Abstract
INCENP, Borealin, Survivin, and Aurora B kinase comprise the chromosomal passenger complex, an essential regulator of mitotic events. INCENP (inner centromere protein) binds and activates Aurora B through a feedback loop involving phosphorylation of a Thr-Ser-Ser (TSS) motif near the INCENP C terminus. Here, we have examined the role of the TSS motif in vertebrate cells using an DT40 INCENPON/OFF conditional knock-out cell line in which mutants are expressed in the absence of wild-type INCENP. Our analysis confirms that regulated phosphorylation of the two serine residues (presumably by Aurora B) is critical for full activation of the kinase and is essential for cell viability. Cells expressing INCENP mutants bearing either phospho-null (TAA) or phospho-mimetic (TEE) mutations exhibit significant levels of Aurora B kinase activity but fail to undergo normal spindle elongation or complete cytokinesis. This work confirms previous suggestions that INCENP can act as a rheostat, with different INCENP mutants promoting differing degrees of kinase activation. Our results also reveal that mitotic progression is accompanied by a requirement for progressively higher levels of Aurora B kinase activity.
Keywords: Cell Cycle, Cell Division, Gene Knockout, Microtubules, Mitosis, Aurora B Kinase, DT40, INCENP, Chromosome Passenger Complex, Cytokinesis
Introduction
The chromosomal passenger complex (CPC)4 regulates numerous mitotic events, including chromatin modification, correction of kinetochore-microtubule misattachments, regulation of sister chromatid cohesion, and completion of cytokinesis (1, 2). The active subunit of this complex is the Aurora B kinase (3), with INCENP exerting a regulatory/scaffolding role and Survivin and Borealin involved in targeting of the complex to its various sites of action during mitosis.
INCENP acts as a scaffold for CPC assembly through its N terminus, which forms a three-helix bundle with the N terminus of Borealin and C terminus of Survivin (4, 5). This interaction is sufficient for centromere targeting of the three proteins (4, 6) and does not require complex formation with Aurora B (7). The INCENP C terminus binds Aurora B through its highly conserved IN-Box (8). This activates the Aurora B kinase through a two-step feedback mechanism. When Aurora B binds INCENP, this partially activates the kinase, and it phosphorylates a conserved Thr-Ser-Ser (TSS) motif near the INCENP C terminus (residues 813–815 in human INCENP) (9). This is followed by the autophosphorylation of Aurora B on its T-loop, which elicits full kinase activity (9–12). This feedback loop has been well studied in vitro; however, it remains little studied in living cells, although phospho-proteomics has confirmed that all three residues are phosphorylated in human mitotic cells (13–15).
We recently showed that INCENP mutants can produce a range of levels of Aurora B kinase activity in living chicken DT40 cells. For example, in cells expressing only an INCENP mutant that cannot bind Aurora B (INCENPW766G-chicken INCENP numbering), the levels of kinase activity detected using endogenous H3 as a substrate were similar to those seen in the absence of INCENP (7). In contrast, an INCENP mutant (INCENPF802A) previously suggested to be unable to promote the correct conformation of the Aurora B catalytic cleft (12), did bind Aurora B, but promoted only a very low level of kinase activation (7).
Here, we have conducted a mutational analysis of the INCENP TSS motif using an INCENPON/OFF conditional knock-out in chicken DT40 cells (7). Because the “T” is missing from Drosophila and Caenorhabditis elegans INCENP and the “SS” has been shown to be required for full Aurora B kinase activity (9, 11, 12), we have focused on the two serine residues. We demonstrate that regulated phosphorylation of these serines is essential for cell viability. We confirm our previous suggestion that INCENP can act as a rheostat, with different INCENP mutants promoting differing degrees of kinase activity (7). Our results also reveal that, as recently demonstrated for CyclinB1-Cdk1 (16), mitotic progression is accompanied by a requirement for progressively higher levels of Aurora B kinase activity.
EXPERIMENTAL PROCEDURES
Cell Culture
DT40 cells were grown in suspension in RPMI 1640 medium supplemented with 10% FBS, 1% chicken serum, 100 units/ml penicillin-streptomycin, 100 μg/ml streptomycin, and 300 mg/ml l-glutamine (Invitrogen). Cultures were maintained in 5% CO2 at 39 °C at no more than 106 cells/ml. Doxycycline at a final concentration of 10–500 ng/ml was added to the culture medium to repress transcription of the endogenous promoter-hijacked INCENP allele (7).
Immunoblotting and Antibodies
Whole cell lysates were prepared, and the equivalent to one million cells was loaded onto a polyacrylamide gel. SDS-PAGE and immunoblotting were performed following standard procedures. Anti-α-tubulin antibody (B512), anti-γ-tubulin antibody (AK15) and anti-H3 phosphoserine 10(H3S10ph), anti-H3 phosphoserine 28 (H3S28ph), were purchased from Sigma and Upstate Biotech, respectively. Rabbit polyclonal (WCE1186) and mouse monoclonal anti-INCENP (3D3) and anti-SBP antibodies were described previously (17–19), as were rabbit polyclonal anti-chicken Survivin, Borealin, and Aurora B (7, 20). All samples were subjected to SDS-PAGE and then checked by immunoblotting, silver staining, or Coomassie Blue staining.
Indirect Immunofluorescence Microscopy
Cells were incubated at 39 °C on polylysine-coated slides (PolysineTM from VWR International) for 15 min before fixation in 4% paraformaldehyde/CB buffer (1.1 mm Na2HPO4, 0.4 mm KH2PO4, 5 mm PIPES, 137 mm NaCl, 5 mm KCl, 2 mm MgCl2, 2 mm EGTA, 5.5 mm glucose, pH 6.1) at 37 °C and permeabilization in 0.15% Triton X-100 in CB buffer. After blocking in 1% BSA/PBS, cells were probed with the antibodies described above, and slides were mounted using Vectashield (Vector Laboratories). Image stacks were taken using an Olympus IX-70 microscope controlled by DeltaVision SoftWorx (Applied Precision) and a 100× objective (NA 1.4). Image stacks were deconvolved, and maximum projections were generated.
Site-directed Mutagenesis
INCENP point mutants were generated by site-directed mutagenesis (QuikChangeTM Site-directed Mutagenesis kit; Stratagene) using the plasmid pTrAP-GgINCENP Class I and transferred into the conditional knock-out cells by electroporation. Primer sequences were as follows. INCENP Class I W766G mutant has been described (7): TS814A/S815A forward primer, gctacttcaagcgcaccgccgctgctgtgtggaactcc and reverse primer, ggagttccacacagcagcggcggtgcgcttgaagtagc; TS814E/S815E forward primer, ccacgctacttcaagcgcaccgaggaggctgtgtggaactccccacca and reverse primer, tggtggggagttccacacagcctcctcggtgcgcttgaagtagcgtgg.
Stable transformants were selected by Zeocin 400 μg/ml. Stable knock-out lines homogeneously expressing the TrAP-tagged fusion protein at levels comparable with the endogenous INCENP in wild-type cells were isolated and grown at 39 °C.
Quantitative Immunoblotting
Membranes were first incubated with primary antibodies recognizing Aurora B, α-tubulin, H3S10ph, and subsequently with IRDye®-labeled secondary antibodies (LI-COR). Fluorescence intensities were subsequently determined using a LI-COR Odyssey CCD scanner according to the manufacturer's instructions.
Spindle Checkpoint Assay
Exponentially growing cells were grown with doxycycline to remove endogenous INCENP, followed by treatment with Taxol (10 or 100 nm), nocodazole (0.5 μg/ml), or ZM447439 (2 μm) for 9 h. Cells were plated on polylysine-coated slides, fixed with 4% paraformaldeyde, then stained with DAPI. A total of 200 cells was scored from each sample to determine the mitotic index. Experiments were performed at least three times.
Quantitative Analysis
Growth curves were generated by seeding the various cell lines at 2 × 105 cells/ml at 39 °C (unless indicated otherwise) and counting the cell number every 12 h until 48 h. To avoid the effects of overgrowth, cells were diluted to 4 × 105 cells/ml whenever the number exceeded 1 × 106 cells/ml. The cell number at each time point was multiplied by the appropriate cumulative dilution factor to get a true count.
To determine the multinucleation and mitotic indices of untreated cultures, a total of 500 cells was scored at each time point for each individual experiment (N > 3). The multinucleation index was calculated by dividing the number of multinucleated interphase cells by the total number (500) of interphase cells. The mitotic index was calculated by dividing the number of mitotic cells by the number of interphase and mitotic cells.
To assess the distribution of mitotic phases, cells were subjected to immunofluorescence using appropriate antibodies (e.g. Aurora B, α-tubulin, γ-tubulin). A total of at least 200 mitotic cells was scored at each time point. Each quantitation was performed independently at least three times.
Measurements of Pole-to-pole Distances
To measure the distance between spindle poles in anaphase control and mutant cells, cells were first stained for γ-tubulin plus α-tubulin or lamin B2 and counterstained with DAPI for DNA. Image stacks were acquired using an Olympus IX-70 microscope controlled by SoftWorx (Applied Precision) using a 100× objective (NA 1.4). Images were all taken using the same magnification. Image stacks were deconvolved, and maximum projections were generated. Measurements were performed only for those anaphase cells with two spindle poles appearing in the same single image plane. The distance (micrometers) was measured with three-dimensional measurement software (Image-Pro Plus.v.7.0; Media Cybernetics Image Analysis Software) and/or two-dimensional measurement (DV SoftWorx). Comparison between three-dimensional and two-dimensional measurements yielded similar results. Measurements were performed in three independent experiments.
Live Cell Imaging
Live cell imaging was performed with a Delta-Vision microscope in a sealed chamber at 39 °C. One single plane was imaged on each channel every 2 min until anaphase onset, then five optical sections of 0.4-μm spacing were collected every minute. Cells were transiently transfected as described previously (21) with H2B-mRFP and Lamin A-GFP constructs, and live cell analyses were conducted 20 h after transfection.
RESULTS AND DISCUSSION
We previously described a conditional genetic knock-out of the incenp1 gene in chicken DT40 cells (7). In those cells, the promoter of one allele was replaced by a tetracycline-regulated promoter controlled by the tTA transcriptional regulator expressed under control of a fragment of the Kif4A promoter. The second allele was disrupted and can only produce a peptide of 28 amino acids. INCENP is overproduced in these cells (Fig. 1, D and E, lanes 0), but its levels drop rapidly following the addition of doxycycline, which represses transcription of the hijacked endogenous allele (19). INCENP becomes undetectable by immunoblotting by 26 h (7). We refer to this condition as INCENPOFF. This system allowed us to create stable cell lines in which we tested the role of different structural mutants of the INCENP protein in an INCENP-null background (7) (supplemental Fig. 1A).
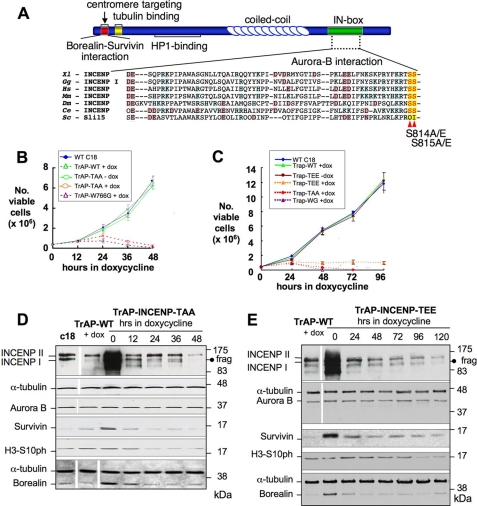
FIGURE 1.
Generation of INCENP TSS motif structural mutants to dissect INCENP-Aurora B Regulation. A, schematic representation of the main domains of INCENP, with a sequence alignment of the INCENP IN-box in several model organisms. Red arrows point to residues Ser814 and Ser815 mutated in this study. B and C, growth curves of DT40 wild-type, INCENPON/OFF, and INCENPON/OFF cells expressing TrAP-INCENPWT-class I plus TrAP-INCENPTS814AS815A (B) or TrAP-INCENPWT-class I plus TrAP-INCENPTS814ES815E (C). D and E, immunoblots showing levels of chromosomal passenger proteins in INCENPON/OFF cells expressing TrAP-INCENPTS814AS815A (D) and TrAP-INCENPTS814ES815E (E). INCENPOFF cells expressing TrAP-INCENPWT are shown as controls. Lane c18 in D shows the levels of the endogenous CPC proteins in wild-type DT40 clone 18. Levels of H3S10ph are shown as a measure of Aurora B kinase activity. α-Tubulin is shown as a loading control.
Three previously published clonal sub-lines were used in these studies. INCENPOFF cells expressing exogenous INCENPWT behave as wild-type cells in all assays (Fig. 1, B and C). INCENPW766G and INCENPF802A both fail to activate Aurora B kinase to a significant level (see Introduction). Cell lines expressing only these mutants start to die within 24 h following the addition of doxycycline (Fig. 1B). All mutants of INCENP were based upon the class I splice variant, and all were tagged with the N-terminal TrAP (His-SBP-S) tag (19).
To determine the role of the INCENP C-terminal TSS motif in vivo, we prepared two mutants of INCENP class I. INCENPTS814AS815A (INCENPTAA, Fig. 1A) mimics the nonphosphorylated state of INCENP. INCENPTS814ES815E (INCENPTEE, Fig. 1A) mimics its constitutively phosphorylated state. For all mutants, we isolated independent clones that stably expressed the exogenous INCENP at a level close to that of endogenous INCENP (Fig. 1D, lane c18). Note that the TrAP tag cloned in front of the INCENP class I ORF caused a minor upward shift in the immunoblots (7) (Fig. 1, D and E). All tagged exogenous INCENP molecules give rise to a fragment in DT40 cells that migrates close to the endogenous class I INCENP protein (Fig. 1, D and E). We showed previously that this fragment is not due to the endogenous protein and can be resolved from it by SDS-PAGE (7).
In the presence of endogenous INCENP, the INCENPTAA and INCENPTEE mutants had growth properties indistinguishable from control cells (Fig. 1, B and C). The endogenous passenger proteins exhibited their typical dynamic localization in these cells (data not shown). The fact that the mutants did not exert detectable dominant negative effects in the INCENP conditional knock-out cells may be due to the overexpression of endogenous INCENP from the hijacked allele (Fig. 1, D and E, lanes 0).
Cultures expressing the nonphosphorylated INCENPTAA mutant died by ∼48 h growth in doxycycline (Fig. 1B). This is significantly delayed relative to the cell death observed for INCENPOFF cells expressing the Aurora B-nonbinding mutant TrAP-INCENPW766G (Fig. 1B).
INCENP mutants mimicking constitutive phosphorylation (INCENPTEE) died even more slowly, between 96 and 120 h after the addition of doxycycline (Fig. 1C and data not shown). It thus appears that regulated INCENP TSS phosphorylation is essential for cells to proliferate normally.
In cell lines where INCENP was overexpressed, Survivin and Borealin levels were correspondingly elevated. Following shutoff of the hijacked endogenous allele, the levels of these proteins fell in parallel with the drop in INCENP levels (Fig. 1, D and E). In contrast, Aurora B protein levels were unaffected by INCENP overexpression or shutoff.
As shown previously (7), Aurora B kinase activity was significantly decreased in INCENPOFF cells as determined by quantitative immunoblotting for histone H3 phosphorylated on serine 10 (H3S10ph; Fig. 2E and F, and supplemental Fig. 1). H3 phosphorylation was rescued in INCENPOFF cells expressing INCENPWT but essentially abolished following addition to ZM447439 (Fig. 2, E and F).
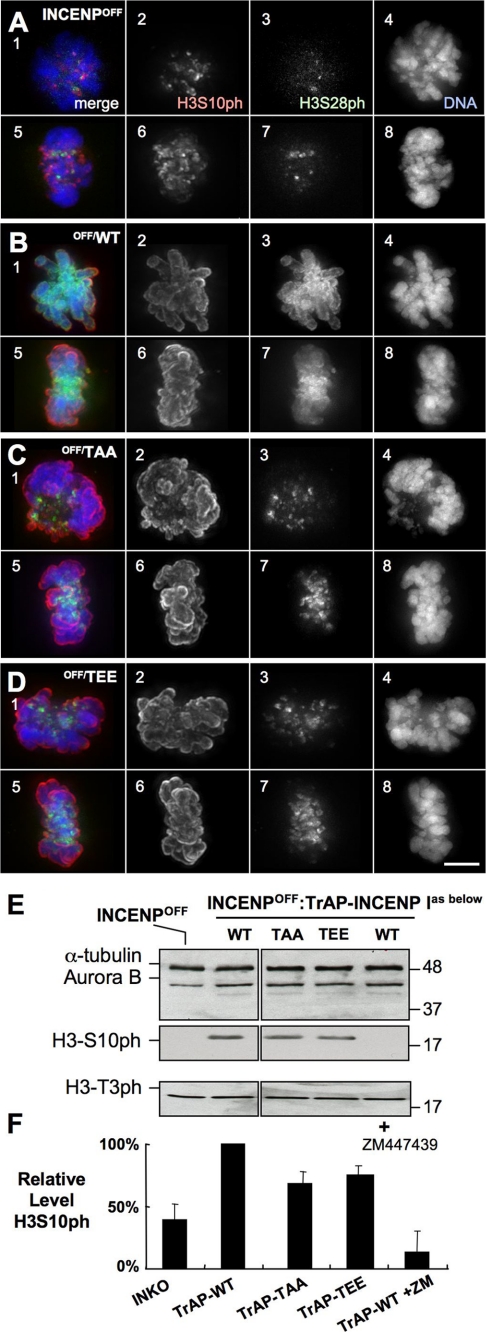
FIGURE 2.
Quantitation of Aurora B kinase activity in INCENP mutants. A–D, staining for H3S10ph and H3S28ph, markers of Aurora B kinase activity: H3S10ph (panels 2 and 6, red), H3S28ph (panels 3 and 7, green), plus DNA (panels 4 and 8, blue) in INCENPOFF cells (A) or INCENPOFF cells expressing TrAP-INCENPWT (B), TrAP-INCENPTS814AS815A (C), or TrAP-INCENPTS814ES815E (D). Scale bar, 5 μm. E, estimation of Aurora B kinase activity by immunoblotting. Cells were harvested following treatment with doxycycline for 28 h as described (7), and lysates were subjected to immunoblotting with the antibodies indicated. α-Tubulin and Haspin kinase substrate H3T3ph are shown as controls. F, measurement of H3S10ph levels in the same samples using the LI-COR Odyssey.
With both TSS mutants the drop in Aurora B kinase activity was relatively modest, remaining at 68–75% of that seen in INCENPOFF cells expressing INCENPWT. These results with quantitative immunoblotting of whole cultures were readily confirmed at the level of single cells by indirect immunofluorescence staining for H3S10ph and H3S28ph (Fig. 2, A–D).
Expression of these mutants had no significant effect on the entry of cells into mitosis (supplemental Fig. 2, A and B), and the cells exhibited normal checkpoint responses to nocodazole as well as low and high dose Taxol (Fig. 3E). However, mitotic progression was delayed, and the mutant cells accumulated in prometaphase/metaphase (Fig. 3, A and C).
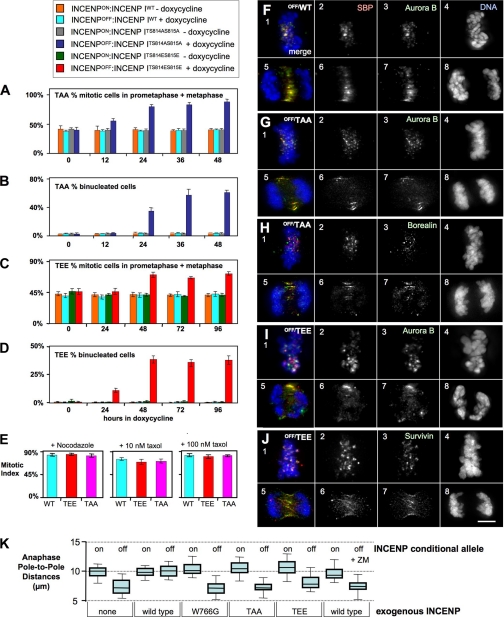
FIGURE 3.
Phenotypic analysis of INCENPOFF cells expressing mutants of the INCENP TSS motif. A–E, analysis of mitotic parameters in INCENPOFF cells expressing INCENPTSS mutants. Cells were plated on coverslips, stained with antibodies to Aurora B, microtubules, or DAPI for DNA, and scored for the percentage of mitotic cells in prometaphase or metaphase (A and C) or the percentage of binucleated cells (B and D). E, INCENPOFF cells expressing TrAP-INCENPWT or TrAP-INCENPTSS mutants exhibit a robust spindle checkpoint response to nocodazole and 10 or 100 nm Taxol. Three independent experiments were performed. Error bars indicate S.D. F–J, immunofluorescence analysis of INCENPOFF cells expressing nonphosphorylatable mutant TrAP-INCENPTS814AS815A or phospho-mimetic mutant TrAP-INCENPTS814ES815E. Cells were harvested after incubation for 28 h in doxycycline. F, in INCENPOFF cells, exogenous TrAP-INCENPWT (panels 2 and 6, red) shown co-localized with endogenous Aurora B (panels 3 and 7, green), with a typical chromosomal passenger dynamic localization throughout mitosis. Cells were also stained with DAPI to label DNA (panels 4 and 8, blue). G–J, INCENPOFF cells expressing the indicated TrAP-INCENPTSS mutant and stained with anti-SBP to show the localization of the exogenous INCENP (panels 2 and 6, red) or with antibodies to Aurora B (F, G, and I, panels 3 and 7, green), Borealin (H, panels 3 and 7, green), or Survivin (J, panels 3 and 7, green) plus DAPI for DNA (panels 4 and 8, blue). Scale bar, 5 μm. K, measurement of the distance between poles of bipolar spindles in INCENPOFF cells and INCENPOFF cells expressing TrAP-INCENPWT or the indicated INCENP mutants. Anaphase cells were measured only when both poles of a bipolar spindle were in the same plane and in a straight line.
In INCENPOFF cells expressing either INCENPTAA or INCENPTEE, the endogenous CPC components behaved normally, localizing to centromeres early in mitosis and to the spindle midzone during mitotic exit (Fig. 3, F–J, and supplemental Fig. 1, B–H). However, the percentage of binucleated (>2n) cells increased dramatically (Fig. 3, B and D), suggesting that the mutant cells failed to undergo cytokinesis. Similar phenotypes were seen with cells expressing both the INCENPTAA and INCENPTEE mutants, although the latter exhibited a modest increase in mitotic index at later time points (supplemental Fig. 2B).
Despite the normal movements of the CPC and normal spindle checkpoint response of INCENP-TSS mutants, these cultures accumulate binucleated cells and ultimately die. We therefore decided to examine the events of anaphase in greater detail.
In a previous study, microinjection of rabbit polyclonal antibody against a C-terminal fragment of INCENP 677–874 caused a 30∼40% decrease of Aurora B kinase activity (22). These cells had shortened spindles at anaphase. Although the INCENP immunogen contains the TSS motif, the mechanism of kinase inhibition and spindle elongation by the antibody was not known.
To investigate the role of INCENP in spindle elongation, we measured the distance between the poles of bipolar spindles stained with antibody to γ-tubulin. Anaphase spindles were significantly shorter in INCENP-depleted cells and in INCENPOFF cells expressing the Aurora B nonbinding mutant INCENPW766G (Fig. 3K). This phenotype was rescued by the expression of exogenous INCENPWT, but not in the presence of ZM447439, where the spindles were again short. Interestingly, despite having up to 75% of the wild-type level of Aurora B kinase activity, INCENPOFF cells expressing either unphosphorylated INCENPTAA or phospho-mimic INCENPTEE both exhibited short spindles in anaphase (Fig. 3K).
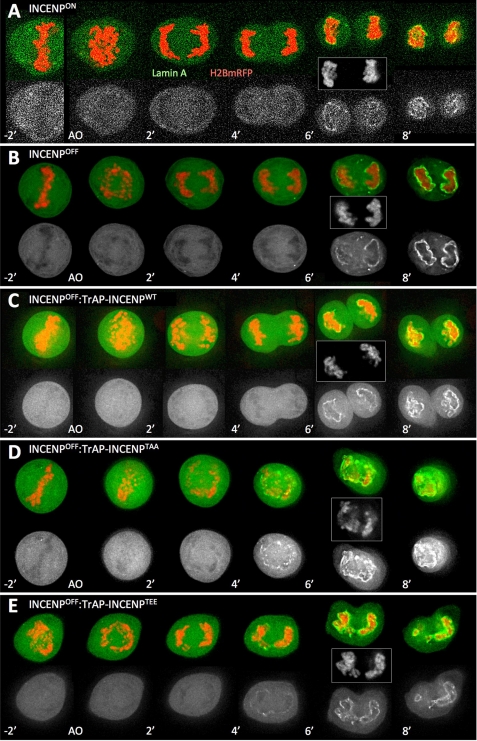
We used live cell imaging to visualize mitotic exit in INCENP-depleted cells expressing various INCENP mutants and verify the above observations. For these experiments, we co-expressed H2B-mRFP to visualize the DNA, and GFP-Lamin A as a marker of nuclear envelope reformation. Consistent with results obtained by imaging fixed cells, DT40 INCENPOFF cells were able to progress to metaphase and enter anaphase (Fig. 4B). However, at the transition from anaphase A to anaphase B when the spindle normally starts to elongate, sister chromatid separation stalled in the mutant cells, which appeared unable to undergo anaphase B (Fig. 4, C–E).
FIGURE 4.
INCENP-mediated Aurora B kinase activation is required to maintain proper spindle elongation. Stills from movies of cells transfected with H2B-mRFP (red) and Lamin A-GFP (green) are shown. A, INCENPON; B, INCENPOFF; C, INCENPOFF:TrAP-INCENPwt; D, INCENPOFF:TrAP-INCENPTAA; E, INCENPOFF:TrAP-INCENPTEE. The lower gray scale images show Lamin A-GFP only. Transiently transfected cells were imaged 20 h after transfection every minute from anaphase onset (AO). DNA is also shown in gray scale for the 6-min time point after anaphase onset.
Current thinking is that spindle structure and dynamics are determined by a balance of forces generated by the kinetochores, microtubule dynamics, and the action of mitotic motors and depolymerases (23, 24). The involvement of the CPC in this process is an important question for the future, with the largely neglected ability of INCENP to bind to microtubules being one possible avenue for study. In fact, recent data support this concept: persistence of the CPC at centromeres in anaphase is accompanied by defects in spindle elongation and erratic chromosome movements (25–27).
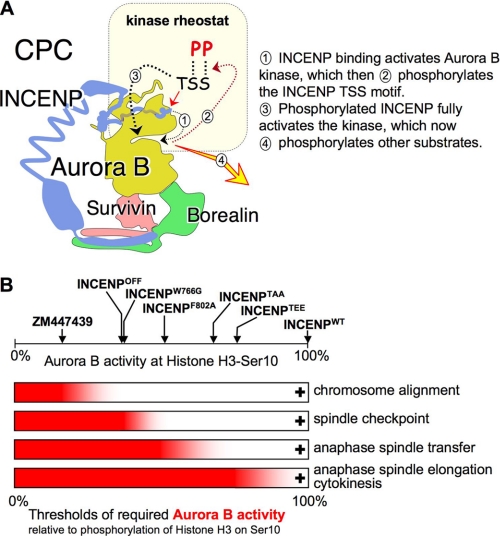
The results presented here extend our previous work suggesting that Aurora B activation by INCENP can be modeled as a rheostat rather than a simple on/off switch (Fig. 5). Furthermore, our results show that progressively higher levels of Aurora B kinase activity are required for the CPC to accomplish its successive tasks as cells traverse mitosis.
FIGURE 5.
An INCENP-mediated Aurora B gradient pool is required to control different mitotic processes. A, nonenzymatic subunits of the CPC regulate Aurora B localization and kinase activity. The INCENP C terminus regulates the level of Aurora B kinase activity through a feedback loop (9). B, functional consequences of the differing levels of Aurora B kinase activity observed with different mutants of the INCENP C terminus. Levels of Aurora B kinase activity against histone H3 Ser10 measured with the LiCor odyssey shown at the top include measurements from Ref. 7. Below are shown the four thresholds for Aurora B kinase activity identified in this and previous work (7, 29). In each case, the mitotic event indicated at the right only occurs when the level of Aurora B kinase activity exceeds that shown by the red bar.
At 15% maximal Aurora B kinase activity (measured as level of phosphorylation of histone H3 on Ser10, a widely accepted measure of Aurora B kinase activity (28)), chromosome alignment is seriously impaired following exposure of cells to ZM447439 (29). In contrast, alignment is delayed, but does occur in INCENPOFF cells, which have ∼35% of the wild-type kinase activity level (7). Another threshold occurs between 35 and 50% of maximal Aurora B kinase activity, as defined by the INCENPW766G and INCENPF802A mutants, respectively. The lower level of kinase activity is insufficient for a robust spindle checkpoint response against low dose Taxol, whereas the higher level is sufficient (Fig. 5).
A third threshold lies between 50 and 68% of maximal Aurora B kinase activity. This is defined by INCENPF802A and the INCENP-TSS mutants described here. Below this threshold, endogenous CPC components target apparently normally to centromeres, but are unable to transfer to the spindle midzone at anaphase. Above it, transfer to the spindle midzone does occur. The present work suggests that even higher levels of Aurora B kinase activity are required for anaphase B spindle elongation, normal timing of nuclear envelope reassembly, and the completion of cytokinesis, although we cannot exclude the less likely possibility that the INCENP TSS motif plays some direct roles in those events.
A recent study used a Förster resonance energy transfer (FRET)-based biosensor to monitor CyclinB1-Cdk1 activity during early mitosis. This study demonstrated that events during early prophase (cell rounding, nuclear import of CyclinB1-Cdk1, AC/C activation) require lower levels of kinase activity than later events (nucleolar disassembly, nuclear envelope breakdown) (16). Our demonstration that events driven by the CPC require ever-higher levels of Aurora B kinase activity as cells traverse mitosis suggests a similar regulation of this kinase as well.
A recent study of the phospho-regulation of microtubule binding by the kinetochore revealed for the KMN network that phosphorylation of three different proteins can act progressively to exert a graded effect on microtubule binding (30). It could be that successful execution of late events in mitosis requires simultaneous phosphorylation of a number of different targets and that this is only achievable with high levels of Aurora B kinase activity. Alternatively, it could be that activation of protein phosphatases during mitotic exit increases the back reaction for CPC-mediated phosphorylation events, requiring higher levels of kinase activity for the phosphorylation to dominate. It is interesting to speculate that this requirement for a gradient of kinase activity might somehow favor error correction. The mutant DT40 cells described here provide a powerful system with which to study this novel pattern of phospho-regulation by the CPC.
Supplementary Material
This work was supported by the Wellcome Trust (to the W. C. E. laboratory).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- CPC
- chromosomal passenger complex
- INCENP
- inner centromere protein.
REFERENCES
- 1.Vagnarelli P., Earnshaw W. C. (2004) Chromosoma 113, 211–222 [DOI] [PubMed] [Google Scholar]
- 2.Ruchaud S., Carmena M., Earnshaw W. C. (2007) Nat. Rev. Mol. Cell Biol. 8, 798–812 [DOI] [PubMed] [Google Scholar]
- 3.Carmena M., Ruchaud S., Earnshaw W. C. (2009) Curr. Opin. Cell Biol. 21, 796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein U. R., Nigg E. A., Gruneberg U. (2006) Mol. Biol. Cell 17, 2547–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyaprakash A. A., Klein U. R., Lindner D., Ebert J., Nigg E. A., Conti E. (2007) Cell 131, 271–285 [DOI] [PubMed] [Google Scholar]
- 6.Vader G., Kauw J. J., Medema R. H., Lens S. M. (2006) EMBO Rep. 7, 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., Ogawa H., Vagnarelli P., Bergmann J. H., Hudson D. F., Ruchaud S., Fukagawa T., Earnshaw W. C., Samejima K. (2009) J. Cell Biol. 187, 637–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams R. R., Wheatley S. P., Gouldsworthy A. M., Kandels-Lewis S. E., Carmena M., Smythe C., Gerloff D. L., Earnshaw W. C. (2000) Curr. Biol. 10, 1075–1078 [DOI] [PubMed] [Google Scholar]
- 9.Bishop J. D., Schumacher J. M. (2002) J. Biol. Chem. 277, 27577–27580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolton M. A., Lan W., Powers S. E., McCleland M. L., Kuang J., Stukenberg P. T. (2002) Mol. Biol. Cell 13, 3064–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda R., Körner R., Nigg E. A. (2003) Mol. Biol. Cell 14, 3325–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sessa F., Mapelli M., Ciferri C., Tarricone C., Areces L. B., Schneider T. R., Stukenberg P. T., Musacchio A. (2005) Mol. Cell 18, 379–391 [DOI] [PubMed] [Google Scholar]
- 13.Nousiainen M., Silljé H. H., Sauer G., Nigg E. A., Körner R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5391–5396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daub H., Olsen J. V., Bairlein M., Gnad F., Oppermann F. S., Körner R., Greff Z., Kéri G., Stemmann O., Mann M. (2008) Mol. Cell 31, 438–448 [DOI] [PubMed] [Google Scholar]
- 16.Gavet O., Pines J. (2010) Dev. Cell 18, 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earnshaw W. C., Bernat R. L. (1991) Chromosoma 100, 139–146 [DOI] [PubMed] [Google Scholar]
- 18.Cooke C. A., Heck M. M., Earnshaw W. C. (1987) J. Cell Biol. 105, 2053–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samejima K., Ogawa H., Cooke C. A., Hudson D. F., Macisaac F., Ribeiro S. A., Vagnarelli P., Cardinale S., Kerr A., Lai F., Ruchaud S., Yue Z., Earnshaw W. C. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 2457–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho A., Carmena M., Sambade C., Earnshaw W. C., Wheatley S. P. (2003) J. Cell Sci. 116, 2987–2998 [DOI] [PubMed] [Google Scholar]
- 21.Vagnarelli P., Hudson D. F., Ribeiro S. A., Trinkle-Mulcahy L., Spence J. M., Lai F., Farr C. J., Lamond A. I., Earnshaw W. C. (2006) Nat. Cell Biol. 8, 1133–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahonen L. J., Kukkonen A. M., Pouwels J., Bolton M. A., Jingle C. D., Stukenberg P. T., Kallio M. J. (2009) Chromosoma 118, 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Civelekoglu-Scholey G., Sharp D. J., Mogilner A., Scholey J. M. (2006) Biophys. J. 90, 3966–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumont S., Mitchison T. J. (2009) Curr. Biol. 19, R749–R761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira R. A., Hamilton R. S., Pauli A., Davis I., Nasmyth K. (2010) Nat. Cell Biol. 12, 185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirchenko L., Uhlmann F. (2010) Curr. Biol. 20, 1396–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vázquez-Novelle M. D., Petronczki M. (2010) Curr. Biol. 20, 1402–1407 [DOI] [PubMed] [Google Scholar]
- 28.Murnion M. E., Adams R. R., Callister D. M., Allis C. D., Earnshaw W. C., Swedlow J. R. (2001) J. Biol. Chem. 276, 26656–26665 [DOI] [PubMed] [Google Scholar]
- 29.Ditchfield C., Johnson V. L., Tighe A., Ellston R., Haworth C., Johnson T., Mortlock A., Keen N., Taylor S. S. (2003) J. Cell Biol. 161, 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welburn J. P., Vleugel M., Liu D., Yates J. R., 3rd, Lampson M. A., Fukagawa T., Cheeseman I. M. (2010) Mol. Cell 38, 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.