Abstract
An approach to automated acquisition of cryoEM image data from lacey carbon grids using the Leginon program is described. Automated liquid nitrogen top up of the specimen holder dewar was used as a step towards full automation, without operator intervention during the course of data collection. During cryoEM studies of actin labelled with myosin V, we have found it necessary to work with lacey grids rather than Quantifoil or C-flat grids due to interaction of myosin V with the support film. Lacey grids have irregular holes of variable shape and size, in contrast to Quantifoil or C-flat grids which have a regular array of similar circular holes on each grid square. Other laboratories also prefer to work with grids with irregular holes for a variety of reasons. Therefore, it was necessary to develop a different strategy from normal Leginon usage for working with lacey grids for targetting holes for image acquisition and suitable areas for focussing prior to image acquisition. This approach was implemented by using the extensible framework provided by Leginon and by developing a new MSI application within that framework which includes a new Leginon node (for a novel method for finding focus targets).
Keywords: Electronmicroscopy, Data collection, Single-particle reconstruction, High throughput
INTRODUCTION
Cryo-electron microscopy and single-particle image analysis are powerful methods for studying large protein complexes. In particular, these techniques can be applied to assemblies lacking symmetry, which are difficult to study by other methods. The techniques are also useful for studying heterogeneous specimens or complexes which may occur in the specimen in a variety of conformational forms (as is usual in dynamic studies (Burgess et al., 2003; Chen et al., 1994; Roberts et al., 2009; Roseman et al., 1996; Thirumurugan et al., 2006; Walker et al., 2000)).
Ever improving resolution is currently being achieved for a wide variety of macromolecular complexes. However, radiation damage limits information recovery from protein cryoEM specimens, necessitating use of low electron dose in recording images and resulting in low signal-to-noise ratio which can only be overcome by averaging large numbers of images. The number of particle images required for 3D reconstruction increases dramatically with the desired resolution - more than 100,000 particles were used in recent reconstructions at between 4.2 and 6.8 Angstroms resolution (Becker et al., 2010; Ludtke et al., 2008; Seidelt et al., 2009). It is estimated that the number must be increased to about 1 million before it will be possible to reach “atomic” resolutions, i.e. better than 4 Angstroms (Glaeser, 1999; Henderson, 1995), when using images of the currently available quality of particles without high symmetry. Therefore, there is a requirement to obtain large numbers of micrographs each containing many particle images in order to achieve high resolution.
Manual cryoEM is a skilled, time consuming and labour intensive task. A number of software packages have been developed for varying levels of automation of image acquisition with the aim of facilitating acquisition of large numbers of micrographs. These include Leginon (Carragher et al., 2000; Potter et al., 1999; Stagg et al., 2006; Suloway et al., 2005) which supports a substantial level of automation. Leginon also has options for collecting data from difficult specimens with some manual operation and user intervention. Manual intervention may be chosen at various stages and there are options that facilitate manual screening of targets in low magnification images of grid squares and/or targets in intermediate magnification images of holes at the beginning of data collection, so that Leginon can subsequently continue to acquire images automatically (Carragher et al., 2010). The main Leginon package does not include code with a dependence on a specific microscope/camera platform and is used with a separate library (pyScope) that facilitates porting Leginon to different platforms (Kisseberth et al., 1998). At present, Leginon has largely been used on Tecnai microscopes with either Gatan or Tietz CCD cameras. (Work has been carried out to allow Leginon to be used with JEOL microscopes, but this is not available in an open source version (Glaeser et al., 2006).) Leginon also provides a framework for building applications for novel data collection protocols (or potentially for other new purposes making use of control of the microscope, such as automated alignments). This is done by linking together existing implementations of functionality in Leginon - called “nodes” - or by developing new nodes (in the source language of Leginon, Python). Various other packages have been developed for automated data collection including JAMES (Marsh et al., 2007), JADAS (Zhang et al., 2009), AutoEM (Zhang et al., 2001; Zhang et al., 2003) and AutoEMation (Lei and Frank, 2005). Recently, the SAM software was developed for semi-automated image collection and is suitable for use with lacey grids (Shi et al., 2008).
Previous work (Cheng et al., 2006; Robinson et al., 2000) describes automated liquid nitrogen top up of the specimen holder during cryoEM. However, it remains usual to top up the liquid nitrogen manually during automated data collection using of the different software packages. In this paper, an approach is described using the Leginon package that makes use of automated liquid nitrogen top up and some of the technical problems that were solved in order to achieve this. Also described is an approach for automated data collection from lacey grids.
METHODS AND RESULTS
(i) Automated liquid nitrogen top up
As a step towards greater levels of automation for image data acquisition, we wished to eliminate the need for manual liquid nitrogen refill of the specimen holder, which must be done every 1.5 – 2 hours. We also wished to extend the time between refills of the liquid nitrogen dewar that cools the anti-contaminator. It is not convenient to have someone regularly top up the liquid nitrogen at night, as is required for round the clock automated image data acquisition, although this is indeed what is done in a number of labs. Automation opens the possibility of collecting tens of thousands of micrographs over a single session of several days, but manual top up will make this increasingly inconvenient with longer times.
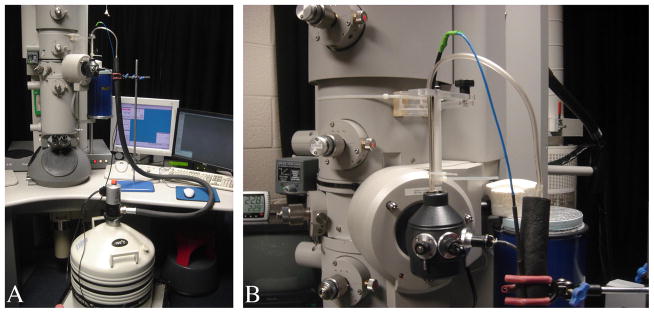
Increasing the interval between anti-contaminator dewar refills was achieved in a straightforward way using a substantially larger dewar (Robinson et al., 2000) and fitting a larger platform on the microscope column to support the new dewar. The original dewar was a KGW Isotherm type 9C flask with an internal capacity of 1 litre, which lasted up to 9.5 hours between refills. This time was just long enough for an operator to leave automatic data collection running unattended overnight. Switching to the larger dewar meant that an operator on the microscope could leave automatic data collection running unattended overnight. The new larger dewar is a KGW Isotherm type 20C flask with an internal capacity of 3 litres and lasts up to 15 hours. Fig 1a shows the new larger dewar in place on a new supporting platform on the microscope.
Fig. 1.
(a) F20 microscope set up with microscope PC and liquid nitrogen pump. The large dewar on the custom platform is also visible.(b) Close-up of sample holder dewar and fill tubes from liquid nitrogen pump with support and retort clamp.
The problem of refilling liquid nitrogen is more difficult in the case of the specimen holder, which must be topped up more frequently. Also, any system for automated top up must avoid transferring vibration to the specimen holder. A Norhof liquid nitrogen pump and software (model #905) was used to regularly top up the specimen holder. The fill tube and sensor from the Norhof pump were held in place by a clamp and retort stand (fig. 1a). An additional support was fitted on the microscope consisting of an articulated arm and a rod pointing down towards the specimen holder dewar. This facilitates correctly aligning the fill tube and sensor with the specimen holder dewar, while avoiding these contacting the sides of dewar mouth, which would introduce vibrations. Cable ties held the fill tube and sensor in place on the perspex rod (fig. 1b). The fill tube was kept above the dewar, pointing down into the mouth opening. The sensor was aligned down the centre of the dewar with its tip lower than the fill tube and marks the level for liquid nitrogen top up.
A further difficulty with top up of the specimen holder is that there are vibrations during top up and for a few minutes afterwards. The vibrations will adversely affect image quality if Leginon continues to acquire images during these times. Leginon functionality detects drift and this may be capable of detecting some vibration under some circumstances. Before focussing, consecutive images of the same field are acquired and, depending on the amount of shift between the images, as determined by the displacement of the peak in a cross-correlation function over a given time, the amount of drift is determined. However, if there is substantial movement of the specimen holder - such as occur may during and shortly after liquid nitrogen top up - then the consecutive images may be of entirely dissimilar fields and the highest peak in the cross-correlation function will not be a reliable indicator of the displacement. Therefore, it is useful to have another way of pausing data collection during and shortly after liquid nitrogen top up and then subsequently re-starting data collection. When manual top up is used, the person responsible can also pause and restart Leginon. Otherwise some other automatic method for synchronizing the pausing and restarting of data collection must be used.
We developed software that works in conjunction with Norhof’s pump monitor software and Leginon to solve this problem. The pump was interfaced to a separate laptop Windows PC. Norhof’s pump monitor software ran on the laptop and was used to change the frequency of top up. Also, the user interface of the pump monitor software had a clock that counts to the next scheduled top up. Our software consisted of a client-server pair written in Perl that had a client that eavesdropped on the Norhof pump monitor software’s user interface, so that the client could itself monitor the state of the pump. The Leginon pause client made uses of a Perl library Win32::GuiTest (available from CPAN) in order to monitor Norhof’s software in this way. The server ran on the same Linux system as Leginon. Depending on Leginon’s state at the time of a request to pause, Leginon may either pause immediately or take a number of minutes to respond to the request; so it is necessary to send a pause request to Leginon a number of minutes (chosen by the user) before a liquid nitrogen top up is expected to occur. The client and server communicated over the network using a simple protocol implemented using socket programming. After top up completed, the client waited a number of minutes (chosen by the user) to request for Leginon to restart. (Pausing and restarting was implemented using the Perl library X11::GUITest by sending X11 requests that simulate a user clicking to change between Leginon nodes and press the pause button in order to pause or restart.) The Leginon pause client was started on the laptop with the command line below (from a DOS prompt): lpclient.pl <server-host> <prefill-time-mins> <postfill-time-mins> where <server-host> is the server hostname, <prefill-time-mins> is the number of minutes before liquid nitrogen top up that the request to pause Leginon will be made and <postfill-time-mins> is the number of minutes after a completed liquid nitrogen top up that the request to restart will be made. (The time between fills is not chosen at the command line for lpclient.pl; because this is controlled either directly at the pump or in the Norhof software.) The Leginon pause server was started on the Linux system that Leginon ran on (after Leginon) with the command line: lpserver.pl >& lpserver.log &
An earlier approach did not use a client-server pair of programs and merely used a program on the Linux system running Leginon to pause Leginon at regular intervals, after synchronizing the timing for the fill times with the Norhof software. The problem with this system was that the potential existed for the timing of the pump to drift out of synchronization with the timing of the program that paused and restarted Leginon, due to variable durations for top up to be completed.
(ii) Automated imaging from lacey carbon grids
Leginon provides an extensible framework for implementing new protocols for data collection and other tasks on the electron microscope by creating new applications. Applications are created by using the Leginon Application Editor to link together existing nodes - modules with some basic functionality - to work together in a novel way (Carragher et al., 2010). Typically, for automated data collection of cryoEM images, the Calibration application and either the MSI-Edge or MSI-T applications would be used. (MSI-Edge and MSI-T differ in the approach used for template-matching to detect holes in the EM support grids.) MSI-Edge and MSI-T both require Quantifoil (or C-flat) grids with a regular array of circular holes on the grid squares for data collection protocol to work correctly.
Unfortunately, in a study of myosin V bound to actin by cryoEM we encountered difficulties in the preparation of Quantifoil and C-flat grids. The actin was found in the holes on these grids (where the protein is required for successful imaging by cryoEM); but the myosin V tended to detach from the actin and bind to the carbon. A change to the cryoEM grid preparation protocol that did not first glow discharge the grids avoided the problem of dissociation, but there was little ice for cryoEM imaging in the holes. It was found that using lacey grids avoids these problems, but as lacey grids have holes with random size and shape, MSI-Edge and MSI-T do not provide a suitable data collection protocol for these grids. However, we found some success in collecting data with the existing MSI-Raster application in Leginon (Carragher et al., 2010) at least with respect to targetting of suitable areas within a square for image acquisition. MSI-Raster has the same nodes linked together in Leginon as MSI-Edge and MSI-T, except the nodes for hole targetting and exposure targetting are different. MSI-Raster is mainly suitable for collecting data from negatively-stained specimens on grids with a continuous carbon support film (without holes). Initial acquisition targets are chosen on a regular array of points with user controlled parameters (orientation and extent of the array of points and the spacing of the points). This is a suitable choice of initial acquisition targets on a lacey grid, given that a large proportion of the area of a grid square is ice and potentially suitable for imaging. Subsequent refinement of the targets by user-controlled parameters allow targetting of areas with a suitable ice thickness for imaging.
The strategy for selecting focus targets in the existing MSI-Raster application is similar to that used for acquisition targets, in that a point (or points) with a fixed position is used for focus targetting. Unfortunately, this was not successful as a method for selecting a focus target prior to focussing before acquiring the final high magnification image, because of the low chance of locating an area of carbon suitable for focussing in this way. Therefore, it was necessary to develop a new node for exposure targetting that finds suitable focus targets in a different way.
The new node - RasterFCFinder - replaced the RasterFinder node in the existing MSI-Raster application in the new application, MSI-LaceyFC. The code for the RasterFCFinder was originally based on the RasterFinder node’s code. The RasterFCFinder code was later re-written to eliminate code replicated from the RasterFinder code where possible (in order to facilitate code maintenance), so the RasterFCFinder node’s code was re-factored so that the rasterfcfinder class inherited from the rasterfinder (using object-oriented programming techniques supported by the Python language that Leginon is written in).
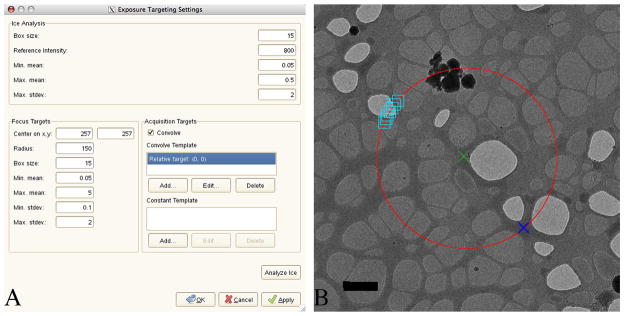
Fig. 2a shows the exposure targetting settings dialogue in the RasterFCFinder (which otherwise is similar in appearance and usage to the original RasterFinder node). Fig. 2b shows a typical intermediate magnification image that would be used for finding suitable targets for focussing and then obtaining one or more high magnification images. The image in fig. 2b has a schematic superimposed in order to illustrate the strategy used to find a suitable focus target. The dialogue in fig. 2a controls how the focus target is selected. The radius in pixels and the coordinates of the centre of a circle are given in the dialogue, and a box size is given. The local mean and standard deviation of the pixels within a box of the given size is computed at each point on the circumference of the circle and the dialogue specifies maximum and minimum values. The focus target chosen is the point on the circumference of the circle with the highest local mean, subject to the constraints (for the maximum and minimum values of the local mean and standard deviation). It is preferred to use areas of carbon for focussing, rather than ice, and targetting the darker areas will generally obtain the carbon areas. However, the additional constraints are useful for avoiding erroneously targetting other types of dark area (such as contaminants or areas outside of the grid square). Based on a survey of an automated data set, 72% intermediate magnification images targeted from square images were of areas suitable for later data acquisition and in 73% of cases correct focus targets were found in test calculations with the intermediate magnification images. The pseudocode below describes the algorithm used to choose the focus target:
Fig. 2.
(a) Dialogue for RasterFCFinder node with alternative focus targetting strategy. (b) Schematic superimposed on a cryoEM image of an intermediate magnification image (using the “hl” preset) explaining alternative focus targetting strategy. The green cross marks the centre of a 150 pixel circle that is used for finding focus targets. Typically data collection will be from one or more targets at or near the centre of the circle (after focussing has occurred). The local mean and standard deviation are computed in boxes centred on the points on the circumference of the circle. In order to avoid the schematic becoming cluttered only a number of boxes (larger than used in the application of the algorithm in this example) are shown. In practice, smaller boxes may be used and are closely spaced about 1 pixel apart from each other. The focus target that is chosen has the highest local mean that satisfies the constraints (that the local mean and standard deviation are between user chosen minimum and maximum values). A blue cross shows the location of the focus target found, using a 15 pixel side box. The scale bar is 1 μm.
initialize list of points to an empty list
initialize list of local means to an empty list
-
loop over points in the circle of radius R:
compute local mean in a square with side B for current point
-
compute local standard deviation in a square
with side B for current point
-
if the current local mean is between the user-chosen
minimum and maximum values
and also the current local standard deviation is between
the user-chosen minimum and maximum values for
standard deviation then add the current point to the list of points
and add the current local mean to the list of local means
(end of loop)
find the largest value, bestFocusMean, in the list of local means
-
find the index of the this largest value in the list of local means and
then look up the point in the list of points with this index -
this gives the chosen point for focus targetting (and has the
local mean bestFocusMean)
The approach described has been useful for obtaining a number of datasets from unattended overnight runs of the automatic image acquisition software. The new node with the above strategy of focussing and an application using the new node will be made available in a future version of Leginon.
CONCLUSIONS
We have described an approach, built on the infrastructure provided by Leginon, for full automatic image data acquisition using an electron microscope (including automatic liquid nitrogen top up) from lacey electron microscope grids (rather than lithographically manufactured Quantifoil or C-flat grids). We developed the ability to automatically collect data from lacey electron microscope grids because of specimen-dependent problems with Quantifoil and C-flat grids. Other laboratories (Shi et al., 2008) have also noticed improved imaging from home-made electron microscope grids that do not have a regular array of circular holes, perhaps because of the high area of carbon compared to holes in lithographed grids.
Acknowledgments
We thank Peiyi Wang for assisting with the platform for the larger ACD dewar, David Parcej for assisting with the design of the scaffolding to hold up the liquid nitrogen fill tubes, Matt Walker for providing the actomyosin V cryoEM grids which were used for part of this work, and also Anchi Cheng and Jim Pulokas for invaluable support and discussions about Leginon. This work was supported by NIH Grant EB00209.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker T, Bhushan S, Jarasch A, Armache JP, Funes S, Jossinet F, Gumbart J, Mielke T, Berninghausen O, Schulten K, Westhof E, Gilmore R, Mandon EC, Beckmann R. Structure of Monomeric Yeast and Mammalian Sec61 Complexes Interacting with the Translating Ribosome. Science. 2010;326:1369–1373. doi: 10.1126/science.1178535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–718. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- Carragher B, Kisseberg N, Kriegman D, Milligan RA, Potter CS, Pulokas J, Reilein A. Leginon: an automated system for acquisition of images from vitreous ice specimens. Journal of Structural Biology. 2000;132:33–45. doi: 10.1006/jsbi.2000.4314. [DOI] [PubMed] [Google Scholar]
- Carragher B, Cheng A, Fellmann D, Guerra F, Irving C, Lander G, Mecurio P, Potter C, Pulokas J, Quispe J, Sheehan B, Stagg S, Suloway C, Voss N, Yoshioka C. Leginon Manual. 2010. [Google Scholar]
- Chen S, Roseman AM, Hunter AS, Wood SP, Burston SG, Ranson NA, Clarke AR, Saibil HR. Location of a folding protein and shape changes in GroEL-GroES complexes imaged by cryo-electron microscopy. Nature. 1994;371:261–264. doi: 10.1038/371261a0. [DOI] [PubMed] [Google Scholar]
- Cheng A, Fellmann D, Pulokas J, Potter CS, Carragher B. Does contamination buildup limit throughput for automated cryoEM? Journal of Structural Biology. 2006;154:303–311. doi: 10.1016/j.jsb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Glaeser RM. Electron crystallography: present excitement, a nod to the past, anticipating the future. Journal of Structural Biology. 1999;128:3–14. doi: 10.1006/jsbi.1999.4172. [DOI] [PubMed] [Google Scholar]
- Glaeser RM, Lee J, Typke D. Microscopy and Microanalysis. Chicago 2006. Chicago: 2006. Advantages and Objectives of High-throughput Data Collection in Single-particle Cryo-EM. [Google Scholar]
- Henderson R. The potential and limitations of neutrons, electrons and X-rays for atomic resolution microscopy of unstained biological molecules. Quarterly Review of Biophysics. 1995;28:171–193. doi: 10.1017/s003358350000305x. [DOI] [PubMed] [Google Scholar]
- Kisseberth N, Whittaker M, Weber D, Potter CS, Carragher B. emScope: A Toolkit for Control and Automation of a Remote Electron Microscope. Journal of Structural Biology. 1998;120:309–319. doi: 10.1006/jsbi.1997.3918. [DOI] [PubMed] [Google Scholar]
- Lei J, Frank J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. Journal of Structural Biology. 2005;150:69–80. doi: 10.1016/j.jsb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Ludtke SJ, Baker ML, Chen DH, Song JL, Chuang DT, Chiu W. De Novo Backbone Trace of GroEL from Single Particle Electron Cryomicroscopy. Structure. 2008;16:441–448. doi: 10.1016/j.str.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Marsh MP, Chang JT, Booth CR, Liang NL, Schmid MF, Chiu W. Modular software platform for low-dose electron microscopy and tomography. Journal of Microscopy. 2007;228:384–389. doi: 10.1111/j.1365-2818.2007.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CS, Chu H, Frey B, Green C, Kisseberth N, Madden TJ, Miller KL, Nahrstedt K, Pulokas J, Reilein A, Tcheng D, Weber D, Carragher B. A system for fully automated acquisition of 1000 electron micrographs a day. Ultramicroscopy. 1999;77:153–161. doi: 10.1016/s0304-3991(99)00043-1. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Numata N, Walker ML, Kato YS, Malkova B, Kon T, Ohkura R, Arisaka F, Knight PJ, Sutoh K, Burgess SA. AAA+ Ring and Linker Swing Mechanism in the Dynein Motor. Cell. 2009;136:485–495. doi: 10.1016/j.cell.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SJ, Fried G, Pulokas J. An automated system for maintaining liquid nitrogen levels in the Gatan cryostage UIUC Technical Report. 2000. [Google Scholar]
- Roseman AM, Chen S, White H, Braig K, Saibil HR. The Chaperonin ATPase Cycle: Mechanism of Allosteric Switching and Movements of Substrate-Binding Domains in GroEL. Cell. 1996;87:241–251. doi: 10.1016/s0092-8674(00)81342-2. [DOI] [PubMed] [Google Scholar]
- Seidelt B, Innis CA, Wilson DN, Gartmann M, Armache JP, Villa E, Trabuco LG, Becker T, Mielke T, Schulten K, Steitz TA, Beckmann R. Structural Insight into Nascent Polypeptide Chain-Mediated Translational Stalling. Science. 2009;326:1412–1415. doi: 10.1126/science.1177662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Williams DR, Stewart PL. A Script-Assisted Microscopy (SAM) package to improve data acquisition rates on FEI Tecnai electron microscopes equipped with Gatan CCD camera. Journal of Structural Biology. 2008;164:166–169. doi: 10.1016/j.jsb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg SM, Lander GC, Pulokas J, Fellmann D, Cheng A, Quispe JD, Mallick SP, Avila RM, Carragher B, Potter CS. Automated cryoEM data acquisition and analysis of 284742 particles of GroEL. Journal of Structural Biology. 2006;155:470–481. doi: 10.1016/j.jsb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: The new Leginon system. Journal of Structural Biology. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Thirumurugan K, Sakamoto T, Hammer JA, III, Sellers JR, Knight PJ. The cargo-binding domain regulates structure and activity of myosin 5. Nature. 2006;442:212–215. doi: 10.1038/nature04865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Burgess S, Sellers J, Wang F, Hammer J, Trinick J, Knight P. Two-headed binding of a processive myosin to F-actin. Nature. 2000;405:804–807. doi: 10.1038/35015592. [DOI] [PubMed] [Google Scholar]
- Zhang J, Nakamura N, Shimizu Y, Liang N, Liu X, Jakana J, Marsh MP, Booth CR, Shinkawa T, Nakata M, Chiu W. JADAS: A Customizable Automated Data Acquisition System and its Application to Ice-embedded Single Particles. Journal of Structural Biology. 2009;165:1–9. doi: 10.1016/j.jsb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Beatty A, Milne JLS, Subramaniam S. Automated Data Collection with a Tecnai 12 Electron Microscope: Applications for Molecular Imaging by Cryomicroscopy. Journal of Structural Biology. 2001;135:251–261. doi: 10.1006/jsbi.2001.4404. [DOI] [PubMed] [Google Scholar]
- Zhang P, Borgnia MJ, Mooney P, Shi D, Pan M, O’Herron P, Mao A, Brogan D, Milne JLS, Subramaniam S. Automated image acquisition and processing using a new generation of 4K CCD cameras for cryo electron microscopic studies of macromolecular assemblies. Journal of Structural Biology. 2003;143:135–144. doi: 10.1016/s1047-8477(03)00124-2. [DOI] [PubMed] [Google Scholar]