for levels of total and phosphorylated Cav-1 (20 μg/lane). Cav-1 and pCav-1 bands were quantified by densitometry analysis, and the pCav-1 signal is expressed as a pCav-1/Cav-1 ratio. Values are the mean ± S.D. of four clones (only two shown) and represent -fold changes relative to untransfected cells (α1KO). B, integrin α1-null mesangial cells were incubated for 18 h with spermidine at the concentrations indicated, and total cell lysates (20 μg/lane) were analyzed by Western blot for levels of total and phosphorylated Cav-1.
To further confirm that activation of full-length TCPTP is required to mediate Cav-1 dephosphorylation, we treated integrin α1-null mesangial cells with different concentrations of spermidine, a newly identified activator of TCPTP (29). As shown in Fig. 7B, integrin α1-null mesangial cells treated with spermidine showed dose-dependent decreased levels of pCav-1, clearly suggesting that activation of TCPTP, either by integrin α1β1 or spermidine, is a key step for TCPTP-mediated dephosphorylation of Cav-1.
DISCUSSION
The goal of this study was to determine whether integrin α1β1 regulates the levels of phosphorylated Cav-1 because phosphorylated Cav-1 has been implicated in oxidative stress-mediated injury, and loss of integrin α1β1 leads to increased basal levels of ROS. We provide evidence that integrin α1β1 negatively regulates Cav-1 phosphorylation by activating the tyrosine phosphatase TCPTP. This was demonstrated by showing that 1) Cav-1 phosphorylation is significantly higher in cells lacking integrin α1β1 both at base line and following oxidative stress; 2) overexpression of TCPTP leads to reduced pCav-1 levels only in cells expressing integrin α1β1; 3) purified Cav-1 directly interacts with TCPTP and the integrin α1 subunit; 4) pCav-1 is a substrate of TCPTP in vitro; and 5) the extent of TCPTP-mediated Cav-1 dephosphorylation is greatly increased by the addition of purified integrin α1β1 or the integrin α1 cytoplasmic peptide to which TCPTP has been shown to bind (26).
Cav-1 phosphorylation on tyrosine 14 in response to various stimuli, including cellular stress and growth factor receptor activation, is reversible, which indicates that PTPs play a critical role in regulating the levels of pCav-1. Therefore, identification of the PTPs that dephosphorylate Cav-1 is critical for understanding how pCav-1 levels are regulated. Prior to our study, only one classical PTP was shown to dephosphorylate Cav-1, namely the endoplasmic reticulum-localized PTP1B (21). The catalytic domain of other PTPs tested, such as hematopoietic PTP, leukocyte antigen-related PTP, and SHP2 (Src homology domain-containing phosphatase 2) had no effect on Cav-1 dephosphorylation (21). Here we provide evidence that pCav-1 is a specific substrate of another tyrosine phosphatase, namely TCPTP. This was demonstrated by in vitro dephosphorylation of pCav-1 using purified TCPTP and by showing a direct interaction between TCPTP and Cav-1. Interestingly, we show that TCPTP-mediated Cav-1 dephosphorylation is increased by the presence of integrin α1β1 or the integrin α1 cytoplasmic peptide to which TCPTP has been shown to bind (26). PTP activity is regulated by cellular localization and/or protein-protein interactions. Integrin α1β1 is a key component in TCTPT-mediated dephosphorylation activity because it both binds and activates TCPTP (26).
Cav-1 has been shown to negatively regulate and/or inhibit the activity of both serine/threonine and tyrosine phosphatases. In this context, Cav-1 inhibits the serine/threonine phosphatase PP2A by sequestering the catalytic subunit PP2A-C in caveolae (37). This results in activation of ataxia telangiectasia-mutated protein kinase and consequent premature senescence of lung fibroblasts after oxidative stress (37). Moreover, Cav-1 down-regulates the activation of the tyrosine phosphatases low molecular weight PTP and TCTPT (38). In this context, Cav-1 has been shown to down-regulate TCPTP-mediated dephosphorylation of the substrate p-nitrophenyl phosphate in vitro (38). This finding agrees with our ELISA, demonstrating that full-length TCPTP, despite directly binding to Cav-1, poorly dephosphorylates this scaffold protein. However, when TCPTP is activated by exogenously added purified integrin α1β1 or in cells expressing integrin α1β1 (i.e. CHO-α1), this is sufficient to prevent and/or overcome Cav-1-mediated inhibition of TCPTP, thus promoting Cav-1 dephosphorylation.
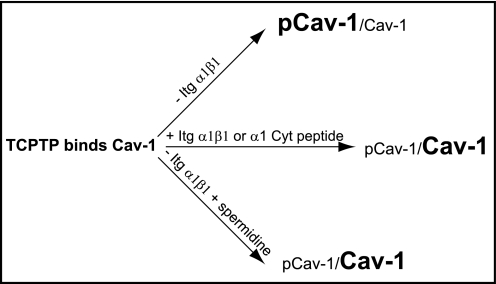
Consistent with the hypothesis that TCPTP needs to be activated in order to dephosphorylate Cav-1, we show that overexpression of TCPTP in cells lacking integrin α1β1 is not sufficient to promote Cav-1 dephosphorylation. However, treating integrin α1-null cells with spermidine is sufficient to promote Cav-1 dephosphorylation. Spermidine, a positively charged polyamine, was identified through a screen for small molecule inhibitors for tyrosine kinase receptors by activating TCPTP, and is specific for TCPTP because its effects were reduced by ∼43-fold in TCPTP-null cells (29). These results indicate that activation of TCPTP either by integrin α1β1 or by agonists (i.e. spermidine) may be viewed as a valid tool to control the levels of pCav-1 (see also Fig. 8 for details).
FIGURE 8.
Schematic representation of factors involved in TCPTP-mediated Cav-1 dephosphorylation. TCPTP can directly bind Cav-1; however, its Cav-1 dephosphorylation activity is significantly enhanced by integrin α1β1, integrin α1 cytoplasmic peptide, or activators such as spermidine.
Another key finding of this study is that the integrin α1 subunit directly binds Cav-1. Our results are consistent with the observation that Cav-1 binds to the transmembrane domain of integrin α1 subunit (36). However, given that this binding was determined based on immunoprecipitation of total cell lysates, this did not exclude the possibility of an indirect interaction. In this context, it was shown recently that the integrin β1 subunit, the partner of the α1 subunit, co-immunoprecipitates with Cav-1 in fibroblast cell lysates (39). This raises the question of whether Cav-1 interacts directly with the integrin α1 subunit. Using purified recombinant protein constructs, we show that a portion of the integrin α1 subunit carrying the first 10 membrane-proximal amino acids of the extracellular domain, the transmembrane domain, and the cytoplasmic tail interacts directly with Cav-1. We also confirmed that, unlike TCPTP, Cav-1 does not bind to the non-conserved region of the integrin α1 subunit cytoplasmic tail, therefore showing that TCPTP and Cav-1 bind distinct domains on the integrin α1 subunit. A key question is whether integrin α1β1 promotes Cav-1 dephosphorylation by binding and activating TCPTP and Cav-1 at the same time. The spermidine experiment in integrin α1-null cells seems to indicate that integrin α1β1, despite binding both proteins, is primarily required for activation of TCPTP. However, future studies comparing the kinetics and efficacy of TCPTP-mediated Cav-1 dephosphorylation in the presence of integrin α1β1 versus spermidine are necessary to better clarify this issue.
We show that the levels of pCav-1 are higher in integrin α1-null mesangial cells both at base line and following oxidative stress, and antioxidant treatment decreases the basal and ROS-induced levels of pCav-1. This finding, together with our previously published data indicating that integrin α1-null mesangial cells have increased ROS levels at base line and following oxidative injury (31, 40), suggests that pCav-1 is a mediator of oxidative injury. In this context, it has been shown that pCav-1 is necessary to promote activation of the EGF receptor (9, 41), which acts as a profibrotic player by positively regulating ROS production (30, 31).
In contrast to the levels of pCav-1, the total levels of Cav-1 indirectly correlate to the activation of the EGF receptor. We have recently shown that loss of integrin α1β1 leads to decreased basal levels of Cav-1, via a PPARγ/ERK pathway, with consequent up-regulation of EGF receptor-mediated ROS production (35). Altogether, these data indicate that integrin α1β1 might negatively regulate oxidative stress by positively regulating the total Cav-1 levels and, at the same time, negatively regulating the levels of pCav-1. If this is the case, down-regulation of Cav-1 and integrin α1β1 and/or up-regulation of pCav-1 should correlate to pathological conditions characterized by oxidative stress and/or fibrosis. In support of this hypothesis, patients with systemic sclerosis or idiopathic pulmonary fibrosis show decreased expression of Cav-1 levels (3). Moreover, mice lacking Cav-1 have a decreased life span due to pulmonary collagen fibril deposition and lung fibrosis (42). Finally, decreased Cav-1 expression occurs in kidneys of patients with focal segmental glomerulosclerosis and lupus glomerulonephritis (43). Down-regulation of integrin α1β1 expression is observed in fibroblasts isolated from patients with systemic scleroderma (44), and exposure to high glucose results in decreased expression of integrin α1β1 in mesangial cells (45). Moreover, integrin α1-null mice develop exacerbated glomerulosclerosis following injury (31, 40). With respect to pCav-1 levels, there is evidence that increased pCav-1 contribute to oxidative stress-induced acute lung injury (19) as well as mechanical strain-induced Akt activation in mesangial cells (9), a key step in promoting collagen synthesis (46).
Although Cav-1 is an essential element of caveolae, it has been proposed that the levels of pCav-1 control caveolae formation, caveolae-mediated endocytosis, and receptor-mediated functions. In this context, radiation-induced Cav-1 phosphorylation is a key step for caveolae-mediated EGF receptor endocytosis, nuclear shuttling, and EGF receptor-mediated DNA repair (47). In addition, enhancement of Cav-1 phosphorylation following treatment with phosphatase inhibitors stimulates caveolae-mediated endocytosis while reducing pinocytosis (48). In adherent cells, integrins sequester pCav-1 at focal adhesions and prevent caveolae-mediated endocytosis. In contrast, in the absence of integrin engagement, pCav-1 moves to caveolae, inducing their internalization (49). Interestingly, we show that in cells lacking integrin α1β1, loss of this collagen receptor engagement leads to increased pCav-1 levels with consequent increased EGF receptor-mediated functions. However, whether this activation is due to pCav-1-mediated formation of caveolae or receptor stabilization needs to be determined.
In contrast to our data showing that integrin α1β1 is a negative regulator of pCav-1 levels, there is evidence that integrins can promote transient Cav-1 phosphorylation. In endothelial cells, for example, integrin β1 activation by acute shear stress induces transient Cav-1 phosphorylation and consequent cytoskeletal rearrangements (50). In mouse embryonic stem cells, fibronectin-mediated integrin activation leads to Cav-1 phosphorylation, thus promoting cell proliferation (51). Thus, it is clear that factors such as ligands, type of integrins, interactions with growth factor receptors, and type of injury can profoundly affect the ability of integrins to differentially regulate Cav-1 phosphorylation.
In conclusion, because integrin α1β1 is required for 1) down-regulation of pCav-1 levels (present study), 2) down-regulation of the profibrotic EGF receptor signaling (30, 35), and 3) TCPTP recruitment and activation (26), we propose that TCPTP might act as an anti-ROS phosphatase by negatively regulating both EGF receptor activation and pCav-1. In conclusion, we demonstrate that pCav-1 is a novel substrate of TCPTP and that integrin α1β1 controls the levels of pCav-1 by activating TCPTP. The increased Cav-1 phosphorylation in the absence of integrin α1β1 may contribute to oxidative stress-mediated injury and thus explain the protective function of integrin α1β1.
Supplementary Material
Acknowledgments
We thank Laura Mizoue in the Vanderbilt Center for Structural Biology for providing pBG100. We especially thank to Dr. Brent Eichman for supplying the His-C-LOV-1 protein and Cathy Alford at the Department of Veterans Affairs for help with the FACS analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants 2P01DK065123 (to A. P. and R. Z.), DK075594 and DK65123 (to R. Z.), and DK083187 (to R. Z. and C. R. S.). These studies were also supported by a Merit Review from the Department of Veterans Affairs (to A. P. and R. Z.) and an American Heart Association established investigator award (to R. Z.); and O'Brien Center Grant P30DK79341-01 (to A. P. and R. Z.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- PTP
- protein-tyrosine phosphatase
- TCPTP
- T cell protein-tyrosine phosphatase
- ROS
- reactive oxygen species
- HEK
- human embryonic kidney
- MC
- mesangial cell
- pCav-1
- phosphorylated Cav-1.
REFERENCES
- 1.Thomas C. M., Smart E. J. (2008) J. Cell. Mol. Med. 12, 796–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Laurentiis A., Donovan L., Arcaro A. (2007) Open Biochem. J. 1, 12–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Galdo F., Lisanti M. P., Jimenez S. A. (2008) Curr. Opin. Rheumatol. 20, 713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lajoie P., Partridge E. A., Guay G., Goetz J. G., Pawling J., Lagana A., Joshi B., Dennis J. W., Nabi I. R. (2007) J. Cell Biol. 179, 341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi F., Sottile J. (2008) J. Cell Sci. 121, 2360–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H., Volonte D., Galbiati F., Iyengar P., Lublin D. M., Bregman D. B., Wilson M. T., Campos-Gonzalez R., Bouzahzah B., Pestell R. G., Scherer P. E., Lisanti M. P. (2000) Mol. Endocrinol. 14, 1750–1775 [DOI] [PubMed] [Google Scholar]
- 7.Sanguinetti A. R., Cao H., Corley Mastick C. (2003) Biochem. J. 376, 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ushio-Fukai M., Zuo L., Ikeda S., Tojo T., Patrushev N. A., Alexander R. W. (2005) Circ. Res. 97, 829–836 [DOI] [PubMed] [Google Scholar]
- 9.Zhang B., Peng F., Wu D., Ingram A. J., Gao B., Krepinsky J. C. (2007) Cell Signal 19, 1690–1700 [DOI] [PubMed] [Google Scholar]
- 10.Volonté D., Galbiati F., Pestell R. G., Lisanti M. P. (2001) J. Biol. Chem. 276, 8094–8103 [DOI] [PubMed] [Google Scholar]
- 11.Parat M. O., Stachowicz R. Z., Fox P. L. (2002) Biochem. J. 361, 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki J., Katoh H., Yasui H., Yamaguchi Y., Nakamura K., Hasegawa H., Ichikawa A., Negishi M. (1999) Biochem. J. 340, 365–369 [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D. B., Li S. M., Qian X. X., Moon C., Zheng J. (2005) Biol. Reprod. 73, 761–772 [DOI] [PubMed] [Google Scholar]
- 14.Chrétien A., Piront N., Delaive E., Demazy C., Ninane N., Toussaint O. (2008) FEBS Lett. 582, 1685–1692 [DOI] [PubMed] [Google Scholar]
- 15.Percy C. J., Pat B. K., Healy H., Johnson D. W., Gobe G. C. (2008) Pathology 40, 694–701 [DOI] [PubMed] [Google Scholar]
- 16.Nag S., Manias J. L., Stewart D. J. (2009) Neuropathol. Appl. Neurobiol. 35, 417–426 [DOI] [PubMed] [Google Scholar]
- 17.Kim H., Ahn M., Lee J., Moon C., Matsumoto Y., Koh C. S., Shin T. (2006) Neurosci. Lett. 402, 76–80 [DOI] [PubMed] [Google Scholar]
- 18.Shajahan A. N., Wang A., Decker M., Minshall R. D., Liu M. C., Clarke R. (2007) J. Biol. Chem. 282, 5934–5943 [DOI] [PubMed] [Google Scholar]
- 19.Sun Y., Hu G., Zhang X., Minshall R. D. (2009) Circ. Res. 105, 676–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caselli A., Taddei M. L., Manao G., Camici G., Ramponi G. (2001) J. Biol. Chem. 276, 18849–18854 [DOI] [PubMed] [Google Scholar]
- 21.Lee H., Xie L., Luo Y., Lee S. Y., Lawrence D. S., Wang X. B., Sotgia F., Lisanti M. P., Zhang Z. Y. (2006) Biochemistry 45, 234–240 [DOI] [PubMed] [Google Scholar]
- 22.Shin J., Jo H., Park H. (2006) Biochem. Biophys. Res. Commun. 339, 737–741 [DOI] [PubMed] [Google Scholar]
- 23.Stuible M., Doody K. M., Tremblay M. L. (2008) Cancer Metastasis Rev. 27, 215–230 [DOI] [PubMed] [Google Scholar]
- 24.Lorenzen J. A., Dadabay C. Y., Fischer E. H. (1995) J. Cell Biol. 131, 631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam M. H., Michell B. J., Fodero-Tavoletti M. T., Kemp B. E., Tonks N. K., Tiganis T. (2001) J. Biol. Chem. 276, 37700–37707 [DOI] [PubMed] [Google Scholar]
- 26.Mattila E., Pellinen T., Nevo J., Vuoriluoto K., Arjonen A., Ivaska J. (2005) Nat. Cell Biol. 7, 78–85 [DOI] [PubMed] [Google Scholar]
- 27.Hao L., Tiganis T., Tonks N. K., Charbonneau H. (1997) J. Biol. Chem. 272, 29322–29329 [DOI] [PubMed] [Google Scholar]
- 28.Mattila E., Auvinen K., Salmi M., Ivaska J. (2008) J. Cell Sci. 121, 3570–3580 [DOI] [PubMed] [Google Scholar]
- 29.Mattila E., Marttila H., Sahlberg N., Kohonen P., Tähtinen S., Halonen P., Perälä M., Ivaska J. (2010) BMC Cancer 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X., Abair T. D., Ibanez M. R., Su Y., Frey M. R., Dise R. S., Polk D. B., Singh A. B., Harris R. C., Zent R., Pozzi A. (2007) Mol. Cell. Biol. 27, 3313–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X., Moeckel G., Morrow J. D., Cosgrove D., Harris R. C., Fogo A. B., Zent R., Pozzi A. (2004) Am. J. Pathol. 165, 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 7489–7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abair T. D., Bulus N., Borza C., Sundaramoorthy M., Zent R., Pozzi A. (2008) Blood 112, 3242–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanguinetti A. R., Mastick C. C. (2003) Cell. Signal. 15, 289–298 [DOI] [PubMed] [Google Scholar]
- 35.Chen X., Whiting C., Borza C., Hu W., Mont S., Bulus N., Zhang M. Z., Harris R. C., Zent R., Pozzi A. (2010) Mol. Cell. Biol. 30, 3048–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wary K. K., Mariotti A., Zurzolo C., Giancotti F. G. (1998) Cell 94, 625–634 [DOI] [PubMed] [Google Scholar]
- 37.Volonte D., Kahkonen B., Shapiro S., Di Y., Galbiati F. (2009) J. Biol. Chem. 284, 5462–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caselli A., Taddei M. L., Bini C., Paoli P., Camici G., Manao G., Cirri P., Ramponi G. (2007) Biochemistry 46, 6383–6392 [DOI] [PubMed] [Google Scholar]
- 39.Xia H., Khalil W., Kahm J., Jessurun J., Kleidon J., Henke C. A. (2010) Am. J. Pathol. 176, 2626–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zent R., Yan X., Su Y., Hudson B. G., Borza D. B., Moeckel G. W., Qi Z., Sado Y., Breyer M. D., Voziyan P., Pozzi A. (2006) Kidney Int. 70, 460–470 [DOI] [PubMed] [Google Scholar]
- 41.Khan E. M., Heidinger J. M., Levy M., Lisanti M. P., Ravid T., Goldkorn T. (2006) J. Biol. Chem. 281, 14486–14493 [DOI] [PubMed] [Google Scholar]
- 42.Park D. S., Cohen A. W., Frank P. G., Razani B., Lee H., Williams T. M., Chandra M., Shirani J., De Souza A. P., Tang B., Jelicks L. A., Factor S. M., Weiss L. M., Tanowitz H. B., Lisanti M. P. (2003) Biochemistry 42, 15124–15131 [DOI] [PubMed] [Google Scholar]
- 43.Ostalska-Nowicka D., Nowicki M., Zachwieja J., Kasper M., Witt M. (2007) Histopathology 51, 611–621 [DOI] [PubMed] [Google Scholar]
- 44.Ivarsson M., McWhirter A., Black C. M., Rubin K. (1993) J. Investig. Dermatol. 101, 216–221 [DOI] [PubMed] [Google Scholar]
- 45.Setty S., Anderson S. S., Wayner E. A., Kim Y., Clegg D. O., Tsilibary E. C. (1995) Cell Adhes. Commun. 3, 187–200 [DOI] [PubMed] [Google Scholar]
- 46.Krepinsky J. C., Li Y., Chang Y., Liu L., Peng F., Wu D., Tang D., Scholey J., Ingram A. J. (2005) J. Am. Soc. Nephrol. 16, 1661–1672 [DOI] [PubMed] [Google Scholar]
- 47.Dittmann K., Mayer C., Kehlbach R., Rodemann H. P. (2008) Mol. Cancer 7, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng Z. J., Singh R. D., Holicky E. L., Wheatley C. L., Marks D. L., Pagano R. E. (2010) J. Biol. Chem. 285, 15119–15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Echarri A., Muriel O., Del Pozo M. A. (2007) Semin. Cell Dev. Biol. 18, 627–637 [DOI] [PubMed] [Google Scholar]
- 50.Radel C., Rizzo V. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H936–945 [DOI] [PubMed] [Google Scholar]
- 51.Park J. H., Ryu J. M., Han H. J. (2011) J. Cell. Physiol. 226, 267–275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.