Abstract
Oncogenic BRAF is a critical driver of proliferation and survival and is thus a validated therapeutic target in cancer. We have developed a potent inhibitor, termed 1t (CCT239065), of the mutant protein kinase, V600EBRAF. 1t inhibits signaling downstream of V600EBRAF in cancer cells, blocking DNA synthesis, and inhibiting proliferation. Importantly, we show that 1t is considerably more selective for mutated BRAF cancer cell lines compared to wildtype BRAF lines. The inhibitor is well tolerated in mice and exhibits excellent oral bioavailability (F=71%). Suppression of V600EBRAF-mediated signaling in human tumor xenografts was observed following oral administration of a single dose of 1t. As expected, the growth rate in vivo of a wildtype BRAF human tumor xenograft model is unaffected by inhibitor 1t. In contrast 1t elicits significant therapeutic responses in mutant BRAF driven human melanoma xenografts.
Keywords: BRAF, kinase inhibitor, melanoma, colorectal carcinoma
Introduction
The RAS–ERK signaling pathway regulates several cell functions, including differentiation, senescence, proliferation and survival (1). In normal cells this pathway is activated by receptor tyrosine kinases, and by hormone and cytokine receptors. However, in approximately 30% of human cancers, the pathway is constitutively activated because its components are either over-expressed or have acquired gain-of-function mutations. One constituent that is mutated in approximately 7-8% of human cancers is BRAF (2), with mutations in this serine/threonine specific protein kinase being particularly common in melanoma (~45%), and thyroid (~40%), ovarian (~10%) and colorectal cancers (~15%) (www.sanger.ac.uk/genetics/CGP/cosmic/). BRAF, together with its close relatives ARAF and CRAF, is responsible for coupling signaling from the small G-protein RAS to the dual specificity kinase MEK, which in turn activates ERK, the third kinase in this cascade. ERK regulates the activity of many cellular proteins to control the cells' biological behavior. However, when BRAF is mutated, the pathway is constitutively activated in a RAS-independent manner (1).
Over 100 different mutations have been described in BRAF in human cancer, but a glutamic acid for valine substitution at position 600 (V600E) is the most common and accounts for over 90% of the mutations that occur in cancer. V600EBRAF can induce transformation of mammalian cells, allowing them to grow in a growth factor-independent manner in vitro and as tumors in nude mice (2, 3). Importantly, inhibition of V600EBRAF signaling blocks ERK activity and proliferation in vitro, and in vivo it blocks the growth of tumor xenografts in nude mice (4, 5). These data validate V600EBRAF as an important therapeutic target in melanoma and the other cancers in which BRAF is mutated. Consequently, a number of drug discovery programs have been initiated to develop inhibitors of this mutant protein kinase.
Initial attempts to target V600EBRAF in melanoma proved disappointing, because although the multi-kinase inhibitor sorafenib was shown to inhibit V600EBRAF signaling in vitro, it failed to yield significant responses in patients in phase I/II clinical trials (4-8). However, sorafenib is approximately 100-fold less active against V600EBRAF in cells (2-5 μM) than it is against the purified kinase (40 nM) in vitro (5). Furthermore, sorafenib has been approved for use in renal and hepatocellular carcinomas (9, 10), where its clinical activity is attributed to its anti-angiogenic effects, thought to be mediated through inhibition of the receptor tyrosine kinases VEGFR2 and PDGFR (7). Indeed, there is a paucity of evidence to show that sorafenib selectively targets oncogenic BRAF in clinical samples. Together these data suggest that sorafenib does not target oncogenic BRAF in human cancer and so there is a pressing need to develop more potent and selective cellular inhibitors of oncogenic BRAF to enable rigorous assessment of the consequences of BRAF inhibition in tumor xenografts and ultimately in patients.
An inhibitor of V600EBRAF, SB590885, was described as a potent type I (active conformation binder) inhibitor of purified V600EBRAF in vitro and to have excellent cellular activity but poor pharmacokinetic/pharmacodynamic (PK/PD) characteristics (11). Other inhibitors include, RAF265, a pan RAF inhibitor which is in phase I/II clinical trials (www.clinicaltrials.gov) and PLX4720, a potent and selective type I inhibitor of mutant BRAF-driven cell proliferation in vitro and of melanoma xenograft growth in mice (12). Its close analogue, PLX4032, is currently in phase II/III clinical trials following promising phase I results (13).
Here we describe and characterize a new pyridopyrazinone V600EBRAF inhibitor, called 1t (CCT239065). This compound is a type II inhibitor (inactive conformation binder) and we describe its activity in vitro and in vivo and demonstrate its potential for development as a therapeutic inhibitor that targets oncogenic BRAF.
Materials and methods
Cell culture
WM266.4, SW620, A375M and Ba/F3 cell lines were obtained from ATCC/LGC standards (Teddington, UK) and D35 cells were a kind gift from Dr Nick Hayward (Queensland Institute of Medical Research, Australia). All lines were re-authenticated by short tandem repeat (STR) and array comparative genomic hybridization (aCGH) analysis within the six months prior to submission of the manuscript. The cells were cultured in RPMI1640 (Ba/F3) or DMEM (WM266.4, SW620, A375M, D35) supplemented with 10% FBS (Invitrogen, Paisley, UK) at 37 °C in 10% CO2. The BRAF and RAS mutation status of the cell lines was determined (Supplemental Table 1). Inhibitor 1t (CCT239065) was synthesized as described (14). Drugs were dissolved in DMSO at 10 mM and diluted as required.
Molecular modeling
Inhibitor 1t was docked into BRAF (PDB code: UWH) using GOLD version 3.1.1 (15). In order to prepare the receptor for docking, the crystal structure was protonated using the Protonate3D tool of MOE (Chemical Computing Group, Cambridge, UK), and the ligand and water molecules were then removed. The active site was defined using a radius of 10 Å from the backbone oxygen atom of Asp594 of the ATP binding pocket. Partial charges of the ligand were derived using the Charge-2 CORINA 3D package in TSAR 3.3, and their geometries optimized using the COSMIC module of TSAR. Ten docking solutions were generated per docking run with GOLD, and the best three stored for analysis.
Cell lysis and Western blotting
Cells lysates were prepared as described (16) for Western blotting using standard approaches and quantification using an Odyssey infrared scanner (Li-Cor Biosciences, Cambridge, UK). The following primary antibodies were used: phospho-MEK1/2, PKB/AKTα (Cell Signalling Technologies, Hitchin, UK), MEK1 (BD Biosciences, Oxford, UK), phospho-ERK1/2, (Sigma, Poole, UK), Cyclin D1 and ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies were goat anti-mouse Alexa Fluor 680 (Invitrogen, Paisley, UK) and goat anti-rabbit 800CW (Li-Cor Biosciences, Cambridge, UK).
Phospho-ERK cell-based ELISA
WM266.4 cells were seeded at 3×104 per well of a 96 well plate, treated with an 11-point titration of compound after 24 h and after a further 6 h fixed in 4% formaldehyde, 0.1% triton in PBS. Non-specific sites were blocked with 5% milk/PBS and incubated with an anti-phospho-ERK antibody (Sigma, Poole, UK) for 2 h, washed with 0.1% Tween 20 and incubated with an anti-mouse-Europium conjugated antibody for 1 h. Time-resolved fluorescence was measured in the presence of enhancement solution (Perkin Elmer, Amersham, UK) using a Spectramax M5 plate reader (Molecular Devices, Wokingham, UK). Fluorescence values were normalised to protein concentration as determined by the BCA assay (Sigma, Poole, UK). IC50 values for ERK inhibition were determined with GraphPad Prism software (GraphPad Software, San Diego, CA) and are the mean of 3 independent assays.
Kinase assays
V600EBRAF protein was expressed, purified and kinase activity measured as described using 96 well-format assays and DELFIA detection (17). This assay measures the direct phosphorylation of bacterially produced GST-MEK by BRAF at an ATP concentration of 100 μM. Duplicate assays were performed within the linear range of the assay (45 min at 20°C), with an 11-concentration response curve to generate IC50 values using GraphPad Prism software. Each IC50 value was derived from the mean of 3 independent assays. Profiling of 1t against selected kinases using SelectScreen Panel technology was performed according to the commercial provider's protocols (Invitrogen, Paisley, UK).
Proliferation assays
The growth inhibitory activity of 1t in a panel of melanoma, colon and breast cancer cell lines was determined using sulforhodamine B (SRB) reagent following a 5 d exposure to the compound (18). Cell proliferation was also assessed using the MTS reagent (Promega, Southampton, UK). Assays were performed in quadruplicate with 10-point dilution series and IC50 values were calculated using GraphPad Prism software (GraphPad Software, San Diego, CA). The number of cells seeded was optimized for each cell line to ensure logarithmic growth could occur over the duration of treatment. DNA synthesis was assessed by measuring tritium-labelled thymidine incorporation. 1-5 × 104 Ba/F3 cells were seeded into the wells of 96-well plates and compounds were added to the desired concentration. After 20 h, 0.08 μCi of [3H]-thymidine (GE Healthcare, Little Chalfont, UK) was added to each well and after a further 4 h the cells were captured onto Multiscreen glass fibre 96 well plates (Millipore, Watford, UK), washed twice with PBS and twice with methanol using a vacuum manifold. 25 μl of Microscint 20 (Perkin-Elmer, Amersham, UK) was added to the wells prior to counting on a TopCount NXT (Perkin-Elmer, Amersham, UK). For adherent cells, 105 cells were seeded into 6 well plates and 0.8 μCi [3H]-thymine added per well. Cells were harvested by trypsinization and an aliquot analyzed as above.
Pharmacokinetics (PK)
All procedures involving animals were performed in accordance with national Home Office regulations under the Animals (Scientific Procedures) Act 1986 and within guidelines set out by the Institute's Animal Ethics Committee and the UK Coordinating Committee for Cancer Research Committee on the Welfare of Animals in Experimental Neoplasia (19).
PK analyses were performed in female BALB/cAnNCrl mice > 6 weeks old, dosed intravenously (i.v. 2 mg/kg, 10 ml/kg, in DMSO:Tween 20:water 10:1:89 v/v) or orally (p.o.) by gavage (10 mg/kg, 10 ml/kg, in DMSO:water 1:19 v/v). At intervals of 5 (i.v. route only), 15, 30 min, 1, 3, 6 and 18 h after dosing, 3 mice were placed under isoflurane anaesthesia and blood for plasma preparation was taken into heparinized syringes. Femoral muscle was also taken following i.v. and p.o. administration. Plasma and tissue storage, extractions and analysis were performed as described (20, 21).
Therapy studies
Tolerability studies were performed by dosing mice with 10 or 20 mg/kg 1t p.o. daily for 4 d and monitoring body weight for a further 27 d. Female Crl:CD1-Foxn1nu mice > 6 weeks old were inoculated subcutaneously (s.c.) with a suspension of human tumor cell lines. For a p.o. therapy, after inoculation of either 107 A375M human melanoma cells or 7 × 106 SW620 human colorectal carcinoma cancer cells, the xenografts were allowed to grow to 50–150 mm3. Groups of 8 mice were then allocated to treatments using stratified distribution of tumor volumes. Inhibitor 1t (20 mg/kg/d, 10 ml/kg, in DMSO:water 1:19 v:v) or control vehicle was given by gavage. Tumors were measured with calipers at least twice per week.
Pharmacodynamics (PD)
Mice bearing established, A375M or SW620 xenografts were prepared as for the therapy studies above. For WM266.4 tumors, 8 × 106 cells were inoculated. 3-4 animals were dosed p.o. by gavage with 1t (20 mg/kg) and 3-4 with control vehicle. After 1 dose, mice were culled by cervical dislocation 4 h post dosing. Tumors were halved and snap-frozen using liquid nitrogen. Control mice were processed similarly approximately 4 h after dosing. Tumors were lyzed in NP40 buffer and homogenised using a Precellys 24 (Stretton Scientific, Stretton, UK). Equal amounts of protein were analyzed by quantitative Western blotting as described above.
Results
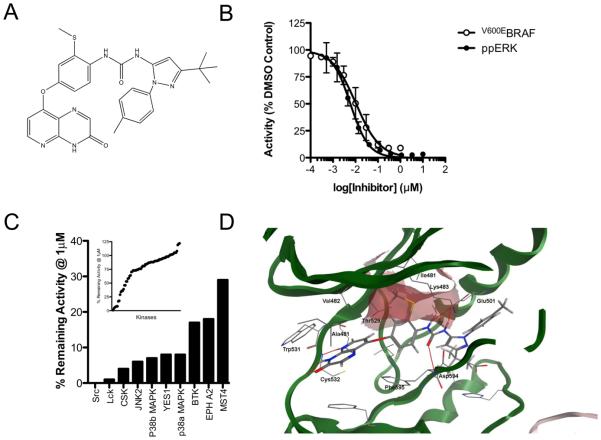
We have developed a series of novel BRAF inhibitors (14, 22-25). One such compound called CCT239065 (henceforth referred to as 1t) with the formula 1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(2-(methylthio)-4-(3-oxo-3,4-dihydropyrido[2,3-b]pyrazin-8-yloxy)phenyl)urea]; Fig 1A) potently inhibits the kinase activity of recombinant, full-length V600EBRAF in vitro with an IC50 of 0.019 ± 0.004 μM (Fig 1B, Table 1). To demonstrate that 1t is active against oncogenic BRAF in cells, we show that it inhibits ERK1/2 phosphorylation at 0.005 ± 0.002 μM in WM266.4 cells (Fig 1B), a melanoma line in which we previously established this pathway to be driven by oncogenic V600DBRAF (5). We also show that 1t achieves high levels of selectivity in vitro and at 1 μM, a concentration that is approximately 50 times higher than its IC50 value against purified V600EBRAF, it failed to inhibit most of the kinases in an 80 kinase panel that represents all branches of the human kinome (Fig 1C; Supplemental Table 2). Profiling of 1t against 16 kinases in the SelectScreen Panel (Invitrogen) demonstrated that the most sensitive kinases are LCK (IC50 6 nM), CRAF (IC50 12 nM) V600EBRAF (13 nM) and SRC (23 nM), but importantly 1t is more than 6 fold less active against wildtype BRAF (81 nM) and more than 50 fold less active against VEGFR2/KDR than against V600EBRAF (Table 1).
Figure 1. Compound 1t is a potent and selective inhibitor of oncogenic BRAF.
(A) Chemical structure of 1t/CCT239065 1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(2-(methylthio)-4-(3-oxo-3,4-dihydropyrido[2,3-b]pyrazin-8-yloxy)phenyl)urea (B) Inhibition of recombinant full-length V600EBRAF in vitro by 1t (○) and inhibition of ERK (measured by activation segment phosphorylation) in WM266.4 (V600DBRAF) melanoma cells following a 6 h exposure to increasing concentrations of 1t (●). (C) Selectivity profile of 1 μM 1t tested against a panel of 80 kinases (inset). Kinases inhibited by >70% are presented in the main histogram. (D) Docking of 1t into the co-crystal structure of BRAF and sorafenib (pdb code UWH). The BPI pocket formed by residues Val471, Ala481, Lys483 and Ile527 is indicated by the red surface.
Table 1.
In vitro kinase selectivity of 1t. The potency of 1t was assessed by performing a 10-point titration of the compound in the presence of 100 μM ATP and each individual kinase using the Z′-LYTE system (Invitrogen, Paisley, UK). Note when analyzed using our in-house DELFIA assay format, we observe an IC5 for V600EBRAF of 0.019 μM.
| Kinase | IC50 (μM) |
|---|---|
| V600EBRAF | 0.013 |
| BRAF | 0.081 |
| CRAF | 0.012 |
| LCK | 0.006 |
| SRC | 0.023 |
| p38α | 0.285 |
| p38γ | 1.02 |
| PDGFRα | 0.439 |
| PDGFRβ | 2.43 |
| VEGFR1 | 5.16 |
| VEGFR2 | 1 |
| FGFR1 | 2.58 |
| RET | 4.86 |
| KIT | >10 |
| COT | >10 |
| MET | >10 |
We have shown that close analogs of compound 1t are type II inhibitors and so bind to the inactive conformation of BRAF (24, 25). Docking studies suggest that 1t also binds to the inactive conformation of BRAF, with the pyridopyrazin-3(4H)-one moiety forming two hydrogen bonds with the backbone of Cys532 of the hinge region (Fig 1D). Three more H bonds are predicted to be formed by the urea moiety of the inhibitor, two between the NH groups and the Glu501 side chain and one between the carbonyl moiety and the backbone of Asp594 of the DFG motif. The tert-butyl pyrazole of the terminal pyrazole ring of 1t resides in a kinase pocket beyond the gatekeeper residue, termed the BPIII pocket by Liao (26). Importantly, the thiomethyl group of the middle aromatic ring elaborates into the BPI pocket (26) and forms Van der Waals contact with the aliphatic side chains of Ile527, Val471, Lys483, Ala481 and Thr529. We previously reported how elaboration into the BPI pocket improves the selectivity of BRAF inhibitors (22, 24), so the thiomethyl group is likely to contribute to both potency and selectivity of 1t.
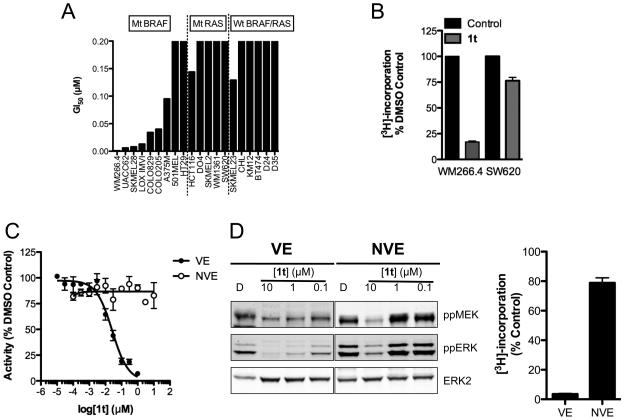
In accordance with its in vitro selectivity, 1t inhibits the growth of cancer cell lines harboring V600D/EBRAF mutations, but is relatively ineffective in cell lines in which BRAF is wildtype (Fig 2A). Concordant with this, 1t induces a profound inhibition of DNA synthesis in mutant V600DBRAF cells but not in mutant KRAS cells (Fig 2B). To characterize further the BRAF-selective activity of 1t, we generated a mutant of V600EBRAF in which the gatekeeper threonine at position 529 is mutated to asparagine (T529N,V600EBRAF). This mutant is resistant to a panel of RAF inhibitors due to steric hindrance within the ATP-binding pocket (27) and we confirm that it is resistant to 1t in vitro and is not inhibited by this compound at up to 10 μM (Fig 2C). Ba/F3 cells normally grow in an IL-3-dependent manner, but their growth can be rendered IL-3-independent by enforced expression of V600EBRAF or T529N,V600EBRAF (27). Notably, ERK phosphorylation is considerably more sensitive to 1t in V600EBRAF-expressing Ba/F3 cells than in the T529N,V600EBRAF-expressing Ba/F3 cells (Fig 2D) and this is reflected in their growth, with theV600EBRAF-expressing Ba/F3 cells being inhibited by 96% following a 24 h treatment with 1 μM 1t compared to only 21% in the T529N,V600EBRAF-expressing cells (Fig 2D).
Figure 2. Antiproliferative activity of 1t is associated with the presence of mutant BRAF.
(A) Antiproliferative effect of 1t following a 5-d exposure across a panel of human melanoma, colon and breast cancer cell lines, determined by SRB assay. (B) WM266.4 (V600DBRAF) and SW620 (G12VKRAS) cells were treated with 0.1 μM of 1t for 24 h and DNA synthesis determined using [3H]-thymidine. (C) Kinase activity of recombinant V600EBRAF (VE) and T529N,V600EBRAF (NVE) in the presence of 1t measured using DELFIA assay. (D) Inhibition of BRAF-mediated MEK/ERK activation in Ba/F3 cells expressing V600EBRAF or T529N,V600EBRAF following a 2 h exposure. DNA synthesis in Ba/F3 cells driven by V600EBRAF (VE) or T529N,V600EBRAF (NVE) following a 24 h exposure to 1t.
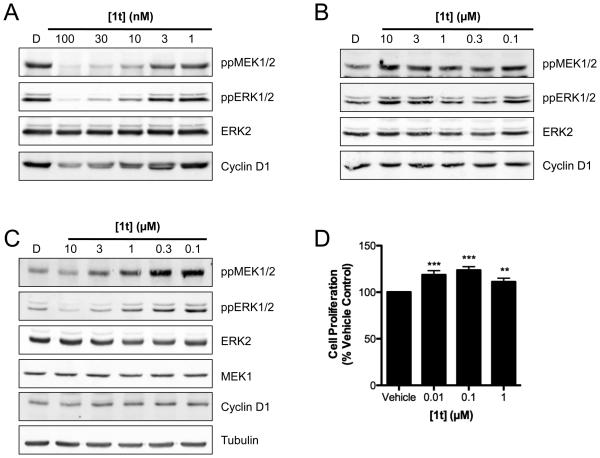
To demonstrate its selectivity further, we show that after 24 h, 1t potently inhibits MEK and ERK phosphorylation in V600DBRAF WM266.4 cells (10-100 nM) and this is accompanied by decreased expression of cyclin D1 (Fig 3A), the transcription of which is regulated by the MAPK pathway (28). In contrast, no such responses are observed in BRAF wild-type D35 melanoma cells at concentrations up to 10 μM (Fig 3B). Furthermore, in KRAS mutant SW620 colorectal carcinoma cells, 1t induces a profound increase in MEK and ERK phosphorylation and this is accompanied by increased cyclin D1 expression (Fig 3C). We attribute this effect to the transactivation of CRAF by BRAF through a mechanism involving RAS-dependent BRAF:CRAF hetero-dimerization, which promotes activation of the downstream signaling cascade as we and others recently reported (29, 30). Notably, the increase in pathway activation is accompanied by a small increase in proliferation driven by 1t in SW620 cells (Fig 3D).
Figure 3. 1t blocks MAPK signaling in BRAF mutant cells.
V600DBRAF WM266.4 cells (A), wildtype BRAF/RAS D35 cells (B) and BRAF wildtype, G12VKRAS mutant SW620 cells (C) were treated with the indicated concentrations of 1t for 24 h and cell lysates analysed by Western blotting. (D) SW620 cells were treated with the indicated concentrations of 1t for 72 h and cell proliferation quantified using MTS assay. *** p<0.0001, ** p<0.005, t-test.
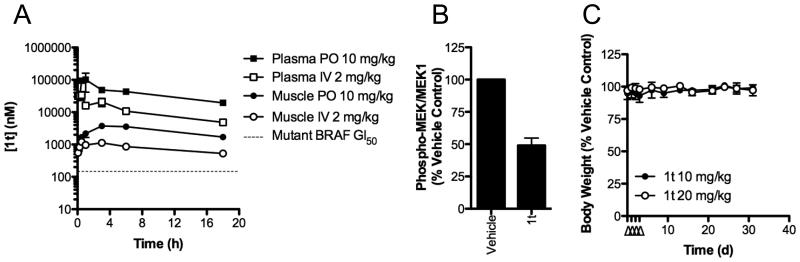
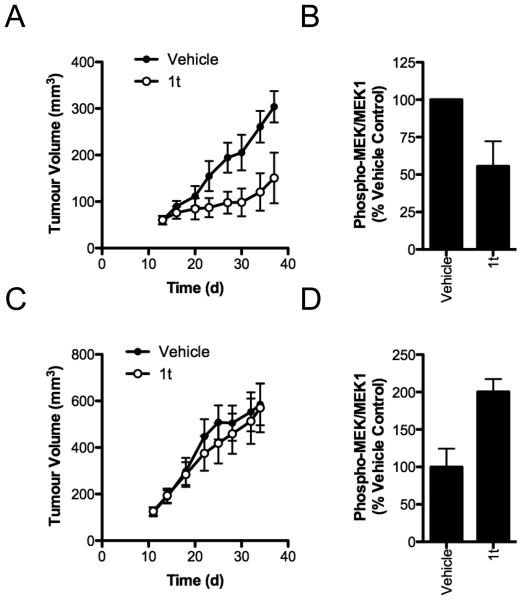
We next examined the efficacy of 1t in vivo. When administered by i.v. injection, 1t shows a very low plasma clearance (0.4 ml/h) consistent with the absence of metabolism and a terminal half-life of 6.8 h (Fig 4A; Supplemental Table 3). Plasma concentrations of 1t achieve over 100-fold greater than the average GI50 value we observe for BRAF mutant cancer cell lines in vitro (Fig 4A, Supplemental Table 3) and are sustained above the average GI50 in plasma and muscle (used as a tumor tissue surrogate) for over 18 h. 1t has excellent oral bioavailability of 71% and a single oral dose of 10 mg/kg maintained plasma and muscle concentrations above 19 and 3 μM respectively for at least 18 h (Fig 4A, Supplemental Table 3). Given these excellent PK properties, we assessed 1t for biomarker modulation in vivo to demonstrate on-target activity of the compound. A single p.o. dose of 20 mg/kg suppresses the phosphorylation of MEK by over 50% in mutant BRAF human WM266.4 melanoma xenografts, relative to vehicle treated mice (Fig 4B). We therefore determined the tolerability of 1t following multiple oral dosing of 10 and 20 mg/kg/d in mice for 4 d and measured the effect on body weight (Fig 4C). No adverse effects were observed. The growth of established V600EBRAF A375M melanoma xenografts is reduced by p.o. administration of 1t (20 mg/kg q.d.) for 24 d, with a significant growth inhibition of 50% (p<0.05) on completion of the experiment (Fig 5A). Inhibition of MEK phosphorylation following a single dose of 1t is also observed in this tumor model (Fig 5B). To demonstrate the dependency upon BRAF inhibition for anti-tumor efficacy of 1t, we also treated mice bearing the G12VKRAS mutant human colorectal carcinoma SW620 xenografts for 23 d. No inhibition of tumor growth is observed in this model, consistent with the in vitro data for this cell line (Fig 5C). Curiously, we also do not see enhanced tumor growth in this model, despite the increase in MEK phosphorylation induced in these tumors (Fig 5D). Importantly, 1t is well tolerated as judged by the observation that the continuous daily dosing used in these therapy experiments does not cause any deaths and causes less than 10% body weight loss over the course of the treatment (Supplemental Figure 1).
Figure 4. 1t is well tolerated and displays excellent PK/PD characteristics.
(A) PK profiles of 1t administered by i.v. injection or p.o. gavage to mice. The dotted line represents the average GI50 value for BRAF mutant tumors (Fig 2A). (B) Mice bearing WM266.4 xenografts received a single p.o. dose of vehicle or 1t (20 mg/kg) and tumors were harvested at the indicated times. Tumor lysates were analyzed by quantitative fluorescent Western blotting for MEK phosphorylation and total MEK1. The level of MEK phosphorylation was normalized to the amount of detectable MEK1. (C) Mice were treated with 10 or 20 mg/kg/d 1t p.o. for 4 d (indicated by Δ) and body weight was monitored for a further 27 d. Mean body weight as a percentage of vehicle-treated animals is plotted ± SEM (n=2 mice per group).
Figure 5. 1t selectively suppresses the growth of BRAF mutant tumors in vivo.
(A) Mice bearing established A375M xenografts were treated with 1t p.o. with daily dosing (20 mg/kg) for 24 d and tumor size was monitored every 3-4 d. Mean tumor volume is plotted ± SEM (n=8 mice per group). (B) A375M tumors were established as above and mice were treated with a single oral dose of 20 mg/kg 1t. After 4 h, tumors were harvested and lysates were analyzed by quantitative fluorescent Western blotting for MEK1/2 phosphorylation and total MEK1. (C) Mice bearing SW620 xenografts were treated as in (A) for 23 d and tumor size was monitored every 3-4 d. (D) Mice bearing SW620 tumors received a single p.o. dose of vehicle or 1t (20 mg/kg) and tumors were harvested 4 h post-dosing. Tumor lysates were analysed for MEK phosphorylation as in (B).
Discussion
Herein we describe the activity of a novel highly selective small molecule inhibitor of oncogenic BRAF. In vitro, this compound does not inhibit the majority of kinases in a panel of 80 receptor and non-receptor kinases and selectively inhibits the proliferation of cancer cell lines harboring oncogenic mutations in BRAF. In-silico docking shows that the thiomethyl group on the central ring of 1t (CCT239065) extends into the BPI cavity of BRAF and may therefore contribute to 1t selectivity. We previously demonstrated that oncogenic RAS signals exclusively through CRAF and does not require BRAF for ERK activation (31) and notably, 1t is also relatively ineffective against cancer lines harboring mutations in RAS genes, as observed for other selective BRAF inhibitors (11, 12). Interestingly, given the equipotent activity of 1t against V600EBRAF and CRAF in vitro, it is surprising that CRAF inhibition is not achieved in RAS mutant cells. However, like many other RAF inhibitors, 1t is ATP competitive and it has recently been shown that V600EBRAF has considerably lower affinity for ATP than wildtype BRAF or wildtype CRAF, providing an elegant explanation of why wildtype BRAF and CRAF may not be efficiently inhibited by 1t in cells (29).
Our data also reveal that sensitivity to BRAF drugs may not be determined by BRAF mutation status alone. For example, V600EBRAF mutant HT29 cells were less sensitive to 1t than the majority of the other BRAF mutant cell lines, whereas SKMEL23 cells were considerably more sensitive to 1t than the other BRAF/RAS wildtype cells. Similar responses have been previously reported in these lines using another BRAF inhibitor, GDC-0879 (32). It has been suggested that HT29 cells are resistant to drugs of this class because they express high levels of glucuronosyltransferase that could metabolize these drugs (33). Conversely, it is feasible that SKMEL23 cells have, as yet unidentified, genetic alterations that confer sensitivity to this class of drug. These observations highlight the fact that sensitivity to specific drugs may not always be determined by a single mutation, and that other genetic aberrations in specific cancer cells can modify cell responses (29). Nevertheless, together, our data suggest that in the cellular context, 1t selectively inhibits oncogenic BRAF over CRAF or the other kinases that are critical for proliferation of BRAF wildtype or RAS mutant cells.
Consistent with the selective nature of 1t, there is a close correlation between the inhibition of ERK phosphorylation and the inhibition of growth in V600D/EBRAF mutant cells and analysis of the ERK pathway gives direct evidence of V600D/EBRAF inhibition, resulting in loss of MEK and ERK phosphorylation and loss of cyclin D1 expression. 1t therefore induces collapse of signaling downstream of oncogenic BRAF and importantly this leads to an inhibition of DNA synthesis and growth arrest. It is interesting to note that the cellular potency of 1t is approximately 4-fold greater than the ability of 1t to inhibit recombinant V600EBRAF in vitro. The reasons for this are unclear but may reflect the complex nature of the interactions between BRAF and other proteins in the cell, such as the molecular chaperone HSP90, which may improve drug access to BRAF in cells, but not in vitro. Alternatively, it is possible that the drug accumulates in cells. To address this, and demonstrate that the therapeutic activity of 1t is dependent on its ability to target mutant BRAF, we generated a gatekeeper mutant of V600EBRAF that is resistant to 1t. This was used to transform Ba/F3 cells and we show that T529N,V600EBRAF resistance to 1t translates into a dramatic reduction in antiproliferative activity. These data demonstrate that off-target effects, such as those against SRC, LCK or p38α that were suggested by the in vitro kinase screens do not appear to contribute to the compound's activity in BRAF mutant cell lines. Clearly however, we cannot completely exclude the possibility that in some genetic backgrounds, such as is present in SKMEL23 cells, other kinases/proteins could be targeted by 1t.
1t demonstrates excellent oral bioavailability of 71% and dosing via this route led to a 50% inhibition of MEK phosphorylation in tumors following a single dose, confirming that 1t targets oncogenic BRAF in vivo. Notably, daily p.o. dosing of 1t elicits a therapeutic response in V600EBRAF human A375M melanoma tumor xenografts. Furthermore, 1t does not affect the growth of G12VKRAS mutant SW620 tumors, consistent with mutant BRAF being the primary target of the compound. Interestingly, treatment of KRAS mutant tumors with 1t causes a 2-fold increase in MEK phosphorylation, which we attribute to enhanced activation of CRAF in response to selective BRAF inhibition (29, 30). Importantly, we do not, however, observe drug-induced accelerated tumor growth in vivo in contrast to observations made with GDC-0879 (29). 1t is also well tolerated, with no adverse effects observed following daily drug treatment for extended periods, and we also did not observe any skin lesions of the type described with another BRAF inhibitor, GDC-0879 (29). This also shows that off-target activity against kinases such as SRC, LCK or p38α inhibition was not inherently toxic.
Our modelling data suggest that 1t binds to the inactive conformation (type II inhibitor) of BRAF. In this, 1t is similar to sorafenib and RAF265, but distinct from agents such as SB590885 and PLX4720/PLX4032 that bind to the active or ‘active-like' conformation (type I inhibitors) (11, 12, 34, 35). From the clinical perspective, it is likely to be important to have drugs that bind to BRAF through distinct mechanisms. Clinical experience with kinase inhibitors shows that clinical resistance often emerges through the acquisition of secondary mutations within the catalytic cleft of the target that prevent drug binding, including but not limited to gatekeeper mutations (36-38). In these cases, the availability of drugs with different binding modes provides an important alternative treatment option for patient and we have recently shown that type I binders are more sensitive to gatekeeper changes than type II binders (27). Given the potency, selectivity and efficacy of 1t both in cell culture models and in human tumor xenograft models, our aim now is to assess the potential of agents such as 1t in melanoma patients whose tumors are driven by oncogenic BRAF.
Supplementary Material
Acknowledgements
This work is supported by The Wellcome Trust (refs: 071487/Z/03/A and 080333/Z/06/Z), Cancer Research UK (refs: C309/A2187, C107/A3096 and C107/A10433), The Institute of Cancer Research and The Isle of Man Anti-Cancer Association. We thank Professors Paul Workman, Chris Marshall and Julian Blagg for helpful discussions and support. Authors who are, or have been, employed by The Institute of Cancer Research are subject to a 'Rewards to Inventors Scheme', which may reward contributors to a programme that is subsequently licensed.
References
- 1.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Wellbrock C, Ogilvie L, Hedley D, Karasarides M, Martin J, Niculescu-Duvaz D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–42. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 4.Hoeflich KP, Gray DC, Eby MT, Tien JY, Wong L, Bower J, et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66:999–1006. doi: 10.1158/0008-5472.CAN-05-2720. [DOI] [PubMed] [Google Scholar]
- 5.Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–8. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 6.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 8.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–12. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 9.Kane RC, Farrell AT, Madabushi R, Booth B, Chattopadhyay S, Sridhara R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14:95–100. doi: 10.1634/theoncologist.2008-0185. [DOI] [PubMed] [Google Scholar]
- 10.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–8. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 11.King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 2006;66:11100–5. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]
- 12.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaherty K, Puzanov I, Sosman J, Kim K, Ribas A, McArthur G, et al. Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol (Meeting Abstracts) 2009;27:9000. [Google Scholar]
- 14.Springer CJ, Niculescu-Duvaz D, Niculescu-Duvaz I, Marais R, Suijkerbuijk BMJM, Zambon A, et al. Cancer Research Technology Limited; UK: Application: WO WO patent 2008-GB4208 2009077766. 2009 20081219. Preparation of pyrido[2,3-b]pyrazine-8-substituted compounds as RAF inhibitors. inventors. Institute of Cancer ResearchRoyal Cancer Hospital). assignee.
- 15.Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and validation of a genetic algorithm for flexible docking. J Mol Biol. 1997;267:727–48. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 16.Whittaker SR, Walton MI, Garrett MD, Workman P. The Cyclin-dependent kinase inhibitor CYC202 (R-roscovitine) inhibits retinoblastoma protein phosphorylation, causes loss of Cyclin D1, and activates the mitogen-activated protein kinase pathway. Cancer Res. 2004;64:262–72. doi: 10.1158/0008-5472.can-03-0110. [DOI] [PubMed] [Google Scholar]
- 17.Niculescu-Duvaz I, Roman E, Whittaker SR, Friedlos F, Kirk R, Scanlon IJ, et al. Novel inhibitors of B-RAF based on a disubstituted pyrazine scaffold. Generation of a nanomolar lead. J Med Chem. 2006;49:407–16. doi: 10.1021/jm050983g. [DOI] [PubMed] [Google Scholar]
- 18.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–66. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 19.Workman P, Balmain A, Hickman JA, McNally NJ, Rohas AM, Mitchison NA, et al. UKCCCR guidelines for the welfare of animals in experimental neoplasia. Lab Anim. 1988;22:195–201. doi: 10.1258/002367788780746467. [DOI] [PubMed] [Google Scholar]
- 20.Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–60. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 21.Raynaud FI, Whittaker SR, Fischer PM, McClue S, Walton MI, Barrie SE, et al. In vitro and in vivo pharmacokinetic-pharmacodynamic relationships for the trisubstituted aminopurine cyclin-dependent kinase inhibitors olomoucine, bohemine and CYC202. Clin Cancer Res. 2005;11:4875–87. doi: 10.1158/1078-0432.CCR-04-2264. [DOI] [PubMed] [Google Scholar]
- 22.Menard D, Niculescu-Duvaz I, Dijkstra HP, Niculescu-Duvaz D, Suijkerbuijk BM, Zambon A, et al. Novel potent BRAF inhibitors: toward 1 nM compounds through optimization of the central phenyl ring. J Med Chem. 2009;52:3881–91. doi: 10.1021/jm900242c. [DOI] [PubMed] [Google Scholar]
- 23.Niculescu-Duvaz D, Gaulon C, Dijkstra HP, Niculescu-Duvaz I, Zambon A, Menard D, et al. Pyridoimidazolones as novel potent inhibitors of v-Raf murine sarcoma viral oncogene homologue B1 (BRAF) J Med Chem. 2009;52:2255–64. doi: 10.1021/jm801509w. [DOI] [PubMed] [Google Scholar]
- 24.Nourry A, Zambon A, Davies L, Niculescu-Duvaz I, Dijkstra HP, Menard D, et al. BRAF inhibitors based on an imidazo[4,5]pyridin-2-one scaffold and a meta substituted middle ring. J Med Chem. 53:1964–78. doi: 10.1021/jm901509a. [DOI] [PubMed] [Google Scholar]
- 25.Suijkerbuijk BM, Niculescu-Duvaz I, Gaulon C, Dijkstra HP, Niculescu-Duvaz D, Menard D, et al. Development of novel, highly potent inhibitors of V-RAF murine sarcoma viral oncogene homologue B1 (BRAF): increasing cellular potency through optimization of a distal heteroaromatic group. J Med Chem. 53:2741–56. doi: 10.1021/jm900607f. [DOI] [PubMed] [Google Scholar]
- 26.Liao JJ-L. Molecular Recognition of Protein Kinase Binding Pockets for Design of Potent and Selective Kinase Inhibitors. Journal of Medicinal Chemistry. 2007;50:409–24. doi: 10.1021/jm0608107. [DOI] [PubMed] [Google Scholar]
- 27.Whittaker S, Kirk R, Hayward R, Zambon A, Viros A, Cantarino N, et al. Gatekeeper Mutations Mediate Resistance to BRAF-Targeted Therapies. Sci Transl Med. 2:35ra41. doi: 10.1126/scitranslmed.3000758. [DOI] [PubMed] [Google Scholar]
- 28.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, et al. Transforming p21 Mutants and c-Ets-2 Activate the Cyclin D1 Promoter through Distinguishable Regions. Journal of Biological Chemistry. 1995;270:23589–97. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 29.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 30.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell. 140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, et al. In Melanoma, RAS Mutations Are Accompanied by Switching Signaling from BRAF to CRAF and Disrupted Cyclic AMP Signaling. Cancer Res. 2006;66:9483–91. doi: 10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 32.Hoeflich KP, Herter S, Tien J, Wong L, Berry L, Chan J, et al. Antitumor efficacy of the novel RAF inhibitor GDC-0879 is predicted by BRAFV600E mutational status and sustained extracellular signal-regulated kinase/mitogen-activated protein kinase pathway suppression. Cancer Res. 2009;69:3042–51. doi: 10.1158/0008-5472.CAN-08-3563. [DOI] [PubMed] [Google Scholar]
- 33.Cummings J, Zelcer N, Allen JD, Yao D, Boyd G, Maliepaard M, et al. Glucuronidation as a mechanism of intrinsic drug resistance in colon cancer cells: contribution of drug transport proteins. Biochemical Pharmacology. 2004;67:31–9. doi: 10.1016/j.bcp.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Ramurthy S, Subramanian S, Aikawa M, Amiri P, Costales A, Dove J, et al. Design and synthesis of orally bioavailable benzimidazoles as Raf kinase inhibitors. J Med Chem. 2008;51:7049–52. doi: 10.1021/jm801050k. [DOI] [PubMed] [Google Scholar]
- 35.Wan PTC, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Structural basis for activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 36.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 37.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamborini E, Bonadiman L, Greco A, Albertini V, Negri T, Gronchi A, et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology. 2004;127:294–9. doi: 10.1053/j.gastro.2004.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.