Abstract
Previous research has shown that the basolateral amygdala (BLA) mediates stimulus-reward learning, including drug-cue associations, while the dorsolateral caudate-putamen (dlCPu) primarily mediates stimulus-response (habit) learning. Recent evidence has indicated that the dlCPu may be critical in cocaine-seeking following extended self-administration, but it remains unknown whether the dlCPu plays a role in early formation of drug-cue associations. The current study used a model of Pavlovian learning to compare the roles of the BLA and dlCPu in the consolidation of cocaine-cue associations that maintain cocaine-seeking during cue-induced reinstatement. Male, Sprague-Dawley rats self-administered cocaine (0.2 mg/50 µl infusion, IV) in the absence of cues for 6 days (2-h/day). Immediately following a single 1-h classical conditioning (CC) session in which passive cocaine infusions were paired with a light+tone cue, animals received bilateral infusions of the GABA receptor agonists, baclofen+muscimol (B/M, 1.0/0.1 mM), or vehicle into the BLA or dlCPu. Following additional cocaine self-administration (5 days) and subsequent extinction (no cocaine or cues, 7 days), the ability of the previously cocaine-paired cues to reinstate cocaine-seeking was assessed. Inactivation of the BLA, but not the dlCPu, immediately following the CC session impaired the consolidation of cocaine-cue associations as seen by decreased cue-induced reinstatement. These results extend previous findings that the BLA mediates the consolidation of learned associations that drive cocaine-seeking during subsequent reinstatement and indicate that the dlCPu does not play a role during initial stimulus-drug associative learning.
Keywords: rat, relapse, self-administration, stimulus-reward, stimulus-response
Introduction
Relapse to drug use is a significant problem for the treatment of addiction (Dackis & O'Brien, 2001). Cues previously associated with drugs of abuse can trigger craving after long periods of abstinence in human addicts (Childress et al., 1988; Volkow et al., 2006) and animal research demonstrates that previously drug paired cues can robustly reinstate drug-seeking behavior (de Wit & Stewart, 1981; See, 2002). Therefore, investigating the neural substrates that underlie the formation of these associations is imperative to understanding cue-induced relapse.
The basolateral amygdala (BLA) has an established role in the reinstatement of drug-seeking, particularly to drug associated cues (See, 2005). Both excitotoxic lesions (Meil & See, 1997) and pharmacological inactivation (Grimm & See, 2000; Kantak et al., 2002a) of the BLA impair conditioned cued reinstatement. However, BLA lesions do not impair cocaine self-administration (Meil & See, 1997) or cocaine-primed reinstatement (McFarland & Kalivas, 2001), demonstrating the specific involvement of the BLA in attaching incentive value to conditioned reinforcers, and not primary reinforcement itself. These findings are congruent with the recognized role of the BLA in other forms of stimulus-affect learning, such as fear conditioning (LeDoux, 2003) and conditioned place preference (Everitt et al., 1991; Fuchs et al., 2002).
We have previously reported a critical role of the BLA in the acquisition and consolidation of early drug-cue associations using a modified cocaine self-administration model, where cue-cocaine associations are formed during a single classical conditioning (CC) session (Kruzich et al., 2001). Inactivation or pharmacological blockade of the BLA impairs both the acquisition (Kruzich & See, 2001; See et al., 2003; Berglind et al., 2006) and consolidation (Fuchs et al., 2006b; Feltenstein & See, 2007) of cocaine-cue associations, demonstrated by subsequent attenuation of the ability of the conditioned cues to reinstate cocaine-seeking.
Recent research indicates the dorsal striatum, particularly the dorsolateral caudate putamen (dlCPu), is involved in drug-seeking. Human cocaine addicts show increased CPu activity in response to cocaine-associated cues (Garavan et al., 2000; Volkow et al., 2006) and studies in rhesus monkeys have demonstrated increasing involvement of the CPu as cocaine self-administration progresses (Porrino et al., 2004). Finally, dlCPu inactivation impairs contextual reinstatement of cocaine-seeking (Fuchs et al., 2006a; See et al., 2007).
Stimulus-reward learning occurs when a previously neutral stimulus acquires incentive properties when paired with a reward. During the CC session described above, the cues acquire incentive value that later drives reinstatement of cocaine-seeking. In contrast, stimulus-response associations occur when a motor response is reinforced in the presence of a neutral stimulus. The reinforcer strengthens the association between the stimulus and the response, but it is not part of the association (Thorndike, 1933; Hull, 1943). While the BLA plays a significant role in stimulus-reward learning (Cador et al., 1989; Everitt et al., 1991), the dlCPu mediates stimulus-response learning (Mishkin & Petri, 1984; Packard & Knowlton, 2002). The present study examined the respective contribution of these two systems in the consolidation of cocaine-cue associations and subsequent cue-induced reinstatement. Given our previous research demonstrating the involvement of the BLA in this type of learning (See et al., 2003), we predicted that BLA inactivation would impair subsequent cued reinstatement of cocaine-seeking. Little is known about the contribution of the dlCPu to early learning about drug-cue associations. Therefore, the current study utilized a behavioral paradigm well established in our laboratory to investigate this question. However, given the known role of the dlCPu in mediating stimulus-response learning (Packard & Knowlton, 2002), we predicted that dlCPu inactivation would not affect consolidation of early cocaine-cue associations, and their subsequent ability to drive reinstatement of drug-seeking.
Materials and methods
Subjects
Male, Sprague-Dawley rats (Charles-River; 250–275 g) were individually housed on a 12:12 h reverse light-dark cycle, with lights off from 6:00 a.m. to 6:00 p.m. All animals received water ad libitum, and were maintained on 25 g of standard rat chow (Harlan, Indianapolis, IN, USA) per day for the duration of the experiment, unless otherwise noted below. Housing and care of the rats were carried out in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and all experimental procedures were approved by the MUSC IACUC.
Apparatus
Testing was conducted in standard self-administration chambers (30×20×20 cm, Med-Associates, St Albans, VT, USA) linked to a computerized data collection program (MED PC). Each chamber was equipped with two retractable levers, a white stimulus light, a tone generator (ENV-223HAM, Med Associates), and a house light on the wall opposite the levers. Each chamber was contained within a sound-attenuating cubicle equipped with a ventilation fan.
Surgery
Animals were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, IP), followed by equithesin (0.5 ml/kg with a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution, IP). Ketorolac (2.0 mg/kg, IP) was administered immediately prior to surgery for analgesia. For jugular catheter implantation, an indwelling catheter (Silastic tubing; 0.51 mm i.d. and 0.94 mm o.d.; Dow Corning, Midland, MI, USA) was inserted into the right jugular vein and securely sutured. The other end was led subcutaneously to a back incision, where it was connected to an external silicone harness (Plastics One, Roanoke, VA). Stylets were inserted into the catheters when the rats were not connected to infusion pumps. Immediately following catheter surgery, animals were placed into a stereotaxic frame (Stoelting, Wood Dale, IL, USA) and implanted with bilateral stainless steel guide cannulae (26 gauge; Plastics One, Roanoke, VA) directed towards the dlCPu or the BLA using the appropriate coordinates in mm (dlCPu: AP +1.2, ML ±3.8, DV − 3.4; BLA: AP: −2.7, ML: ±5.0, DV −6.6). Guide cannulae were secured to the skull using steel jeweler’s screws and dental acrylic. Following surgery, stylets were placed into the guide cannulae to prevent blockage. For five days following surgery, catheters were flushed daily with 0.1 ml each of 70 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, NJ, USA) and an antibiotic solution cefazolin (10 mg/0.1 ml, Schein Pharmaceuticals, Florham Park, NJ, USA) to maintain catheter patency. During the entire self-administration period, rats received an infusion of 10 U heparinized saline immediately prior to each session, and the cefazolin and 70 U/ml heparinized saline regimen following the session. Catheter patency was assessed occasionally by administration of 2% methohexital sodium (10.0 mg/ml IV; Eli Lilly and Co., Indianapolis, IN, USA), a short-acting barbiturate that produces rapid and reversible muscle flaccidity.
Cocaine self-administration and classical conditioning
Five days following surgery, rats began cocaine self-administration. Infusion tubing for cocaine administration was enclosed in a wire coil and screwed to the external catheter mount on the rat’s back. A weighted swivel apparatus (Instech, Plymouth Meeting, PA, USA) allowed for free movement within the chamber. Cocaine hydrochloride (National Institute on Drug Abuse, Research Triangle Park, NC, USA) was mixed in 0.9% sterile saline and filtered (0.45 µm) prior to self-administration, with infusions (0.2 mg/50 µl bolus, IV) delivered by syringe pumps located outside the cubicle. The house light signaled the initiation of the session and remained illuminated throughout the session. Cocaine reinforcement was available along a fixed-ratio 1 (FR-1) schedule for daily 2-h sessions. During each session, a response on the active lever resulted in a 2-s cocaine infusion in the absence of any paired stimulus presentations, followed by a 20-s time-out period. Responding during the time-out or on the inactive lever was recorded, but resulted in no programmed consequences. Daily self-administration sessions progressed until animals reached a criterion of 6 sessions with at least 10 cocaine infusions per session. Upon reaching criterion, each animal experienced a single 1-h classical conditioning (CC) session with levers unavailable, and passive cocaine infusions were paired with a 5-s light-tone stimulus complex. The number of infusions each animal received during the CC session was equal to the mean number of infusions self-administered by that animal during the first hour of the previous two sessions.
Intracranial infusions
To assess the role of the BLA or the dlCPu in the consolidation of cocaine-cue associations and their subsequent ability to reinstate cocaine-seeking, animals received bilateral intra-BLA or intra-dlCPu infusions of either baclofen/muscimol (B/M, 1.0 and 0.1 mM, respectively) or phosphate buffered saline (PBS) vehicle immediately following the CC session. Injection cannulae (33 gauge; Plastics One) were inserted 2 mm below the tip of the guide cannulae. Bilateral infusions (0.6 µl/side dlCPu; 0.5 µl/side - BLA) lasted 2 min, and the cannulae remained for an additional min after the infusion to allow for diffusion. Previous studies have shown that this concentration of B/M and respective bolus amounts impair reinstatement of drug-seeking when infused in the BLA or dlCPu just prior to reinstatement testing (See et al., 2007; Rogers et al., 2008).
Extinction and reinstatement
Following the CC session, animals received an additional 5 days of self-administration sessions on an FR-1 schedule in the absence of conditioned stimulus presentations. After self-administration, rats underwent daily 2-h extinction sessions, during which both the active and inactive lever responses were recorded, but had no programmed consequences. Once extinction criterion was reached (a minimum of 7 sessions with at least 2 consecutive sessions of ≤25 active lever responses), animals underwent a conditioned cue reinstatement test, during which each active lever response resulted in a 5-s presentation of the light-tone stimulus in the absence of cocaine reinforcement.
Histology and data analysis
Following testing, animals were anesthetized and transcardially perfused with PBS and 10% formaldehyde solution. Brains were coronally sectioned at a thickness of 75 µm, and stained with cresyl violet. The sections were examined under light microscopy to determine cannula placement and the most ventral point of each cannula track mapped onto schematics from a rat brain atlas (Paxinos & Watson, 1997). Analyses of active and inactive lever responding and cocaine intake during self-administration, number of CC pairings, and lever responses during extinction and CS-reinstatement testing were conducted using one- or two-way repeated measures ANOVA and one-way ANOVA or t tests, where appropriate. All data were analyzed using SPSS 17.0 (SPSS Inc. Chicago, IL)
Results
Histology
Schematic representations of the most ventral point of bilateral BLA and dlCPu infusion cannulae are indicated in Fig. 1. Infusion cannulae locations (anterior-posterior from bregma) within the BLA ranged from −2.56 to −3.14, and in the dlCPu from 1.60 to 1.00. Animals with one or both infusion cannulae tracks located outside of the target regions (N=3) were not included in data analyses. The final N/group was: BLA B/M (N = 9), BLA Veh (N = 10), dlCPu B/M (N = 8), and dlCPu Veh (N = 9).
Figure 1.
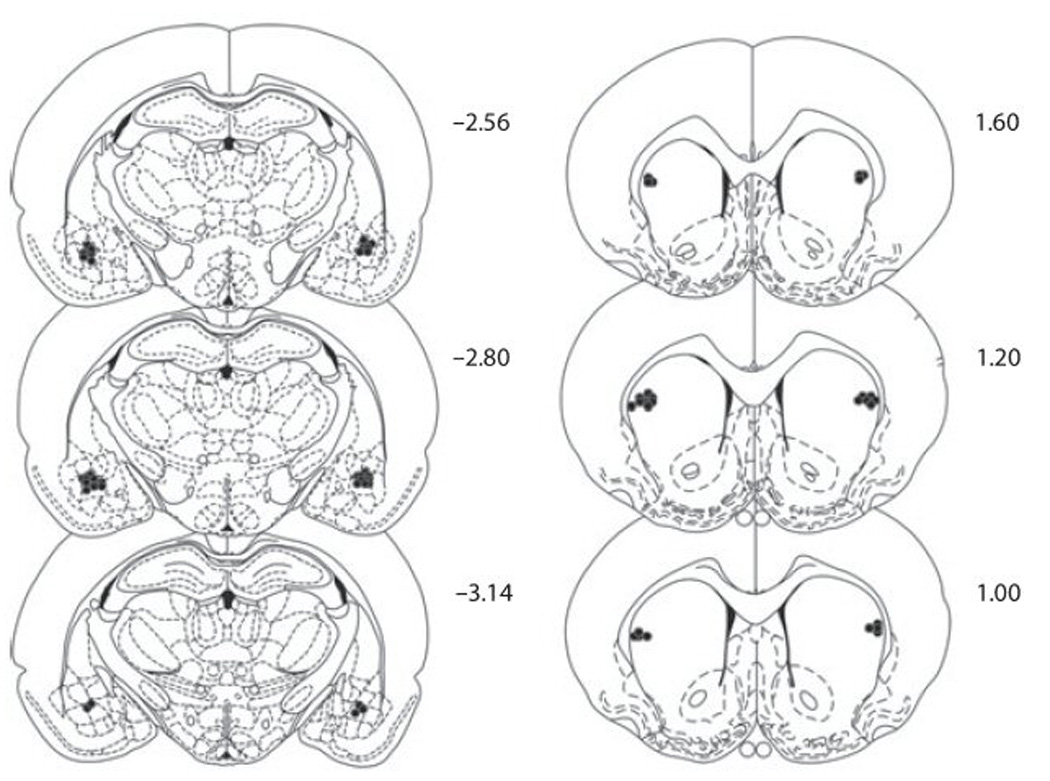
Schematic diagram of coronal sections (anterior–posterior from bregma) of infusion cannulae placements for the basolateral amygdala (left) and the dorsolateral caudate putamen (right) (adapted from Paxinos & Watson, 1997).
BLA
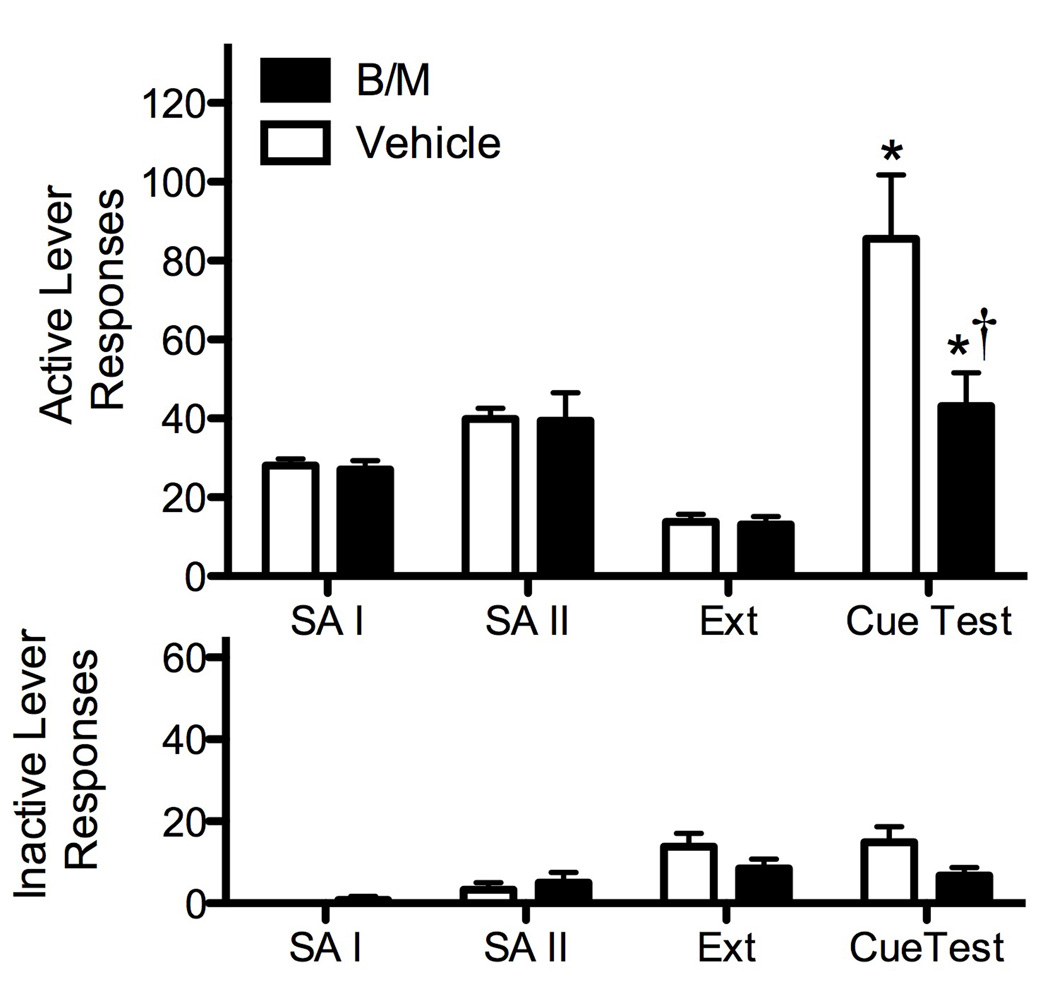
Animals with BLA cannulae readily acquired cocaine self-administration and showed stable responding and cocaine intake by the end of the first 6 days of self-administration (Fig. 2). There were no differences between groups for active and inactive lever responses, or for daily cocaine intake as seen across all days of the self-administration period prior to (F = 0.002–1.65, P = 0.18–0.97) or following (F = 0.08–2.87, P = 0.12–0.99) the CC session. Inactive lever responding across all sessions was routinely very low and did not show any apparent trends. There were no significant differences between groups for the number of cocaine infusions received during the CC session (F1,17 = 0.01, P = 0.92). Additionally, there were no differences between groups for active (F1,17= 0.06, P = 0.81) or inactive lever (F1,17 = 1.71, P = 0.21) responding across the last two days of extinction sessions (Fig. 2).
Figure 2.
Mean (±SEM) active (top) and inactive (bottom) lever responses for the last two days of self-administration (SA) phases I and II, extinction (EXT), and during the CS-reinstatement test. Animals received bilateral intra-BLA infusions of vehicle or B/M immediately following the CC session. Significant differences are indicated as compared to extinction levels (*P < 0.05) or vehicle (†P < 0.05).
Analyses conducted on active lever responding during extinction and the conditioned cued-reinstatement test in animals with BLA cannulae indicated a significant main effect for test (F1,17 = 32.04, P = 0.000), treatment group (F1,17 = 4.492, P = 0.049), and a test × treatment interaction (F1,17 = 5.37, P = 0.033). Post-hoc analyses revealed that both groups showed significant reinstatement responding above extinction levels (Tukey P < 0.05); however, B/M inactivation of the BLA significantly impaired conditioned cued reinstatement responding as compared to PBS vehicle controls (Tukey P <0.05) (Fig. 2). There were no significant differences for inactive lever responding during extinction and the conditioned cued reinstatement test (F = 0.04–2.92, P = 0.11–0.84).
dlCPu
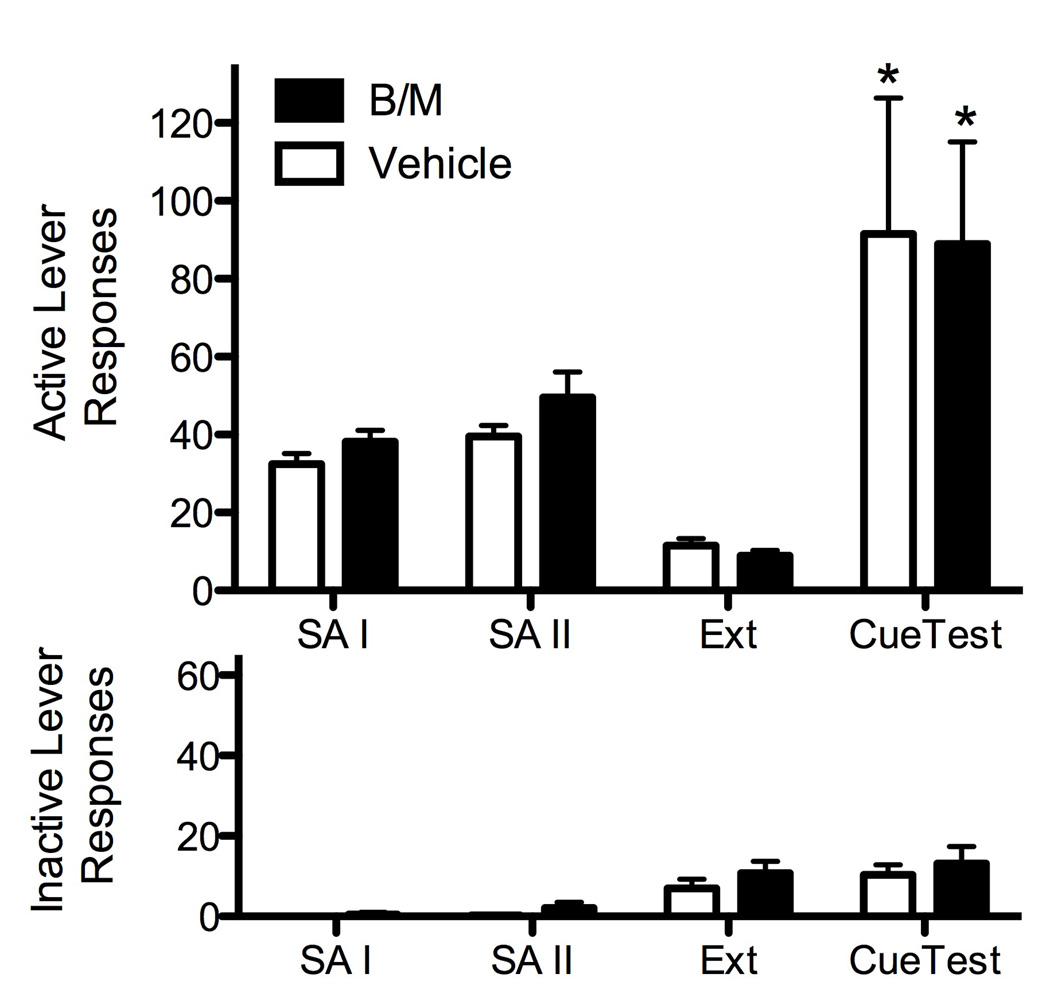
Animals with dlCPu cannulae readily acquired cocaine self-administration and showed stable responding and cocaine intake by the end of the first 6 days of self-administration (Fig. 3). There were no pre-existing differences between groups for active lever responses or for daily cocaine intake as seen across all days of the self-administration period prior to (F = 0.08–1.23, P = 0.30–0.78), or following (F = 0.001–2.09, P = 0.17–0.97) the CC session. In general, inactive lever responding across all sessions was routinely very low (less than 2/session) and did not show consistent trends. However, a significant difference between groups was seen for inactive lever responses post-CC session (F1,15= 4.79, P = 0.045), with group means ± SEM across days as follows (B/M: 1.55±0.46, Veh: 0.16±0.44). There were no significant differences between groups for the number of cocaine infusions received during the CC session (F1,15 = 2.32, P = 0.15). Additionally, there were no difference between groups for active (F1,15= 1.16, P = 0.30) or inactive lever (F1,15= 1.11, P = 0.31) responding across the last two days of extinction training (Fig. 3).
Figure 3.
Mean (±SEM) active (top) and inactive (bottom) lever responses for the last two days of self-administration (SA) phases I and II, extinction (EXT), and during the CS-reinstatement test. Animals received bilateral intra-CPu infusions of vehicle or B/M immediately following the CC session. Significant differences are indicated as compared to extinction levels (*P < 0.01).
Analyses conducted on active lever responding during extinction and the conditioned cued reinstatement test in animals with dlCPu cannulae showed a significant main effect for test (F1,15 = 13.64, P = 0.002), with both groups showing robust reinstatement responding above extinction levels. However, in contrast to animals that received BLA infusions, ANOVA did not reveal a significant main effect for treatment group (F1,15 = 0.01, P = 0.91), or a significant interaction effect (F1,15= 0.00, P = 0.998). Thus, inactivation of the dlCPu failed to impair conditioned cued reinstatement responding as compared to PBS vehicle controls (Fig. 3). For the dlCPu, no significant differences were noted for inactive lever responding during extinction and the conditioned cued reinstatement test (F = 0.18–3.76, P = 0.07–0.67).
Discussion
The results of the current study show that BLA, but not dlCPu, inactivation with B/M immediately following the CC session impaired consolidation of cocaine-cue associations, as demonstrated through attenuated cocaine-seeking during subsequent cue induced reinstatement. BLA inactivation following the CC session did not impact subsequent cocaine self-administration, supporting the selective effect of BLA inactivation on conditioned processes. These results extend and support our previous evidence that the BLA plays an important role in the acquisition and consolidation of cocaine-cue associations that drive subsequent cue induced reinstatement of cocaine-seeking (Kruzich & See, 2001; See et al., 2003; Berglind et al., 2006; Fuchs et al., 2006b; Feltenstein & See, 2007). The role of the BLA in drug-paired cue conditioning in this paradigm is consistent with other evidence of BLA-mediated stimulus-reward learning with cocaine (Cador et al., 1989; Everitt et al., 1991; McDonald & White, 1993; Whitelaw et al., 1996), and the fact that BLA lesions do not impair primary reinforcement by cocaine (Whitelaw et al., 1996; Meil & See, 1997). By demonstrating the involvement of the BLA in the consolidation of drug-cue associations that mediate drug-seeking, these results further support the role of the BLA as a key substrate underlying the process by which cues acquire incentive-motivational properties in addiction (for review, see Everitt et al., 1999; Robbins et al., 2008).
The dlCPu has been well established in habitual stimulus-response learning (Mishkin & Petri, 1984; Knowlton et al., 1996; Packard & Knowlton, 2002), and has recently been shown to be involved in craving and relapse (Garavan et al., 2000; Ito et al., 2002; Porrino et al., 2004; Vanderschuren et al., 2005; Fuchs et al., 2006a; Volkow et al., 2006; See et al., 2007). The striatum receives extensive innervation from the cerebral cortex, and the dlCPu in particular receives inputs from the sensory and motor cortical areas (McGeorge & Faull, 1989). These corticostriatal loops may direct the specific motoric outputs in response to stimuli, such as those formed during stimulus-response associations (Wise et al., 1996). Further, dlCPu dependent stimulus-response learning is incremental and acquired over time as reinforcers strengthen the association between stimuli and response (Mishkin & Petri, 1984; Knowlton et al., 1996; Packard & Knowlton, 2002). Recent theories of compulsive drug use propose the formation of maladaptive stimulus-response habits that maintain drug-seeking behavior. While initial drug use is characterized by complex behaviors that are clearly goal directed, these behaviors persist despite negative consequences associated with drug addiction (White, 1996; Everitt & Robbins, 2005; Everitt et al., 2008). This switch to habitual learning may occur through the ‘spiraling’ loop circuitry in the striatum, in which information progresses in a ventral to dorsal pattern throughout the striatum and associated cortical and midbrain regions (Haber, 2003). Indeed, disconnection between the ventral and dorsolateral striatum through dopamine receptor antagonism in the dorsal striatum contralateral to the unilaterally lesioned nucleus accumbens core impaired cocaine-seeking maintained by a conditioned reinforcer (Belin & Everitt, 2008). In rhesus monkeys trained to self-administer cocaine, chronic cocaine altered striatal glucose utilization in a duration dependent manner, whereby the effects of cocaine were increasingly manifested in the dorsal striatum as the duration of cocaine self-administration progressed (Porrino et al., 2004). Given the connections between the dlCPu and areas such as the BLA (McDonald, 1991) that mediate cued reinstatement (Meil & See, 1997; Grimm & See, 2000; Kantak et al., 2002a), it is possible that information about the cues could be encoded by the dorsal striatum and come to later mediate cue driven drug-seeking behavior. However, the lack of effect of dlCPu inactivation on conditioned cued-reinstatement responding is likely due to the fact that the CC session occurred during a single trial and that the animals never actively responded for the cues during self-administration, rather than learning during repeated sessions of operant responding that comprises a typical stimulus-response task mediated by the dlCPu (Mishkin & Petri, 1984; Knowlton et al., 1996; Packard & Knowlton, 2002).
While the dlCPu does not appear to be necessary for consolidation of initial drug-cue associative learning, drug-induced changes in dlCPu function have been implicated in other aspects of addictive behavior. Brain imaging studies with fMRI or PET studies in human cocaine addicts have demonstrated increased activity (Garavan et al., 2000) and increased dopamine release (Volkow et al., 2006) within the dorsal striatum following presentations of cocaine associated cues. Rats trained on a second order schedule of reinforcement showed increases in extracellular dopamine in the dlCPu in response to cocaine-contingent cue presentation (Ito et al., 2002), while intra-dorsal striatum infusion of dopamine receptor antagonists impaired cocaine-seeking maintained by a conditioned reinforcer (Vanderschuren et al., 2005). While these studies demonstrate that cue motivated responding for cocaine involves the dlCPu following training under a second order schedule of reinforcement, lidocaine inactivation of the dlCPu failed to impair cue induced reinstatement (Kantak et al., 2002b). Similarly, in animals trained to nose poke for cocaine plus a light cue and then trained to maintain a lever response for the light cue alone, B/M inactivation of the dlCPu did not impair cue induced reinstatement of the nose poke response (Di Ciano et al., 2008). Importantly, dlCPu inactivation did impair maintained lever responding for the previously drug-paired reinforcer alone (Di Ciano et al., 2008). These results suggest that the dlCPu may mediate habitual persistent responding for conditioned reinforcers and that conditioned cue-induced reinstatement may involve the retrieval of associations between cues and rewards mediated by the BLA and associated limbic regions (Di Ciano et al., 2008).
While BLA inactivation did not completely block reinstatement responding, response levels were significantly attenuated as compared to the potentiated responding seen in all other groups. The lack of a complete blockade (i.e., down to extinction levels) is consistent with our previous results using sodium channel blockade (Fuchs et al., 2006b) or NMDA receptor antagonism (Feltenstein & See, 2007) at the time of consolidation, and may be due to the limited anatomical extent of the inactivation, particularly along the rostral-caudal extent. Alternatively, inactivation of the BLA at the time of the CC session may not be sufficient to completely block cocaine-cue learning as other pathways (e.g., nucleus accumbens) likely contribute to drug-cue learning (See et al., 2007). Future studies will be directed at other specific areas that may contribute to consolidation of drug-cue learning. Of particular interest is the possible role of the dorsomedial CPu, an area that has been implicated in action-outcome learning (Yin et al., 2005).
A recent study (McDonald & Hong, 2004) examined the dissociating effects of BLA and dlCPu excitotoxic lesions on radial arm maze training to approach a light cue for food reward and a subsequent preference test. BLA-lesioned rats were able to acquire the stimulus-response task normally, but were unable to demonstrate a preference for the lighted arm. Conversely, dlCPu-lesioned rats showed impaired stimulus-response task performance, but showed a preference for the lighted arms. These results suggest that the intact BLA, while unable to maintain the stimulus-response task, still encoded associations between the cue and the reward (McDonald & Hong, 2004). This and similar studies on the roles of the BLA and dlCPu in stimulus-reward and stimulus-response learning, respectively, have demonstrated that these relatively independent systems can be activated simultaneously and in parallel to acquire the appropriate information during any given situation (McDonald & White, 1993).
During prolonged drug use, both amygdalar and dorsal striatal pathways are engaged in the processes that drive drug-taking and drug-seeking behaviors. The current results confirm and extend upon prior studies that demonstrate dissociable roles of the BLA and CPu in appetitive learning, but place this dichotomy within the context of drug-cue learning. While future studies are clearly warranted, our findings support the hypothesis that the dlCPu is less involved in early drug-cue associative processing, but may become more involved in drug-seeking behavior as drug use progresses in an addictive manner (Everitt et al., 2008).
Acknowledgements
The authors thank Shannon Ghee, Bernard Smalls, and Sarah-Wade Boatwright for excellent technical assistance. This research was supported by National Institute on Drug Abuse Grant DA10462 (RES) and NIH grants T32007288 and C06 RR015455.
Abbreviations
- BLA
basolateral amygdala
- dlCPu
dorsolateral caudate putamen
- CC
classical conditioning
- CS
conditioned stimulus
- B/M
baclofen/muscimol
References
- Belin D, Everitt B. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See R. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O'Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res. Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J. Subst. Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Everitt B, Belin D, Economidou D, Pelloux Y, Dalley J, Robbins T. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala- ventral striatal subsystems. Ann. N. Y. Acad. Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol. Learn. Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J. Neurosci. 2006a;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur. J. Neurosci. 2006b;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of Behavior. New York: Appleton-Century Crofts; 1943. [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J. Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J. Neurosci. 2002a;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Stimulus-response functions of the lateral dorsal striatum and regulation of behavior studied in a cocaine maintenance/cue reinstatement model in rats. Psychopharmacology (Berl) 2002b;161:278–287. doi: 10.1007/s00213-002-1036-z. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav. Neurosci. 2001;115:1086–1092. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J. Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS. A dissociation of the dorso-lateral striatum and amygdala function on the same stimulus-response habit task. Neuroscience. 2004;124:507–513. doi: 10.1016/j.neuroscience.2003.11.041. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav. Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Petri HL. Memories and habits: some implications for the analysis of learning and retention. In: Squire LR, B N, editors. Neuropsychology of Memory. New York: Guilford; 1984. pp. 287–296. [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J. Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug Addiction and the Memory Systems of the Brain. Ann. N. Y. Acad. Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol. Biochem. Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur. J. Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Thorndike EL. A Proof of the Law of Effect. Science. 1933;77:173–175. doi: 10.1126/science.77.1989.173-a. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J. Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 1996;127:213–224. [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit. Rev. Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]