Abstract
Although the phosphatidylinositol 3-kinase to Akt to mammalian target of rapamycin (PI3K-Akt-mTOR) pathway promotes survival signaling, inhibitors of PI3K and mTOR induce minimal cell death in PTEN (phosphatase and tensin homolog deleted from chromosome 10 ) mutant glioma. Here, we show that the dual PI3K-mTOR inhibitor PI-103 induces autophagy in a form of glioma that is resistant to therapy. Inhibitors of autophagosome maturation cooperated with PI-103 to induce apoptosis through the mitochondrial pathway, indicating that the cellular self-digestion process of autophagy acted as a survival signal in this setting. Not all inhibitors of mTOR synergized with inhibitors of autophagy. Rapamycin delivered alone induced autophagy, yet cells survived inhibition of autophagosome maturation because of rapamycin-mediated activation of Akt. In contrast, adenosine 5′-triphosphate–competitive inhibitors of mTOR stimulated autophagy more potently than did rapamycin, with inhibition of mTOR complexes 1 and 2 contributing independently to induction of autophagy. We show that combined inhibition of PI3K and mTOR, which activates autophagy without activating Akt, cooperated with inhibition of autophagy to cause glioma cells to undergo apoptosis. Moreover, the PI3K-mTOR inhibitor NVP-BEZ235, which is in clinical use, synergized with the lysosomotropic inhibitor of autophagy, chloroquine, another agent in clinical use, to induce apoptosis in glioma xenografts in vivo, providing a therapeutic approach potentially translatable to humans.
INTRODUCTION
The ability of cells to sense and respond to growth factors and nutrients represents a fundamental requirement for survival. Under nutrient- and growth factor–poor conditions, decreased activation of the kinases Akt and mammalian target of rapamycin (mTOR), two key integrators of growth factor and nutrient signaling, leads to initiation of a catabolic program that enables cells to survive periods of starvation or stress [reviewed in (1)]. Under nutrient- and growth factor–rich conditions, growth factors signal through receptor tyrosine kinases (RTKs) to activate downstream kinases such as class IA phosphatidylinositol 3-kinases [principally PI3K α and β, as reviewed in (2)]. The PI3Ks in turn propagate downstream signals, including activation of Akt and mTOR, stimulating an anabolic program of protein synthesis and cell growth.
Tight regulation of the Akt-mTOR pathway enables cells to sense changes in their environment and survive both minor and major perturbations in the abundance of nutrients and growth factors. Akt signaling stimulates the activity of numerous downstream targets, including the proapoptotic proteins BAD (Bcl-2/Bcl-XL–associated death promotor), caspases 3 and 9, and FoxO (forkhead) family transcription factors, that act to tip the balance from survival toward apoptosis during periods of growth factor deprivation. Given the central role for Akt in cell survival, it is not surprising that Akt overactivation has been implicated in cancer. For example, malignant glioma, the most common primary brain tumor, is frequently associated with deletion or silencing of the gene encoding the lipid phosphatase PTEN (phosphatase and tensin homolog deleted from chromosome 10), which antagonizes Akt signaling [reviewed in (2)]. In both clinical and preclinical trials, PTEN deletion has been associated with resistance to therapy (3–5), supporting a role for the RTK-PI3K-Akt-mTOR axis in mediating cancer cell survival.
The initial enthusiasm for using inhibitors of PI3Ks, Akt, or mTOR as antineoplastic agents has been tempered by observations that inhibition of these kinases typically promotes growth arrest rather than cell death in solid tumors [reviewed in (6)]. Because mTOR is a target of both growth factor and nutrient signaling, its blockade is likely to activate one or more survival pathways that act to enable cells to endure periods of starvation or stress. Macroautophagy (hereafter called autophagy), a cellular self-digestion process that provides energy and nutrients during stress (7), is a good candidate for such a survival pathway (8). Indeed, experiments in the yeast Saccharomyces cerevisiae suggest that Tor is a key node central to control of autophagy (9).
Autophagy is an evolutionarily conserved process through which organelles and proteins are sequestered into autophagic vesicles (autophagosomes) within the cytosol [reviewed in (8)]. These vesicles then fuse with the lysosome, forming autophagolysosomes, which promote the degradation of intracellular contents. Microtubule-associated protein light chain 3 (LC3-I) is an abundant cytoplasmic protein that is cleaved and lipidated during initiation of autophagy (forming LC3-II), translocating to and associating with the autophagosome in a punctate pattern (10). Autophagy thus enables the cell to eliminate and recycle proteins or organelles to sustain metabolism and can be recognized in part by formation of LC3-II punctae.
Inhibition of autophagy promotes cancer cell death (11–13) and potentiates various anticancer therapies (14–24), implicating autophagy as a mechanism that enables tumor cells to survive antineoplastic therapy. The antimalarial drug chloroquine inhibits autophagy of glioma cells and has been tested as an antineoplastic agent in a small clinical study (25). The related molecule hydroxychloroquine is the subject of an ongoing Phase II study (14) and is a much-discussed option among patients who may self-medicate during therapy for glioma (26). Although chloroquine’s use in glioma was not predicated on the basis of its ability to inhibit autophagic degradation, this compound, like hydroxychloroquine, blocks lysosomal functions required for the terminal steps of autophagy (15).
Here, we showed that dual inhibitors of PI3K and mTOR signaling activated autophagy in glioma, and that inhibition of two distinct mTOR protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), induced autophagy in an additive fashion. Because the allosteric mTORC1 inhibitor rapamycin induces autophagy, we were surprised to find that inhibition of autophagosome maturation in the presence of rapamycin did not promote apoptosis. Rather, apoptosis was induced only when rapamycin was combined with inhibitors of both autophagosome maturation and PI3K. To understand why blockade of PI3K itself does not induce apoptosis but was critical to the induction of apoptosis by the combination of rapamycin and inhibitors of autophagosome maturation, we investigated the ability of rapamycin to induce autophagy and concurrently activate Akt. We found that rapamycin induced both autophagy and Akt phosphorylation as separate survival signals. Combining rapamycin with inhibitors of autophagy or of PI3K blocked only one of these, allowing cells to survive. In contrast, combining rapamycin with inhibitors of autophagy and of PI3K blocked both survival signals, resulting in apoptosis.
Furthermore, we showed that NVP-BEZ235, which inhibits both PI3K and mTOR signaling and is currently in Phase I/II clinical trials in solid tumors (27), cooperated with chloroquine to promote cell death in glioma. Because inhibitors of PI3K, mTOR, and autophagosome maturation are all in clinical trials or clinical use, this combination of agents represents a promising and translatable approach to cancer therapy.
RESULTS
A dual inhibitor of PI3K and mTOR induces autophagosome formation in glioma cells
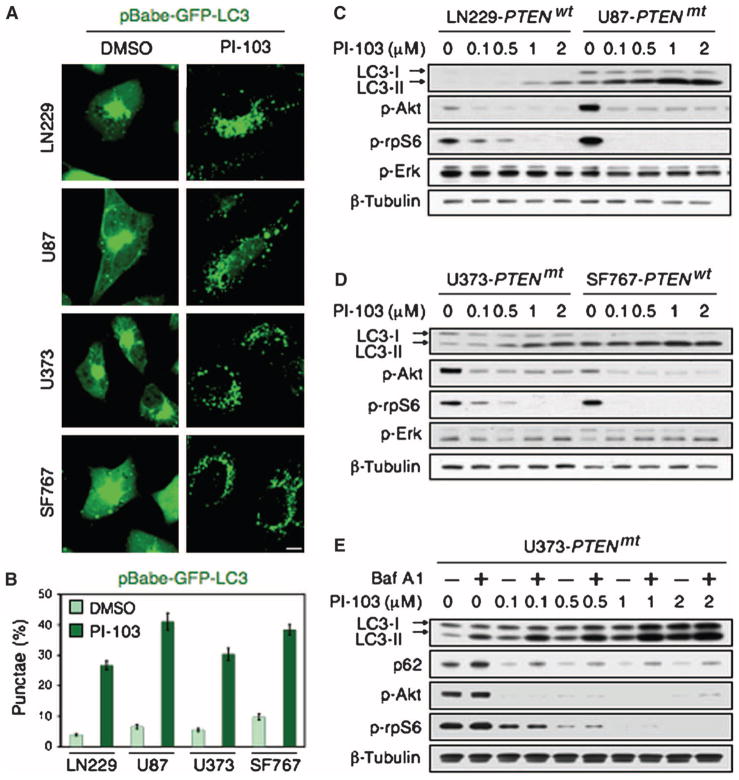
We found that PI-103, a small molecule that acts as a direct inhibitor of both PI3K and mTOR (28–30), induced autophagosome formation, as measured by punctate fluorescence of a GFP (green fluorescent protein)–LC3 fusion protein, in both PTEN wild-type (PTEN wt-LN229, SF767) and PTEN-deficient (PTEN mt-U87, U373) glioma cell lines (Fig. 1, A and B). Immunoblot analysis revealed that PI-103 induced the conversion of LC3-I to LC3-II in a dose-dependent manner. Furthermore, this conversion was independent of PTEN, because LC3-II was apparent in all cell lines tested (Fig. 1, C and D).
Fig. 1.
The dual PI3K and mTOR inhibitor PI-103 induces autophagy in PTENwt and PTENmt glioma cell lines. (A) PTENwt (LN229), PTENwt (SF767), PTENmt (U87), and PTENmt (U373) cell lines were transduced with pBabe-GFP-LC3 and treated with PI-103 (1 μM) for 48 hours. PI-103–treated cells show punctate patterns of GFP staining, typical of autophagy. (B) Quantification of punctate foci from (A). The percentage of cells accumulating LC3-GFP in vacuoles was quantified as the mean ± SD (five high-power microscopic fields were counted for each value). (C and D) Glioma cells wild type (LN229, SF767) or mutant (U87, U373) for PTEN were treated with PI-103 at concentrations shown, lysed, and analyzed by immunoblot. PI-103 decreased phosphorylation of Akt and that of the mTOR target p-rpS6 in all cell lines. Activation of autophagy was dose-dependent, as indicated by increased amounts of the autophagy marker LC3-II. (E) U373 cells were treated with PI-103 at concentrations shown for 48 hours, treated with 10 nM Baf A1 for 2 hours, lysed, and analyzed by immunoblot for p62, an LC3-binding protein and marker of autophagic flux. Degradation of p62 indicates induction of autophagy. β-Tubulin shown as a loading control.
We next treated U373 PTEN mt glioma cells with PI-103, followed by brief exposure to bafilomycin A1 (Baf A1), which inhibits vacuolar-type H+-ATPase (H+-dependent adenosine triphosphatase) and thereby blocks autophagosome maturation (31). Baf A1–treated cells showed increased conversion of LC3-I to LC3-II (Fig. 1E), likely due to autophagosome accumulation (32). PI-103 also induced degradation of the protein p62 (Fig. 1E), a process specific to autophagy (33, 34).
Inhibition of PI3K, mTOR, and autophagosome maturation induces apoptosis in PTENmt glioma
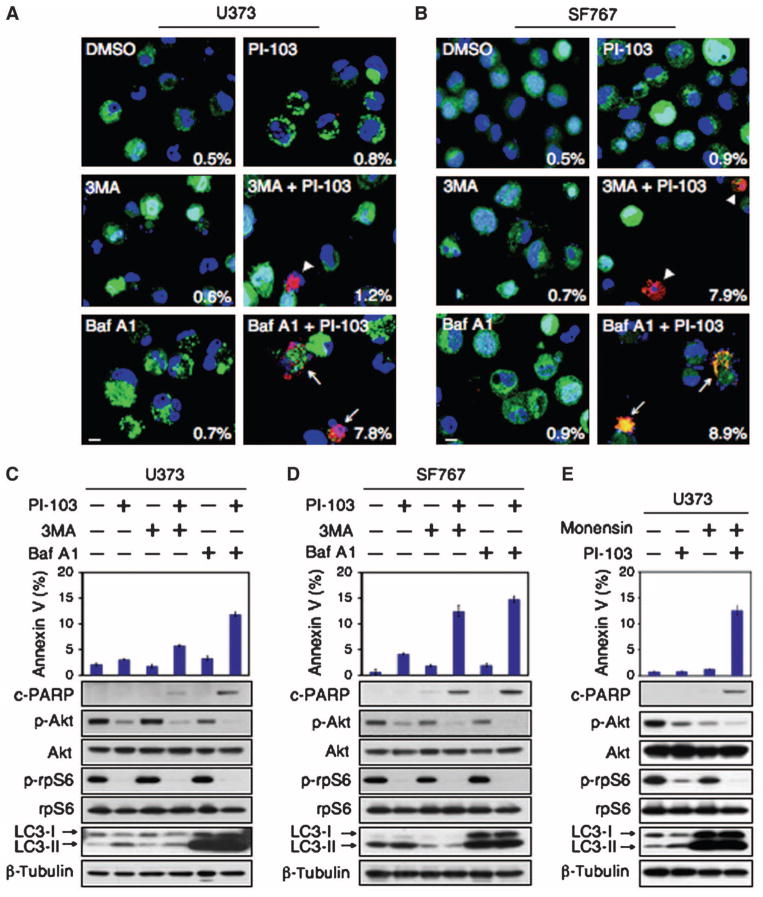
Inhibition of autophagy with lysosomotropic agents enhances the anti-neoplastic activity of radiation, chemotherapy, and targeted agents (15–24). We therefore wondered whether blocking the induction or progression of autophagy could promote cell death when combined with inhibition of PI3K and mTOR. No appreciable cell death was observed in PTEN wild-type or mutant glioma cells treated individually with PI-103, 3-methyladenine (3MA), which inhibits early stages of autophagosome formation (35), or Baf A1, which inhibits later stages of autophagosome maturation (Fig. 2, A to D, and fig. S1). In contrast, combining PI-103 with 3MA or Baf A1 led to significant apoptosis, measured by quantification of cells in the sub-G1 fraction, an indicator of DNA fragmentation (36), cleavage of caspase 3 and poly(adenosine 5′-diphosphate–ribose) polymerase (PARP), or annexin V flow cytometry (Fig. 2, A to D, and fig. S1).
Fig. 2.
PI-103 synergizes with Baf A1, an inhibitor of autophagosome maturation, to induce apoptosis in glioma. Glioma cells (U373, PTEN mt and SF767, PTEN wt) were transduced with pBabe-GFP-LC3, treated with DMSO or PI-103 (1 μM) for 24 hours, and then treated for 48 hours with either 3MA (5 mM) or Baf A1 (10 nM). (A and B) Cytospin preparations were stained with antibody against cleaved caspase 3 (red) or GFP-LC3 (green). Nuclei stained in blue (Hoechst dye). Arrowheads indicate cleaved caspase 3–positive cells, with percentages indicated. Arrows show cleaved caspase 3–positive cells with condensed GFP-LC3 punctate dots. Scale bar, 100 μm. (C and D) Apoptotic cells were analyzed by flow cytometry. Percentages of cells positive for annexin V are the mean ± SE for triplicate samples (U373: P < 0.0001 by Student’s t test for PI-103 plus 3MA versus DMSO; P < 0.0001 for PI-103 plus Baf A1 versus DMSO; SF767: P < 0.0001 by Student’s t test for PI-103 plus 3MA versus DMSO; P < 0.0001 for PI-103 plus Baf A1 versus DMSO). Lysed cells were analyzed by immunoblot with antisera indicated. (E) To exclude off-target effects of Baf A1 independent of lysosomal trafficking, we treated U373 PTEN mt glioma cells with 1 μM PI-103 or DMSO for 24 hours. Monensin (3 μM) was added where indicated (24 hours), and cells were analyzed by flow cytometry for annexin V. Data show error among triplicate measurements for each value (top panel). An aliquot of cells was analyzed by immunoblot with antisera indicated (bottom panel).
In PTENwt SF767 cells, apoptosis was equivalent when PI-103 was combined with either Baf A1 or 3MA. In contrast, PTEN mt U373 cells were more susceptible to combination therapy with PI-103 and Baf A1 than to PI-103 and 3MA (Fig. 2, A to D). To exclude off-target effects of Baf A1 independent of lysosomal trafficking, we treated cells with small interfering RNA (siRNA) directed against lysosome-associated membrane protein-2 (LAMP2), which is required for autophagosome maturation (37). PI-103 cooperated with LAMP2 siRNA to induce apoptosis, measured both by annexin V flow cytometry and by PARP cleavage (fig. S4). We next analyzed the effects of monensin, an antibiotic that inhibits autophagy by blocking fusion of the autophagosome with the lysosome (38). Like Baf A1, monensin synergized with PI-103 to induce apoptosis (Fig. 2E).
We also assessed the effects of PI-103 on mouse embryonic fibroblasts (MEFs) deleted for Atg5, which affects early steps of autophagosome formation (39, 40). PI-103 treatment induced apoptosis more frequently in Atg5 knockout MEFS than it did in wild-type controls (fig. S2). Together, these data indicate that blocking autophagy contributes to apoptosis when combined with PI-103. The combination of small-molecule inhibitors that was most effective at eliciting apoptosis in PTEN mt glioma cells used anti-autophagic agents that target late rather than early stages of autophagy.
Apoptosis proceeds through the mitochondria-dependent intrinsic pathway
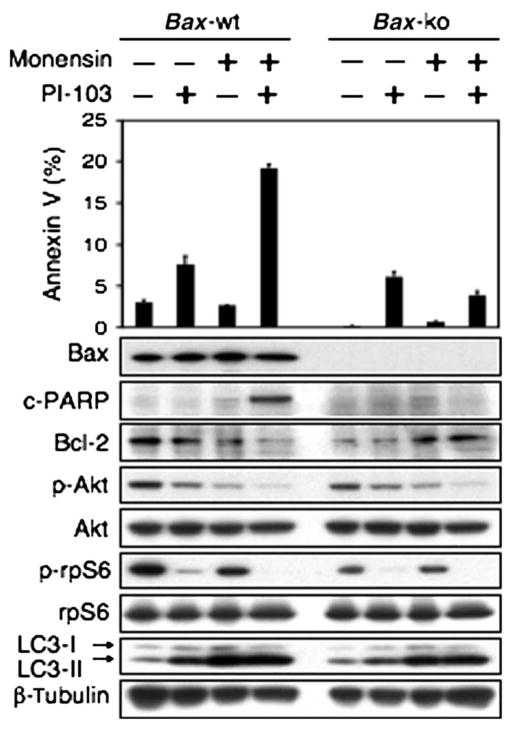
Apoptosis can be induced through stimulation of the transmembrane death receptors (extrinsic pathway) or through release of signal factors by mitochondria within the cell (intrinsic pathway). To clarify which of these pathways was activated in response to combination treatment with PI-103 and the lysosomal agent monensin, we used Bax wild-type or Bax-deficient MEFs in components of the apoptotic machinery, because Bax is a mitochondrial protein required for the intrinsic pathway of apoptosis (41). We tested the ability of PI-103 and monensin or a combination of the two to induce apoptosis in Bax wild-type or Bax-deficient MEFs. Basal apoptosis was decreased in Bax-deficient MEFs compared with that in wild-type MEFs. Treatment with PI-103 alone induced modest degrees of apoptosis in Bax wild-type or Bax-deficient MEFs, whereas monensin alone did not. Combination therapy with PI-103 and monensin led to apoptosis only in MEFs wild type for Bax as measured by annexin V flow cytometry. Induction of apoptosis in these experiments was correlated with decreased abundance of the antiapoptotic protein Bcl-2, as evidenced by 190% decreased abundance of Bcl-2 in Bax wild-type MEFs treated with PI-103 and monensin when compared with vehicle controls (Fig. 3). Although Bax is often redundant with Bak (leading many investigators to test MEFs deficient in both Bax and Bak) (41), a nonredundant role for Bax as an apoptotic regulator in neural cells has been demonstrated (8, 42), and we found that Bax deficiency alone was sufficient to block cell death induced by PI-103 plus monensin (Fig. 3). We conclude that PI-103 cooperates with monensin to elicit apoptosis through the intrinsic mitochondrial pathway that requires Bax.
Fig. 3.
Apoptosis induced by combined inhibition of PI3K and mTOR signaling and of lysosomal trafficking proceeds through the intrinsic mitochondrial pathway. MEFs wild type for Bax show modest apoptosis in response to PI-103 (1 μM) but not to monensin (3 μM). Combination therapy with monensin and PI-103 led to apoptosis only in cells wild type for Bax, demonstrating that apoptosis induced by monensin and PI-103 depends on the mitochondrial pathway. Data show error among triplicate measurements for each value (top panel). An aliquot of cells was lysed and analyzed by immunoblot with antisera indicated (bottom panel). A blot representative of two independent experiments is shown.
Inhibition of PI3K, mTORC1, mTORC2, and autophagy contributes to induction of apoptosis
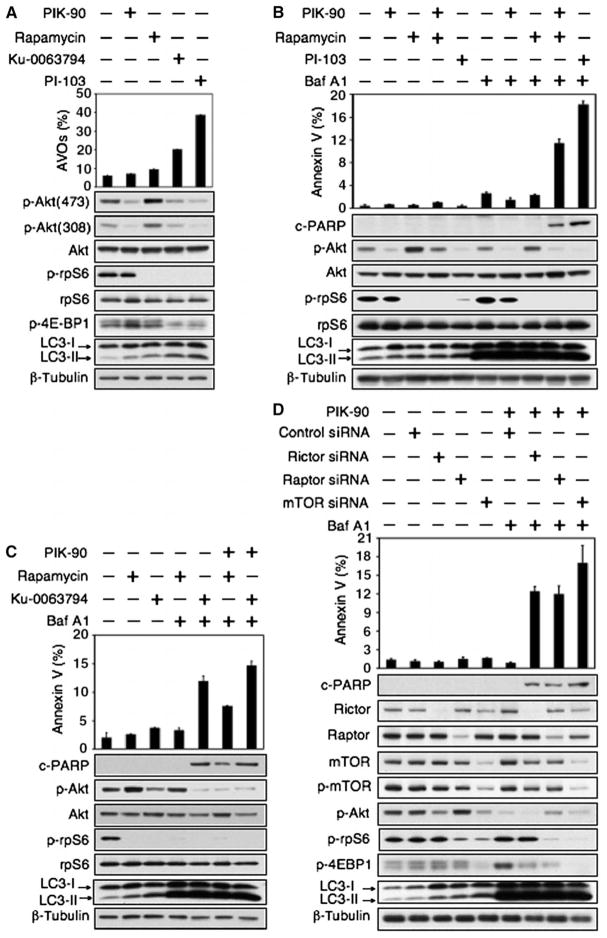
In addition to inhibitors that block both PI3K and mTOR, small-molecule inhibitors are also being developed against specific kinases, including PI3K, Akt, and mTOR (28, 29, 43, 44). To clarify whether representative inhibitors targeting these kinases induce autophagy, and whether autophagy inhibitors induce apoptosis in combination with inhibitors of PI3K, Akt, or mTOR, we extended our studies to analyze inhibitors of these kinases. Inhibitors of mTOR that bind to the catalytic site (mTOR kinase inhibitors) induce autophagy more potently than does rapamycin (44). Therefore, to separately probe roles for inhibition of PI3K and mTOR in the induction of autophagy by PI-103, we analyzed the effects of the PI3Kα inhibitor PIK-90, the allosteric mTORC1 inhibitor rapamycin (45), and the mTOR kinase inhibitor Ku-0063794 (46). We measured induction of autophagy in response to PIK-90, rapamycin, Ku-0063794, and PI-103 by immunoblot and by staining for acridine orange, which moves freely across biological membranes and accumulates in acidic vesicle organelles (AVOs) associated with autophagy (16, 47, 48). Consistent with a central role for mTOR blockade in the induction of autophagy, PIK-90 did not block phosphorylation of the mTOR target rpS6 (49) and only minimally induced either appreciable AVOs or LC3-II conversion (Fig. 4A and fig. S5). In contrast, rapamycin, Ku-0063794, and PI-103 all blocked p-rpS6, induced AVOs, and more efficiently induced LC3-II conversion (Fig. 4, A and B, and fig. S5).
Fig. 4.
Combined inhibition of mTOR and PI3K signaling and of autophagosome maturation leads to apoptosis in U373 PTEN mt glioma. (A) The mTOR kinase inhibitor Ku-0063794 induced autophagy more potently than did the allosteric mTORC1 inhibitor rapamycin or the PI3Kα inhibitor PIK-90. Cells were treated with DMSO, PIK-90 (1 μM), rapamycin (100 nM), Ku-0063794 (5 μM), or PI-103 (1 μM) for 48 hours and stained with acridine orange (1 μg/ml) for 15 min. Acidic vesicular organelles were quantified (top panel). Cells treated for 24 hours were examined by immunoblot with antisera shown (bottom panel). (B) Apoptosis induced by Baf A1 requires concurrent inhibition of PI3K and mTOR. U373 glioma cells were treated with PIK-90 (1 μM), rapamycin (100 nM), PIK-90 plus rapamycin, or PI-103 (1 μM) for 24 hours. Baf A1 (10 nM) was added for 48 hours, and cells were analyzed by flow cytometry for annexin V and by immunoblot. (C) Blockade of autophagosome maturation induces apoptosis when combined with Ku-0063794, or with rapamycin and PIK-90 in combination. U373 glioma cells were treated with 1 μM PIK-90, 5 μM Ku-0063794, 1 μM PIK-90 plus 100 nM rapamycin, or 1 μM PIK-90 plus 5 μM Ku-0063794 for 24 hours. Baf A1 (10 nM) was added for 48 hours, and cells were analyzed as in (B). (D) To probe a role for mTORC1 and mTORC2 in the induction of autophagy and in apoptosis, we transfected U373 glioma cells with control siRNA, or siRNA against rictor, raptor, or to mTOR itself (24 hours). Cells were subsequently treated with PIK-90 (1 μM) and Baf A1 (10 nM) for 48 hours and analyzed as in (B). A blot representative of two independent experiments is shown. Data shown are means ± SD for triplicate measurements in (A), (B), (C), and (D) (top panel).
Having established that mTOR blockade is necessary to induce autophagosome formation, and that an inhibitor of PI3K affected neither mTOR nor autophagy, we looked to see whether inhibition of PI3K or of mTOR could cooperate with Baf A1 to induce apoptosis. Single-agent treatment with Baf A1, rapamycin, PIK-90, Ku-0063794, or PI-103 failed to induce apoptosis in the PTEN mt cell line U373MG (Fig. 4, B and C). However, blockade of PI3K and mTOR with PIK-90 and rapamycin induced apoptosis in combination with Baf A1, as did the combinations of Ku-0063794 and Baf A1; Ku-0063794, PIK-90, and Baf A1; and PI-103 and Baf A1 (Fig. 4, B and C).
To determine whether mTORC1 and mTORC2 have independent roles in the induction of autophagy, we treated U373 glioma cells with siRNA directed against components of mTORC1 [raptor (50, 51)], mTORC2 [rictor (52)], or both [mTOR itself (45, 53, 54)], analyzing the effects of these siRNAs alone or in combination with the PI3K inhibitor PIK-90 and the lysosomal agent Baf A1. Knockdown of raptor, rictor, or mTOR each induced autophagy, measured by the appearance of LC3-II (Fig. 4D). The amount of LC3-II produced in response to siRNA directed against mTOR was greater than that observed with siRNA directed against either raptor or rictor; similarly, there was increased apoptosis upon addition of PIK-90 and Baf A1 to siRNA directed against mTOR, in comparison with addition of PIK-90 and Baf A1 to siRNA directed against either raptor or rictor (Fig. 4D). We conclude that both mTORC1 and mTORC2 contribute to the formation of autophagosomes.
We evaluated the importance of Akt blockade by comparing the effects of the PI3K inhibitor PIK-90 with those of AktI-1/2, a PH (pleckstrin homology) domain–dependent isozyme-selective inhibitor of Akt1 and Akt2 (43). Using U373 PTEN mt glioma cells, we analyzed the effects of PIK-90 and AktI-1/2 alone or in combination with rapamycin and Baf A1 (fig. S3). Glioma cells generally uncouple signaling between Akt and mTOR (49); consistent with this, both PIK-90 and AktI-1/2 blocked phosphorylation of Akt without affecting that of the mTOR target rpS6 (fig. S3). Although neither agent induced cell death in isolation, both synergized with rapamycin and Baf A1 to induce apoptosis (fig. S3).
Because the class III PI3K Vps34 links nutrient sensing to mTOR (55, 56), we tested the ability of siRNA directed against Vps34 to inhibit mTOR activity and to affect autophagy. Knockdown of Vps34 only slightly reduced phosphorylation of the downstream mTOR target rpS6, modestly blocked conversion of LC3-I to LC3-II, and induced a small degree of apoptosis in combination with PI-103 (fig. S4B).
Rapamycin induces both autophagosome formation and p-Akt as separate survival signals
Inhibition of PI3K was required for induction of cell death by the combination of Baf A1 and PI-103 (Fig. 4). Consistent with this, the combination of Baf A1, rapamycin, and PIK-90 also induced apoptosis (Fig. 4, B and C). However, inhibition of autophagosome maturation with Baf A1 failed to induce apoptosis in combination with either rapamycin or PIK-90 alone. If rapamycin alone induces autophagosome formation, why does apoptosis require the combined inhibition of autophagy, mTOR, and PI3K? In investigating the basis for this conundrum, we were struck by the ability of rapamycin to induce Akt activation, as evidenced by a 170% increase in phosphorylated Akt (p-Akt) in cells treated with rapamycin versus dimethyl sulfoxide (DMSO), P = 0.021, Student’s t test or a 130% increase with siRNA directed against raptor when compared with vehicle controls (Fig. 4).
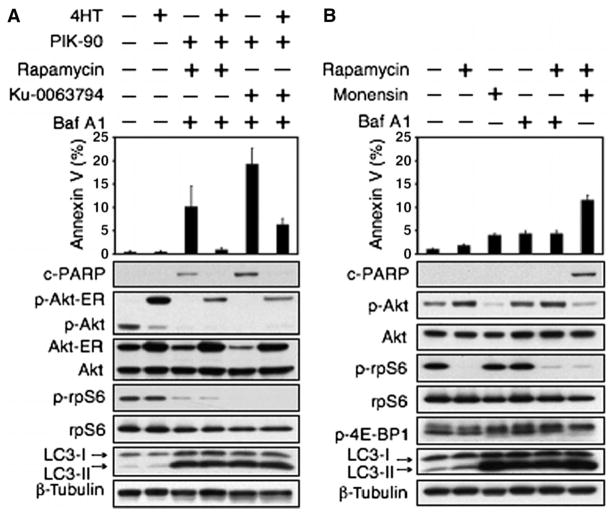
To determine whether feedback activation of Akt contributed to the failure of rapamycin plus Baf A1 to induce apoptosis, we generated a PTEN mt glioma cell line (U373) in which the activity of Akt could be regulated independently of small-molecule inhibitors of PI3K and mTOR. Using cells carrying an allele of Akt fused to the steroid-binding domain of the estrogen receptor (Akt-ER) (57), an agent that activates known Akt targets (58–62), we showed that combining Baf A1 and PIK-90 with Ku-0063794 or rapamycin, without activating Akt-ER, induced PARP cleavage and increased the abundance of annexin V–fluorescein isothiocyanate (FITC) (Fig. 5A). Addition of the estrogen antagonist 4-hydroxytamoxifen (4HT) activated Akt-ER in these cells and blocked apoptosis driven by Baf A1, rapamycin, and PIK-90, and by Baf A1, PIK-90, and Ku-0063794 (Fig. 5A). These results confirm that apoptosis (observed in response to combining inhibitors of mTOR with inhibitors of autophagosome maturation) also requires inhibition of Akt.
Fig. 5.
Activation of Akt cooperates with induction of autophagy to promote therapeutic resistance in glioma. (A) U373 glioma cells were transduced with Akt-ER, an allele of Akt fused to the steroid-binding domain of the ER (57 ). Cells were treated with PIK-90 (1 μM), rapamycin (100 nM), or Ku-0063794 (5 μM) for 24 hours. Baf A1 (10 nM) was added in the presence or absence of 4HT (500 nM) for 48 hours. Cells were analyzed by annexin V–FITC flow cytometry and by immunoblot. Activation of Akt-ER blocked apoptosis in cells treated with Baf A1, rapamycin, and PIK-90 or with Baf A1, Ku-0063794, and PIK-90. These data suggest that the induction of p-Akt serves as a survival signal, promoting resistance to apoptosis in glioma. (B) Monensin also blocks activation of Akt (Figs. 2E and 3). To address whether monensin could synergize with rapamycin to induce death, we treated U373 glioma cells with monensin (3 μM), rapamycin (100 nM), or both agents in combination (48 hours). Cells were analyzed by annexin V–FITC flow cytometry and by immunoblot. Monensin cooperated with rapamycin to induce cell death.
That inhibition of both Akt signaling and autophagy might contribute to apoptosis has previously been shown by others (20) and is supported by data in Fig. 5B, which shows apoptosis only in laneswith little p-Akt. Because monensin blocked both autophagy and Akt phosphorylation (Fig. 2E), we treated U373 glioma cells with monensin and rapamycin and found that monensin cooperated with rapamycin to induce apoptosis, bypassing the need for a third agent that targeted either PI3K or Akt (Fig. 5B). We conclude that dual inhibitors of PI3K and mTOR induce autophagy as a survival signal, and blockade of autophagosome maturation in this setting leads to apoptosis. In contrast, rapamycin induces both autophagy and activation of Akt as separate survival signals. This Akt-dependent survival signal blocks the cytotoxic effect of inhibitors of autophagosome maturation in rapamycin-treated cells. Subsequent blockade of PI3K (through inhibitors of PI3Kor Akt, or through use of monensin, a lysosomotropic agent that blocks both autophagy and Akt activation) (Fig. 5B) abrogates this second survival signal, leading to apoptosis.
Clinical inhibitors of PI3K and mTOR synergize with clinical inhibitors of autophagosome maturation to induce apoptosis in vivo
Dual inhibitors of PI3K and of mTOR are now being tested in cancer patients (27), whereas chloroquine, a drug that blocks autophagosome maturation (63), is a well-established clinical antimalarial agent. To test whether clinically used inhibitors of PI3K and mTOR and autophagosome maturation can induce apoptosis in glioma, we treated glioma cells with the Novartis compound NVP-BEZ235 (64), which is now being tested in clinical trials, and with the generic antimalarial agent chloroquine, which raises lysosomal pH, thereby impairing degradation of proteins in the autophagosome (63). NVP-BEZ235 induces autophagy in glioma cell lines and promotes survival in mice bearing U87 intracranial glioma xenografts (65).
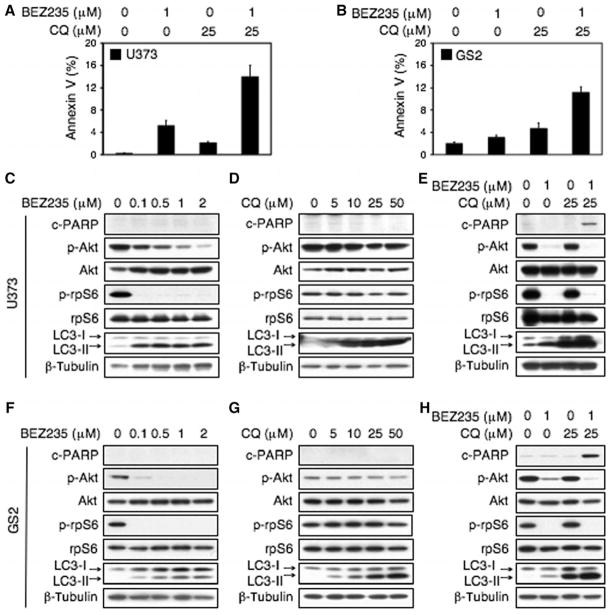
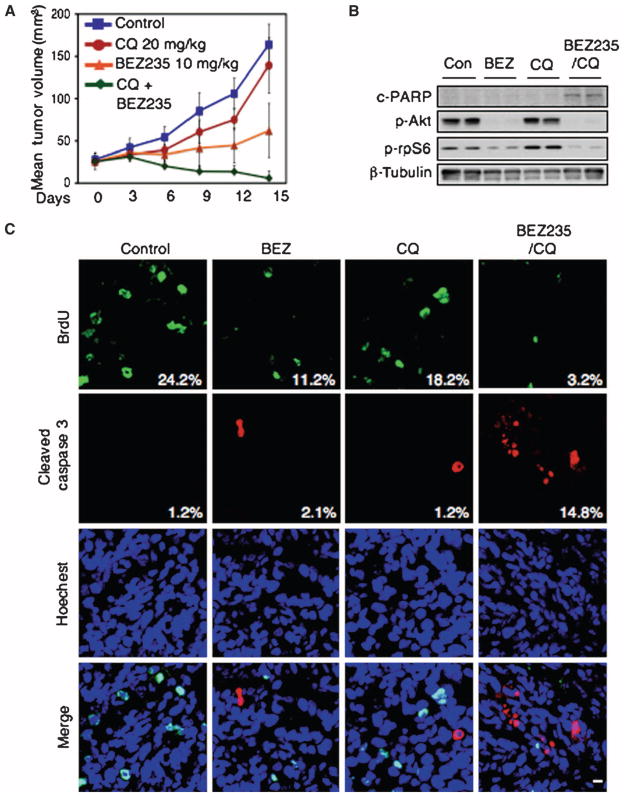
Using U373 and GS2 cell lines, we demonstrated that NVP-BEZ235 and chloroquine could cooperate to induce apoptosis (annexin Vand PARP cleavage) compared with either agent alone (Fig. 6). To translate these results to an in vivo setting, we established xenografts from GS2 (66, 67). All animals with established xenografts of GS2 survived treatment with NVP-BEZ235, chloroquine, or combination treatment without significant changes in overall body weight or behavior. The combination of NVP-BEZ235 and chloroquine caused tumor regression, whereas monotherapy with NVP-BEZ235 or chloroquine slowed tumor growth (Fig. 7A). Necropsies revealed no obvious toxicity of mono- or combination therapies. Analyses of treated tumors confirmed that the combination of NVP-BEZ235 and chloroquine induced a marked increase in apoptosis (Fig. 7, B and C). Quantification of five high-power microscopic fields per animal, five animals per group, demonstrated an increase in cleaved caspase 3 from 1.2% of cells showing staining for cleaved caspase 3 (chloroquine monotherapy) to 14.8% (NVP-BEZ235 plus chloroquine, P < 0.0001, Student’s t test). Apoptosis (caspase cleavage) was similar in animals treated with monotherapy: 1.2% control versus 2.1% for NVP-BEZ235 monotherapy (P = 0.4793, Student’s t test) and 1.2% control versus 1.2% for chloroquine monotherapy (P = 0.8822, Student’s t test).
Fig. 6.
The clinically used drugs BEZ235 and chloroquine cooperate to cause apoptosis in PTEN mt glioma cell lines. U373 PTENmt cells and cells derived from a PTEN mt xenograft of glioblastoma multiforme (GS2) were treated with the PI3K-mTOR inhibitor NVP-BEZ235 (BEZ) or the antimalarial autophagy and lysosomal trafficking inhibitor chloroquine (CQ) or both drugs. (A and B) BEZ and CQ cooperate to induce apoptosis (annexin V flow cytometry) in U373 and GS2 cells. Cells were treated with BEZ or vehicle (24 hours); CQ was then added where indicated (48 hours). (C to H) Dose response with single agents and effects of combination treatment in U373 and GS2 cells. The combination of BEZ and CQ induced apoptosis in both cell lines, whereas neither agent alone induced apoptosis (assessed by PARP cleavage). (C, D, F, and G) BEZ and CQ treatments were for 48 hours. (E and H) Treatments were as in (A) and (B).
Fig. 7.
NVP-BEZ235 and chloroquine cause apoptosis and induce regression of established GS2 flank xenografts. (A) Animals with established tumors were treated by daily intraperitoneal injection as shown, n = 5 per group. BrdU was added 2 hours before animals were killed (day 16). Animals were killed 30 min after their last treatment with vehicle, chloroquine, BEZ, or combination therapy. (B and C) Residual tumors were analyzed by immunoblot (B) and by immunofluorescence staining for BrdU (a measure of proliferation) and cleaved caspase 3, a measure of apoptosis (C). Cells positive for BrdU and cleaved caspase 3 were counted in five high-power microscopic fields. Numbers denote the percentage of BrdU-and cleaved caspase 3–positive cells. Quantification of five high-power microscopic fields from five animals per group demonstrated an increase in cleaved caspase 3 levels from 1.2% (chloroquine monotherapy) to 14.8% (NVP-BEZ235 plus chloroquine; P < 0.0001, Student’s t test). The amount of apoptosis was similar in animals treated with monotherapy, 1.2% control versus 2.1% for NVP-BEZ235 monotherapy (P = 0.4793, Student’s t test), 1.2% control versus 1.2% for chloroquine monotherapy (P = 0.8822, Student’s t test). Scale bar, 100 μm.
DISCUSSION
Autophagy is a cellular process of cannibalization that, depending on context, can promote or block cell death. It provides a mechanism through which cancer cells can survive stress, including stresses imposed by therapy. In glioma in particular, the alkylating agent temozolomide and the mTOR inhibitor rapamycin both induce autophagy (17, 68), although whether autophagy promotes cell survival or death in response to these agents remains unclear.
PI3K and mTOR are individually central to survival and to autophagy. Inhibition of mTORC1 and mTORC2 blocks glucose uptake and glycolysis (69), slowing tumor growth, and inducing autophagy as a survival pathway (12, 70). Given interest from both scientists and patients in understanding whether autophagy induced by agents that inhibit both PI3K and mTOR promotes or blocks cancer growth (71), we documented induction of autophagy in glioma cell lines by the dual PI3K and mTOR inhibitor PI-103. We demonstrated further that blockade of autophagy at the level of lysosomal trafficking led to enhanced cell death in response to PI-103. These observations highlight the importance of autophagy as a survival signal in response to targeting the PI3K-Akt-mTOR axis in glioma (Table 1).
Table 1.
Summary of combination therapies targeting the PI3K-Akt-mTOR and autophagic pathways to induce apoptosis in glioma.
| Agent | Target | Reference | Agent status | Results |
|---|---|---|---|---|
| PI-103 | PI3K and mTOR catalytic site | (28–30) | Preclinical | Induced autophagy. Cooperated with Baf A1 and monensin individually to induce apoptosis. |
| PIK-90 | PI3K | (29) | Preclinical | Induced autophagy weakly. Cytostatic in combination with rapamycin or Baf A1. Apoptosis required rapamycin, PIK-90, and Baf A1 in combination. |
| AktI-1/2 | PH domain of Akt1 and Akt2 | (43) | Preclinical | Induced autophagy weakly. Cytostatic in combination with Baf A1. Apoptosis required AktI-1/2, rapamycin, and Baf A1 in combination. |
| Rapamycin | mTORC1 | (45) | Clinical trials | Induced autophagy. Stimulated Akt phosphorylation. Cytostatic in combination with PIK-90 or Baf A1. Apoptosis required rapamycin, PIK-90, and Baf A1 in combination. |
| NVP-BEZ235 | PI3K and mTOR catalytic site | (64) | Clinical trials | Induced autophagy. Cooperated with chloroquine to induce apoptosis. |
| Ku-0063794 | mTOR catalytic site | (46) | Preclinical | Induced autophagy. Cooperated with Baf A1 to induce apoptosis. |
| 3MA | hVps34 (blocks autophagosome formation) | (35) | Preclinical | Cooperated with PI-103 to induce apoptosis. |
| Baf A1 | Vacuolar type H+-ATPase (blocks autophagosome maturation) | (31) | Preclinical | Cooperated with PI-103 to induce apoptosis. |
| Monensin | Na+/H+ antiporter (blocks autophagosome maturation) | (38) | Preclinical | Cooperated with PI-103 to induce apoptosis. |
| Chloroquine | Heme crystallization (blocks autophagosome maturation) | (63) | Clinical | Cooperated with NVP-BEZ235 to induce apoptosis. |
To dissect the importance of mTORC1 and mTORC2 to autophagy, we compared the allosteric mTORC1 inhibitor rapamycin, the ATP-competitive mTOR inhibitor Ku-0063794, and the ATP-competitive PI3K-mTOR kinase inhibitor PI-103. Both PI-103 and Ku-0063794 induced AVOs more potently than did rapamycin. As a likely consequence, blockade of autophagosome maturation promoted apoptosis more effectively in response to knockdown of components of mTORC1 and mTORC2 in combination, when compared to knockdown of components specific to mTORC1 (raptor) or mTORC2 (rictor). These data indicate a role for mTORC2 as well as one for mTORC1 in the induction of autophagy in glioma.
Rapamycin also induced autophagy in glioma; however, blockade of autophagosome maturation in conjunction with rapamycin did not result in cell death. We showed that Akt signaling plays a central role in promoting resistance to the combination of rapamycin with inhibitors of autophagy. We demonstrated that a feedback loop linking allosteric inhibitors of mTOR to Akt activation blocked apoptosis independently of autophagy. Although the existence of this feedback loop has been the subject of intense study in cancer (28, 72, 73), our data document a functional role for rapamycin-driven feedback activation of Akt. Activation of Akt phosphorylation blocked the induction of apoptosis that might otherwise be observed in combining inhibitors of autophagy with rapamycin. The concurrent use of a PI3K inhibitor in combination with rapamycin blocked this feedback loop and—in conjunction with inhibition of autophagosome maturation—promoted apoptosis in glioma.
The observation that PI-103 cooperates with lysosomal agents to induce apoptosis has been made in the prostate cancer cell line PC3 (20). Our study provides mechanistic insights into these earlier observations, delineating how perturbations in signaling through PI3K, Akt, and mTOR influence both autophagy and the ability of small-molecule inhibitors selective among these three kinases to cooperate with lysosomal agents. First, we clarified the roles of mTORC1 and mTORC2 as independent regulators of autophagy. Second, we demonstrated that a feedback loop driven by rapamycin activates Akt, abrogating the ability of lysosomal agents to cooperate with rapamycin and promote apoptosis. Finally, we extended these observations to a wide panel of glioma cell lines and to the use of a PI3K-mTOR inhibitor now in clinical trials in combination with a lysosomal agent now in clinical use. Although mutation of PTEN is generally associated with therapeutic resistance in glioma and other cancers (3), we found that the combination of NVP-BEZ235 and chloroquine led to apoptosis of PTEN mt glioma in an in vivo xenograft model, offering a translatable approach to therapy of patients with this generally lethal tumor.
MATERIALS AND METHODS
Cell lines and reagents
Human glioma cell lines LN229, SF763, U373, and U87; human primary glioma GS2 cells; and Atg-5-wt, Atg-ko, Bax-wt, and Bax-ko MEFs were grown in 1 or 10% fetal bovine serum (FBS). 3MA, Baf A1, acridine orange, monensin, and chloroquine were purchased from Sigma Chemical Co. Rapamycin was purchased from Cell Signaling. Akt inhibitor VIII was purchased from EMD Biosciences. PIK-90, PI-103, and Ku-0063794 were synthesized as described (29, 74). NVP-BEZ235 was a gift from Novartis Pharma AG.
Detection and quantification of AVOs
Cells were treated with the indicated inhibitors for 48 hours, stained with acridine orange (1 μg/ml) for 15 min, washed with phosphate-buffered saline (PBS), trypsinized, and then collected in phenol red–free growth medium. Green (510 to 530 nm) and red (650 nm) fluorescence emissions from 1 × 105 cells illuminated with blue (488 nm) excitation light were measured with a FACSCalibur from Becton-Dickinson with CellQuest software. To quantify GFP-LC3 punctae, we counted five random fields in five high-power microscopic fields; cells with more than 10 GFP-LC3 punctate dots were considered to be GFP-LC3–positive cells.
Immunoblotting
Membranes were blotted with antibodies directed against p-Akt (Ser473), p-Akt (Thr308), Akt (pan), p-S6 ribosomal protein (Ser235/236), S6 ribosomal protein, rictor, raptor, p-mTOR (Ser2448), mTOR, p-4E-BP1 (Thr37/46), p-Erk (Thr202/Tyr204), Bcl-2, cleaved PARP (Asp214), Bax, Vps34 (all from Cell Signaling), p62 (Progen), LC3 (Novus), LAMP2 (Santa Cruz Biotechnology), or β-tubulin (Upstate Biotechnology). Bound antibodies were detected with horseradish peroxidase–linked antibody against mouse or antibody against rabbit immunoglobulin G (IgG; Amersham), followed by ECL (Amersham).
Apoptosis detection
Apoptosis was detected by measurement of sub-G1 fraction (75), by staining for cleaved caspase 3 (28), or by flow cytometry for annexin V–FITC per the manufacturer’s protocol (annexin V–FITC detection kit, BioVision). Percentages of cells positive for cleaved caspase 3 were quantified; cells were transferred onto slides by means of a cytospin apparatus, fixed in 4% paraformaldehyde, permeabilized for 5 min (0.1% Triton X-100 in PBS), incubated overnight at 4°C with rabbit polyclonal antibody against cleaved caspase 3 (1:200, Cell Signaling), and then incubated at room temperature (RT) for 1 hour with Alexa Fluor 555–conjugated secondary antibody against rabbit (Molecular Probes). Nuclei were labeled with Hoechst. Cells were mounted with Vectashield media (Vector Laboratories Inc.) and counted in five high-power fields with a Zeiss 510 LSM confocal microscope.
pBabe-GFP-LC3 transduction and siRNA transfection
To generate retrovirus, we cotransfected the packaging cell line 293Twith plasmids expressing gag/pol and VSVg (vesicular stomatitis virus glycoprotein), using Effectene transfection reagent (Qiagen). High-titer virus was collected at 48 hours and used to infect cells as previously described (76). pBabe-GFP-LC3 (77)–transduced glioma cells were treated with DMSO or 1 μM PI-103 for 48 hours and visualized by confocal laser scanning microscopy. Control siRNA was purchased from Santa Cruz Biotechnology. siRNAs against LAMP2, Vps34, rictor, raptor, and mTOR were purchased from Dharmacon and transfected with Lipofectamine 2000 (Invitrogen) as previously described (76).
Histological and immunohistochemical analyses
For indirect immunofluorescence, mice were injected with a single dose of bromodeoxyuridine (BrdU; 100 mg/kg), and tumors were harvested 2 hours later. Sections were incubated in 60% formamide in 2× SSC at 54°C for 30 min. DNA was denatured in 2 N HCl in 0.1% Triton X-100 (v/v) for 30 min and neutralized with 0.1 M Na2B4O7·10H2O (pH 8.5). Sections were washed in PBS, and then blocked in PBS containing 0.1% Triton X-100 and 5% normal goat serum for 30 min. Sections were incubated overnight at 4°C with rat monoclonal antibody against BrdU (1:200, Accurate Chemical) and then with Cy2-conjugated donkey antibody against rat IgG (H + L; 1:200, Jackson ImmunoResearch Laboratories) at RT for 1 hour. For cleaved caspase 3 staining, sections were permeabilized, incubated with antibody against cleaved caspase 3 (Cell Signaling), washed, and incubated with Alexa Fluor 555–conjugated antibody against rabbit (1:200; Molecular Probes). Nuclei were labeled with Hoechst.
Sections and cells were mounted with Vectashield mounting media (Vector Laboratories) and analyzed by confocal microscopy.
Xenografts
Human primary GS2 cells (106) (67) were injected subcutaneously just caudal to the left forelimb in 4- to 6-week-old female Balb/c nu/nu mice (Harlan Sprague Dawley). After tumors were established (50 to 100 mm3), five mice per group were randomly allocated to treatment with chloroquine (20 mg/kg; Sigma) in PBS, NVP-BEZ235 (10 mg/kg; Novartis) in 70% DMSO, chloroquine (20 mg/kg) plus NVP-BEZ235 (10 mg/kg), and 70% DMSO alone (control), delivered by daily intraperitoneal injection. Tumor diameters were measured with calipers at 3-day intervals, and tumor volumes (in cubic millimeters) were calculated by the following formula: volume = width2 × length/2. Each value represented the mean tumor volume ± SE obtained from five mice.
Supplementary Material
Acknowledgments
We thank N. Chen, M. Prados, and J.-P. Upton for useful discussions; C. Garcia-Echeverria, W. HackI, E. Harney, and S.-M. Maira for NVP-BEZ235; and H. Phillips for GS2 cells.
This work was supported by grants from Accelerate Brain Cancer Cure, the Brain Tumor Society, Burroughs Wellcome Fund, the Howard Hughes Medical Institute, and NIH Specialized Programs of Research Excellence grant P50CA097257, and the Samuel Waxman Cancer Research Foundation.
Footnotes
Author contributions: Q.-W.F., C.C., W.A.W., and C.H. designed and performed the experiments and analyzed the data. All authors were involved in the interpretation of data and manuscript content. Q.-W.F. and W.A.W. wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 2.Kok K, Geering B, Vanhaesebroeck B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem Sci. 2009;34:115–127. doi: 10.1016/j.tibs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma—animal models and therapeutic challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 10.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 12.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 13.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.http://clinicaltrials.gov/ct2/show/NCT00486603.
- 15.Bursch W, Ellinger A, Kienzl H, Torök L, Pandey S, Sikorska M, Walker R, Hermann RS. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: The role of autophagy. Carcinogenesis. 1996;17:1595–1607. doi: 10.1093/carcin/17.8.1595. [DOI] [PubMed] [Google Scholar]
- 16.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 17.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 18.Górka M, Daniewski WM, Gajkowska B, Lusakowska E, Godlewski MM, Motyl T. Autophagy is the dominant type of programmed cell death in breast cancer MCF-7 cells exposed to AGS 115 and EFDAC, new sesquiterpene analogs of paclitaxel. Anticancer Drugs. 2005;16:777–788. doi: 10.1097/01.cad.0000171514.50310.85. [DOI] [PubMed] [Google Scholar]
- 19.Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, Cleveland JL. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl–mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degtyarev M, De Maziere A, Orr C, Lin J, Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, Davis DP, Stern HM, Murray LJ, Hoeflich KP, Klumperman J, Friedman LS, Lin K. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68:1485–1494. doi: 10.1158/0008-5472.CAN-07-0562. [DOI] [PubMed] [Google Scholar]
- 22.Qadir MA, Kwok B, Dragowska WH, To KH, Le D, Bally MB, Gorski SM. Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res Treat. 2008;112:389–403. doi: 10.1007/s10549-007-9873-4. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H. Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann Surg Oncol. 2009;16:761–771. doi: 10.1245/s10434-008-0260-0. [DOI] [PubMed] [Google Scholar]
- 24.Shingu T, Fujiwara K, Bögler O, Akiyama Y, Moritake K, Shinojima N, Tamada Y, Yokoyama T, Kondo S. Inhibition of autophagy at a late stage enhances imatinib-induced cytotoxicity in human malignant glioma cells. Int J Cancer. 2009;124:1060–1071. doi: 10.1002/ijc.24030. [DOI] [PubMed] [Google Scholar]
- 25.Sotelo J, Briceño E, López-González MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 26.http://csn.cancer.org.
- 27.http://clinicaltrials.gov/ct2/show/NCT00620594?term=bez235&rank=1.
- 28.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, Bjerke LM, Kelland L, Valenti M, Patterson L, Gowan S, de Haven Brandon A, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Patel S, Saghir N, Parker P, Waterfield M, Workman P. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 32.Rubinsztein DC, Cuervo AM, Ravikumar B, Sarkar S, Korolchuk V, Kaushik S, Klionsky DJ. In search of an “autophagomometer”. Autophagy. 2009;5:585–589. doi: 10.4161/auto.5.5.8823. [DOI] [PubMed] [Google Scholar]
- 33.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 35.Seglen PO, Gordon PB. 3-Methyladenine: Specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G Nomenclature Committee on Cell Death 2009. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 38.Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 40.Mizushima N, Sugita H, Yoshimori T, Ohsumi Y. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J Biol Chem. 1998;273:33889–33892. doi: 10.1074/jbc.273.51.33889. [DOI] [PubMed] [Google Scholar]
- 41.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shindler KS, Latham CB, Roth KA. bax deficiency prevents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci. 1997;17:3112–3119. doi: 10.1523/JNEUROSCI.17-09-03112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: Discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 46.García-Martínez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traganos F, Darzynkiewicz Z. Lysosomal proton pump activity: Supravital cell staining with acridine orange differentiates leukocyte subpopulations. Methods Cell Biol. 1994;41:185–194. doi: 10.1016/s0091-679x(08)61717-3. [DOI] [PubMed] [Google Scholar]
- 48.Stankiewicz M, Jonas W, Hadas E, Cabaj W, Douch PG. Supravital staining of eosinophils. Int J Parasitol. 1996;26:445–446. doi: 10.1016/0020-7519(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 49.Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, McCormick F, Shokat KM, Weiss WA. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 51.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 52.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 53.Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci USA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 55.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 57.Mirza AM, Kohn AD, Roth RA, McMahon M. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 2000;11:279–292. [PubMed] [Google Scholar]
- 58.Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, Shelley SA, Jove R, Tsichlis PN, Nicosia SV, Cheng JQ. AKT1/PKBα kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of ανβ3 and α5β1 integrins. Mol Cell Biol. 2004;24:1505–1515. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He YY, Council SE, Feng L, Chignell CF. UVA-induced cell cycle progression is mediated by a disintegrin and metalloprotease/epidermal growth factor receptor/AKT/cyclin D1 pathways in keratinocytes. Cancer Res. 2008;68:3752–3758. doi: 10.1158/0008-5472.CAN-07-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janes SM, Watt FM. Switch from ανβ5 to ανβ6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol. 2004;166:419–431. doi: 10.1083/jcb.200312074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981;90:665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, García-Echeverría C. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 65.Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, Maira SM, Garcia-Echevrria C, Yung WK. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 67.Günther HS, Schmidt NO, Phillips HS, Kemming D, Kharbanda S, Soriano R, Modrusan Z, Meissner H, Westphal M, Lamszus K. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27:2897–2909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 68.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 69.Edinger AL, Linardic CM, Chiang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63:8451–8460. [PubMed] [Google Scholar]
- 70.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kroemer G, Levine B. Autophagic cell death: The story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 74.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan QW, Specht KM, Zhang C, Goldenberg DD, Shokat KM, Weiss WA. Combinatorial efficacy achieved through two-point blockade within a signaling pathway—a chemical genetic approach. Cancer Res. 2003;63:8930–8938. [PubMed] [Google Scholar]
- 76.Fan QW, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM, Weiss WA. A dual phosphoinositide-3-kinase α/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morgenstern JP, Land H. Advanced mammalian gene transfer: High titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.