Abstract
Background
Medication non-adherence is common and results in preventable disease complications. This study assesses the effectiveness of a multifactorial intervention to improve both medication adherence and blood pressure control and to reduce cardiovascular events.
Methods and Results
In this multi-center, cluster-randomized trial, physicians from hospital-based hypertension clinics and primary care centers across Spain were randomized to receive and provide the intervention to their high-risk patients. Eligible patients were ≥50 years of age, had uncontrolled hypertension, and had an estimated 10-year cardiovascular risk greater than 30%. Physicians randomized to the intervention group counted patients’ pills, designated a family member to support adherence behavior, and provided educational information to patients. The primary outcome was blood pressure control at 6 months. Secondary outcomes included both medication adherence and a composite end-point of all cause mortality and cardiovascular-related hospitalizations. Seventy-nine physicians and 877 patients participated in the trial. The mean duration of follow-up was 39 months. Intervention patients were less likely to have an uncontrolled systolic blood pressure (odds ratio 0.62; 95% confidence interval [CI] 0.50–0.78) and were more likely to be adherent (OR 1.91; 95% CI 1.19–3.05) when compared with control group patients at 6 months. After five years 16% of the patients in the intervention group and 19% in the control group met the composite end-point (hazard ratio 0.97; 95% CI 0.67–1.39).
Conclusions
A multifactorial intervention to improve adherence to antihypertensive medication was effective in improving both adherence and blood pressure control, but it did not appear to improve long-term cardiovascular events.
Keywords: hypertension, medication adherence, blood pressure, intervention studies
Hypertension is a major but modifiable contributory factor to cardiovascular diseases (CVD) such as stroke and coronary heart disease.1;2 According to the Seventh Report of the Joint National Committee on Prevention, Detection and Treatment of High Blood Pressure in the United States (JNC-7), the percentage of patients whose blood pressure (BP) is under control (i.e., < 140/90 mmHg) increased from 10% in 1976–80 to 34% in the period 1999–2000. Nevertheless, BP control rates are far below the Healthy People 2000 goal of 50%.3–5 The situation in Spain is similar with at best 40% of patients with hypertension under control.3;6
A major modifiable reason for the lack of BP control is medication nonadherence, where adherence is defined as the extent to which a person’s behavior corresponds with the recommendations of their health care provider.7 In general, poor adherence to medications is associated with the development of complications, disease progression, avoidable hospitalizations, premature disability and death.7–10 The same is true for adherence to antihypertensive medications.11;12
Most previous adherence intervention studies share the following methodological limitations: a lack of patient-centered outcomes; limited statistical power; short duration of follow-up; unreliable measures of adherence; and limited generalizibility due to their intensity and complexity.13;14 The objective of this study was to assess the effectiveness of a simple, multi-factorial intervention aimed at increasing antihypertensive medication adherence, improving BP control, and reducing cardiovascular events in high-risk patients with uncontrolled hypertension. The intervention was designed to be implemented in resource-poor settings and combined behavioral, cognitive, and social support components. The behavioral component included counting pills in front of the patients. Counting pills by doctors is a routine activity in most clinical trials and patients tend to adhere better in clinical trials than in routine clinical practice.15;16 Based on prior research, we hypothesized that this Hawthorne effect could be used to improve medication adherence in routine practice.15;17 In this study, adherence was monitored electronically, and study participants were followed for up to five years. A cluster-randomized design randomizing physicians was chosen to avoid within-physician contamination.
METHODS
Study design
The study was approved by the Ethics Committees of the Hospital General de Vic (Barcelona, Spain) and Hospital de la Princesa (Madrid, Spain). In this multicenter, cluster-randomized trial, participating physicians from hospital-based hypertension clinics and primary care centers across Spain were randomized in blocks of two within their site of practice. Block randomization was used to insure that physicians were balanced across intervention and control groups within hospitals and primary care clinics. Randomization was centralized through a single coordinating center and the sequence was concealed until interventions were assigned. A computer generated random number list was used to randomize physicians, and investigators were not aware of the randomization scheme. The trial was actively monitored by both internal and external quality control auditors.
Participants
Patients were ≥50 years of age; they had uncontrolled essential hypertension, as defined by an average systolic blood pressure ≥140 mmHg and/or an average diastolic blood pressure ≥90 mmHg; and they had a calculated 10-year cardiovascular risk >30% as defined by the WHO/ISH 1999 guidelines.18 There were only two exclusion criteria – participation in another clinical trial in the preceding 3 months and refusal/incapacity to provide informed consent. There were two coordinating centers (in Catalonia and Madrid, Spain), which separately recruited physicians. Patients were recruited by their physicians after assessing eligibiliy. The coordinating center in Catalonia was also in charge of randomization, the validation of patients eligibility criteria, the provision and analysis of the Medical Events Monitoring Systems (MEMS) containers (Aardex Ltd., Zug, Switzerland), the provision and calibration of semiautomatic sphygmomanometers, data entry, and study quality control. Patients were recruited between January 2000 and July 2002, and were followed until December 2005.
We administered a survey to all study physicians to assess their characteristics across intervention and control groups. Sixty-nine (87%) out of 79 physicians who actively recruited patients answered the survey. Characteristics of recruiting physicians are depicted in Table 1. There were no differences in the survey response rate between control and intervention groups.
Table 1.
Distribution of physician related characteristics in control and intervention groups. Numbers are mean ± SD[N], and N (%).
| Variable | Control group (39, 36 physicians with survey data) | Intervention group (40, 33 physicians with survey data) | P value |
|---|---|---|---|
| Variables with data available for all 79 analyzed physicians | |||
| Physicians under the coordination of the Catalan coordinating center | 21/39 (53.8%) | 22/40 (55.0%) | 0.918 |
| Female sex | 18/39 (46.2%) | 15/40 (37.5%) | 0.498 |
| Working place | |||
| Hospital | 12/39 (30.8%) | 13/40 (32.5%) | |
| Primary care center | 27/39 (69.2%) | 27/40 (67.5%) | 0.869 |
| Average number of patients per physician | 11.8 ± 8.3 [39] | 10.6 ± 7.9 [40] | 0.529 |
| Variables with data available only for the 69 physicians who completed the survey* | |||
| MD degree year | 1982.9 ± 5.3 [35] | 1982.8 ± 6.4 [33] | 0.834 |
| Age | 43.1 ± 5.2 [35] | 43.6 ± 6.5 [33] | 0.749 |
| Working in a Teaching Center | 21/36 (58.3%) | 20/33 (60.6%) | 0.848 |
| Previous participation in clinical trials | 28/35 (80%) | 25/33 (75.8%) | 0.773 |
| Specialty | |||
| Internal medicine | 6/34 (17.6%) | 7/33 (21.2%) | |
| Family medicine | 22/34 (64.7%) | 19/33 (57.6%) | |
| Nephrology | 6/34 (17.6%) | 7/33 (21.2%) | 0.836 |
| Practice size in primary care centers (number of patients) | 1629.8 ± 490.3 [24] | 1745.5± 493.3 [22] | 0.930 |
| Hours a week with direct contact with patients | 28.6 ± 8.5 [35] | 27.4 ± 9.0 [31] | 0.649 |
| Average visit time in primary care centers (in minutes) | 7.5 ± 3.2 | 7.0 ± 2.2 | 0.755 |
| Average time visit for follow-up visits in hospital (in minutes) | 15.0 ± 4.4 | 16.25 ± 5.3 | 0.722 |
| Holding a full time job in the health center | 27/36 (75.0%) | 23/33 (69.7%) | 0.788 |
Of 79 physicians who actively recruit patients, 69 (87%) completed the survey
Study Intervention
Patients in the control group received standard care but they followed the same schedule of visits as the intervention group. Patient in both groups had two baseline visits a week apart and prior to study initiation so as to insure that patient met study criteria. These baseline visits were followed by a study initiation visit in the same month during which patients in both groups received their MEMS devices; this visit consitituted the start of the study. Follow-up visits occured at 1, 3, and 6 months following study initiation, and thereafter every 6 months. Medication changes were not allowed in both groups until the 3-month follow-up visit unless there was intervening severe hypertension. The intervention to improve adherence in the treatment group lasted 6 months and consisted of three main components: 1) counting pills during physician visits; 2) designating a family member to support adherence behavior, and 3) providing an information sheet to patients at the start of the intervention. Patients were supposed to bring back the information sheet at each follow-up visit. If they did not, they were provided a new sheet at the start of the follow-up visit.
The information sheet included information on each BP medication dose and frequency, potential medication side effects, what to do if a dose was missed, what to do when the medication was running low, and how different types of antihypertensive medication could be taken together. It also included two questions for the patient to complete prior to each visit – one question on any problems taking the medication since the last visit and the other question on whether the patient experienced any side effects. Space was provided on the information sheet for patients to record BP readings, including self-measurements, and their current antihypertensive regimen. Patients were encouraged to measure their BP every other week,19 and they were given calendars to mark the day that they took their medication. All intervention physicians underwent an initial 2-hour session on motivational interviewing techniques to promote patient adherence.20 Physicians were advised to avoid confrontation and to respect patients’ autonomy. Case vignettes representing confrontational and motivational interviewing scenarios were used during the training.
Outcome measures
The primary outcome was systolic and diastolic BP control at the end of the first 6 months of follow-up. Medication adherence over 6 months of follow-up was the secondary outcome. As an additional, exploratory outcome we included a composite end point of all cause mortality and admission to a hospital for any cardiovascular event at 5 years of follow-up. The end points were defined identically to those in the VALUE trial.21 The end-points were adjudicated by a clinical events committee which was blinded to the patients’ treatment group.
Three BP readings were obtained at each visit with a semiautomatic oscillometric BP monitor (OMRON 705-CP), which could print the readings. Each reading was printed and attached to the data collection instruments. The average of the three readings was used in the analysis.
Adherence was measured electronically using MEMS bottles (AARDEX Ltd., Zug, Switerland) during the first 6 months of follow-up. Patients were informed of the MEMS funcionality. Because of cost limitations, only one MEMS bottle was provided per patient, and fixed dose combination antihypertensive medications were encouraged when a patient required more than one BP medication. Therefore, when a patient was taking a fixed dose combination antihypertensive medication, that was the one introduced in the MEMS bottle. If the patient was not using combination medications, the drug the patient had been taking for the longest period of time was the one monitored electronically. The average number of antihypertensive drugs used was 2.2 (1.0, standard deviation [SD]) and the average number of drugs monitored electronically was 1.3 (0.4, SD). Accordingly, the average percentage of antihypertensive drugs monitored electronically per patient was 67% of those taken. For the medications monitored, we defined adherence as the percentage of days in which the correct number of doses was taken. Two additional adherence measures were assessed – medication-taking adherence and medication-timing adherence. Medication-taking adherence was defined as the percentage of prescribed doses taken, whereas medication-timing adherence was the percentage of prescribed doses taken on time. We allowed a 12 hour window for medication taken once daily and a 6 hour window for medications taken twice daily.
Statistical Analysis
Sample size estimates accounted for the cluster-randomized design. Due to low initial enrollment, sample size estimates had to be downwardly adjusted from a cluster size of 25 patients per physician to 11 patients per physician. Assuming an intraclass correlation coefficient (ICC) of 0.05 and 12% of patients lost to follow-up, an enrolled sample size of 900 patients (i.e., 450 per arm) equated to an effective sample size of 264 patients per arm. Therefore, with 264 patients per arm and a type I error of 0.05, we estimated 80% power to detect a 3.9 mmHg change in SBP and a 2.7 mmHg change in DBP.
Continuous baseline variables were compared using either a two sample t-test or the Wilcoxon test. Categorical variables were compared using a chi-square test.
Outcomes were analyzed as intention-to-treat. Mean BP was analyzed using generalized estimating equation (GEE) repeated measures ANOVA. The GEE clusters were defined by multiple patients sharing the same physician and the up to three repeated measures (i.e., for the 1, 3, and 6-month visits) for each patient. As the distribution of adherence measures appeared to be exponential, GEE gamma and logistic regression models were used to analyze the continuous and binary measures of medication adherence, respectively. Non-adherence was defined as measured adherence of less than 80%. This cutoff is widely used and has been shown to be associated with hypertensive clinical outcomes.22 The composite cardiovascular outcome was analyzed as time-to-event using Kaplan-Meier estimation and Cox proportional hazards regression models. Physician clusters were accounted for using the COVSANDWICH (AGGREGATE) option in Proc PHREG in SAS v9.1 (SAS Institute Inc, Cary, NC).23 Statistical analyses were performed by an independent group blinded to group assignment. A two-sided alpha level of 0.05 was considered statistically significant.
RESULTS
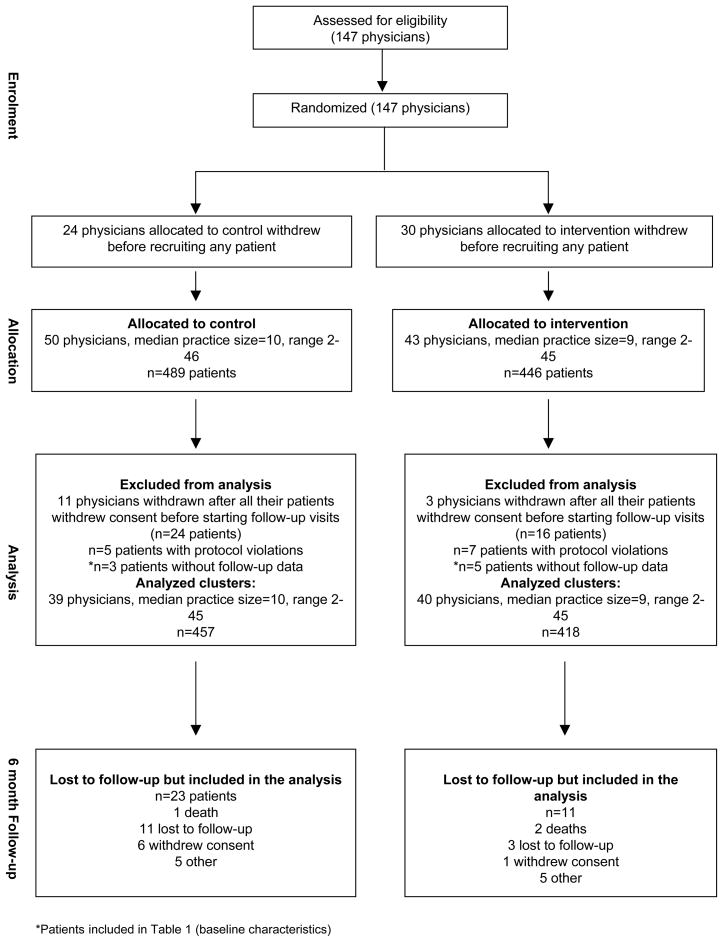
Between January 2000 and July 2002, 147 physicians were recruited and randomized to the control and intervention groups (Figure 1). Fifty-four physicians (24 in the control arm and 30 in the intervention arm) withdrew before recruiting any patients. Fourteen physicians (11 in the control arm and 3 in the intervention arm) withdrew study consent prior to their 40 patients starting follow-up. Twelve patients (5 in the control arm and 7 in the intervention arm) were excluded because of protocol violations. Eight patients (3 in the control arm and 5 in the intervention arm) did not have follow-up data. Therefore, 79 physicians (39 in the control arm and 40 in the intervention arm) and 875 of their patients (457 in the control arm and 418 in the intervention arm) were available for complete analysis. Among those, MEMS data were not available for 78 patients (8.9%) for different reasons (14 devices were lost, 2 patients never used the MEMS, 4 MEMS were defective, 58 MEMS were not returned by the patient or the investigator). There were no statistically significant differences in the distribution of those patients between the intervention and the control group.
Figure 1.
Flow of patients and physicians during recruitment and the first 6 months of follow-up.*Patients also included in table 1 (Baseline characteristics).
Table 1 and Table 2 show the characteristics of physicians and patients, respectively, in the control and intervention groups. All characteristics were similar with the exception that baseline DBP, heart rate, and self-reported medication nonadherence were significantly higher among patients in the intervention group when compared with control group patients.
Table 2.
Distribution of patient related characteristics in control and intervention groups. Numbers are mean ± SD[N], and Ns (%).
| Variable | Control group (N= 460) | Intervention group (N= 423) | P value |
|---|---|---|---|
| Geographical area | 0.943 | ||
| Madrid coordinating center | 154 (33%) | 140 (33%) | |
| Barcelona coordinating center | 306 (67%) | 283 (67%) | |
| Baseline SBP | 160.8 ± 15.0 [460] | 162.1 ± 16.3 [423] | 0.222 |
| Baseline DBP | 86.1 ± 9.8 [460] | 88.6 ± 11.1 [423] | <0.001 |
| Baseline heart rate | 74.3 ± 12.0 [459] | 76.2 ± 11.6 [421] | 0.016 |
| Baseline SBP ≥ 140 | 441/460 (96%) | 398/423 (94%) | 0.228 |
| Baseline DBP ≥ 90 | 146/460 (32%) | 193/423 (46%) | <0.001 |
| Severe hypertension | 90/460 (20%) | 103/423 (24%) | 0.086 |
| Age | 66.8 ± 8.6 [459] | 66.3 ± 8.1 [422] | 0.391 |
| Male gender | 237/460 (52%) | 199/422 (47%) | 0.195 |
| BMI>30 | 221/456 (48%) | 220/422 (52%) | 0.277 |
| Current smoker | 62/458 (14%) | 62/422 (15%) | 0.623 |
| History of smoking | 122/375 (32%) | 104/339 (31%) | 0.553 |
| HT duration (years) | 10.5 ± 8.7 [460] | 11.0 ± 9.3 [420] | 0.454 |
| Number of hypotensive drugs at baseline | 2.24 ± 1.10 [460] | 2.20 ± 1.06 [423] | 0.670 |
| Number of hypotensive drugs grouped | |||
| 0 | 2 (0.4) | 2 (0.5) | |
| 1 | 137 (29.8) | 125 (29.6) | |
| 2 | 147 (32.0) | 138 (32.6) | |
| 3–4 | 164 (35.7) | 150 (35.5) | |
| >4 | 10 (2.2) | 8 (1.9) | 0.998 |
| Number of drugs adherence was monitored with MEMS system | 1.24 ± 0.43 [458] | 1.28 ± 0.45 [420] | 0.196 |
| Patients with combinations of two drugs adherence being monitored with MEMS system | 109/458 (23.8%) | 116/420 (27.6%) | 0.195 |
| Percentage of total number of drugs monitored with MEMS system | 66.2 ± 29.4 [457] | 67.9 ± 28.7 [420] | 0.295 |
| Number of risk factors | 1.9 ± 1.2 [460] | 1.8 ± 1.1 [423] | 0.769 |
| Left ventricular hypertrohpy | 128/460 (28%) | 120/422 (28%) | 0.840 |
| Consumption of alcohol (grs) | 40.4 ± 117.3 [460] | 35.2 ± 86.6 [422] | 0.447 |
| Diabetes | 289/460 (63%) | 266/422 (63%) | 0.949 |
| Severe hypertensive retinopathy (III–IV) | 12/460 (3%) | 11/422 (3%) | 0.998 |
| Peripheral artheriopathy | 33/460 (7%) | 23/422 (5%) | 0.294 |
| Stroke history | 58/460 (13%) | 60/422 (14%) | 0.467 |
| Coronary heart disease | 43/460 (9%) | 43/422 (10%) | 0.674 |
| Congestive heart failure | 20/460 (4%) | 12/422 (3%) | 0.233 |
| Proteinuria | 57/460 (12%) | 46/422 (11%) | 0.491 |
| Abnormal creatinine | 44/460 (10%) | 35/422 (8%) | 0.509 |
| Self-reported nonadherence (Haynes-Sackett test)24 | 142/456 (31%) | 162/414 (39%) | 0.014 |
| History of not attending scheduled visits in the previous 6 months* (self-reported)24 | 123/415 (30%) | 95/383 (25%) | 0.126 |
Patients without visits in the previous 6 months were coded missing.
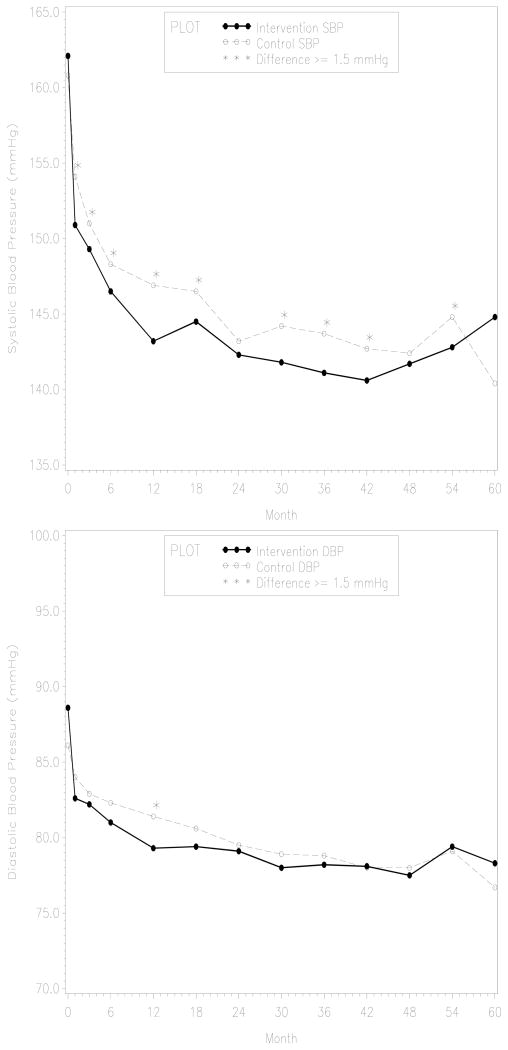
The mean duration of patient follow-up was 39 months, and 67% of the patients had at least 36 months of follow-up. The ICCs for the outcomes of interest were as follows: 0.197 for SBP, 0.194 for DBP, 0.107 for adherence, and 0.063 for combined cardiovascular events. Table 3 shows the study results for BP and adherence outcomes in the first 6 months of follow-up. At six months, intervention patients had significantly lower mean SBP (148.9 mmHg vs. 151.1 mmHg; P=0.008) and lower mean DBP (81.9 mmHg vs. 83.0 mmHg; P=0.013) when compared with control patients. Moreover, intervention patients were less likely to have an uncontrolled SBP (i.e., ≥ 140mmHg) when compared with control patients (OR 0.62; 95% CI 0.50, 0.78). On the other hand, intervention patients were not less likely to have an uncontrolled DBP (i.e., ≥ 90mmHg) when compared with control patients (OR 0.94; 95% CI 0.73, 1.20). Differences around 2mm of Hg in SBP between groups persisted over the 5-years of follow-up, whereas differences in DBP between groups were < 1mmHg after 18 months of follow-up (Figure 2). Only a few of the SBP differences after the 6-month visit were statistically significant.
Table 3.
Comparison of blood pressure and adherence outcomes between the control and the intervention group during the first 6 months of follow-up.
| Repeated BP Measures (1, 3 & 6 Months visits)* | Unadjusted Analysis | Adjusted analysis | ||
|---|---|---|---|---|
| Intervention (n=418) | Control (n=457) | Intervention (n=417) | Control (n=453) | |
| SBP mmHg [mean (SE)] | 149.7 (0.82) | 151.1 (0.70) | 148.9 (0.69)‡ | 151.1 (0.57) |
| Uncontrolled SBP >= 140 mmHg (%) | 65.6‡ | 73.3 | 66.6§ | 76.3 |
| Uncontrolled SBP >= 140 mmHg [OR (95% CI)] | 0.70 (0.56, 0.87) | 1.00 (Ref. group) | 0.62 (0.50, 0.78) | 1.00 (Ref. group) |
| DBP mmHg [mean (SE)] | 82.8 (0.49) | 82.3 (0.42) | 81.9 (0.35)|| | 83.0 (0.30) |
| Uncontrolled DBP >= 90 mmHg (%) | 26.1 | 22.4 | 18.1 | 19.0 |
| Uncontrolled DBP >= 90 mmHg [OR (95% CI)] | 1.22 (0.97, 1.55) | 1.00 (Ref. group) | 0.94 (0.73, 1.20) | 1.00 (Ref. group) |
| 6 Month Cumulative main Adherence Outcomes† | Intervention (n=374) | Control (n=423) | Intervention (n=332) | Control (n=377) |
| Adherence, % days correct dose taken [mean (SE)] | 91.2 (0.11)|| | 88.2 (0.09) | 92.2 (0.09)‡ | 89.0 (0.07) |
| Adherence, % of patients with at least 80% adherence | 88.2 | 83.5 | 91.9‡ | 85.6 |
| Adherence ≥ 80% [OR (95% CI)] | 1.49 (0.87, 2.54) | 1.00 (Ref. group) | 1.91 (1.19, 3.05) | 1.00 (Ref. group) |
Analysis for BP outcomes were adjusted for geographic area (Catalonia versus Madrid), number of risk factors, body mass index, age, and the baseline level of the outcome variable.
Analysis for adherence outcomes were adjusted for baseline diastolic blood pressure, geographic area, self-reported measure of adherence at baseline, self-reported measure of not showing up at the scheduled doctor visits.
P<0.01
P<0.0001
P<0.05
Figure 2.
Systolic and diastolic blood pressure differences between the intervention and the control group during the 60 months follow-up. Asterisks mark the visits in which differences in BP favoring the intervention group were of 1.5 mm of Hg or more.
Patients in the intervention group also appeared to be more adherent over the six months of the intervention, as they took their correct dose on a greater proportion of days when compared with patients in the control group (92.2% vs. 89.0%, respectively; P=0.002) and were more likely to be at least 80% adherent (OR 1.91; 95% CI 1.19, 3.05) (Table 3). For the other adherence measures, intervention patients were also more likely to achieve a value ≥ 80% adherence over the six-month period. Expanded Tables 1, 2, and 3 and additional methods descriptions are available on-line.
During the study it was discovered that the MEMS chips of 45 containers malfunctioned at least once in the study, resulting in inflated measurements of between 4–8 uses per day. The malfunctioning MEMS containers were replaced by the manufacturer who also developed an algorithm to identify and delete invalid readings. Through this algorithm we estimated that approximately 5% of all readings on the defective devices were invalid. As a sensitivity analysis, we repeated the analysis excluding the 45 patients who had defective devices and the results did not change (data not shown).
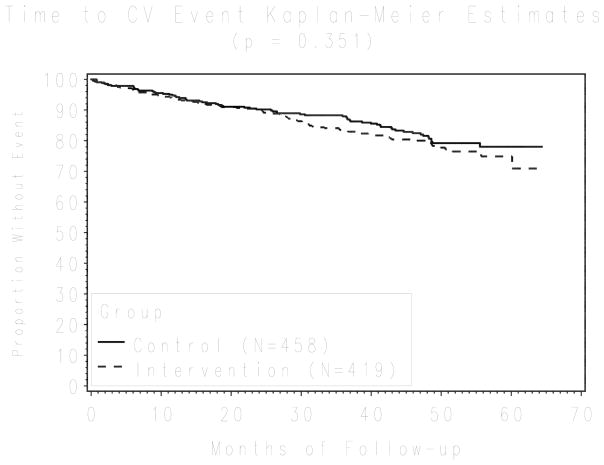
After five years of follow-up, 153 patients had at least one of the composite cardiovascular events - 67 (16%) in the intervention group and 86 (19%) in the control group (Figure 3). Although intervention patients had fewer events when compared with control patients, after adjusting for DBP, age, gender, self-reported measures of adherence, and cardiovascular risk profile, this difference was not statistically significant (hazard ratio 0.97; 95% CI 0.67,1.39).
Figure 3.
Kaplan-Meier survival curve for cardiovascular end-points comparing the intervention and the control groups (log-rank test, P = 0.351). The plot shows unadjusted results. Survival results after adjusting, using a multivariable survival Cox model accounting for physician cluster effects, for diastolic blood pressure, age, gender, self-reported measures of adherence, and cardiovascular risk profile, were not statistically significant either (HR 0.97; 95% CI 0.67, 1.39)
DISCUSSION
This multi-component, low-intensity intervention to improve adherence to antihypertensive medication was effective in improving both BP control and adherence. While the magnitude of the SBP effect appeared small (a difference of 2 mm Hg between groups), one large meta-analysis has suggested that such differences could be associated with a 10% reduction in stroke and a 7% reduction in coronary heart disease mortality.1
The strengths of the study include the cluster-randomized design, which avoided study contamination resulting from individual physicians treating patients in both study arms. We also assessed adherence objectively, used valid methods to measure BP, and had adequate power for both the BP and adherence study outcomes. Moreover, our findings are consistent with the findings of other recent studies. For example, a smaller study by Qureshi et al.25 tested a physician-targeted educational intervention. The intervention consisted of a day of training, which reviewed the seventh report of the Joint National Committee (JNC VII) and the report of the Fourth Working Party of the British Hypertension Society modified for the Indo-Asian population. The intervention was effective at improving patient adherence when compared to the usual care groups (48% vs. 32%, respectively; p<0.05). Adherent patients experienced greater decreases in SBP when compared with non-adherent patients (P<0.05), but the differences were not statistically significant for DBP. Another study by Ogedegbe et al.26 tested an intervention consisting of motivational interviewing sessions every three months (x4) targeting medication adherence behavior among low-income African-American women with uncontrolled BP. In that study, baseline adherence was measured with MEMS during a 3-month run-in period, and patients were followed afterward for an additional 9 months. The intervention was associated with a nearly 20% absolute increase in adherence levels at 12 months, but not with statistically significant changes in BP. Blood pressure decreased in both groups, but the study did not report results on the association between adherence levels and BP control.
The fact that mean adherence was close to 90% in both groups in our study could suggest a Hawthorne effect on both groups, whereby patients knowing that their adherence was being monitored changed their behavior accordingly. Periods longer than 6-months might be required to observe a significant attenuation of the Hawthorne effect.27–29 Although we hypothesized that counting pills could promote a Hawthorne effect in the intervention group, the MEMS bottles may also have resulted in a Hawthorne effect in both study arms, resulting in smaller than expected differences in adherence between groups. On the other hand, high antihypertensive medication adherence rates have also been found in studies measuring adherence with pharmacy refill methods, thus indicating that high levels of adherence might not always be attributable to the Hawthorne effect.30 Regardless of whether there was a Hawthorne effect, we found clinically and statistically significant differences between the intervention and control groups in our study outcomes.
We did not, however, observe a direct association between adherence and blood pressure control (data not shown). Therefore, it is possible that the study intervention improved BP control through mechanisms other than improved adherence. For example, the intervention may have helped overcome clinical inertia (i.e., clinicians’ reluctance to intensify therapy), resulting in better overall BP control.31 However, changes in BP medication were not allowed by protocol until the third month of follow-up, suggesting that BP improvements were not the result of overcoming clinical inertia. Although other factors may account for the BP improvement, we were likely underpowered to detect the association between adherence and BP control due to the overall high levels of adherence in both groups with limited variability.15;32;33 In addition, approximately 37% of the patients were using at least one antihypertensive medication for which adherence was not monitored electronically. Perhaps with the additional assessment of adherence for these medications we would have seen a stronger association between adherence and blood pressure control.
This study has additional limitations. As mentioned previously, we had a problem with some of the MEMS containers resulting in invalid readings. However, including or excluding data from the affected participants did not substantively influence our findings. Next, although we instructed physicians to keep a log of how many patients were offered enrollment in the study, many physicians did not complete these logs. Therefore, we are unable to comment on how our study population differed from the larger, eligible patient population. A similar situation occurs with the physicians. An important proportion withdrew without recruiting patients and no information is available on them. Thus, we have a limitation to evaluate the generalizability of our results to the larger population of both patients and physicians. On the other hand, patients included met the eligibility criteria of high cardiovascular risk and complete clinical information was available at baseline. Information on participating physicians was also available from the physicians survey. Finally, adherence is not the only reason explaining poor rates of BP control. As mentioned above, clinician factors, such as clinical inertia, may also play an important role in the unsatisfactory rates of BP control.31 Therefore, future studies should assess and address both patient adherence and the need for a provider intensification of antihypertensive medication simultaneously. Future studies will also be needed to elucidate the impact of this type of intervention on cardiovascular morbidity and mortality, as this study was underpowered for those outcomes.
In summary, we conclude that a multi-factorial intervention to improve adherence to antihypertensive medication was effective in improving both adherence and BP control. While the effect size was small, we did observe very high levels of adherence in both arms. This suggests that in addition to the intervention described, other factors, such as the Hawthorne effect may have contributed to these results. The relative importance of various components of this intervention, including a potential Hawthorne effect, in improving adherence and BP control has yet to be determined.
Supplementary Material
Acknowledgments
Fondo de Investigación Sanitaria (FIS00/0045-01 and FIS00/0045-02) y Fondos Europeos de Desarrollo Regional; Catalan Agency for Health Technology Assessment and Research. The following drug companies funded the study but were not involved in the design, analysis, and manuscript preparation: Novartis, Almirall Prodesfarma, and Aventis. Drs Pladevall and Williams were funded in part through the Fund for Henry Ford Hospital, and grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK64695) and the National Heart Lung and Blood Institute (R01HL079055), NIH.
We tank Dr R Brian Haynes from McMaster University for his advice and suggestions during the design phase of the trial.
Footnotes
Writing committee on behalf of the COM99 Study Group. See appendix available on line for a complete list of investigators.
Conflicy of Interest Disclosures: None.
Reference List
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High Blood Pressure and Cardiovascular Disease Mortality Risk Among U.S. Adults: The Third National Health and Nutrition Examination Survey Mortality Follow-up Study. Annals of Epidemiology. 2008;18:302–309. doi: 10.1016/j.annepidem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, Poulter N, Primatesta P, Stegmayr B, Thamm M. Hypertension Treatment and Control in Five European Countries, Canada, and the United States. Hypertension. 2004;43:10–17. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- 4.Jones DW, Hall JE. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and Evidence From New Hypertension Trials. Hypertension. 2004;43:1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- 5.Wong ND, Lopez VA, L’italien G, Chen R, Kline SE, Franklin SS. Inadequate Control of Hypertension in US Adults With Cardiovascular Disease Comorbidities in 2003–2004. Arch Intern Med. 2007;167:2431–2436. doi: 10.1001/archinte.167.22.2431. [DOI] [PubMed] [Google Scholar]
- 6.Barrios V, Banegas JR, Ruilope LM, Rodicio JL. Evolution of blood pressure control in Spain. J Hypertens. 2007;25:1975–1977. doi: 10.1097/HJH.0b013e32829fb3ec. [DOI] [PubMed] [Google Scholar]
- 7.Sabaté E, World Health Organization. Evidence for action. Geneva: World Health Organization; [Accessibility verified April 29, 2008]. 2003. Adherence to long-term therapies. Available at http://whqlibdoc.who.int/publications/2003/9241545992.pdf. [Google Scholar]
- 8.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 9.National Council on Patient Information and Education. Enhancing Prescription Medicine Adherence: A National Action Plan. National Council on Patient Information and Education; [Accessibility verified November 23, 2008]. 2007. Available at http://www.talkaboutrx.org/documents/enhancing_prescription_medicine_adherence.pdf. [Google Scholar]
- 10.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Elston Lafata J. Clinical Outcomes and Adherence to Medications Measured by Claims Data in Patients With Diabetes. Diabetes Care. 2004;27:2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung V, Huang J, Brand R, Newhouse JP, Hsu J. Hypertension treatment in a medicare population: adherence and systolic blood pressure control. Clin Ther. 2007;29:972–984. doi: 10.1016/j.clinthera.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Muszbek N, Brixner D, Benedict A, Keskinaslan A, Khan ZM. The economic consequences of noncompliance in cardiovascular disease and related conditions: a literature review. Int J Clin Pract. 2008;62:338–351. doi: 10.1111/j.1742-1241.2007.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008 April 16;:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004;164:722–732. doi: 10.1001/archinte.164.7.722. [DOI] [PubMed] [Google Scholar]
- 15.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol. 2001;54:217–224. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 17.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, Yusuf S, Michelson EL, Pfeffer MA. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 18.1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. 1999;17:151–83. [PubMed] [Google Scholar]
- 19.Marquez-Contreras E, Martell-Claros N, Gil-Guillen V, de la Figuera-Von Wichmann, Casado-Martinez JJ, Martin-de Pablos JL, Figueras M, Galera J, Serra A. Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM-HTA study. J Hypertens. 2006;24:169–175. doi: 10.1097/01.hjh.0000198023.53859.a2. [DOI] [PubMed] [Google Scholar]
- 20.Resnicow K, Dilorio C, Soet J, et al. Motivational Interviewing in medical and public health settings. In: Miller WR, Rollnick S, editors. Motivational interviewing: preparing people for change. New York: Guilford Press; 2002. pp. 251–269. [Google Scholar]
- 21.Mann J, Julius S. The Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial of cardiovascular events in hypertension. Rationale and design. Blood Press. 1998;7:176–83. doi: 10.1080/080370598437394. [DOI] [PubMed] [Google Scholar]
- 22.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of Medication Nonadherence on Hospitalization and Mortality Among Patients With Diabetes Mellitus. Arch Intern Med. 2006;166:1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 23.SAS Institute Inc. SAS/STAT Users Guide. Cary, NC: SAS Institute Inc; 2004. Version 9.1 ed. [Google Scholar]
- 24.Stephenson BJ, Rowe BH, Haynes RB, Macharia WM, Leon G. Is this patient taking the treatment as prescribed? JAMA. 1993;269:2779–81. [PubMed] [Google Scholar]
- 25.Qureshi NN, Hatcher J, Chaturvedi N, Jafar TH. Effect of general practitioner education on adherence to antihypertensive drugs: cluster randomised controlled trial. BMJ. 2007;335:1030. doi: 10.1136/bmj.39360.617986.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogedegbe G, Chaplin W, Schoenthaler A, Statman D, Berger D, Richardson T, Phillips E, Spencer J, Allegrante JP. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21:1137–1143. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wetzels GE, Nelemans PJ, Schouten JS, Dirksen CD, van der WT, Stoffers HE, Janknegt R, de Leeuw PW, Prins MH. Electronic monitoring of adherence as a tool to improve blood pressure control. A randomized controlled trial. Am J Hypertens. 2007;20:119–125. doi: 10.1016/j.amjhyper.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Wetzels GE, Nelemans P, Schouten JS, Prins MH. Facts and fiction of poor compliance as a cause of inadequate blood pressure control: a systematic review. J Hypertens. 2004;22:1849–1855. doi: 10.1097/00004872-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Wetzels GE, Nelemans PJ, Schouten JS, Van Wijk BL, Prins MH. All that glisters is not gold: a comparison of electronic monitoring versus filled prescriptions--an observational study. BMC Health Serv Res. 2006;6:8. doi: 10.1186/1472-6963-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120:26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Heisler M, Hogan MM, Hofer TP, Schmittdiel JA, Pladevall M, Kerr EA. When More Is Not Better: Treatment Intensification Among Hypertensive Patients With Poor Medication Adherence. Circulation. 2008;117:2884–2892. doi: 10.1161/CIRCULATIONAHA.107.724104. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder K, Fahey T, Hay AD, Montgomery A, Peters TJ. Relationship between medication adherence and blood pressure in primary care: prospective study. J Hum Hypertens. 2006;20:625–627. doi: 10.1038/sj.jhh.1002011. [DOI] [PubMed] [Google Scholar]
- 33.Mant J, McManus RJ. Does it matter whether patients take their antihypertensive medication as prescribed? The complex relationship between adherence and blood pressure control. J Hum Hypertens. 2006;20:551–553. doi: 10.1038/sj.jhh.1002046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.