Abstract
Terminal differentiation of mammalian erythroid progenitors involves 4–5 cell divisions and induction of many erythroid important genes followed by chromatin and nuclear condensation and enucleation. The protein levels of c-Myc (Myc) are reduced dramatically during late stage erythroid maturation, coinciding with cell cycle arrest in G1 phase and enucleation, suggesting possible roles for c-Myc in either or both of these processes. Here we demonstrate that ectopic Myc expression affects terminal erythroid maturation in a dose-dependent manner. Expression of Myc at physiological levels did not affect erythroid differentiation or cell cycle shutdown but specifically blocked erythroid nuclear condensation and enucleation. Continued Myc expression prevented deacetylation of several lysine residues in histones H3 and H4 that are normally deacetylated during erythroid maturation. The histone acetyltransferase Gcn5 was up-regulated by Myc, and ectopic Gcn5 expression partially blocked enucleation and inhibited the late stage erythroid nuclear condensation and histone deacetylation. When overexpressed at levels higher than the physiological range, Myc blocked erythroid differentiation, and the cells continued to proliferate in cytokine-free, serum-containing culture medium with an early erythroblast morphology. Gene expression analysis demonstrated the dysregulation of erythropoietin signaling pathway and the up-regulation of several positive regulators of G1-S cell cycle checkpoint by supraphysiological levels of Myc. These results reveal an important dose-dependent function of Myc in regulating terminal maturation in mammalian erythroid cells.
Keywords: Chromatin Histone Modification, Erythropoiesis, Hematopoiesis, Histone Acetylase, Myc, Erythroid Enucleation
Introduction
Mammalian terminal erythroid development is a precisely regulated process involving rapid proliferation and serial morphological changes of committed erythroid progenitors (colony forming units-erythroid, or CFU-E)5 to form mature erythrocytes. The initial stages of terminal erythroid maturation are highly dependent on erythropoietin (1), whose roles in activation of erythroid specific genes, terminal proliferation, and protection against apoptosis are well understood (2). This is followed by an erythropoietin-independent, fibronectin-dependent phase where survival and proliferation of the erythroblasts require signaling by α4β1 integrins (3). Upon erythropoietin stimulation, CFU-E progenitors undergo 4–5 cell divisions accompanied by a decrease in cell size, increase in hemoglobin content, and nuclear condensation followed by withdrawal from the cell cycle (4). Late stage mammalian erythroblasts undergo nuclear condensation and enucleate by extrusion of the pycnotic nucleus surrounded by a thin layer of cytoplasm and cell membrane (5–7). The molecular mechanisms that regulate this hallmark process remain to be fully elucidated. We previously reported that Rac GTPases and their downstream effector mDia2 play important roles in the cytoskeletal reorganization leading to the extrusion of the pycnotic nucleus from late stage erythroblasts (8). Nevertheless, the mechanisms regulating condensation of the erythroid nucleus preceding its extrusion remain unclear.
The proto-oncogene c-Myc (Myc) has been widely studied since its identification as a cellular homolog of the retroviral v-myc oncogene (9), and its functions in the genesis and maintenance of tumors are well established (10, 11). The Myc transcription factor plays important roles in normal, non-transformed cells in regulating proliferation, differentiation, cell growth and apoptosis (12–14) and is critical for several aspects of hematopoietic development and function (15). Studies of epiblast-specific Myc deletion in mice suggest that both primitive and definitive hematopoiesis are impaired in the absence of Myc (16). Forced expression of Myc promoted differentiation of hematopoietic stem cells at the expense of their self-renewal ability (17), indicating that Myc functions in the hematopoietic lineages are not limited to stimulating proliferation. Myc expression is rapidly induced upon erythropoietin stimulation of committed erythroid progenitor cells (18), and its levels are substantially reduced during the final stages of erythroid maturation (19). The physiological relevance of these noticeable changes in Myc expression during terminal erythroid maturation remains unclear due to conflicting reports in literature. Studies in G1E erythroid cell lines report that forced Myc expression prevented cell cycle arrest but had minimal effects on erythroid maturation (20). In contrast, Myc expression blocked erythroid differentiation in human leukemia K562 cells without preventing the cell cycle exit (21). These discrepancies are possibly a result of the different cell lines used as models of terminal erythroid maturation. Retroviral overexpression of Myc in unfractionated murine fetal liver cells resulted in continuously growing erythroblast cell lines, suggesting that Myc overexpression enhanced the self- renewal ability of early erythroblasts at the expense of differentiation (22). Here we use a recently developed in vitro culture system in which the proliferation and differentiation of purified TER119-negative mouse fetal liver erythroblasts can be monitored quantitatively in a step-by-step manner (23) to investigate the role of Myc in terminal erythroid maturation.
We demonstrate that ectopic Myc expression has a dose-dependent effect on terminal erythroid differentiation of purified mouse fetal liver erythroblasts cultured in vitro. Ectopic Myc expression at physiological levels specifically blocks enucleation without affecting terminal erythroid maturation. In contrast, overexpression of Myc at levels above the physiological range promotes proliferation of early erythroblasts at the expense of differentiation and also evokes an apoptotic response. Our study indicates that Myc levels play a critical role in regulating the balance between proliferation and differentiation in the erythroid lineage. Our data uncover an important role for Myc in regulating erythroid nuclear condensation and enucleation.
EXPERIMENTAL PROCEDURES
Purification and Culture of Murine Fetal Liver Erythroid Progenitors
All mouse work was carried out with approved Institutional Animal Care and Use Committee protocols at the Biological Research Centre mouse facility at Biopolis, Singapore. Fetal livers were isolated from E13.5 C57BL/6 mice embryos and mechanically dissociated by pipetting in PBS containing 20% fetal bovine serum (FBS). The dissociated cells were passed through 70- and 30-μm strainers to obtain single-cell suspensions. For negative selection, cells were labeled with magnetic microbead-conjugated antibodies for TER119 and CD11b (to remove macrophages) and passed through LD columns according to the manufacturer's instructions (Miltenyi Biotec). The purified TER119-negative erythroblasts were seeded in fibronectin-coated plates and cultured in Iscove's modified Dulbecco's medium (Invitrogen) containing 15% FBS (StemCell Technologies), 1% detoxified bovine serum albumin (StemCell Technologies), 200 μg/ml holotransferrin (Sigma),10 μg/ml recombinant human insulin (Sigma), 2 mm l-glutamine (Invitrogen), 10−4 m β-mercaptoethanol (Invitrogen), and 2 units/ml erythropoietin (R&D systems). For p27 knock-out studies, E13.5 embryos harvested from intercrosses of p27+/− mice (24) were genotyped as described previously (25) followed by purification of fetal liver erythroid progenitors.
Retroviral Constructs
The murine stem cell retroviral vector (MSCV) construct that co-expresses Myc and GFP (MSCV-Myc-IRES-GFP) was a gift from Dr. Michael H. Tomasson (26). The MSCV-Gcn5-IRES-GFP construct to overexpress Gcn5 was made by cloning the full-length Gcn5 open reading frame (ORF) into the EcoRI-NotI sites of the MSCV-IRES-GFP vector. The full-length GCN5 ORF was PCR-amplified from cDNA clones kindly provided by Dr. Sharon Y.R. Dent (27). The retroviral construct expressing shRNA against c-Myc was purchased from Open Biosystems. The target sequence for the c-Myc shRNA is 5′-AGCCTTGAAATGTAAATAACTT-3′, and the target sequence of the non-silencing negative control (incomplete luciferase shRNA) is 5′-GTGCGTTGCTAGTACCAACTTCAAGAGA-3′.
Retrovirus Generation and Infection
Replication-incompetent retroviruses were produced by co-transfection of 293T cells with the MSCV constructs and the ecotropic packaging vector pCL-Eco using Lipofectamine 2000 (Invitrogen). Retroviral supernatants were harvested 48 h after transfection, passed through 0.45-μm filters, and stored in aliquots at −80 °C. The virus titers for the MSCV retroviral supernatants were estimated by transduction of NIH3T3 cells and quantifying the percentage of GFP-positive cells by FACS analysis. For infection of purified TER119-negative erythroid progenitor cells, 5 × 105 cells were resuspended in 1 ml of retroviral supernatant containing 8 μg/ml Polybrene (Sigma) and centrifuged at 2000 rpm for 1 h at 37 °C. The virus supernatant was then replaced by erythropoietin-containing medium.
Immunostaining and Flow Cytometry
Cells were washed and resuspended in 200 μl of PBS containing 4% FBS for immunostaining. For analysis of erythroid differentiation, cells were incubated with 1:200 dilution of phycoerythrin-conjugated anti-CD71 (BD Biosciences) and allophycocyanin-conjugated anti-Ter-119 antibody (BD Biosciences) for 15 min at room temperature. For enucleation analysis, cells were additionally stained with 10 μg/ml Hoechst 33342 (Sigma) at room temperature for 15 min. Propidium iodide (BD Biosciences) at a final concentration of 0.2 μg/ml was added to exclude dead cells from the analysis. Apoptosis was evaluated by co-staining with allophycocyanin-conjugated annexin V (BD Biosciences) and propidium iodide. To perform cell cycle analysis, the cells were washed and resuspended in 50 μl of PBS, fixed in 1 ml of cold 90% ethanol, and stored at 4 °C overnight. The fixed cells were washed and incubated in PBS containing propidium iodide (20 μg/ml) and RNase-A (200 μg/ml) for 1 h at room temperature. Flow cytometry was performed using BD LSRII (BD Biosciences), and data analysis was carried out using BD FACSDiva (BD Biosciences).
Benzidine-Giemsa Staining and Hemoglobin Assay
5 × 104 cells were centrifuged onto glass slides using Cytospin 4 (Shandon). The cells were air-dried completely and fixed in −20 °C methanol for 2 min. Benzidine solution was prepared by dissolving one benzidine tablet (Sigma, D5905) in 10 ml of PBS and filtered through a 0.22-μm filter. 10 μl of 30% Hydrogen peroxide solution (Calbiochem) was added to the benzidine solution just before staining and placed as drops directly on fixed cells for 2 h at room temperature. The cells were rinsed briefly with PBS and stained with 1:20 dilution of Giemsa (Sigma, GS500) in water for 15 min at room temperature. Excess Giemsa stain was removed by washing with PBS. Images were captured on an Olympus BX51 microscope equipped with an Olympus DP70 digital camera using DPController image capture software (Olympus). For hemoglobin assay, 1 × 106 erythroid cells were lysed in 200 μl of Drabkin's reagent (Sigma), and hemoglobin content was quantified by spectrophotometric measurement of absorbance at 540 nm on a Tecan Sunrise Reader.
Measurement of Nuclear Size by High Content Screening
Erythroid cells were fixed in 3.7% formaldehyde in PBS for 7 min at room temperature, then incubated in 10 μg/ml solution of Hoechst 33342 in PBS for 15 min to stain the nucleus followed by 2 washes with PBS. The cells were resuspended in PBS, transferred to flat-bottom 96-well cell culture plates, and centrifuged at 1000 rpm for 3 min to deposit the cells as a single layer at the bottom of the wells. Images were acquired by the automated Arrayscan VTI high content screening reader (Cellomics) using a 20× objective lens from 16 different fields in each well. The images were analyzed by Cellomics Target Activation BioApplication software. Cell clumps and debris were excluded from analysis by setting appropriate cut-off values after visual inspection of the images, and data acquired consisted of at least 5000 objects (cells) per sample. The nuclear size (nuclear area per cell) for each sample was determined as the average Hoechst area per object in the sample.
Quantitative Real-time (qRT-PCR)
Total RNA was extracted from cells using RNeasy mini kits (Qiagen). 1 μg of total RNA for each sample was reverse-transcribed using the High Capacity cDNA Archive kit (Applied Biosystems). Relative transcript levels of different genes were quantified by SYBR Green real time PCR using ABI Prism 7900HT Sequence Detection System 2.2 (Applied Biosystems). The results were normalized to GAPDH and analyzed using SDS 2.2.2 software. The primer sequences are provided in supplemental Table S2.
Gene Expression Microarrays and Data Analysis
Total RNA was extracted from 3 biological replicates of the cells under each condition with RNeasy mini kits, and 500 ng was reverse-transcribed into cDNA followed by in vitro transcription to biotin-labeled cRNA using the Illumina TotalPrep RNA Amplification kit (Applied Biosystems). 750 ng of each cRNA sample was hybridized to MouseRef-8 Version 2.0 Expression BeadChip microarrays (Illumina) and scanned on the BeadArray Reader (Illumina) at scan factor 1. Background subtraction was applied on raw intensity values, and subsequent data were subjected to quantile normalization on the Beadstudio Data Analysis platform (Illumina) with a normalized expression value cutoff at 100. Differentially expressed genes were identified based on an at least a 2-fold change in at least one condition compared with controls (D0 WT). Individual gene lists were uploaded into Ingenuity Pathway Analysis software (Ingenuity System) to identify the significantly enriched gene ontology categories based on molecular and cellular functions. Microarray data were deposited into Gene Expression Omnibus (accession number GSE18558).
Western Blots
Cells were lysed in radioimmune precipitation assay buffer (Pierce) containing protease inhibitors (Roche Applied Science). 20 μg of total protein for each sample was resolved on a 10% SDS-polyacrylamide gel and transferred to methanol-activated polyvinylidene difluoride membranes (GE Healthcare). After washing once with TBST (20 mm Tris, pH 7.5, 500 mm NaCl, 0.1% Tween 20), the membranes were blocked for 1 h at room temperature in 5% bovine serum albumin (BSA) in TBST with mild shaking. The blots were then incubated with primary antibody at appropriate dilution in TBST with 2% BSA overnight with gentle shaking at 4 °C, washed 3 times for 15 min with TBST, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The blots were washed with TBST 3 times for 5 min and visualized using a chemiluminescent substrate (Luminol reagent, Santa Cruz Biotechnologies). Histones were purified by acid extraction for Western blot analysis of histones H3 and H4 acetylation. Cells were washed once with ice-cold PBS, and cell pellets were incubated in PBS containing 0.5% Triton X-100 and protease inhibitors for 10 min on ice. Pellets were resuspended in 0.2 n HCl at 4 °C overnight for extraction of histones. The acidic pH of histone extracts was neutralized with 1 m Tris, pH 8. Equal loading of histones was ensured by Ponceau staining before proceeding for immunoblotting with acetylation-specific antibodies. Primary antibodies used for Western blotting were as follows: Myc (SC-764, Santa Cruz); p27 (610241, BD Biosciences); Gcn5 (#3305, Cell Signaling); GAPDH (ab37187, Abcam); total histone H4 (ab10158, Abcam). The antibodies for H3K9Ac, H3K18Ac, H3K23Ac, and H3 (total) were purchased from Cell Signaling (#9927). Antibodies for H4K5Ac, H4K8Ac, H4K12Ac, and H4K16Ac were purchased from Millipore (#17–211).
Chromatin Immunoprecipitation (ChIP) Assay
TER119-negative mouse fetal erythroid progenitor cells were cross-linked with 1% formaldehyde for 10 min at room temperature, and chromatin immunoprecipitation was performed according to Agilent ChIP-on-chip protocol (version 9.1, Nov 2006) using anti-c-Myc antibody (SC-764, Santa Cruz). The enrichments of binding sites in the immunoprecipitated DNA were quantified using qRT-PCR. The -fold enrichment values were calculated by determining the apparent immunoprecipitation efficiency (ratios of the amount of immunoprecipitated DNA to that of the input sample) and normalized to the level observed at a control region, which was defined as 1.0. The ChIP qRT-PCR primer sequences are available in supplemental Table S2.
Statistical Analysis
Student's t test was used to determine the significance of differences between treated samples and controls. Statistical analysis was performed using Microsoft Office Excel 2003.
RESULTS
Ectopic Myc Expression Has a Dose-dependent Effect on Terminal Erythroid Maturation
As a first step in addressing the role of Myc in terminal erythropoiesis, we monitored the changes in Myc protein levels during differentiation of TER119-negative erythroid progenitor cells purified from E13.5 mouse fetal livers and cultured for 2 days on fibronectin-coated plates in medium containing serum and erythropoietin. These culture conditions support proper terminal proliferation and differentiation of CFU-E progenitors in a manner mimicking erythropoiesis in vivo (23). Myc protein levels decreased substantially during the 2-day in vitro culture (Fig. 1A, lanes 1–3). We also quantified changes in p27Kip1 (p27) protein levels, as in many cells p27 is repressed by Myc (28), and changes in p27 levels have been suggested to play a critical role in the withdrawal from cell cycle during terminal erythroid maturation (29). As expected, p27 protein levels were strongly induced during the later stages of erythroid differentiation (Fig. 1A, lanes 1–3).
FIGURE 1.
Dose-dependent effect of ectopic Myc on terminal erythroid maturation. A, Western blot analysis of Myc and p27 protein levels is shown. TER119-negative fetal liver erythroblasts purified from E13.5 mouse embryos and cultured in erythropoietin-containing medium in fibronectin-coated plates and cells were harvested at 0 h (D0), 24 h (D1), or 48 h (D2) in culture. Purified erythroblasts were infected with bicistronic retroviruses encoding GFP only as control or both Myc and GFP at 0 h in culture. At 24 h post-infection, cells were sorted based on GFP intensity into low (Myc-GFPlow) and high (Myc-GFPhigh) intensity populations, then cultured for another 24 h to allow for differentiation. Cells were harvested at indicated time points, and lysates were subjected to Western blotting using the indicated antibodies. GAPDH protein levels were determined as a loading control. The Myc bands in the Western blot image were quantified using ImageJ, normalized to the GAPDH bands, and are presented as -fold change relative to D0 levels. Error bars represent S.D. (n = 3). B, flow cytometric analysis of terminal erythroid maturation is shown. At 48 h post-infection, control, Myc-GFPlow, and Myc-GFPhigh cells were analyzed by flow cytometry. Erythroid differentiation was assayed by quantifying the CD71+TER119+ population, which represents the late stage erythroid cells (top). Enucleation was assayed by quantifying the TER119+Hoechst− populations, which correspond to the enucleated reticulocytes (middle). Apoptosis and cell death were assessed by co-staining with annexin V and propidium iodide (PI, bottom).
We then examined differentiation of TER119-negative erythroid progenitor cells ectopically expressing Myc. A bicistronic retroviral vector (MSCV-Myc-IRES-GFP) that also encodes green fluorescent protein (GFP) was used to express Myc in purified TER119-negative erythroid progenitor cells. This allowed for GFP intensity measured by flow cytometry to serve as a reporter for the level of ectopic Myc expression (30). At 24 h post-retroviral infection, the cells were sorted based on GFP intensity into low GFP (Myc-GFPlow) and high GFP populations (Myc-GFPhigh) followed by culture for another 24 h to allow for complete terminal maturation. Cells infected with retroviruses encoding GFP only (MIG) served as control. Western blot analysis of retrovirus-transduced cells was performed at 24 and 48 h post-infection to measure Myc and p27 levels (Fig. 1A, lanes 4–9) and were compared with those during differentiation of uninfected erythroblasts (Fig. 1A, lanes 1–3). The Myc-GFPlow cells showed continued Myc expression at levels similar to those of normal cells at 1 day of differentiation (Fig. 1A, lanes 5 and 8) and, thus, within a physiological range. In contrast, the Myc-GFPhigh cells showed Myc expression at levels above the physiological range of Myc expression in differentiating erythroblasts (Fig. 1A, lanes 6 and 9). Myc-GFPhigh cells demonstrated Myc protein levels greater than early erythroid progenitors (freshly purified TER119-negative fetal liver erythroblasts) at both 24 and 48 h in culture, indicating that these cells represented high level Myc-overexpressing erythroblasts. At 48 h in culture, the induction of p27 in Myc-GFPlow cells was similar to that of control cells, but p27 induction was significantly reduced in Myc-GFPhigh cells (Fig. 1A, lanes 7–9).
The terminal differentiation states of Myc-GFPlow, Myc-GFPhigh, and control (MIG) cells were examined by flow cytometry at 48 h in culture. The percentage of CD71+TER119+ populations was similar in Myc-GFPlow and control cells but was significantly reduced in the Myc-GFPhigh cells, suggesting that overexpression of Myc at high levels blocked erythroid differentiation (Fig. 1B, top panel). Enucleation was quantified by measuring the reticulocytes formed in culture, which stain positive for the erythroid differentiation marker TER119 and negative for Hoechst 33342 (Hoechst). The TER119+ Hoechst− reticulocyte population seen in the control cells was absent in both Myc-GFPlow and Myc-GFPhigh cells (Fig. 1B, middle panel), indicating that sustained expression of Myc at a physiological level was sufficient to block erythroid enucleation. Myc-GFPlow cells did not show any significant change in the levels of apoptosis compared with control cells, whereas Myc-GFPhigh cells showed significantly high levels of apoptosis (Fig. 1B, bottom panel). Taken together, continued expression of Myc at a physiological level blocked enucleation specifically without affecting the terminal differentiation or survival of primary erythroblasts, whereas overexpression of Myc at supraphysiological levels additionally blocked erythroid differentiation and induced apoptosis.
Ectopic Myc Expression at Supraphysiological Levels Inhibits Hemoglobin Expression and Exit from the Cell Cycle
To confirm the inhibition of terminal erythroid differentiation by overexpression of Myc at supraphysiological levels, we measured the hemoglobin content of the cultured erythroblasts at 48 h post-infection. The hemoglobin content of Myc-GFPhigh cells was significantly lower than that of control cells, whereas the hemoglobin level of Myc-GFPlow cells was normal (Fig. 2A). We next measured total cell counts at 48 h in culture relative to an equal number of sorted cells at 24 h to evaluate the effect of ectopic Myc on terminal proliferation (Fig. 2B). We normalized the absolute cell numbers to account for enucleation, where one erythroblast forms a nucleus and a reticulocyte, and demonstrated that continued Myc expression at physiological levels had no effect on terminal proliferation. The absolute cell counts for Myc-GFPhigh cells at 48 h were significantly lower than that of control (Fig. 2B), and this reduction was explained by the significant induction of apoptosis by supraphysiological levels of Myc (Fig. 1B). Cell cycle analysis was performed at 48 h in culture to investigate the effect of ectopic Myc expression on the withdrawal from cell cycle observed during the final stages of normal erythroid maturation (Fig. 2C). Myc-GFPlow cells accumulated in the G1 phase of the cell cycle similar to the control cells. In contrast, Myc-GFPhigh cells showed a signification population of cells in the S-phase, indicating that the cells continue to cycle (Fig. 2C). The Myc-GFPhigh cells continued to proliferate in erythropoietin-free culture conditions (DMEM containing 10% serum and antibiotics) for at least 8 weeks, prompting us to test whether these cells have been transformed. Mice transplanted with these cultured Myc-GFPhigh erythroblasts did not show evidence of erythroleukemia development even after 6 months post-transplant, suggesting that ectopic expression of Myc was not sufficient to fully transform erythroblasts into erythroleukemias (data not shown). This is consistent with the conclusions from a previous study (22), where retroviral overexpression of Myc in murine fetal liver cells gave rise to continuously growing erythroblast cell lines but did not fully transform them. These results indicate that Myc overexpression at supraphysiological levels in early erythroblasts promotes proliferation at the expense of differentiation.
FIGURE 2.
Ectopic Myc expression at supraphysiological levels inhibits hemoglobin expression and exit from cell cycle. TER119-negative fetal liver erythroblasts were infected with bicistronic retroviruses encoding GFP only as control or both Myc and GFP at 0 h in culture. At 24 h post-infection, cells were sorted based on GFP intensity into low (Myc-GFPlow) and high (Myc-GFPhigh) intensity populations and then cultured for another 24 h to allow for differentiation. A, relative hemoglobin content was quantified at 48 h post-infection by cell lysis in Drabkin's reagent followed by measurement of absorbance at 540 nm. B, cell counts were measured at 48 h post-infection for an equal starting cell number (25 × 104) at 24 h after sorting based on GFP intensity. The total cell numbers are represented as black bars. The numbers were normalized to account for enucleation using the equation n = total cell number × (1 − percentage enucleation/100) and are shown as white bars. C, cell cycle analysis by flow cytometry at 48 h post-retroviral infection is shown. All error bars represent S.D. (n = 3). In all panels, two-tailed t test are indicated by as asterisk, p < 0.01.
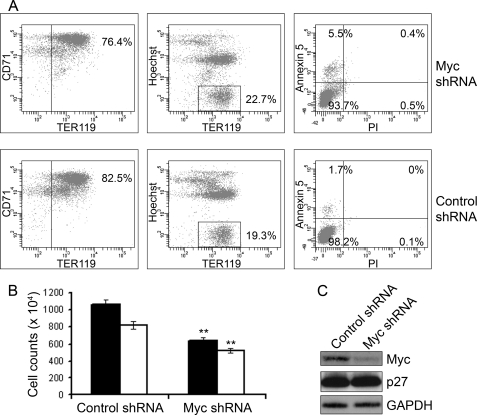
To test the role of Myc in the rapid terminal proliferation of erythroid cells, we infected purified TER119-negative erythroblasts with retroviruses harboring shRNAs against Myc or with retroviruses encoding non-silencing negative control shRNA followed by culture for 48 h. There was no significant effect of knockdown of Myc on erythroid differentiation, enucleation, or apoptosis (Fig. 3A), but proliferation was significantly reduced in cells expressing Myc shRNA (Fig. 3B). Western blot analysis confirmed the reduction of Myc protein levels in cells expressing Myc shRNA (Fig. 3C). The p27 protein was not increased upon knockdown of Myc, presumably because p27 protein expression is already at its maximum in late stage erythroblasts. Because Myc levels are normally down-regulated during terminal erythroid maturation, it was not surprising that knockdown of Myc had no adverse effects on terminal differentiation or enucleation. Interestingly, accelerated depletion of Myc significantly reduced the proliferation rates of differentiating erythroid cells, suggesting that Myc levels may regulate the number of cell divisions during terminal erythroid differentiation.
FIGURE 3.
Depletion of Myc by shRNA inhibits terminal proliferation of erythroblasts without affecting terminal differentiation. A, purified TER119-negative erythroblasts were infected with retroviruses encoding shRNA against Myc or control retroviruses encoding non-silencing negative control shRNA followed by culture in erythropoietin containing medium. The cells were analyzed by flow cytometry after 48 h in culture. Erythroid differentiation was assayed by quantifying the CD71+TER119+ population, which represents the late stage erythroid cells (left). Enucleation was assayed by quantifying the TER119+Hoechst− populations, which corresponds to the enucleated reticulocytes (middle). Apoptosis and cell death were assessed by co-staining with annexin V and propidium iodide (PI, right). B, cell counts were measured at 48 h in culture for the same cells as in panel A, for an equal starting cell number (50 × 104). The total cell numbers are represented as black bars. The numbers were normalized to account for enucleation using the equation n = total cell number × (1 − percentage enucleation/100) and are shown as white bars. Error bars represent S.D. (n = 3). Two-tailed t test are indicated by double asterisks, p < 0.01. C, shown is Western blot analysis of the same cells as in panel A using antibodies against Myc and p27. GAPDH protein levels were determined as a loading control.
Terminal Erythroid Differentiation and Cell Cycle Arrest Are Not Affected by Deficiency of p27
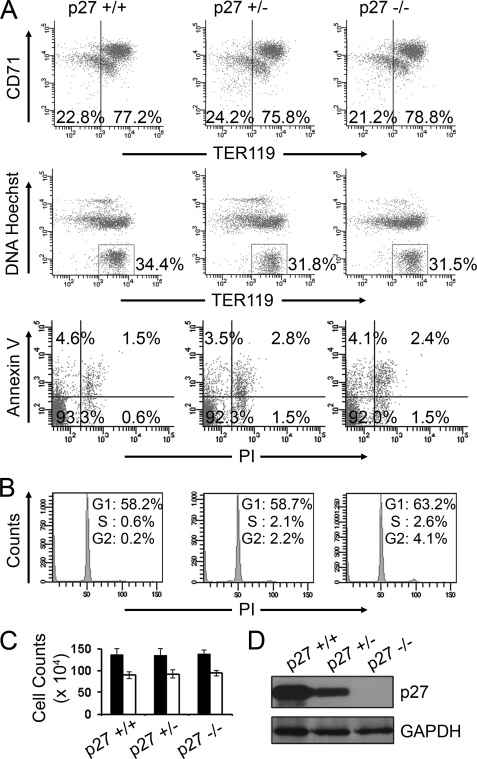
Myc overexpression at supraphysiological levels in erythroid cells prevented their withdrawal from the cell cycle (Fig. 2C) and also diminished the induction of p27 during late stage erythroid maturation (Fig. 1A, lanes 7 and 9). p27 has been implicated in the cell cycle arrest during the final stages of erythroid maturation (29). This prompted us to investigate whether p27 protein levels were the critical mediators of cell cycle arrest during terminal erythroid differentiation and whether the effect of Myc on cell cycle in erythroid cells was mediated through its regulation of p27 protein levels. To this end, we purified TER119-negative fetal liver erythroblasts from E13.5 transgenic embryos that were wild-type (p27+/+), heterozygous (p27+/−) or nullizygous (p27−/−) for the p27 locus and compared their terminal differentiation in vitro to test if p27 was necessary for normal terminal erythroid maturation and cell cycle arrest. Differentiation, enucleation, and apoptosis were quantified by flow cytometry at 48 h of culture, and no significant difference was observed between the p27+/+, p27+/−, and p27−/− cells (Fig. 4A). There was also no significant difference in the extent of G1 arrest (Fig. 4B) or terminal proliferation (Fig. 4C) in the absence of one or both alleles of p27. Western blot analysis was performed to confirm the complete absence of p27 protein in the p27−/− erythroblasts at 48 h in culture (Fig. 4D), and the protein levels were intermediate in p27+/− cells consistent with previous studies (24, 31, 32). Adult p27 knock-out mice are larger in body size than their wild-type counterparts, but there are no apparent defects in the proportions of various cell types in peripheral blood (32). These results demonstrate that terminal erythroid differentiation and cell cycle arrest occurred normally even in the absence of p27 and suggest that the effect of Myc overexpression on cell cycle progression in erythroid cells may not be mediated by p27 alone.
FIGURE 4.
Terminal erythroid differentiation is not affected by deficiency of p27. TER119-negative fetal liver erythroblasts were purified from p27+/+, p27+/−, and p27−/− E13.5 mouse embryos and cultured in erythropoietin-containing medium in fibronectin-coated plates for 48 h. A, differentiation (top), enucleation (middle), and apoptosis (bottom) were quantified by flow cytometry at 48 h in culture, as described in Fig. 1B. PI, propidium iodide. B, shown is cell cycle analysis of cells harvested at 48 h in culture by flow cytometry. C, cell counts were measured at 48 h in culture for an equal starting cell number (1 × 105). The total cell numbers are represented as black bars. The numbers were normalized to account for enucleation as in Fig. 2C and are shown as white bars. All error bars represent S.D. (n = 3). D, Western blot analysis to determine p27 protein levels is shown. GAPDH protein levels were determined as a loading control.
Gene Expression Analysis Demonstrates That Myc-GFPhigh Cells Are Similar to Early Erythroblasts, and Myc-GFPlow Cells Resemble Late Stage Erythroid Cells
Because Myc is a pleiotropic transcription factor regulating several diverse cellular functions (12–14), we used microarrays to investigate the genes and pathways perturbed by ectopic Myc expression at physiological and supraphysiological levels in erythroid cells. Total RNA was extracted from Myc-GFPlow and Myc-GFPhigh cells at 48 h in culture post retroviral infection and from uninfected erythroblasts in culture at 0 (D0 WT), 24 (D1 WT), and 48 h (D2 WT) as controls. RNA was then processed for hybridization to Illumina microarrays. A hierarchical clustering analysis of the 2795 differentially expressed genes, identified based on an at least 2-fold change in at least one condition compared with D0 WT, was performed, and the results are shown in Fig. 5A. Each sample contained 3 biological replicates (termed 1, 2, and 3) that correlated well with each other. Cluster analysis showed that the mRNA expression pattern of Myc-GFPhigh cells at 48 h in culture was most similar to that of uncultured TER119-negative erythroblasts (D0 WT). The expression pattern of wild-type cells changed markedly during differentiation, comparing erythroblasts cultured for 48 h (D2 WT) against uncultured TER119-negative erythroblasts (D0 WT). Importantly, the expression pattern of Myc-GFPlow cells at 48 h in culture was most similar to that of differentiated erythroblasts (D2 WT).
FIGURE 5.
Microarray analysis demonstrates that global gene expression of Myc-GFPhigh cells is similar to early erythroblasts and Myc-GFPlow cells resemble differentiated late stage erythroid cells. A, purified TER119-negative erythroblasts were cultured in erythropoietin-containing medium, and cells were harvested at 0 h (D0 WT), 24 h (D1 (WT), or 48 h (D2 WT) culture or were infected with bicistronic retroviruses encoding Myc and GFP at 0 h in culture and sorted based on GFP fluorescence into low (Myc-GFPlow) and high (Myc-GFPhigh) expression populations at 24 h post-infection followed by culturing for another 24 h. Total RNA was extracted from three biological replicates under each condition and processed for hybridization on Illumina microarrays. Hierarchical clustering of all treatments and replicates was performed on Beadstudio software using Pearson correlation with nesting by average linkage method. The numerical scale shows the 1-r distance metric, where r is the Pearson correlation coefficient, and shorter branches denote greater similarity. The heatmap shows 2795 differentially expressed genes that are up-regulated (red) or down-regulated (green) by at least 2-fold in at least 1 condition compared with the average of the D0 WT replicate controls. The color scale bar indicates relative -fold change in gene expression compared with the average of D0 WT replicate controls on a Log2 scale. B, qRT-PCR analysis of the expression of key erythroid differentiation genes (top), genes downstream of erythropoietin signaling (middle), and cell cycle genes (bottom) in Myc-GFPlow and Myc high cells at 48 h in culture relative to control cells infected with retroviruses encoding GFP only (D2 Control). All error bars represent S.D. (n = 3). The two-tailed t test results are indicated by an asterisks, p < 0.01.
The genes that are up-regulated, unchanged, or down-regulated significantly in differentiated control erythroblasts (D2 WT) relative to early erythroblasts (D0 WT) and are significantly perturbed upon ectopic Myc expression are denoted as gene subsets i, ii, and iii, respectively (Fig. 5A). The significantly enriched gene ontology categories for each of these gene subsets were identified using Ingenuity Pathway Analysis software (supplemental Table S1). Gene subset i includes genes that are normally up-regulated during erythroid differentiation. The up-regulation of these erythroid important genes is blocked in Myc-GFPhigh cells, consistent with the observed differentiation block in these cells. A significant number of these genes were also perturbed in Myc-GFPlow cells, suggesting that differentiation is not completely normal in these cells, although hemoglobin levels and TER119 expression were normal (Figs. 1 and 2). The genes that are not significantly induced or repressed during terminal erythroid maturation but are significantly perturbed upon ectopic Myc expression (Gene subset ii) were enriched for gene ontology categories such as cell-to-cell signaling, cellular movement, general cellular maintenance, and metabolism (supplemental Table S1). The genes down-regulated during normal erythroid maturation (Gene subset iii) control cellular processes that are shut down during late stage erythroid maturation; that is, gene expression, RNA post-transcriptional modification, cell death, and cell cycle and DNA replication and repair (supplemental Table S1). The expression pattern of these genes in Myc-GFPhigh cells was similar to that of early erythroblasts (D0 WT), and that of Myc-GFPlow cells was closer to differentiated late stage erythroblasts (D2 WT).
The expression of several key erythroid transcripts such as the hemoglobin isoforms (Hbb-b1, Hbb-b2, and Hba-a1), the erythroid-specific membrane protein Spectrin α-chain (Spna1), and the erythroid-specific cell surface protein glycophorin A (GypA) were repressed in Myc-GFPhigh cells but were normal in Myc-GFPlow cells, as confirmed by qRT-PCR (Fig. 5B, top panel). The expression of the master regulator of terminal erythroid maturation GATA1 was not significantly changed in either Myc-GFPlow or Myc-GFPhigh cells at 48 h in culture, suggesting that the differentiation block observed in Myc-GFPhigh cells is not due to repression of GATA1 expression by Myc. However, the expression of several genes downstream of the erythropoietin signaling pathway that have been implicated to be critical for normal terminal erythroid differentiation, such as protein kinase Cδ (Prkcd) (33), Janus kinase 2 (Jak2) (34), protein kinase Bγ (Akt3) (35, 36), the anti-apoptotic protein Bcl-XL (37, 38), the small GTPase Rac2 (8), and the inhibitory cyclin G2 (ccng2) (39), were significantly repressed in Myc-GFPhigh cells but were only modestly repressed or remained relatively normal in Myc-GFPlow cells (Fig. 5B, middle panel). This suggests that the dysregulation of the erythropoietin signaling pathway in Myc-GFPhigh cells leads to the observed block in differentiation in Myc-GFPhigh cells. The expression of these genes changes modestly in Myc-GFPlow cells, suggesting that Myc-GFPlow cells may have other minor defects in differentiation that are not immediately apparent in our assays. We also analyzed the expression of positive regulators of the G1-S transition cell cycle checkpoint in Myc-GFPlow and Myc-GFPhigh cells. The expression of Cyclin E (25, 40) and cdc25a (41) was up-regulated significantly in Myc-GFPhigh cells but not in Myc-GFPlow cells, whereas the expression of H-ras (23) and Ebp1 (42) increased in a dose-dependent manner upon increase in ectopic Myc expression (Fig. 5B, bottom panel). The expression of p27, a negative regulator of G1-S transition, was significantly repressed in Myc-GFPhigh cells but only mildly reduced in Myc-GFPlow cells (Fig. 5B, bottom panel). Because we demonstrated that p27 is dispensable for normal terminal erythroid differentiation and cell cycle exit (Fig. 4), this result suggests that the induction of several positive regulators of the G1-S transition by ectopic expression of Myc at supraphysiological levels abrogates the normal G1-phase cell cycle arrest, whereas the changes in expression of these genes induced by continued Myc expression at physiological levels was insufficient to overcome the normal erythroid cell cycle arrest.
In summary, gene expression analysis demonstrated that Myc-GFPlow cells resembled differentiated erythroid cells and Myc-GFPhigh cells were similar to undifferentiated early erythroblasts. Ectopic Myc expression even at a physiological level had significant effects on the gene expression patterns observed in late stage erythroid cells, suggesting that although overall terminal differentiation was normal in terms of hemoglobin levels and expression of major red cell proteins, there are other abnormal changes in these cells besides a block in enucleation. Ectopic Myc expression at supraphysiological levels prevented the induction of several key erythroid genes and disrupted genes involved in the erythropoietin signaling pathway, leading to a block in terminal erythroid differentiation, and also induced the expression of proliferation-promoting genes preventing normal erythroid cell cycle exit.
Ectopic Myc Expression Inhibits Nuclear Condensation and Histone Deacetylation in Late Stage Erythroid Cells
To further characterize the block in the ability of the Myc-expressing cells to undergo enucleation, we performed benzidine-Giemsa staining on the cultured cells (Fig. 6A). The Myc-GFPhigh cells did not stain strongly with benzidine, showing a lack of hemoglobinization, and were similar in appearance to early erythroblasts; that is, large cells with large nuclei. This further confirmed that Myc expression at supraphysiological levels arrested terminal differentiation at an early erythroblast stage. There was no apparent difference in benzidine staining between Myc-GFPlow cells and control cells at 48 h in culture, confirming that ectopic Myc expression at physiological levels had no effect on erythroid maturation and hemoglobinization (Fig. 6A). Importantly, the nuclei in the late stage Myc-GFPlow erythroblasts were larger and more centralized compared with the control cells at 48 h in culture but were smaller than the nuclei of erythroblasts in earlier stages of development harvested at 0 and 24 h in culture (Fig. 6A). To confirm this observation, we used Cellomics high content screening to measure and compare the erythroid nuclear size in a quantitative manner (Fig. 6B). The nuclear area per cell for Myc-GFPhigh cells at 48 h was similar to that of control erythroblasts at 24 h in culture. The nuclear area per cell was significantly higher for Myc-GFPlow cells compared with control cells at 48 h in culture, and both values were significantly lower than those of erythroblasts in earlier stages of development harvested at 0 and 24 h in culture (Fig. 6B). These results demonstrate that ectopic Myc expression inhibits nuclear condensation in late stage erythroid cells and suggests that the observed block in enucleation due to continued Myc expression at physiological levels was a result of inhibition of nuclear condensation.
FIGURE 6.
Ectopic Myc expression inhibits nuclear condensation and histone deacetylation in late stage erythroid cells. Purified TER119-negative mouse fetal erythroblasts were infected with retroviruses encoding GFP only (MIG) or with retroviruses encoding both Myc and GFP at 0 h in culture and sorted based on GFP intensity into low (Myc-GFPlow) and high (Myc-GFPhigh) intensity populations at 24 h post infection followed by culture for another 24 h to allow for differentiation in vitro. Uninfected erythroblasts were harvested at 0 and 24 h in culture as earlier time point controls for comparison. A, cytospin preparations of the harvested cells were stained with benzidine and Giemsa. The arrowhead indicates an enucleated reticulocyte. Arrows indicate erythroblasts with condensed nuclei that are at an early stage of enucleation. B, shown is measurement of nuclear area to quantify the degree of nuclear condensation. The harvested cells were fixed with formaldehyde and incubated with Hoechst 33342 to stain the nucleus. Nuclear area per cell was determined for each sample using high content screening, as described under “Experimental Procedures.” Error bars represent S.D. across three biological replicates. The two-tailed t test are indicated by asterisks, p < 0.01. C, Western blot analysis of acetylation states of histones H3 and H4 was performed using the indicated acetylation-specific antibodies. Equal loading of samples was confirmed by stripping and reprobing the membranes with total histone H3 or H4 antibodies.
We, thus, investigated the molecular mechanism underlying the inhibition of nuclear condensation and enucleation by Myc. Pharmacological inhibition of histone deacetylases (HDACs) has been reported to block chromatin condensation and enucleation in erythroid cells, demonstrating that global histone deacetylation observed during late stage erythroid maturation is necessary for erythroid nuclear condensation and enucleation (43, 44). Recent studies have shown that Myc regulates global chromatin structure by influencing widespread histone modifications (45–47). We, thus, sought to determine whether Myc expression induces changes in global histone acetylation in erythroid cells, possibly leading to the observed block in nuclear condensation. Western blot analysis of histone acetylation was performed using antibodies detecting histones H3 and H4 acetylated at specific lysine residues (Fig. 6C). Global levels of acetylation of histone H3 at Lys-9 was maximal at 24 h in culture and decreased slightly at 48 h in culture. Ectopic Myc expression reversed the observed decrease in acetylation at H3K9 during late stage erythroid maturation. There was no change in levels of H3K18 and H3K23 acetylation during the course of erythroid maturation with or without ectopic Myc expression. Global levels of histone H4 acetylation at Lys-5, -8, -12, and -16 decreased at 48 h in culture concomitant with the nuclear condensation observed during late stage erythroid maturation, and this decrease did not occur after ectopic Myc expression at either physiological or supraphysiological levels. Hence, continued Myc expression even at a physiological level inhibited the global decrease in histone acetylation observed during late stage erythroid maturation, suggesting that histone deacetylation mediated by down-regulation of Myc during terminal erythroid differentiation regulates nuclear condensation and enucleation.
Because changes in global histone acetylation accompanied the block in erythroid nuclear condensation by Myc, we investigated the changes in expression of the major histone acetyltranferases (HATs) and HDACs during terminal erythroid maturation and upon ectopic Myc expression (Fig. 7). The expression of the HATs Elp3, Gcn5, Hat1, and Tip60 decreased during the course of erythroid maturation (Fig. 7A), suggesting that this decrease in expression of HATs may contribute to the decrease in global histone acetylation observed during late stages of erythroid maturation. The expression of the various HDACs was down-regulated in general during the course of erythroid maturation (Fig. 7B), except for HDAC5, which was up-regulated during late stages of erythroid maturation, as has been reported previously (43). Because ectopic Myc expression increased global histone acetylation, we analyzed the expression changes of the HATs and HDACs upon ectopic Myc expression to identify and select HATs that were significantly (at least 2-fold change relative to D2 MIG control) up-regulated or HDACs that were significantly down-regulated for further functional studies. Ectopic Myc expression led to a significant and dose-dependent increase in Gcn5 expression at 48 h relative to control (D2 MIG), whereas the increase in expression of the other HATs upon ectopic Myc expression was not significant (Fig. 7A), and none of the HDACs tested was significantly down-regulated upon ectopic Myc expression (Fig. 7B). Gcn5 has been reported to function downstream of Myc in the regulation of global chromatin structure (46). This prompted us to investigate the role of Gcn5 in terminal erythroid maturation and enucleation.
FIGURE 7.
Effect of ectopic Myc on the expression of HATs and HDACs. Cells were treated and harvested as described in Fig. 6, qRT-PCR analysis of the expression of the indicated HATs (A) and HDACs (B) was performed, and the relative expression levels normalized to D2 MIG control cells are shown. All error bars represent S.D. (n = 3). The two-tailed t test results are indicated by asterisks, p < 0.01.
Ectopic Gcn5 Expression Inhibits Late Stage Erythroid Nuclear Condensation and Enucleation
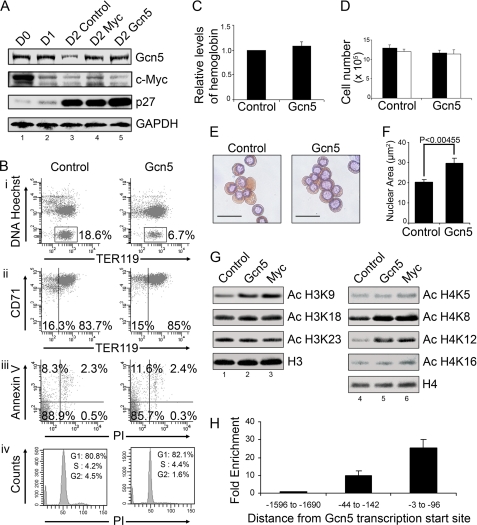
To directly investigate the role of Gcn5 in terminal erythroid maturation and enucleation, we infected TER119-negative mouse fetal erythroblasts with retroviruses encoding Gcn5. The levels of Gcn5, Myc, and p27 proteins in cells infected with retroviruses encoding Gcn5 were compared with the expression levels during normal erythroid maturation and also with Myc-expressing cells using Western blot analysis (Fig. 8A). Gcn5 protein levels normally decreased substantially during late stage erythroid maturation, coinciding with the onset of nuclear condensation, similar to the decrease in Myc protein levels at the same stage (Fig. 8A, lanes 1–3). Cells infected with retroviruses encoding Myc showed overexpression of Myc protein at levels within the physiological range (Fig. 8A, lanes 1–4) and also displayed significantly increased Gcn5 protein expression (Fig. 8A, lanes 3 and 4), consistent with the induction of Gcn5 transcripts by Myc (Fig. 7A). Cells infected with retroviruses encoding Gcn5 displayed elevated levels of Gcn5 protein expression relative to controls, but the levels of Myc and p27 were not affected (Fig. 8A, lanes 3 and 5). Ectopic Gcn5 expression inhibited erythroid enucleation significantly without affecting the differentiation, apoptotic status, or cell cycle arrest of the cultured erythroid cells (Fig. 8B). The hemoglobin content of Gcn5 expressing cells was similar to those of the controls (Fig. 8C), further confirming that the inhibition of enucleation was not due to a block in erythroid differentiation. Ectopic Gcn5 expression also did not affect the terminal proliferation rates of the differentiating erythroblasts (Fig. 8D). Benzidine-Giemsa staining of Gcn5-expressing cells at 48 h in culture indicated late stage erythroblasts with larger nuclei relative to controls, with no apparent difference in hemoglobin content (Fig. 8E). High content screening-based measurement of nuclear area confirmed that the average nuclear size of Gcn5-expressing erythroblasts was significantly higher than those of control cells at 48 h in culture (Fig. 8F). Furthermore, Western blot analysis of global acetylation states of histones H3 and H4 demonstrated that Gcn5 expression significantly reversed the histone deacetylation at H3K9, H4K8, and H4K12 normally observed during late stage erythroid maturation, similar to the effects of ectopic Myc expression (Fig. 8G). Gcn5, unlike Myc, did not affect the acetylation at H4K5 and H4K16 significantly. The acetylation states of H3K18 and H3K23, which did not change during the course of normal terminal erythroid maturation (Fig. 6C), remained unchanged upon Gcn5 expression (Fig. 8G). Because Gcn5 expression was induced by ectopic Myc expression in erythroid cells, and because both were down-regulated substantially and simultaneously in normal late stage erythroblasts (Fig. 8A), we reasoned that Gcn5 could be a direct transcriptional target of Myc in erythroid cells. Gcn5 has been reported to be a direct Myc target gene in human fibroblasts (46). Thus, ChIP assays were performed on purified TER119-negative mouse fetal erythroblasts using an antibody against Myc. The immunoprecipitated DNA was significantly enriched for Gcn5 promoter sequences relative to negative control regions chosen 1500 bp away from the Gcn5 transcriptional start site (Fig. 8H), indicating that Gcn5 is transcriptionally regulated by Myc in erythroid cells. Taken together, these results suggest that Gcn5 functions downstream of Myc in regulating histone deacetylation, nuclear condensation, and enucleation in erythroid cells.
FIGURE 8.
Ectopic Gcn5 expression inhibits late stage erythroid nuclear condensation and enucleation. A, TER119-negative mouse fetal erythroblasts were cultured for 0 h (D0) and 24 h (D1) or were cultured for 48 h after infection with bicistronic retroviruses encoding GFP and Myc (D2 Myc), GFP, and Gcn5 (D2 Gcn5) or GFP alone (D2 control). Total protein lysates from the cells were subjected to Western blot analysis using the indicated antibodies. B, TER119-negative mouse fetal erythroblasts were infected with bicistronic retroviruses encoding Gcn5 and GFP (Gcn5) or GFP alone (Control). The cells were analyzed by flow cytometry after 48 h in culture to quantify enucleation (i), differentiation (ii), and apoptosis (iii). The cells were fixed in ethanol, and cell cycle analysis was performed by flow cytometry (iv). The percentage of cells in G1, S, and G2 phase of cell cycle are shown. C, relative hemoglobin content was quantified by lysing cells in Drabkin's reagent followed by measurement of absorbance at 540 nm. D, cell counts were measured at 48 h in culture for the same cells as in panel B, with an equal starting cell number (1 × 105) at 0 h. The total cell numbers are represented as black bars. The number was normalized to account for enucleation and is shown as white bars. E, benzidine-Giemsa staining images of cells harvested at 48 h in culture are shown. Scale bars represent 20 μm. F, cells were harvested at 48 h in culture, fixed, and stained with Hoechst 33342, and nuclear area per cell was measured by high content screening. G, TER119-negative fetal erythroblasts were cultured for 48 h after infection with retroviral vectors encoding GFP and Myc, GFP and Gcn5, or GFP alone (Control), and Western blot analysis of acetylation states of histones H3 and H4 was performed using the indicated antibodies. H, a ChIP assay shows Myc binding to the Gcn5 promoter. ChIP was performed on purified TER119-negative mouse fetal liver erythroblasts using an antibody against c-Myc. PCR primers were designed for prospective Myc binding regions in the Gcn5 promoter at −3 to −96 and −44 to −142 bp upstream of the Gcn5 transcriptional start site. The enrichment for Gcn5 promoter sequences in the immunoprecipitated DNA was quantified using qRT-PCR, relative to a negative control region at −1596 to −1690 bp upstream of the Gcn5 transcriptional start site. All error bars represent S.D. (n = 3).
DISCUSSION
The present study uncovers the significance of changes in Myc expression in controlling mammalian terminal erythroid differentiation. Myc is normally down-regulated beginning at the CFU-E stage. We show that overexpression of Myc at levels above that of these early erythroblasts promotes proliferation at the expense of differentiation and is accompanied by induction of apoptosis. In contrast, continued Myc expression at physiological levels blocks enucleation specifically and inhibits histone deacetylation and nuclear condensation in late stage erythroid cells. Our results support a model whereby down-regulation of Myc from the high levels seen in early erythroblasts is essential for the progression of normal terminal erythroid differentiation.
Upon erythropoietin induction of terminal differentiation, the mouse erythroblasts undergo about 4–5 terminal cell divisions in 48 h followed by a G1-phase cell cycle arrest. The mechanism of regulation of these rapid terminal cell divisions followed by a G1 arrest is not well understood. Up-regulation of p27 during late stage erythroid differentiation has been implicated in regulating the withdrawal of erythroid cells from cell cycle (29). However, we demonstrated using p27 knock-out erythroblasts that terminal erythroid differentiation is unaffected by the absence of p27, suggesting that p27 alone is not sufficient to regulate the late stage G1 arrest in erythroid cells. We provide evidence suggesting that Myc plays a critical role in controlling the number of terminal cell divisions and the withdrawal from the cell cycle. shRNA-mediated depletion of Myc in cultured erythroblasts significantly inhibited terminal cell proliferation without affecting terminal differentiation, indicating that a precisely regulated decrease in Myc levels during late stage erythroid maturation may control the exact number of terminal cell divisions before the cell cycle arrest. Myc levels were normally dramatically reduced during late stages of erythroid maturation, coinciding with the arrest in G1-phase of the cell cycle. Ectopic Myc expression at levels above that of early erythroblasts promotes their proliferation in culture for much longer times in an undifferentiated state. This suggests that down-regulation of Myc levels below a critical threshold might be necessary for commitment toward both terminal erythroid maturation and the withdrawal from the cell cycle.
Because of its well established roles in driving cell proliferation, repression of Myc during late stage erythroid maturation has been suggested to be involved mainly in the withdrawal from cell cycle (20). Our data demonstrates that normal down-regulation of Myc in late stage erythroid cells is essential for nuclear condensation and enucleation, revealing a novel role for Myc in mammalian erythropoiesis. Myc is down-regulated during the late stages of erythroid maturation, concomitant with the global histone deacetylation that is critical for erythroid nuclear condensation and enucleation (43, 44). Ectopic Myc expression at physiological levels that did not induce cell cycle re-entry was sufficient to block the global histone deacetylation and inhibit nuclear condensation and enucleation. These findings strongly support the emerging notion that Myc regulates global chromatin structure by influencing genome-wide histone modifications (45–47). We showed that the HAT Gcn5 was down-regulated during normal terminal erythroid maturation and was induced upon ectopic Myc expression and demonstrated its important role in the regulation of the balance between global histone acetylation and deacetylation and nuclear condensation in late stage erythroblasts. This function of Gcn5 is consistent with previous studies in yeast showing that Gcn5 was required either directly or indirectly for the acetylation of several sites in histones H3 and H4 (48). The inhibition of enucleation by Gcn5 expression was not as complete as that of Myc, suggesting that there are additional mechanisms other than induction of Gcn5 by which Myc can regulate erythroid chromatin condensation and enucleation. We previously reported that Rac GTPases regulate the cytoskeletal reorganization leading to the extrusion of the condensed nucleus from late stage erythroblasts (8). Ectopic Myc expression led to a modest, dose-dependent decrease in the expression of Rac2, suggesting that this may additionally contribute to the block in enucleation by Myc.
Epigenetic regulation of nuclear condensation can explain the selective transcription of certain critical genes such as Bcl-XL (49) during late stage erythroid maturation when DNA replication and RNA transcription in the condensed nucleus are largely shut down. It also allows for a rapid condensation and general inactivation of the erythroid nucleus in a manner similar to apoptotic nuclear condensation while also leaving the general nuclear architecture and chromosomal DNA intact (50). Although our results are strongly consistent with epigenetic regulation of erythroid chromatin condensation (43), they do not exclude the possibility that other genes activated by repression of Myc during late stage erythroid maturation may also act as important regulators of erythroid nuclear condensation and/or enucleation.
In many cell types ectopic Myc expression promotes proliferation and blocks terminal differentiation, which are essential outcomes leading to tumor formation (51). Myc also induces apoptosis through the Arf-Mdm2-p53 tumor suppressor pathway (52, 53), and additional mutations that overcome the induction of apoptosis by Myc lead to tumorigenesis. We demonstrate here that the dosage of Myc is critical in determining the phenotypic outcome of ectopic Myc expression on differentiation, proliferation, and survival of erythroid cells. Continued Myc expression at physiological levels did not elicit an apoptotic response in cultured erythroid cells and broadly allowed for terminal maturation and exit from the cell cycle, with mainly nuclear condensation and enucleation being affected. High levels of ectopic Myc induced several proliferation-promoting genes and repressed the expression of several genes in the erythropoietin signaling pathway, leading to continued proliferation at the expense of differentiation. Supraphysiological levels of Myc expression also induced significant levels of apoptosis. This supports the theory that the apoptotic response is a safeguard against the abnormal proliferation mediated by oncogene activation as in the case of high level ectopic Myc overexpression and that low levels of ectopic Myc that do not induce cell cycle re-entry do not evoke the apoptotic response either. An elegant study using an in vivo model of Myc-induced tumorigenesis previously showed that activation of the apoptotic pathways required high level Myc overexpression, and low level deregulated Myc was potentially oncogenic in several but not all tissue types by avoiding the engagement of tumor suppression pathways (54). In our study using primary erythroid cells cultured in vitro, low-dose Myc expression did not induce cell cycle re-entry or a complete block in differentiation. Hence, expression levels of Myc protein together with other factors such as cell type (26) and developmental stage and context (55) control the phenotypic outcome of ectopic or deregulated Myc expression. A detailed understanding of the functioning of all these factors in combination would lead to better therapeutic intervention in cancers that involve deregulated Myc expression.
Supplementary Material
Acknowledgments
We thank all our colleagues in Biopolis and the Whitehead Institute for valuable discussions and the staff at Biopolis Shared Facilities for providing the flow cytometry and High Content Screening services and advice. We are thankful to Dr. Motomi Osato and Dr. Bindya Jacob for help with mice bone marrow transplants.
This work was supported, in whole or in part, by National Institutes of Health Grants DK047636 and AI54973 (to B. L.) and P01 HL32262 (to H. F. L.). This study was also supported by Singapore-MIT alliance Grant C-382-641-001-091 (to B. L. and H. F. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
- CFU-E
- colony forming units-erythroid
- MSCV
- murine stem cell retroviral vector
- qRT
- quantitative real-time
- HDAC
- histone deacetylase
- HAT
- histone acetyltransferase
- MIG
- MSCV-IRES-GFP retroviral vector.
REFERENCES
- 1.Koury M. J., Bondurant M. C. (1988) J. Cell. Physiol. 137, 65–74 [DOI] [PubMed] [Google Scholar]
- 2.Richmond T. D., Chohan M., Barber D. L. (2005) Trends Cell Biol. 15, 146–155 [DOI] [PubMed] [Google Scholar]
- 3.Eshghi S., Vogelezang M. G., Hynes R. O., Griffith L. G., Lodish H. F. (2007) J. Cell Biol. 177, 871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koury S. T., Koury M. J., Bondurant M. C. (1988) Exp. Hematol. 16, 758–763 [PubMed] [Google Scholar]
- 5.Skutelsky E., Danon D. (1967) J. Cell Biol. 33, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson C. F., Kling J. M. (1967) J. Cell Biol. 35, 237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Repasky E. A., Eckert B. S. (1981) Prog. Clin. Biol. Res. 55, 679–692 [PubMed] [Google Scholar]
- 8.Ji P., Jayapal S. R., Lodish H. F. (2008) Nat. Cell Biol. 10, 314–321 [DOI] [PubMed] [Google Scholar]
- 9.Vennstrom B., Sheiness D., Zabielski J., Bishop J. M. (1982) J. Virol. 42, 773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oster S. K., Ho C. S., Soucie E. L., Penn L. Z. (2002) Adv. Cancer Res. 84, 81–154 [DOI] [PubMed] [Google Scholar]
- 11.Meyer N., Penn L. Z. (2008) Nat. Rev. Cancer 8, 976–990 [DOI] [PubMed] [Google Scholar]
- 12.Dang C. V., O'Donnell K. A., Zeller K. I., Nguyen T., Osthus R. C., Li F. (2006) Semin. Cancer Biol. 16, 253–264 [DOI] [PubMed] [Google Scholar]
- 13.Hoffman B., Liebermann D. A. (2008) Oncogene 27, 6462–6472 [DOI] [PubMed] [Google Scholar]
- 14.Iritani B. M., Eisenman R. N. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman B., Amanullah A., Shafarenko M., Liebermann D. A. (2002) Oncogene 21, 3414–3421 [DOI] [PubMed] [Google Scholar]
- 16.Dubois N. C., Adolphe C., Ehninger A., Wang R. A., Robertson E. J., Trumpp A. (2008) Development 135, 2455–2465 [DOI] [PubMed] [Google Scholar]
- 17.Wilson A., Murphy M. J., Oskarsson T., Kaloulis K., Bettess M. D., Oser G. M., Pasche A. C., Knabenhans C., Macdonald H. R., Trumpp A. (2004) Genes Dev. 18, 2747–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spangler R., Sytkowski A. J. (1992) Blood 79, 52–57 [PubMed] [Google Scholar]
- 19.Spotts G. D., Hann S. R. (1990) Mol. Cell. Biol. 10, 3952–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rylski M., Welch J. J., Chen Y. Y., Letting D. L., Diehl J. A., Chodosh L. A., Blobel G. A., Weiss M. J. (2003) Mol. Cell. Biol. 23, 5031–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta J. C., Ferrándiz N., Bretones G., Torrano V., Blanco R., Richard C., O'Connell B., Sedivy J., Delgado M. D., León J. (2008) Mol. Cell. Biol. 28, 7286–7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cory S., Maekawa T., McNeall J., Metcalf D. (1991) Cell Growth Differ. 2, 165–172 [PubMed] [Google Scholar]
- 23.Zhang J., Socolovsky M., Gross A. W., Lodish H. F. (2003) Blood 102, 3938–3946 [DOI] [PubMed] [Google Scholar]
- 24.Kiyokawa H., Kineman R. D., Manova-Todorova K. O., Soares V. C., Hoffman E. S., Ono M., Khanam D., Hayday A. C., Frohman L. A., Koff A. (1996) Cell 85, 721–732 [DOI] [PubMed] [Google Scholar]
- 25.Aleem E., Kiyokawa H., Kaldis P. (2005) Nat. Cell Biol. 7, 831–836 [DOI] [PubMed] [Google Scholar]
- 26.Luo H., Li Q., O'Neal J., Kreisel F., Le Beau M. M., Tomasson M. H. (2005) Blood 106, 2452–2461 [DOI] [PubMed] [Google Scholar]
- 27.Xu W., Edmondson D. G., Roth S. Y. (1998) Mol. Cell. Biol. 18, 5659–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W., Shen J., Wu M., Arsura M., FitzGerald M., Suldan Z., Kim D. W., Hofmann C. S., Pianetti S., Romieu-Mourez R., Freedman L. P., Sonenshein G. E. (2001) Oncogene 20, 1688–1702 [DOI] [PubMed] [Google Scholar]
- 29.Hsieh F. F., Barnett L. A., Green W. F., Freedman K., Matushansky I., Skoultchi A. I., Kelley L. L. (2000) Blood 96, 2746–2754 [PubMed] [Google Scholar]
- 30.Liu X., Constantinescu S. N., Sun Y., Bogan J. S., Hirsch D., Weinberg R. A., Lodish H. F. (2000) Anal. Biochem. 280, 20–28 [DOI] [PubMed] [Google Scholar]
- 31.Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D. Y., Nakayama K. (1996) Cell 85, 707–720 [DOI] [PubMed] [Google Scholar]
- 32.Fero M. L., Rivkin M., Tasch M., Porter P., Carow C. E., Firpo E., Polyak K., Tsai L. H., Broudy V., Perlmutter R. M., Kaushansky K., Roberts J. M. (1996) Cell 85, 733–744 [DOI] [PubMed] [Google Scholar]
- 33.Leng L., Yu F., Dong L., Busquets X., Osada S., Richon V. M., Marks P. A., Rifkind R. A. (1993) Cancer Res. 53, 5554–5558 [PubMed] [Google Scholar]
- 34.Neubauer H., Cumano A., Müller M., Wu H., Huffstadt U., Pfeffer K. (1998) Cell 93, 397–409 [DOI] [PubMed] [Google Scholar]
- 35.Ghaffari S., Kitidis C., Zhao W., Marinkovic D., Fleming M. D., Luo B., Marszalek J., Lodish H. F. (2006) Blood 107, 1888–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W., Kitidis C., Fleming M. D., Lodish H. F., Ghaffari S. (2006) Blood 107, 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Socolovsky M., Fallon A. E., Wang S., Brugnara C., Lodish H. F. (1999) Cell 98, 181–191 [DOI] [PubMed] [Google Scholar]
- 38.Dolznig H., Habermann B., Stangl K., Deiner E. M., Moriggl R., Beug H., Müllner E. W. (2002) Curr. Biol. 12, 1076–1085 [DOI] [PubMed] [Google Scholar]
- 39.Fang J., Menon M., Kapelle W., Bogacheva O., Bogachev O., Houde E., Browne S., Sathyanarayana P., Wojchowski D. M. (2007) Blood 110, 2361–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koff A., Giordano A., Desai D., Yamashita K., Harper J. W., Elledge S., Nishimoto T., Morgan D. O., Franza B. R., Roberts J. M. (1992) Science 257, 1689–1694 [DOI] [PubMed] [Google Scholar]
- 41.Ray D., Kiyokawa H. (2007) Cell Cycle 6, 3039–3042 [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Lu Y., Zhou H., Lee M., Liu Z., Hassel B. A., Hamburger A. W. (2008) BMC Cell Biol. 9, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popova E. Y., Krauss S. W., Short S. A., Lee G., Villalobos J., Etzell J., Koury M. J., Ney P. A., Chasis J. A., Grigoryev S. A. (2009) Chromosome Res 17, 47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji P., Yeh V., Ramirez T., Murata-Hori M., Lodish H. F.Haematologica (2010) doi: 10.3324/haematol.2010.029827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knoepfler P. S. (2007) Cancer Res. 67, 5061–5063 [DOI] [PubMed] [Google Scholar]
- 46.Knoepfler P. S., Zhang X. Y., Cheng P. F., Gafken P. R., McMahon S. B., Eisenman R. N. (2006) EMBO J. 25, 2723–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guccione E., Martinato F., Finocchiaro G., Luzi L., Tizzoni L., Dall' Olio V., Zardo G., Nervi C., Bernard L., Amati B. (2006) Nat. Cell Biol. 8, 764–770 [DOI] [PubMed] [Google Scholar]
- 48.Zhang W., Bone J. R., Edmondson D. G., Turner B. M., Roth S. Y. (1998) EMBO J. 17, 3155–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregoli P. A., Bondurant M. C. (1997) Blood 90, 630–640 [PubMed] [Google Scholar]
- 50.Krauss S. W., Lo A. J., Short S. A., Koury M. J., Mohandas N., Chasis J. A. (2005) Blood 106, 2200–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janz S. (2005) Oncogene 24, 3541–3543 [DOI] [PubMed] [Google Scholar]
- 52.Hermeking H., Eick D. (1994) Science 265, 2091–2093 [DOI] [PubMed] [Google Scholar]
- 53.Eischen C. M., Weber J. D., Roussel M. F., Sherr C. J., Cleveland J. L. (1999) Genes Dev. 13, 2658–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy D. J., Junttila M. R., Pouyet L., Karnezis A., Shchors K., Bui D. A., Brown-Swigart L., Johnson L., Evan G. I. (2008) Cancer Cell 14, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beer S., Zetterberg A., Ihrie R. A., McTaggart R. A., Yang Q., Bradon N., Arvanitis C., Attardi L. D., Feng S., Ruebner B., Cardiff R. D., Felsher D. W. (2004) PLoS Biol. 2, e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.