Abstract
IFNα exerts potent inhibitory activities against malignant melanoma cells in vitro and in vivo, but the mechanisms by which it generates its antitumor effects remain unknown. We examined the effects of interferon α (IFNα) on the expression of human members of the Schlafen (SLFN) family of genes, a group of cell cycle regulators that mediate growth-inhibitory responses. Using quantitative RT-real time PCR, we found detectable basal expression of all the different human SLFN genes examined (SLFN5, SLFN11, SLFN12, SLFN13, and SLFN14), in malignant melanoma cells and primary normal human melanocytes, but SLFN5 basal expression was suppressed in all analyzed melanoma cell lines. Treatment of melanoma cells with IFNα resulted in induction of expression of SLFN5 in malignant cells, suggesting a potential involvement of this gene in the antitumor effects of IFNα. Importantly, stable knockdown of SLFN5 in malignant melanoma cells resulted in increased anchorage-independent growth, as evidenced by enhanced colony formation in soft agar assays. Moreover, SLFN5 knockdown also resulted in increased invasion in three-dimensional collagen, suggesting a dual role for SLFN5 in the regulation of invasion and anchorage-independent growth of melanoma cells. Altogether, our findings suggest an important role for the SLFN family of proteins in the generation of the anti-melanoma effects of IFNα and for the first time directly implicate a member of the human SLFN family in the regulation of cell invasion.
Keywords: Antiviral Agents, Interferon, JAK Kinase, Signal Transduction, Tumor Suppressor, Melanoma
Introduction
The interferons (IFNs) are cytokines with important pleiotropic biological effects, including generation of antitumor responses and antiviral activities (1–3). The ability of IFNs to induce antitumor responses in selective systems is highly relevant and, over the years, has had a major impact in the management of certain leukemias and solid tumors in humans. Malignant melanoma is one of the most IFN-sensitive solid tumors. There has been extensive clinical evidence on the ability of IFNα to generate antitumor effects in vitro in subset groups of patients with advanced metastatic malignant melanoma (4–7), and IFNα is now a Food and Drug Administration-approved agent for the treatment of this malignancy. An important outstanding issue in the IFN research field has been the identification of specific mechanisms that account for differential sensitivity to the effects of IFNs. Despite the advances in the IFN-signaling field over the last 2 decades, the precise mechanisms and specific signals that account for the unique IFN sensitivity that some tumors exhibit remain largely unknown.
It is now well established that IFNs regulate transcription of target genes with important functional relevance via engagement of the JAK-STAT pathway (8–11). In recent years, additional levels of cellular regulation of IFN-inducible genes and their products have been identified, such as involvement of members of the PKC family (12–16), the MAPK cascades (17–22), translational regulation via mammalian target of rapamycin and 4EBP1 (23–28), modulation of histone acetylation (29–33), and ISGylation (34–36). Although the identification of these pathways has established critical information on the mechanisms by which IFNs control transcription of distinct target genes and regulation of mRNA translation, the identity of specific genes that may account for the induction of the antiproliferative and antitumor effects of IFNs remains to be established. We have previously shown that IFNs induce mouse SLFN genes in murine hematopoietic progenitors and fibroblasts in a STAT1- and/or p38 MAPK-dependent manner and that the SLFN2 gene, which is mouse IFNα-inducible, mediates growth-inhibitory effects and anchorage-independent growth of NIH3T3 and L929 fibroblasts (37), suggesting that this SLFN protein may be a mediator of the generation of antineoplastic responses by type I IFNs in the mouse system.
In this study, we examined the patterns of expression of human SLFN proteins in human malignant melanoma cells and the effects of type I IFN treatment on their expression. Basal expression and induction of human SLFN genes in response to IFNα was analyzed in normal human primary melanocytes and IFN-sensitive or IFN-resistant malignant melanoma cells. Our results demonstrate that SLFN5, and to a lesser degree SLFN11 and SLFN12, are induced by IFNα in normal melanocytes, although in IFN-sensitive melanoma cells (SKMEL2, SKMEL5, and SKMEL28) only SLFN5 is induced. On the contrary, none of the human SLFN genes is induced in interferon-resistant melanoma D10 cells. Importantly, basal mRNA expression of SLFN5 is consistently suppressed in all melanoma cell lines tested when compared with normal human primary melanocytes, suggesting a selection against SLFN5 in malignant melanoma cells. Knockdown of SLFN5 increases the ability of individual SKMEL28 melanoma cells to invade through three-dimensional collagen and enhances anchorage-independent growth, suggesting a mechanism by which regulation of SLFN5 by human IFNα may mediate induction of the antitumor effects of IFNα in malignant melanoma.
MATERIALS AND METHODS
Cells Lines, Reagents, and Antibodies
SKMEL2, SKMEL5 and SKMEL28 cells were grown in ATTC Eagle's minimum essential medium, supplemented with 10% fetal calf serum and antibiotics. D10 cells were grown in Roswell Park Memorial Institute medium 1640 (RPMI 1640), supplemented with 10% fetal calf serum and antibiotics. Normal human epidermal melanocytes (HEMa-LP) were obtained from Invitrogen Cascade Biologics and were grown in medium 254 supplemented with human melanocyte growth supplement-2 and antibiotics, according to the manufacturer's instructions. Recombinant human IFNα was obtained from Hoffmann-La Roche. Recombinant human IFNβ was obtained from Biogen Idec. The antibody against SLFN5 was purchased from Sigma Atlas antibodies. U1A and U4A cells were kindly provided by Dr. George Stark (Cleveland Clinic Research Foundation, Cleveland, OH). The antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from Millipore. The antibody against lamin A was purchased from Santa Cruz Biotechnology, and the antibody against tubulin was purchased from Abcam.
Cell Lysis and Immunoblotting, Isolation of Nuclear and Cytosolic Fractions
Cell lysis in phosphorylation lysis buffer and immunoblotting using an ECL kit for protein detection were performed as described previously (21, 38). For the detection of SLFN5 protein expression in response to IFNα or IFNβ treatment, cells were treated with 2 × 103 IU/ml for 24 or 48 h, as indicated. Nuclear and cytosolic fractions were isolated using the NE-PER kit from Pierce Thermo Fisher Scientific, according to the manufacturer's instructions. For the detection of short-timed SLFN5 redistribution within the cells following IFNα treatment, melanoma cells were either left untreated or were treated with 1 × 104 IU/ml of IFNα for the indicated times.
Cell Proliferation Assays
Cell proliferation assays using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method were performed as described previously (39, 40).
Soft Agar Assays
Anchorage-independent cell growth was analyzed in soft agar assays, as described previously (37, 41). For these studies, cells were plated in the absence or in the presence of 1–2 × 103 IU/ml IFNα, as indicated, and colony formation was assessed after 7 days of culture.
Three-dimensional Collagen Invasion Assay
Invasion in three-dimensional collagen was analyzed in a radial invasion assay, essentially as described previously (42). Briefly, 100,000 cells were suspended in 2.2% w/v collagen and allowed to form a cylinder-shaped plug. The plug was then inserted in an additional layer of collagen to allow formation of a core with a clean border between cells and the surrounding collagen. Wells were overlaid with media, and the distance of invading cells leaving the core was determined at the indicated times, using the Carl-Zeiss AxioVision software tool.
Immunofluorescence Detection of SLFN5 Subcellular Localization
Cells were grown onto glass coverslips in the presence or absence of IFNα. For SLFN5 detection, cells were treated for 48 h with 2 × 103 IU/ml IFNα, fixed with formaldehyde, permeabilized, and incubated with an antibody against SLFN5 followed by Alexa Fluor 488 secondary antibody. Additional staining was performed with DAPI (nuclei) and phalloidin (actin). Cells were examined with a fluorescence microscope.
RNA Isolation and Real Time PCR Probes and Primers
Cells were either treated with 5 × 103 IU/ml IFNα for the indicated times, as indicated. Isolation of RNA and conversion into cDNA was performed using the respective kits from Qiagen, according to the manufacturer's instructions. Validated, inventoried probes and primers for real time PCR and TaqMan PCR master mix were purchased from Applied Biosystems and used according to the manufacturer's instructions. The probes and primers used were as follows: SLFN5, Hs00376501_m1; SLFN11, Hs00430118_m1; SLFN12, Hs00430118_m1; SLFN13, Hs0043118 7_m1; and SLFN14, Hs03967006_cn. GAPDH (Hs99999905_m1) was used as an internal control. For the comparison of basal SLFN expression in various cells, a validated universal human reference RNA from Agilent Technologies-Stratagene was used.
Generation of Stable SLFN5 Knockdown Cells
Generation of stable SLFN5 knockdown SKMEL28 cells was performed using standard approaches (37). Briefly, two SLFN5 silencer select siRNA and control-scrambled sequences were used as templates in the shRNA sequence designer tool for pSIREN vectors (Clontech). Plasmids were digested with restriction enzymes to verify the presence of the siRNA-encoding insert and then used for retroviral infection of SKMEL28 cells. Cells harboring pSIREN SLFN5-siRNA or pSIREN control-siRNA were green fluorescent and were selected by flow cytometry.
RESULTS
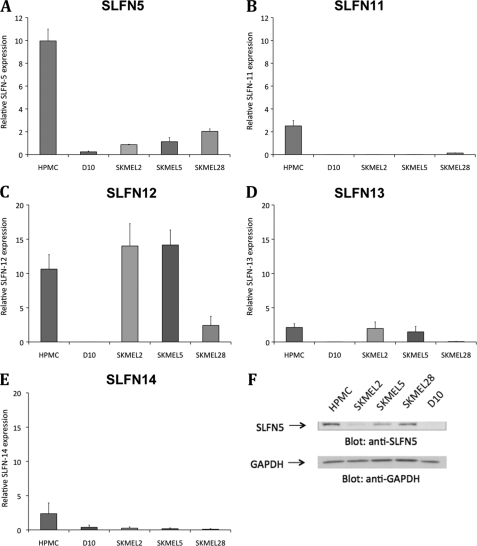
We sought to determine the patterns of expression of different human SLFN mRNAs in normal human melanocytes and malignant melanoma cells and the effects of IFNα on such expression. In initial experiments, the expression of different SLFN genes in the IFN-responsive SKMEL2, SKMEL5, and SKMEL28 and the IFN-resistant D10 melanoma cell lines, as well as normal human primary melanocytes, was examined. Basal expression levels of SLFN5 (Fig. 1A), SLFN11 (Fig. 1B), SLFN12 (Fig. 1C), SLFN13 (Fig. 1D), and SLFN14 (Fig. 1E) were first assessed. As shown in Fig. 1A, SLFN5 was expressed at a higher level of about 10-fold over reference in human primary melanocytes, compared with a 1–2-fold expression over reference in the various melanoma lines. The expression of all SLFN genes was generally at the lowest level in D10 cells (Fig. 1, A–E), although SLFN12 and SLFN13 were expressed at similar levels in both primary melanocytes and in the melanoma cell lines SKMEL2 and SKMEL5 (Fig. 1, C and D). Altogether, these results established that SLFN5 expression is suppressed in the different melanoma cell lines, as compared with normal human melanocytes. Consistent with these findings, immunoblotting using an anti-SLFN5 antibody demonstrated clearly lower levels of expression of SLFN5 protein in the malignant melanoma cell lines as compared with primary human melanocytes (Fig. 1F).
FIGURE 1.
Relative expression of human SLFN genes in primary melanocytes and melanoma cell lines. A–E, RNA from human primary melanocytes (HPMC) and the malignant melanoma cell lines D10, SKMEL2, SKMEL5, and SKMEL28 was isolated, and the expression of SLFN5 (A), SLFN11 (B), SLFN12 (C), SLFN13 (D), and SLFN 14 (E) over GAPDH was analyzed via RT-real time PCR using specific primers and GAPDH as an internal control. To be able to compare the relative expression of SLFN genes in different cells, a universal human Stratagene reference RNA was used, which was also normalized to GAPDH in the samples. Means ± S.E. of three independent experiments are shown. F, lysates from human primary melanocytes or the indicated cell lines were separated by SDS-PAGE and immunoblotted with antibodies against SLFN5 or GAPDH, as indicated.
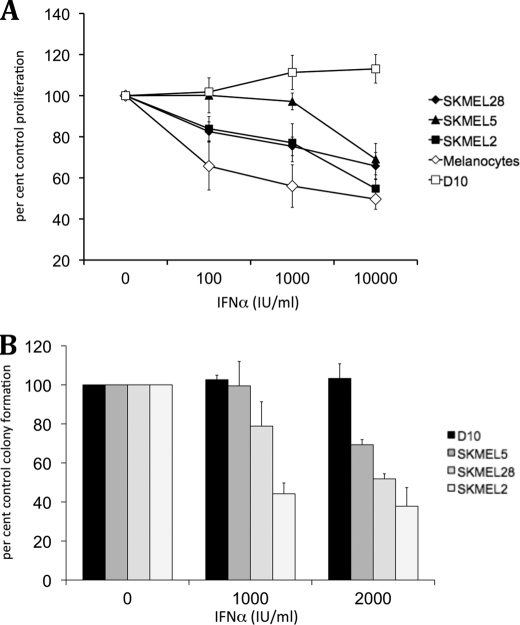
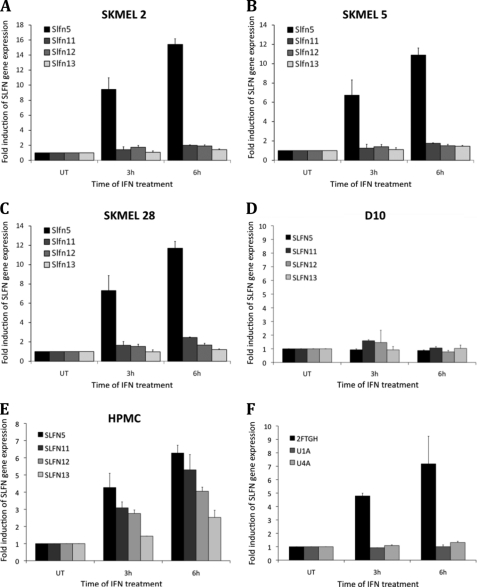
Previous work has established that the melanoma cell lines SKMEL2, SKMEL5, and SKMEL28 show variable responsiveness to IFNα (43–45), although D10 melanoma cells are IFN-resistant (46). We obtained similar findings in experiments in which the effects of IFNα on cell proliferation were examined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays (Fig. 2A), as well as in soft agar assays examining the effects of IFNα on anchorage-independent growth of malignant melanoma cells (Fig. 2B). To determine whether IFNα-inducible SLFN gene expression correlates with sensitivity to the inhibitory effects of IFNα, SKMEL2, SKMEL5, SKMEL28, D10, and primary melanocytes were treated with IFNα, and induction of SLFN5, SLFN11, SLFN12, and SLFN13 mRNA expression was determined using quantitative real time RT-PCR. IFNα treatment only resulted in induction of SLFN5 in SKMEL2 (Fig. 3A), SKMEL5 (Fig. 3B), and SKMEL28 (Fig. 3C) cells, although none of the other SLFN genes was significantly inducible during the time points tested. No IFN-dependent induction of SLFN5 was observed in the resistant D10 cells (Fig. 3D), although all analyzed SLFN genes were inducible by IFNα treatment of normal melanocytes (Fig. 3E). SLFN5 induction was most prominent at about 6-fold over base line after 6 h of treatment, followed by SLFN11 at about 5-fold over base line, SLFN12 at 4-fold over base line, and SLFN13 with a modest but consistent 2.5-fold induction (Fig. 3E). To determine whether IFN-inducible SLFN5 expression required upstream engagement of type I IFN-associated JAK kinases, we utilized Tyk2-deficient U1A cells, JAK1-deficient U4A cells, and parental 2FTGH fibrosarcoma cells that express both type I IFNR-associated JAK kinases (47). Cells were treated with IFNα or were left untreated, and SLFN5 expression was analyzed by quantitative real time RT-PCR. As shown in Fig. 3F, SLFN5 was clearly inducible in 2FTGH cells after 6 h of IFNα treatment, although no induction was visible in either U1A or U4A cells, indicating a requirement for JAK kinases in the process.
FIGURE 2.
Effects of IFNα on proliferation and anchorage-independent growth of malignant melanoma cells. A, normal melanocytes, SKMEL2, SKMEL5, SKMEL28, and D10 melanoma cells were incubated with the indicated concentrations (IU/ml) of IFNα, and cellular proliferation was assessed after 5 days using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays. Data shown represent means ± S.E. of three independent experiments. B, SKMEL2, SKMEL28, SKMEL5, and D10 cells were plated in soft agar in the presence or absence of the indicated concentrations (IU/ml) of IFNα. Colony formation was scored after 7 days. Data are expressed as percent control colony formation for untreated cells. Means ± S.E. of three independent experiments are shown.
FIGURE 3.
IFNα-inducible human SLFN gene expression. A–E, melanoma cell lines SKMEL2 (A), SKMEL5 (B), SKMEL28 (C), D10 (D), and normal human primary melanocytes (HPMC) (E) were treated with IFNα for 3 or 6 h, as indicated. RNA was isolated, and induction of SLFN5, SLFN11, SLFN12, and SLFN13 mRNA gene expression was analyzed by quantitative RT-real time PCR. Data are expressed as fold increase over untreated (UT) controls. F, U1A, U4A, or parental 2FTGH cells were treated with IFNα, and SLFN5 mRNA gene expression was analyzed, using GAPDH as an internal control. Data shown represent means ± S.E. of three independent experiments for A–C and two independent experiments for D, E, and F.
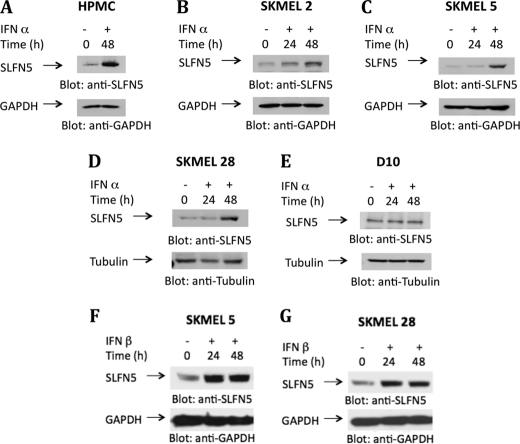
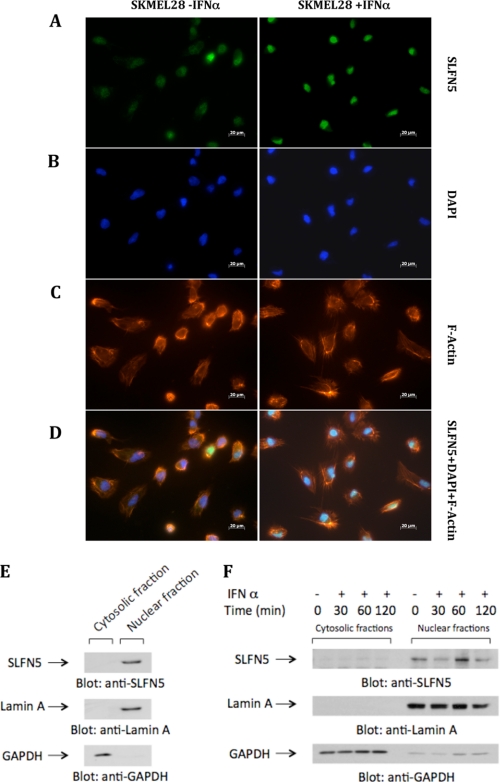
In further studies, we used a specific antibody against SLFN5, to determine IFNα-dependent induction SLFN5 protein expression in primary melanocytes and various IFN-sensitive and -resistant malignant melanoma cell lines. The levels of SLFN5 protein increased after IFNα treatment in primary melanocytes (Fig. 4A) and the IFN-responsive SKMEL2 (Fig. 4B), SKMEL5 (Fig. 4C), and SKMEL28 (Fig. 4D) lines, although there was no induction in IFN-resistant D10 cells (Fig. 4E). Similarly, treatment with IFNβ also resulted in up-regulation of SLFN5 protein expression in the IFN-responsive SKMEL5 or SKMEL28 cell lines (Fig. 4, F and G). We then performed studies to determine the subcellular localization of IFN-inducible SLFN5. In a previous study in which the subcellular localization of murine HA-tagged SLFN proteins was analyzed, groups I and II SLFNs were found to be localized in the cytosol, although all group III SLFNs were localized in the nucleus (48). To determine whether a similar pattern of localization of human SLFN5 occurs, we performed studies using melanoma cells and primary melanocytes. We examined the subcellular localization of SLFN5 protein in SKMEL28 cells by immunofluorescence of whole cells (Fig. 5, A–D). SKMEL28 cells were incubated in the presence or absence of IFNα, and after 48 h of treatment, SLFN5 protein abundance and localization were detected by immunofluorescence. As shown in Fig. 5A, SLFN5 expression was clearly enhanced in IFNα-treated SKMEL28 cells (right panel) compared with untreated cells (left panel), and there were IFN-inducible expression and nuclear localization of the SLFN5 protein (Fig. 5, B–D). In other studies, nuclear and cytosolic fractions were isolated from primary melanocytes or SKMEL5 cells, and equal amounts of protein were analyzed for the presence of SLFN5. As shown in Fig. 5E, SLFN5 was clearly seen in the nucleus of primary melanocytes (Fig. 5E). It was also seen in nuclear fractions of SKMEL5 cells that were either not treated or treated with IFNα for short times (Fig. 5F).
FIGURE 4.
Type I IFNα-inducible human SLFN protein expression. Human primary melanocytes (HPMC) (A) and the melanoma cell lines SKMEL2 (B), SKMEL5 (C and F), SKMEL28 (D and G), and D10 (E) were treated with IFNα or IFNβ or were left untreated, as indicated. Cells were lysed, proteins resolved by SDS-PAGE, and immunoblotted with an antibody against SLFN5. The same blots were re-probed with anti-GAPDH or anti-tubulin antibodies, as indicated, to control for protein loading.
FIGURE 5.
Subcellular localization of SLFN5, as shown by immunofluorescence and immunoblotting after separation of nuclear and cytosolic fractions. A–D, SKMEL28 cells were incubated in the presence or absence of IFNα for 48 h, as indicated. Cells were fixed, permeabilized, and incubated with an antibody against SLFN5 followed by Alexa Fluor 488 secondary antibody (A), DAPI (B), or phalloidin 586 (C). Overlay of the fluorescence signals is shown in D. E and F, nuclear and cytosolic fractions were isolated from lysates of primary melanocytes (E) or from lysates of SKMEL5 cells that had been treated with IFNα for the indicated times (F). Equal amounts of protein were separated by SDS-PAGE, and fractions were analyzed for subcellular localization of SLFN5 via immunoblotting. GAPDH and nuclear lamin A were analyzed in parallel to confirm proper separation of fractions.
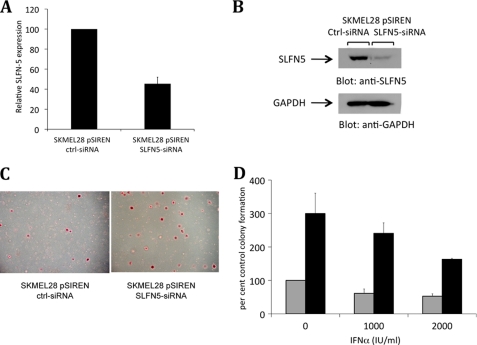
To determine the functional relevance of base-line and IFN-inducible SLFN5 expression in human malignant melanoma cells, we generated SKMEL28 cells in which SLFN5 was stably knocked down using the pSIREN ZsGreen retroviral vector (Fig. 6, A and B). SKMEL28-ctrl-siRNA and SKMEL28-SLFN5-siRNA cells were plated in soft agar in the presence or absence of IFNα, and colony formation was determined after crystal violet staining. Knockdown of SLFN5 clearly enhanced soft agar colony formation of SKMEL28 cells (Fig. 6, C and D). There were also clearly higher numbers of colonies from IFNα-treated SKMEL28-SLFN5-siRNA cells versus IFNα-treated SKMEL28-ctrl-siRNA cells, suggesting that knockdown of SLFN5 expression has a negative impact on the ability of IFNα to act as a suppressor on the growth potential of malignant melanoma cells.
FIGURE 6.
Stable knockdown of SLFN5 enhances anchorage-independent growth of SKMEL28 melanoma cells. A, expression of SLFN5 mRNA in pSIREN-SLFN5 siRNA or pSIREN-control-siRNA SKMEL28 cells was determined by real time RT-PCR using GAPDH as an internal control. The data are presented as percentage of SLFN5 expression in pSIREN ZsGreen control-siRNA cells and represent the means ± S.E. of four experiments. B, cell lysates from pSIREN SLFN5-siRNA or control-siRNA SKMEL28 cells were resolved by SDS-PAGE and immunoblotted with an anti-SLFN5 antibody. The same blot was then re-probed with an anti-GAPDH antibody to control for protein loading. C, equal numbers of SKMEL28-pSIREN SLFN5-siRNA or SKMEL28-pSIREN ctrl-siRNA cells were plated in soft agar, and colony formation was assessed after 7 days of culture in the presence or absence of IFNα. Representative areas show colony formation of SKMEL28-pSIREN SLFN5-siRNA and SKMEL28-pSIREN ctrl-siRNA cells in soft agar plates stained with crystal violet dye. D, colonies derived from untreated or IFNα-treated cells were counted, and results are expressed as percentages of colony formation of untreated SKMEL28 pSIREN-ctrl-siRNA cells. Data shown represent means ± S.E. of three independent experiments, including the one shown in C. Gray bars, pSIREN-ctrl-siRNA SKMEL28 cells; black bars, pSIREN-SLFN5-siRNA SKMEL28 cells.
Because invasion through collagen is essential for melanoma progression (49, 50), we sought to determine whether SLFN5 knockdown would also enhance the ability of melanoma cells to invade through type I collagen. SKMEL28-ctrl-siRNA (control cells) and SKMEL28-SLFN5-siRNA cells were plated in a three-dimensional collagen invasion assay system, and the ability of individual melanoma cells to invade was assessed. Individual SKMEL28 cells in which SLFN5 expression was knocked down invaded almost twice as far into the surrounding collagen as compared with control cells (Fig. 7, A and B), indicating that malignant cell invasion is suppressed by SLFN5. To determine whether increased invasion was due to SLFN5 regulation of matrix metalloproteinases, we examined the effects of SLFN5 knockdown on matrix metalloproteinases that are expressed by SKMEL28 cells. SKMEL28 cells express MMP-2, MMP-14, and MMP-15, and as shown in supplemental Fig. S1, SLFN5 knockdown either had no significant effect or repressed matrix metalloproteinase mRNA expression, suggesting a different mechanism for SLFN5-mediated suppression of cell invasion.
FIGURE 7.
Stable knockdown of SLFN5 enhances invasion of SKMEL28 melanoma cells in collagen. Equal numbers of SKMEL28 pSIREN SLFN5-siRNA and SKMEL28 pSIREN ctrl-siRNA cells were used in a collagen invasion assay. Cells were plated in a collagen core surrounded by an additional layer of collagen. Invading cells actively invaded away from the core into the surrounding collagen. Single cell invasion of SKMEL28 pSIREN SLFN5-siRNA and SKMEL28 pSIREN ctrl-siRNA cells was analyzed by measuring the distance between the cell border forming the core and the individual cells that invaded the furthest after 2–4 days of incubation. A, representative areas showing cell invasion of SKMEL28 pSIREN SLFN5-siRNA and SKMEL28 pSIREN ctrl-siRNA cells in collagen stained with crystal violet dye are shown. B, invasion distance was measured using the Carl-Zeiss AxioVision software tool. Mean distance ± S.D. from the cell border is shown in μm (B).
DISCUSSION
The SLFN family of proteins includes groups of genes that were originally identified for their growth regulatory properties (51). The first Slfn gene to be identified, mouse Slfn1, was found to exhibit important regulatory effects on T-lymphocyte growth and development (51). Subsequently, several additional mouse members of the family were identified that can be divided into the following three major groups based on sequence homology and domain structure (51, 52): group I (Slfn1 and -2), group II (Slfn3 and -4), and group III (Slfn5, -8–10, and -14) (51, 52). All SLFN proteins share a highly conserved N-terminal (AAA) domain involved in ATP/GTP binding (52, 53); however, only group III SLFNs harbor a motif found in superfamily I DNA/RNA helicases (52). Group I and II SLFNs have been linked to growth suppression, differentiation, and apoptosis (37, 48, 45, 51–58), although group III SLFNs are associated with differentiation in various cells (52). Interestingly, there is significant variability in the evolution and distribution of SLFN genes among species, and not all SLFN genes can be found in primates or other mammals (59). In fact, data base analysis shows that humans only have group II and group III SLFNs, a fact that raises several questions. For instance, it has been recently shown that SLFN2 is a major contributor of T-cell quiescence and that an inactivating mutation in the Slfn2 gene results in immunodeficiency in mice harboring the elektra mutation (58, 60). Because humans have no homologue to SLFN2, at this time it is unknown which of the human SLFN genes, if any, may have similar effects on T-cell quiescence. Similar questions may apply for other functions of group I SLFN genes, especially as more information on the function and properties of these genes accumulates with time.
In this study, we sought to define the functional relevance of human members of the SLFN family of proteins in the generation of the inhibitory effects of human IFNα in malignant melanoma. Previous work from our laboratory (37) had established that several mouse SLFN members are induced in a type I IFN-dependent manner and that mouse SLFN2 plays a key role in the regulation of normal mouse hematopoiesis. Importantly, our previous studies had shown that gene transcription for all murine members of the SLFN family was IFN-inducible (37), suggesting that other members of that family may be participating in the generation of other biological functions of IFNs. Because of the heterogeneity and differences between mouse and human systems, we analyzed gene expression for human members of the SLFN family, SLFN5 and -11–13. Surprisingly, only human SLFN5 was found to be inducible by human IFNα in malignant melanoma cells, suggesting a specific role for this member in the generation of IFNα responses. Interestingly, when the basal expression of group II (SLFN12) or group III (SLFN5 and 11, 13, and 14) SLFN genes in primary melanocytes and melanoma cells was examined, SLFN5 was found to be highly abundant in normal melanocytes and consistently suppressed in all different malignant melanoma cell types studied. Other SLFN genes were either not suppressed in all different malignant melanoma cell lines (SLFN12) or were not expressed at significant levels at base line in normal melanocytes (SLFN11, -13, and -14). This raises the possibility that suppression of SLFN5 expression in malignant melanoma cells may provide them with a growth advantage and promote tumorigenesis. To address the functional role of SLFN5 in melanoma cell growth and invasion, we generated SKMEL28 cells with stable SLFN5 knockdown and examined their ability to grow in soft agar and invade through collagen. Our data establish that decreased expression of SLFN5 correlates with enhanced anchorage-independent growth, as well as significantly increased invasion through collagen. As the protein is type I IFN-dependent and the suppressive effects of IFNα cannot be optimally achieved in malignant melanoma cells with stable suppression of SLFN5 expression, these findings suggest an important role for SLFN5 in the suppressive effects of type I IFNs on malignant melanoma cell growth and invasion.
To the best of our knowledge, this is the first study directly implicating a member of the human Schlafen protein family in the control of cell invasion. These findings provide valuable new information that advances our understanding of the mechanisms of action of IFNα in malignancies. Generally, the molecular mechanisms by which IFNα is effective against malignant melanoma are not known. In particular, very little is known on the effects of IFNs on cellular events that promote growth and survival of malignant melanoma cells. The precise mechanism by which SLFN5 suppresses growth and invasion of malignant melanoma cells remains to be established. It is possible that SLFN5 regulates expression of other tumor suppressor genes, cell cycle regulators, or genes maintaining senescence, and this remains to be determined in future studies. We have also shown that human SLFN5 is localized in the nucleus of human melanocytes and melanoma cells, consistent with what has been shown before for group III SLFNs (48). Interestingly, like some other SLFNs, SLFN5 harbors a COG2865 region containing a helix-turn-helix motif (52), suggesting DNA binding activity. It will be interesting to examine in future studies if SLFN5 participates in transcriptional regulation of IFN-sensitive genes and, if so, to identify its targets that may mediate its effects on anchorage-independent growth and invasion in collagen.
Supplementary Material
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grants CA77816, CA121192, C100579, and CA126888. This work was also supported by a merit review grant from the Department of Veterans Affairs.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
REFERENCES
- 1.Pestka S., Langer J. A., Zoon K. C., Samuel C. E. (1987) Annu. Rev. Biochem. 56, 727–777 [DOI] [PubMed] [Google Scholar]
- 2.Katze M. G., He Y., Gale M., Jr. (2002) Nat. Rev. Immunol. 2, 675–687 [DOI] [PubMed] [Google Scholar]
- 3.Parmar S., Platanias L. C. (2003) Curr. Opin Oncol. 15, 431–439 [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood J. M., Strawderman M. H., Ernstoff M. S., Smith T. J., Borden E. C., Blum R. H. (1996) J. Clin. Oncol. 14, 7–17 [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood J. M., Ibrahim J. G., Sondak V. K., Richards J., Flaherty L. E., Ernstoff M. S., Smith T. J., Rao U., Steele M., Blum R. H. (2000) J. Clin. Oncol. 18, 2444–2458 [DOI] [PubMed] [Google Scholar]
- 6.Hancock B. W., Wheatley K., Harris S., Ives N., Harrison G., Horsman J. M., Middleton M. R., Thatcher N., Lorigan P. C., Marsden J. R., Burrows L., Gore M. (2004) J. Clin. Oncol. 22, 53–61 [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood J. M., Ibrahim J. G., Sosman J. A., Sondak V. K., Agarwala S. S., Ernstoff M. S., Rao U. (2001) J. Clin. Oncol. 19, 2370–2380 [DOI] [PubMed] [Google Scholar]
- 8.Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. (1998) Annu. Rev. Biochem. 67, 227–264 [DOI] [PubMed] [Google Scholar]
- 9.van Boxel-Dezaire A. H., Rani M. R., Stark G. R. (2006) Immunity 25, 361–372 [DOI] [PubMed] [Google Scholar]
- 10.Darnell J. E., Jr., Kerr I. M., Stark G. R. (1994) Science 264, 1415–1421 [DOI] [PubMed] [Google Scholar]
- 11.Platanias L. C. (2005) Nat. Rev. Immunol. 5, 375–386 [DOI] [PubMed] [Google Scholar]
- 12.Uddin S., Sassano A., Deb D. K., Verma A., Majchrzak B., Rahman A., Malik A. B., Fish E. N., Platanias L. C. (2002) J. Biol. Chem. 277, 14408–14416 [DOI] [PubMed] [Google Scholar]
- 13.Deb D. K., Sassano A., Lekmine F., Majchrzak B., Verma A., Kambhampati S., Uddin S., Rahman A., Fish E. N., Platanias L. C. (2003) J. Immunol. 171, 267–273 [DOI] [PubMed] [Google Scholar]
- 14.Redig A. J., Sassano A., Majchrzak-Kita B., Katsoulidis E., Liu H., Altman J. K., Fish E. N., Wickrema A., Platanias L. C. (2009) J. Biol. Chem. 284, 10301–10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur S., Parmar S., Smith J., Katsoulidis E., Li Y., Sassano A., Majchrzak B., Uddin S., Tallman M. S., Fish E. N., Platanias L. C. (2005) Exp. Hematol. 33, 550–557 [DOI] [PubMed] [Google Scholar]
- 16.Srivastava K. K., Batra S., Sassano A., Li Y., Majchrzak B., Kiyokawa H., Altman A., Fish E. N., Platanias L. C. (2004) J. Biol. Chem. 279, 29911–29920 [DOI] [PubMed] [Google Scholar]
- 17.Katsoulidis E., Li Y., Mears H., Platanias L. C. (2005) J. Interferon Cytokine Res. 25, 749–756 [DOI] [PubMed] [Google Scholar]
- 18.David M., Petricoin E., 3rd, Benjamin C., Pine R., Weber M. J., Larner A. C. (1995) Science 269, 1721–1723 [DOI] [PubMed] [Google Scholar]
- 19.Uddin S., Majchrzak B., Woodson J., Arunkumar P., Alsayed Y., Pine R., Young P. R., Fish E. N., Platanias L. C. (1999) J. Biol. Chem. 274, 30127–30131 [DOI] [PubMed] [Google Scholar]
- 20.Uddin S., Lekmine F., Sharma N., Majchrzak B., Mayer I., Young P. R., Bokoch G. M., Fish E. N., Platanias L. C. (2000) J. Biol. Chem. 275, 27634–27640 [DOI] [PubMed] [Google Scholar]
- 21.Verma A., Deb D. K., Sassano A., Uddin S., Varga J., Wickrema A., Platanias L. C. (2002) J. Biol. Chem. 277, 7726–7735 [DOI] [PubMed] [Google Scholar]
- 22.Mayer I. A., Verma A., Grumbach I. M., Uddin S., Lekmine F., Ravandi F., Majchrzak B., Fujita S., Fish E. N., Platanias L. C. (2001) J. Biol. Chem. 276, 28570–28577 [DOI] [PubMed] [Google Scholar]
- 23.Lekmine F., Uddin S., Sassano A., Parmar S., Brachmann S. M., Majchrzak B., Sonenberg N., Hay N., Fish E. N., Platanias L. C. (2003) J. Biol. Chem. 278, 27772–27780 [DOI] [PubMed] [Google Scholar]
- 24.Lekmine F., Sassano A., Uddin S., Smith J., Majchrzak B., Brachmann S. M., Hay N., Fish E. N., Platanias L. C. (2004) Exp. Cell Res. 295, 173–182 [DOI] [PubMed] [Google Scholar]
- 25.Thyrell L., Hjortsberg L., Arulampalam V., Panaretakis T., Uhles S., Dagnell M., Zhivotovsky B., Leibiger I., Grandér D., Pokrovskaja K. (2004) J. Biol. Chem. 279, 24152–24162 [DOI] [PubMed] [Google Scholar]
- 26.Kaur S., Lal L., Sassano A., Majchrzak-Kita B., Srikanth M., Baker D. P., Petroulakis E., Hay N., Sonenberg N., Fish E. N., Platanias L. C. (2007) J. Biol. Chem. 282, 1757–1768 [DOI] [PubMed] [Google Scholar]
- 27.Kaur S., Sassano A., Dolniak B., Joshi S., Majchrzak-Kita B., Baker D. P., Hay N., Fish E. N., Platanias L. C. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4808–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi S., Kaur S., Redig A. J., Goldsborough K., David K., Ueda T., Watanabe-Fukunaga R., Baker D. P., Fish E. N., Fukunaga R., Platanias L. C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlasáková J., Nováková Z., Rossmeislová L., Kahle M., Hozák P., Hodny Z. (2007) Blood 109, 1373–1380 [DOI] [PubMed] [Google Scholar]
- 30.Chang H. M., Paulson M., Holko M., Rice C. M., Williams B. R., Marié I., Levy D. E. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 9578–9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamoto S., Potla R., Larner A. C. (2004) J. Biol. Chem. 279, 40362–40367 [DOI] [PubMed] [Google Scholar]
- 32.Klampfer L., Huang J., Swaby L. A., Augenlicht L. (2004) J. Biol. Chem. 279, 30358–30368 [DOI] [PubMed] [Google Scholar]
- 33.Nusinzon I., Horvath C. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14742–14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie K. J., Hahn C. S., Kim K. I., Yan M., Rosario D., Li L., de la Torre J. C., Zhang D. E. (2004) Nat. Med. 10, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 35.Malakhova O. A., Yan M., Malakhov M. P., Yuan Y., Ritchie K. J., Kim K. I., Peterson L. F., Shuai K., Zhang D. E. (2003) Genes Dev. 17, 455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malakhov M. P., Kim K. I., Malakhova O. A., Jacobs B. S., Borden E. C., Zhang D. E. (2003) J. Biol. Chem. 278, 16608–16613 [DOI] [PubMed] [Google Scholar]
- 37.Katsoulidis E., Carayol N., Woodard J., Konieczna I., Majchrzak-Kita B., Jordan A., Sassano A., Eklund E. A., Fish E. N., Platanias L. C. (2009) J. Biol. Chem. 284, 25051–25064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin S., Fish E. N., Sher D. A., Gardziola C., White M. F., Platanias L. C. (1997) J. Immunol. 158, 2390–2397 [PubMed] [Google Scholar]
- 39.Kannan-Thulasiraman P., Katsoulidis E., Tallman M. S., Arthur J. S., Platanias L. C. (2006) J. Biol. Chem. 281, 22446–22452 [DOI] [PubMed] [Google Scholar]
- 40.Giafis N., Katsoulidis E., Sassano A., Tallman M. S., Higgins L. S., Nebreda A. R., Davis R. J., Platanias L. C. (2006) Cancer Res. 66, 6763–6771 [DOI] [PubMed] [Google Scholar]
- 41.Hamburger A. W., Salmon S. E. (1977) Science 197, 461–463 [DOI] [PubMed] [Google Scholar]
- 42.Rowe R. G., Li X. Y., Hu Y., Saunders T. L., Virtanen I., Garcia de Herreros A., Becker K. F., Ingvarsen S., Engelholm L. H., Bommer G. T., Fearon E. R., Weiss S. J. (2009) J. Cell Biol. 184, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong L. H., Krauer K. G., Hatzinisiriou I., Estcourt M. J., Hersey P., Tam N. D., Edmondson S., Devenish R. J., Ralph S. J. (1997) J. Biol. Chem. 272, 28779–28785 [DOI] [PubMed] [Google Scholar]
- 44.Ralph S. J., Wines B. D., Payne M. J., Grubb D., Hatzinisiriou I., Linnane A. W., Devenish R. J. (1995) J. Immunol. 154, 2248–2256 [PubMed] [Google Scholar]
- 45.Lesinski G. B., Trefry J., Brasdovich M., Kondadasula S. V., Sackey K., Zimmerer J. M., Chaudhury A. R., Yu L., Zhang X., Crespin T. R., Walker M. J., Carson W. E., 3rd. (2007) Clin. Cancer Res. 13, 5010–5019 [DOI] [PubMed] [Google Scholar]
- 46.Certa U., Seiler M., Padovan E., Spagnoli G. C. (2001) Br. J. Cancer 85, 107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKendry R., John J., Flavell D., Müller M., Kerr I. M., Stark G. R. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 11455–11459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neumann B., Zhao L., Murphy K., Gonda T. J. (2008) Biochem. Biophys. Res. Commun. 370, 62–66 [DOI] [PubMed] [Google Scholar]
- 49.Miller A. J., Mihm M. C., Jr. (2006) N. Engl. J. Med. 355, 51–65 [DOI] [PubMed] [Google Scholar]
- 50.Smith S. C., Theodorescu D. (2009) Nat. Rev. Cancer 9, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz D. A., Katayama C. D., Hedrick S. M. (1998) Immunity 9, 657–668 [DOI] [PubMed] [Google Scholar]
- 52.Geserick P., Kaiser F., Klemm U., Kaufmann S. H., Zerrahn J. (2004) Int. Immunol. 16, 1535–1548 [DOI] [PubMed] [Google Scholar]
- 53.Brady G., Boggan L., Bowie A., O'Neill L. A. (2005) J. Biol. Chem. 280, 30723–30734 [DOI] [PubMed] [Google Scholar]
- 54.Bell T. A., de la Casa-Esperón E., Doherty H. E., Ideraabdullah F., Kim K., Wang Y., Lange L. A., Wilhemsen K., Lange E. M., Sapienza C., de Villena F. P. (2006) Genetics 172, 411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sohn W. J., Kim D., Lee K. W., Kim M. S., Kwon S., Lee Y., Kim D. S., Kwon H. J. (2007) Mol. Immunol. 44, 3273–3282 [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y., Yang Z., Cao Y., Zhang S., Li H., Huang Y., Ding Y. Q., Liu X. (2008) Biochem. J. 413, 239–250 [DOI] [PubMed] [Google Scholar]
- 57.Patel V. B., Yu Y., Das J. K., Patel B. B., Majumdar A. P. (2009) Biochem. Biophys. Res. Commun. 388, 752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berger M., Krebs P., Crozat K., Li X., Croker B. A., Siggs O. M., Popkin D., Du X., Lawson B. R., Theofilopoulos A. N., Xia Y., Khovananth K. Y., Moresco E. M., Satoh T., Takeuchi O., Akira S., Beutler B. (2010) Nat. Immunol. 11, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bustos O., Naik S., Ayers G., Casola C., Perez-Lamigueiro M. A., Chippindale P. T., Pritham E. J., de la Casa-Esperón E. (2009) Gene 447, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horton M. R., Powell J. D. (2010) Nat. Immunol. 11, 281–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.