Abstract
Acid sphingomyelinase (A-SMase) is an important enzyme in sphingolipid metabolism and plays key roles in apoptosis, immunity, development, and cancer. In addition, it mediates cytotoxicity of cisplatin and some other chemotherapeutic drugs. The mechanism of A-SMase activation is still undefined. We now demonstrate that, upon CD95 stimulation, A-SMase is activated through translocation from intracellular compartments to the plasma membrane in an exocytic pathway requiring the t-SNARE protein syntaxin 4. Indeed, down-regulation of syntaxin 4 inhibits A-SMase translocation and activation induced by CD95 stimulation. This leads to inhibition of the CD95-triggered signaling events, including caspase 3 and 9 activation and apoptosis, activation of the survival pathway involving the protein kinase Akt, and important changes in cell cycle and proliferation. The molecular interaction between A-SMase and syntaxin 4 was not known and clarifies the mechanism of A-SMase activation. The novel actions of syntaxin 4 in sphingolipid metabolism and exocytosis we describe here define signaling mechanisms of broad relevance in cell pathophysiology.
Keywords: Apoptosis, Exocytosis, Receptors, Signal transduction, Sphingolipid, CD95, Acid Sphingomyelinase, Syntaxin 4
Introduction
Acid sphingomyelinase (A-SMase)3 (EC 3.1.4.12) is a phosphodiesterase that catalyzes the hydrolysis of membrane lipid sphingomyelin to ceramide and phosphorylcholine. The enzyme plays important roles in pathophysiology, as it mediates the action of several apoptogenic molecules, cytokines, and neurotrophins, regulating neuronal function, immunity, and infections (1–3).
Enzymatic dysfunction of A-SMase leads to Niemann-Pick diseases types A and B (4). The enzyme is also important in cancer development and therapy; tumor growth is enhanced in A-SMase knock-out mice (5), and the enzyme contributes significantly to the cytotoxic effects of several anticancer drugs (6–9). The biology of A-SMase under resting conditions, including its localization to lysosomal compartments, has been clarified (6, 9). Less clear is how A-SMase is activated. Activation has been suggested to require translocation from intracellular compartments to the extracellular surface of the cell through pathways as yet unknown (3, 9) or to occur within the lysosomes (10). Elucidating the molecular mechanisms of A-SMase activation is of biological relevance and might reveal novel candidate targets for therapy, including cancer therapy.
Using U373, a human glioma cell line expressing the death receptor CD95, we have now established that translocation of A-SMase to the plasma membrane is required for its early activation, that this process takes place by exocytosis, and that a key role in it is played by syntaxin 4, an ubiquitously expressed t-SNARE implicated in several regulated exocytic pathways including the translocation of Glut4 to the plasma membrane, exocytosis of secretory granules, and the calcium-dependent release of lysosomes (11, 12). Furthermore, we demonstrate that the blockade of syntaxin 4-dependent exocytosis inhibits CD95 receptor clustering and internalization, caspase activation and loss of mitochondrial membrane potential, leading to inhibition of apoptosis and maintenance of the cells in a proliferative state. The identification of syntaxin 4 as responsible for A-SMase activation is therefore of biological relevance and suggests new functional roles for this SNARE protein.
EXPERIMENTAL PROCEDURES
Materials
The following reagents were purchased as indicated: anti-CD95 monoclonal antibody (Ab) CH-11, IgM from Upstate Biotechnology; polyclonal Abs against A-SMase from Eurogentec (Liege, Belgium) and from Areta international s.r.l. (Gerenzano, Italy); the monoclonal Ab against cathepsin D (CatD) from Calbiochem; the monoclonal Abs against syntaxin 3 and syntaxin 4 from Synaptic System (Gottingen, Germany); the monoclonal Abs against syntaxin 4, early endosome antigen 1 (EEA1), lysosomal-associated membrane protein 1 (Lamp-1), and sorting nexin 1 (snx1) from BD Pharmingen; the monoclonal Ab against transferrin receptor (TfR) from Zymed Laboratories Inc. (South San Francisco, CA); the monoclonal Ab against the Na+/K+ ATPase from ABR Affinity Bioreagent (Rockford, IL); the monoclonal Ab against caspase 9 and the polyclonal Ab against cleaved caspase 3, phospho-Akt (Ser473) and Akt from Cell Signaling Technology (Beverly, MA); and the monoclonal Ab against proliferating cell nuclear antigen (PCNA) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Reagents for cell cultures were from Euroclone (Milan, Italy). EZ-Link Sulfo-NHS-LC-LC-Biotin, immobilized streptavidin, the bicinchonic acid kit, and the enhanced chemiluminescence (ECL) kit were from Thermo Scientific Inc. (Waltham, MA). FITC-labeled human recombinant annexin was from Bender MedSystem (Wien, Austria). Syntaxin 4 and 3 siRNAs and the scrambled control siRNA were from Ambion (Austin, TX). RNAiMAX transfection reagent was from Invitrogen. Cycloheximide, N-ethylmaleimide (NEM), and all other chemicals were from Sigma-Aldrich.
Cell Cultures
U373 cells derived from a human astroglioma were routinely grown at 37 °C, 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mm glutamine, 100 units/ml penicillin, and 100 units/ml streptomycin.
A-SMase Activity
Cells (2 × 106 cells/ml) incubated at 37 °C in culture medium were treated with CH11 (100 ng/ml). At the time points indicated, the incubations were terminated by rapid immersion of reaction tubes in a methanol/dry ice bath. The suspensions were centrifuged, and the cell pellets were washed once with ice-cold PBS. Pellets were homogenized, supplemented with N-[methyl-14C]sphingomyelin (55 mCi/mmol; 50,000 dpm/assay; 0.3 mmol/assay), and A-SMase activity was determined by measuring the conversion of sphingomyelin to phosphorylcholine without added Zn2+ as previously described (13).
Apoptosis and Cell Cycle Analysis
Apoptosis and cell cycle were analyzed by flow cytometry using a Fluorescence-activated Cell Sorter (FC500 Dual Laser system; Beckman Coulter, Brea, CA) as described previously (14, 15). Phosphatidylserine exposure on the outer leaflet of the plasma membrane in propidium iodide-excluding cells was detected by analysis of cells stained for 15 min with FITC-labeled annexin V (1 μg/ml) and analyzed by the FCS Express software, version 3 (Los Angeles, CA). For cell cycle analysis, cells were harvested and resuspended in 0.1% sodium citrate containing 75 mm propidium iodide at a density of 1 × 106 cells ml−1 and treated for 30 min at 48 °C in the dark with 2.5 units/ml RNase A and 0.012% Nonidet P-40. Finally, the cells were filtered to remove aggregates and analyzed for DNA content by quantifying the red fluorescence. The percentage of cells in G0/G1, S, or G2/M phases of cell cycle were determined by analysis of the results using the FlowJo software version 7.5.5 (Tree Star Inc., Ashland, OR).
LC-MS Analysis of Sphingolipids
Cells were pelleted, washed in PBS, and transferred to glass vials. Sphingolipid extracts, fortified with internal standards (N-dodecanoylsphingosine, N-dodecanoylglucosylsphingosine, and N-dodecanoylsphingosylphosphorylcholine, 0.2 nmol each), were prepared and analyzed as described (16). The LC-mass spectrometer consisted of a Waters Aquity UPLC system connected to a Waters LCT Premier orthogonal accelerated time-of-flight mass spectrometer (Waters, Millford, MA), operated in positive electrospray ionization mode. Full scan spectra from 50 to 1,500 Da were acquired, and individual spectra were summed to produce data points each 0.2 s. Mass accuracy and reproducibility were maintained by using an independent reference spray by the LockSpray interference. The analytical column was a 100-mm ×2.1-mm inner diameter, 1.7-mm C8 Acquity UPLC BEH (Waters). The two mobile phases were: phase A, methanol/water/formic acid (74/25/1 v/v/v); phase B, methanol/formic acid (99/1 v/v), both containing also 5 mm ammonium formate. A linear gradient was programmed as follows: 0.0 min, 80% B; 3 min, 90% B; 6 min, 90% B; 15 min, 99% B; 18 min, 99% B; 20 min, 80% B. The flow rate was 0.3 ml/min. The column was held at 308 °C. Quantitation was carried out using the extracted ion chromatogram of each compound, using 50-mDa windows. The linear dynamic range was determined by injecting standard mixtures. Positive identification of compounds was based on the accurate mass measurement with an error <5 ppm and its LC retention time compared with that of a standard (<2%).
Immunofluorescence
For confocal immunofluorescence studies, cells were seeded at the 70–80% of confluence on glass coverslips coated with poly-l-lysine. After treatment, cells were fixed with methanol for 5 min at −20 °C, incubated for 5 min with 1% glycine-PBS, and blocked for 30 min with 10% goat serum, 1% BSA-PBS at room temperature. Cells were then incubated with specific Abs against A-SMase, CatD, Lamp-1 syntaxin 3, syntaxin 4, snx1, EEA1, or TfR in 1% BSA, 0.1% saponin in PBS for 1 h at room temperature. For fluorescent detection appropriate secondary Abs conjugated with Alexa Fluor 488 (green), Alexa Fluor 546 (red) or Alexa Fluor 647 (blue) were used. Fluorescence staining was analyzed with a Bio-Rad MRC 1024 confocal microscope. To quantify the degree of co-localization, we used ImageJ software and the JACoP plug-in to determine the Pearson's correlation coefficient as described previously (17). Pearson's coefficient was expressed as the mean ± S.D.
A-SMase Exposure on the Cell Surface
Translocation of A-SMase from intracellular compartments to the plasma membrane was investigated by the following three methods:
Cell Surface Biotinylation Assay
Cells were stimulated with 100 ng/ml CH11 at the indicated times in culture medium at 37 °C. Stimulation was stopped with ice-cold PBS. Cells were washed twice with PBS and then incubated twice with 0.5 mg/ml Sulfo-NHS-LC-Biotin in DMEM without serum for 10 min at 4 °C. After washing with serum-free DMEM for 10 min and three times with PBS for 5 min at 4 °C, cells were solubilized in lysis buffer (10 mm Tris, 150 mm NaCl, 1 mm EDTA, 0.1% SDS, 1% Triton X-100 with protease inhibitor mixture, pH 7.4) for 30 min at 4 °C. Lysates were then centrifuged for 5 min at 1,500 × g, and streptavidin beads were added to the supernatant to isolate cell membrane proteins. After incubation of the mixture for 16 h at 4 °C, biotin-streptavidin beads complexes were sedimented at 13,000 rpm for 3 min. The supernatant was used as control, and, after two washes with PBS, bead-bound proteins were denatured in Laemmli buffer and analyzed by SDS-PAGE followed by Western blotting with the anti-A-SMase Ab as described (18). Cell surface exposure of A-SMase was normalized to 25 μg of total lysate for each sample.
Flow Cytometry and Immunofluorescence
Cells were trypsinized, resuspended in medium, and stimulated for A-SMase exposure as described above. After stimulation, cells were washed twice with ice-cold PBS, incubated with the anti-A-SMase Ab in 1% BSA-PBS for 1 h at 4 °C, and then stained with the appropriate secondary Abs conjugated with Alexa Fluor 488 (green) for 1 h at 4 °C followed by fixation with 4% paraformaldehyde for 5 min at room temperature. Cells were divided into two aliquots and analyzed either by flow cytometry or by confocal microscopy after cytospinning at 170 × g (Cytospin 3; Shandon, Cheshire, UK) for 3 min.
Cell Fractionation on Density Gradient
Cell fractionation was carried out essentially as described (19). Briefly, cells were homogenized in a homogenization buffer (0.25 m sucrose, 1 mm Mg CH3COO2, 1 mm EDTA, 10 mm Hepes, pH 7.4) plus protease inhibitors. The total homogenate was centrifuged for 5 min at 1,500 × g, and the postnuclear supernatant was loaded on the top of a continuous sucrose gradient (0.4–2.2 m) and centrifuged at 25,000 rpm for 18 h in a SW41 rotor (Beckman). After centrifugation, 24 fractions of 500 μl were collected. Equal amounts of proteins from each fraction were separated on SDS-polyacrylamide gels and analyzed by Western blotting.
Western Blotting
Cells were homogenized in 50 mm Tris-HCl, pH 7.4, 1 mm EGTA, 1 mm EDTA, 1% Triton X-100, and protease inhibitor mixture and centrifuged at 1,500 × g for 5 min at 4 °C to discard cellular debris. After separation by SDS-PAGE, polypeptides were electrophoretically transferred to nitrocellulose filters (Whatman), and antigens were revealed by the respective primary Abs and the appropriate secondary HRP-conjugated goat anti-rabbit or anti-mouse Abs (from Cell Signaling, Beverly, CA and Bio-Rad, respectively). Proteins were visualized by ECL.
RNA Interference
Syntaxin 4 and syntaxin 3 siRNA (5′-AAGGAGGAAGCTGATGAGAAC-3′) (5′-AACGTCCGGAACAAACTGAAG-3′) and the scrambled control siRNA (5′-AAGGAGTAAGATGATGCGAAC-3′) were designed according to Fu et al. (20). A-SMase siRNA (5′-AACTCCTTTGGATGGGCCTGG-3′) and its scrambled control siRNA (5′-GCAGTCTCTCGCTGGATGTGA-3′) were designed according to Zeidan and Hannun (9).
U373 seeded at 40% confluence were transfected when at 60% confluence with the siRNAs using RNAiMAX Lipofectamine according to the manufacturer's protocol. Silencing was assayed after 48 h of incubation by Western blotting with the specific Abs against A-SMase and syntaxin 4.
CatD Release
CatD was assayed by concentrating culture supernatants by speed vacuum concentration (Christ vacuum concentrator SpeedDry 2–33IR) and analyzing concentrated supernatants and remaining cell extracts by SDS-PAGE and immunoblotting with the anti-CatD Ab. The amount of the fully processed (34 kDa) form of mature CatD present in cells or released into the medium was determined by densitometry. In all experiments, the amount of release was calculated as a percentage of the total amount of CatD secreted into the medium compared with the total amount of CatD secreted into the medium plus that remaining cell-associated.
CD95 Receptor Capping and Internalization
After transfection, 106 cells/ml were treated with 1 μg/ml CH11 for 45 min on ice. Unbound Ab was removed by washing with medium at 4 °C, and cells were warmed, resuspended in medium, and kept at 37 °C for the indicated time points to trigger CD95 stimulation or were kept on ice (time [t] = 0). Cells were then stained with 1 μg/ml FITC-conjugated goat anti-mouse IgM for 45 min on ice and fixed with 2% paraformaldehyde for 5 min at room temperature. CD95 capping was analyzed by confocal microscopy on cells centrifuged on a Cytospin 3 for 3 min on a coverslip. CD95 internalization was analyzed by flow cytometry.
Statistical Analysis
The results are expressed as means ± S.E.; n represents the number of individual experiments. Statistical analysis was carried out using the Student's t test for unpaired variables (two-tailed). The asterisks *, **, and *** or crosses +, ++, and +++ in the figure panels refer to statistical probabilities (p) of <0.5, <0.01, and <0.001, respectively, measured for the cells treated in the various experimental conditions as described in details in figure legends.
RESULTS
A-SMase Translocation to the Plasma Membrane and Activation Induced by CD95 Depends on SNARE-mediated Exocytosis
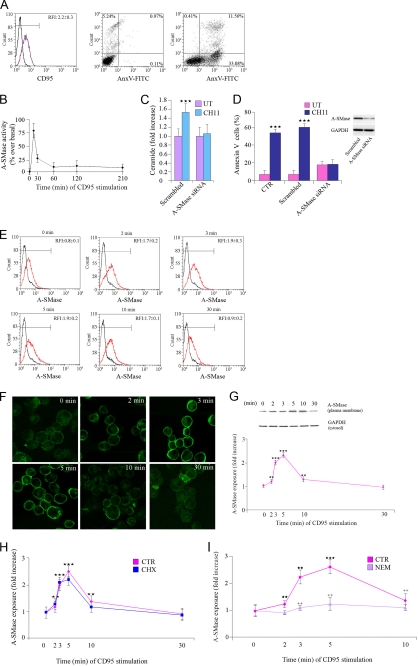
Death receptors stimulation induces apoptosis through the increase of ceramide levels, and this is due to the activation of A-SMase (21). In this study we used the U373 human glioma cell line, which expresses the A-SMase-activating, apoptogenic receptor CD95, and thus constitutes an ideal experimental model (Fig. 1A). U373 cell treatment with the CD95-activating Ab CH11 induced a transient activation of A-SMase that peaked at 5 min and returned to basal levels after 30 min (Fig. 1B) accompanied by increases in ceramide levels (Fig. 1C). A siRNA that specifically silenced the expression of the human form of A-SMase inhibited CD95-induced apoptosis (Fig. 1D).
FIGURE 1.
A-SMase translocation and activation depend on SNARE-mediated exocytosis. A, CD95 expression and induction of apoptosis. CD95 expression on the cell surface of U373 cells was revealed by FACS analysis of unpermeabilized cells with a specific Ab against CD95. Apoptosis was evaluated by flow cytometry measuring annexin V (AnxV) exposure to the plasma membrane in propidium iodide-excluding U373 cells, treated or not (UT) with CH11 (100 ng/ml). Results shown are representative of five reproducible experiments. B–D, A-SMase activation, ceramide generation, and induction of apoptosis by CD95 stimulation. B, A-SMase activity determined in cell lysates by measuring sphingomyelin hydrolysis to phosphorylcholine at pH 5.5. Values are expressed as % ± S.E. over basal A-SMase activity (1.28 ± 0.6 and 0.52 ± 0.3 nmol/mg h−1) (n = 5). C, ceramide levels measured by mass spectroscopy in U373 transfected with a specific siRNA against A-SMase and with its scrambled control after treatment with CH11. Untreated cells were analyzed in parallel (UT). Values are expressed as fold increase over the basal ± S.E. (n = 3). D, cells not transfected (CTR) or transfected with either scrambled or with A-SMase siRNA treated or not (UT) with CH11 for 16 h. Apoptosis was assessed by measuring phosphatidylserine exposure on the outer leaflet of the plasma membrane by staining with Annexin V. E–H, exposure of A-SMase onto the plasma membrane induced by CH11. A-SMase translocation was evaluated, at the indicated time points after CD95 activation, by flow cytometry (E, red trace), immunofluorescence on unpermeabilized cells (F), and assessment of biotinylated plasma membrane A-SMase using the A-SMase Ab and assessing cytosolic GAPDH expression in parallel as a loading control (G). The results of one of three reproducible experiments are shown. The graph shows the densitometric values ± S.E. (n = 3). H, time course of A-SMase translocation evaluated as in E after stimulation with CH11 in the presence or absence (CTR) of the protein synthesis inhibitor cycloheximide (CHX) (1 μg/ml) (n = 3). I, NEM inhibition of A-SMase translocation. Cells were treated with or without (CTR) NEM (1 mm), and A-SMase exposure onto plasma membrane was evaluated by flow cytometry. Values are expressed as fold increases over cells not receiving CH11 (n = 4). Asterisks and crosses in E–G indicate statistical significance, measured as indicated under “Experimental Procedures,” versus untreated cells and CH11-treated cells, respectively.
We examined whether CD95 activation induced translocation of A-SMase to the plasma membrane. A-SMase exposure to the cell surface was assessed by flow cytometry, immunofluorescence, and biotinylation of proteins exposed at the plasma membrane outer leaflet, using a specific anti-A-SMase polyclonal antiserum that we previously characterized (18).
The small fraction of A-SMase localized at the plasma membrane under resting conditions was significantly increased by CD95 stimulation, with a peak of exposure occurring between 3 and 5 min after stimulation and with a return to basal levels after 30 min (Fig. 1, E–G), consistent with the time course of A-SMase activation (Fig. 1B). The fraction of A-SMase thus exposed upon receptor activation represented 10.4 ± 1.1% (n = 5) of the total enzyme, as judged by densitometric analyses of plasma membrane versus cytosolic fractions, indicating the existence of two pools of the enzyme. In addition, the amount of A-SMase exposed on the plasma membrane was not influenced by cell treatment with the protein synthesis inhibitor cycloheximide (1 μg/ml) (Fig. 1H), indicating that activation and translocation of A-SMase induced by CD95 did not depend on changes in protein synthesis.
Next, we investigated the nature of the transport system involved in A-SMase translocation. Considering the organellar distribution of A-SMAse and the fact that CD95 has been reported to increase cytosolic calcium concentrations (22), we treated U373 cells with NEM, a drug that inhibits the function of the SNAREs complex, the machinery that accomplishes the final fusion of a transport carrier with its target membrane, including the fusion of intracellular vesicles with the plasma membrane (12). NEM inhibited A-SMase translocation to the plasma membrane triggered by CD95 activation (Fig. 1I), suggesting that this event occurs through a SNARE-mediated exocytic pathway.
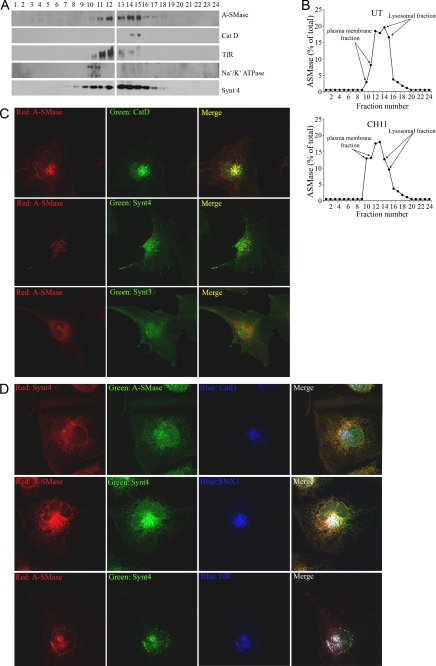
A-SMase Co-localizes with Syntaxin 4
We analyzed the exocytic pathway of A-SMase further, comparing its distribution with that of plasma membrane and lysosomal markers and with syntaxins. Immunoblot analysis of fractions obtained by density gradient centrifugation showed that in unstimulated cells A-SMase sediments in CatD-containing fractions, consistent with its described lysosomal localization, which was further confirmed by co-localization with the lysosomal marker Lamp-1 (Table 1 and supplemental Fig. 1), as well as in fractions containing the recycling endosome marker TfR (Fig. 2, A and B). Only a small amount of A-SMase was found in the fractions enriched in the plasma membrane, as confirmed by its localization with the specific marker Na+/K+ATPase, consistently with the immunofluorescence, flow cytometry and biotinylation experiments (see Fig. 1). After a 5-min challenge with CH11, i.e. when the peak of A-SMase activation occurs (see Fig. 1), the enzyme was found to be significantly reduced in lysosomal fractions while enriched in the plasma membrane (Fig. 2C). We observed that syntaxin 4, in addition to its plasma membrane localization, was also enriched in the A-SMase-containing fractions (Fig. 2A) and co-localized with A-SMase (see Fig. 2C and supplemental Fig. 1 for representative images and Table 1 for quantitation). Interestingly, A-SMase did not co-localize with syntaxin 3, a t-SNARE also involved in exocytosis, however of different types of vesicles (11, 12), thus highlighting the specificity of the A-SMase exocytic pathway we define here. A-SMase and syntaxin 4 co-localized also with the endosomal markers TfR, snx1, and EEA1 (see Fig. 2D and supplemental Fig. 1 for representative images and Table 1 for quantitation) (23). Of importance, although both syntaxin 4 and CatD co-localized with A-SMase (see Fig. 2B and supplemental Fig. 1 for representative images and Table 1 for quantitation), they did not co-localize with each other (Fig. 2D). Syntaxin 4 is known to shuttle between the plasma membrane and recycling endosomes (24); it appears therefore that A-SMase resides not only in CatD-positive lysosomes but also in recycling endosomes, a localization not yet described for this enzyme.
TABLE 1.
Quantitation of A-SMase co-localization with markers of cell organelles by the Pearson's correlation coefficient-analyzing images with the ImageJ software
Pearson's coefficient is expressed as the mean ± S.D. (n = 4).
| Marker | Pearson's coefficient |
|---|---|
| Syntaxin 4 | 0.78 ± 0.0048 |
| CatD | 0.83 ± 0.0036 |
| Syntaxin 3 | 0.19 ± 0.0103 |
| Lamp1 | 0.87 ± 0.0096 |
| TfR | 0.74 ± 0.0119 |
| EEA1 | 0.37 ± 0.0016 |
FIGURE 2.
A-SMase co-localizes with syntaxin 4. A and B, subcellular fractionation on sucrose density gradient U373 cells. A, representative Western blots of unstimulated U373 in which A-SMase distribution is evaluated in relation to that of the markers of lysosomes CatD, recycling endosomes TfR, plasma membrane Na+/K+ ATPase, and the SNARE protein syntaxin 4 (Synt 4). B, distribution of A-SMase in the fractions of sucrose density gradient in unstimulated (UT) and in CH11-stimulated cells (100 ng/ml for 5 min). C and D, confocal immunofluorescence images of U373 stained with an anti-A-SMase Ab and with anti-CatD, TfR, snx-1, syntaxin 4, and syntaxin 3 (Synt 3) Abs. All images shown are representative of one of three reproducible experiments.
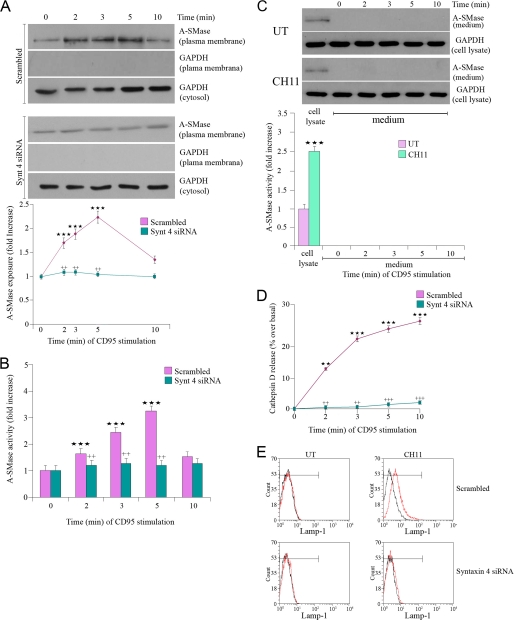
Syntaxin 4 Is Needed for A-SMase Plasma Membrane Localization and Activation
Because plasma membrane syntaxin 4 is a key player in exocytosis, we investigated whether it plays a role in plasma membrane docking of A-SMase/CatD-containing vesicles and in enzyme activation. A siRNA that specifically silenced the expression of the human form of syntaxin 4 (supplemental Fig. 2A) (25), significantly inhibited A-SMase enzymatic activity (Fig. 3B) and translocation induced by CD95 (Fig. 3A), but not its levels of expression (supplemental Fig. 2A). Specific silencing of syntaxin 3 did not affect A-SMase activation indicating the specific role of syntaxin 4 (supplemental Fig. 2, A and B). In parallel to A-SMase translocation, we observed the appearance of Lamp-1 at the plasma membrane and release of CatD in the extracellular space. Both CatD release and Lamp-1 plasma membrane localization required syntaxin 4, as demonstrated by the inhibition of these events in cells transfected with the syntaxin 4 siRNA (Fig. 3, C and D). We did not detect release or activity of A-SMase in the medium at all time points (Fig. 3E). These results suggest that Lamp-1-positive vesicles containing A-SMase and CatD fuse with the plasma membrane upon CD95 stimulation in a syntaxin 4-dependent way, leading to both A-SMase membrane translocation and CatD release.
FIGURE 3.
Syntaxin 4 is essential for A-SMase function. U373 cells were transfected for 48 h with scrambled or syntaxin 4 siRNA and treated with CH11 at the indicated time points, and then A-SMase translocation and activity and A-SMase and CatD release as well as Lamp-1 exposure were evaluated. A, A-SMase translocation was evaluated by Western blotting of biotinylated plasma membrane proteins with an anti-A-SMase Ab. The results of one of three reproducible experiments are shown; the purity of the plasma membrane preparation was evaluated by the absence of GAPDH; cytosolic GAPDH expression was revealed in parallel as a loading control. The graph shows the densitometric values of plasma membrane A-SMase normalized on cytosolic GAPDH ± S.E. (n = 3). B, A-SMase activity was determined in cell lysates by measuring sphingomyelin hydrolysis to phosphorylcholine at pH 5.5. Values are expressed as % ± S.E. over basal A-SMase activity (1.28 ± 0.6 and 0.52 ± 0.3 nmol/mg h−1) (n = 4). C, secretion of A-SMase was evaluated by Western blotting and activity assay on cell culture medium of cells treated with or without (UT) CH11; cytosolic GAPDH expression was revealed in parallel as control. Reported is also the activity of A-SMase in cell lysates after 5 min of stimulation with or without CH11. D, CatD release was measured by the analysis of supernatant and lysates by Western blotting by using anti-CatD Abs. Values are expressed as % over basal ± S.E., considering the amount of CatD released with respect to the total amount of CatD (supernatant plus cell lysate) (n = 4). E, Lamp-1 exposure onto the plasma membrane was determined by flow cytometry in nonpermeabilized cells after 5-min treatment with or without CH11. Asterisks in A, B, and C and crosses in A and B indicate statistical significance, measured as indicated under “Experimental Procedures,” versus cells not receiving CH11 and versus cells receiving CH11, respectively.
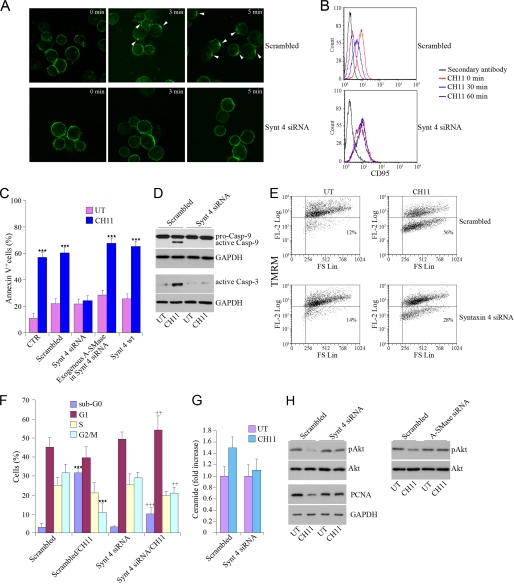
Down-regulation of Syntaxin 4 Inhibits A-SMase-dependent Apoptosis and Stimulates Cell Survival and Proliferation
The functional consequences of the syntaxin 4-dependent A-SMase activation in the context of CD95 induced apoptosis were then investigated. Capping of CD95 and its internalization, two early events in apoptosis, clearly observed in cells transfected with the scrambled siRNA were significantly reduced by a specific syntaxin 4 siRNA (Fig. 4, A and B). In agreement, knock down of syntaxin 4 inhibited also the downstream apoptotic events induced by CD95 activation, including exposure of phosphatidylserine onto the cell surface (Fig. 4C), activity of caspases 3 and 9 (Fig. 4D), the decrease of the mitochondrial membrane potential assessed by staining with tetramethyl rhodamine methyl ester (Fig. 4E), and the percentage of cells in the hypodiploid, sub-G0 phase of cell cycle (Fig. 4F). Interestingly, the degree of inhibition of apoptosis, as well as of ceramide generation, which was observed after syntaxin 4 silencing was similar in extent to the one observed after A-SMase silencing (Cfr. Fig. 1, C and D, with Fig. 4, C and G). Moreover, administration of exogenous A-SMase reversed inhibition of apoptosis (Fig. 4C). This observation demonstrates further the relevance of syntaxin 4-dependent exposure of A-SMase at the plasma membrane in mediating CD95-induced apoptosis. Apoptosis induced by CD95 activation was not affected by silencing of syntaxin 3, indicating the specific role of syntaxin 4 (supplemental Fig. 2C). In addition, syntaxin 4 down-regulation maintained the prosurvival protein Akt into its phosphorylated, active state, preventing dephosphorylation induced by CD95 activation (Fig. 4H). Of importance, this effect was similar to that induced by transfection with the A-SMase siRNA, demonstrating that loss of syntaxin is equivalent to loss of A-SMase with respect to this downstream signaling event. Syntaxin 4 siRNA transfection modified another process affected by A-SMase activation, i.e. cell progression through cell cycle. Indeed, although CH11 administration in scrambled-transfected cells induced a strong reduction of dividing cells (Fig. 4F), down-regulation of syntaxin 4 stimulated proliferation, as demonstrated by the increased expression of the marker of proliferation, PCNA (Fig. 4H).
FIGURE 4.
Down-regulation of syntaxin 4 inhibits A-SMase-dependent apoptosis. A and B, CD95 capping and internalization are shown. U373 cells transfected with scrambled siRNA or with the syntaxin 4 siRNA were incubated with CH11 for 45 min on ice. Cells were then washed and warmed to 37 °C for the indicated periods. Cells were then stained with FITC-conjugated secondary Abs for 45 min on ice, fixed with 2% paraformaldehyde, and attached to poly-l-lysine-coated slides (A), or left in suspension (B). A, samples were analyzed by confocal microscopy. White arrows indicate capping of the receptor. The images shown are representative of one of three reproducible experiments in which cells in several fields per slide were counted for each time point. B, CD95 staining onto the plasma membrane was analyzed by flow cytometry. The result shown is one of three reproducible experiments. C, apoptosis was evaluated. U373 cells were mock transfected (CTR) or transfected with syntaxin 4 siRNA or its scrambled control, or with syntaxin 4 wild type and then stimulated for 16 h with or without (UT) CH11, in the presence or absence of exogenous human placental A-SMase (2.0 unit/ml, added 1 h before CH11). Apoptosis was evaluated by flow cytometry measuring annexin V (AnxV) exposure to the plasma membrane in propidium iodide-excluding cells. Values reported in the diagrams represent the % of annexin V+ cells ± S.E. (n = 5). D–H, apoptosis, cell cycle, and proliferation hallmarks were determined. Cells were stimulated for 16 h with or without CH11 after transfection with syntaxin 4 siRNA, A-SMase siRNA, or the scrambled control as indicated. D, caspases (Casp) 9 and 3 activities were evaluated by Western blotting with specific Abs recognizing the inactive (pro) and active forms (caspase 9) or the active form (caspase 3) of the enzymes. E, mitochondrial membrane potential was measured using the potential-sensitive mitochondrial dye tetramethyl rhodamine methyl ester (TMRM). F, percentages of cells in G0/G1, S, or G2/M phases of cell cycle were determined by flow cytometry after staining with propidium iodide. Values reported in the diagrams represent the % cells in each phase ± S.E. (n = 4). G, amount of total ceramide was measured by mass spectrometry. Values are expressed as fold increase over the basal ± S.E. (n = 3). H, phosphorylated and total Akt and PCNA were evaluated by Western blotting with specific Abs on cell lysates. Asterisks and crosses in C and F indicate statistical significance, measured as indicated under “Experimental Procedures” versus untreated cells and CH11-treated cells, respectively. Images in D, E, and G are from one of three reproducible experiments.
DISCUSSION
In this study we clarify the pathway of A-SMase activation, identifying its endo/exocytic nature, and the key role in it of syntaxin 4, demonstrating an important link between exocytosis and apoptosis.
Previous studies had shown that A-SMase undergoes translocation to the plasma membrane during its activation triggered by a variety of stimuli, including UV and activators of apoptogenic receptors (6, 9). These studies had also shown that A-SMase translocation is required to induce receptor capping and initiate apoptosis (26). How A-SMase translocates to the plasma membrane and whether this is a consequence or a trigger of its activation were, however, unknown. In addition, this mechanism of activation had been questioned by other studies that suggested an intralysosomal activation of the enzyme (10, 27).
We now show that A-SMase is localized under resting conditions in (CatD- and Lamp-1-positive) vesicles, as already established, and in (snx-1-, EEA1-, and TfR-positive) early/recycling endosomes, where it co-localizes with syntaxin 4, a protein known to shuttle between these organelles and the plasma membrane (24). Using CD95 as the A-SMase-activating receptor, we then demonstrate by independent experimental approaches that A-SMase translocates to the cell surface in the first minutes after receptor activation. Such translocation involves a fraction of the A-SMase contained in CatD- (and Lamp-1)-positive vesicles, is transient, mediated by an exocytic pathway in which syntaxin 4 is required, and leads to release of the soluble lysosomal protease CatD in the medium. In addition, we demonstrate that translocation of A-SMase is required for its activation because both the blockade of exocytosis with NEM and the silencing of syntaxin 4 inhibited A-SMase activation. Syntaxin 4 silencing inhibited also CD95 capping, its internalization, and its ability to induce apoptosis, indicating that the pathway of A-SMase activation we describe here is necessary to full receptor stimulation.
The pathways leading to CD95-induced apoptosis are tightly regulated, in view of the pathophysiological relevance of this receptor in a variety of tissues and organs (28–31). A key pathway regulating CD95 apoptogenic function is the one activated by PI3K/Akt. Once activated by phosphorylation, Akt is recruited at the inner leaflet of the plasma membrane and prevents the formation of plasma membrane lipid platforms containing CD95, which are fundamental to initiate apoptotic signaling (23). Ceramide inhibits Akt activation through its dephosphorylation in Ser473 by the ceramide-activated protein phosphatase, thus potentiating the apoptotic pathway (32). In addition, ceramide inhibits cell proliferation by reducing the expression levels of PCNA and inhibiting the nuclear transport of key proteins of cell cycle progression (33). Here, we show that these functions are critically regulated by syntaxin 4. Silencing of syntaxin 4, with inhibition of A-SMase function, prevents the dephosphorylation of Akt and the decrease of PCNA, restoring the normal condition in terms of survival and proliferation. This confirms the central role of syntaxin 4 in apoptosis induction by CD95.
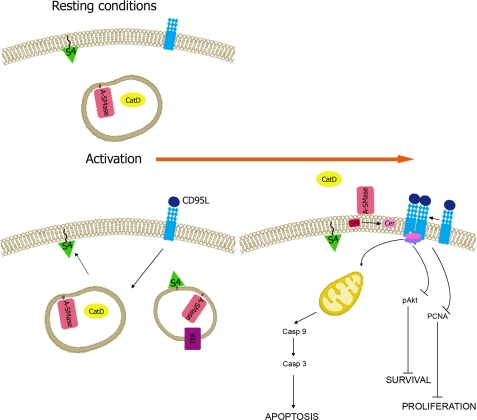
Based on our and previous findings, we propose a model of the trafficking leading to activation of A-SMase that explains the existing information and solves some contradictory aspects. According to this model (Fig. 5) A-SMase under resting condition is contained within lysosomes and exocytic vesicles, possibly secretory lysosomes. Upon stimulation, these vesicles reach the plasma membrane and dock to syntaxin 4 in a pathway of unconventional exocytosis used also by other types of vesicles (11, 12). CatD is then released into the extracellular milieu. The localization under resting conditions of A-SMase in vesicles positive for syntaxin 4/snx1 syntaxin 4/TfR and Lamp-1/CatD suggests that the enzyme is sorted back to lysosomes and recycling endosomes, although specific studies are needed to confirm this issue. Of importance, a similar route of internalization had been described for the A-SMase-activating receptors after their stimulation (10), indicating that the receptor and the enzyme may localize at the end of the activation process together in lysosomes. This model would therefore explain why activation of A-SMase occurs not only in the initial phases of receptor stimulation at the plasma membrane (6, 9, 14) but also at later phases in the lysosomes (10, 27). It remains to be established whether and how these two mechanisms of activation of A-SMase trigger different cell signaling events and/or cooperate to generate complex biological responses, as observed with other signal transduction events (34).
FIGURE 5.
Model of A-SMase trafficking upon CD95 stimulation. Under resting conditions A-SMase is stored in CatD-positive vesicles. Upon stimulation of CD95 with its physiological ligand (CD95L), a fraction of these vesicles fuses with the plasma membrane in a syntaxin 4-dependent pathway. Whereas CatD is released in the extracellular milieu, A-SMase is exposed at the cell surface, where it hydrolyzes sphingomyelin to ceramide, inducing CD95 clustering and leading to caspases activation, Akt and PCNA pathway inhibition, and cell death. Shown is also the vesicular localization of syntaxin 4 we observed, which is consistent with its localization to recycling endosomes. See “Discussion” for further details.
Previous studies showed that activation of A-SMase by death receptors depends upon recruitment of FADD and stimulation of caspase 8; however, the molecular mechanism through which this is accomplished is unknown (35, 36). Interestingly, it had been reported that CD95 activation induces profound changes in intracellular membrane trafficking, dependent on caspase 8 activation, including also the secretory pathway (37). Our identification of a secretory/exocytic pathway of A-SMase activation offers a link between these events.
The relationship between syntaxin 4-dependent intracellular trafficking and A-SMase activation is relevant also beyond apoptosis and may provide a molecular basis for other relevant biological processes in which translocation of A-SMase has been suggested to play a role, such as in plasma membrane fusion of cells required to form multinucleated giant cells (38), an event occurring in macrophages and cancer cells (39). In addition, recent evidence showed that A-SMase is a key regulator in the process of the cytotoxic granule secretion by primary T lymphocytes (2), an event dependent on SNAREs (40). In this context, A-SMase regulates the release of contents of cytotoxic granules after formation of a fusion pore (2). Finally, A-SMase translocation is needed for plasma membrane shedding of microvesicles, a relevant mechanism of intercellular communication (18).
Supplementary Material
Acknowledgments
We thank Jacopo Meldolesi (Milano) and Nica Borgese (Catanzaro) for helpful suggestions and reading of the manuscript.
This work was supported by grants from the Italian Association of Cancer Research (to E. C.), the Italian Ministry of Research Programmi di Ricerca di Rilevante Interesse Nazionale 2007 (to E. C.), Fondazione Cariplo (to E. C.), Fondazione Romeo and Enrica Invernizzi (to E. C.), Deutsche Forschungsgemeinschaft Grant GU335/21-1 (to E. G.), Italian Ministry of Health Ricerca Finalizzata 2007 (to M. T. B. and E. C.), and Ricerca Corrente 2010 (to M. T. B. and E. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- A-SMase
- acid sphingomyelinase
- CatD
- cathepsin D
- EEA1
- early endosome antigen 1
- Lamp-1
- lysosomal-associated membrane protein 1
- NEM
- N-ethylmaleimide
- PCNA
- proliferating cell nuclear antigen
- snx1
- sorting nexin 1
- TfR
- transferrin receptor.
REFERENCES
- 1.Ng C. G., Griffin D. E. (2006) J. Virol. 80, 10989–10999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herz J., Pardo J., Kashkar H., Schramm M., Kuzmenkina E., Bos E., Wiegmann K., Wallich R., Peters P. J., Herzig S., Schmelzer E., Krönke M., Simon M. M., Utermöhlen O. (2009) Nat. Immunol 10, 761–768 [DOI] [PubMed] [Google Scholar]
- 3.Grassmé H., Jendrossek V., Riehle A., von Kürthy G., Berger J., Schwarz H., Weller M., Kolesnick R., Gulbins E. (2003) Nat. Med 9, 322–330 [DOI] [PubMed] [Google Scholar]
- 4.Smith E. L., Schuchman E. H. (2008) FASEB J. 22, 3419–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Barros M., Paris F., Cordon-Cardo C., Lyden D., Rafii S., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2003) Science 300, 1155–1159 [DOI] [PubMed] [Google Scholar]
- 6.Gulbins E., Kolesnick R. (2003) Oncogene 22, 7070–7077 [DOI] [PubMed] [Google Scholar]
- 7.Lacour S., Hammann A., Grazide S., Lagadic-Gossmann D., Athias A., Sergent O., Laurent G., Gambert P., Solary E., Dimanche-Boitrel M. T. (2004) Cancer Res. 64, 3593–3598 [DOI] [PubMed] [Google Scholar]
- 8.Perrotta C., Bizzozero L., Falcone S., Rovere-Querini P., Prinetti A., Schuchman E. H., Sonnino S., Manfredi A. A., Clementi E. (2007) Cancer Res. 67, 7559–7564 [DOI] [PubMed] [Google Scholar]
- 9.Zeidan Y. H., Hannun Y. A. (2007) J. Biol. Chem. 282, 11549–11561 [DOI] [PubMed] [Google Scholar]
- 10.Schneider-Brachert W., Tchikov V., Neumeyer J., Jakob M., Winoto-Morbach S., Held-Feindt J., Heinrich M., Merkel O., Ehrenschwender M., Adam D., Mentlein R., Kabelitz D., Schütze S. (2004) Immunity 21, 415–428 [DOI] [PubMed] [Google Scholar]
- 11.Chieregatti E., Meldolesi J. (2005) Nat. Rev. Mol. Cell Biol. 6, 181–187 [DOI] [PubMed] [Google Scholar]
- 12.Nickel W., Rabouille C. (2009) Nat. Rev. Mol. Cell Biol. 10, 148–155 [DOI] [PubMed] [Google Scholar]
- 13.Falcone S., Perrotta C., De Palma C., Pisconti A., Sciorati C., Capobianco A., Rovere-Querini P., Manfredi A. A., Clementi E. (2004) J. Immunol. 173, 4452–4463 [DOI] [PubMed] [Google Scholar]
- 14.Barsacchi R., Perrotta C., Sestili P., Cantoni O., Moncada S., Clementi E. (2002) Cell Death Differ. 9, 1248–1255 [DOI] [PubMed] [Google Scholar]
- 15.Perrotta C., Falcone S., Capobianco A., Camporeale A., Sciorati C., De Palma C., Pisconti A., Rovere-Querini P., Bellone M., Manfredi A. A., Clementi E. (2004) Cancer Res. 64, 3767–3771 [DOI] [PubMed] [Google Scholar]
- 16.Merrill A. H., Jr., Sullards M. C., Allegood J. C., Kelly S., Wang E. (2005) Methods 36, 207–224 [DOI] [PubMed] [Google Scholar]
- 17.Bolte S., Cordelières F. P. (2006) J. Microsc. 224, 213–232 [DOI] [PubMed] [Google Scholar]
- 18.Bianco F., Perrotta C., Novellino L., Francolini M., Riganti L., Menna E., Saglietti L., Schuchman E. H., Furlan R., Clementi E., Matteoli M., Verderio C. (2009) EMBO J. 28, 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calegari F., Coco S., Taverna E., Bassetti M., Verderio C., Corradi N., Matteoli M., Rosa P. (1999) J. Biol. Chem. 274, 22539–22547 [DOI] [PubMed] [Google Scholar]
- 20.Fu J., Naren A. P., Gao X., Ahmmed G. U., Malik A. B. (2005) J. Biol. Chem. 280, 3178–3184 [DOI] [PubMed] [Google Scholar]
- 21.Marchesini N., Hannun Y. A. (2004) Biochem. Cell Biol. 82, 27–44 [DOI] [PubMed] [Google Scholar]
- 22.Sciorati C., Rovere P., Ferrarini M., Heltai S., Manfredi A. A., Clementi E. (1997) J. Biol. Chem. 272, 23211–23215 [DOI] [PubMed] [Google Scholar]
- 23.Bénéteau M., Pizon M., Chaigne-Delalande B., Daburon S., Moreau P., De Giorgi F., Ichas F., Rebillard A., Dimanche-Boitrel M. T., Taupin J. L., Moreau J. F., Legembre P. (2008) Mol. Cancer Res. 6, 604–613 [DOI] [PubMed] [Google Scholar]
- 24.Band A. M., Ali H., Vartiainen M. K., Welti S., Lappalainen P., Olkkonen V. M., Kuismanen E. (2002) FEBS Lett. 531, 513–519 [DOI] [PubMed] [Google Scholar]
- 25.Brunelli S., Sciorati C., D'Antona G., Innocenzi A., Covarello D., Galvez B. G., Perrotta C., Monopoli A., Sanvito F., Bottinelli R., Ongini E., Cossu G., Clementi E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grassmé H., Cremesti A., Kolesnick R., Gulbins E. (2003) Oncogene 22, 5457–5470 [DOI] [PubMed] [Google Scholar]
- 27.Algeciras-Schimnich A., Shen L., Barnhart B. C., Murmann A. E., Burkhardt J. K., Peter M. E. (2002) Mol. Cell. Biol. 22, 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guicciardi M. E., Gores G. J. (2009) FASEB J. 23, 1625–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henriques-Pons A., de Oliveira G. M. (2009) J. Cardiovasc. Pharmacol. 53, 94–99 [DOI] [PubMed] [Google Scholar]
- 30.Poonia B., Pauza C. D., Salvato M. S. (2009) Retrovirology 6, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouillet P., O'Reilly L. A. (2009) Nat. Rev. Immunol. 9, 514–519 [DOI] [PubMed] [Google Scholar]
- 32.Schubert K. M., Scheid M. P., Duronio V. (2000) J. Biol. Chem. 275, 13330–13335 [DOI] [PubMed] [Google Scholar]
- 33.Faustino R. S., Cheung P., Richard M. N., Dibrov E., Kneesch A. L., Deniset J. F., Chahine M. N., Lee K., Blackwood D., Pierce G. N. (2008) J. Lipid Res. 49, 654–662 [DOI] [PubMed] [Google Scholar]
- 34.Pizzo P., Pozzan T. (2007) Trends Cell Biol. 17, 511–517 [DOI] [PubMed] [Google Scholar]
- 35.Wiegmann K., Schwandner R., Krut O., Yeh W. C., Mak T. W., Krönke M. (1999) J. Biol. Chem. 274, 5267–5270 [DOI] [PubMed] [Google Scholar]
- 36.Rotolo J. A., Zhang J., Donepudi M., Lee H., Fuks Z., Kolesnick R. (2005) J. Biol. Chem. 280, 26425–26434 [DOI] [PubMed] [Google Scholar]
- 37.Degli Esposti M., Tour J., Ouasti S., Ivanova S., Matarrese P., Malorni W., Khosravi-Far R. (2009) Mol. Biol. Cell 20, 600–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utermöhlen O., Herz J., Schramm M., Krönke M. (2008) Immunobiology 213, 307–314 [DOI] [PubMed] [Google Scholar]
- 39.Peraud A., Watanabe K., Schwechheimer K., Yonekawa Y., Kleihues P., Ohgaki H. (1999) Lab. Invest. 79, 123–129 [PubMed] [Google Scholar]
- 40.Hong W. (2005) Trends Cell Biol. 15, 644–650 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.