Abstract
Objectives/Hypothesis
Ischemic necrosis of the tongue is a rare entity generally associated with vasculitis. Critically ill patients with shock might experience hypoperfusion of head and neck end organs including the tongue.
Study Design
Retrospective analysis of hospital charts.
Methods
Case histories and photographs of five patients who developed ischemic tongue necrosis in the context of cardiogenic shock.
Results
Five critically ill patients in our institution’s cardiothoracic intensive care unit developed ischemic necrosis of the tongue. All five patients experienced protracted courses of profound cardiogenic shock requiring high-dose vasopressor support and urgent cardiac surgery. Three patients required intraaortic balloon pumps. All patients had concomitant signs of poor end organ perfusion, including lower extremity ischemia and renal and hepatic failure. Ultimately, four of five patients died, with one patient surviving after sloughing of the entire oral tongue.
Conclusions
Ischemic necrosis of the oral tongue is an uncommon but perhaps under-reported manifestation of end organ hypoperfusion in shock, likely signifying poor prognosis.
Keywords: Tongue, necrosis, gangrene, ischemic, shock
INTRODUCTION
Ischemic tongue necrosis is a rare manifestation of systemic disease. Essentially all reports of this entity have been associated with giant cell arteritis (GCA), with a total of 21 cases reported since 1961.1–12 This diagnosis was usually made in patients with known GCA and a history of progressive head and neck symptoms. In almost all cases, tongue necrosis is minimal and limited to the tip of the oral tongue, and the condition is generally treated successfully with high-dose corticosteroids.5 None of the reported cases required surgical intervention, although rarely, autoamputation of the necrosed tongue tip did occur.4,11
Tongue necrosis as a sequela of shock has not been widely reported, with the exception of one case of ischemic tongue necrosis resulting from profound cardiogenic shock, reported by our group in 2007.13 We now present an updated case series of an additional four patients at a single institution developing this entity over 3 years. Altogether, the five patients developing ischemic tongue necrosis were on the cardiothoracic surgery service, and required high-dose vasopressor therapy to maintain blood pressure. Three required intra-aortic balloon pumps (IABP). All five developed extensive necrosis of the oral tongue concurrently with hypoperfusion of multiple organ systems. Four of the five patients died shortly after the finding of tongue necrosis.
We present clinical information and photographs of these patients and discuss the factors likely contributing to the entity of ischemic tongue necrosis in shock.
MATERIALS AND METHODS
We retrospectively analyzed hospital charts. Between 2007 and 2009, the otolaryngology service evaluated five critically ill patients on the cardiothoracic surgery service who had been noted to have dusky or black tongues. The first case was previously reported by our group in an earlier publication, and abbreviated clinical data is presented here for completeness.13 This study was deemed exempt from review by the institutional review board.
CASE SERIES
Case details are summarized in Table I.
TABLE I.
Patient Characteristics.
| Patient | Age, yr | Sex | Cause of Cardiogenic Shock | Length of Pressor Treatment, d |
Use of IABP | Outcome |
|---|---|---|---|---|---|---|
| 1 | 79 | F | Flail mitral valve | 3 | Concurrent with pressors | Survived, entire oral tongue sloughed |
| 2 | 77 | M | Acute MI | 3 | — | Died, MODS |
| 3 | 88 | F | Acute MI, atrial fibrillation, and occlusive carotid artery disease |
5 | Removed 9 days before tongue necrosis |
Died, acute cerebral edema |
| 4 | 91 | F | Acute MI | 2 | Concurrent with pressors | Died, cardiac arrest |
| 5 | 59 | M | Acute MI and ascending aortic aneurysm | 32 | — | Died, MODS |
All five subjects were on high-dose vasopressive agents at the time that distal tongue ischemia was noted. All had concurrent hepatic or renal failure and/or distal extremity ischemia.
IABP = intra-aortic balloon pump; F = female; M = male; MI = myocardial infarction; MODS = multiple organ dysfunction syndrome.
Patient 1
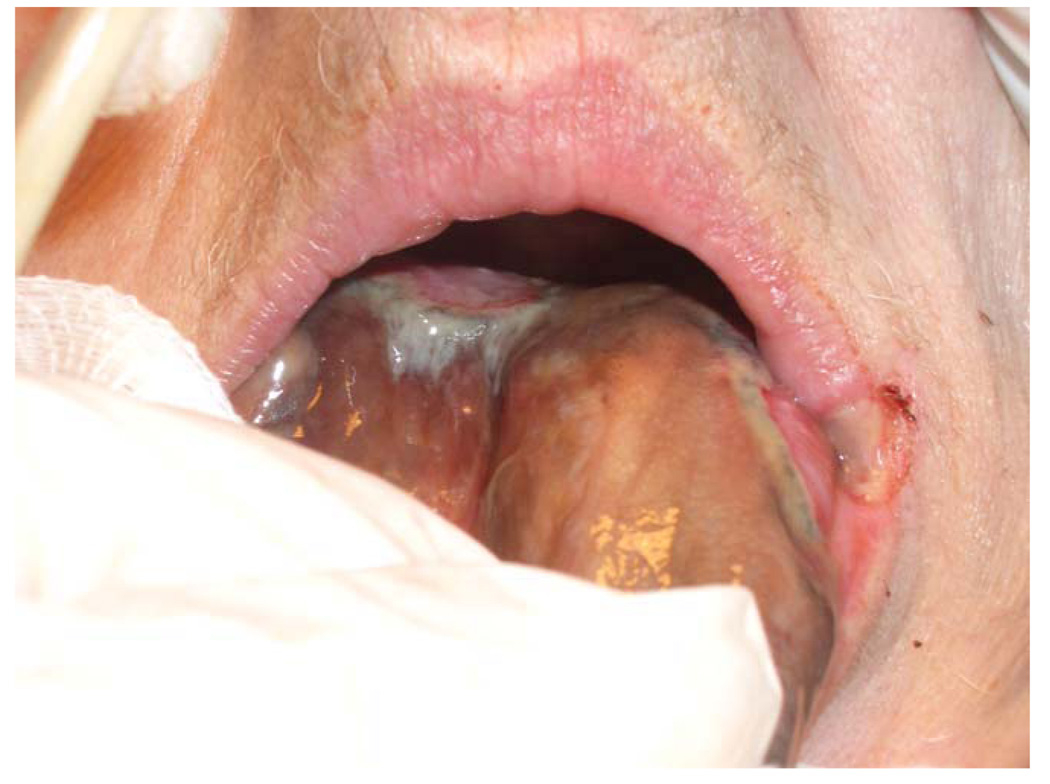
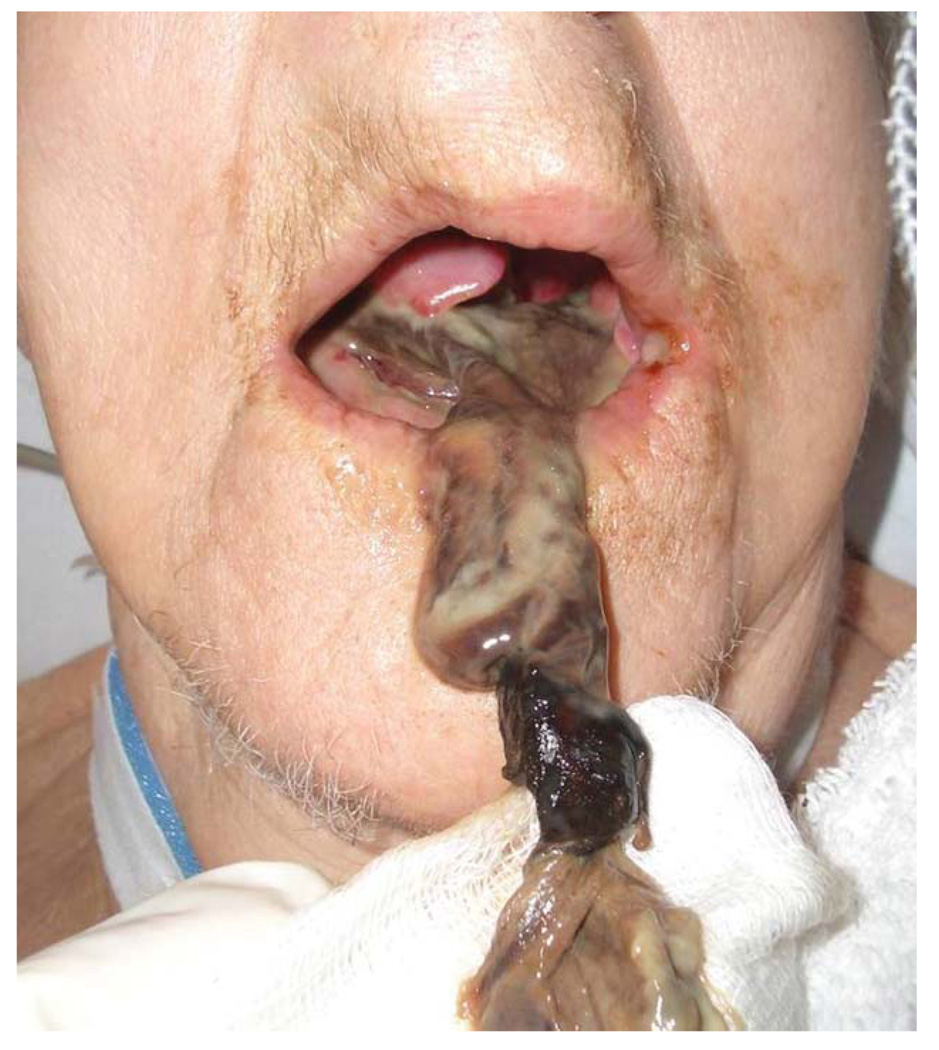
A 79-year-old woman with hypertension and congestive heart failure presented in severe cardiogenic shock with a flail mitral valve leaflet. Vasopressor and inotrope support were initiated, and an IABP was placed after 12 hours. The tip of the oral tongue was noted to be dusky the next day. The patient underwent emergent valve replacement, the IABP was removed, and the otolaryngology service consulted on hospital day 3. At this point, the patient had developed multiple organ failure, and the tip of the tongue was noted to be black. There was a sharp line of demarcation between black tissue and healthy tongue mucosa, and there was no evidence of pressure or venous congestion from the endotracheal tube or teeth (the patient was edentulous). The erythrocyte sedimentation rate, checked for concern for giant cell arteritis, was not elevated. A tracheotomy was performed, meticulous oral hygiene was initiated, and broad-spectrum antibiotics continued, as well as aspirin and Coumadin for cardiac purposes. Over the following week, the area of necrosis progressed. On day 11, the entire oral tongue had liquefied and began to separate from the base of tongue (Fig. 1). The entire oral tongue sloughed on day 18 (Fig. 2). The patient was ultimately discharged to an acute nursing facility on hospital day 30. Some details of this case have been reported by our group in a previous publication.13
Fig. 1.
Case 1, during partial slough of the oral tongue. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 2.
Case 1, after complete loss of the oral tongue. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Patient 2
A 77-year-old man requiring urgent coronary artery bypass (CAB) surgery after an acute myocardial infarction developed profound cardiogenic shock postoperatively, requiring prolonged high-dose vasopressor support. The anterior third of the oral tongue was noted to be black 3 days later. There was no evidence of compression by teeth or the endotracheal tube. The patient died the same day as a result of multiple organ failure.
Patient 3
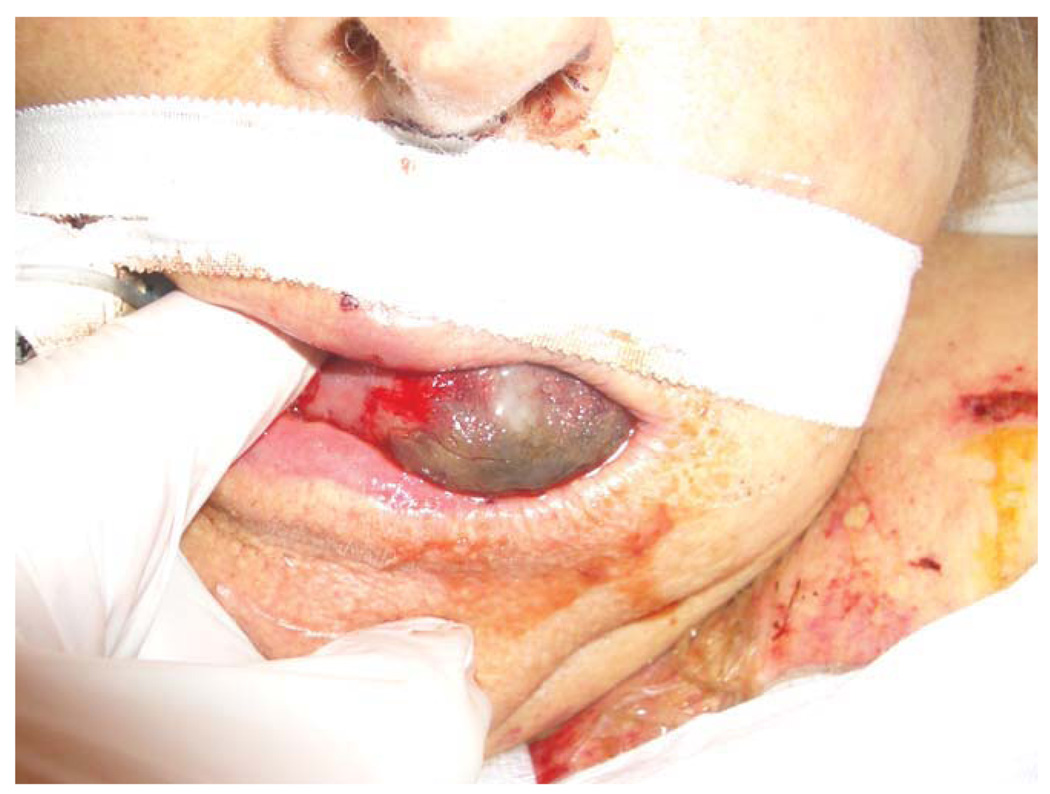
An 88-year-old woman with coronary artery disease underwent cardiac catheterization, which revealed obstructive left main coronary artery disease. During the procedure, she developed severe hypotension and bradycardia requiring emergent placement of an IABP and transvenous pacemaker. The following day a two-vessel CAB was performed and the IABP removed postoperatively. The patient was extubated the next day, but on postoperative day 4 developed atrial fibrillation and a massive stroke due to occlusive carotid arterial disease, requiring urgent carotid embolectomy and subsequent vasopressor support. The patient developed critical lower extremity ischemia 5 days later, at which time the anterior aspect of the oral tongue was noted to be black (Fig. 3). There was no evidence of compression by the teeth or endotracheal tube. The patient ultimately died from acute cerebral edema 2 days later.
Fig. 3.
Case 3. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Patient 4
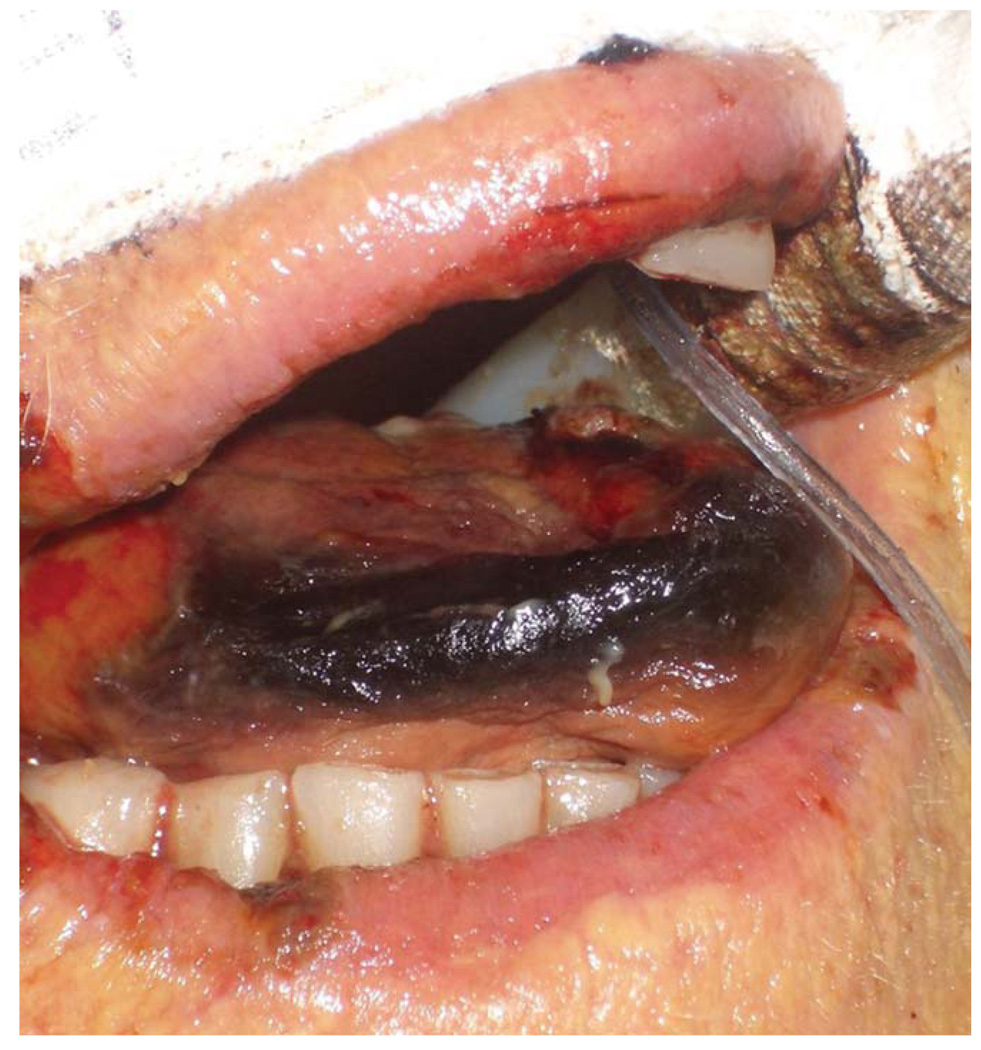
A 91-year-old woman with coronary artery disease experienced profound cardiogenic shock 8 days after urgent CAB. An IABP was placed for 48 hours. Two days later, the otolaryngology service was consulted for a black tip of the tongue (Fig. 4). There was no evidence of compression by the teeth or endotracheal tube. The patient simultaneously developed multiorgan failure, including renal and hepatic failure. Meticulous oral hygiene was instituted, and antiplatelet therapy was continued. The patient experienced a fatal cardiac arrest the following day.
Fig. 4.
Case 4. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Patient 5
A 59-year-old man with a history of hypertension, peripheral vascular disease, and coronary artery disease presented with an acute myocardial infarction and was also found to have an aneurysm of the ascending aorta. Emergent CAB and ascending aortic root replacement were performed. Postoperatively, the patient required re-exploration for mediastinal bleeding on three occasions over 3 weeks. He remained intubated and on vasopressors during the entire postoperative course, and a tracheotomy was performed on postoperative day 27. The otolaryngology service was consulted on postoperative day 32 for evaluation of a necrotic tongue, which developed on the same day as hepatic failure and lower extremity ischemia. The tongue was noted to be black and firm from the tip to the circumvallate papillae. There was no evidence of trauma from the teeth, and the endotracheal tube had been replaced by a tracheotomy 5 days prior. The ischemic tongue remained stable without separation from the tongue base for 6 days until the patient expired on postoperative day 38 from multiple organ failure.
DISCUSSION
Gangrene of the oral tongue has been rarely reported, mostly in the context of GCA. This chronic necrotizing vasculitis usually manifests in the temporal artery, and typically presents with headache, visual changes, and jaw claudication in patients older than 50 years. GCA-associated tongue necrosis has been attributed to lingual artery vasculitis.1–12 Of 21 reported cases of tongue gangrene related to GCA, the majority have been limited to the tip of the tongue, with only one case reported to involve the entire oral tongue.1 Surgical intervention is not described for this entity, but the tongue has been reported to occasionally autoamputate.4,11
Several other vasculitis-associated cases of oral tongue necrosis have been reported in patients with Kawasaki disease and Wegener’s granulomatosis. Kawasaki disease is usually self-limited, but can cause inflammatory coronary artery aneurysms, as in the reported case with associated tongue necrosis.14 Wegener’s granulomatosis, a necrotizing vasculitis affecting the upper and lower respiratory tracts and kidneys, can be indolent or rapidly progressive. In the three reported cases of associated tongue necrosis, two were rapidly progressive and ultimately fatal.15
The differential diagnoses of tongue necrosis also include several hypercoagulable disease states. One case has been reported of tongue necrosis in a patient with disseminated intravascular coagulation, during the early consumptive coagulation phase.16 Another case of ischemic tongue necrosis occurred in a patient with essential thrombocytosis.17 A third reported entity was rheumatoid hyperviscosity syndrome, characterized by a high rheumatoid factor and a polyclonal gammopathy that led to a hypercoagulable state.18
We report here the first case series of total oral tongue necrosis as a sequela of shock. All five patients had experienced protracted courses of profound cardiogenic shock requiring high-dose vasopressor support and urgent or emergent cardiac surgery. All patients had other physical manifestations of end organ and distal extremity hypoperfusion, including distal lower extremity (toe) or upper extremity (finger) gangrene, renal failure, and hepatic failure. One patient developed synchronous carotid arterial occlusive disease requiring intervention. GCA was unlikely to be a contributing factor in these patients as none had a history, signs, or symptoms of any vasculitis. Ultimately, four of five patients died, with one patient surviving after sloughing of the entire oral tongue.
In low-flow states, such as cardiogenic shock, decreased distal and mesenteric perfusion might be exacerbated by the vasoconstrictive properties of alpha-1 adrenergic vasopressor agents. However, the structures of the head and neck are normally protected from hypoperfusion due to extensive collateral blood supply. Nevertheless, the tongue, although supplied by multiple branches of the external carotid artery, is a potentially susceptible muscular end organ. Furthermore, in severe shock, blood flow to the internal carotid artery is protected at the expense of the external carotid system because of shunting to the lower resistance, less muscular internal carotid, and cerebrovascular system.
Three of the five reported patients required IABP counterpulsation therapy, which increases coronary perfusion and decreases afterload. However, recent evidence suggests that the IABP does not augment carotid arterial flow because the pump operates distal to the takeoffs of the carotid arteries.19,20 As a result, perfusion of the head, neck, and brain might not be improved with IABP support.
We are aware of one other reported case, in addition to our prior reported case, in which prolonged hypotension requiring vasopressors resulted in tongue ischemia.21 In this case, terlipressin, a long-acting anti-diuretic hormone analog, was used for maintenance of blood pressure in a patient with severe septic shock and eventual pseudohepatorenal syndrome.
Our findings should be interpreted with caution. Because this report is a case series, it is a hypothesis-generating, not hypothesis-confirming, report. There are several caveats to our conclusions. First, an alternative explanation for these cases would be local pressure from teeth or the endotracheal tube. Low lingual artery pressures in a state of shock might make the tongue more susceptible to external compression. However, local pressure is unlikely to be the sole cause of the tongue ischemia, as no cases of tongue ischemia following endotracheal intubation alone have been reported in the literature. All of the cases we observed were bilaterally symmetric and distal, consistent with involvement of both lingual arteries. The pattern of necrosis invariably progressed from distal to proximal, less consistent with the distribution expected from compression by teeth or the endotracheal tube, and more consistent with a systemic process. In fact, one patient (case 5) developed tongue necrosis 5 days after tracheotomy. Additionally, the cases of tongue necrosis in our critical care units have been limited to patients with profound cardiogenic shock; other intubated patients in the same unit, cared for by the same medical team, have not developed tongue necrosis.
A second caveat is the low number of prior reported cases of this entity. We suspect that ischemic tongue necrosis in shock might be more common, but because it is an end-stage manifestation, it might not be noticed in critically ill patients with multiple organ system failure. After our institution’s cardiac surgery and critical care teams became aware of this entity, more cases were identified. Finally, a case series cannot prove causality. Although impaired perfusion of the tongue, in concert with distal extremity gangrene, seems to be the most parsimonious explanation, we do not have sufficient evidence to make definitive statements about causation.
CONCLUSION
Patients in shock who require long-term vasopressors support should be carefully monitored for signs of head and neck hypoperfusion. With a high mortality rate in this case series, ischemic tongue necrosis due to shock is an end organ sign carrying a grave prognosis. The cluster of cases in our institution might be indicative of the acuity of critical illness in patients referred for cardiac surgery. This case series is intended as an early hypothesis-generating report, and we anticipate that as critically ill patients with shock experience longer survival, this entity might be reported more frequently, improving understanding of pathogenesis.
If tongue necrosis does develop, it should be managed similarly to dry gangrene of the extremities. Supportive wound care should be administered, with debridement of necrotic tissue only if infection develops. Antibiotics should be considered prophylactically to cover oral flora. Adequate pain control is also important. Similar to distal extremity ischemia, anticoagulation is unlikely to have a positive effect on tongue necrosis. If compression by the endotracheal tube is thought to be a contributing factor, an early tracheotomy should be considered.
Footnotes
Presented at the 2009 Triological Society Annual Meeting at COSM, Phoenix, Arizona, U.S.A., May 28–31, 2009.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Missen GAK. Gangrene of the tongue. Br Med J. 1961;1:1393–1394. [Google Scholar]
- 2.Reed C, Inglis MJ. Acute massive gangrene of the tongue. Br Med J. 1965;2:575–576. doi: 10.1136/bmj.2.5461.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sofferman RA. Lingual infarction in cranial arteritis. JAMA. 1980;243:2422–2423. [PubMed] [Google Scholar]
- 4.Ginzburg E, Evans WE, Smith W. Lingual infarction: a review of the literature. Ann Vasc Surg. 1992;6:450–452. doi: 10.1007/BF02007001. [DOI] [PubMed] [Google Scholar]
- 5.McRorie ER, Chalmers J, Campbell IW. Lingual infarction in cranial arteritis. Br J Clin Pract. 1994;48:280. [PubMed] [Google Scholar]
- 6.Navarro M, Niembro E, Scola B, Scola E, del Pozo A. Necrosis of the tongue secondary to Horton’s disease. Acta Otorrinolaringol Esp. 1995;46:227–229. [PubMed] [Google Scholar]
- 7.Kleinjung T, Strutz J. Spontaneous, unilateral necrosis of the tongue. Temporal arteritis [in German] HNO. 1998;46:274–275. doi: 10.1007/s001060050238. [DOI] [PubMed] [Google Scholar]
- 8.Hellmann DB. Temporal arteritis: a cough, toothache, and tongue infarction. JAMA. 2002;287:2996–3000. doi: 10.1001/jama.287.22.2996. [DOI] [PubMed] [Google Scholar]
- 9.Biebl MO, Hugl B, Posch L, et al. Subtotal tongue necrosis in delayed diagnosed giant-cell arteritis: a case report. Am J Otolaryngol. 2004;25:438–441. doi: 10.1016/j.amjoto.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Goicochea M, Correale J, Bonamico L, et al. Tongue necrosis in temporal arteritis. Headache. 2007;47:1213–1215. doi: 10.1111/j.1526-4610.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann AT, Brown M. Tongue infarction in giant cell (temporal) arteritis. Intern Med J. 2008;38:376. doi: 10.1111/j.1445-5994.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 12.Brodmann M, Dorr A, Hafner F, Gary T, Pilger E. Tongue necrosis as first symptom of giant cell arteritis (GCA) Clin Rheumatol. 2009;28:S47–S49. doi: 10.1007/s10067-009-1141-z. [DOI] [PubMed] [Google Scholar]
- 13.Morris LG, Komisar A, Liberatore LA. Total gangrene of the oral tongue following intra-aortic balloon pump for cardiogenic shock. Otolaryngol Head Neck Surg. 2007;137:358–359. doi: 10.1016/j.otohns.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Scardina GA, Fuca G, Carini F, et al. Oral necrotizing microvasculitis in a patient affected by Kawasaki disease. Med Oral Patol Oral Cir Bucal. 2007;12:E560–E564. [PubMed] [Google Scholar]
- 15.Carter LM, Brizman E. Lingual infarction in Wegener’s granulomatosis: a case report and review of the literature. Head Face Med. 2008;4:19. doi: 10.1186/1746-160X-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamatani T, Yamashita K, Okabayashi T, et al. Bilateral ischemic necrosis of the tongue due to disseminated intravascular coagulation. Int J Oral Maxillofac Surg. 2008;37:777–779. doi: 10.1016/j.ijom.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Casado A, Sanchez-Gonzalez B. Images in clinical medicine. Tongue necrosis in a patient with essential thrombocytosis. N Engl J Med. 2009;360:e28. doi: 10.1056/NEJMicm0807709. [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer J, Ridder GJ. Spontaneous tongue necrosis consecutive to rheumatoid hyperviscosity syndrome. A case report and literature review [in German] Laryngorhinootologie. 2008;87:43–48. doi: 10.1055/s-2007-966779. [DOI] [PubMed] [Google Scholar]
- 19.Applebaum RM, Wun HH, Katz ES, et al. Effects of intraaortic balloon counterpulsation on carotid artery blood flow. Am Heart J. 1998;135:850–854. doi: 10.1016/s0002-8703(98)70045-6. [DOI] [PubMed] [Google Scholar]
- 20.Reesink KD, Dekker AL, Van Ommen V, et al. Miniature intracardiac assist device provides more effective cardiac unloading and circulatory support during severe left heart failure than intraaortic balloon pumping. Chest. 2004;126:896–902. doi: 10.1378/chest.126.3.896. [DOI] [PubMed] [Google Scholar]
- 21.Megarbane H, Barete S, Khosrotehrani K, et al. Two observations raising questions about risk factors of cutaneous necrosis induced by terlipressin (Glypressin) Dermatology. 2009;218:334–337. doi: 10.1159/000195676. [DOI] [PubMed] [Google Scholar]