Abstract
Reperfusion of ischemic tissue induces significant tissue damage in multiple conditions, including myocardial infarctions, stroke and transplantation. Although not as common, the mortality rate of mesenteric ischemia/reperfusion (IR) remains over 70%. Although complement and naturally occurring antibodies are known to mediate significant damage during IR, the target antigens are intracellular molecules. We investigated the role of the serum protein, β2-glycoprotein I as an initiating antigen for antibody recognition and β2-GPI peptides as a therapeutic for mesenteric IR. The time course of β2-GPI binding to the tissue indicated binding and complement activation within 15 min post-reperfusion. Treatment of wildtype mice with peptides corresponding to the lipid binding domain V of β2-GPI blocked intestinal injury and inflammation, including cellular influx and cytokine and eicosanoid production. The optimal therapeutic peptide (peptide 296) contained the lysine rich region of domain V. In addition, damage and most inflammation was also blocked by peptide 305 which overlaps with peptide 296 but does not contain the lysine rich, phospholipid binding region. Importantly, peptide 296 retained efficacy after replacement of Cys residues with Ser. In addition, infusion of wildtype serum containing reduced levels of anti-β2-GPI antibodies into Rag-1−/− mice prevented IR-induced intestinal damage and inflammation. Together these data suggest that the serum protein,β2-GPI initiates the IR-induced intestinal damage and inflammatory response and as such is a critical therapeutic target for IR-induced damage and inflammation. This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org
Keywords: mouse, intestine, complement, antibodies
Introduction
During an ischemic event, the lack of blood flow to an organ induces tissue damage. However, return of blood flow during reperfusion enhances pathology significantly. The inflammatory response to ischemia/reperfusion (IR)-induced organ damage may subsequently lead to a systemic inflammatory response with multiple organ failure. Intestinal IR results in severe inflammatory-induced mucosal damage, barrier dysfunction, and subsequent bacterial translocation leading to sepsis (1) and frequently results in liver and lung damage (2).
Mesenteric IR-induced tissue injury is mediated by at least two components of the innate immune response, neutrophil infiltration and complement activation (3–5). Initial studies demonstrated that neutrophil depletion attenuated intestinal IR-induced injury (4–5). However, the presence of neutrophils was not sufficient for tissue damage when complement activation was inhibited (6). Complement activation increased adhesion molecule expression after IR and released a cascade of inflammatory mediators including leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) which also contributed to tissue damage (7–9). Additionally, depletion or inhibition of complement activation products prevented both local and remote organ injury in response to intestinal IR (10–13).
Cells subjected to hypoxic conditions express cryptic antigens on the plasma membrane (14–15). Cryptic antigens expressed on apoptotic cells are recognized by natural antibodies which frequently exhibit low affinity binding (16). Previous studies indicated that administering naturally occurring monoclonal antibodies (mAb) reconstituted IR-induced intestinal damage in antibody-deficient Rag-1−/− mice (15, 17–19). Multiple natural antibodies which recognized intracellular antigens, DNA, non-muscle myosin and ribonucleoprotein, and cardiolipin induced damage in the IR-resistant Rag-1−/− mouse suggesting that the antibodies and antigens are critical to IR-induced damage (18–21). In conjunction with anti-phospholipid mAb, antibodies to the serum protein, β2-glycoprotein I (β2-GPI) also restored tissue damage in Rag-1−/−, IR resistant mice (19). These data suggest that ischemia induces a cellular response resulting in expression of multiple cryptic antigens targeted by low-affinity, naturally occurring antibodies also found in autoimmune diseases.
The serum protein, β2-GPI, also known as apolipoprotein H, is a member of the complement control protein family (22–23) but has no known complement regulating function (24). However, β2-GPI is a cofactor for plasminogen activation (25) and an opsonin for the clearance of apoptotic cells by phagocytes (26). By binding to anionic phospholipids, DNA or other negatively charged molecules (22), β2-GPI is the major antigenic target for anti-phospholipid antibodies found in the serum of anti-phospholipid antibody syndrome (APLS) patients (27). Increased anti-β2-GPI antibody titer also correlated with increased risk of ischemic stroke or heart disease in APLS or systemic lupus erythematosus patients, respectively (28–29). Together, these data suggest anti-β2-GPI antibodies are involved in ischemic events.
Based on these data, we hypothesized that during reperfusion, serum protein β2-GPI binds ischemic cell membranes and is recognized by naturally occurring antibodies which leads to complement activation and inflammation. Using an in vitro model, our findings demonstrate that anti-β2-GPI antibodies recognized β2-GPI bound to the surface of hypoxic endothelial cells. In a mouse model of intestinal ischemia, β2-GPI binding to damaged ischemic intestinal tissue correlated with tissue injury and reduction of anti-β2-GPI antibodies mitigated intestinal damage and inflammation. As reduction of antibodies in vivo is difficult, we injected β2-GPI peptides to compete with β2-GPI binding to the tissue. Importantly, injection of peptides specific for the lipid binding domain of β2-GPI blocked intestinal injury as well as eicosanoid and cytokine production. Administration of peptides containing the phospholipid binding, lysine rich region and adjacent regions were most effective. Together these data suggest that β2-GPI initiates the IR-induced intestinal damage and inflammatory response and as such is a critical therapeutic target for IR-induced damage and inflammation.
Materials and methods
Mice
C57Bl/6 and Rag-1−/− (backcrossed to C57Bl/6 for 10 generations) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred and maintained under 12 h light/dark cycles at Kansas State University, Division of Biology (Manhattan, KS). All mice were allowed access to food and water ad libitum and maintained under specific pathogen free conditions. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and was approved by the Institutional Animal Care and Use Committee.
Ischemia/reperfusion Procedure
Animals were subjected to IR similar to previously described studies (30). Briefly, ketamine (16mg/kg) and xylazine (80 mg/kg) anesthetized mice were administered buprenorphine (0.06mg/kg) for pain. After laparotomy and a 30 min equilibration period, a small vascular clamp (Roboz Surgical Instruments) was applied to the isolated superior mesenteric artery. After 30 min of ischemia, the clamp was removed and the intestines reperfused for up to 2 h. Sham animals sustained the same surgical intervention without superior mesenteric artery occlusion. Mice treated with the various β2-GPI peptides underwent the same procedure with i.v. administration of the peptides (40 μM) 5 min prior to artery occlusion. Peptides 296, 305, and 296Cys to Ser were soluble in N. Saline and injected i.v. in 100ul volumes. Peptides 100 and 181 were dissolved in DMSO prior to diluting 1/100 in N. Saline. Additional mice received peptides prior to Sham treatment.
In some experiments, 200μl of C57Bl/6 sera with or without the reduction of anti-β2-GPI Ab was administered i.v. to Rag-1−/− mice 20 min prior to clamp application. After euthanization, mid-jejunum 10–20cm distal to the gastroduodenal junction was removed for analysis. Survival was not significantly different between treatment groups.
β2-GPI peptides
To decrease β2-GPI binding to the cells and tissue, peptides from mouse β2-GPI were designed. As Domain V is proposed to contain the lipid binding site (31), we designed three overlapping 24–25 aa peptides from domain V (peptides 296, 305 and 322) using the NCBI sequence, AAB30789 (32). Peptide 296 contains the lysine rich region previously identified as the critical lipid binding region (31, 33). The overlapping peptide 305 contains the final 3 residues of the lysine rich region and continues into the tail region which is proposed to insert into the lipid membrane. Peptide 322 is contained within the tail region. Additional control peptides 100 and 181 are contained within domains II and III respectively. Most peptides used in this study were purchased from Invitrogen with purity (>90%) and sequence determined by the manufacturer. Production of β2-GPI peptide 296Cys to Ser was generated by solid-phase synthesis with 9-fluorenylmethoxycarbonyl chemistry, as described in detail previously (34). The peptides were purified by reversed-phase HPLC and characterized by matrix-assisted laser desorption time-of-flight mass spectroscopy. All lyophilized peptides were stored at −20°C until time of use.
Histology and Injury Scoring
Immediately after euthanasia, a 2 cm mid-jejunum tissue section was immediately fixed in 10% buffered formalin, embedded in paraffin, and 8μm sections were cut transversely and H&E stained. Mucosal injury was graded on a six-tiered scale adapted from Chiu et al. (35) as described previously (36). Briefly, the average damage score of the intestinal section (75–150 villi) was determined after grading each villus from 0–6. Normal villi were assigned a score of zero; villi with tip distortion were assigned a score of 1; a score of 2 was assigned when Guggenheims’ spaces were present; villi with patchy disruption of the epithelial cells were assigned a score of 3; a score of 4 was assigned to villi with exposed but intact lamina propria with epithelial sloughing; a score of 5 was assigned when the lamina propria was exuding; last, villi that displayed hemorrhage or were denuded were assigned a score of 6. Photomicrographs were obtained from H&E stained slides using a 20X, 0.5 Plan Fluor objective on Nikon 80i microscope and images acquired at room temperature using a Nikon DS-5M camera with DS-L2 software.
Ex vivo eicosanoid and cytokine generation
Ex vivo generation of eicosanoids by mid-jejunal tissue was determined as previously described (30). Immediately after collection, a 2 cm intestinal section was minced, washed, resuspended in 37°C oxygenated Tyrode’s buffer (Sigma-Aldrich), incubated at 37°C for 20 minutes and the supernatants collected. PGE2 and LTB4 concentrations were determined using enzyme immunoassay kits (Cayman Chemical). IL-6 and IL-12 concentrations were determined using a Milliplex MAP immunoassay kit (Millipore) and read on a Milliplex Analyzer (Millipore). All eicosanoid and cytokine concentrations were standardized to the total tissue protein content.
C3 deposition and immunohistochemistry
After euthanasia, a 2 cm intestinal section was immediately snap frozen in O.C.T. freezing medium and 8 μm sections were placed on slides for immunohistochemistry. The C3 deposition and F4/80 expression on the tissue sections was detected by staining with a purified rat-anti-mouse C3 (Hycult Biotechnologies) or F4/80 (eBioscience) antibody followed by a Texas-red conjugated donkey-anti-rat IgG secondary antibody (Jackson Immunoresearch). CD31 (PECAM-1), CD106 (VCAM-1) were detected by FITC conjugated rat anti-mouse CD31 or CD106 (Biolegend) antibodies. Each experiment contained serial sections stained with the appropriate isotype control antibodies. All slides were mounted with ProLong Gold (Invitrogen). Images were obtained at room temperature using a Nikon eclipse 80i microscope equipped with a CoolSnap CF camera (Photometrics) and analyzed using Metavue software (Molecular Devices).
Immunoprecipitation of β2-GPI complexes from tissue
Mid-jejunum (25–30 mm) was longitudinally opened, adhered to a 6-well plate and incubated at 4°C for 2 h in freshly oxygenated Tyrodes buffer containing 15μg/ml FC1 mAb (mouseIgG1, anti-β2-GPI)(37). The crosslinker, DTSSP (Pierce), was added to the Ab solution at a final concentration of 1.5mM and incubated at 4°C for an additional 2 h. The reaction was stopped with Tris, pH 7.5 and the washed mucosa was lysed in 1 ml of MES/Brij58 (145 mM NaCl, 0.2 mM EDTA, 0.5% w/v Brij58 (Sigma), 25 mM MES (Sigma), pH 6.5). The lysate was incubated for 30 min on ice, with periodic vortexing and clarified by centrifuging at 5,000×g for 10 min at 4°C. Antibody was immunoprecipitated overnight at 4°C with Protein G beads (Pierce) and the samples were boiled in non-reducing Laemmli sample buffer prior to SDS-PAGE (10%) and Western blot analysis. Human β2-GPI (Fitzgerald) was used as a positive control. The blots were probed with anti-β2-GPI antibody, MAB1066 (Chemicon) followed by goat anti-mouse IgG HRP conjugate (Pierce). Protein was visualized using SuperSignal Detection Kit (Pierce) according to the manufacturer’s protocol.
Hypoxia and immunocytochemistry
Hypoxia was conducted similar to previous studies with the following modifications (38). Hypoxic MS-1 endothelial cells (ATCC) received degassed, serum-free DMEM and were placed in a hypoxia chamber containing 94% nitrogen and 5% CO2. Normoxic cells received DMEM supplemented with 10% heat-inactivated sera from Rag-1−/− mice in 8% CO2. After 4 h at 37°C, all cells received fresh medium containing 10% heat-inactivated Rag-1−/− sera and were incubated in normoxic conditions for 1 hr at 37°C. Additional peptide studies were performed by addition of peptides (40 uM final concentration) during the hypoxic period. Cells were methanol fixed and stained with the anti-β2-GPI mAb (Millipore) followed by an anti-mouse IgG antibody to determine β2-GPI binding. Anti-β2-GPI binding was determined by allowing anti-β2-GPI mAb (Millipore) to bind during the 1 h normoxic period. The cells were then stained with anti-mouse IgG antibodies (Jackson ImmunoResearch) as previously described (19). The fluorescence was determined in a blinded manner using a Nikon 80i fluorescent microscope with a 40x Plan Fluor objective and images acquired using a CoolSnap Cf camera (Photometrics) and MetaVue Imaging software (Molecular Devices).
Anti-β2-GPI concentrations and isotyping
Anti-β2-GPI concentrations were determined based on optimal conditions previously described (39–40). The specific isotypes of anti-β2-GPI antibodies were determined after binding serum in duplicate to coated and blocked wells and incubating for 1 h. After washing, the appropriate biotinylated anti-mouse Ig isotype antibodies were added to each well for 1 h at RT while gently shaking. After incubation with avidin peroxidase (Sigma) the plate was developed using TMB (Kirkegaard).
Reduction of anti-β2-GPI activity from C57Bl/6 serum
An ELISA plate was coated for 2 h at room temperature with 2μg of β2-GPI (Fitzgerald) in PBS. After blocking for 2 h with 100μl of 3% bovine serum albumin in PBS, 50μl of heat-inactivated C57Bl/6 sera was added to half of the coated wells for 2 h at room temperature. The sera was then transferred to the remaining coated set of wells and incubated for an additional 2 h at room temperature. The reduced serum was removed, pooled and then administered as described above. The reduction procedure removed approximately 50% of the anti-β2-GPI antibodies as determined by ELISA.
Statistical analysis
Data are presented as mean ± SEM and significance (p <0.05) determined by one-way ANOVA with Newman-Keuls post hoc analysis (GraphPad/Instat Software).
Results
Mucosal injury and β2-GPI binding to ischemic or hypoxic tissue occurs early in reperfusion
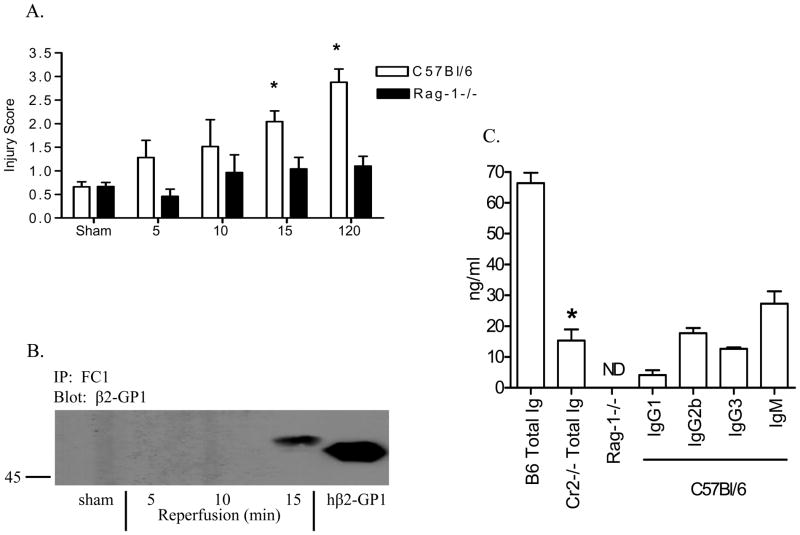
C57Bl/6 mice were subjected to ischemic injury followed by 5, 10, or 15 min of reperfusion. Compared to pooled sham-treated animals, significant mid-jejunal mucosal injury was observed after 15 min of reperfusion and increased up to 2 h post-reperfusion (Fig. 1A). In contrast, Rag-1−/− mice did not sustain intestinal damage at any time point analyzed (Fig. 1A). When analyzed for β2-GPI, sera from both C57Bl/6 and Rag-1−/− mice contained similar concentrations of β2-GPI (data not shown). As previously shown, anti-β2-GPI binds ischemic-damaged tissue within 2 h following reperfusion (19); however, we were interested in determining the early kinetics of β2-GPI binding to tissue following ischemia. To examine the kinetics, tissue harvested after 5, 10, or 15 min of reperfusion was probed with the anti-β2-GPI mAb, FC1. The antibody/antigen complexes were cross-linked to the surface of the villi prior to immunoprecipitation and Western blotting. Immunoprecipitation indicated the presence of β2-GPI bound to the cell surface at 15 min post-reperfusion but not at the earlier time points (Fig. 1B). The apparent molecular weight difference between human and mouse is likely due to differential glycosylation and different isoelectric points (41). Additionally the presence of detectable levels of tissue-bound β2-GPI correlates positively with the earliest time point when significant damage was observed (Fig 1A).
Figure 1. The presence of β2-GPI and anti-β2-GPI correlates with IR-induced intestinal damage.
A. Mid-jejunal sections collected from C57Bl/6 or Rag-1−/− mice at 5, 10 and 15 min after reperfusion or from Sham-treated mice were scored for intestinal injury (75–150 villi per animal with 3–10 animals/treatment and each treatment was performed on at least 2 separate days). B. β2-GPI was immunoprecipitated with FC1 from tissue sections collected at 5, 10 and 15 min post-reperfusion or from Sham-treated mice and subjected to Western blot analysis. Human β2-GPI (50 kDa) was run as a control for mouse β2-GPI (54 kDa). Blot is representative of 4 experiments. C. Serum concentrations of anti-β2-GPI antibodies in C57Bl/6, C3−/−, CR2−/− or Rag-1−/− mice as determined by ELISA. Isotypes of the specific antibodies bound to β2-GPI were determined using specific rat anti-mouse isotyping antibodies. Each bar represents the mean ±SEM of 3 independent experiments. * = p ≤ 0.05 compared to Sham.
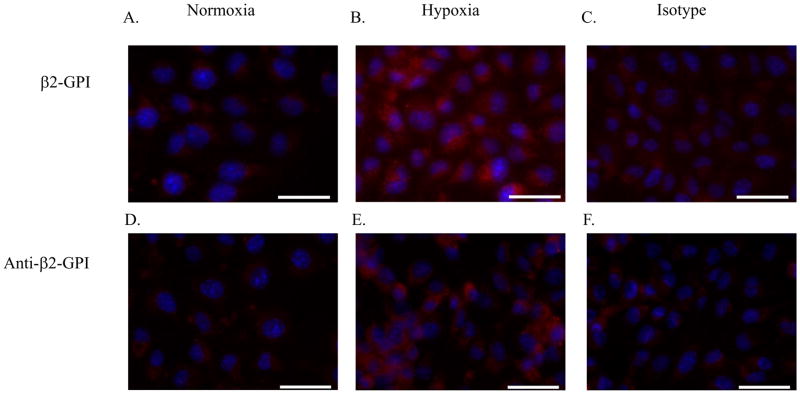
MS-1 endothelial cells were subjected to hypoxia or normoxia to validate β2-GPI binding in vitro. Addition of sera from Rag-1−/− mice during the subsequent normoxia (reperfusion) stage provided the β2-GPI. After hypoxic but not normoxic treatment, cells were positive for β2-GPI (Fig. 2A–C). The addition of anti-β2-GPI mAb to the cells during reperfusion, again showed that only hypoxic but not normoxic-treated cells stained positively for anti-β2-GPI antibodies (Fig. 2D–F). Similar to the in vivo results, in vitro studies showed that hypoxia-induced cellular changes facilitated the binding of both β2-GPI and anti-β2-GPI antibodies to the surface of ischemic cells.
Figure 2. β2-GPI and anti-β2-GPI antibodies bind to MS-1 cells following hypoxia.
Cells were subjected to 4 hours of normoxia in media containing 10% heat-inactivated Rag-1−/− sera (A, D) or hypoxia under serum-free conditions (B–C, E–F), followed by 1 hour of normoxia in media containing 10% Rag-1−/− serum in the absence (A–C) or presence of anti-β2-GPI (D–E) or isotype control (F) antibody. The cells were fixed with methanol, probed with a primary anti-β2-GPI antibody (A–B) or isotype control antibody (C) then stained with an anti-mouse secondary or stained with secondary antibody only (Red; D–F). Slides were mounted with DAPI (Blue) to identify the nuclei. Each photomicrograph is representative of 3 experiments with 4–6 photomicrographs per treatment group in each experiment. Bar = 40μm.
Characterization of the anti-β2-GPI activity in C57Bl/6 serum
To further understand the role of anti-β2-GPI antibodies, we examined the presence of these antibodies in wildtype and Rag-1−/− mice. As shown in Figure 1C, we determined that approximately 60 ng/ml anti-β2-GPI antibody (total Ig) is present in C57Bl/6 serum but as expected Rag-1−/− serum contained no detectable antibodies (Fig. 1C). Interestingly, serum from IR-resistant, Cr2−/− mice contains significantly less anti-β2-GPI antibody (Fig. 1C). These results indicate that naturally occurring antibodies against β2-GPI exist in wildtype mice. The anti-β2-GPI antibody concentration in wildtype sera was determined to be primarily of the IgM and IgG2b isotypes with minor amounts of IgG3 and IgG1 isotypes (Fig. 1C). The presence of IgG2b, IgG3 and IgM isotypes is consistent with complement activation. Therefore, β2-GPI represents a significant target for forming antibody/antigen complexes capable of facilitating complement-mediated tissue damage.
Reduction of serum anti-β2-GPI activity attenuated intestinal damage and inflammation
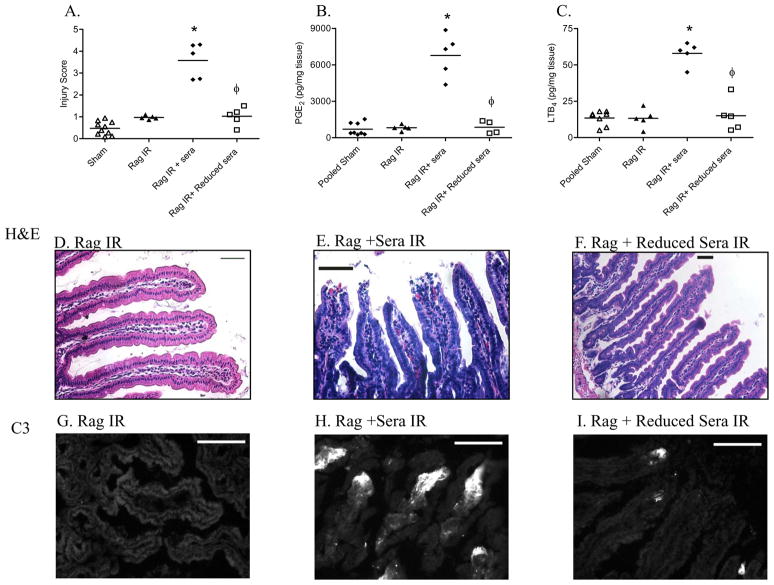
The effects of anti-β2-GPI antibody reduction on IR-mediated damage were assessed by subjecting Rag-1−/− mice to IR after reconstitution with wildtype serum after 2 rounds of adsorption to bound β2-GPI. When Rag-1−/− mice were reconstituted with non-adsorbed C57Bl/6 serum, significant damage was observed after 2 h reperfusion (Fig. 3A) similar to previous results for C57Bl/6 mice (Fig. 1A). However, when mice were administered anti-β2-GPI reduced serum, no damage was observed similar to that seen in Rag-1−/− IR control mice (Fig. 3A, D–F). Moreover, the effects of anti-β2-GPI reduction extended to dramatically decreasing the intestinal inflammatory response. The IR-induced increase in PGE2 and LTB4 production was abrogated with the antibody-reduced serum to concentrations similar to Rag-1−/− IR controls (Fig. 3B, C). These data suggest that inhibition of anti-β2-GPI antibodies may provide a therapeutic target for IR-induced tissue damage.
Figure 3. Reduction of anti-β2-GPI antibody attenuates tissue injury and inflammation.
A. Mid-jejunal sections were scored (75–150 villi per animal) from Rag-1−/− mice with or without injection of C57Bl/6 sera or anti-β2-GPI antibody reduced C57Bl/6 serum prior to Sham or IR treatment. PGE2 (B) or LTB4 (C) production was measured in Rag-1−/− mice injected with C57Bl/6 sera or anti-β2-GPI antibody reduced C57Bl/6 serum prior to Sham or IR treatment. Values are represented as pg/mg of intestinal protein. * = p ≤ 0.05 compared to Sham, φ = p ≤ 0.05 compared to animals receiving non-reduced sera. Each animal is represented by an individual point with the bar representing the average. Each treatment was performed on at least 2 separate days. D–I. Representative intestinal sections H&E stained (D–F) or stained for C3 deposition (G–I) from IR-treated Rag-1−/− mice (D,G), IR-treated Rag-1−/− mice receiving C57Bl/6 serum (E, H), or IR-treated Rag-1−/− mice receiving anti-β2-GPI antibody reduced C57Bl/6 serum are shown (F, I). Microphotographs are representative of 3–4 animals stained in at least 3 independent experiments. H&E bar = 50μm and immunohistochemistry bar = 40μm.
Domain V β2-GPI peptides block IR-induced intestinal damage
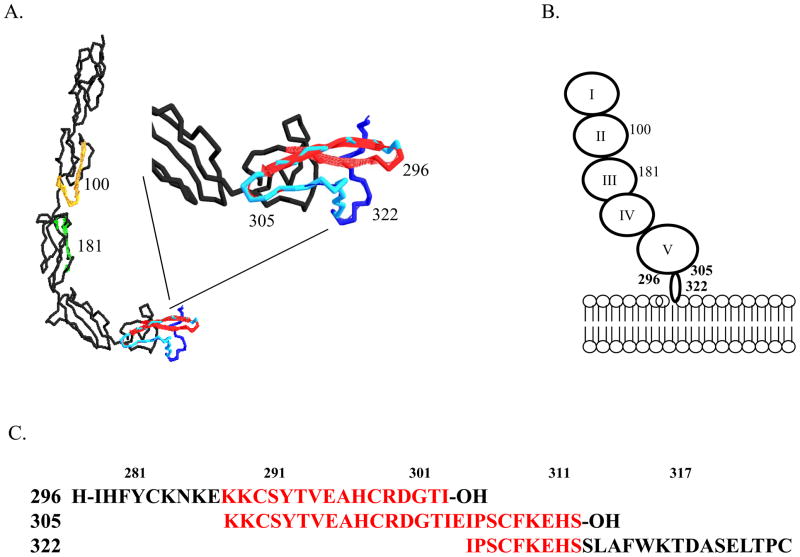
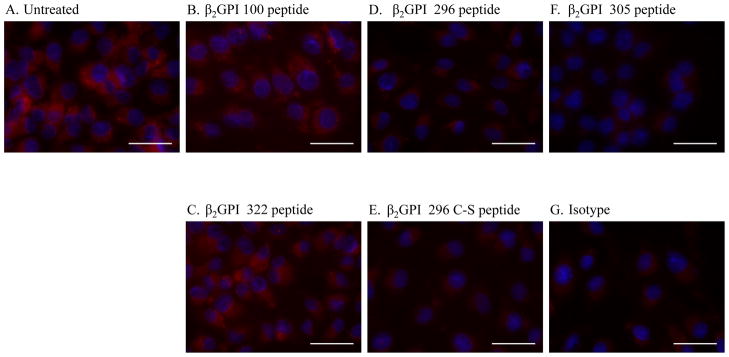
We hypothesized that if inhibition of antibody binding to β2-GPI on the tissue attenuated injury, then peptides which block the lipid-binding domain of β2-GPI may inhibit β2-GPI binding and attenuate IR-induced intestinal damage and inflammation as well. Peptides were designed to match sequences from multiple domains of mouse β2-GPI, including domains II, III and lipid-binding domain V as indicated in Table 1 and Fig 4. Within domain V, three overlapping peptides were created, 296, 305 and 322 (Fig. 4 and Table 1) to cover the lysine rich domain (296) and the tail which is inserted into the lipid membrane (322) with peptide 305 spanning the intervening region. Additional peptides from Domains II and III were used as controls. Initial in vitro studies tested the ability of the peptides to block β2-GPI binding to hypoxic endothelial cells. As indicated in Fig. 5, after 4 h hypoxia, anti-β2-GPI mAb bound to untreated MS-1 cells significantly more than isotype control mAb. β2-GPI peptides 100 or 322 did not inhibit antibody binding to the hypoxic endothelial cell line. In contrast, anti-β2-GPI mAb did not bind to hypoxic MS-1 cells, which were pre-treated with peptides 296 or 305. Together these data indicated that the overlapping peptides 296 and 305 were capable of preventing β2-GPI from binding to hypoxic endothelial cells. As these two peptides contain 3 Cys and may bind non-specifically, the Cys of peptide 296 were changed to Ser and used in the in vitro hypoxia assay. Similar to peptide 296, the Cys-Ser substituted peptide also attenuated β2-GPI binding to the hypoxic cells.
Table I.
β2-GPI peptide sequences
| Peptide name | Sequencea | Residue numbers | MWb (Da) |
|---|---|---|---|
| 100 | H-KNISFACNPGFFLNG-NH2 | 105–118 | 1627 |
| 181 | H-GNDTVMCTEQQN-NH2 | 182–193 | 1338 |
| 296 | H-IHFYCKNKEKKCSYTVEAHCRDGTI-OH | 296–320 | 2974 |
| 296 Cys-Ser | H-IHFYSKNKEKKSSYTVEAHSRDGTI-OH | 296–320 | 2925 |
| 305 | H-KKCSYTVEAHCRDGTIEIPSCFKEHS-OH | 305–330 | 2969 |
| 322 | H-IPSCFKEHSSLAFWKTDASELTPC-NH2 | 322–345 | 2629 |
Amino acid sequence based on NCBI sequence, AAB30789, as described in the Materials and Methods.
MW is Molecular weight
Figure 4. Location of overlapping β2-GPI peptides.
A. Ribbon diagram of human β2-GPI with peptide locations identified by color, peptide 100 (gold), peptide 181 (green), peptide 296 (red), peptide 322 (dark blue) and overlapping peptide 305 (light blue). Inset is magnification of Domain V. B. Cartoon of β2-GPI binding to lipid membrane with peptide locations indicated. C. Sequence identification of overlapping regions of peptides 296, 305 and 322. Red indicates regions of overlap. Peptides were designed based on the published sequences (32) to mimic the lipid binding domain and tail inserted into the lipid bilayer.
Figure 5. β2-GPI peptides inhibit anti-β2-GPI staining of hypoxic MS-1 cells.
Cells were subjected to 4 hours of hypoxia under serum-free conditions with (A) or without (B–G) β2-GPI peptides prior to 1 hour normoxia in media containing 10% heat-inactivated Rag-1−/− sera. The cells were fixed with methanol, probed with a primary anti-β2-GPI antibody (A–F) or isotype control antibody (G) followed by a Texas red labeled, anti-mouse secondary antibody. Slides were mounted with DAPI (Blue) to identify the nuclei. Each photomicrograph is representative of 3 experiments with 4–6 photomicrographs per treatment in each experiment. Bar = 40μm.
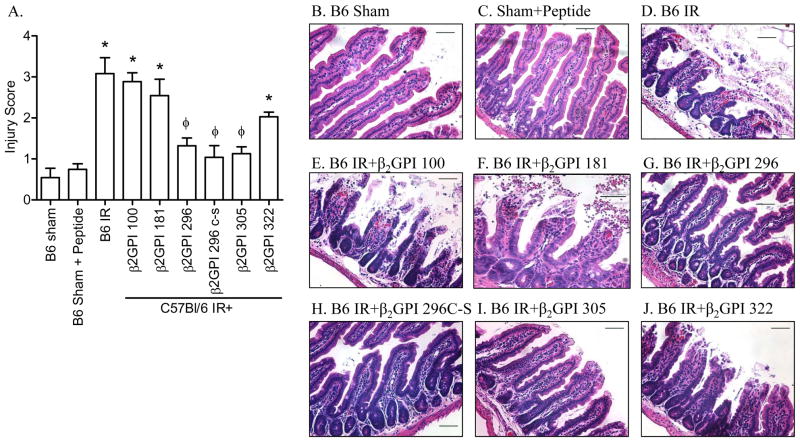
The in vitro hypoxia studies suggested that peptides 296 and 305 may attenuate IR-induced tissue damage. To test this hypothesis, peptides were infused into C57Bl/6 mice 5 min prior to intestinal IR and mucosal damage and inflammation evaluated. Similar to in vitro results, mice which received peptides 296, 305 or 296 Cys to Ser sustained attenuated mucosal damage in response to IR (Fig. 6). In contrast, peptides 100, 181, and 322 sustained IR-induced intestinal damage similar to untreated mice (Fig. 6). Thus, peptide inhibition of β2-GPI attenuates IR-induced intestinal damage.
Figure 6. β2-GPI peptides attenuate IR-induced mucosal damage in wildtype mice.
A. Mid-jejunal sections were scored (75–150 villi per animal) from C57Bl/6 mice with or without injection of β2-GPI peptides prior to Sham or IR treatment. B-G. Representative intestinal sections H&E stained from C57Bl/6 Sham-treated mice (B), IR-treated C57Bl/6 mice in the absence of peptide (C) or receiving β2-GPI peptide 100 (D), peptide 296 (E), peptide 206Cys to Ser (F), peptide 305 (G), and peptide 322 (H). Microphotographs are representative of 3–4 animals stained in at least 3 independent experiments. Bar = 50μm. * = p ≤ 0.05 compared to Sham + peptide, φ = p ≤ 0.05 compared to IR treatment animals not receiving peptides. Each bar is representative of 3–4 animals and each treatment was performed on at least 2 separate days.
Domain V β2-GPI peptides block IR-induced intestinal inflammation
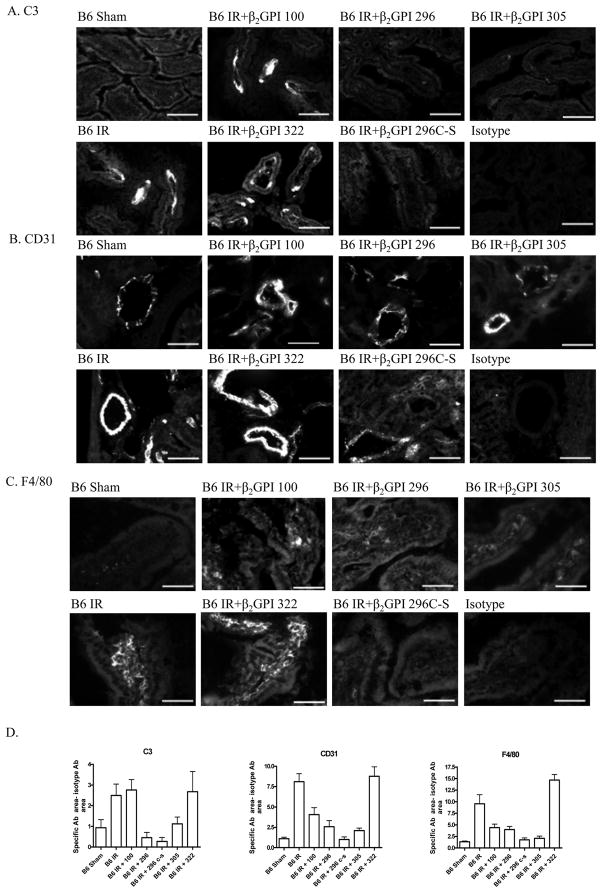
To examine the multiple pathways of inflammation involved in IR-induced damage, intestinal tissues from the peptide treated mice were examined for complement deposition, adhesion molecule expression and the macrophage marker, F4/80. As expected, IR induced C3 deposition on the intestines of C57Bl/6 mice in response to IR but not Sham treatment (Fig. 7A). Similar to injury results, peptides 100 and 322 did not significantly inhibit C3 deposition (Fig. 7A). In addition, infusion of peptides 296, 305 and 296Cys to Ser prior to IR significantly decreased C3 deposition (Fig. 7A). Similarly, the expression of adhesion molecules, CD31and VCAM, was inhibited after treatment with peptides 296 and 296Cys to Ser but not after treatment with peptides 100 or 322 (Fig. 7B, data not shown). Expression of the mature macrophage marker increased in response to IR with or without peptide 100 or peptide 322 (Fig. 7C). Treatment with peptides 296 and 296 Cys to Ser reduced macrophage to Sham levels after treatment with peptide 296Cys to Ser (Fig. 7C). In contrast, peptide 305 which prevented injury and complement deposition did not attenuate CD31 or VCAM expression or macrophage infiltration.
Figure 7. β2-GPI peptides attenuate IR-induced complement deposition, adhesion molecule expression and macrophage infiltration.
Representative intestinal sections stained for C3 (A), CD31 (B), or F4/80 (C) from Sham-treated C57Bl/6 mice, IR-treated C57Bl/6 in the absence or presence of β2-GPI peptides as indicated. Microphotographs are representative of 3–4 animals stained in at least 3 independent experiments. Bar = 40μm.
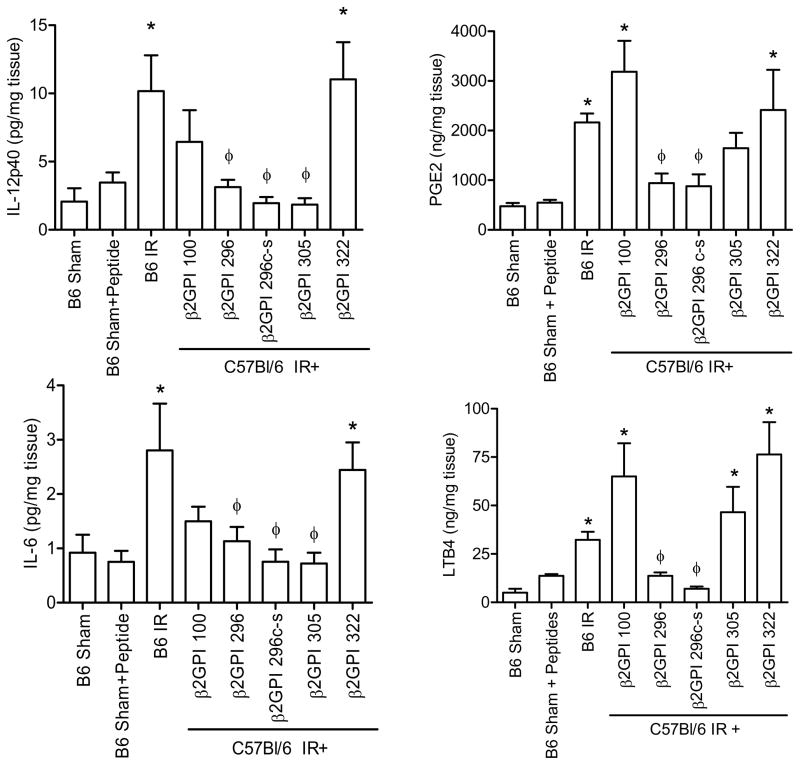
The pro-inflammatory cytokines, IL-12 and IL-6, and eicosanoids, LTB4 and PGE2, increase rapidly in response IR (42). Therefore, we examined the ability of peptides 296, 305 and 296 Cys to Ser to attenuate production of these inflammatory molecules. Similar to previous results, IR induced IL-12 and IL-6 production which was attenuated by protective peptides 296, 305 and 296Cys to Ser (Fig. 8A, B). Interestingly, peptide 100 also attenuated IL-6 production (Fig. 8B). However, peptide 322 did not inhibit IR-induced cytokine production (Fig. 8A,B). Thus, β2-GPI binding occurs prior to IR-induced, pro-inflammatory cytokine production.
Figure 8. β2-GPI peptides attenuate IR-induced pro-inflammatory cytokine and eicosanoid production.
IL-12 (A), IL-6 (B), PGE2 (C) or LTB4 (D) production was measured in C57Bl/6 mice with or without injection of β2-GPI peptides prior to Sham or IR treatment. Values are represented as pg/mg of intestinal protein. * = p ≤ 0.05 compared to Sham, φ = p ≤ 0.05 compared to animals not receiving peptide. Each bar is representative of 3–4 animals and each treatment was performed on at least 2 separate days.
Previous studies demonstrated that IR also induces eicosanoid production within 2 hr post ischemia (36). To determine if β2-GPI initiation of intestinal damage contributes to eicosanoid production, intestinal LTB4 and PGE2 production within the intestine was examined in mice subjected to Sham or IR in the presence or absence of the various β2-GPI peptides. As demonstrated in injury, peptides 296 and 296Cys to Ser attenuated IR-induced production of both eicosanoids while mice treated with peptides 100 and 322 sustained inflammation similar to untreated mice (Fig. 8C–D). Despite the ability to attenuate IR-induced intestinal damage, peptide 305 did not attenuate intestinal eicosanoid production (Fig. 8C–D). Together, these data indicate that β2-GPI has a role in IR-induced tissue damage and initiation of inflammation. Further studies are required to determine if administration ofβ2-GPI peptides at later time points attenuate injury and as such may provide clinically relevant therapeutics for a condition with a high mortality rate.
Discussion
Antibody-dependent complement activation is required for initiation of IR-induced tissue damage (1, 10). Although antibodies against multiple intracellular antigens have been implicated in initiating damage in response to IR, the identification of an extracellular antigen remained unclear (14, 20). We hypothesized that a serum protein, β2-GPI, binding to ischemic tissues is likely responsible for initiating the complement cascade. Both peptide inhibition of β2-GPI activity in wildtype mice (Table II) and infusion of wildtype serum containing reduced levels of anti-β2-GPI antibodies into Rag-1−/− mice prevented IR-induced intestinal damage and inflammation. Thus, our results demonstrate that natural antibodies targeting β2-GPI play a critical role in initiating antibody/antigen complexes required for subsequent complement activation in response to IR. In addition, these data suggest that binding of β2-GPI to ischemic cells is critical for IR-induced damage and inflammation. Although many studies have associated anti-phospholipid antibodies with autoimmunity and acute graft rejection (reviewed in (43)), β2-GPI and anti-β2-GPI antibodies also mediate reperfusion-induced organ damage.
Table II.
Summary of IR-induced injury and inflammation C57Bl/6 mice with or without peptide treatment
| B6 IRa | B6 + β2-100 | B6 + β2-296 | B6 + β2-296c-s | B6 + β2-305 | B6 + β2-322 | |
|---|---|---|---|---|---|---|
| Injuryb | +c | + | − | − | − | + |
| C3 Deposition | + | + | − | − | − | + |
| CD31 Deposition | + | + | − | − | + | + |
| F4/80 Deposition | + | +/− | +/− | − | − | + |
| IL-12p40 Induction | + | +/− | − | − | − | + |
| IL-6 Induction | + | +/− | − | − | − | + |
| PGE2 Production | + | + | − | − | +/− | + |
| LTB4 Production | + | + | − | − | + | + |
C57Bl/6 mice subjected to IR with or without peptide treatment.
Measure of injury or inflammation.
+ indicates significant difference from Sham treated mice; − indicates not significantly different from C57Bl/6 sham treated mice; +/− indicates no significant difference from either C57Bl/6 mice subjected to either Sham or IR
We showed that β2-GPI binding to the tissue within 15 min of reperfusion correlates with initiation of tissue damage. The binding to injured tissue also correlates with previously determined IR-induced lipid changes (44). Domain V of β2-GPI is responsible for binding negatively charged substrates such as membranes containing phosphatidylserine and/or cardiolipin (45–47) which are significantly increased in response to IR (44). Thus, peptides 100 or 181 from domains II and III, respectively would not be expected to reduce damage in the IR model of tissue damage. In the analysis of domain V, mutagenesis studies suggested that the Lys286 in the CKNKEKKC sequence is critical for in vitro binding of β2-GPI to cardiolipin (48). In addition, mutation of Lys284, Lys 286 and Lys287 abolished anti-β2-GPI binding as detected by ELISA (48). Peptide 296 contains this lysine rich sequence with three cysteine residues. Correlating with previous findings that the Cys residues were not as critical as lysine residues, peptide 296Cys to Ser also inhibited IR-induced damage and inflammation. A recent in vivo study found that a related lysine rich sequence from cytomegalovirus (KEKRKKK) inhibited antibody-induced thrombosis by competing with β2-GPI (49). It is likely that a similar mechanism may be occurring in the peptide treated mice. However, protective peptide 305 contains only the last the last three amino acids in the CKNKEKKC sequence suggesting that additional residues are capable of binding to cells as well. Interestingly, peptide 305 did not prevent eicosanoid production indicating that distinct residues may be critical for the inflammatory response and intestinal damage.
Reperfusion is accompanied by the production of inflammatory mediators and immune cell infiltration (50) which are believed to be responsible for the subsequent systemic pathologies (1). IR-induced lipid changes result in increased arachidonic acid and subsequent production of the inflammatory mediators, LTB4 and PGE2 (44) which may contribute to cellular infiltration. Interestingly, anti-β2-GPI antibodies binding of β2-GPI induced cellular infiltration and eicosanoid generation. Importantly, all these inflammatory mediators and the IR-induced pro-inflammatory cytokines were blocked by peptides 296 and 296Cys to Ser while peptide 305 inhibited IL-12 and IL-6 production but not eicosanoid production (summarized in Table II). Together these data suggest that the inflammatory response is controlled by a larger sequence than the CKNKEKKC sequence of the lipid binding domain. Activation of complement also initiates immune cell infiltration and activation as treatment with C5a receptor antagonists attenuated neutrophil infiltration (4, 6–7, 50). Despite containing four complement regulatory domains, β2-GPI exhibits no known complement regulating function (24). However, antibody reduction or treatment with peptides 296, 305 and 296Cys to Ser attenuated complement activation suggesting that antibody recognition of the serum protein, β2-GPI initiates complement activation. As the inflammatory responses are also induced by LPS, TLR pathways involvement is also possible.
The mechanism of β2-GPI binding to cells is not fully understood. Previous studies demonstrated that mice lacking specific immune regulatory proteins such as toll-like receptor 4 (TLR4) also render mice resistant to IR-induced damage (30). Despite having the proper antibody repertoire, TLR4lpsd mice remain resistant to intestinal IR-induced damage (30). One possible explanation is that anti-β2-GPI antibodies recognize β2-GPI in conjunction with TLR4 (51–52) resulting in signaling through TLR4. This hypothesis is supported by the fact that antiβ2-GPI antibodies induce phosphorylation, NF-κB activation and TNF production by monocytes (53). Another possibility is presented by recent evidence illustrating that β2-GPI binds TLR2 on endothelial cells (54). Correlating with these data, we demonstrated that peptides 296 and 296Cys to Ser inhibit IR-induced IL-12 and IL-6 as well as upregulation of adhesion molecules and subsequent increases in cellular infiltration. Thus, the lack of TLR expression may prevent intestinal damage by interfering with antibody recognition of β2-GPI. In contrast, other studies indicate that β2-GPI-antibody complexes interact with other proteins including Ro60 on apoptotic cells (55) or annexin II (Ma et al., 2000). Binding to either of these proteins would suggest the antigen/antibody complexes resulted in monocyte stimulation, as limited evidence exists for a transmembrane domain in either Ro60 or annexin II. Although the nature of the interaction remains unclear, binding of the serum protein, β2-GPI, appears to initiate the subsequent inflammatory response during IR.
Similar to the TLR4 deficient mice, Cr2−/− mice are also resistant to IR-induced tissue damage. Despite having normal serum levels of antibodies, Cr2−/− mice do not produce the necessary antibody repertoire required for IR-mediated tissue damage (19, 36, 56). The lack of anti-β2-GPI antibodies in the Cr2−/− mice correlates with initial studies indicating that infusion of an anti-β2-GPI mAb was sufficient to restore injury (19). Although the exact role that CR2 plays in generating β2-GPI reactive antibodies is unclear, CR2 is associated with the B cell Ig receptor and therefore may influence the selection of β2-GPI reactive B cells (57). Thus, the interactions of TLR4 and CR2 with β2-GPI and/or anti-β2-GPI antibodies remain unclear and require additional investigation.
It has been proposed that binding of β2-GPI to the cell membrane exposes cryptic epitope(s) recognized by natural antibodies (58). Natural antibody recognition of β2-GPI is a characteristic of APLS and results in tissue damage and fetal loss (59–61). When compared to IR in normal patients, APLS patients have significantly higher anti-β2-GPI antibody titers and the antibodies exhibit greater affinity for the target antigen which is suggested to result in damage (62). By recognizing stressed or damaged tissue, β2-GPI recognition by anti-β2-GPI antibodies appears to lead to IR-induced pathology. Although CR2 may play a role in generating antibodies against β2-GPI, it is unclear why, under normal immunological functioning, β2-GPI elicits autoantibodies. The generation of anti-β2-GPI antibodies may be for removal of apoptotic cells by phagocytes. This hypothesis suggests that β2-GPI binds to ischemic tissue because the membrane changes are similar to early apoptotic cells and that the process will facilitate clearance of the damaged cells (26, 63). When significantly lower concentrations of anti-β2-GPI antibodies exist, such as in Cr2−/− mice or in the reduced serum, the hypoxic cells are not targeted and complement activation is significantly reduced. Similarly, transfer of Cr2−/− serum or antibodies to Rag-1−/− mice did not restore IR-induced intestinal damage (36). Although the exact nature of the alterations occurring in IR or apoptotic tissues is not fully characterized, ischemia exposes changes in the lipid and/or protein composition of the membrane allowing β2-GPI binding and subsequent natural antibody recognition during reperfusion.
Our previous studies indicated that IR-induced damage in Rag-1−/− mice required a combination of two IgG monoclonal antibodies recognizing β2-GPI and negatively charged phospholipids (19). Importantly, neither mAb alone was sufficient to induce damage. These data suggest that IR-induced damage requires a complex of antibodies recognizing multiple antigens, including β2-GPI bound to phospholipids. Based on these results, prevention of either the phospholipid changes or β2-GPI binding would attenuate injury. Recently, we demonstrated that IR-induced lipid changes occur in both Rag-1−/− and C57Bl/6 wildtype mice within 15 min post-reperfusion (44). As lipid mobility is critical to cellular signaling, blocking the lipid changes may produce significant side effects. In contrast, peptide inhibition of either β2-GPI binding to the lipids or antibody binding to β2-GPI would prevent binding by one mAb and subsequently prevent intestinal damage. In addition, as a natural serum protein, the expected side effects may be significantly lower than lipid blockade.
Previous studies indicated that IR-induced damage is due to natural antibodies with reactivity to non-muscle myosin, glycogen phosphorylase or annexin IV. However, attenuated damage following peptide inhibition of β2-GPI binding suggests that these additional target antigens may be exposed after β2-GPI binding. It is possible that β2-GPI binding induces a signal which leads to either apoptosis with annexin IV expression or necrosis and non-muscle myosin exposure. As specific β2-GPI peptides reduced IR-induced tissue damage to Sham levels, β2-GPI appears to be a critical therapeutic target for mesenteric IR. In addition, reperfusion-induced tissue damage in response to myocardial infarction, stroke, and transplantation appears to use similar mechanisms (42, 64). Thus, understanding the exact role of β2-GPI itself or the natural antibodies recognizing β2-GPI in mediating tissue damage may lead to effective strategies of preventing reperfusion injury in multiple organs.
Acknowledgments
The authors wish to thank Mr. Andrew Fritze for excellent technical assistance and Dr. Maurizio Tomasi for insightful discussions.
Non-standard abbreviations include
- IR
ischemia/reperfusion
- LTB4
leukotriene B4
- β2-GPI
β2-glycoprotein I (apolipoprotein H)
- APLS
anti-phospholipid antibody syndrome
Footnotes
This work was supported by NIH Grants AI061691, P20 RR017686 and RR016475 from the Institutional Development Award (IDeA) Program of the NCRR and Kansas State University.
The authors have no conflicting financial interests.
References
- 1.Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336–343. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- 2.Burns BJ, Brandt LJ. Intestinal ischemia. Gastroenterol Clin North Am. 2003;32:1127–1143. doi: 10.1016/s0889-8553(03)00093-1. [DOI] [PubMed] [Google Scholar]
- 3.Crawford MH, Grover FL, Kolb WP, McMahan CA, O’Rourke RA, McManus LM, Pinckard RN. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circ. 1988;78:1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 5.Simpson R, Alon R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann Surg. 1993;218:444–453. doi: 10.1097/00000658-199310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehrig S, Fleming SD, Anderson J, Guthridge JM, Rakstang J, McQueen CE, Holers VM, Tsokos GC, Shea-Donohue T. Complement inhibitor, complement receptor 1-related gene/protein y-Ig attenuates intestinal damage after the onset of mesenteric ischemia/reperfusion injury in mice. J Immunol. 2001;167:5921–5927. doi: 10.4049/jimmunol.167.10.5921. [DOI] [PubMed] [Google Scholar]
- 7.Fleming SD, Anderson J, Wilson F, Shea-Donohue T, Tsokos GC. C5 is required for CD49d expression on neutrophils and VCAM expression on vascular endothelial cells following mesenteric ischemia/reperfusion. Clin Immunol. 2003;106:55–64. doi: 10.1016/s1521-6616(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 8.Fleming SD, Mastellos D, Karpel-Massler G, Shea-Donohue T, Lambris JD, Tsokos GC. C5a causes limited, polymorphonuclear cell-independent, mesenteric ischemia/reperfusion-induced injury. Clin Immunol. 2003;108:263–273. doi: 10.1016/s1521-6616(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 9.Lappegard KT, Fung M, Bergseth G, Reiesenfeld J, Lambris JD, Videm V, Mollnes TE. Effect of Complement Inhibition and Heparin Coating on Artificial Surface-Induced Leukocyte and Platelet Activation. Cardiovascular. 2004;77:932–944. doi: 10.1016/S0003-4975(03)01519-4. [DOI] [PubMed] [Google Scholar]
- 10.Eror AT, Stojadinovic A, Starnes BW, Makrides SC, Tsokos GC, Shea-Donohue T. Anti-inflammatory effects of soluble complement receptor type 1 promote rapid recovery of ischemia/reperfusion injury in rat small intestine. Clin Immunol. 1999;90:266–275. doi: 10.1006/clim.1998.4635. [DOI] [PubMed] [Google Scholar]
- 11.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Complement inhbition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998;124:782–792. doi: 10.1067/msy.1998.91489. [DOI] [PubMed] [Google Scholar]
- 12.Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD. Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149:1723–1728. [PubMed] [Google Scholar]
- 13.Pemberton M, Anderson G, Vetvicka V, Justus DE, Ross GD. Microvascular effects of complement blockade with soluble recombinant CR1 on ischemia/reperfusion injury of skeletal muscle. J Immunol. 1993;150:5104–5113. [PubMed] [Google Scholar]
- 14.Holers VM, Kulik L. Complement receptor 2, natural antibodies and innate immunity: Inter-relationships in B cell selection and activation. Mol Immunol. 2006 doi: 10.1016/j.molimm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Weiser MR, Williams JP, Moore FD, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunology Today. 2000;21:624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- 17.Williams JP, Pechet TTV, Weiser MR, Reid R, Kobzik L, Moore FD, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Austen WG, Chiu I, Alicot EM, Humg R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming SD, Egan RP, Chai C, Girardi G, Holers VM, Salmon J, Monestier M, Tsokos GC. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J Immunol. 2004;173:7055–7061. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keith MP, Moratz C, Egan R, Zacharia A, Greidinger EL, Hoffman RW, Tsokos GC. Anti-ribonucleoprotein antibodies mediate enhanced lung injury following mesenteric ischemia/reperfusion in Rag-1(−/−) mice. Autoimmunity. 2007;40:208–216. doi: 10.1080/08916930701262986. [DOI] [PubMed] [Google Scholar]
- 22.Hagihara Y, Hong DP, Hoshino M, Enjyoji K, Kato H, Goto Y. Aggregation of beta(2)-glycoprotein I induced by sodium lauryl sulfate and lysophospholipids. Biochemistry. 2002;41:1020–1026. doi: 10.1021/bi015693q. [DOI] [PubMed] [Google Scholar]
- 23.Atsumi T, Amengual O, Yasuda S, Matsuura E, Koike T. Research around B2-glycoprotein I: a major target for antiphospholipid antibodies. Autoimmunity. 2005;38:377–381. doi: 10.1080/08916930500124312. [DOI] [PubMed] [Google Scholar]
- 24.Valesini G, Shoenfeld Y. A new player in the antiphospholipid syndrome: the beta 2 glycoprotein I cofactor. Autoimmunity. 1992;14:105–110. doi: 10.3109/08916939209083128. [DOI] [PubMed] [Google Scholar]
- 25.Bu C, Gao L, Xie W, Zhang J, He Y, Cai G, McCrae KR. β2-glycoprotein I is a cofactor for tissue plasminogen activator-mediated plasminogen activation. Arthritis Rheum. 2009;60:559–568. doi: 10.1002/art.24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manfredi AA, Rovere P, Heltai S, Galati G, Nebbia G, Tincani A, Balestrieri G, Sabbadini MG. Apoptotic cell clearance in systemic lupus erythematosus. II. Role of beta2-glycoprotein I. Arthritis Rheum. 1998;41:215–223. doi: 10.1002/1529-0131(199802)41:2<215::AID-ART5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Cabiedes J, Cabral AR, Alarcon-Segovia D. Clinical manifestations of the antiphospholipid syndrome in patients with systemic lupus erythematosus associate more strongly with anti-beta 2-glycoprotein-I than with antiphospholipid antibodies. J Rheumatol. 1995;22:1899–1906. [PubMed] [Google Scholar]
- 28.Ali HY, Abdullah ZA. Anti-beta(2)-glycoprotein I autoantibody expression as a potential biomarker for strokes in patients with anti-phospholipid syndrome. J Immunotoxicol. 2008;5:173–177. doi: 10.1080/15476910802129638. [DOI] [PubMed] [Google Scholar]
- 29.Nojima J, Masuda Y, Iwatani Y, Kuratsune H, Watanabe Y, Suehisa E, Takano T, Hidaka Y, Kanakura Y. Arteriosclerosis obliterans associated with anti-cardiolipin antibody/beta2-glycoprotein I antibodies as a strong risk factor for ischaemic heart disease in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2008;47:684–689. doi: 10.1093/rheumatology/ken124. [DOI] [PubMed] [Google Scholar]
- 30.Moses T, Wagner LM, Fleming SD. Tlr4 mediated cox-2 expression increases intestinal ischemia/reperfusion induced damage. J Leukoc Biol. 2009 doi: 10.1189/jlb.0708396. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt J, Krilis S. The fifth domain of beta 2-glycoprotein I contains a phospholipid binding site (Cys281-Cys288) and a region recognized by anticardiolipin antibodies. J Immunol. 1994;152:653–659. [PubMed] [Google Scholar]
- 32.Sellar GC, Steel DM, Zafiropoulos A, Seery LT, Whitehead AS. Characterization, expression and evolution of mouse beta 2-glycoprotein I (apolipoprotein H) Biochem Biophys Res Commun. 1994;200:1521–1528. doi: 10.1006/bbrc.1994.1623. [DOI] [PubMed] [Google Scholar]
- 33.Steinkasserer A, Barlow PN, Willis AC, Kertesz Z, Campbell ID, Sim RB, Norman DG. Activity, disulphide mapping and structural modelling of the fifth domain of human beta 2-glycoprotein I. FEBS Lett. 1992;313:193–197. doi: 10.1016/0014-5793(92)81442-o. [DOI] [PubMed] [Google Scholar]
- 34.Iwamoto T, Grove A, Montal MO, Montal M, Tomich JM. Chemical synthesis and characterization of peptides and oligomeric proteins designed to form transmembrane ion channels. Int J Peptide Protein Res. 1996;43:597–607. doi: 10.1111/j.1399-3011.1994.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 35.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 36.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 37.Monestier M, Kandiah DA, Kouts S, Novick KE, Ong GL, Radic MZ, Krilis SA. Monoclonal antibodies from NZW x BXSB F1 mice to β2-glycoprotien I and cardiolipin. J Immunol. 1996;156:2631–2641. [PubMed] [Google Scholar]
- 38.Banerjee D, Gakhar G, Madgwick D, Hurt A, Takemoto D, Nguyen TA. A novel role of gap junction connexin46 protein to protect breast tumors from hypoxia. Int J Cancer. 2009 doi: 10.1002/ijc.25107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavazzana A, Ruffatti A, Tonello M, Bortolati M, De Moerloose P, Reber G. An analysis of experimental conditions influencing the anti-beta2-glycoprotein I ELISA assay results. Annals of the New York Academy of Sciences. 2007;1109:484–492. doi: 10.1196/annals.1398.054. [DOI] [PubMed] [Google Scholar]
- 40.Wong RC, Favaloro EJ, Adelstein S, Baumgart K, Bird R, Brighton TA, Empson M, Gillis D, Hendle MJ, Laurent R, Mallon D, Pollock W, Smith S, Steele RH, Wilson RJ. Consensus guidelines on anti-beta 2 glycoprotein I testing and reporting. Pathology. 2008;40:58–63. doi: 10.1080/00313020701717720. [DOI] [PubMed] [Google Scholar]
- 41.Gries A, Nimpf J, Wurm H, Kostner GM, Kenner T. Characterization of isoelectric subspecies of asialo-beta 2-glycoprotein I. Biochem J. 1989;260:531–534. doi: 10.1042/bj2600531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 43.McIntyre JA, Wagenknecht DR, Faulk WP. Antiphospholipid antibodies: discovery, definitions, detection and disease. Progress in Lipid Research. 2003;42:176–237. doi: 10.1016/s0163-7827(02)00048-6. [DOI] [PubMed] [Google Scholar]
- 44.Sparkes BL, Slone EE, Roth M, Welti R, Fleming SD. Intestinal lipid alterations occur prior to antibody-induced prostaglandin E2 production in a mouse model of ischemia/reperfusion. Biochim Biophys Acta. 2010;1801:517–525. doi: 10.1016/j.bbalip.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouma B, de Groot PG, van den Elsen JM, Ravelli RB, Schouten A, Simmelink MJ, Derksen RH, Kroon J, Gros P. Adhesion mechanism of human beta(2)-glycoprotein I to phospholipids based on its crystal structure. Embo J. 1999;18:5166–5174. doi: 10.1093/emboj/18.19.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunt JV, Bailey JR, Schultz DL, McKay AG, Mitchinson MJ. Apolipoprotein oxidation in the absence of lipid peroxidation enhances LDL uptake by macrophages. FEBS Lett. 1994;349:375–379. doi: 10.1016/0014-5793(94)00706-3. [DOI] [PubMed] [Google Scholar]
- 47.Subang R, Levine JS, Janoff AS, Davidson SM, Taraschi TF, Koike T, Minchey SR, Whiteside M, Tannenbaum M, Rauch J. Phospholipid-bound beta 2-glycoprotein I induces the production of anti-phospholipid antibodies. J Autoimmun. 2000;15:21–32. doi: 10.1006/jaut.2000.0382. [DOI] [PubMed] [Google Scholar]
- 48.Sheng Y, Sali A, Herzog H, Lahnstein J, Krilis SA. Site-directed mutagenesis of recombinant human beta 2-glycoprotein I identifies a cluster of lysine residues that are critical for phospholipid binding and anti-cardiolipin antibody activity. J Immunol. 1996;157:3744–3751. [PubMed] [Google Scholar]
- 49.Ostertag MV, Liu X, Henderson V, Pierangeli SS. A peptide that mimics the Vth region of beta-2-glycoprotein I reverses antiphospholipid-mediated thrombosis in mice. Lupus. 2006;15:358–365. doi: 10.1191/0961203306lu2315oa. [DOI] [PubMed] [Google Scholar]
- 50.Riedemann NC, Ward PA. Complement in ischemia reperfusion injury. Am J Pathol. 2003;162:363–367. doi: 10.1016/S0002-9440(10)63830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raschi E, Testoni C, Bosisio D, Borghi MO, Koike T, Mantovani A, Meroni PL. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101:3495–3500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 52.Raschi E, Borghi MO, Grossi C, Broggini V, Pierangeli S, Meroni PL. Toll-like receptors: another player in the pathogenesis of the anti-phospholipid syndrome. Lupus. 2008;17:937–942. doi: 10.1177/0961203308095140. [DOI] [PubMed] [Google Scholar]
- 53.Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, Conti F, Buttari B, Rigano R, Ortona E, Valesini G. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis and Rheumatism. 2007;56:2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 54.Alard JE, Gaillard F, Daridon C, Shoenfeld Y, Jamin C, Youinou P. TLR2 Is One of the Endothelial Receptors for {beta}2-Glycoprotein I. J Immunol. 2010 doi: 10.4049/jimmunol.1000526. [DOI] [PubMed] [Google Scholar]
- 55.Reed JH, Giannakopoulos B, Jackson MW, Krilis SA, Gordon TP. Ro 60 functions as a receptor for beta(2)-glycoprotein I on apoptotic cells. Arthritis Rheum. 2009;60:860–869. doi: 10.1002/art.24361. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M, Alicot EM, Carroll MC. Human natural IgM can induce ischemia/reperfusion injury in a murine intestinal model. Mol Immunol. 2008;45:4036–4039. doi: 10.1016/j.molimm.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zabel MD, Weis JH. Cell-specific regulation of the CD21 gene. International Immunopharmacology. 2001;1:483–493. doi: 10.1016/s1567-5769(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 58.Pengo V, Biasiolo A, Fior MG. Autoimmune antiphospholipid antibodies are directed against a cryptic epitope expressed when beta 2-glycoprotein I is bound to a suitable surface. Thromb Haemost. 1995;73:29–34. [PubMed] [Google Scholar]
- 59.Cabral AR, Amigo MC, Cabiedes J, Alarcon-Segovia D. The antiphospholipid/cofactor syndromes: a primary variant with antibodies to beta 2-glycoprotein-I but no antibodies detectable in standard antiphospholipid assays. Am J Med. 1996;101:472–481. doi: 10.1016/s0002-9343(96)00254-9. [DOI] [PubMed] [Google Scholar]
- 60.Cabral AR, Cabiedes J, Alarcon-Segovia D. Antibodies to phospholipid-free beta 2-glycoprotein-I in patients with primary antiphospholipid syndrome. J Rheumatol. 1995;22:1894–1898. [PubMed] [Google Scholar]
- 61.Roubey RA. Antigenic specificities of antiphospholipid autoantibodies: implications for clinical laboratory testing and diagnosis of the antiphospholipid syndrome. Lupus. 1996;5:425–430. doi: 10.1177/096120339600500518. [DOI] [PubMed] [Google Scholar]
- 62.Cucnik S, Kveder T, Krizaj I, Rozman B, Bozic B. High avidity anti-beta 2-glycoprotein I antibodies in patients with antiphospholipid syndrome. Ann Rheum Dis. 2004;63:1478–1482. doi: 10.1136/ard.2003.017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balasubramanian K, Chandra J, Schroit AJ. Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of beta2-glycoprotein I in macrophage recognition. J Biol Chem. 1997;272:31113–31117. doi: 10.1074/jbc.272.49.31113. [DOI] [PubMed] [Google Scholar]
- 64.Arumugam TV, Magnus T, Woodruff M, Proctor LM, Shiels IA, Taylor SM. Complement mediators in ischemia-reperfusion injury. Clinica Chimica Acta. 2006 doi: 10.1016/j.cca.2006.06.010. [DOI] [PubMed] [Google Scholar]