Abstract
Interferon Regulatory Factor-1 (IRF-1) is a transcription factor that regulates gene expression during immunity. We hypothesize that IRF-1 plays a pivotal role in liver transplant (LTx) ischemia/reperfusion (I/R) injury. Mouse orthotopic LTx was conducted after 24 hours cold storage in UW solution in wild type (WT) C57BL/6 and IRF-1 knock out (KO) mice. IRF-1 deficiency in liver grafts, but not in recipients, resulted in significant reduction of hepatocyte apoptosis and liver injury, as well as improved survival. IRF-1 mRNA upregulation was typically seen in graft hepatocytes in WT→WT LTx. Deficiency of IRF-1 signaling in graft resulted in significantly reduced mRNA levels for death ligands and death receptors in hepatocytes, as well as decreased caspase-8 activities, indicating that IRF-1 mediates death ligand-induced hepatocyte death. Further, a smaller but significant IRF-1 mRNA upregulation was seen in WT graft non-parenchymal cells (NPC) and associated with IFN-γ mRNA upregulation exclusively in NPC. IFN-γ mRNA was significantly reduced in IRF-1 KO graft. Thus, IRF-1 in graft hepatocytes and NPC has distinct effects in hepatic I/R injury. However, LTx with chimeric liver grafts showed that grafts lacking hepatocellular IRF-1 had better protection compared to those lacking IRF-1 in NPC. The study identifies a critical role for IRF-1 in liver transplant I/R injury.
Keywords: IRF-1, TRAIL, transplantation, ischemia, Fas
Introduction
Graft injury due to hypothermic storage and warm reperfusion is a major problem complicating liver transplantation (LTx). Although all liver grafts exhibit some degree of preservation damage, patients receiving grafts with severe preservation injury suffer poor early liver function and are more susceptible to a variety of complications (1, 2). However, the initiating events that account for early graft damage are only partially understood (3, 4).
Interferon regulatory factor (IRF)-1, a transcription factor originally identified as a regulator of IFN-α/β, is known to be involved in many aspects of innate and adaptive immune responses (5–7). IRF-1 mRNA is expressed constitutively in all cell types, and IRF-1 protein levels are regulated at the transcriptional level. IRF-1 is upregulated in response to various stimuli such as IFNs (type I and II), double stranded RNA, cytokines, and hormones (8). Nuclear translocation of IRF-1 results in activation of not only IFNs (7), but also various type of immune-active genes such as iNOS and IL-12, particularly in immune cells (9, 10).
IRF-1 is also known as a regulator of apoptosis induced in various cell types with a variety of mechanisms (11, 12). IRF-1 induces a ligand-independent caspase-8-mediated apoptosis in breast cancer cells (12). Importance of IRF-1 in apoptosis is also shown in the liver, and hepatocytes from IRF-1 deficient mice are resistant to apoptosis induction by IFN-γ (11). Further, IRF-1 deficient mice show less liver injury in concanavalin A-induced hepatitis model and LPS/D-Galactosamine hepatitis model (13, 14).
In the context of the hepatic ischemic injury, we have previously reported that IRF-1 was upregulated in hepatic warm ischemia model and IRF-1 deficient mice have less hepatic damage (15). However, the role of endogenous IRF-1 in liver transplant ischemia reperfusion (I/R) injury has not been examined. Further, the pathophysiology of “cold” I/R injury during liver transplantation is not identical to warm I/R injury, and the signaling mechanisms of IRF-1 -mediated liver injury have not been defined. Therefore, we tested the hypothesis that IRF-1 plays a pivotal role in hepatic I/R injury associated with LTx. Using IRF-1 deficient mice as donors and/or recipients, the study demonstrates that IRF-1 expression in graft cells, but not in recipient cells, is responsible for liver transplant I/R injury, in part by promoting hepatocyte apoptosis. We also show that both hepatocytes and non-parenchymal cells (NPC) express IRF-1 during hepatic I/R injury. However, using bone marrow (BM) chimeras to generate chimeric donor liver grafts, IRF-1 expression in graft hepatocytes is more crucial to liver I/R injury.
Materials and Methods
Animals
Both C57BL/6 (IRF-1 +/+; WT) and C57BL/6 background IRF-1 deficient mice (IRF-1 −/−; KO) were purchased from the Jackson Laboratory. Animals were maintained in a laminar flow, specific pathogen-free atmosphere at the University of Pittsburgh.
Orthotopic Liver Transplantation (LTx)
The basic techniques of liver harvesting and syngeneic orthotopic liver transplantation without hepatic artery reconstruction were based on the method described by Qian et al (16). Liver grafts were perfused with 1.0 ml of University of Wisconsin (UW) solution via the portal vein, and stored in UW solution for 24 hours at 4 °C. Anhepatic time averaged 19.8±1.7 min.
WT and KO mice were used for both donors and recipients in this study. Recipient animals were sacrificed at 1 – 24 hours after reperfusion for serum and liver graft samples. Separate groups of animals were followed for 7 days to determine the roles of IRF-1 on graft survival. All procedures in this study were performed according to the guidelines of the National Research Council’s Guide for Care and Use of Laboratory animals and approved by the Council on Animal Care at the University of Pittsburgh.
BM Chimeras
BM chimeras were created by BM transplantation between WT and KO mice. BM cells were collected from long bones of the extremities of WT or KO mice, and 2 × 107 cells were intravenously injected into lethally (9.5 Gy) irradiated WT or KO mice via the penile vein. Animals were used as liver graft donors >2 month after BM transplantation.
Alanine Aminotransferase (ALT) Levels
Serum ALT levels were measured using the Opera Clinical Chemistry System (Bayer Co., Tarrytown, NY).
Routine and Immunohistopathology
Liver graft tissues were fixed in 10% formalin, embedded in paraffin, sectioned and stained with H&E. The percentage of necrotic area was estimated by random evaluation of 5 low power fields (× 40) per each H&E section.
Apoptosis was determined in formalin-fixed paraffin-embedded graft sections using the in situ end-labeling technique (Apop Tag Peroxidase Kit, Intergen Co., Purchase, NY) as described previously (17). For cleaved caspase-3, formalin-fixed paraffin-embedded sections of liver grafts were stained (17) using anti-cleaved caspase-3 antibody (Asp175: Cell Signaling, Danvers, MA).
Real-time RT-PCR
mRNA expression was quantified by SYBR Green real-time RT-PCR using ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) as previously described (17) using primers shown in supplementary Table 1. The expression of each gene was normalized to β-actin mRNA content and calculated relative to normal liver.
Serum IFN-γ Levels
Serum samples were analyzed for IFN-γ levels using ELISA kit (R&D Systems, Minneapolis, MN).
Western Blot
Western blot was performed using cytoplasmic protein and nuclear protein (20 µg) as previously described (17). Membranes were incubated with primary antibody for IRF-1 (Santa Cruz, Santa Cruz, CA), Sam68, cleaved caspase-3 (Cell Signaling), and actin (Sigma Aldrich, Saint Louis, MO), and developed with the SuperSignal detection systems (Thermo Scientific, Rockford, IL).
Caspase Activity Assay
Caspase-8 activity was measured using Caspase-Glo 8 assay systems (Promega, Madison, WI), following the method previously described (18).
Isolation of Hepatocytes and NPC
Hepatocytes and hepatic NPC were isolated from the liver grafts by the collagenase digestion method of Berry and Friend (19). The initial cell suspension was filtrated through a 70 µm nylon mesh, and hepatocytes and NPC in a crude fraction were separated by low-speed centrifugation (five times at 45 g for 5 min).
Statistical Analysis
Data are represented as the mean ± SEM. Comparisons between the groups at different time points were performed by using the Student’s t test or ANOVA. Logrank test was performed to evaluate survival study. Differences were considered significant at a P value less than 0.05.
Results
IRF-1 deficiency in the graft, but not in the host, ameliorated transplant induced hepatic I/R injury
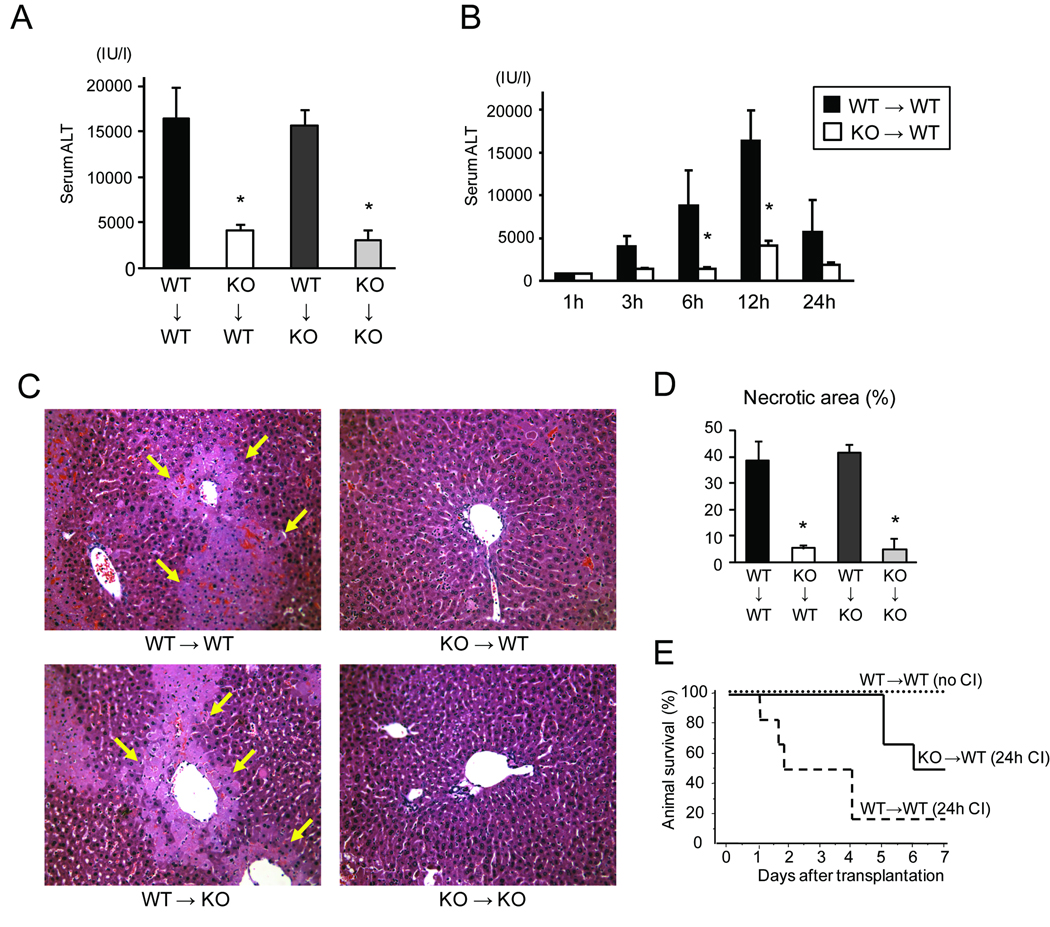
To examine the roles of IRF-1 in transplant-induced liver I/R injury, LTx between IRF-1 KO and WT mice were performed with 24 hours cold storage in UW solution which is known to produce a severe preservation injury (17, 20). Serum ALT levels increased to >15,000 IU/L at 12 hours after WT→WT LTx with significant hepatic necrosis mainly in zone 2 and 3 in H&E sections (Figure 1A, C, and D). In contrast, KO→KO LTx resulted in significantly lower ALT levels and necrotic area when compared to WT→WT LTx. Interestingly, IRF-1 KO→WT LTx showed serum ALT levels and necrotic areas similar to those in KO→KO LTx. In contrast, WT→KO LTx was not protected, and the degree of I/R injury was similar to those seen in the WT→WT LTx. Sequential analysis of serum ALT levels confirmed that KO→WT LTx showed lower ALT levels than WT→WT at all time points (Figure 1B). These results clearly indicate that IRF-1 signaling in the donor liver graft (but not the recipient) is crucial in mediating liver damage post-transplant.
Figure 1. Hepatic injury after liver transplantation (LTx) between IRF-1 +/+ (WT) and IRF-1 −/− (KO) mice with 24 hours cold preservation.
(A) Serum ALT levels at 12 hours after LTx in WT→WT (n=7), KO→WT (n=4), WT→KO (n=4), and KO→KO (n=3) combinations. KO→WT and KO→KO groups showed significantly lower ALT levels (*<0.05), compared to those in WT→WT at 12 hours.
(B) Serum ALT levels at different time points after reperfusion in WT→WT and KO→WT LTx groups. ALT levels in KO→WT LTx were significantly lower (*p<0.05) at 6 and 12 hours after reperfusion, compared to those in WT→WT LTx at the same time points. (1h WT n=2, 1h KO n=2, 3h WT n=4, 3h KO n=3, 6h WT n=6, 6h KO n=5, 12h WT n=7, 12h KO n=4, 24h WT n=3, 24h KO n=2)
(C) Representative histopathological images of liver grafts at 12 hours after reperfusion. Arrows indicate necrotic area. H&E stain, original magnification ×200.
(D) Percentages of necrotic area at 12 hours in liver grafts. KO→WT and KO→KO, but not WT→KO, LTx group showed significantly less hepatic necrotic area at 12 hours after reperfusion (*p<0.05), compared to WT→WT (n=4 for each group).
(E) Data shown are survival rates of animals in WT→WT LTx without cold ischemia (no CI) (n=4), WT→WT LTx with 24 hours cold ischemia (24h CI) (n=6), and KO→WT LTx with 24 hours CI (n=6). Recipient survival after LTx with 24 hours CI was significantly better in KO→WT than WT→WT group (p<0.05, Logrank test).
IRF-1 deficiency in the graft improved animal survival after hepatic I/R injury
To further examine the extent of hepatic protection in IRF-1 KO liver grafts, we tested liver graft survival in WT→WT and KO→WT LTx. WT→WT LTx without cold storage resulted in 100% recipient survival for 7 days (Figure 1E). However, when WT animals received WT liver grafts with 24 hours cold storage, only 17% survived for 7 days. In contrast, WT animals that received IRF-1 KO grafts with 24 hours cold storage showed significantly improved animal survival of 50%. These results further support a critical role for liver graft IRF-1 in mediating transplant I/R injury, and show that absence of endogenous IRF-1 in the liver graft during a severe preservation injury ultimately improves survival.
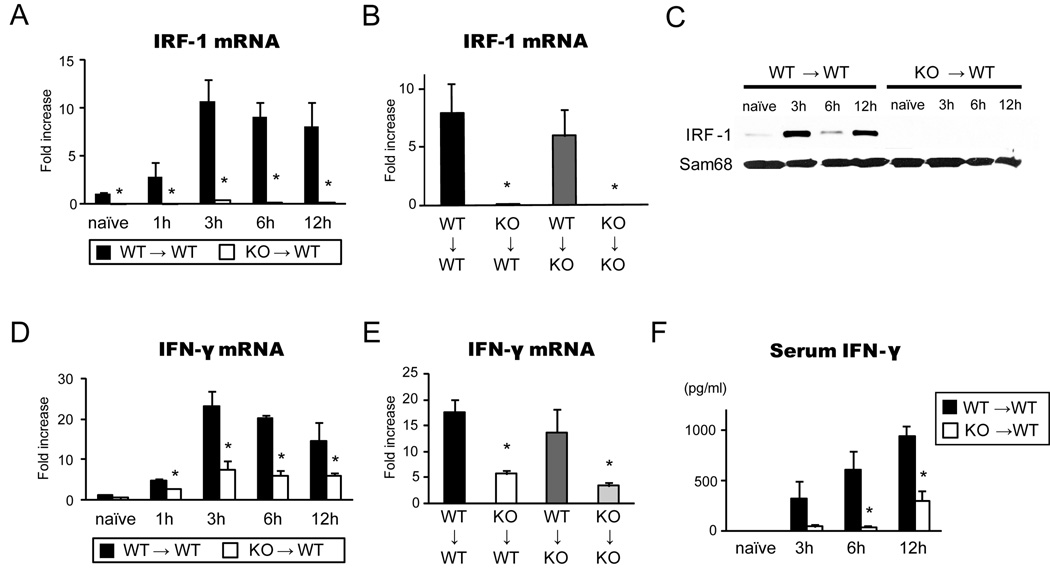
Hepatic IRF-1 mRNA and protein expression were induced after LTx
As IRF-1 is transcriptionally regulated (5, 6), we examined the expression of endogenous hepatic IRF-1 mRNA in transplant-induced liver I/R injury. Hepatic IRF-1 mRNA levels promptly increased (>10-fold) after WT→WT LTx, reached peak levels at 3 hours, and were maintained at high levels for 12 hours (Figure 2A). As expected, hepatic IRF-1 mRNA levels were barely detectable after KO→WT LTx. Small increases in IRF-1 mRNA in IRF-1 KO grafts at 3–12 hours appeared to be caused by WT host infiltrating cells. When WT grafts were transplanted into KO recipients, the degree of IRF-1 mRNA induction in the liver graft was similar to the WT→WT group, while KO→KO LTx did not induce IRF-1 mRNA upregulation (Figure 2B). Nuclear IRF-1 protein was also markedly increased at 3 – 12 hours after WT→WT, but not in KO→WT, LTx (Figure 2C).
Figure 2. IRF-1 and IFN-γ levels in liver grafts during hepatic I/R injury.
(A) Time course of graft IRF-1 mRNA levels in WT→WT and KO→WT LTx. Liver grafts were stored for 24 hours in UW solution and transplanted into relevant recipients. Liver graft samples were obtained at 1–12 hours of reperfusion (n=3 for each time point) and analyzed by SYBR Green real-time RT-PCR. *p<0.05 vs. WT→WT
(B) IRF-1 mRNA levels in liver graft at 12 hours in 4 different groups of LTx (n=3 for each group). *p<0.05 vs. WT→WT
(C) IRF-1 protein expression in the nuclear extracts of liver grafts at different time points. IRF-1 protein expression increased in WT→WT, but not in KO→WT, LTx. Representative images of Western blot of liver samples (n=3). Sam68 was used as internal controls of nuclear proteins.
(D) Time course of IFN-γ mRNA levels in liver grafts from WT→WT (n=3 for each time point) and KO→WT LTx (n=3 for each time point). *p<0.05 vs. WT→WT
(E) IFN-γ mRNA expression in liver grafts in 4 different groups at 12 hours after reperfusion (n=3 for each group). *p<0.05 vs. WT→WT
(F) Time course of serum IFN-γ levels in WT→WT (n=3) and KO→WT (n=3) LTx. Serum IFN-γ was significantly reduced in KO→WT LTx group at 3, 6 and 12 hours after reperfusion when compared to WT→WT group (* p<0.05 vs. WT→WT).
IFN-γ mRNA and protein were reduced in IRF-1 KO graft
IFN-γ is known to be a crucial cytokine activating IRF-1 gene transcription (6, 15, 21), and also is a downstream molecule of IRF-1 (21, 22). Hence we examined the hepatic graft IFN-γ mRNA levels as well as circulating serum IFN-γ protein levels. Hepatic I/R injury strongly upregulated IFN-γ mRNA (~20-fold) at 3–12 hours after WT→WT LTx (Figure 2D). In contrast, in KO→WT LTx IFN-γ̣ mRNA levels were significantly reduced at all time points examined. Among 4 different LTx groups, IFN-γ mRNA expression was significantly suppressed in KO→WT and KO→KO groups, while both WT→WT and WT→KO groups showed comparable IFN-γ mRNA upregulation at 12 hours (Figure 2E). Serum IFN-γ levels correspondingly increased after WT→WT LTx. In KO→WT LTx, serum IFN-γ levels were significantly low compared to WT→WT LTx (Figure 2F). Thus, deficiency in IRF-1 significantly decreased (but did not totally eliminate) IFN-γ mRNA and serum IFN-γ release. This suggests there are IRF-1- independent, as well as IRF-1–dependent pathways to produce IFN-γ.
Since production of type I IFNs and IL-12 have been shown to be regulated by IRF-1 (7, 10), we also examined for type I IFNs (IFN-α and IFN-β) and IL-12 in the liver grafts at 1–12 hrs after LTx. Using RT-PCR, all three cytokines showed significant hepatic mRNA up-regulation after LTx, peaking 3 hrs after reperfusion, and decreasing by 12 hrs after LTx (data not shown). Interestingly, there was no significant difference in mRNA levels of these molecules comparing WT→WT vs KO→WT LTx groups (data not shown), suggesting that production of type I IFN and IL-12 are not strongly regulated by IRF-1 in this model.
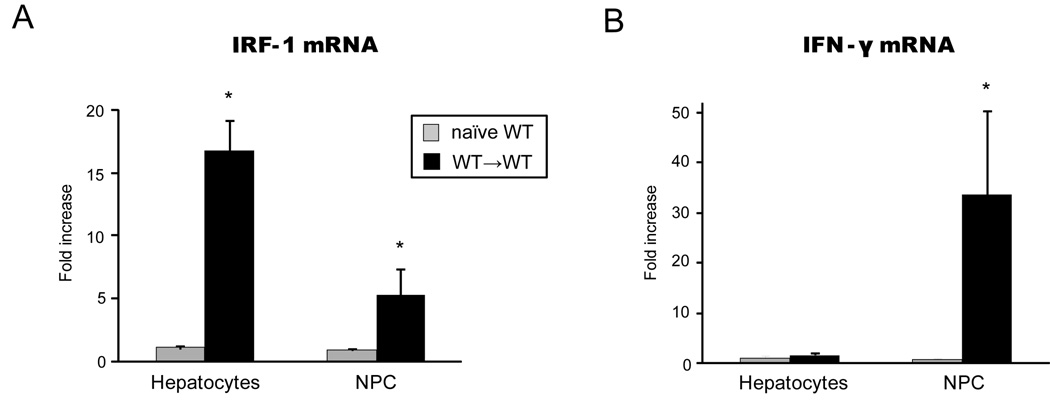
IRF-1 and IFN-γ were differently upregulated in graft hepatocytes vs. NPC
To determine the specific hepatic cell fractions responsible for IRF-1 and IFN-γ upregulation in I/R injury, we isolated hepatocytes and NPC from liver grafts in WT→WT LTx for RT-PCR analysis. Hepatocyte fractions isolated from liver grafts at 3 hours after reperfusion showed >15-fold increase in IRF-1 mRNA levels compared to those in naïve hepatocytes isolated from normal liver (Figure 3A). On the other hand, NPC after LTx showed ~5-fold increase over naïve NPC (Figure 3A). Thus, IRF-1 mRNA induction after LTx was seen in both hepatocytes and NPC cells, although to a much greater degree in hepatocytes. In contrast, IFN-γ mRNA was upregulated exclusively in the NPC fraction (Figure 3B). Thus graft hepatocytes and NPC have distinctive roles in hepatic I/R injury by upregulating mRNA for IRF-1 and IFN-γ, respectively.
Figure 3. IRF-1 and IFN-γ mRNA expression in hepatocytes and NPC.
(A) IRF-1 mRNA expression in hepatocytes and NPC in hepatic I/R injury. Liver grafts were obtained at 3 hours after WT→WT LTx. Hepatocytes and NPC fractions were isolated and analyzed by SYBR Green real-time PCR. Data were shown as fold increases compared to naïve hepatocytes and NPC, respectively (n=3 each). IRF-1 mRNA levels were largely increased in hepatocyte fraction (>15 folds), but IRF-1 mRNA was also upregulated to a lesser extent in graft NPC. (*p<0.05 vs. naïve WT)
(B) IFN-γ mRNA expression in hepatocytes and NPC at 3 hours after WT→WT LTx. Data were shown as fold increases compared to naïve hepatocytes and NPC, respectively (n=3 each). Upregulation of IFN-γ mRNA expression was only seen in NPC at 3 hours after reperfusion. (*p<0.05 vs. naïve WT)
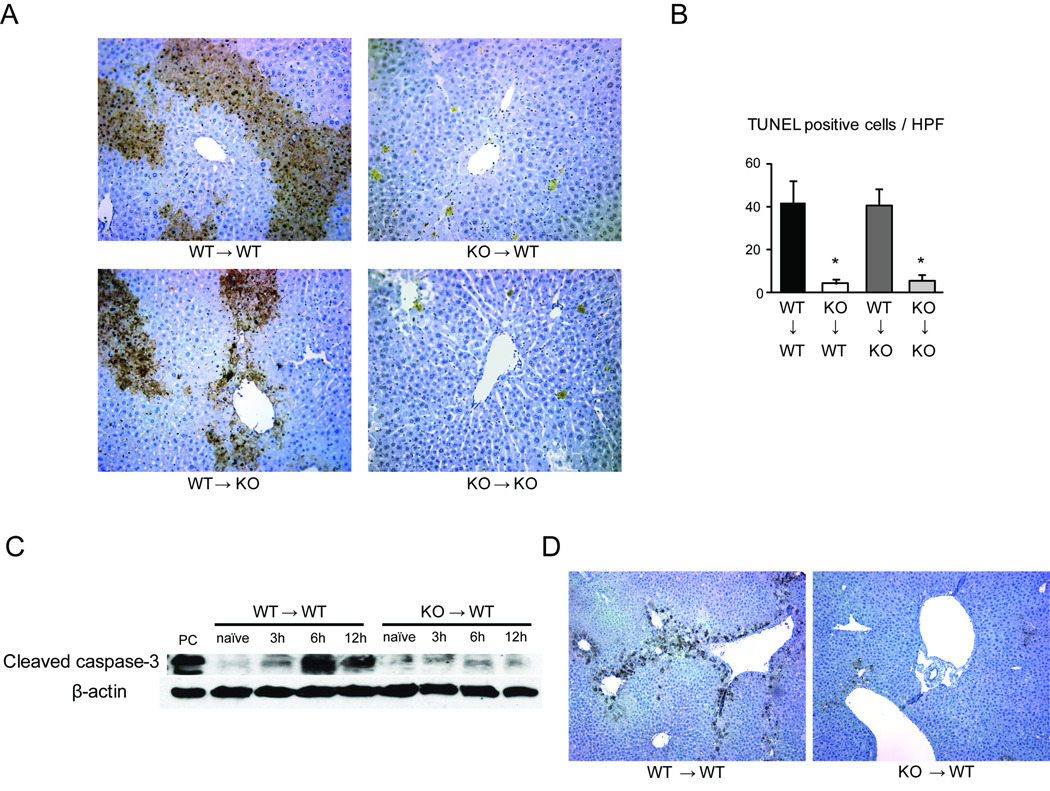
Lack of IRF-1 in the liver graft significantly decreased hepatocyte apoptosis
As IRF-1 signaling has been known to contribute to apoptosis, we next assessed hepatocyte apoptosis in this model. The number of TUNEL+ hepatocytes significantly increased by 12 hours after LTx in the WT→WT and WT→KO groups (Figure 4A and 4B). In contrast, only small numbers of TUNEL+ hepatocytes were seen in KO→WT and KO→KO LTx. These results correlated with the hepatocellular necrosis pattern and serum ALT levels, and suggested a role for IRF-1 in promoting hepatocyte apoptosis during LTx I/R injury. The significant reduction of hepatocyte apoptosis in KO liver grafts correlated with significantly lower cleaved caspase-3 protein expression in KO→WT, compared to WT→WT at 6 and 12 hours after LTx (Figure 4C). By immunohistochemistry, cleaved caspase-3 positive hepatocytes were seen mainly in zone 3 at 6 hours after reperfusion in WT→WT LTx (Figure 4D). In contrast, KO→WT LTx showed substantially less cleaved caspase-3 staining.
Figure 4. IRF-1 deficiency in liver grafts associated with suppression of hepatocyte apoptosis.
(A) TUNEL staining of liver samples at 12 hours after 4 different LTx. Abundant TUNEL positive hepatocytes (brown) were found in WT→WT and WT→KO LTx. KO→WT and KO→KO LTx showed only few TUNEL positive cells (original magnification ×200).
(B) The numbers of TUNEL positive cells were quantified (n=3 for each group). *p<0.05 vs. WT→WT
(C) Cleaved caspase-3 protein expression in liver grafts. After WT→WT and KO→WT LTx, liver grafts obtained at 3, 6, and 12 hours were analyzed by Western blot. Cleaved caspase-3 expression was significantly less in KO→WT than in WT→WT LTx. Representative images of 3 experiments.
(D) Cleaved caspase-3 staining of liver grafts at 6 hours after reperfusion. Cleaved caspase-3 was expressed on hepatocytes in WT→WT LTx. KO→WT LTx show few positive hepatocytes (original magnification ×100).
LTx-mediated activation of the extrinsic apoptotic pathway was inhibited in IRF-1 deficient grafts
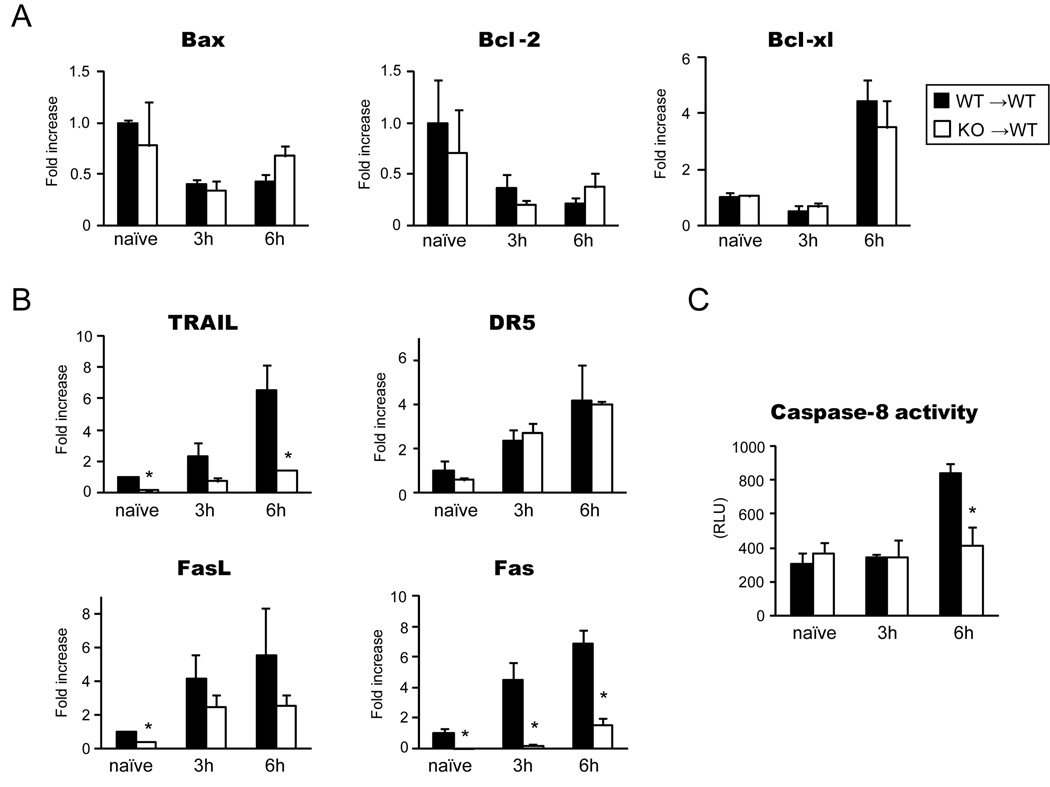
To define the mechanisms of hepatocyte apoptosis mediated by IRF-1 during hepatic I/R injury, we first conducted RT-PCR for molecules involved in the intrinsic pathway of apoptosis. However, Bcl-2–associated X protein (Bax), Bcl-2 and Bcl-xL mRNA expressions were not significantly different between WT→WT and KO→WT LTx (Figure 5A).
Figure 5. Influence of IRF-1 deficiency on intrinsic and extrinsic pathways of apoptosis.
(A) mRNA expression of genes related to the intrinsic apoptotic pathway. RT-PCR for Bax, Bcl-2 and Bcl-xL using liver grafts obtained at 3 and 6 hours of WT→WT and KO→WT LTx showed similar mRNA levels (n=3 for each group at each time point). Naïve liver tissues were obtained from untreated WT or IRF-1 KO animals (n=3 for each). Data were shown as fold increase compared to WT naïve liver.
(B) mRNA expression of genes in the extrinsic apoptotic pathway. TRAIL, DR5, FasL, Fas mRNA expression were analyzed by quantitative RT-PCR using liver grafts from WT→WT and KO→WT LTx at 3 and 6 hours (n=3 for each group at each time point). Data were shown as fold increase compared to WT naïve liver. (* p<0.05 vs. WT→WT LTx)
(C) Caspase-8 activity was measured using Caspase-Glo 8 assay systems. Data were shown as Relative Luminescence Units (RLU). Significant decreases of caspase-8 activities were seen at 6 hour after reperfusion in KO→WT LTx when compared to WT→WT control. (n=3 for each group at each time point) *p<0.05 vs. WT→WT LTx
Next we assessed mRNA expression of genes in the extrinsic pathway of apoptosis. TRAIL, a death ligand with high homology to FasL, triggers extensive apoptosis via the binding to TRAIL receptors, Death receptor 4 (DR4) and DR5, recruiting Fas-associated protein with death domain (FADD), and cleaving caspase-8 (23). TRAIL mRNA was significantly increased at 3 and 6 hours after WT→WT LTx, but was suppressed in KO→WT LTx. Basal TRAIL mRNA expression was noticeably lower in naïve IRF-1 KO livers than in WT (Figure 5B). On the other hand, TRAIL receptor DR5 mRNA levels were not different between the two groups. Likewise, Fas mRNA levels were significantly reduced in KO grafts at 3 and 6 hours after LTx compared to WT grafts. FasL mRNA had a trend toward decreased expression in the IRF-1 KO grafts. Furthermore, caspase-8 activities were significantly reduced in KO→WT than in WT→WT LTx at 6 hours after reperfusion. These results indicate that IRF-1 signaling regulates hepatocyte apoptosis via the extrinsic pathway during hepatic I/R injury (Figure 5C).
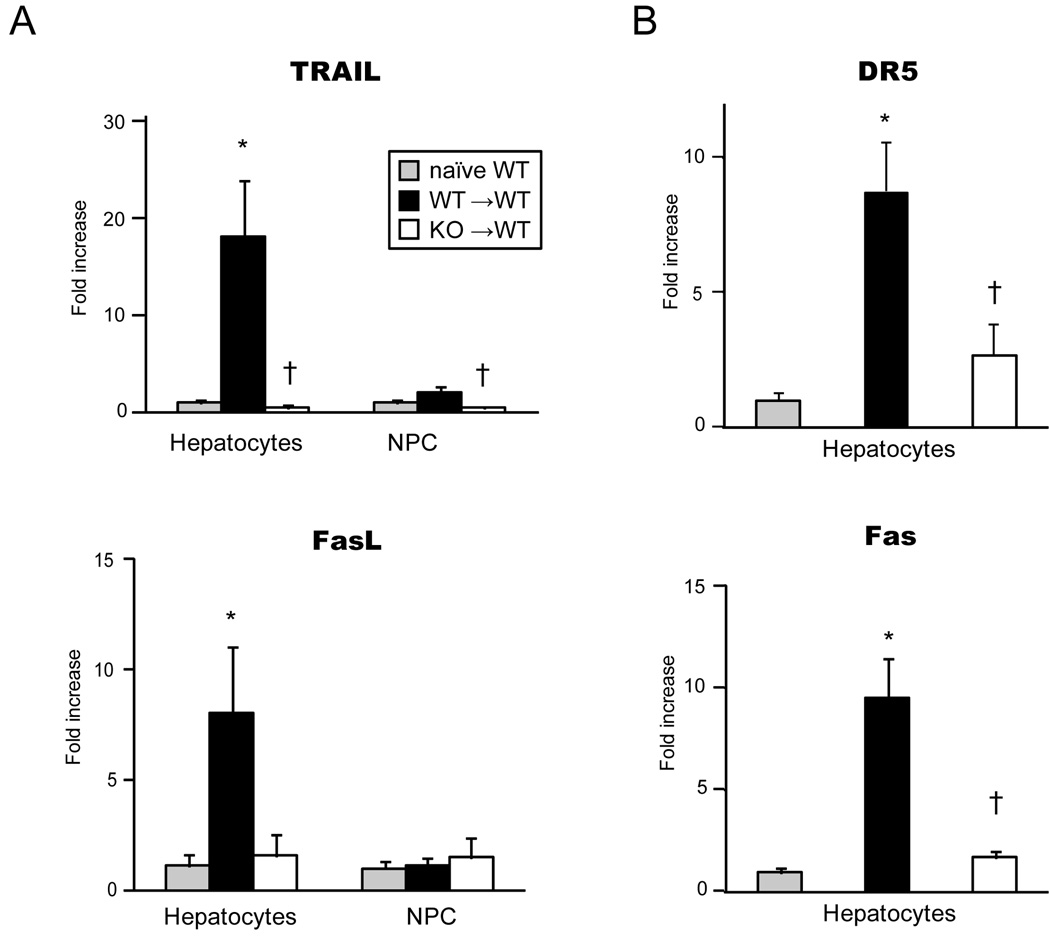
Liver graft IRF-1 deficiency inhibited expression of both death ligands and receptors in hepatocytes after LTx
To determine the site of death ligand and receptor upregulation in liver grafts during I/R injury, we analyzed mRNA expression for death molecules in graft hepatocyte and NPC fractions following LTx. Interestingly, the elevation of TRAIL and FasL mRNA levels were mainly seen in hepatocytes in WT→WT 6 hours after LTx. In contrast, TRAIL mRNA upregulation was completely inhibited in hepatocytes from IRF-1 KO grafts transplanted into WT recipients. FasL mRNA levels also were lower in the IRF-1 KO hepatocytes (Figure 7A). In NPC fractions, marginal increases of TRAIL mRNA were seen after WT→WT LTx, which was inhibited in KO→WT LTx (Figure 6A upper). FasL mRNA levels did not increase in NPC fractions obtained from WT→WT or KO→WT LTx (Figure 6A lower). NPC have been considered as the source of death ligands; however, our data suggests a role for hepatocytes in producing TRAIL and FasL during I/R injury.
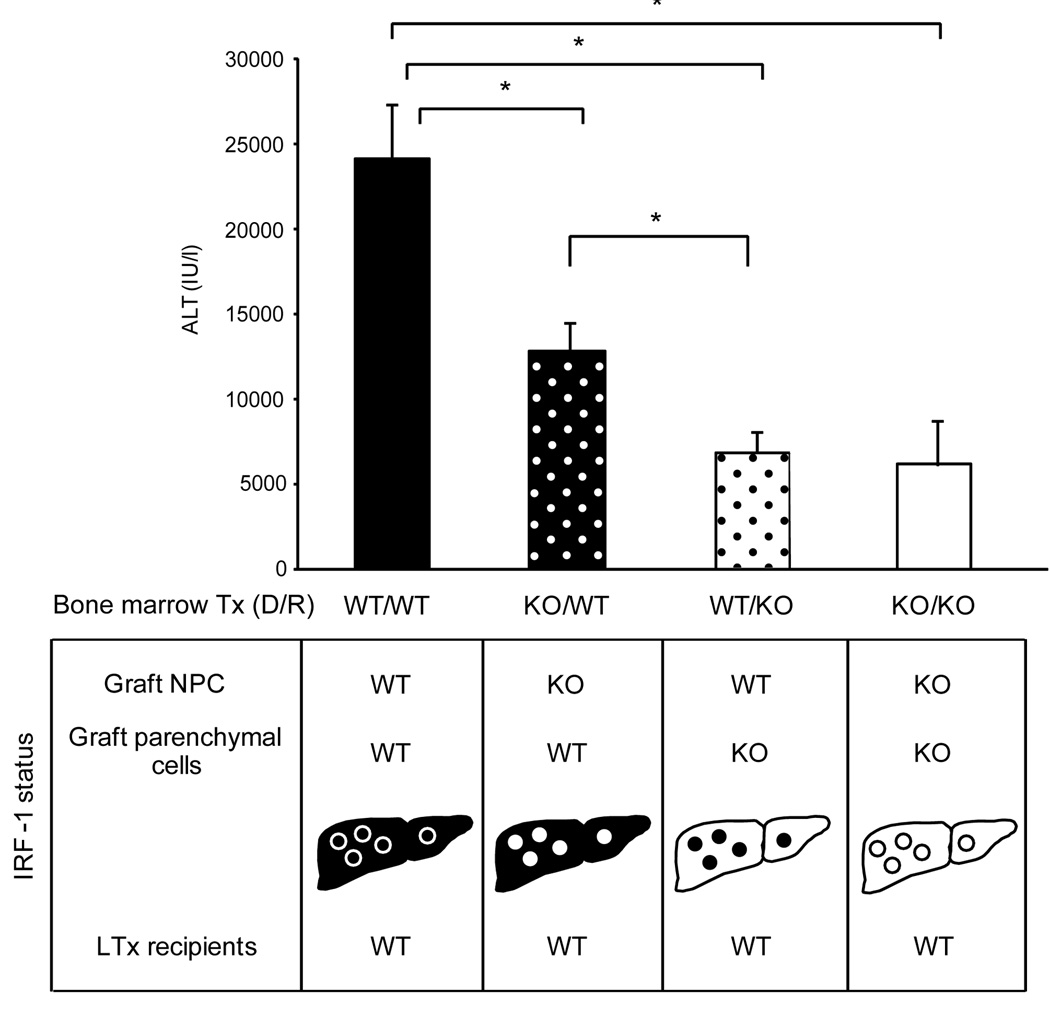
Figure 7. Serum ALT levels of recipients that received liver grafts from bone marrow (BM) chimeras.
Four types of BM chimeras were created in mice. Liver grafts were obtained >2 months later and transplanted into WT recipients, and serum ALT levels of recipients were determined at 12 hours after reperfusion. Liver grafts were from; WT/WT (solid bar): WT BM into irradiated WT mice, KO/WT (black bar with white dots): KO BM into irradiated WT mice, WT/KO (white bar with black dots): WT BM into irradiated KO mice, and KO/KO (white bar): KO BM into irradiated KO mice. IRF-1 status in parenchymal cells (hepatocytes) and NPC was shown for each group. IRF-1 deficiency in NPC (KO/WT) showed significantly lower ALT levels, compared to WT/WT group; however, the lack of IRF-1 in hepatocytes (WT/KO) resulted in further substantial reduction comparable to KO/KO group. (* p<0.05)
Figure 6. Death ligands in hepatocytes and NPC fractions (A) and death receptors in hepatocytes (B) during hepatic I/R injury.
(A) WT (solid bars) and KO (open bars) liver grafts were obtained at 6 hours after LTx into WT, and hepatocyte and NPC fractions were isolated for RT-PCR analysis to determine mRNA expression of death ligands. Data were shown as fold increases over WT naïve hepatocytes or NPC (gray bars), respectively (n=3 for each group). The elevation of TRAIL and FasL mRNA levels during I/R were mainly seen in hepatocytes. TRAIL mRNA was completely inhibited in IRF-1 KO hepatocytes. FasL mRNA levels tended to be lower in the IRF-1 KO hepatocytes. (* p<0.05 vs. naïve WT, † p<0.05 vs. WT→ WT LTx)
(B) Death receptor mRNA expression in hepatocyte fraction was analyzed using WT (solid bars) and KO (open bars) liver grafts at 6 hours after LTx. Data were shown as fold increases over WT naïve hepatocytes (gray bars), (n=3 for each group). DR5 and Fas were upregulated in hepatocytes during I/R, and were significantly inhibited in hepatocytes obtained from KO→WT LTx. (* p<0.05 vs. naïve WT, † p<0.05 vs. WT→WT LTx)
As expected, death receptors, DR5 and Fas, were strongly expressed on injured hepatocytes after WT→WT LTx. These receptor expressions were significantly inhibited in hepatocytes obtained from KO→WT LTx (Figure 6B).
BM chimeric mice indicated a role for both hepatocyte and NPC IRF-1 in mediating LTx injury
To determine the specific role of IRF-1 in hepatocytes and NPC in mediating liver damage, we created BM radiation chimeric mice using IRF-1 KO or WT BM, and produced liver grafts lacking IRF-1 exclusively in either the parenchymal cells (hepatocytes) or NPC. These chimeric donor liver grafts were then transplanted into WT recipients with 24 hour cold storage (Figure 7, bottom). Liver injury was greatest when both hepatocytes and NPC were WT (Figure 7, top, WT/WT). Liver grafts from WT animals reconstituted with IRF-1 KO BM had IRF-1 KO NPC and WT hepatocytes (KO/WT). Recipients of these liver grafts exhibited moderately decreased ALT levels compared to WT/WT, indicating that IRF-1 expression in the NPC does contribute to liver injury.
In contrast, liver grafts from IRF-1 KO mice reconstituted with WT BM had WT NPC and KO hepatocytes (WT/KO), and showed significantly decreased ALT levels compared to either the WT/WT or KO/WT group, which were comparable to liver grafts totally lacking IRF-1 (KO/KO). The results of IRF-1, IFN-γ and TRAIL mRNA expression in the liver grafts from the same chimeric LTx animals are shown in supplementary figure 1. The results are consistent with the pattern of liver injury. Taken together, these results suggest that although IRF-1 in hepatocytes and NPC contributes to transplant-induced liver I/R injury, IRF-1 in hepatocytes is more crucial.
Discussion
Transplant induced liver I/R injury is characterized by initial tissue damage during cold ischemic period, followed by progressive injury during the reperfusion period. Hypothermic and hypoxic stress during cold preservation leads to ATP depletion and deterioration of the intracellular homeostasis, resulting in disruption of intercellular contact and denudation in sinusoidal endothelial cells and glycolysis in hepatocytes (24–27). Subsequent warm reperfusion period has more complex features including impairment of microcirculation, ROS production, cytokine production, and expression of adhesion molecules and extravasation of host leukocytes. However, the eventual dysfunction of grafts is caused by parenchymal cell injury involving both apoptosis and necrosis of hepatocytes. The major and novel findings of this paper are: 1) Using IRF-1 deficient mice, this study demonstrates that IRF-1 expression in hepatic grafts plays a critical role in mediating liver transplant preservation injury, 2) IRF-1-mediated apoptosis involves activation of caspase-3 and -8, as well as induction of TRAIL/DR5 and FasL/Fas pathways in hepatocytes, 3) Cell isolation experiment and chimeric liver donor LTx confirm that IRF-1 expression in both cell fractions, but to a larger extent in hepatocytes, contributes to liver damage, and 4) IRF-1 signaling in hepatic graft NPC appears to regulate proinflammatory cytokine production of IFN-γ during hepatic I/R injury. To our knowledge, this is the first study showing a role for IRF-1 in liver transplant I/R injury.
Hepatic IRF-1 expression is known to be transcriptionally regulated (5, 6), and time course studies showed a marked induction of IRF-1 mRNA and protein in WT→WT liver grafts by 3 hours after transplant (Figure 2). Because both donor and recipient cells participate in transplant induced liver I/R injury, we first addressed if IRF-1 expression in donor or recipient cells was critical in mediating liver I/R injury. Our results show that a significant reduction in graft damage occurs only when liver grafts are obtained from IRF-1 deficient animals, while the IRF-1 status in the recipient does not influence liver injury (Figure 1). These results indicate that IRF-1 expression in liver grafts, but not host infiltrates, is responsible for liver I/R injury.
We next investigated the specific liver cell types that expressed IRF-1 in the liver transplant setting by isolating hepatocyte and NPC fractions from liver grafts. RT-PCR assay showed that IRF-1 mRNA levels were largely increased in hepatocyte fraction (>15 folds) at 3 hours after reperfusion in WT→WT LTx, but IRF-1 mRNA was also upregulated to a lesser extent in graft NPC. To further support a specific role for IRF-1 in hepatocytes and NPC in causing liver transplant graft damage, chimeric donors were generated by BM transplant of WT or IRF-1 KO donors yielding specific KO of IRF-1 in either the NPC or hepatocytes in the liver grafts which were then transplanted into WT recipient animals (Figure 7). The absence of IRF-1 in the donor hepatocytes, and to a lesser extent in the donor NPC, resulted in decreased ALT levels, confirming a functional role for hepatocyte and NPC IRF-1 in mediating transplant I/R injury.
To address the mechanism of IRF-1 mediated liver I/R injury, we analyzed apoptosis related genes in whole liver graft samples as well as isolated hepatocyte fractions. Previous work has shown that IRF-1 expression in hepatocytes is a key inducer of apoptosis (11). In our LTx model, TUNEL staining, as well as cleaved caspase-3 expression were significantly increased in WT→WT LTx, but were abolished when IRF-1 KO liver grafts were utilized. Hepatic mRNA levels for intrinsic pathway related genes (Bax, Bcl-2 and Bcl-xL) were not different between WT and KO grafts; however, mRNA upregulation for extrinsic pathway related genes for death receptors and ligands seen in WT grafts was significantly reduced in IRF-1 KO grafts. Further, reduction of caspase-8 activity in IRF-1 KO grafts supports that IRF-1 deficiency suppresses the extrinsic pathway of hepatocyte apoptosis. As shown in Figure 6, hepatocytes from WT, but not IRF-1 KO, grafts actively transcribe mRNA for death ligands and death receptors after reperfusion, supporting critical roles for hepatocyte IRF-1 expression in I/R injury-induced hepatocyte apoptosis via the extrinsic pathway. Although membrane binding TRAIL and FasL are well known to be expressed on T, NK, or NKT cells, and are crucial in several models of liver injury, accumulating evidence indicates that hepatocytes also can actively produce soluble TRAIL and FasL in response to various stimuli to promote cell death in an autocrine/paracrine manner (28–30).
The importance of death receptor/ligand interaction in I/R injury has been shown in previous studies. Lack of Fas/FasL pathway strongly suppressed myocardial ischemia (31). Small interfering RNA targeting Fas protected mice from renal I/R injury (32), and administration of anti-FasL or anti-Fas antibodies suppressed liver I/R in rats (33). Caspase-8 small interfering RNA has also been shown to decrease liver I/R in mice (34).
IRF-1, a ubiquitous nuclear factor, is induced by both type I (IFN-α/β) and type II (IFN-γ) IFNs (5, 15) and regulates the transcription of IFN-responsive genes (6). Among IFNs, IFN-γ is a potent antiviral, immunoregulatory, and anti-tumor cytokine critically involved in innate and adaptive immune responses. Interestingly, IFN-γ or IFN-γ receptor deficient mice have been reported to have no reduction in liver injury following hepatic warm ischemia, while type I IFN receptor KO mice were protected from liver damage (3, 35). However, a recent study shows that Rag KO mice reconstituted with WT cells, but not with IFN-γ KO cells, develop hepatic injury in the warm I/R injury model, suggesting the importance of IFN-γ in liver I/R injury (36). In our study, IFN-γ mRNA is upregulated exclusively in NPC at 3 hours after reperfusion when IRF-1 nuclear protein shows peak nuclear translocation. Considering that graft IFN-γ mRNA and serum IFN-γ protein levels are significantly reduced in KO→WT LTx group, it is tempting to assume that IFN-γ is produced by graft NPC, at least in part, via IRF-1 upregulation, and further stimulates hepatocyte IRF-1 expression to augment liver I/R injury.
It is known that IRF-1 KO mice have less numbers of NK and NKT cells in the liver (37). NKT cells are the major population (30%) in mouse liver NPC compared to NK cells (8%) (38). They are the key producer of IFN-γ and have important roles in liver I/R injury (36, 39). The altered immune cell phenotype in the liver of IRF-1 KO mice might contribute to the resistance of IRF-1KO grafts against hepatic I/R injury. While our study did not address the role of NKT cells during hepatic I/R injury, we have unpublished preliminary data using liver grafts from CD1d KO mice with 24 hours cold storage showing no reduction in serum ALT levels in WT recipients (19561±5285 IU/l: n=3), indicating that the protective effects of IRF-1 deficiency in liver grafts are not likely due to the reduction of NKT cells.
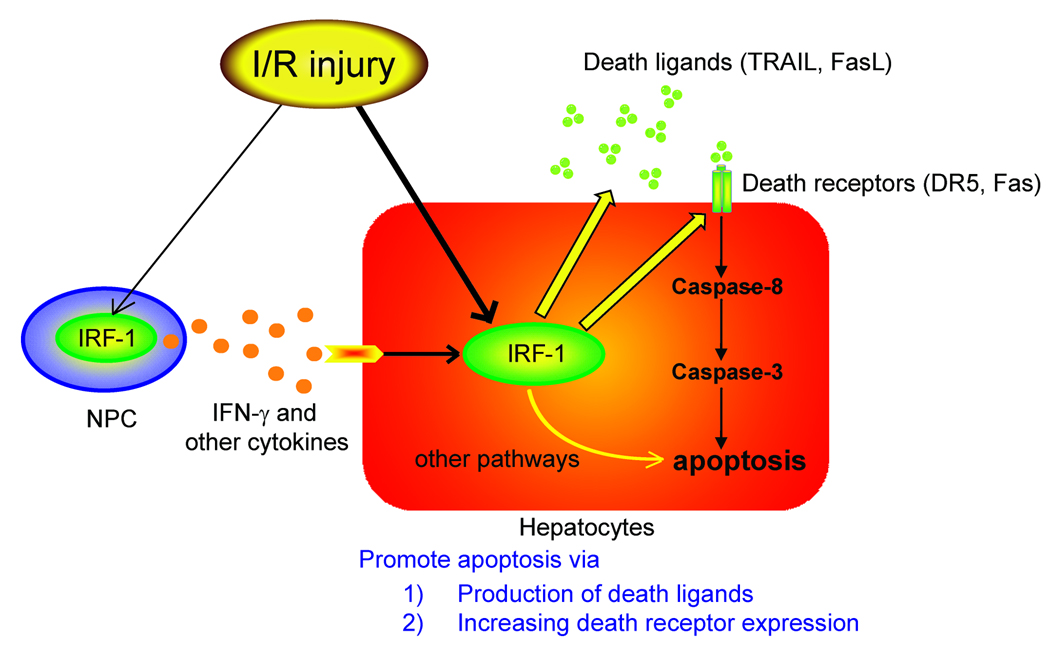
Based on findings in this study, our current understanding of the role of IRF-1 in hepatic I/R injury is shown in Figure 8. Liver I/R injury upregulates IRF-1 signals in hepatocytes and promotes hepatocyte apoptosis through the increased expression of death receptors (DR5 and Fas) and active production of death ligands (TRAIL and FasL). In addition, I/R injury also increases IRF-1 signals in hepatic NPC and promotes production of IFN-γ and other cytokines, which can further augment IRF-1 signal in hepatocytes and can influence the magnitude of host cell infiltration into the graft. Antagonism or silencing of IRF-1 gene expression may be a potential strategy to ameliorate liver damage associated with transplant preservation injury.
Figure 8. Schematic explanation of our hypothesis in the role of IRF-1 in transplant induced I/R injury.
I/R injury induce IRF-1 upregulation in graft hepatocytes and NPC. IRF-1 in hepatocytes plays roles in producing death ligands (TRAIL and FasL) and upregulating death receptors (DR5 and Fas) during I/R injury, resulting in caspase activation and hepatocyte apoptosis. In contrast, IRF-1 in NPC has a role to produce IFN-γ and other cytokines, which can further augment IRF-1 upregulation in hepatocytes.
Supplementary Material
Liver graft tissues were collected from BM chimera LTx mice at 12 hours after reperfusion and analyzed RT-PCR for IRF-1, IFN-γ and TRAIL mRNA. Liver grafts were from; WT/WT (solid bar): WT BM into irradiated WT mice, KO/WT (black bar with white dots): KO BM into irradiated WT mice, WT/KO (white bar with black dots): WT BM into irradiated KO mice, and KO/KO (white bar): KO BM into irradiated KO mice. Data were shown as fold increase compared to WT naïve liver (n=3 for each group).
Acknowledgement
We thank Mike Tabacek, Lisa Chedwick, Lifang Shao, Nicole Martik, and Carla Forsythe for assistance.
Financial Support
This work was supported by the National Institutes of Health Grants DK071753 (Murase), GM52021 (Geller), DK62313 (Geller), P01AI-81678 (Thomson/Geller/Murase). S. Ueki was supported by a Postdoctoral Fellowship Grant from the Thomas E. Starzl Transplantation Institute.
Abbreviations
- IRF-1
Interferon Regulatory Factor-1
- LTx
Liver transplantation
- I/R
Ischemia/reperfusion
- BM
bone marrow
- UW
University of Wisconsin solution
- ALT
alanine aminotransferase
- NPC
non-parenchymal cells
- Bax
Bcl-2–associated X protein
- TRAIL
TNF-related apoptosis-inducing ligand
- DR5
Death Receptor 5
- FADD
Fas-associated protein with death domain
- RLU
Relative Luminescence Units
Contributor Information
Shinya Ueki, Email: uekis@upmc.edu.
Rajeev Dhupar, Email: dhuparr@upmc.edu.
Jon Cardinal, Email: cardinaljs@upmc.edu.
Allan Tsung, Email: tsung@upmc.edu.
Junichi Yoshida, Email: jun1yosh@gmail.com.
Kikumi S. Ozaki, Email: ozakiks@upmc.edu.
John Robert Klune, Email: klunejr@upmc.edu.
Noriko Murase, Email: murase+@pitt.edu.
David A. Geller, Email: gellerda@upmc.edu.
References
- 1.Furukawa H, Todo S, Imventarza O, Casavilla A, Wu YM, Scotti-Foglieni C, et al. Effect of cold ischemia time on the early outcome of human hepatic allografts preserved with UW solution. Transplantation. 1991;51:1000–1004. doi: 10.1097/00007890-199105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 3.Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 4.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 7.Fujita T, Sakakibara J, Sudo Y, Miyamoto M, Kimura Y, Taniguchi T. Evidence for a nuclear factor(s), IRF-1, mediating induction and silencing properties to human IFN-beta gene regulatory elements. EMBO J. 1988;7:3397–3405. doi: 10.1002/j.1460-2075.1988.tb03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22:5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- 9.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 10.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, et al. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 11.Kano A, Haruyama T, Akaike T, Watanabe Y. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem Biophys Res Commun. 1999;257:672–677. doi: 10.1006/bbrc.1999.0276. [DOI] [PubMed] [Google Scholar]
- 12.Stang MT, Armstrong MJ, Watson GA, Sung KY, Liu Y, Ren B, et al. Interferon regulatory factor-1-induced apoptosis mediated by a ligand-independent fas-associated death domain pathway in breast cancer cells. Oncogene. 2007;26:6420–6430. doi: 10.1038/sj.onc.1210470. [DOI] [PubMed] [Google Scholar]
- 13.Lee HJ, Oh YK, Rhee M, Lim JY, Hwang JY, Park YS, et al. The role of STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial transmembrane potential during hepatic cell death induced by LPS/d-GalN. J Mol Biol. 2007;369:967–984. doi: 10.1016/j.jmb.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 14.Jaruga B, Hong F, Kim WH, Gao B. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1044–G1052. doi: 10.1152/ajpgi.00184.2004. [DOI] [PubMed] [Google Scholar]
- 15.Tsung A, Stang MT, Ikeda A, Critchlow ND, Izuishi K, Nakao A, et al. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1261–G1268. doi: 10.1152/ajpgi.00460.2005. [DOI] [PubMed] [Google Scholar]
- 16.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaizu T, Ikeda A, Nakao A, Takahashi Y, Tsung A, Kohmoto J, et al. Donor graft adenoviral iNOS gene transfer ameliorates rat liver transplant preservation injury and improves survival. Hepatology. 2006;43:464–473. doi: 10.1002/hep.21067. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Li C, Chen Y, Burnett C, Liu XY, Downs S, et al. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem. 2004;279:48434–48442. doi: 10.1074/jbc.M407190200. [DOI] [PubMed] [Google Scholar]
- 19.Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11:1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 21.Salkowski CA, Thomas KE, Cody MJ, Vogel SN. Impaired IFN-gamma production in IFN regulatory factor-1 knockout mice during endotoxemia is secondary to a loss of both IL-12 and IL-12 receptor expression. J Immunol. 2000;165:3970–3977. doi: 10.4049/jimmunol.165.7.3970. [DOI] [PubMed] [Google Scholar]
- 22.Kano S, Sato K, Morishita Y, Vollstedt S, Kim S, Bishop K, et al. The contribution of transcription factor IRF1 to the interferon-gamma-interleukin 12 signaling axis and TH1 versus TH-17 differentiation of CD4+ T cells. Nat Immunol. 2008;9:34–41. doi: 10.1038/ni1538. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 24.Trocha SD, Kevil CG, Mancini MC, Alexander JS. Organ preservation solutions increase endothelial permeability and promote loss of junctional proteins. Ann Surg. 1999;30:105–113. doi: 10.1097/00000658-199907000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolz DB, Ross MA, Ikeda A, Tomiyama K, Kaizu T, Geller DA, et al. Sinusoidal endothelial cell repopulation following ischemia/reperfusion injury in rat liver transplantation. Hepatology. 2007;46:1464–1475. doi: 10.1002/hep.21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cywes R, Greig PD, Morgan GR, Sanabria JR, Clavien PA, Harvey PR, et al. Rapid donor liver nutritional enhancement in a large animal model. Hepatology. 1992;16:1271–1279. [PubMed] [Google Scholar]
- 27.Churchill TA, Cheetham KM, Simpkin S, Green CJ, Wang LC, Fuller BJ. Liver metabolism in cold hypoxia: a comparison of energy metabolism and glycolysis in cold-sensitive and cold-resistant mammals. J Comp Physiol [B] 1994;164:396–404. doi: 10.1007/BF00302556. [DOI] [PubMed] [Google Scholar]
- 28.Strand S, Hofmann WJ, Grambihler A, Hug H, Volkmann M, Otto G, et al. Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat Med. 1998;4:588–593. doi: 10.1038/nm0598-588. [DOI] [PubMed] [Google Scholar]
- 29.Guy CS, Wang J, Michalak TI. Hepatocytes as cytotoxic effector cells can induce cell death by CD95 ligand-mediated pathway. Hepatology. 2006;43:1231–1240. doi: 10.1002/hep.21201. [DOI] [PubMed] [Google Scholar]
- 30.Mundt B, Kuhnel F, Zender L, Paul Y, Tillmann H, Trautwein C, et al. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94–96. doi: 10.1096/fj.02-0537fje. [DOI] [PubMed] [Google Scholar]
- 31.Jeremias I, Kupatt C, Martin-Villalba A, Habazettl H, Schenkel J, Boekstegers P, et al. Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation. 2000;102:915–920. doi: 10.1161/01.cir.102.8.915. [DOI] [PubMed] [Google Scholar]
- 32.Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:14883–14888. doi: 10.1073/pnas.0406421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima H, Mizuta N, Fujiwara I, Sakaguchi K, Ogata H, Magae J, et al. Blockade of the Fas/Fas ligand interaction suppresses hepatocyte apoptosis in ischemia-reperfusion rat liver. Apoptosis. 2008;13:1013–1021. doi: 10.1007/s10495-008-0234-5. [DOI] [PubMed] [Google Scholar]
- 34.Contreras JL, Vilatoba M, Eckstein C, Bilbao G, Anthony Thompson J, Eckhoff DE. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery. 2004;136:390–400. doi: 10.1016/j.surg.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, et al. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology. 1999;30:1448–1453. doi: 10.1002/hep.510300615. [DOI] [PubMed] [Google Scholar]
- 36.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, et al. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 38.Hammond KJ, Pellicci DG, Poulton LD, Naidenko OV, Scalzo AA, Baxter AG, et al. CD1d-restricted NKT cells: an interstrain comparison. J Immunol. 2001;167:1164–1173. doi: 10.4049/jimmunol.167.3.1164. [DOI] [PubMed] [Google Scholar]
- 39.Shimamura K, Kawamura H, Nagura T, Kato T, Naito T, Kameyama H, et al. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell Immunol. 2005;234:31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Liver graft tissues were collected from BM chimera LTx mice at 12 hours after reperfusion and analyzed RT-PCR for IRF-1, IFN-γ and TRAIL mRNA. Liver grafts were from; WT/WT (solid bar): WT BM into irradiated WT mice, KO/WT (black bar with white dots): KO BM into irradiated WT mice, WT/KO (white bar with black dots): WT BM into irradiated KO mice, and KO/KO (white bar): KO BM into irradiated KO mice. Data were shown as fold increase compared to WT naïve liver (n=3 for each group).