Abstract
Hematopoietic cell transplantation (HCT) from a matched related donor (MRD) benefits many adults with acute myeloid leukemia (AML) in first complete remission (CR1). The majority of patients do not have such a donor, however, requiring use of an alternative donor if HCT is undertaken. We retrospectively analyzed 226 adult AML CR1 patients undergoing myeloablative unrelated donor (URD) (10/10 match, n=62; ≤9/10, n=29) or MRD (n=135) HCT from 1996–2007. Five-year estimates of overall survival (OS), relapse, and non-relapse mortality (NRM) were 57.9%, 29.7%, and 16.0%, respectively. Failure for each of these outcomes was slightly higher for 10/10 URD than MRD HCT, although statistical significance was not reached for any endpoint. The adjusted hazard ratios (HR) were 1.43 (0.89–2.30, p=0.14) for overall mortality, 1.17 (0.66–2.08, p=0.60) for relapse, and 1.79 (0.86–3.74, p=0.12) for NRM, respectively, and the adjusted odds ratio (OR) for grades 2–4 acute graft-versus-host disease was 1.50 (0.70–3.24, p=0.30). Overall mortality among 9/10 and 10/10 URD recipients was similar (adjusted HR=1.16 [0.52–2.61], p=0.71). These data indicate that URD HCT can provide long-term survival for CR1 AML; outcomes for 10/10 URD HCT, and possibly 9/10 URD HCT, suggest that this modality should be considered in the absence of a suitable MRD.
Keywords: acute myeloid leukemia (AML), first complete remission, hematopoietic stem cell transplantation, unrelated donor, matched related donor
INTRODUCTION
Recent advances in the understanding of the molecular pathogenesis of adult acute myeloid leukemia (AML) have refined our ability to provide prognostic information and risk determination for subgroups of patients and have helped in the development of risk-adapted therapeutic strategies (1–3). Nevertheless, the optimal treatment approach remains controversial in many clinical situations. This is particularly true for patients in first complete remission (CR1), where treatment-related mortality has the potential to offset or exceed therapeutic benefits. Recent meta-analyses of prospective trials of adults with AML in CR1 that assigned participants to undergo allogeneic hematopoietic cell transplantation (HCT) from a human leukocyte antigen (HLA)-identical (“matched”) related donor (MRD) vs alternative treatments suggested that allogeneic HCT offers a statistically significant advantage with regard to overall survival (OS) and relapse-free survival (RFS) compared to chemotherapy or autologous HCT. This benefit is most obvious for patients with poor-risk cytogenetics, is less impressive for patients with intermediate-risk cytogenetics, and is lost for patients with good-risk cytogenetics (4–6). Together, these analyses suggest an important role of MRD HCT for many patients in CR1 (7–9).
Overall, only 30% of patients who are candidates for allogeneic HCT have a MRD to serve as a source for stem cells. With the growth of unrelated donor (URD) registries worldwide, the probability that a patient will successfully identify a suitable donor has dramatically increased (10, 11). Yet, the benefit of URD HCT for the treatment of CR1 AML is unknown. Furthermore, the indications for the use of HLA mismatched donors when matched donors are not available for the treatment of patients in CR1 with low-, intermediate-, or high-risk disease remain to be defined. Recent retrospective studies on URD HCT from two large registries indicate that a significant graft-versus-leukemia effect may be evident and prolonged RFS might be achieved in some patients who are unlikely to be cured with chemotherapy alone; however, in both studies, non-relapse mortality (NRM) appeared relatively high, possibly negating a net benefit (12, 13). In addition, preliminary analyses from a prospective trial suggested a benefit of matched URD HCT compared to autologous HCT for patients with high-risk disease based on adverse cytogenetics or persistent disease after induction therapy (14). These findings have prompted the use of URD HCT primarily as an option for AML patients in CR1 with high-risk features (10, 11, 13, 15). It is conceivable, however, that patients with normal risk features could also benefit from this approach if NRM were relatively low. Therefore, the outcome of URD HCT for AML in CR1 deserves further investigation, as is the study of predictors for adverse outcome in this clinical setting. To this end, we have retrospectively analyzed clinical outcome after URD HCT from a single center between 1996 and 2007, and compared it with our MRD HCT experience in the same time period.
PATIENTS AND METHODS
Study Cohort
Patients ≥18 years of age who underwent HCT were identified from the computerized database at the Fred Hutchinson Cancer Research Center (Seattle, WA) for this retrospective analysis. Patients were included if they had AML in CR1 at the time of HCT, underwent myeloablative conditioning, had either a matched sibling or unrelated donor with available high-resolution HLA typing data, and received a first transplant between 1996 and 2007. For our analysis, AML at diagnosis was classified according to the 2001 World Health Organization (WHO) classification (16). Therefore, patients undergoing HCT before 2001 with a French-American-British (FAB) Cooperative Group diagnosis of Refractory Anemia with Excess Blasts in Transformation (RAEB-T) were reclassified as having AML and included in this analysis. CR was defined according to standard criteria proposed by an International Working Group (17). Cytogenetic risk-group assignment was done according to the Southwest Oncology Group/Eastern Cooperative Oncology Group (SWOG/ECOG) criteria (2). Pretransplantation comorbidities were assessed retrospectively using the HCT-specific comorbidity index (HCT-CI) (18, 19). All patients were treated on protocols that were approved by the FHCRC Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki.
HLA Typing and Matching
All related donors were HLA-matched siblings based on family studies. Histocompatibility testing and selection of unrelated donors are described in detail elsewhere (20). In brief, high resolution typing methods for discriminating nucleotide differences encoded in exons 2 and 3 of class I HLA-A, C and B, and exon 2 of HLA-DRB1 and DQB1 included sequencing-based and oligonucleotide probe hybridization methods for human genomic DNA. Recipients and donors were defined as HLA-A, C, B, DRB1, and DQB1 allele matched (“10/10”), or mismatched in the graft-versus-host vector, the host-versus-graft vector, or bidirectionally. In the absence of matched donors, preference was given to donors with a single HLA locus disparity (“9/10”) over multi-locus mismatches (“8/10” or greater) (21).
Acute and Chronic Graft-Versus-Host Disease (GVHD)
Criteria for diagnosis and grading of acute and chronic GVHD have been reported previously (22, 23).
Statistical Analysis
Unadjusted probabilities of OS and RFS were estimated using the Kaplan-Meier method, and probabilities of NRM, relapse, and GVHD were summarized using cumulative incidence estimates. NRM was defined as death without prior relapse and was considered a competing risk for the endpoint of relapse. Death without GVHD was considered a competing risk for GVHD, and relapse a competing risk for NRM. Outcomes for time-to-event endpoints (overall mortality, relapse/death [failure for DFS], relapse, NRM, chronic GVHD) were compared between the matched-related and unrelated donor groups using Cox regression, while logistic regression was used for acute GVHD. In the URD group, risk factors for overall mortality, relapse, and NRM were evaluated using Cox regression. No adjustments were made for multiple comparisons, and all p-values derived from these regression models are two-sided. All statistical analyses were performed using STATA 10 (StataCorp LP, College Station, TX).
RESULTS
Patient characteristics
Between January 1996 and December 2007, a total of 226 patients with primary or secondary AML in CR1 met our study inclusion criteria: 135 patients received MRD HCT, 62 patients had a 10/10 matched URD HCT, 23 patients had a 9/10 matched URD HCT, and 6 patients had a <9/10 matched URD HCT. The median duration of CR1 prior to HCT was 122 days (range: 10–526 days). More than one course of chemotherapy was required to achieve remission in 39.2% of patients required (2 cycles in 30.4%, 3 or more cycles in 8.8%). Seventy-six percent of patients received consolidation chemotherapy before HCT, including 41.6% receiving more than 2 cycles of consolidation therapy; high-dose cytarabine was used as consolidative therapy in 60.8% of patients. Other characteristics of the patients, conditioning regimens, and GVHD prophylaxis are provided in Table 1.
Table 1. Pre-Transplant and Transplant Characteristics of Study Cohort.
| Parameter | Matched-related Donor HCT n = 135 |
10/10 Unrelated Donor HCT n = 62 |
9/10 Unrelated Donor HCT n = 23 |
|---|---|---|---|
| Patient Pre-Transplant Characteristics | |||
| Median Age (range), years | 42.5 (18.7–69.3) | 41.9 (18.2–66.8) | 44.8 (22.0–57.9) |
| Sex (male/female) | 58/77 | 30/32 | 13/10 |
| Median WBC (range) at diagnosis, ×103/µL | 5.2 (0.1–310.0) | 3.7 (0.7–192.0) | 7.7 (0.8–63.5) |
| Cytogenetic Risk Group, n (%) | |||
| “Favorable” | 5 (3.7%) | 2 (3.2%) | 0 (0%) |
| “Intermediate” | 83 (61.5%) | 44 (71.0%) | 14 (60.9%) |
| “Unfavorable” | 41 (30.4%) | 15 (24.2%) | 4 (17.4%) |
| “Unknown” or missing | 6 (4.4%) | 1 (1.6%) | 5 (21.7%) |
| Secondary AML | 20.7% | 42.6% | 30.4% |
| Median Time Diagnosis to HCT (range), days | 150.5 (43–363) | 179 (30–556) | 191 (51–332) |
| Median HCT Comorbidity Index (range) | 1 (0–8) | 2 (0–7) | 1 (0–6) |
| Source of Stem Cells, n (%) | |||
| Bone marrow | 45 (33.3%) | 20 (32.3%) | 7 (30.4%) |
| Peripheral blood | 90 (66.7%) | 42 (67.7%) | 16 (69.6%) |
| CMV Seropositive Pre-HCT | 61.8% | 51.6% | 47.8% |
| Donor and Transplant Characteristics | |||
| Median Donor Age (range), years | 41.6 (11.5–76.6) | 34.8 (19.1–56.1) | 38.0 (22.2–52.1) |
| Donor Sex (male/female/unknown) | 73/62 | 50/12 | 13/10 |
| Patient / Donor Sex, n (%) | |||
| Male / Male | 30 (22.2%) | 25 (40.3%) | 9 (39.1%) |
| Female / Female | 34 (25.2%) | 7 (11.3%) | 6 (26.1%) |
| Male / Female | 28 (20.7%) | 5 (8.1%) | 4 (17.4%) |
| Female / Male | 43 (31.9%) | 25 (40.3%) | 4 (17.4%) |
| Donor CMV Seropositive | 47.0% | 29.0% | 30.4% |
| Patient / Donor CMV Serostatus, n (%) | |||
| Positive / Positive | 44 (32.6%) | 14 (22.6%) | 3 (13.0%) |
| Negative / Negative | 33 (24.4%) | 26 (41.9%) | 8 (34.8%) |
| Positive / Negative | 37 (27.4%) | 18 (29.0%) | 8 (34.8%) |
| Negative / Positive | 17 (12.6%) | 4 (6.5%) | 4 (17.4%) |
| Unknown | 4 (3.0%) | 0 (0%) | 0 (0%) |
| Conditioning Regimen, n (%) | |||
| Chemotherapy ± radiolabeled antibody | 104 (77.0%) | 35 (56.5%) | 15 (65.2%) |
| TBI ± radiolabeled antibody | 31 (23.0%) | 27 (43.5%) | 8 (34.8%) |
| T Cell Depletion with ATG, n (%) | 8 (5.9%) | 8 (12.9%) | 0 (0%) |
| GVHD Prophylaxis, n (%) | |||
| Calcineurin inhibitor + methotrexate | 130 (96.3%) | 53 (85.5%) | 23 (100%) |
| Calcineurin inhibitor + MMF | 4 (3.0%) | 5 (8.1%) | 0 (0%) |
| Other | 1 (0.7%) | 4 (6.5%) | 0 (0%) |
| Median Nucleated Cell Dose (range), ×108/kg | |||
| Bone marrow | 1.9 (0.5–5.9) | 3.8 (1.6–7.0) | 2.5 (0.9–5.6) |
| Peripheral blood | 11.1 (4.6–45.0) | 9.5 (4.0–18.4) | 9.1 (6.0–19.1) |
Abbreviations: ATG, anti-thymocyte globulin; MMF, mycophenolate mofetil.
Acute and Chronic GVHD
In the entire cohort, the incidence of grades 2–4 acute GVHD was 70.9% post-HCT among 220 with available data, while the incidence of grades 3–4 acute GVHD was 20.9%. By two years following HCT, the estimated probability of chronic GVHD was 49.6%. The incidences of acute and chronic GVHD at 2 years for the various donor groups, MRD, 10/10 URD, and 9/10 URD, are shown in Table 2. In unadjusted models among all patients in the MRD, 10/10 URD, and 9/10 URD groups, patients undergoing 10/10 URD tended to have higher odds of developing grades 2–4 acute GVHD than patients undergoing MRD (odds ratio (OR): 1.90 [95% confidence interval: 0.95–3.81], p=0.07). In contrast, the probability of grades 3–4 GVHD tended to be lower in the 10/10 URD group (OR 0.72 [0.33–1.61], p=0.43) and the risk of chronic GVHD was similar (HR 1.16 [0.76–1.78], p=0.49). After adjustment for various covariates (conditioning regimen, age, source of stem cells, year of HCT, and CMV status), comparing 10/10 URD to MRD HCT, the ORs were 1.50 (0.70–3.24, p=0.30) for grades 2–4 acute GVHD, 0.79 (0.33–1.93, p=0.61) for grades 3–4 acute GVHD, while the HR was 1.42 (0.90–2.24, p=0.13) for extensive chronic GVHD (Table 3). Similar univariate and multivariable models were fit for the comparison of 10/10 URD vs 9/10 URD HCT among only patients in these groups. These models indicated that patients undergoing 9/10 URD HCT may have a higher risk for development of grades 3–4 acute GVHD (OR: 2.55 [0.78–8.33], p=0.12) and, to a lesser degree, grades 2–4 acute GVHD (OR: 1.48 [0.37–5.86], p=0.58) as well as extensive chronic GVHD (HR: 1.45 [0.76–2.76], p=0.26), although the small sample sizes limited the power to observe a statistical significant difference. Given these results, a comparison of the 9/10 URD group with the MRD group results in similar HRs and ORs relative to the 10/10 URD group compared to the MRD group (Table 3).
Table 2. Incidence and Degree of Acute and Chronic GVHD.
| Parameter | Matched-related Donor HCT n = 135 |
10/10 Unrelated Donor HCT n = 62 |
9/10 Unrelated Donor HCT n = 23 |
|---|---|---|---|
| Acute GVHD overall | |||
| 0 | 31.0% | 17.7% | 13.0% |
| 1 | 4.7% | 4.8% | 4.4% |
| 2 | 43.4% | 61.3% | 52.2% |
| 3 | 20.2% | 14.5% | 21.7% |
| 4 | 0.8% | 1.6% | 8.7% |
| Grades 2–4 | 64.3% | 77.4% | 82.6% |
| Severe (grades 3+4) | 20.9% | 16.1% | 30.4% |
| Missing, n | 6 (4.4%) | 0 (0%) | 0 (0%) |
| Chronic extensive GVHD | 49.7% | 50.0% | 60.9% |
Table 3 . Adjusted (Multivariate) Regression Models for Risk of Grades 2–4 Acute GVHD, Grades 3–4 Acute GVHD, and Extensive Chronic GVHD in 10/10 URD, 9/10 URD, and MRD HCT.
Odds ratios (for acute GVHD) and hazard ratios (for chronic GVHD) are provided with 95% confidence intervals and p-values.
| Factor | Acute GVHD Grade 2–4 | Acute GVHD Grade 3–4 | Extensive Chronic GVHD |
|---|---|---|---|
|
Transplant Type MRD (n=135) 10/10 URD (n=62) 9/10 URD (n=23) |
1 1.50 (0.70–3.24), p=0.297 2.05 (0.62–6.80), p=0.241 |
1 0.79 (0.33–1.93), p=0.610 2.31 (0.77–6.89), p=0.135 |
1 1.42 (0.90–2.24), p=0.134 1.94 (1.04–3.62), p=0.037 |
| Year of Transplant (n=220)* | Not used in the model | 0.84 (0.72–0.97), p=0.020 | 0.90 (0.85–0.96), p=0.001 |
|
Patient/Donor CMV −/− (n=67) +/+ (n=61) +/− (n=63) −/+ (n=25) |
Not used in the model | Not used in the model |
1 1.27 (0.75–2.15), p=0.383 1.99 (1.21–3.28), p=0.007 1.53 (0.81–2.87), p=0.191 |
|
Conditioning No TBI (n=154) TBI (n=66) |
1 7.05 (2.57–19.32), p<0.001 |
1 1.53 (0.69–3.42), p=0.297 |
Not used in the model |
| Patient Age (n=220)* | 1.04 (1.01–1.07), p=0.003 | 1.04 (1.01–1.08), p=0.015 | 1.02 (1.00–1.04), p=0.030 |
|
Source of Stem Cells PBSC (n=148) BM (n=72) |
1 1.38 (0.68–2.79), p=0.367 |
1 0.75 (0.30–1.88), p=0.537 |
Not used in the model |
modeled as continuous linear variable
OS, RFS, Relapse, and NRM
Among the entire study cohort, 67 patients (29.6%) relapsed and 95 patients (42.0%) died as of July 25, 2009. Neither median OS nor median DFS was reached for the entire cohort. The estimates for 5- and 10-year OS were 57.9% (95% confidence interval, 51.0–64.2%) and 54.3% (46.5–61.4%), respectively. The estimates for 5- and 10-year DFS were only slightly lower at 54.3% (47.4–60.8%) and 51.1% (43.6–58.1%), respectively. The 2- and 5-year estimates of relapse were 26.8% (21.0–32.6%) and 29.7% (23.6–35.8%), while the 2- and 5-year estimates of NRM were 13.8% (9.3–18.3%) and 16.0% (11.1–20.9%), respectively. In univariate analyses among all patients in the MRD, 10/10 URD, and 9/10 URD groups, patients undergoing 10/10 URD HCT had trends for higher risks of overall mortality (hazard ratio [HR]: 1.48 [0.94–2.34], p=0.09; Figure 1A), failure for DFS (HR: 1.37 [0.89–2.13], p=0.16; Figure 1B), and NRM (HR 1.88 [0.91–3.87], p=0.09) when compared to patients undergoing MRD HCT. In contrast, the risk of relapse was comparable (HR: 1.15 [0.66–2.01], p=0.62; Figure 1C) among these 2 groups. After adjustment for various covariates (cytogenetics, conditioning regimen, age, source of stem cells, and comorbidities as expressed by the HCT-CI (18, 19)), the HRs were 1.43 (0.89–2.30, p=0.14) for overall mortality, 1.33 (0.85–2.10, p=0.21) for failure of DFS, 1.17 (0.66–2.08, p=0.60) for relapse, and 1.79 (0.86–3.74, p=0.12) for NRM (Table 4).
Figure 1. Comparison of 10/10 URD, 9/10 URD, and MRD HCT.



Estimate of the probability of OS (A), DFS (B) and relapse (C), for patients undergoing MRD HCT as compared to those undergoing 10/10 URD or 9/10 URD HCT.
Table 4. Adjusted (Multivariate) Regression Models for Risk of Overall Mortality, Relapse, and Non-Relapse Mortality (NRM) in 10/10 URD, 9/10 URD, and MRD HCT.
Hazard ratios are provided with 95% confidence intervals and p-values.
| Factor | Overall Mortality | Failure for DFS | Relapse | NRM |
|---|---|---|---|---|
|
Transplant Type MRD (n=135) 10/10 URD (n=62) 9/10 URD (n=23) |
1 1.43 (0.89–2.30), p=0.137 1.66 (0.84–3.27), p=0.146 |
1 1.33 (0.85–2.10), p=0.213 1.45 (0.74–2.87), p=0.278 |
1 1.17 (0.66–2.08), p=0.595 1.63 (0.70–3.82), p=0.259 |
1 1.79 (0.86–3.74), p=0.122 1.38 (0.48–4.01), p=0.551 |
|
Cytogenetics Unfavorable (n=60) Favor/Intermed (n=148) |
1 0.61 (0.39–0.97), p=0.035 |
1 0.50 (0.33–0.77), p=0.002 |
1 0.35 (0.20–0.59), p<0.001 |
Not used in the model |
|
Conditioning No TBI (n=154) TBI (n=66) |
1 1.43 (0.92–2.22), p=0.111 |
1 1.45 (0.95–2.21), p=0.084 |
1 1.52 (0.90–2.56), p=0.116 |
1 1.36 (0.67–2.74), p=0.391 |
| Patient Age (n=220)* | 1.01 (0.99–1.03), p=0.170 | 1.01 (0.99–1.02), p=0.563 | 1.00 (0.97–1.02), p=0.675 | Not used in the model |
|
Source of Stem Cells PBSC (n=148) BM (n=72) |
1 1.48 (0.95–2.30), p=0.083 |
1 1.26 (0.82–1.94), p=0.284 |
1 0.72 (0.40–1.29), p=0.268 |
1 3.00 (1.50–6.01), p=0.002 |
|
HCT-CI 0 (n=71) 1–2 (n=49) 3+ (n=43) |
1 1.80 (0.97–3.33), p=0.062 3.02 (1.68–5.41), p<0.001 |
1 1.76 (0.98–3.15), p=0.059 2.66 (1.53–4.63), p=0.001 |
1 1.42 (0.69–2.92), p=0.336 2.20 (1.14–4.25), p=0.019 |
1 3.02 (1.09–8.39), p=0.034 4.14 (1.50–11.44), p=0.006 |
modeled as continuous linear variable
Similar univariate models for these main outcome measures were fit among patients who received 10/10 URD vs 9/10 URD HCT. The univariate models indicated that patients undergoing 9/10 URD HCT had higher, but not statistically significantly higher, failure rates for overall mortality (HR: 1.24 [0.65–2.40], p=0.51; Figure 1A), DFS (HR: 1.19 [0.62–2.27], p=0.61; Figure 1B), relapse (HR: 1.27 [0.55–2.93], p=0.57; Figure 1C), and NRM (HR: 1.07 [0.38–3.01], p=0.89) when compared to patients undergoing 10/10 URD HCT. After adjustment for covariates (cytogenetics, comorbidities, primary vs secondary AML), the multivariable models indicated that the outcomes of patients undergoing 9/10 URD HCT were, for the most part, similar to those undergoing 10/10 URD HCT with regard to overall mortality (HR: 1.16 [0.52–2.61], p=0.71), failure for DFS (HR: 1.09 [0.48–2.44], p=0.84), risk of relapse (HR: 1.43 [0.56–3.64], p=0.45), and NRM (HR: 0.95 [0.32–2.78], p=0.92). Given these results, a comparison of the 9/10 URD group with the MRD group leads to similar HRs relative to the comparison of the 10/10 URD group with the MRD group (Table 4).
In light of previous studies that have dictated the indication for HCT in this patient population, we examined the effect of donor according to cytogenetic risk. There was no evidence to suggest that the difference in outcome between 10/10 URD and MRD was influenced by cytogenetic risk (Figure 2), although this essentially amounted to comparing the donor effect in patients with intermediate-risk cytogenetics to that among patients with unfavorable cytogenetics due to the small number with favorable cytogenetics. Similarly, there was no evidence to suggest dependence of donor effect on either presence of secondary AML or HCT-CI (data not shown).
Figure 2. Survival Following 10/10 URD or MRD HCT According to Cytogenetic Risk Group.
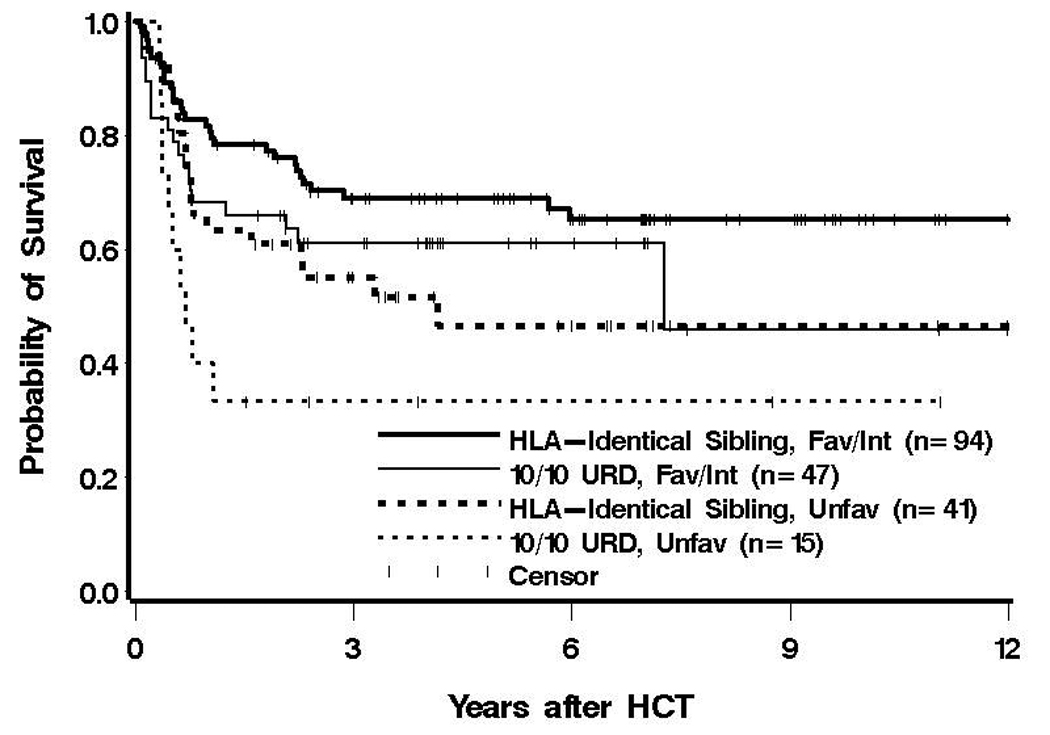
Estimate of the probability of OS after 10/10 URD or MRD HCT for patients with either favorable/intermediate or unfavorable cytogenetics.
DISCUSSION
The findings from our retrospective analysis presented in this report support three major conclusions. First, the likelihood of overall survival and disease-free survival at 5 and 10 years following myeloablative allogeneic HCT in our patient population exceed 50%, suggesting that more than half of adult patients with AML in first complete remission are or will likely be cured with this treatment modality regardless of donor stem cell source. Second, compared with patients undergoing MRD HCT, the probability of an unfavorable outcome was somewhat higher for patients undergoing URD HCT, although chance remains a plausible explanation for this observation. And third, patients undergoing 9/10 URD HCT had, for the most part, similar outcomes with regard to overall mortality, failure for DFS, relapse, and NRM when compared to patients undergoing 10/10 URD HCT, although the risk of developing grades 3–4 GVHD and chronic extensive GVHD was suggestively higher in the 9/10 URD group.
The optimal treatment of AML patients in CR1 has been a question of great debate over the last two decades. A large number of prospective trials have been conducted with the intent to define the indications of allogeneic HCT in this clinical situation. Recent studies and meta-analyses have suggested that allogeneic HCT from a MRD donor should be considered for patients with poor-risk cytogenetics, intermediate-risk cytogenetics with the exception of the nucleophosmin-1 (NPM1)-positive/fms-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD)-negative subgroup, and favorable-risk cytogenetics if certain receptor tyrosine kinase mutations are present (4–6, 9, 24, 25). By comparison, the indications for allogeneic HCT using URDs are less well defined, and there is currently considerable uncertainty about the appropriate patient selection for this treatment approach.
Our results are consistent with those of recent Australian and German studies reporting statistically similar outcomes for matched URD and sibling donor HCT for adult patients with AML (26, 27); in both studies, however, only a minority of patients received HCT for AML in CR1 while the majority of patients had more advanced stages of AML (mostly first relapse or second complete remission). An additional prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy showed similar outcomes of MRD and fully matched URD allogeneic HCT for patients with AML, chronic myeloid leukemia (CML), and myelodysplastic syndrome (MDS) (28). While the studies by Moore et al. and Schetelig et al. differed with regard to the stage of disease at time of HCT (26, 27), and the study by Yakoub-Agha et al. included different types and stages of diseases (28), the combined findings from these studies and the results reported in this manuscript are nevertheless very similar.
The impact of HLA disparity on outcome of URD HCT has recently been reported in a large study from the National Marrow Donor Program (NMDP) (29). In 3,857 myeloablative transplantations performed from 1988 to 2003 for AML, acute lymphoblastic leukemia (ALL), CML, and MDS, high-resolution DNA matching for HLA-A, -B, -C, and -DRB1 (8/8 match) was the minimum level of matching associated with the highest survival. By comparison, HCT from a donor with even a single mismatch (“7/8”) was associated with lower OS and DFS, higher treatment-related mortality, and more acute GVHD (29). While our data did not yield a statistically significant difference in outcome between 9/10 and 10/10 URD HCT, the relatively small number of patients in each group severely limited the power to detect such a difference, and precluded measurements of locus-specific effects on clinical outcome. However, the hazard ratios for 9/10 vs. 10/10 URD HCT for the endpoints of overall mortality and failure for disease-free survival observed in our study were similar to those obtained by Lee et al. (29). Together, these data suggest that while the outcome following 9/10 may be worse than that following 10/10 URD HCT, the magnitude of the difference is likely relatively small.
An important limitation of our study is its non-randomized nature, which offers the potential for the introduction of bias. Such bias could work both ways; only healthier patients are referred for URD transplants, or only those perceived to be at highest risk might be referred. To minimize this bias, we developed multivariate models to adjust for baseline differences in the various patient cohorts. A further limitation is the number of patients analyzed in this study, which limits to a degree the power that we had to detect differences. Acknowledging these limitations, our data indicate allogeneic HCT provides significant long-term survival for patients receiving grafts from matched (10 of 10 alleles) or nearly matched (9 of 10 alleles) URDs. The observation that the outcome for these patients is nearly as good as for patients for whom an HLA-identical related donor is available suggest that HCT may be advantageous over non-HCT approaches using chemotherapy alone even in the event that a suitable MRD cannot be identified. Until prospective studies are completed, this conclusion supports the rationale for the recommendation to consider matched or nearly matched URD HCT for similar indications as currently put forward for matched related donor HCT for AML patients in first complete remission.
ACKNOWLEDGEMENTS
The authors acknowledge the excellent care provided to these patients by the physicians and nurses of the HCT teams, as well as the work of the staff in the Long Term Follow-up office at the Fred Hutchinson Cancer Research Center. We are greatly indebted to Lacey M. Hedin and Amanda M. Axtman for help with data collection.
This work was supported by NIH Grants (P01-CA18029, P01-AI33484, K08-CA95448, K23-CA137161, and K99-HL088021) and the Frederick Kullman Memorial Fund. J.M.P. is supported by Career Development Awards from the Lymphoma Research Foundation and is a clinical scholar of the Damon Runyon Cancer Foundation.
Footnotes
Presented in part at the 2007 Annual Meetings of the American Society of Clinical Oncology (ASCO; abstract #7000) and the American Society of Hematology (ASH; abstract #330)
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Fröhling S, Scholl C, Gilliland DG, Levine RL. Genetics of myeloid malignancies: pathogenetic and clinical implications. J Clin Oncol. 2005;23:6285–6295. doi: 10.1200/JCO.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood reviews. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 3.Mrózek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. Jama. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109:3658–3666. doi: 10.1182/blood-2006-06-025627. [DOI] [PubMed] [Google Scholar]
- 6.Yanada M, Matsuo K, Emi N, Naoe T. Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a metaanalysis. Cancer. 2005;103:1652–1658. doi: 10.1002/cncr.20945. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum FR, Pearce SF. Hematopoietic cell transplantation in first complete remission versus early relapse. Best practice & research. 2006;19:333–339. doi: 10.1016/j.beha.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Appelbaum FR. Incorporating hematopoietic cell transplantation (HCT) into the management of adults aged under 60 years with acute myeloid leukemia (AML) Best practice & research. 2008;21:85–92. doi: 10.1016/j.beha.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Appelbaum FR. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia when a matched related donor is not available. Hematology / the Education Program of the American Society of Hematology American Society of Hematology. 2008:412–417. doi: 10.1182/asheducation-2008.1.412. [DOI] [PubMed] [Google Scholar]
- 10.Anasetti C, Perkins J, Nieder ML, Field T. Are matched unrelated donor transplants justified for AML in CR1? Best practice & research. 2006;19:321–328. doi: 10.1016/j.beha.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Zuckerman T, Rowe JM. Alternative donor transplantation in acute myeloid leukemia: which source and when? Current opinion in hematology. 2007;14:152–161. doi: 10.1097/MOH.0b013e328017f64d. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus HM, Perez WS, Klein JP, Kollman C, Bate-Boyle B, Bredeson CN, et al. Autotransplantation versus HLA-matched unrelated donor transplantation for acute myeloid leukaemia: a retrospective analysis from the Center for International Blood and Marrow Transplant Research. British journal of haematology. 2006;132:755–769. doi: 10.1111/j.1365-2141.2005.05947.x. [DOI] [PubMed] [Google Scholar]
- 13.Tallman MS, Dewald GW, Gandham S, Logan BR, Keating A, Lazarus HM, et al. Impact of cytogenetics on outcome of matched unrelated donor hematopoietic stem cell transplantation for acute myeloid leukemia in first or second complete remission. Blood. 2007;110:409–417. doi: 10.1182/blood-2006-10-043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauter J, Heil G, Hoelzer D, Ottmann OG, Martin H, Lubbert M, et al. Treatment of patients up to 60 years with high risk AML: final results of the AML SHG-Hannover 01/99 trial. Blood. 2006;108:132a. (abstract #433) [Google Scholar]
- 15.Appelbaum FR. Hematopoietic cell transplantation from unrelated donors for treatment of patients with acute myeloid leukemia in first complete remission. Best practice & research. 2007;20:67–75. doi: 10.1016/j.beha.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersdorf EW, Anasetti C, Martin PJ, Gooley T, Radich J, Malkki M, et al. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104:2976–2980. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 21.Petersdorf EW, Hansen JA, Martin PJ, Woolfrey A, Malkki M, Gooley T, et al. Major-histocompatibility-complex class I alleles and antigens in hematopoietic-cell transplantation. The New England journal of medicine. 2001;345:1794–1800. doi: 10.1056/NEJMoa011826. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Paschka P, Marcucci G, Ruppert AS, Mrozek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24:3904–3911. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 25.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. The New England journal of medicine. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 26.Moore J, Nivison-Smith I, Goh K, Ma D, Bradstock K, Szer J, et al. Equivalent survival for sibling and unrelated donor allogeneic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2007;13:601–607. doi: 10.1016/j.bbmt.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 27.Schetelig J, Bornhauser M, Schmid C, Hertenstein B, Schwerdtfeger R, Martin H, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26:5183–5191. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- 28.Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]


