Abstract
The generation of reactive oxygen species (ROS) plays a major role in endothelial signaling and function. Of the several potential sources of ROS in the vasculature, the endothelial NADPH Oxidase (Nox) family of proteins, Nox1, Nox2, Nox4 and Nox5, are major contributors of ROS. Excess generation of ROS contributes to the development and progression of vascular disease. While hyperoxia stimulates ROS production through Nox proteins, hypoxia appears to involve mitochondrial electron transport in the generation of superoxide. ROS generated from Nox proteins and mitochondria are important for oxygen sensing mechanisms. Physiological concentrations of ROS function as signaling molecule in the endothelium; however, excess ROS production leads to pathological disorders like inflammation, atherosclerosis, and lung injury. Regulation of Nox proteins is unclear; however, antioxidants, MAP Kinases, STATs, and Nrf2 regulate Nox under normal physiological and pathological conditions. Studies related to redox regulation of Nox should provide a better understanding of ROS and its role in the pathophysiology of vascular diseases.
Keywords: NADPH Oxidase, ROS, Endothelium, Redox, Nox proteins
1. Introduction
Reactive oxygen species (ROS) are widely recognized as important mediators of cell growth, adhesion, differentiation, senescence, and apoptosis (Droge 2002). ROS refers to a group of highly reactive molecules that includes oxygen anions and free radicals like superoxide anion (O2−.), hydroxyl radical (OH−), and hydrogen peroxide (H2O2) (Mandal, Fu et al 2010.). ROS are generated endogenously in response to agonists, cytokines, growth factors, hyperoxia, hypoxia, and shear stress (Frey, Ushio-Fukai et al. 2009), and mediate signal transduction through reversible regulation of protein-tyrosine phosphatases, cytosolic and receptor protein-tyrosine kinases, and cytoskeletal proteins (Pendyala, Usatyuk et al. 2009). ROS, as secondary messengers, are predominantly generated by the NAPDH oxidase (Nox)/Dual Oxidase (Duox) family of proteins (Lambeth, Kawahara et al. 2007) consisting of seven members: Nox-1 through 5, Duox-1 and Duox-2. Although they share common structural similarities with six transmembrane domains and the cytoplasmic domain that comprises NADPH- and flavin adenine dinucleotide-binding sites, each member appears to exert a specific biological role (Pendyala, Usatyuk et al. 2009). Recent studies suggest that low levels of ROS modulate protein phosphorylation mediated by protein kinases and phosphatases, alter intracellular calcium levels, stimulate phospholipases, and regulate transcription factors and growth factors/growth factor receptors (Cai, Griendling et al. 2003; Cave, Brewer et al. 2006). However, excessive production and accumulation of ROS and/or reactive nitrogen species are detrimental to cells and tissues, resulting in injury and ultimately loss of viability and death through oxidative damage to cellular macromolecules. Increased ROS production has been directly linked to development and progression of inflammatory vascular diseases. The imbalance in the ratio of oxidants produced to oxidants detoxified (alteration in the redox equilibrium) seems to play an important role in the development of various inflammatory diseases (Ryter, Kim et al. 2007). A major source of the variability in vascular regulation seen in arteries and veins, and the microcirculation of different organ systems during the progression of aging and disease processes, originates from changes in ROS and their interaction with systems controlling release of endothelial mediators and vascular function (Wolin 2009). The vascular endothelium predominantly expresses Nox2 and Nox4. In our studies in lung Nox1 was also detected with relatively lower expression compared to Nox2 and Nox4 (Pendyala, Gorshkova et al. 2009). Here, we address redox regulation of Nox proteins in vascular endothelium and the role of ROS in mediating oxidative damage to endothelium and modulating endothelial function.
2. NADPH Oxidase (Nox) is a major source of vascular ROS
The generation of ROS by aerobic cells occurs through enzymatic and non-enzymatic reactions (Thannickal and Fanburg 2000). Sub-cellular organelles such as mitochondria, endoplasmic reticulum, nuclear membranes, peroxisomes, plasma membranes, and the cytoplasm have enzymatic systems to transfer electrons from NADH or NADPH to molecular O2. In mammalian cells, in addition to mitochondrial electron transport, the other potential enzymatic sources of ROS include arachidonic acid metabolism by cyclooxygenase/lipoxygenase, cytochrome P450, xanthine oxidase, Nox, NO synthase, peroxidase and other hemoproteins. Phagocytic cells of the immune system (neutrophils, eosinophils, monocytes, and macrophages) generate O2.−, instrumental in the killing of invading pathogens, solely by the NADPH oxidase (Cave, Brewer et al. 2006; Griendling 2006; Ushio-Fukai 2006). Deficiency of O2.− results in the genetically inherited disorder chronic granulamatous disease, a condition in which the affected individuals are susceptible to infection (Allen, DeLeo et al. 1999).
2.1 Structure Nox Proteins
Since the identification of mox1 as a homolog of gp91phox, and based on the current database from human genome seven Nox protein family members [Nox1, Nox2 (gp91phox), Nox3, Nox4, Nox5, Duox1 and Duox2] have been described (Geiszt and Leto 2004; Krause 2004). All the family members share a common core structure made up of 6 transmembrane domains (contains 2 heme binding domains) and a long cytoplasmic C-terminus (contains FAD and NADPH binding domains). On the other hand, Nox5, Duox1 and Duox2 have an N-terminal extension and in the case of Nox 5, this extension consists of 4 EF-hands (Geiszt and Leto 2004). Furthermore, consistent with the ability of the EF hand domain to bind calcium, Nox5, Duox1 and Duox2 are activated by Ca2+ (De Deken, Wang et al. 2000; Banfi, Molnar et al. 2001).
2.2 Tissue distribution and expression of Nox proteins
Tissue distribution of Nox proteins in tissues and cells are somewhat contradictory, and limited due to a lack of specific antibodies, species variations, and differences in passage numbers of cultured mammalian cells.Nox1 is predominantly found in colon and smooth muscle cells (SMCs) while Nox2 is not only expressed in phagocytic cells but also in vascular cells including ECs and SMCs. Nox3 is primarily expressed in fetal tissues and almost exclusively in inner ear. Nox4 is widely expressed in kidney, vascular cells osteoclasts and placenta. Nox5 is also highly expressed in fetal tissues as well as in adult spleen and uterus Nox isoforms are aberrantly expressed in cancer cells with Nox4 being the predominant isoform most highly expressed in tumors (Krause 2004; Griendling 2006; Kawahara, Quinn et al. 2007) Interestingly,, mRNA expression profiles of Nox isoforms do not correlate with protein expression and oxidase activity. For example, Nox1 mRNA is highly expressed in vascular smooth muscle cells (SMCs) as compared to Nox4 mRNA, which is the predominant isoform in lung endothelial cells (ECs). In the endothelium, the Nox4 isoform, in addition to Nox2, is emerging as a key regulator of non-mitochondrial sources of ROS production. Nox1 and Nox2 genes have almost identical numbers and lengths of exons and exhibit (~60%) sequence homology. In addition to its constitutive expression, Nox1 message is induced by angiotensin II, PDGF, and PGF2α in vascular SMCs. Originally described as a renal oxidase, Nox4 is highly expressed in the kidney, and recent studies have shown that all of the oxidase components are present in ECs from macro- and micro-vascular beds (Geiszt and Leto 2004). The EC oxidase is constitutively active and generates low levels of ROS under basal conditions and activation of the oxidase by hyperoxia or TNF-α or other stimuli stimulates moderately higher ROS production. Thus, the oxidative burst by vascular Nox is much smaller compared to the phagocytic oxidative burst. While it is well established that Nox2 is expressed in ECs including human pulmonary artery endothelial cells (HPAECs) and human lung microvascular endothelial cells (HLMVECs) (Pendyala, Gorshkova et al. 2009), it appears that the mRNA levels for Nox4 in some of the ECs from rat and human is much higher compared to Nox2. Interestingly, in HPAECs and HLMVECs, expression of p22phox under unstimulated conditions, is several fold higher (~10 to 50 fold) compared to Nox4 expression (Pendyala, Gorshkova et al. 2009). In phagocytes, the expression levels of Nox2 and p22phox are much higher with relatively low expression of Nox4.
3. Contribution of Nox1, Nox2 and Nox4 to O2.− / ROS production in the vasculature under normal and pathological conditions
With the identification of seven Nox homologues in non-phagocytes, there is increasing evidence that Noxes in non-phagocytes serve as a major source of ROS that play a key role in cell signaling and function. Each of the seven isoform has its unique expression profile, sub-cellular localization and regulation; thus increasing the complexity of regulating ROS generation in a specific tissue or cell type. It is apparent that signaling specificity of Nox protein derived ROS may be modulated by the specific sub-cellular localization and post-translational modification(s) of Nox isoforms. Among the Nox isoforms, the sub-cellular distribution of Nox4 is more complex. In the endothelium, expression of native Nox4 is different from that of the over-expressed protein. Recent studies show that the human umbilical vein and lung ECs, Nox4 is predominantly localized in the nucleus as compared to endoplasmic transduction reticulum, mitochondria or plasma membrane (Kuroda, Nakagawa et al. 2005; Pendyala, Gorshkova et al. 2009). However, over-expression of adenoviral V5-tagged Nox4 in human aortic ECs resulted in predominant localization to ER (Chen, Kirber et al. 2008). This differential localization of native vs. over-expressed Nox4 protein necessitates careful interpretation on the specificity of intracellular ROS generation and signal.
3.1 Role of Nox1-derived ROS generation and lung injury
Nox1 generates O2.− at very low levels under basal and stimulated conditions and depends on cytosolic Noxo1 and Noxa1 organizing proteins for activity (Kawahara, Quinn et al. 2007). Nox1−/−, but not Nox2−/−, null mice showed reduced ROS generation and cell death of the alveolocapillary barrier during hyperoxia compared to wild type mice involving JNK and ERK pathways (Carnesecchi, Deffert et al. 2009). Similarly, Nox1 deficiency protected angiotensin II-dependent aortic dissection in mice via suppression of tissue inhibitor of metalloproteinase 1 expression (Gavazzi, Deffert et al. 2007) These studies provide evidence for the role of Nox1 in lung injury and aortic dissection.
3.2 Nox2 vs. Nox4 in ROS generation, lung inflammation and injury
Nox2 is a highly glycosylated protein and its activation requires interaction with other membrane (p22phox) and cytosolic (p47phox, p67phox, p40phox and Rac1/2) components. Although Nox2 is a critical component of phagocytic NADPH oxidase mediated O2.− / ROS production, the role of Nox2 in vascular NADPH oxidase activity is controversial and may depend on cell type(s) involved within the vessel wall (Krause 2004; Ray and Shah 2005; Sumimoto, Miyano et al. 2005). For example, expression of Nox2 has been reported in arteriolar, but not in aortic, SMCs (Chose, Sansilvestri-Morel et al. 2008). As ECs express higher levels of Nox4 compared to Nox2, some recent data using anti-sense oligonucleotides to Nox4 suggest that Nox4 is involved in O2.− / ROS formation in vascular SMCs and ECs (Ellmark, Dusting et al. 2005). In human pulmonary artery smooth muscle cells (PASMCs), human Urotensin II (hU-II) activated NADPH oxidase was abrogated by p22phox or Nox4 anti-sense oligonucleotides (Djordjevic, BelAiba et al. 2005). Furthermore, depletion of Nox4 or p22phox blocked hU-II, but not S1P, mediated cell proliferation of PASMCs indicating an involvement of Nox4 or p22phox in mitogenesis (Dhaunsi, Paintlia et al. 2004). In 3T3-L1 adipocytes, Nox4, but not Nox2, appears to be a major mediator of insulin-induced ROS production that was associated with oxidative inhibition of protein tyrosine phosphatase (PTP), PTP1B activity (Mahadev, Motoshima et al. 2004; Goldstein, Mahadev et al. 2005). In human aortic SMCs, 7-ketocholesterol, a component of oxidized LDL, triggers NADPH oxidase activation and over-production of ROS via Nox4 and JNK signaling (Pedruzzi, Guichard et al. 2004). Similarly, exogenous H2O2, oleoylacetyglycerol and free arachidonic acid (AA) stimulated ROS production in cardiac fibroblasts that was sensitive to diphenyleneiodonium and antioxidants through a pathway involving phospholipase A2 and Nox4.
3.3 Compensatory mechanisms between Nox2 and Nox4 in ROS production
In many of the studies concerning Nox4 and ROS production, it is unclear if Nox4 is subjected to a post-translational modification as a pre-requisite for activation or whether it is constitutively active like iNOS. Studies with HPAECs showed that generation of O2.− / ROS by hyperoxia (3-24 h) does not involve mitochondrial electron transport and was dependent on NADPH oxidase activation. Furthermore, hyperoxia- or TNF-α- mediated ROS production was attenuated by Nox2 or Nox4 siRNA suggesting a role for Nox2 and Nox4 isoforms in ROS production in these cells. Interestingly, although Nox4 siRNA did not alter the expression of Nox1 and Nox3 levels, expression of Nox2 mRNA was up-regulated by silencing Nox4 while Nox4 mRNA and protein expression were enhanced after knockdown of Nox2. Similarly, siRNA-mediated knockdown of p22phox increased Nox4 mRNA levels by ~2 fold (Pendyala, Gorshkova et al. 2009). A similar compensatory mechanism between Nox4 and Nox2 after siRNA treatment was observed in human cardiac ECs (Petry, Djordjevic et al. 2006); however, in primary human bronchial epithelial cells and the adenocarcinoma cell line A549, knockdown of Nox2 and Nox4 by siRNA did not up-regulate the expression of Nox4 or Nox2, respectively (Mochizuki, Furuta et al. 2006). These data suggest the ability of lung ECs to reciprocally compensate for Nox2 or Nox4 deficiency.
3.4 Nox4, HIF-1α, and ROS generation
While Nox2 and Nox4 seem to regulate hyperoxia-induced ROS production in lung ECs (Pendyala, Usatyuk et al. 2009), a role for Nox2 in ROS generation from lungs and ECs derived from Nox2 gene-targeted mice in response to normoxic lung ischemia has been demonstrated. .There is accumulating evidence that Nox isoforms, and in particular, Nox2 and Nox4 are involved in chronic responses of the pulmonary vasculature to hypoxia. Hypoxia-induced endothelial dysfunction of intrapulmonary arteries was mediated via Nox2/ROS pathway in mice (Mittal, Roth et al. 2007). However, TGF-β induced Nox4 expression and ROS production was implicated in proliferation of human pulmonary artery SMCs, a hall mark of pulmonary hypertension (Ismail, Sturrock et al. 2009). A recent study on hypoxia-dependent development of pulmonary arterial hypertension in mice demonstrated substantially increased Nox4 expression in medial pulmonary artery SMCs supporting a key role for Nox4 in vascular remodeling associated with the development of hypoxia-induced pulmonary arterial hypertension (Nisbet, Graves et al. 2009). Hypoxia increases the expression of TGF-β, production of the TGF-β activating protein furin, and Nox4 expression. TGF-β in turn induces HIF-1α; thereby raising the potential link between hypoxia, HIF-1α, TGF-β and Nox4 in pulmonary arterial hypertension. Furthermore, Nox4 has been shown to be critical for HIF-2α transcriptional activity in von Hippel-Lindau renal carcinoma suggesting a potential role for HIF-1α/HIF-2α and Nox4 in hypoxia-induced pulmonary arterial hypertension (Maranchie and Zhan 2005). In addition to involvement in hyperoxia- and hypoxia-induced lung injury, a role for Nox4 in LPS-induced pro-inflammatory responses by human aortic ECs has been described. Down-regulation of Nox4 by transfection of Nox4 siRNA resulted in a failure to induce ROS generation and subsequent expression of ICAM-1, MCP-1 and IL-8 secretion in response to LPS (Carnesecchi, Deffert et al.2009). It is also shown that Nox1 is an important contributor to ROS production and cell death of the alveolocapillary barrier during hyperoxia and is an upstream actor in oxidative stress-induced acute lung injury involving JNK and ERK pathways in mice (Carnesecchi, Deffert et al. 2009).
4.0 Implication of Nox proteins in vascular diseases
Inflammatory conditions and vascular diseases are being studied in relation to oxidative stress caused by Nox mediated ROS production. Role of ROS and function of redox-regulated systems are attributed in cardiovascular diseases and aging. In cardiovascular diseases like hypertension and heart failure, a direct link between increased O2−. generation and attenuation of vascular smooth muscle relaxation by nitric oxide and the ability by vascular endothelium to generate hydrogen peroxide has been demonstrated (Wolin 2009). Lung injury represents a wide spectrum of pathologic conditions, the most severe being the acute respiratory distress syndrome (ARDS). Acute lung injury is a syndrome that includes pulmonary vasoconstriction, inflammation, and increased permeability of both the alveolar capillary endothelium and epithelium, resulting in arterial hypoxemia, and the presence of diffuse infiltrates on chest radiograph (Pendyala, Usatyuk et al. 2009).
4.1 Nox proteins and acute lung injury
Various studies have implicated involvement of ROS generated by Nox activation in the pathobiology of acute lung injury. Although Nox2 and Nox4 seem to regulate hyperoxia-induced ROS production in lung ECs (Pendyala, Gorshkova et al. 2009), a role for Nox2 in ROS generation from lungs and endothelial cells derived from Nox2 gene-targeted mice in response to normoxic lung ischemia has been demonstrated (Zhang, Matsuzaki et al. 2005; Milovanova, Chatterjee et al. 2006). Prolonged exposure to low O2 tension induces pulmonary hypertension (PAH), which is characterized by vascular remodeling and enhanced vasoreactivity. Accumulating evidence suggests that Nox isoforms, and in particular, Nox2 and Nox4, are involved in long-term responses of the pulmonary vasculature to hypoxia (Liu, Zelko et al. 2006). In addition to involvement in hyperoxia- and hypoxia induced lung injury, a role for Nox4 in LPS-induced pro-inflammatory responses by human aortic ECs has been described. Down-regulation of Nox4 by transfection of Nox4 siRNA resulted in a failure to induce ROS generation and subsequent expression of ICAM-1, MCP-1, and IL-8 secretion in response to lipopolysaccharide LPS (Park, Chun et al. 2006). Cigarette smoke (CS) is a major risk factor for the development of COPD, and prolonged exposure to CS induces lung inflammation and injury involving enhanced recruitment of inflammatory cells in the lungs and generation of ROS via Nox. Interestingly, exposure of mice with targeted genetic ablation of NADPH oxidase components (p47phox and gp91phox) to CS showed decreased ROS generation; however, the mice were more susceptible to CS-induced lung inflammation, airspace enlargement, and alveolar damage (Yao, Edirisinghe et al. 2008). This pathologic abnormality was linked to enhance TLR4/NF-kB signaling in response to CS in p47phox −/− and gp91phox −/− knockout mice.
4.2 Nox4 and pulmonary fibrosis
Persistence of myofibroblasts is believed to contribute to the development of fibrosis in idiopathic pulmonary fibrosis (IPF), and transforming growth factor beta1 (TGFbeta1) irreversibly converts fibroblasts into pathological myofibroblasts. Reactive oxygen species produced by Noxes have been shown to regulate cell differentiation and increased expression of NADPH oxidase components, namely, p47phox and p67phox, and ROS production in the development of bleomycin-induced pulmonary fibrosis have been demonstrated (Wang, Kang et al. 2007). Recent studies implicate a role for the Nox4 isoform in tissue repair functions of myofibroblasts and fibrogenesis (Amara, Goven et al.; Hecker, Vittal et al. 2009) Transforming growth factor-beta1 (TGF-beta1) induces Nox4 expression in lung mesenchymal cells via SMAD-3, a receptor-regulated protein that modulates gene transcription. Expression of Nox4 was upregulated in lungs of mice subjected to noninfectious injury and in cases of human idiopathic pulmonary fibrosis (IPF). Furthermore, genetic or pharmacologic targeting of Nox4 attenuated fibrogenesis in murine models of lung injury (Hecker, Vittal et al. 2009). These studies support a function for Nox4 in tissue fibrogenesis and phenotype in idiopathic pulmonary fibrosis.
4.3 Role of Nox in diabetes and atherosclerosis
Several Nox proteins are also expressed in the liver and pancreatic β-cells. There is now evidence that inappropriate activation of Nox proteins may damage the liver and pancreatic β-cells. In the context of the metabolic syndrome, the emerging epidemic of non-alcoholic steatohepatitis is thought to be Nox/ROS-dependent and of particular medical relevance. Nox/ROS-dependent β-cell damage is thought to be involved in glucolipotoxicity and thereby leads to progression from the metabolic syndrome to Type 2 diabetes (Guichard, Moreau et al. 2008). The molecular basis of this element of diabetogenesis has been closely linked to oxidative stress. Under conditions of elevated metabolism, many tissue-specific cells are continuously subject to insult from ROS. This is probably a common feature for elements of the metabolic syndrome such as hypertension, hyper-triglyceridemia, diabetes and obesity (Griendling and FitzGerald 2003; Sakai, Matsumoto et al. 2003; Robertson 2006). Chronic elevation of ROS leads to impaired insulin signaling by a complex mechanism. This involves increased JNK (c-Jun N-terminal kinase) 1-mediated-serine/threonine phosphorylation of IRS1, increased proteasomal degradation of IRS1, impaired insulin-stimulated redistribution of IRS1 and PI3K (phosphoinositide 3-kinase) activity causing reduced Akt/PKB (protein kinase B) phosphorylation (Newsholme, Haber et al. 2007). Thus NADPH oxidase, especially Nox2 and Nox4, seem to be involved in pathways leading to insulin resistance and steatosis in the liver. Atherosclerosis is another vascular disorder constituting mainly two processes of oxidative stress and inflammation. The role of Nox proteins in atherosclerosis has been studied with the role of NF-κB in the regulation of Nox1/4 expression in TNF-α-exposed human aortic SMCs (Manea, Tanase et al.2010).
5.0 Regulation of Nox proteins
Many studies suggest that cellular reduction/oxidation (redox) status regulates various aspects of cellular function. Oxidative stress can elicit positive responses such as cellular proliferation or activation, as well as negative responses such as growth inhibition or cell death. Cellular redox status is maintained by intracellular redox-regulating molecules, including thioredoxin (TRX) (Nakamura, Nakamura et al. 1997).
5.1 Regulation of Nox by phospholipaseD, Src and cytoskeleton
In vascular endothelium, Nox1, Nox2 and Nox4 are regulated through different mechanisms. In lung vasculature, the work from various laboratories including ours have clearly established increased ROS production during hyperoxia, (Usatyuk, Gorshkova et al. 2009) and ROS generated through NADPH oxidase to be a key component of hyperoxia-induced lung inflammation and injury. Mechanisms that regulate hyperoxia-induced NADPH oxidase activation are complex involving phospholipase D-mediated and Src-dependent tyrosine phosphorylation of p47phox and cortactin (Chowdhury, Watkins et al. 2005) and recruitment of Rac1, IQGAP1 and other cytoskeletal as well as oxidase components to caveolin-1 rich lipid microdomains (Singleton, Pendyala et al. 2009). Also, we have recently reported that hyperoxia up-regulates expression of Nox4 and Nox2, but not Nox1 or Nox3, proteins in human lung ECs, and knockdown of either Nox2 or Nox4 expression by siRNA up-regulates the mRNA and protein expression of either Nox4 or Nox2, respectively (Pendyala, Gorshkova et al. 2009).
5.2 Nrf2 regulates Nox4 expression via ARE in Nox4 promoter
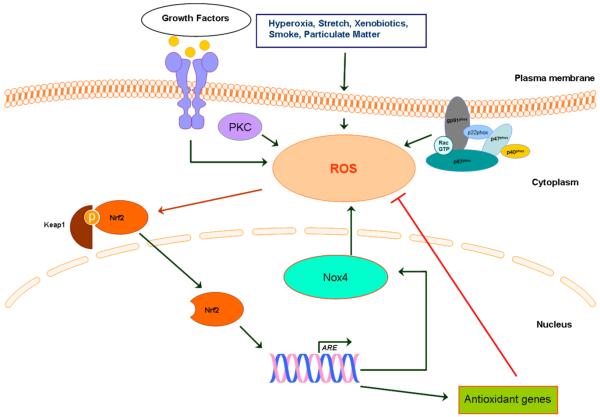
Very little is known on transcriptional regulation of Nox4. We provide evidence for the first time demonstrating a role for Nrf2, a critical transcriptional regulator of antioxidant genes, in up-regulating Nox4 expression in the mouse lung and human lung endothelium in response to hyperoxia (Pendyala, Moitra et al 2010). Results from this study show that: (i) the presence of functional cis-acting ARE sequences at positions -438-458 and -619-636 in the 5′-flanking region of Nox4 gene, (ii) recruitment of Nrf2 to the endogenous Nox4 promoter encompassing ARE in human lung endothelial cells following hyperoxia exposure, and (iii) lack or diminished levels of hyperoxia induced Nox4 expression in human lung endothelial cells or mouse lung with reduced levels of Nrf2 (Figure 1). While redox imbalance due to excess ROS generation has been implicated in the pathogenesis of hyperoxia- and oxidant-induced lung injury (Pendyala, Gorshkova et al. 2009), hyperoxia activates expression of endogenous antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, heme oxygenase 1 and catalase to offset the oxidant burden in the lung (Cho, Reddy et al. 2006). Emerging evidence from in vivo and in vitro studies have identified Nrf2 as a key regulator in mediating the induction of genes encoding several antioxidant enzymes via the ARE in response to stressful stimuli, including hyperoxia. The nuclear localization of Nox4 in human endothelial cells is important for regulation by ARE. Our extensive studies in human lung microvascular endothelial cells and human pulmonary artery endothelial cells show that Nox4 is localized in the nucleus (Pendyala et al. 2009); however Nox4 localization in mitochondria was described in breast cancer epithelial cells (Graham, Kulawiec et al 2010.). Over-expression of Nox4 in normal breast epithelial cells resulted in cellular senescence, resistance to apoptosis, and tumorigenic transformation. Further, over-expression of Nox4 in already transformed breast tumor cells increased tumorigenicity (Graham, Kulawiec et al. 2010). These data suggest an oncogenic function of Nox4, which is localized in mitochondria and ROS production in tumorogenesis. Another finding confirmed the localization of Nox4 in mitochondria. in cultured mesangial cells and kidney cortex (Block, Gorin et al. 2009) of diabetic animals. In this study authors, confirmed that Nox4 is present in mitochondria-enriched heavy fractions, and in purified mitochondria. A recent study shows that Nox4 is localized on ER endomembranes in human umbilical vein endothelial cells (HUVECs) (Wu, Ma et al.). In this study authors have shown that oxidative stress induces specific production of H2O2 by ER localized Nox4. While redox imbalance due to excess ROS generation has been implicated in the pathogenesis of hyperoxia- and oxidant-induced lung injury (Pendyala, Gorshkova et al. 2009), hyperoxia activates expression of endogenous antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, heme oxygenase 1 and catalase to offset the oxidant burden in the lung (Cho, Reddy et al. 2006). Emerging evidence from in vivo and in vitro studies have identified Nrf2 as a key regulator in mediating the induction of genes encoding several antioxidant enzymes via the ARE in response to stressful stimuli, including hyperoxia (Cho and Kleeberger 2004). Genetic disruption of Nrf2 rendered mice highly susceptible to hyperoxia-induced lung injury compared to wild type (Nrf2+/+) mice and this susceptibility was mainly attributed to decreased expression levels of several antioxidant genes (Cho and Kleeberger 2007). We observed that induction of Nox4 expression by hyperoxia was impaired in Nrf2-null mice compared to Nrf2+/+ mice (Pendyala, Moitra et al 2010), suggesting a potential role of Nrf2 in regulating Nox4 expression in response to hyperoxia in vivo. A direct role for Nrf2 in regulating Nox4 expression was confirmed in human lung ECs. Knockdown of Nrf2 expression using siRNA approach markedly attenuated Nox4 expression, without altering Nox2 levels Recent studies have also shown an important role for localized H2O2 in cellular signaling and its regulation of peroxiredoxins that are known to be regulated by Nrf2 regulated cellular stress (Woo, Yim et al 2010.).
Figure 1. Redox regulation of Nox proteins in vascular endothelium.
ROS produced by Nox regulates angiogenesis and signaling pathways. Smoking, stretch, hyperoxia and other environmental factors can induce ROS in endothelial cells. ROS triggers Nrf2 to uncouple Keap1 and translocates to nucleus to stimulate antioxidant genes. The paradoxical mechanism of Nrf2 regulating Nox4 regulation through ARE activation is also shown.
5.3 Redox regulation of Nox proteins
Besides Nrf2, an antioxidant transcriptional factor, two other well defined transcriptional factors, nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) are regulated by the intracellular redox state (Sen and Packer 1996). NF-kB and AP-1 have been implicated in the inducible expression of a wide variety of genes involved in oxidative stress and cellular response mechanisms. Binding sites of the redox-regulated transcription factors NF-kB, AP-1 and Nrf2 are located in the promoter region of a large variety of genes that are directly involved in the pathogenesis of diseases, e.g., ARDS, diabetes, atherosclerosis and cardiovascular diseases. In human lung ECs, hyperoxia-mediated Nox4 expression was attenuated by N-acetylcysteine and other agents that regulate intracellular thiol levels suggesting redox regulation of Nox4 in the endothelium (V. Natarajan and S. Pendyala, unpublished results). Therefore, studying redox regulation of Nox isoforms is key to understanding the role of Nox-derived ROS in signaling and cellular functions.
6.0 Conclusions
ROS have implicated in a number of biological processes under normal and pathological conditions such as acute lung injury, pulmonary hypertension, diabetes, and atherosclerosis. Recent studies have identified a role for Nox derived ROS in intracellular signal transduction and pathophysiology of human diseases. Further, the specificity of intracellular ROS mediated signaling resulting in cellular responses may dependent on differences in the sub-cellular localization of Nox isoforms, which could be tissue or cell specific. Further, expression and regulation of some Nox isoforms may be redox regulated. Future studies on redox regulation and spatial production of ROS by Nox isoforms will provide a better understanding of ROS role in intracellular signal transduction and pathophysiology of human diseases. Development of specific small molecule inhibitors for Nox isoforms should provide novel therapeutic approaches to ameliorate ROS-dependent cardiovascular and other human diseases.
Acknowledgments
Sources of funding: NIH RO1 HL 085553 and PO1 HL58064 to V.N
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen LA, DeLeo FR, Gallois A, Toyoshima S, Suzuki K, Nauseef WM. Transient association of the nicotinamide adenine dinucleotide phosphate oxidase subunits p47phox and p67phox with phagosomes in neutrophils from patients with X-linked chronic granulomatous disease. Blood. 1999;93(10):3521–30. [PubMed] [Google Scholar]
- Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65(8):733–8. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276(40):37594–601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106(34):14385–90. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24(9):471–8. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, Barazzone Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med. 2009;180(10):972–81. doi: 10.1164/rccm.200902-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8(5-6):691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181(7):1129–39. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 244(1):43–56. doi: 10.1016/j.taap.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Cho HY, Kleeberger SR. Genetic mechanisms of susceptibility to oxidative lung injury in mice. Free Radic Biol Med. 2007;42(4):433–45. doi: 10.1016/j.freeradbiomed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8(1-2):76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- Chose O, Sansilvestri-Morel P, Badier-Commander C, Bernhardt F, Fabiani JN, Rupin A, Verbeuren TJ. Distinct role of nox1, nox2, and p47phox in unstimulated versus angiotensin II-induced NADPH oxidase activity in human venous smooth muscle cells. J Cardiovasc Pharmacol. 2008;51(2):131–9. doi: 10.1097/FJC.0b013e31815d781d. [DOI] [PubMed] [Google Scholar]
- Chowdhury AK, Watkins T, Parinandi NL, Saatian B, Kleinberg ME, Usatyuk PV, Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280(21):20700–11. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275(30):23227–33. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- Dhaunsi GS, Paintlia MK, Kaur J, Turner RB. NADPH oxidase in human lung fibroblasts. J Biomed Sci. 2004;11(5):617–22. doi: 10.1007/BF02256127. [DOI] [PubMed] [Google Scholar]
- Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, Gorlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25(3):519–25. doi: 10.1161/01.ATV.0000154279.98244.eb. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65(2):495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11(4):791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension. 2007;50(1):189–96. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem. 2004;279(50):51715–8. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54(2):311–21. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KA, Kulawiec M, Owens KM, Li X, Desouki MM, Chandra D, Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther. 2010;10(3) doi: 10.4161/cbt.10.3.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK. NADPH oxidases: new regulators of old functions. Antioxid Redox Signal. 2006;8(9-10):1443–5. doi: 10.1089/ars.2006.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108(17):2034–40. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- Guichard C, Moreau R, Pessayre D, Epperson TK, Krause KH. NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem Soc Trans. 2008;36(Pt 5):920–9. doi: 10.1042/BST0360920. [DOI] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15(9):1077–81. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296(3):L489–99. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57(5):S28–9. [PubMed] [Google Scholar]
- Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10(12):1139–51. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43(3):319–31. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24(5):1844–54. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal D, Fu P, Levine AD. REDOX regulation of IL-13 signaling in intestinal epithelial cells: usage of alternate pathways mediates distinct gene expression patterns. Cell Signal. 2010;22(10):1485–94. doi: 10.1016/j.cellsig.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Manea A, Tanase LI, Raicu M, Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2010;396(4):901–7. doi: 10.1016/j.bbrc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Maranchie JK, Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65(20):9190–3. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanova T, Chatterjee S, Manevich Y, Kotelnikova I, Debolt K, Madesh M, Moore JS, Fisher AB. Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. Am J Physiol Cell Physiol. 2006;290(1):C66–76. doi: 10.1152/ajpcell.00094.2005. [DOI] [PubMed] [Google Scholar]
- Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101(3):258–67. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25(26):3699–707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583(Pt 1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol. 2009;40(5):601–9. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res. 2006;72(3):447–55. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24(24):10703–17. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2009;11(4):747–64. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal. 2009;11(4):841–60. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8(9-10):1473–84. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- Ray R, Shah AM. NADPH oxidase and endothelial cell function. Clin Sci (Lond) 2005;109(3):217–26. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006;6(6):615–9. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9(1):49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, Kawashima J, Shirotani T, Ichinose K, Brownlee M, Araki E. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun. 2003;300(1):216–22. doi: 10.1016/s0006-291x(02)02832-2. [DOI] [PubMed] [Google Scholar]
- Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. Faseb J. 1996;10(7):709–20. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Pendyala S, Gorshkova IA, Mambetsariev N, Moitra J, Garcia JG, Natarajan V. Dynamin 2 and c-Abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and reactive oxygen species production in caveolin-enriched microdomains of the endothelium. J Biol Chem. 2009;284(50):34964–75. doi: 10.1074/jbc.M109.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338(1):677–86. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Gorshkova IA, He D, Zhao Y, Kalari SK, Garcia JG, Natarajan V. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem. 2009;284(22):15339–52. doi: 10.1074/jbc.M109.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71(2):226–35. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Wang CL, Kang J, Li ZH. [Increased expression of NADPH oxidase p47-PHOX and p67-PHOX factor in idiopathic pulmonary fibrosis] Zhonghua Jie He He Hu Xi Za Zhi. 2007;30(4):265–8. [PubMed] [Google Scholar]
- Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol. 2009;296(3):H539–49. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140(4):517–28. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Wu RF, Ma Z, Liu Z, Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol. 2010;30(14):3553–68. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol. 2008;172(5):1222–37. doi: 10.2353/ajpath.2008.070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Matsuzaki I, Chatterjee S, Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L954–61. doi: 10.1152/ajplung.00210.2005. [DOI] [PubMed] [Google Scholar]