Abstract
Proteasomes degrade most proteins in mammalian cells and are established targets of anti-cancer drugs. The majority of proteasome inhibitors are composed of short peptides with an electrophilic functionality (pharmacophore) at the C terminus. All eukaryotic proteasomes have three types of active sites as follows: chymotrypsin-like, trypsin-like, and caspase-like. It is widely believed that active site specificity of inhibitors is determined primarily by the peptide sequence and not the pharmacophore. Here, we report that active site specificity of inhibitors can also be tuned by the chemical nature of the pharmacophore. Specifically, replacement of the epoxyketone by vinyl sulfone moieties further improves the selectivity of β5-specific inhibitors NC-005, YU-101, and PR-171 (carfilzomib). This increase in specificity is likely the basis of the decreased cytotoxicity of vinyl sulfone-based inhibitors to HeLa cells as compared with that of epoxyketone-based inhibitors.
Keywords: Cysteine Protease, Protease Inhibitor, Proteasome, Protein Degradation, Ubiquitin
Introduction
The ubiquitin-proteasome pathway is essential in the maintenance of protein homeostasis in all eukaryotic cells and is involved in the regulation of numerous biologic processes. Proteasome inhibition causes apoptosis of malignant cells (1, 2). The proteasome inhibitor bortezomib (Velcade, PS-341) is used for the treatment of multiple myeloma and mantle cell lymphoma. Four other proteasome inhibitors are at different stages of clinical trials (3–6).
The 26 S proteasome is a large (1.6–2.4 MDa), hollow cylindrical, and multifunctional particle that consists of a 20 S proteolytic core and one or two 19 S regulatory complexes. Each eukaryotic 20 S core particle has three pairs of proteolytic sites with distinct substrate specificities (7–11). The β5 proteolytic sites are “chymotrypsin-like,” and the β2 sites are “trypsin-like.” The β1 sites cleave after acidic residues (Glu and Asp) and are referred to as “post-acidic,” “post-glutamate peptide hydrolase,” or “caspase-like.” Tissues of the immune system also express immunoproteasomes, in which β5, β1, and β2 catalytic subunits are replaced by their major histocompatibility complex (MHC) locus-encoded counterparts LMP7 (β5i), LMP2 (β1i), and MECL-1 (β2i).
The chymotrypsin-like sites have long been considered the only suitable targets for anti-neoplastic agents and are the primary targets of all these agents. However, our recent work indicates that cytotoxicity of proteasome inhibitors correlates poorly with exclusive inhibition of the chymotrypsin-like sites and that co-inhibition of other sites is usually needed to achieve maximal cytotoxicity (12). In this regard, we have considered it of interest to determine whether inhibitors with increased specificity for β5 display decreased cytotoxicity.
Many structural classes of proteasome inhibitors are known (2, 13). The majority of these are N-terminally capped short peptides (2–4 residues) with an electrophilic trap at the C terminus (e.g. aldehydes, boronates, epoxyketones, and vinyl sulfones). This electrophile reacts with the catalytic N-terminal threonines of the proteasome. The peptide portion binds in substrate-binding pockets and defines the active site specificity of inhibitors. It has long been assumed that the nature of the pharmacophore, while influencing reactivity of the compound, does not affect specificity, at least when it comes to proteasome active sites. However, we have recently discovered that changing pharmacophores without altering the peptide portion of the inhibitor can affect active site specificity (14). For example, in the process of development of active site probes, we have made the surprising observation that changing epoxyketone to vinyl sulfone in the β5-specific inhibitor NC-005 increases the β5 specificity of this agent (15). In the study presented here, we address the question of whether the same is true for other β5-specific (e.g. carfilzomib, YU-101) (3, 16) and β5i-specific (e.g. PR-957) (17) epoxyketones and, if so, whether this increase in specificity leads to a decrease in cytotoxicity of these compounds.
Another indication that the pharmacophore may affect the specificity of inhibitors is a recent report by Marastoni et al. (18) that Hmb5-Val-Ser-Leu-vinyl ester (Hmb-VSL-ve) is a specific inhibitor of the trypsin-like (β2) sites. Trypsin-like sites cut peptide bonds after basic residues, and inhibitors with leucine in the P1 position would not be expected to be specific for the trypsin-like sites (19), unless one assumes that the vinyl ester moiety contributes to β2-specific targeting. To determine whether the β2 specificity of this compound is determined by the vinyl ester pharmacophore or by its peptide fragment, we have swapped the pharmacophores and peptide fragments between this compound and the β5- and β1-specific epoxyketone and vinyl sulfones we synthesized previously (12, 20).
The combined arguments outlined above led to the design of several new peptide-based proteasome inhibitors, on which we report here. Our data reveal the following findings: 1) peptide-based vinyl esters have no inhibitory activity toward proteasomes; 2) replacement of epoxyketones by vinyl sulfones increases the specificity of inhibitors for the β5 sites (but not for the β5i sites); and 3) this increase in specificity decreases cytotoxicity of the compounds, confirming our previously reported observation that inhibition of other sites in conjunction with the chymotrypsin-like sites is a prerequisite for potential anti-tumor activity (12).
EXPERIMENTAL PROCEDURES
Inhibitors and Substrates
NC-005 and NC-001 were synthesized as described previously (12). NC-005-mvs (NAc-mYFL-mvs) and NC-005-pvs (NAc-mYFL-pvs) were synthesized as described previously (15). The synthesis of peptidyl vinyl esters, Hmb-VSL-pvs, Hmb-VSL-mvs, Hmb-VSL-ek, PR-171 (carfilzomib), PR-171-mvs, YU-101, YU-101-mvs, PR-957, PR-957-mvs, and the analytical data for these compounds are described in the supplemental material. MG-132 (Z-LLL-al) and MG-262 (Z-LLL-boronate) were purchased from Boston Biochem. Z-LLL-ek and Z-LLL-vs were synthesized as described previously (14). Suc-LLVY-amc and Z-FR-amc were purchased from Bachem; Ac-RLR-amc, Ac-RQR-amc, and Ac-nLPnLD-amc were custom-synthesized by MP Biomedicals or Gene Script. E-64d (EST) was from Calbiochem.
Purification of 26 S Proteasomes
For the purification of constitutive proteasomes, young rabbit muscles (200 g, Pel-Freeze Biologicals) were homogenized in a blender in 500 ml of buffer containing 50 mm Tris-HCl, pH 7.5, 1 mm DTT, 1 mm EDTA, 0.25 m sucrose, 5 mm MgCl2, and 2 mm ATP. The homogenate was centrifuged for 15 min at 10,000 × g and then for 30 min at 40,000 × g. The supernatant was filtered through a 5-micron filter, and proteasomes were batch-absorbed on 50 ml of DE52 DEAE-cellulose. After 30 min of stirring with the supernatant, the resin was washed on a glass filter with ∼500 ml of buffer A (20 mm Tris-HCl, pH 7.5, 10% glycerol, 1 mm ATP, 5 mm MgCl2, 1 mm DTT, 0.5 mm EDTA) and then with 250 ml of 50 mm NaCl in buffer A. Proteasomes were eluted with 150 mm NaCl in the same buffer, and 40–50-ml fractions were collected. All fractions were monitored for activity using Suc-LLVY-amc as a substrate. Active fractions were pooled (∼200 mg of total protein) and loaded on a 10-ml Source Q (GE Healthcare) column, which was eluted by a gradient of 0.15–0.35 m of NaCl in 120 ml of buffer A at a flow rate of 3 ml/min. Fractions containing proteasome activity (eluting approximately at 0.28 m NaCl) were combined to give ∼40 mg of total protein, diluted 2-fold, and loaded on a 1.3-ml Uno Q column. 26 S proteasome was separated from 20 S proteasome by a gradient of 0.13–0.3 m NaCl in 30 ml of buffer A. 20 S proteasome-containing fractions were distinguished from the 26 S containing fractions based on SDS activation in the peptidase assays (21). The reason for two consecutive high resolution cation exchange chromatography steps is that Source Q column provided better separation from contaminating proteins than the Uno Q column but did not separate 20 S and 26 S proteasomes. Fractions containing 26 S proteasomes (1–2 mg of protein in a total volume of 1–2 ml/per tube) were loaded on a 32-ml 20–40% glycerol gradient (in 20 mm HEPES, pH 7.5, 1 mm DTT, 0.5 mm EDTA, 5 mm MgCl2, 0.5 mm ATP). After 16 h of centrifugation at 130,000 × g, gradients were fractionated and active fractions pooled, concentrated using Centriprep YM-50 devices, aliquoted, and stored at −80 °C. Purification of immunoproteasomes was carried out from frozen rabbit spleen using a similar procedure, except that the amount of tissue was 10 g.
Inhibitor Assays
Purified 26 S proteasomes (∼10 ng/ml) were incubated with various concentrations of inhibitors at 37 °C for 30 min in the assay buffer (50 mm Tris-HCl, pH 7.5, 1 mm ATP, 50 μg/ml BSA, 2 mm EDTA, 40 mm KCl). Immediately after the end of this incubation, an aliquot of the inhibitor-treated proteasome was mixed with the 100 μm substrate (Suc-LLVY-amc for the β5 or β5i sites, Ac-nLPnLD-amc for β1/β1i sites, and Ac-RLR-amc or Ac-RQR-amc for β2/β2i sites), and fluorescence of released amc was measured continuously for 30 min at 37 °C. (Substrate solutions did not contain inhibitors except when reversible inhibitor MG-132 was tested; in this case, MG-132 was added to the substrate at the same concentration as in the enzyme/inhibitor preincubation mixture.) The rate of reaction was determined from the slope of the reaction progress curves. Residual activity was calculated as the slope of reaction in inhibitor-treated sample divided by the slope of reaction in the control sample (i.e. proteasomes incubated under the same conditions but in the absence of inhibitor).
Extracts of HEK-293T cells (10 μg of protein, prepared as described previously (15)) were incubated with inhibitors for 1 h at 37 °C, then with 1 μm MV-151 for an additional hour at 37 °C, and then fractionated on 12.5% SDS-PAGE. Upon completion of electrophoresis, gels were scanned on a Typhoon imager (excitation laser, 532 nm; emission filter, 560 nm).
Tissue Culture Experiments
HeLa S3 cells were cultured in DMEM supplemented with 5% newborn calf serum and penicillin and streptomycin. Proteasome activity in inhibitor-treated cells was measured with luminogenic substrates using Promega ProteasomeGloTM cell-based assay (Promega) (22). Inhibitors were washed out prior to measurements. See the supplemental material in Ref. 12 for details of the procedure. Cell viability measurements were performed using Alamar Blue mitochondrial dye conversion assay (12).
Preparation of Cytosol-depleted Extracts for Cathepsin Activity Measurements
Cells were harvested, washed with PBS, and permeabilized on ice with 0.05% digitonin in 4–5 volumes of 50 mm Tris-HCl, pH 7.5, containing 250 mm sucrose, 5 mm MgCl2, 1 mm DTT, 1 mm ATP, and 0.5 mm EDTA. Cytosol was squeezed out by centrifugation for 15 min at 20,000 × g at 4 °C, and residual cell pellet was lysed with a buffer containing 50 mm BisTris-HCl, pH 5.5, 10% glycerol, 5 mm MgCl2, 1 mm EDTA, 2 mm DTT, and 0.5% CHAPS. These cytosol-depleted acidic extracts were used for measurement of cathepsin activity. Protein concentration in extracts was determined using Pierce 660 nm protein assay reagent.
Measurements of Cathepsin Activity
An aliquot of cytosol-depleted acidic extracts was added to 100 μl of 40 μm pan-cathepsin substrate Z-FR-amc in 100 μm phosphate buffer, pH 6.0, 2 mm EDTA, 4 mm DTT (23). Increase of fluorescence of released amc was recorded continuously for 30 min, and the rate of reaction was calculated from the slopes of the linear reaction progress curves. Cleavage of this substrate was completely blocked in extracts of cells treated with 5 μm E-64d.
RESULTS
Vinyl Esters Do Not Inhibit Proteasomes
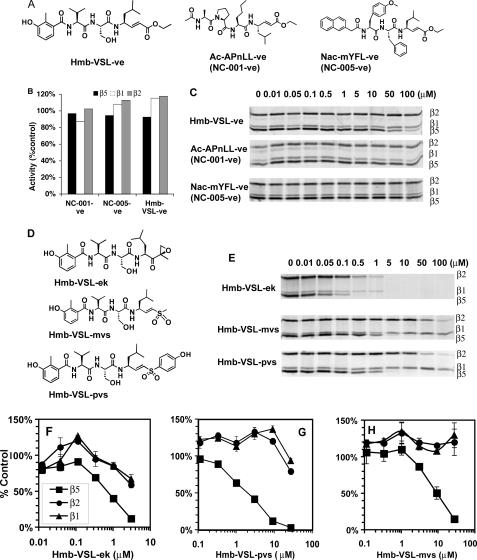
To determine which part of the Hmb-VSL-ve molecule is responsible for the β2 specificity, we synthesized this compound and vinyl ester analogues of the β1- and β5-specific inhibitors we developed earlier, namely NC-001 (Ac-APnLL-ek) and NC-005 (NAc-mYFL-ek) (12), which we designated NC-001-ve (Ac-APnLL-ve) and NC-005-ve (Nac-mYFL-ve) (Fig. 1A). We also synthesized epoxyketone and methyl and hydroxyphenyl vinyl sulfone analogues of Hmb-VSL-ve, Hmb-VSL-ek, Hmb-VSL-mvs, and Hmb-VSL-pvs (Fig. 1D).
FIGURE 1.
Peptidyl vinyl esters do not inhibit proteasomes. A, structures of peptidyl vinyl esters. B, purified 26 S proteasomes from rabbit muscles were incubated with 40 μm vinyl esters for 30 min followed by measurements of activities. Black bars, β5 sites; white bars, β1 sites; gray bars, β2 sites. C, HEK-293T lysates (10 μg total protein) were incubated with the indicated concentrations of vinyl esters for 1 h at 37 °C. Residual proteasome activity was fluorescently labeled by subsequent incubation with 1 μm MV-151 for 1 h at 37 °C. Extract were analyzed by SDS-PAGE, and MV-151-modified active subunits visualized by fluorescent imaging. D, structures of epoxyketone and vinyl sulfone derivatives of Hmb-VSL-ve. E, assays of inhibitors shown in (D) in HEK-293T lysates. F–H, inhibition of purified proteasomes from rabbit muscles by inhibitors shown in D. Squares, β5 activity; triangles, β1 activity; circles, β2 activity. Values are averages ± S.E. of two independent experiments.
All these compounds were tested for their ability to inhibit purified 26 S proteasomes from rabbit muscle and proteasomes in extracts of HEK-293T cells. After 30 min of incubation with 40 μm vinyl esters compounds, none of these inhibited activity of purified proteasomes (Fig. 1B). Vinyl esters were then incubated with extracts of HEK-293T cells, and proteasome inhibition was evaluated based on ability to prevent subsequent modification of the catalytic subunits by the fluorescent activity-based probe MV-151 (24). Except for a weak inhibition of β5 site at 100 μm of Hmb-VSL-ve (instead of expected inhibition of β2 site; Fig. 1C), no inhibition was observed. Thus, in contrast to what has been reported, peptide vinyl esters do not inhibit any proteasome active sites.
In contrast, epoxyketone and vinyl sulfone derivatives of Hmb-VSL-ve (Fig. 1D) inhibited proteasomes in both assays (Fig. 1, E–H) but were not β2-specific. The preferred target of these compounds was the β5 site. The vinyl sulfones (Fig. 1, G and H) were more β5-specific than the epoxyketones (Fig. 1F).
Comparison of β5-specific Vinyl Sulfones and Epoxyketones
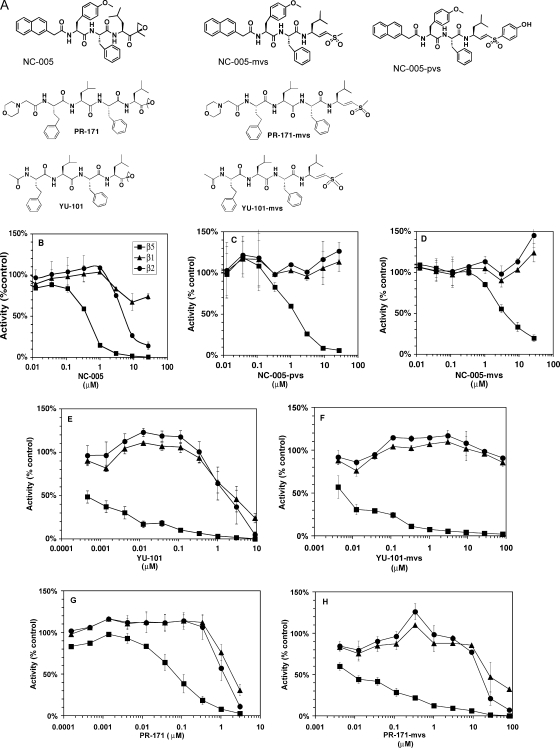
The observation that Hmb-VSL-pvs and Hmb-VSL-mvs are more β5-specific than Hmb-VSL-ek is consistent with the earlier observation that vinyl sulfone derivatives of NC-005 (NAc-mYFL-ek) are more β5-specific than NC-005 itself (15). This effect was originally observed in HEK-293T lysates with the MV-151 activity-based probe (15). Here, we confirm this observation using purified 26 S proteasomes and fluorogenic substrates (Fig. 2, B–D). Although the vinyl sulfones are less potent inhibitors of the β5 sites than the epoxyketone, they do not inhibit β1 and β2 sites. In contrast, the epoxyketones markedly inhibited β2 sites and reduced activity of β1 sites partially (Fig. 2B).
FIGURE 2.
Inhibition of purified 26 S proteasomes from rabbit muscles by epoxyketones and peptidyl vinyl sulfones targeting β5 sites. A, structures of the compounds. B–H, purified 26 S proteasomes from rabbit muscles were incubated with inhibitors at concentrations indicated for 30 min, followed by measurements of all three peptidase activities. Mock-treated proteasomes served as controls. Squares, β5 activity; triangles, β1 activity; circles, β2 activity. All values are averages ± S.E. of 2 or 3 independent measurements.
To test the generality of these findings, we have synthesized methyl vinyl sulfone derivatives of two other β5-specific epoxyketones, YU-101 (16) and PR-171 (carfilzomib) (3). In both cases, vinyl sulfones were more β5-specific than epoxyketones (Fig. 2, E–H). YU-101-vs is the most β5-specific, as it did not inhibit β1 and β2 sites even at 100 μm (Fig. 2F). It should be noted that among parental epoxyketones, YU-101 is also more β5-specific than PR-171 (compare Fig. 2, E and G). Thus, replacement of epoxyketone by vinyl sulfones increases selectivity of inhibitors to the β5 sites (at least in the context of leucine in the P1 position).
Comparison of MG-132 Derivatives with Different Pharmacophores
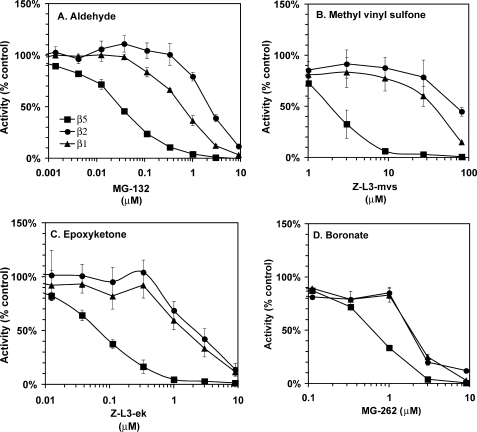
Proteasome inhibitors with different pharmacophores are widely used by the scientific community. Because blocking the β5 site alone is not sufficient to block the bulk of protein degradation (25), the question of how the chemical nature of the pharmacophore affects active site specificity of inhibitors is of great importance to the scientific community. For example, a scientist using MG-132 (Z-L3-aldehyde(al)) or its vinyl sulfone analogue Z-L3-mvs may need to substitute for these an inhibitor that does not block lysosomal proteases, such as MG-262 (Z-L3-boronate) or Z-L3-ek. Information on the impact of this substitution on the active site specificity would be very useful. We have analyzed inhibition of purified 26 S proteasomes by MG-132 and its boronate (MG-262), methyl vinyl sulfone (Z-L3-mvs), and epoxyketone (Z-L3-ek) derivatives. Although the β5 site was the primary target of all four compounds, only vinyl sulfone (Fig. 3B) was truly β5-specific, achieving 95% inhibition of β5 sites before significant inhibition of β1 and β2 sites was observed. The epoxyketone (Fig. 3C) was slightly less specific; 85% inhibition of β5 sites was achieved before inhibition of β1 and β2 sites was observed. MG-262 (Fig. 3D) was β5-specific up to 70% inhibition, after which both β2 and β1 sites were rapidly inhibited. In MG-132 (aldehyde)-treated proteasomes (Fig. 3A), only 50% inhibition of β5 sites could be achieved before inhibition of β1 sites was observed; inhibition of β2 sites was observed at higher inhibitor concentrations. We conclude that the nature of the pharmacophore affects the secondary active site specificity of proteasome inhibitors.
FIGURE 3.
Effect of pharmacophore on inhibition of purified 26 S proteasomes from rabbit muscle by MG-132 derivatives. Squares, β5 activity; triangles, β1 activity; circles, β2 activity. Values are averages ± S.E. of two independent experiments.
Effect of Epoxyketone Replacement by the Vinyl Sulfone on the β5i Subunit of the Immunoproteasomes
As discussed above, replacement of epoxyketone by methyl or 4-hydroxyphenyl vinyl sulfone makes β5-specific inhibitors even more β5-specific (Fig. 2). We asked whether a similar phenomenon happens in the purified immunoproteasomes (i.e. whether vinyl sulfones are more β5i-specific) and analyzed inhibition of different active sites in the purified 26 S immunoproteasomes from rabbit spleens (26) by NC-005, PR-171, YU-101, and β5i-specific inhibitor PR-957 (morpholino-Ac-Ala-(Me)Tyr-Phe-ek (17)) and their methyl vinyl sulfone analogues (supplemental Fig. S1 and Table 1).
TABLE 1.
Effect of inhibitors on the purified immunoproteasomes
26 S proteasomes, purified from rabbit spleens, were incubated with different concentrations of inhibitors for 30 min at 37 °C followed by measurements by activities as described under “Experimental Procedures.” Residual activity was plotted against concentration on semi-log plots (supplemental Fig. S1), which were used to determine IC50 values. (To allow for easy comparison with data on Fig. 2, IC50 values are provided instead of Ki and k2.)
| Active sites |
IC50 ratio |
||||
|---|---|---|---|---|---|
| β5i | β2i | β1i | β2i/β5i | β1i/β5i | |
| IC50 (μm) | -fold | ||||
| NC-005 | 0.044 | 4.6 | 10 | 105 | 225 |
| NC-005-mvs | 1.5 | ∼140 | ∼140 | ∼93 | ∼93 |
| YU-101 | 0.26 | 1.9 | 4.5 | 7.3 | 17.3 |
| YU-101-mvs | 1.9 | ≫100a | ≫100b | ||
| PR-171 | 0.00028 | 0.62 | 2.42 | 2321 | 8643 |
| PR-171-mvs | 0.011 | 5.3 | 22.5 | 481 | 2045 |
| PR-957 | 0.0102 | 1.04 | 6.4 | 102 | 627 |
| PR-957-mvs | 1.0 | 53 | 78 | 53 | 78 |
a 21 ± 1% inhibition was at 81 μm.
b 16 ± 2% inhibition was at 81 μm (values are mean ± S.E. of two independent measurements).
Replacement of the pharmacophore produced results different from those observed in constitutive proteasomes. With the exception of YU-101-mvs (Table 1), which was more β5i-specific than YU-101, all other vinyl sulfones were less β5i-specific than their epoxyketone counterparts. Thus, vinyl sulfones do not improve the targeting of inhibitors to the chymotrypsin-like sites of immunoproteasomes.
Increasing β5 Site Specificity Decreases Inhibitor Cytotoxicity
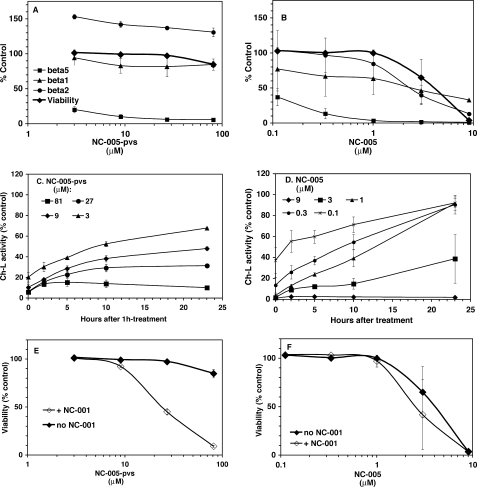
In our previous study, we showed that cytotoxicity of proteasome inhibitors poorly correlates with the inhibition of β5 sites and that co-inhibition of β2 and/or β1 sites is observed under cytotoxic conditions (12). This result predicts that increasing β5 specificity would decrease cytotoxicity of inhibitors. We tested this prediction by comparing effects of NC-005 and homologous phenol vinyl sulfone NC-005-pvs on HeLa cells. This pair of inhibitors was chosen for comparison as they offered more distinct differences in specificity than YU-101- and PR-171-based pairs (Fig. 2). Between the two NC-005-derived vinyl sulfones, 4-hydroxyphenyl vinyl sulfone was chosen over methyl vinyl sulfone as a more potent inhibitor. HeLa cells were chosen over the myeloma cells used in our previous study (12) because they do not express immunoproteasomes, in which differences in active site specificity between vinyl sulfone and epoxyketone would be less dramatic due to the lack of pharmacophore effect on β5i targeting (Table 1).
When HeLa cells were treated with these agents (Fig. 4 and supplemental Fig. S2), differences in potencies and specificities were the same as with purified proteasomes (Fig. 2), with the epoxyketone being an ∼10-fold more potent inhibitor of β5 sites than the methyl vinyl sulfone. The most noticeable difference between purified proteasomes and proteasomes in HeLa cells was activation of β2 activity by vinyl sulfone in cells (Fig. 4 and supplemental Fig. S2).
FIGURE 4.
Effect of 1-h pulse treatment HeLa S3 cells with NC-005-pvs (A, C and E) and NC-005 (B, D and F). HeLa S3 were treated with inhibitors for 1 h and then cultured in the absence of inhibitor for 48 h, whereupon cell viability was measured with an Alamar Blue assay. At times indicated, proteasome peptidase activities were measured in the aliquots of cultures. A and B, Proteasomal peptidase activities immediately after 1 h of treatment plotted together with cell viability 48 h after start of the experiment. Squares, β5 activity; triangles, β1 activity; circles, β2 activity; diamonds, cell viability. C and D, activity of β5 sites was measured at different times after removal of inhibitor. Activity is normalized to the number of cells per sample. Numbers in the legend indicate concentration of the inhibitors used for treatments. See Fig. S3 for β1 and β2 activity values. E and F, following NC-005-pvs and NC-005 treatment, cultures were split in half. One set of cultures was continuously treated with 4 μm NC-001 (open diamonds), the other mock treated (closed diamonds). NC-001 completely inhibited β1 activity but did not inhibit β2 activity and did not alter recovery rate of β5 activity (supplemental Fig. S3). On all graphs, values are average ± S.E. of 2 or 3 independent measurements (i.e. biologic replicates).
As in our previous work (12), we treated cells with inhibitors for 1 h and then removed the inhibitors and cultured cells for an additional 48 h, at which point cell viability was measured with an Alamar Blue mitochondrial dye conversion assay. Immediately after the removal of the drug, inhibition of the proteasome was confirmed by measuring activity of β1, β2, and β5 sites with site-specific luminescent substrates. Recovery of activity was followed throughout the washout period with the same assay.
We observed different effects on cell viability from 1-h exposure with NC-005 and NC-005-pvs. Vinyl sulfone was not cytotoxic at concentrations as high as 80 μm (Fig. 4A), at which β5 activity was inhibited by 90% and remained inhibited by >85% during the 24-h washout period (Fig. 4C). It should be noted that β2 activity was activated by 20–40% by this treatment and stayed activated through the washout period (supplemental Fig. S3A). Contrary to the vinyl sulfone, 1-h exposure to the epoxyketone NC-005 induced cytotoxicity (Fig. 4B). However, induction of cytotoxicity coincided with the inhibition of the β1 and β2 activities but not with the inhibition of the β5 sites (Fig. 4D). Stronger cytotoxicity of NC-005 cannot be explained by the slower recovery of proteasome activity during the washout period as this recovery was in fact faster in NC-005-treated cells (Fig. 4D) than in NC-005-pvs-treated cells (Fig. 4C). Thus, a more specific targeting of proteasome inhibitors to the β5 site decreases their cytotoxic potential.
If stronger cytotoxicity of the epoxyketone is due to its ability to co-inhibit β1 and/or β2 sites, co-inhibiting β1 sites by β1-specific inhibitor NC-001 (12) in NC-005-pvs-treated cells should sensitize them to this agent. Indeed, adding NC-001 to the media during recovery of NC-005-pvs-treated cells led to a dramatic 70–80% decrease in viability under conditions where β5 was almost completely inhibited (Fig. 4E). Contrary to this, the same β1-specific inhibitor did not cause significant sensitization of HeLa cells to the epoxyketone NC-005 (Fig. 4F). (We confirmed by activity measurements that NC-001 treatments inhibited activity of β1 sites by more than 90% but did not change inhibition of β5 and β2 sites, see supplemental Fig. S3.) Thus, complete or nearly complete inhibition of β5 and either β1 or β2 sites is needed to decrease viability of HeLa cells to less than 10%.
We then asked what would be the effects on cells of specific inhibition of β5 sites under the conditions when recovery of proteasome activity is not possible. We treated HeLa cells with NC-005-pvs continuously. Under these conditions, inhibition reaches maximum within 6 h (supplemental Fig. S2) and does not recover (Table 2). We found that specific 70% inhibition of β5 sites at 0.3 μm NC-005-pvs (40% activation of β2 activity was observed at this concentration) did not lead to cytotoxicity (Table 2). 95% inhibition of β5 sites at 1 μm NC-005-pvs (with simultaneous inhibition of β1 sites by 20% and activation of β2 sites by 15%) led to a 65% decrease in viability. Thus, specific inhibition of β5 sites is cytotoxic to HeLa cells only when it is nearly total and is long lasting.
TABLE 2.
Effect of continuous treatment with inhibitors on cell viability
Cells were treated with NC-005-pvs or NC-005 for 48 h, when viability was measured. Peptidase activities were measured 6 and 24 h after the start of treatment. Note that inhibition of active sites did not change from 6 to 24 h. Activities are normalized to the number of cells per sample at time 0 and expressed relative to values in the mock-treated controls. Values are averages ± S.E. of 2 or 3 independent measurements. Negative values indicate activation. Condition where specific inhibition of β5 sites leads to partial loss of viability is highlighted in boldface.
| Inhibitor | Viability | β5 |
β2 |
β1 |
|||
|---|---|---|---|---|---|---|---|
| 6 h | 24 h | 6 h | 24 h | 6 h | 24 h | ||
| % control | % inhibition | % inhibition | % inhibition | ||||
| NC-005-pvs | |||||||
| 0.33 μm | 100 ± 7 | 70 ± 3 | 30 ± 0.05 | −42 ± 7 | −47 ± 1 | 39 ± 5 | −11 ± 5 |
| 1 μm | 35 ± 14 | 95 ± 0.4 | 96 ± 1 | −14 ± 9 | −19 ± 14 | 23 ± 5 | 2 ± 11 |
| 3 μm | 15 ± 4 | 98 ± 0 | 99 ± 0 | 16 ± 7.5 | 2 ± 6 | 7 ± 1 | 11 ± 6 |
| 9 μm | 9 ± 3 | 98 ± 0 | 98 ± 0 | 20 ± 1 | 1 ± 32 | 46 ± 0.25 | 19 ± 28 |
| NC-005 | |||||||
| 0.11 μm | 83 ± 13 | 94.4 ± 0.4 | 74 ± 9 | −8 ± 18 | −55 ± 40 | 23 ± 7 | -31 ± 33 |
| 0.33 μm | 20 ± 4 | 97.4 ± 0.1 | 97 ± 1 | 30 ± 12 | 29 ± 22 | 39 ± 7 | 37 ± 17 |
| 1 μm | 10 ± 2 | 99 ± 0 | 99 ± 0 | 69 ± 1 | 71 ± 9 | 65 ± 1 | 68 ± 8 |
| 3 μm | 4 ± 0 | 99 ± 0 | 99 ± 0 | 88 ± 1 | 90 ± 2 | 84 ± 1 | 86 ± 1 |
As NC-005-pvs concentrations increased, its specificity decreased leading to a slight increase in cytotoxicity, but even at the highest concentration used some cells remained viable. In a parallel experiment, we treated cells continuously with the epoxyketone NC-005 (Table 2), and the percentage of surviving cells appeared to be lower than with the vinyl sulfone treatment.
To determine how increased selective targeting of inhibitors to β5 sites affects residual survival rates, we performed clonogenic survival assay of cells treated with NC-005 and NC-005-pvs for 24 h (Table 3). We used concentrations of compounds that completely inhibited β5 sites but varied in the inhibition of β1 and β2 sites. Of cells treated with 3 μm NC-005, which inhibited β1 and β2 sites by more than 80%, none survived. A smaller percentage of cells treated with 1 μm NC-005, which inhibited β1 and β2 sites by more than 60%, survived than cells treated with 9 μm of vinyl sulfone, which inhibited β1 sites by 46% and β2 sites by 20%. Thus, strong co-inhibition of β1 and β2 sites, as occurs in NC-005 treated cells, is needed to suppress residual survival of HeLa cells.
TABLE 3.
Effect of epoxyketone and vinyl sulfone on residual survival
HeLa S3 cells were treated with inhibitors at the concentrations indicated (or mock-treated) for 24 h. Cells were harvested in fresh media and replated in fresh media on 6-well plates at densities varying from 50,000 to 100,000 cells/well. 21 days after plating, media were removed; colonies were washed with PBS, stained with methylene blue, and counted. Numbers are averages ± S.E. of two independent experiments.
| Concentration | Colonies | Active site |
||
|---|---|---|---|---|
| β5 | β1 | β2 | ||
| μm | % control | % inhibition | ||
| NC-005 | ||||
| 1 | 0.12 ± 0.11 | 98.7 ± 0.05 | 65 ± 1 | 69 ± 1 |
| 3 | 0 | 99.0 ± 0.05 | 84 ± 1 | 88 ± 1 |
| NC-005-pvs | ||||
| 3 | 2.35 ± 1.77 | 98.3 ± 0 | 39 ± 5 | 16 ± 8 |
| 9 | 0.72 ± 0.13 | 98.4 ± 0.05 | 46 ± 0.25 | 20 ± 1 |
Vinyl sulfones can potentially inhibit cysteine proteases such as lysosomal cathepsins (27). To determine whether a 6-h treatment with NC-005-pvs leads to inhibition of these enzymes, we have measured cathepsin activity in extracts of inhibitor-treated cells (Table 4). We used the fluorogenic peptide substrate Z-FR-amc, which is cleaved by the majority of cathepsins (23). Cleavage of this substrate was inhibited in cells treated by EST (E-64d), a cell-permeable precursor of the class-specific inhibitor of cysteine proteases E-64 (Table 5). Indeed, NC-005-pvs but not NC-005 inhibited this activity in a concentration-dependent manner (Table 4). To determine whether inhibition of cathepsin by NC-005-pvs contributes to cytotoxicity of NC-005-pvs, we determined whether E-64d is cytotoxic to cells under similar treatment conditions as used in this experiment for NC-005-pvs. Because E64-d did not cause any reduction in cell viability after 48 h of treatment (Table 5), we conclude that inhibition of cathepsins is unlikely to contribute to the cytotoxicity of NC-005-pvs.
TABLE 4.
Effect of NC-005-pvs and NC-005 on cathepsin activity in HeLa S3 cells
Hydrolysis of pan-cathepsin substrate Z-FR-amc by acidic extracts of cytosol-depleted cells was measured after 6 h of treatment of cells with inhibitors. Values are averages ± S.E. of three independent measurements for NC-005-pvs; results of single measurement for NC-005 are shown.
| Concentration | Inhibitor |
|
|---|---|---|
| NC-005-pvs | NC-005 | |
| μm | cathepsin activity (% control) | |
| 0.1 | 82 ± 42 | 77 |
| 0.33 | 58 ± 24 | 93 |
| 1 | 37 ± 10 | 85 |
| 3 | 15 ± 2 | 93 |
| 9 | 13 ± 3 | |
TABLE 5.
Effect of E-64d on cathepsin activity and viability of HeLa cells
HeLa S3 cells were continuously treated with E-64d (EST), a cell-permeable precursor of inhibitor of cysteine proteases E-64. Activity of cathepsins was measured as in Table 4 in extracts of cells harvested 6 h after the start of the treatment. Cell viability was measured with Alamar Blue 48 h after the treatment. Cathepsin activity is normalized to the amount of protein in extracts used for the measurements of activity and expressed relative to the value in mock-treated controls. Values are averages ± S.E. of two independent measurements.
| E-64d | Cathepsin activity | Cell viability |
|---|---|---|
| μm | % control | % control |
| 0.22 | 52 ± 15 | 99 ± 5 |
| 0.67 | 19 ± 7 | 95 ± 3 |
| 2 | 13 ± 0 | 93 ± 1 |
| 6 | 7 ± 2 | 88 ± 5 |
DISCUSSION
Vinyl Sulfones Are More Specific β5 Inhibitors than Epoxyketones
Although we noticed a few years ago that the nature of the electrophilic group may affect the active site specificity of proteasome inhibitors (14), we report here the first systematic comparison of vinyl sulfones and epoxyketones in specific targeting of inhibitors to the chymotrypsin-like sites of the proteasome. Our conclusion that vinyl sulfone inhibitors are more β5-specific than epoxyketone inhibitors is supported by the data on five series of compounds. 1) Hmb-VSL-mvs and Hmb-VSL-pvs are clearly more specific than Hmb-VSL-ek (Fig. 1). 2) Replacement of epoxyketone in NC-005 by either of the vinyl sulfone pharmacophores dramatically decreases its ability to co-inhibit β2 and β1 sites (Figs. 2 and 4) (15), even with prolonged treatment of cells (Table 2 and supplemental Fig. S2). 3) Conversion of the epoxyketone YU-101 into a vinyl sulfone abolishes inhibition of β1 and β2 sites (Fig. 2). 4) PR-171-mvs is a more specific β5 inhibitor than the parent epoxyketone PR-171. 5) Z-L3-mvs is more β5-specific than Z-L3-ek (Fig. 3). This conclusion does not extend to the immunoproteasomes, as vinyl sulfones do not improve selectivity of inhibitors to the β5i sites (Table 1 and supplemental Fig. S1).
Vinyl Esters Do Not Inhibit Proteasomes
As we clearly demonstrate the effect of a vinyl sulfone pharmacophore on the β5 specificity, we reject the previous claim that vinyl esters composed of the same peptide sequence are selective inhibitors of the β2 sites (18). In fact, we found that said peptidyl vinyl ester does not have any proteasome inhibitory activity at all, at least in our assays (Fig. 1). An explanation for the differences between our results and that of Marastoni et al. (18) might be that the inhibitory activity in the preparation of the vinyl ester used by Marastoni et al. belongs not to a major component but to a minute contaminant (or possibly a contaminating diastereomer) that was not separated by HPLC or detected by NMR. We prepared the vinyl ester via two synthetic routes (see supplemental material), including the reported route, and took care to purify the compound to homogeneity, and we are therefore confident that we have in fact prepared the compound claimed by Marastoni et al. (18) as a β2-specific inhibitor.
Increasing β5 Specificity Decreases Cytotoxicity of Inhibitors
The proteasome inhibitor bortezomib is being used clinically for the treatment of multiple myeloma, and second generation inhibitors are at different stages of development (3–6). Development of all these compounds has been focused on inhibition of β5 sites. However, most of them co-target β1 and/or β2 sites, and it is not completely clear whether co-inhibiting these sites is important for their anti-neoplastic activity. Thus, an important issue for the development of next-generation compounds is whether targeting β5 sites is sufficient to achieve optimal anti-neoplastic activity. In our previous study, we have shown that, for the majority of multiple myeloma cell lines, cytotoxicity of NC-005 poorly correlates with β5 inhibition (12) and that adding a β1-specific inhibitor sensitizes them to NC-005. These data suggest that specific inhibition of β5 sites would not be sufficient to induce cytotoxicity in the majority of cell lines. Development of a more selective β5-specific inhibitor has allowed us to test this prediction in this study. Upon a 1-h pulse treatment of HeLa S3 cells, we found that as β5 specificity increases, cytotoxicity of inhibitors decreases dramatically (Fig. 4). Specific inhibition of β5 sites leads to the loss of viability only if inhibition exceeds 95% and is continuous (Table 2). Even under these conditions, loss of viability is only partial (65%). Strong co-inhibition (80%) of other sites is needed to suppress residual viability (Table 3). These data are consistent with the observations of Parlati et al. (28), who found that specific inhibition of the chymotrypsin-like activity causes partial loss of viability of cell lines derived from hematologic malignancies. It should be noted that the conditions under which we observed that specific inhibition leads to cytotoxicity (e.g. 95% inhibition of β5 sites lasting 20 h, with only 20% inhibition of β1 sites and activation of β2 sites) could not be achieved with any other reported β5-specific inhibitors as they all lose specificity when β5 inhibition is so strong.
A caveat in using vinyl sulfones is their potential for inhibition of cysteine proteases (e.g. cathepsins) (27). We have addressed this concern by measuring cathepsin inhibition (Table 4). Even though we found such an inhibition, these off-target effects of vinyl sulfones are unlikely to contribute to the cytotoxicity of the compounds because the class-specific inhibitor of thiol proteases E-64d was not cytotoxic to HeLa cells (Table 5).
In certain situations, conferring the ability to inhibit cathepsins to proteasome inhibitors may improve their therapeutic utility. Bortezomib was recently shown to have additive effects with a cathepsin S inhibitor in a mouse model of multiple sclerosis (29). Thus, peptide vinyl sulfones that target proteasome chymotrypsin-like activity and cathepsins may find therapeutic applications in the treatment of autoimmune disease.
In summary, this work clearly demonstrates the importance of pharmacophores in determining active site specificity of proteasome inhibitors and provides new tools for highly specific inhibition of proteasome β5 sites.
Supplementary Material
Acknowledgment
We thank Hans van der Elst (Leiden Institute of Chemistry) for assistance with LC-MS.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 Grant from NCI (to A. F. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- Hmb
- 3-hydroxy-2-methylbenzoyl
- al
- aldehyde
- amc
- 7-amido-4-methylcoumarinamide
- ek
- epoxyketone
- mvs
- methyl vinyl sulfone
- mY
- 4-methyltyrosine
- NAc
- (2-naphthyl)-acetyl
- nL
- norleucine
- pvs
- 4-hydroxyphenyl vinyl sulfone
- Suc
- succinyl
- ve
- vinyl ester
- Z
- benzyloxycarbonyl
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1.Adams J. (2004) Nat. Rev. Cancer 4, 349–360 [DOI] [PubMed] [Google Scholar]
- 2.Kisselev A. F., Goldberg A. L. (2001) Chem. Biol. 8, 739–758 [DOI] [PubMed] [Google Scholar]
- 3.Demo S. D., Kirk C. J., Aujay M. A., Buchholz T. J., Dajee M., Ho M. N., Jiang J., Laidig G. J., Lewis E. R., Parlati F., Shenk K. D., Smyth M. S., Sun C. M., Vallone M. K., Woo T. M., Molineaux C. J., Bennett M. K. (2007) Cancer Res. 67, 6383–6391 [DOI] [PubMed] [Google Scholar]
- 4.Chauhan D., Catley L., Li G., Podar K., Hideshima T., Velankar M., Mitsiades C., Mitsiades N., Yasui H., Letai A., Ovaa H., Berkers C., Nicholson B., Chao T. H., Neuteboom S. T., Richardson P., Palladino M. A., Anderson K. C. (2005) Can. Cell 8, 407–419 [DOI] [PubMed] [Google Scholar]
- 5.Piva R., Ruggeri B., Williams M., Costa G., Tamagno I., Ferrero D., Giai V., Coscia M., Peola S., Massaia M., Pezzoni G., Allievi C., Pescalli N., Cassin M., di Giovine S., Nicoli P., de Feudis P., Strepponi I., Roato I., Ferracini R., Bussolati B., Camussi G., Jones-Bolin S., Hunter K., Zhao H., Neri A., Palumbo A., Berkers C., Ovaa H., Bernareggi A., Inghirami G. (2008) Blood 111, 2765–2775 [DOI] [PubMed] [Google Scholar]
- 6.Kupperman E., Lee E. C., Cao Y., Bannerman B., Fitzgerald M., Berger A., Yu J., Yang Y., Hales P., Bruzzese F., Liu J., Blank J., Garcia K., Tsu C., Dick L., Fleming P., Yu L., Manfredi M., Rolfe M., Bolen J. (2010) Cancer Res. 70, 1970–1980 [DOI] [PubMed] [Google Scholar]
- 7.Groll M., Ditzel L., Löwe J., Stock D., Bochtler M., Bartunik H. D., Huber R. (1997) Nature 386, 463–471 [DOI] [PubMed] [Google Scholar]
- 8.Dick T. P., Nussbaum A. K., Deeg M., Heinemeyer W., Groll M., Schirle M., Keilholz W., Stevanoviæ S., Wolf D. H., Huber R., Rammensee H. G., Schild H. (1998) J. Biol. Chem. 273, 25637–25646 [DOI] [PubMed] [Google Scholar]
- 9.Chen P., Hochstrasser M. (1996) Cell 86, 961–972 [DOI] [PubMed] [Google Scholar]
- 10.Arendt C. S., Hochstrasser M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 7156–7161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinemeyer W., Fischer M., Krimmer T., Stachon U., Wolf D. H. (1997) J. Biol. Chem. 272, 25200–25209 [DOI] [PubMed] [Google Scholar]
- 12.Britton M., Lucas M. M., Downey S. L., Screen M., Pletnev A. A., Verdoes M., Tokhunts R. A., Amir O., Goddard A. L., Pelphrey P. M., Wright D. L., Overkleeft H. S., Kisselev A. F. (2009) Chem. Biol. 16, 1278–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groll M., Huber R. (2004) Biochim. Biophys. Acta 1695, 33–44 [DOI] [PubMed] [Google Scholar]
- 14.Verdoes M., Florea B. I., van der Linden W. A., Renou D., van den Nieuwendijk A. M., van der Marel G. A., Overkleeft H. S. (2007) Org. Biomol. Chem. 5, 1416–1426 [DOI] [PubMed] [Google Scholar]
- 15.Verdoes M., Willems L. I., van der Linden W. A., Duivenvoorden B. A., van der Marel G. A., Florea B. I., Kisselev A. F., Overkleeft H. S. (2010) Org. Biomol. Chem. 8, 2719–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elofsson M., Splittgerber U., Myung J., Mohan R., Crews C. M. (1999) Chem. Biol. 6, 811–822 [DOI] [PubMed] [Google Scholar]
- 17.Muchamuel T., Basler M., Aujay M. A., Suzuki E., Kalim K. W., Lauer C., Sylvain C., Ring E. R., Shields J., Jiang J., Shwonek P., Parlati F., Demo S. D., Bennett M. K., Kirk C. J., Groettrup M. (2009) Nat. Med. 15, 781–787 [DOI] [PubMed] [Google Scholar]
- 18.Marastoni M., Baldisserotto A., Cellini S., Gavioli R., Tomatis R. (2005) J. Med. Chem. 48, 5038–5042 [DOI] [PubMed] [Google Scholar]
- 19.Groll M., Nazif T., Huber R., Bogyo M. (2002) Chem. Biol. 9, 655–662 [DOI] [PubMed] [Google Scholar]
- 20.van Swieten P. F., Samuel E., Hernández R. O., van den Nieuwendijk A. M., Leeuwenburgh M. A., van der Marel G. A., Kessler B. M., Overkleeft H. S., Kisselev A. F. (2007) Bioorg. Med. Chem. Lett. 17, 3402–3405 [DOI] [PubMed] [Google Scholar]
- 21.Kisselev A. F., Akopian T. N., Woo K. M., Goldberg A. L. (1999) J. Biol. Chem. 274, 3363–3371 [DOI] [PubMed] [Google Scholar]
- 22.Moravec R. A., O'Brien M. A., Daily W. J., Scurria M. A., Bernad L., Riss T. L. (2009) Anal. Biochem. 387, 294–302 [DOI] [PubMed] [Google Scholar]
- 23.Kirschke H., Wiederanders B. (1994) Methods Enzymol. 244, 500–511 [DOI] [PubMed] [Google Scholar]
- 24.Verdoes M., Florea B. I., Menendez-Benito V., Maynard C. J., Witte M. D., van der Linden W. A., van den Nieuwendijk A. M., Hofmann T., Berkers C. R., van Leeuwen F. W., Groothuis T. A., Leeuwenburgh M. A., Ovaa H., Neefjes J. J., Filippov D. V., van der Marel G. A., Dantuma N. P., Overkleeft H. S. (2006) Chem. Biol. 13, 1217–1226 [DOI] [PubMed] [Google Scholar]
- 25.Kisselev A. F., Callard A., Goldberg A. L. (2006) J. Biol. Chem. 281, 8582–8590 [DOI] [PubMed] [Google Scholar]
- 26.Cascio P., Hilton C., Kisselev A. F., Rock K. L., Goldberg A. L. (2001) EMBO J. 20, 2357–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer J. T., Rasnick D., Klaus J. L., Brömme D. (1995) J. Med. Chem. 38, 3193–3196 [DOI] [PubMed] [Google Scholar]
- 28.Parlati F., Lee S. J., Aujay M., Suzuki E., Levitsky K., Lorens J. B., Micklem D. R., Ruurs P., Sylvain C., Lu Y., Shenk K. D., Bennett M. K. (2009) Blood 114, 3439–3447 [DOI] [PubMed] [Google Scholar]
- 29.Fissolo N., Kraus M., Reich M., Ayturan M., Overkleeft H., Driessen C., Weissert R. (2008) Eur. J. Immunol. 38, 2401–2411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.