Abstract
Melatonin is mainly produced in the mammalian pineal gland during the dark phase. Its secretion from the pineal gland has been classically associated with circadian and circanual rhythm regulation. However, melatonin production is not confined exclusively to the pineal gland, but other tissues including retina, Harderian glands, gut, ovary, testes, bone marrow and lens also produce it. Several studies have shown that melatonin reduces chronic and acute inflammation. The immunomodulatory properties of melatonin are well known; it acts on the immune system by regulating cytokine production of immunocompetent cells. Experimental and clinical data showing that melatonin reduces adhesion molecules and pro-inflammatory cytokines and modifies serum inflammatory parameters. As a consequence, melatonin improves the clinical course of illnesses which have an inflammatory etiology. Moreover, experimental evidence supports its actions as a direct and indirect antioxidant, scavenging free radicals, stimulating antioxidant enzymes, enhancing the activities of other antioxidants or protecting other antioxidant enzymes from oxidative damage. Several encouraging clinical studies suggest that melatonin is a neuroprotective molecule in neurodegenerative disorders where brain oxidative damage has been implicated as a common link. In this review, the authors examine the effect of melatonin on several neurological diseases with inflammatory components, including dementia, Alzheimer disease, Parkinson disease, multiple sclerosis, stroke, and brain ischemia/reperfusion but also in traumatic CNS injuries (traumatic brain and spinal cord injury)
Keywords: Melatonin, inflammation, neurodegeneration, mitochondria, antioxidant, free radical.
INTRODUCTION
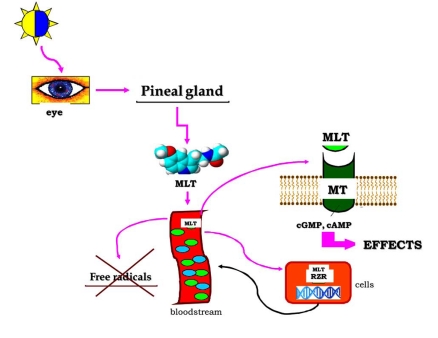
Brain tissue is very vulnerable to free radical damage because of its high oxygen utilization (20% of the total oxygen inspired), high concentrations of polyunsaturated fatty acids [57] and transition metals such as iron, which is involved in the generation of the hydroxyl radical [76], and low concentrations of cytosolic antioxidants [44, 56, 152]. Glutathione (GSH) is the predominant antioxidant in the brain that is present at millimolar concentrations [174]. Melatonin, a secretory product of the pineal gland, is a ubiquitously acting direct free radical scavenger and also an indirect antioxidant [6, 197]. Melatonin, as well as its metabolites, are efficient in detoxifying reactive oxygen species (ROS), e.g., HO• and H2O2 [194], and they also interact directly with reactive nitrogen species (RNS) [32, 68, 146, 196]. Endogenous melatonin is synthesized in the pineal gland from the neurotransmitter serotonin in a circadian manner. In mammals, the melatonin rhythm is generated by an endogenous circadian master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus, which is entrained by the light/dark cycle over a 24-hr period. It participates in several neuroendocrine and physiological processes and is appropriately referred to as a tissue factor, a paracoid, an autocoid, and an anti-oxidant and sometimes as a hormone depending on its physiological actions [192]. It has profoundanti-oxidant actions against oxidative and nitrosative stress [159]. It also exerts significant immunomodulatory influences [189] and modulates angiogenesis and wound healing processes [181]. Melatonin exerts its effects through receptor-mediated and receptor-independent mechanisms, thus, manifesting enormous functional versatility and diversity [160]. The ubiquitous distribution of melatonin receptors in the CNS as well as in the peripheral organs further compliments the fact that melatonin’s actions are not compromised by morpho-physiological barriers such as the blood-brain barrier. It is likely to influence every cell with which it comes into contact. At physiological concentrations, melatonin exerts receptor-mediated actions whereas the receptor-independent actions usually require higher supraphysiological/pharmacological melatonin concentrations because of the circumstances under which it is tested. Even at the higher concentrations, melatonin is well-tolerated by humans [176]. Two melatonin receptors, MT1 and MT2, which belong to the G-protein-coupled receptor superfamily, have been cloned in mammals and share some specific short amino-acid sequences, suggesting that they represent a specific subfamily. A third melatonin-binding site has been purified and characterized as the enzyme quinine reductase 2, and the inhibition of this enzyme by melatonin may contribute to its anti-oxidant properties [138, 195].
Exogenous administration of melatonin can also entrain the circardian clock by a direct action on SCN and, thus, it has potential to treat the disoriented circardian clock in cases such as jet-lag, shift-work, in profoundly blind subjects, and in individuals with delayed or advanced sleep phase syndromes and sleep inefficiency [72]. Besides being used to increase sleep efficiency, treat jet lag, improve the cardiovascular system [177], and as an antiaging drug [21, 71, 167] and a dietary supplement and cancerprotective hormone [149], intensive research has indicated melatonin’s beneficial effects in experimental models of neurodegenerative disorders. Brain oxidative damage has been implicated as a common link in the pathogenesis of such diseases.
MELATONIN AND ITS RECEPTORS
Melatonin (N-acetyl-5-methoxytryptamine) is a natural hormone secreted by the pineal gland. In clinical use for many years, melatonin is safe and well-tolerated even at high doses [214]. Melatonin, or N-acetyl-5-methoxytryptamine, is an indole mainly produced in the mammalian pineal gland during the dark phase. Melatonin secretion from the pineal gland has been classically associated with circadian and circanual rhythm regulation, and with adjustments of physiology of animals to seasonal environmental changes [151]. Melatonin production, however, is not confined exclusively to the pineal gland, but other organs and tissues including retina, Harderian glands, gut, ovary, testes, bone marrow and lens also have been reported to produce it [128, 193]. In recent years, it has become apparent that melatonin’s actions transcended those of a hormonal modulator. Melatonin had been shown to subtly influence the function of a variety of tissues and cells not generally considered in the endocrine category [193]. This led to a suspicion that melatonin had yet undefined actions at the cellular level. Numerous data suggest a role for melatonin and its metabolites function in the antioxidative defence in all organisms that produce this indole [120, 158, 168, 196], particularly, since melatonin crosses all morphophysiological barriers and enters all cells. Several mechanisms have been proposed for the neuroprotective effect of melatonin. Melatonin upregulates antioxidative defensive systems, including the activities of superoxide dismutase and glutathione peroxidase as well as the levels of glutathione [202]. Furthermore, melatonin and its metabolites scavenge free radicals [147, 190] and thus terminate the initiation and propagation of lipid peroxidation. A recent studies show that melatonin binds several metals, including iron [107, 108] and this effect may attenuate the Fenton reaction and the generation of the hydroxyl radical.
It participates crucially in neurogenesis, immunomodulation [182, 200], improving immune defense [118], regulating circadian rhythms and sleep [1], eliminating free radicals [146, 155, 156, 196], intervening in lipid metabolism [119, 173], and also inhibiting cancer growth [61]. Mechanistically, melatonin functions, by acting through G-protein coupled membrane receptors, MT1 and MT2 [61, 119, 173] and through nuclear receptors RZR/ROR [142].
Melatonin also stimulates a host of antioxidative enzymes including SOD, glutathione peroxidase (GPx) and glutathione reductase (GRd); these actions further reduce the oxidation state of cells [6, 122].
Melatonin inhibits the activity of nitric oxide synthase and dopamine release, potentiates the inhibitory effect of GABA in the central nervous system, modulates the serotonin receptors and potentiates the opioid analgesic.
Melatonin possesses an electron-rich aromatic indole ring and functions as an electron-donor, thereby reducing and repairing electrophilic radicals [124]. Of additional interest regarding melatonin, is its reported binding to quinone reductase 2 [217]. This enzyme, which is considered a melatonin receptor, is important in the detoxification of pro-oxidant quinones. Experimental evidence supports its actions as an indirect antioxidant when stimulating antioxidant enzymes [161], its ability to enhance the activities of other antioxidants and its protection of antioxidant enzymes from oxidative damage [126].
Melatonin has two important functional groups which determine its specificity and amphiphilicity: the 5-methoxy group and the N-acetyl side chain. Once synthesized, the majority of melatonin diffuses directly towards the cerebrospinal fluid of the brain's third ventricle, while another fraction is released into the blood stream where it is distributed to all tissues [33]. Circulating melatonin is metabolized by P-450 liver enzymes, which hydroxylate this hormone at the 6-carbon position to yield 6- hydroxymelatonin. This reaction is followed by conjugation with sulfuric or glucuronic acid, to produce the principal urinary metabolite, 6-sulfatoxymelatonin. In the last step, conjugated melatonin and minute quantities of unmetabolized melatonin are eliminated through the kidney. In addition to hepatic metabolism, oxidative pyrrole-ring cleavage appears to be the major metabolic pathway in other tissues, including the CNS [163]. Melatonin exhibits high affinity binding to its receptors, of less than 500 pM [77]. There are four different melatonin receptor subtypes. Two of them are membrane-associated receptors, while the other two are nuclear receptors. Membrane melatonin receptors are classified based upon their kinetic properties and pharmacological profiles into MT1 and MT2 melatonin receptor subtypes [186]. They belong to the seven transmembrane receptor family, have 60% aminoacid homology, and differ in molecular structure and gene chromosomal localization [162]. MT1 andMT2 melatonin receptor subtypes are present in humans and other mammals [162]. The MT2 melatonin receptor has lower affinity (Kd=160 pmol/l) for 125I-melatonin as compared to the human MT1 melatonin receptor (Kd=20-40 pmol/l) [186]. Although several authors proposed the existence of a putative MT3 melatonin receptor subtype, recent studies have provided evidence that the MT3 melatonin receptor is actually a cytosolic quinine reductase 2 enzyme rather than a membrane receptor [117]. For this reason, the MT3 melatonin receptor subtype is no longer recognized in IUPHAR's classification as a G protein-coupled melatonin receptor subtype. Melatonin is also a ligand for retinoid orphan nuclear hormone receptors [12], referred to as RZRα and RZRβ at concentrations in the low nanomolar range. Both receptors are present in the central and peripheral nervous system and have been associated with cell differentiation and immune response regulation [179]. The RZRα melatonin receptor has been implicated in inflammatory reactions. Thus, Steinhilber et al. [183] reported that melatonin can down-regulate the expression of 5-lipooxygenase (5-LOX), an important inflammatory mediator, in B lymphocytes via RZRα receptors.
Several groups have shown that melatonin reverses chronic and acute inflammation [37, 40]. Melatonin treatment also causes an important reduction of nitric oxide (NO) and malondialdehyde (MDA) levels, two compounds that are closely related to inflammation [15]. Communication between the brain and the immune system involves a complex network of bidirectional signals which link the neural, endocrine, and immune systems [104, 131]. Cerebral disorders disrupt the functional interaction between the nervous and immune systems [27]. The immunomodulatory properties of melatonin are well known; it acts on the immune system by regulating cytokine production of immunocompetent cells [26, 67]. Experimental and clinical data showing that melatonin reduces adhesion molecules and pro-inflammatory cytokines including IL-6, IL-8, and tumor necrosis factor (TNF)-α and modifies serum inflammatory parameters. As a consequence, melatonin improves the clinical course of illnesses which have an inflammatory etiology [148].
Melatonin has several additional anti-inflammatory effects, which are probably related to a direct interaction with specific binding sites located in lymphocytes and macrophages [141] (Fig. 1).
Fig. (1).
The regulative pathway of melatonin synthesis in pineal gland and the action sites of melatonin in biological system.
EFFECT OF MELATONIN ON NEURODEGENERATIVE DISORDERS
The inherent biochemical and physiological characteristics of the brain, including high polyunsaturated fatty acids and energy requirements, make it particularly susceptible to free radicals mediated insult [23]. Increasing evidence indicates that accumulation of aberrant or misfolded proteins, protofibril formation, ubiquitin proteasome system dysfunction, excitotoxic insult, oxidative and nitrosative stress, mitochondrial injury and failure of axonal and dendritic transport represent unifying events in many slowly progressive neurodegenerative disorders [171].
Several effects of melatonin through its receptors may account for its ability to prevent oligodendroglial damage: free radical scavenger production by activated microglia [130, 196], improvement of membrane fluidity and reduction of edema and polymorphonuclear cell infiltration into damaged tissue, prevention of translocation of the nuclear factor-kappa-B (NF-κB) to the nucleus and the subsequent reduction of pro-inflammatory cytokines expression, which play a relevant role in the inflammatory reaction [123, 125]. In addition, melatonin could modulate astrocyte reactivity or death through an upregulation of astrocytic anti-oxidative defenses [22]. The neuroprotective pathway appears to implicate both melatonin receptors and inflammatory modulation, leading to a promotion of oligodendrocyte maturation.
NO plays crucial roles in the brain such as neuromodulation, neurotransmission and synaptic plasticity, but is also involved in pathological processes including neurodegeneration and neuroinflammation [62]. It is now well documented that NO and its toxic metabolites can inhibit components of the mitochondrial respiratory chain leading to cellular energy deficiency and, eventually, to cell death [46]. Astroglial cells become “activated” in a wide range of CNS pathologies, leading to the induction of iNOS [17, 48]. Although glial activation can be protective, excess activation causes damage in most occasions [133] since the NO formed may inhibit neuronal respiration. Formation of NO by astrocytes has been suggested to contribute, via impairment of mitochondrial function, to the neurodegenerative process. In addition, a relationship between neuronal injury and NO originated from glial cells has been suggested [41] since activation of iNOS results in the release of large amounts of NO neurotoxic over long periods of time [121, 222]. Several possibilities have been proposed for neurodegenerative damage in CNS, including oxidative stress. Astrocytes are involved in multiple brain functions in physiological conditions participating in neuronal development, synaptic activity and they also actively participate in the processes triggered by brain injuries, aimed at limiting and repairing brain damage [47]. Reactive astrocytes have been implicated in several neurological diseases with inflammatory components, including HIV-1- associated dementia, Alzheimer disease (AD), multiple sclerosis and stroke [69, 129]. Astrocytes contribute to the neuroprotection and survival of neurons; any astrocytic dysfunction seriously affects neuronal viability. In addition, astrocytes preserve neuronal survival through inactivation of ROS. Glia become activated by inflammatory mediators and express new proteins such as the iNOS [121]. NO produced by iNOS seems to be a key mediator of such glial-induced neuronal death.
In recent decades, neuroinflammation has been proposed as a causative factor of neurological diseases and disorders [200]. The chronic inflammatory state would create an activated immune response, including acute phase protein response, cytokines (interferons and interleukins), macrophages, lymphocytes, and other immune system cells as well [191]. Oxidative stress in the brain occurs when the generation of ROS exceeds the ability of the endogenous antioxidant system to remove excess ROS subsequently leading to cellular damage. The presence of oxidative stress in brain mitochondria should cause changes in LPO and GSH and, if ROS damage is prolonged, also changes should occur in enzymes of the redox cycle of GSH [122].
Melatonin, with antioxidant and anti-inflammatory well documented actions [32, 83, 146, 165, 166, 190, 197], showed marked beneficial effects against brain mitochondrial dysfunction with age. These effects, and the fact that melatonin virtually lacks toxicity even at large doses [42, 84, 114], supports its clinical use in preventing the impairments of aging. Due to the lipophilicity of melatonin, it is accumulated by the mitochondria at high concentrations [36], allowing its interaction with mitochondrial membranes [150], possibly locating near the polar heads of membrane phospholipids [90]. In this location, melatonin may easily protect brain mitochondrial membranes from free radical attack, stabilizing them [102]. The ability of melatonin to prevent GSH loss with age probably reflects its effect on the activities of the GSH redox cycle enzymes [122].
Mitochondria have been identified as a target for melatonin [5, 92]. Melatonin promotes mitochondrial homeostasis. Melatonin may be possible to treat neurodegenerative disorders by inhibiting mitochondrial cell death pathways [82, 112, 180, 205]. Studies show that melatonin levels are lower in AD patients compared with that in age-matched control subjects [112, 116, 140]. The great advance has been currently conduced in studies of protection against AD by antioxidant melatonin inhibiting Aβ-induced toxicity [52-55, 85, 100, 225] and attenuating tau hyperphosphorylation [43, 105, 113, 143, 210, 211, 213]. Besides the antioxidant properties, the anti-amyloidogenic properties of melatonin for AD have been studied [86, 144]. Melatonin improved learning and memory deficits in an APP695 transgenic mouse model of AD in vivo [52]. In vitro experiments showed that Aβ-treated cultures exhibited characteristic features of apoptosis, and melatonin attenuated Aβ-induced apoptosis in a number of cellular models of AD including mouse microglial BV2 cells, rat astroglioma C6 cells, and PC12 cells [43, 52, 54, 145, 225]. Studies in transgenic AD mice and cultured cells have suggested that administration of melatonin inhibited the Aβ-induced increase in the levels of mitochondria related Bax [43, 55]. Furthermore, melatonin prevented upregulated expression of Par-4 and suppressed Aβ-induced caspase-3 activity [55]. Another experiment in mouse microglial BV2 cells in vitro showed that melatonin also decreased caspase-3 activity, inhibited NF-κB activation, and reduced the generation of Aβ-induced intracellular ROS (reactive oxygen species) [53]. In addition, in vivo observations showed that melatonin-treated animals had diminished expression of NF-κB compared to untreated animals [209]. Another experiment demonstrated that melatonin inhibited the phosphorylation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase via a PI3K/Akt-dependent signaling pathway in microglia exposed to Aβ1-42 [100]. Taken together, the above-mentioned evidence suggests that melatonin may provide an effective means of treatment for AD through its antiapoptotic activities.
Mounting evidence indicates that melatonin blocks the 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-dependent apoptotic fragmentation of nuclear DNA in rat astrocytes [89], rat mesencephalic cultures [80], and mouse striatal neurons [81]. Besides its traditional role as an antioxidant and free radical scavenger, melatonin proved to target a variety of pathways while its systemic effect correlates with the drug’s disruption of the intrinsic mitochondrial cell death pathway, silencing of the Rip2/caspase-1 pathway, and the activation of survival pathways. These actions may be synchronistic and complementary in models of HD. Effective treatment to prevent neurodegeneration could be achieved using a combination of melatonin and other pharmacological agents that act on different apoptosis targets.
Antiinflammatory actions of melatonin depend on its inhibition of the expression of iNOS and, here, mitochondrial iNOS [38, 49]. It was recently reported that the brain melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK) [163] is a better antioxidant than its precursor AFMK [164], and it is a highly efficient NO scavenger, which forms a stable product that does not easily re-donate NO [73]. AMK was more potent than melatonin in inhibiting in vitro and in vivo striatal NOS activity [103], and both compounds, melatonin and AMK, easily cross the brain-blood barrier after their administration, reaching neuronal and glial cells [16, 19, 103]. Moreover, several of the melatonin metabolites, in addition to AMK, and including 3-hydroxymelatonin, and AFMK are likewise free radical scavengers [146, 196]. With regard to the anti-inflammatory effects of melatonin, the most important feature is its inhibition of iNOS expression [38]. Antioxidant and anti-inflammatory properties of melatonin are relevant in mitochondrial physiology [16], and they may play a neuroprotective role in PD. AMK is a potent inhibitor of mitochondrial iNOS activity and a more efficient NO scavenger than its precursor melatonin [73].
Melatonin is known to be a stabilizer or protector of cell and organelle membranes because of its inhibitory effects on lipid peroxidation [153]. Melatonin has a regulatory effect on the activities of enzymes involved in the generation of free radicals, and on microsomal membrane fluidity in situations of elevated oxidative stress [208]. Moreover, its solubility in lipid and aqueous media, which allows it to cross morphophysiological barriers and enter subcellular compartments, permit melatonin to function as a highly effective inhibitor of oxidative damage. Our results indicate that melatonin stabilizes C6 glioma cell line membranes. Thus, melatonin’s ability to stabilize membranes may contribute to its neuroprotective actions. Recent work shows that melatonin prevents specifically the activation of the pro-inflammatory enzymes COX-2 and iNOS in glioma cells without simultaneous inhibition of COX-1 enzyme, thus indicating an anti-inflammatory action. In fact, COX-2 inhibition by melatonin may account for many protective effects including those observed in neurodegenerative diseases. Importantly, melatonin did not alter COX-1 protein level, one of the major disadvantages of using NSAIDs is their side effect on COX-1 [66]. Due to its lack of effect on COX-1, melatonin could potentially share the benefits of NSAIDs while avoiding their side effects. Moreover, melatonin lowered the nitrite/nitrate production by reducing iNOS expression in LPS/IFN-γ through the inhibition of NF-κB activation. Inhibition of NF-κB by melatonin has been previously reported in other experimental models that show how melatonin prevents NF-κB activation by oxidative stress [24]. Moreover, our study showed an inhibitory effect of melatonin on nNOS expression in glioma cell line, confirming a role of noncompetitive inhibitors of the nNOS. Additional studies are required to define the specific concentrations of melatonin to inhibit oxidative damage in specific disease/conditions. Melatonin protects against NO-induced impairment and this effect is associated with decreased formation of oxidatively modified proteins and with decreased upregulation oxidative stress-responsive genes, such as Hsp70. Expression of stress responsive genes, such Hsp70, used as indicator of astroglial stress, is markedly inhibited, consistent with a protective effect of melatonin.
EFFECT OF MELATONIN ON TRAUMATIC CNS INJURIES
Traumatic CNS injuries include traumatic brain injury (TBI) and SCI, depending on the anatomical region damaged. It is crucial to relate melatonin’s efficacy to the aberrant Ca2+-homeostasis-driven signaling pathways as they form a common denominator to any traumatic CNS injury. Melatonin is highly effective in preventing molecular mutilation due to aberrant Ca2+-homeostasis. A recent experimental study in aged mice found that oral administration of melatonin restored the metabolic function of cells with improvement in several aspects of Ca2+-signaling such as the amplitude and frequency, the size of intracellular Ca2+-pools, capacitative Ca2+-entry, and the mitochondrial potential [102, 202]. Such aberrant Ca2+-homeostasis during aging is undoubtedly a slow and gradual process that causes the accumulation of molecular debris over time. Some benefits of melatonin may also be expected when sudden neurotrauma disturbs tightly regulated Ca2+-signaling processes. This evidence comes from the experimental studies where melatonin alleviated the impaired large conductance of Ca2+-activated K+ channel activity in hippocampal neurons, which were injured as a result of intermittent hypoxia [201] or in ischemia-reperfusion injury in chronically hypoxic rats [219]. Melatonin receptors, in addition to coupling with the heterotrimeric G proteins, also physically associate with other intracellular proteins, e.g., calmodulin [141], and such interactions multiply the modulatory functions of melatonin in cell signaling [87] and cytoskeltal rearrangements [13, 79]. Moreover, melatonin receptors undergo heterodimerization as MT1/MT2, although the functional consequences of this association on receptor signaling and trafficking are currently unknown [87]. The advantages of therapeutic and clinical utilization of melatonin have been repeatedly emphasized [2, 99] and such encouragement may, in due course, lead to an efficient neuroprotective intervention in CNS trauma. One may expect dual benefits of melatonin administration in traumatic CNS injuries. Firstly, it may be important to restore the perturbed endogenous melatonin rhythm if it is disturbed by mechanical interruption of melatonin synthesis due to damage to the neural connections between the SCN and the pineal gland. Secondly, it may be a result of its multiple neuroprotective/neurorestorative actions that melatonin has at the site of injury in the CNS. Progression of traumatic CNS injuries follow an archetypal course through primary and secondary damaging events, which are distinct in their spatiotemporal windows. Because of melatonin’s multiple actions, its use as a treatment may profoundly influence both the short-term primary damaging events as well as preventing some of the long-term secondary damage. The half-life of exogenously administered melatonin in the blood is short; hence, there is a need for stable melatonin mimetics, be they synthetic ligands or receptor modulators. To date, these melatonin analogs have not been tested for their possible benefits in SCI or any CNS injury.
Clinically, SCI involves two components: an initial mechanical instability precipitating into a secondary injury process leading to the final neurological deficit [109]. Traumatic SCI may be a direct lesion that causes focal injury to the neural elements at the site of impact or a lesion due to stretching or compressive forces applied to spinal cord via bones and/or ligaments. In either case, the cord lesion expands with time following injury over adjoining spinal segments causing secondary injury. Primary injuries cause disruption of the blood supply to spinal cord and lead to ischemic damage. A quest for a good neuroprotectant in SCI remains unresolved. A possible correlation between the neuroprotective efficacy of melatonin and SCI originally emerged almost three decades ago as clinical case reports in which the circardian profiles of endogenous melatonin were assessed in SCI patients. Much later the experimental studies on the utility of exogenously-applied melatonin in experimental models of SCI were conducted.
Levels of melatonin in SCI patients differed strictly with respect to the site of injury. SCI disrupting the cervical spinal cord significantly perturbed the levels of endogenous melatonin, whereas, an injury at the levels of thoracic or lumbar did not affect it severely. These differences are explicable in terms of the central neural pathways, which connect the eyes to the pineal gland. Lesions within the cervical spinal column caused decentralization of pineal gland because they perturbed descending sympathetic fibers and led to an absence of significant increment in nocturnal melatonin; this, clearly distinguished quadriplegic subjects from normal males and from the subject with a lesion of the lumbar spinal cord [96]. It was further confirmed that neurologically complete cervical spinal cord transection results in complete loss of the circardian melatonin rhythm [223]. More complete studies on rhythms of serum melatonin in patients with spinal lesions at the cervical, thoracic, or lumbar region revealed that the cervical region of the spinal cord includes the neural pathway, which is essential for the diurnal rhythm of pineal melatonin secretion in human. Retrospectively, such studies clearly discerned the relationship between regional specificity of SCI lesions and changes in the endogenous profile of melatonin [106]. Such a regional bias was reconfirmed in a recent clinical study conducted using tetraplegic patients with bilateral oculo-sympathetic paresis showing a complete loss of the nocturnal production of melatonin [224]. A recent assessment based on the “Basic Nordic Sleep Questionaire" conducted at the Karolinska University Hospital in Stockholm, on a large SCI patient population comprised of 230 patients, inferred that perturbances in the melatonin rhythm contributed to poor subjective sleep quality associated with higher ratings of pain intensity, anxiety, and depression [137]. Another study confirmed the positive correlation of an altered melatonin cycle in cervical SCI patients with reduced sleep quality and documented the need for larger studies on the potential usefulness of melatonin replacement therapy in normalizing sleep in SCI patients [175]. Several encouraging clinical case studies suggest that melatonin may be the neuroprotectant of choice in this devastating injury. There is no neuroprotective drug in clinical practice to date that is highly useful as a treatment for this complex neurological problem. Hence, it is important to assess the outcomes of melatonin’s use in experimental models of SCI with the intent of eventually applying this information for treatment of SCI in humans. The first experimental study to test melatonin’s efficacy in reducing neural damage in experimental SCI in animals is that of Fujimoto and colleagues [59]. They studied an acute to chronic SCI model and showed a significant protection by melatonin against neutrophil-mediated damage including lipid peroxidation, reduced levels of secondary injury, and an earlier recovery [59]. Systemically applied pharmacological doses of melatonin were shown to boost the antioxidant defense system in a variety of ways after acute SCI [198]. Melatonin also protected against autodestruction following SCI by reducing the levels of free iron and the products of lipid peroxidation [111]. When compared with methylprednisolone, melatonin was found to be more effective in an acute SCI model against lipid peroxidation and in preserving the structural integrity of neurons, axons, and intracellular organelles [91]. A later study uncovered the greater efficacy of melatonin compared to methylprednisolone in preserving the ultrastructural histopathological integrity of the spinal cord although the reduction of lipid peroxidation after SCI was less obvious. Furthermore, this study also verified that the neuroprotective effect of melatonin was dose-dependent [70]. A combination therapy of melatonin with methylprednisolone did show an additive effect against the accumulation of lipid peroxidation products in the subacute phase of injury, but did not show any difference in a brief chronic 10-day study following SCI in rats as evidenced by neurobehavioral, ultrastructural, and electrophysiological recovery [29]. However, more recently in a SCI model in mice, the combination therapy with melatonin and dexamethasone had a significant and important beneficial anti-inflammatory effect by blocking the possible progression of secondary injury events after SCI. This study showed that the effective dose of dexamethasone could be reduced 10-fold when it was given in combination with melatonin [63]. In comparison to other anti-oxidants such as oxytetracycline or prostaglandin E1, melatonin was found to be more potent in ameliorating secondary damage in an acute model of SCI [203]. Melatonin reduces the development of inflammation and tissue injury associated with SCI by blocking both oxidative and nitrosative stress [65]. Moreover, melatonin limits the expression and activity of matrix metalloproteinases (MMP-9 and MMP-2) thereby also reducing pro-inflammatory TNF-α expression in a mouse model of traumatic SCI [51]. Moreover, melatonin’s protective role in SCI was related to the regulation of MAPK signaling pathways and the high-mobility group box 1 protein expression (HMGB1) in mice. Melatonin treatment in SCI mice enhanced motor recovery, reduced the activation of p38 MAPK, JNK, and ERK1/2, and the expression of HMGB1 [50]. An earlier report also showed that activation of the endogenous melatonin system in the spinal cord reduced the generation, development, and maintenance of central sensitization, with a resultant inhibition of capsaicin-induced secondary mechanical allodynia and hyperalgesia [204]. It was recently confirmed in an experimental model of SCI that exogenously administered melatonin reduced mechanical allodynia by altering the expression of water channel aquaporins [134]. Complementing the data regarding the neuroprotective effects in rodent SCI models, the beneficial neurobiological effects of melatonin have also been demonstrated in the rabbit SCI models. Further confirmation of neuroprotective efficacy of melatonin in traumatic SCI comes from a study conducted in rabbits wherein pinealectomy retarded the recovery rate after SCI, and administration of melatonin exogenously to the pinealectomized animals augmented the recovery [7]. The multifunctional efficacy of melatonin treatment after SCI has been shown in vivo by many investigations [172]. Calpain, a Ca2+-dependent neutral protease, is known to be a key player in the pathogenesis of SCI. Treatment of SCI animals with melatonin attenuated calpain expression, inflammation, axonal damage, and neuronal death, indicating that melatonin was highly neuroprotective in this situation. Moreover, examination of levels of calpain and caspase-3 expression and activity indicated significant reductions in the proteolytic events in SCI animals after treatment with melatonin.
Neutrophils are the first leukocytes to arrive within the injured spinal cord [74, 127]. In this setting, neutrophils release neutral proteases and ROS [39]. In this regard, the production of ROS such as superoxide anions, hydrogen peroxide, and peroxynitrite, is also associated with local and systemic inflammatory response as well as the neurodegenerative disease [157]. Glucocorticoids (GC) are widely used in the management of inflammatory diseases. Glucocorticoids exert beneficial effects after acute CNS injury in humans and experimental animals [94]. Important data show that GC may have a disease-modifying effect in addition to their well-documented anti-inflammatory actions [18]. In fact, the therapeutic management of long-term pathologies with steroids is often linked to a series of unwanted side effects, involving the hypothalamus-pituitary-adrenal axis, the cardiovascular system, as well as the fat and bone metabolism [8]. Glucocorticoids are among a variety of endogenous compounds that have been suggested to influence melatonin production in various vertebrate species, including humans, and the existence of the mutual relationship between the pineal gland and the hypothalamo-pituitary-adrenal axis has been postulated. This study provides the first evidence that the combination therapy with melatonin (10 mg/kg) and dexamethasone (0.025 mg/kg), used at a dose which are not effective when administered as single treatment, attenuates: (i) the tissue damage, (ii) the infiltration of the spinal cord with PMN, (iii) TNF-a expression, (iv) the nitration of tyrosine residues, (v) iNOS expression, (vi) apoptosis level, and (vii) motor recovery. The anti-inflammatory action observed with the combination therapy is also related to the reduction of the degree of iNOS protein. In addition, the degree of staining for nitrotyrosine was significantly reduced by the treatment with the combination therapy in SCI-treated mice. In this study, it was shown that the pharmacologic combination of melatonin plus dexamethasone reduced the inflammatory cell infiltration as assessed by the specific granulocyte enzyme MPO. Neutrophils, recruited into the tissue [199], can contribute to tissue destruction by the production of reactive oxygen metabolites [31, 127], granule enzymes, and cytokines that further amplify the inflammatory response [206]. Already, within 1 hr after SCI, an increased synthesis and/or secretion of TNF-α, is detectable at the injury site [11, 95, 222]. After SCI, TNF-α might serve as an external signal, initiating apoptosis in neurons and oligodendrocytes [10, 34]. It is well known that Bax, a proapoptotic gene, plays an important role in developmental cell death [9] and in CNS injury [135]. Similarly, it was shown that the administration of Bcl-xL fusion protein (Bcl-xL FP; Bcl-2 is the most expressed antiapoptotic molecule in adult central nervous system) into injured spinal cords significantly increased neuronal survival, suggesting that SCI-induced changes in Bcl-xL contributed considerably to neuronal death [115]. The co-treatment of melatonin with dexamethasone in SCI experimental model record features of apoptotic cell death after SCI, suggesting that protection from apoptosis may be a prerequisite for regenerative approaches to SCI. In particular, the co-administration of melatonin with dexamethasone reduced Bax expression, while Bcl-2 protein was expressed much more in SCI treated mice. This result suggests that melatonin with dexamethasone prevents the loss of the antiapoptotic way and reduces the pro-apoptotic pathway activation with a mechanism still to be discovered. Fas (CD95)-mediated apoptosis is an essential mechanism for the maintenance of homeostasis, and the disruption of this death pathway contributes to many human diseases [28]. Results from our research group, moreover, suggest that co-treatment in the acute phase of SCI may decrease the extent of intramedullary spinal cord hemorrhage and damage likely is demonstrated by the histologic tissue analysis. Thus, we have found in the post-traumatic inflammatory combination therapy that melatonin plus dexamethasone exerts a significant and important beneficial anti-inflammatory effect by blocking the possible progression of secondary damage. Furthermore, our results suggest that in vivo, the combination therapy allowed reducting 10-fold the effective dexamethasone dose. These findings indicate a novel function of a possible combination therapy, which provides a key procedure for the control of the secondary damage development after SCI.
SCI is a highly debilitating pathology. Although innovative medical care has improved patient outcome, advances in pharmacotherapy for the purpose of limiting neuronal injury and promoting regeneration are limited. The complex pathophysiology of SCI may explain the difficulty in finding a suitable therapy [185]. It is known that SCI initiates a series of cellular and molecular cascade events [10, 75, 199] and that a progressive neuronal injury results from a combination of secondary injury factors including ischemia, biochemical alterations, apoptosis, excitotoxicity, neurotransmitter accumulation and lipid peroxidation/free radical injury [25]. It is believed that inflammatory and immune responses are the major component of secondary injury and play a central role in regulating the pathogenesis of acute and chronic SCI [3, 14]. Proteinases and, in particular, matrix metalloproteinases (MMPs), are likely mediators of early secondary vascular pathogenesis after SCI [136]. MMPs are soluble and cell-surface bound zinc-dependent endopeptidases that mediate cellular infiltration, extracellular matrix degradation and release of growth factors and cytokines from the matrix, cell migration, tissue damage, remodeling, and repair [20, 132]. They are synthesized as inactive precursors, which limits their potentially destructive properties, and become activated upon removal of the propeptide [184]. The catalytic activities of MMPs are highly regulated at multiple levels, including transcription, secretion, zymogen activation, and inhibition by tissue inhibitors of metalloproteinases (TIMPs) [188]. In particular, most MMPs are rapidly upregulated by transcription in response to the exposure of a cell to growth factors, cytokines, chemokines, extracellular matrix (ECM) components and other transcriptional regulators. Transcription is followed by translation, and an MMP is first produced in its pro-form that requires subsequent conversion to the active enzyme. Free radicals, serine proteases and other activated MMPs are among the factors that convert a pro-MMP to its active enzyme. MMPs activity is required for the inflammatory cell infiltration that occurs following SCI and most likely contributes to early barrier disruption. The early inflammatory response involves an initial wave of infiltrating neutrophils, followed by migration of monocytes and macrophages into injured segment. Each of these inflammatory cells expresses MMPs, including MMP-2 (gelatinase A), MMP-8 (neutrophil collagenase), MMP-9 (gelatinase B), MMP-11 (stromelysin-3), and MMP-12 (metalloelastase). MMP-2 and -9 degrade types IV and V collagen, and thus have the potential to modify constituents of basal laminae [101, 178]. Expression of MMP-2 and -9 has been demonstrated throughout the nervous system during development [207] and also in disease states, such as painful nerve injury, injured spinal cord, and cerebral ischemia [45]. In the nervous system, these enzymes are directly involved in axonal outgrowth during development [220]. On the other hand, the aberrant expression of MMPs is involved in various disease processes, e.g., cancer metastasis and CNS disorders including multiple sclerosis, stroke, Alzheimer’s disease, and trauma [220]. Recently, various studies using experimental SCI model have demonstrated an involvement of MMPs in the posttraumatic events. In particular, a significant upregulation of MMPs was demonstrated in a mouse SCI compression model [215]. Moreover, it has been also clearly demonstrated that the pharmacological blockade of MMPs, limited to the first several days after SCI, improves locomotor recovery [78]. Recently, it has been pointed out that reactive oxygen and nitrogen species regulate MMP activity in vitro, and disrupt the balance of MMP activation and inactivation [58]. Melatonin exerts protective effects reducing SCI-induced MMP-9 and MMP-2 activity and expression.
Much of the damage that occurs in the spinal cord following traumatic injury is due to the secondary effects of glutamate excitotoxicity, Ca2+ overload, and oxidative stress, three mechanisms that take part in a spiraling interactive cascade ending in neuronal dysfunction and death [4, 64]. The role of reactive oxygen-induced damage to spinal cord lipids (i.e., lipid peroxidation) and proteins has been strongly supported in various studies [217]. Antioxidative mechanisms of melatonin as well as its metabolites have been suggested to be singlet oxygen quenching and free radical scavenging during prevention of lipid peroxidation [5, 139]. Decreasing oxidative stress reduces the amount of secondary damage due to trauma. The regulation of MMPs identifies another mechanism whereby the melatonin minimizes oxidative damage and inflammation involved in SCI. In the CNS, MMPs participate in injury and disease states. In brain and spinal cord injury, MMPs, including MMP-2 and MMP-9, contribute to early secondary pathogenesis by disrupting the blood-brain/spinal cord barrier and promoting inflammation [169, 170]. Consistent with other studies, we show that MMP-2, like MMP-9, is upregulated during SCI [78, 136]. Various combinations of proinflammatory molecules may provide the signal for induction of MMPs, especially MMP-9 activity. The anti-inflammatory activity of melatonin involves a significant downregulation of MMP-9 activity and a modest depression MMP-2 activity. Therefore, the inhibition of the MMP-2, and MMP-9 by melatonin is most likely attributed to the suppressive effect on TNF-α production.
The inhibition of mitogen-activated protein kinase family members, including extracellular signal-regulated kinases ½ (ERK1/2), c-jun N-terminal protein kinases, and p38 kinases, is believed to be beneficial in a number of experimental models of neurodegenerative diseases, diabetes type II, bipolar disorders, stroke, cancer, sepsis, and chronic inflammatory disease. The protective effect of melatonin might largely be accounted for by inhibition of p38 and ERK1/2 MAPK. Evidence indicates that ERK1/2 and especially p38 play an important role in NO-mediated degeneration of neurons in the spinal cord following SCI [218]. Previous studies also showed that the expression of activated ERK1/2 and p38 MAPK in microglia/macrophages may play a key role in production of CNS inflammatory cytokines and free radicals, such as NO [110]. Inhibition of phosphorylation of p38 MAPK and to a lesser extent of ERK1/2 reduces iNOS mRNA expression and rescues neurons from apoptosis after acute traumatic SCI [218]. We also here report that 24 hr after SCI, melatonin treatment significantly inhibit HMGB1 expression. In this study, our research group demonstrate that melatonin treatment significantly reduced the SCI-induced spinal cord tissue alterations as well as improved motor function.
Like other forms of neurotrauma, TBI involves multifactorial etiologies including free radical generation, which culminates in oxidative and nitrosative damage, disrupted macrocirculation and microcirculation in the vicinity of the injury, lymphocytopenia, opportunistic infections, perturbed sleep-wake cycles, suppression of nonspecific resistance, and toxicity caused by therapeutic agents. Together, these factors precipitate into the development of heterogenous clinical symptoms in the secondary phase. Melatonin has been adjudged as a protective agent against damage following TBI in several in vitro and in vivo studies [118]. Enhanced vulnerability of brain to such oxidative or nitrosative stress that escalates the damage following TBI may be handled more deftly by melatonin compared to other antioxidants. Infection and inflammation complicates TBI in surviving patients. Melatonin also aids in inhibition of pro-inflammatory cytokines and activation of adhesion molecules. It would consequently reduce lymphocytopenia and infections by opportunistic organisms. Moreover, the chronobiotic capacity of melatonin may also reset the natural circadian rhythm of sleep and wakefulness, which may serve as a major advantage of melatonin’s use as a therapeutic molecule after traumatic injury. It may also reduce the toxicity and enhance the efficacy of drugs used in clinical management of TBI such as steroidal or nonsteroidal anti-inflammatory agents, anti-ulcer agents, anti-psychotics/antidepressants, anti-epileptics and also anti-anemic drugs. Similar benefits of melatonin may be seen in other traumatic injuries to CNS as well. Finally, melatonin, not being restricted by the blood-brain barrier, reportedly reduces the contusion volume and stabilizes cellular membranes preventing vasospasm and apoptosis of endothelial cells that occurs as a result of TBI [118, 156].
EFFICACY OF MELATONIN ON BRAIN ISCHEMIA/REPERFUSION
The integrity of the CNS is highly vulnerable to a transitory interruption of its blood supply; this is specially true of the brain which is susceptible to focal or global ischemia. Unless ischemia is promptly reversed, reperfusion produces further cerebral damage. Ischemia/reperfusion injury is a subset of CNS damage that is unfortunately common but also occurs with equal frequency in nonneural tissues. In the CNS, interruption of the blood supply followed by reperfusion may not always be a result of traumatic assault.
Melatonin effectively attenuated ischemic brain injury via the Bcl-2-related survival pathway by increasing the expression of Bcl-2 [110] and Bcl-xL [93] in the ischemic brain.
Several recent reviews [30, 155, 212] summarize the reports that have documented the neuroprotective effects of melatonin against ischemia/reperfusion injury to the brain. Ischemia (focal or global) causes extensive destruction of neural tissue and the primary objective in the clinic is to promptly reverse it. Procedures such as acute thrombolysis or defibrinogenation, however, are effective only in selective patients, and are associated with a significant risk of complications due to bleeding. To date, a number of neuroprotectants, which were initially found effective in experimental studies for stroke therapy, failed in clinical trials. In vivo and in vitro evidence in favor of the use of melatonin to protect against focal and global cerebral ischemia/reperfusion injury has been reviewed [212]. The benefits of conducting further experimental studies to examine any synergistic protective action by combining melatonin with thrombolysis, defibrinogenation or other neuroprotectants are emphasized. The planning of phase II and phase III trials in an attempt to define the potential benefits of melatonin as an acute stroke treatment in humans is suggested [212]. When the results of all the reports are considered, it is clear that endogenously-produced and exogenously-administered melatonin reduces the degree of tissue damage and limits the behavioral deficits associated with experimental models of stroke [155]. The most recent updates on melatonin’s efficacy in models of stroke come from another review where the potentially widespread applications of melatonin have been showed [30]. These studies provide the preclinical tests for melatonin in acute stroke therapy, and its application at different treatment schedules. Advantageous characteristics of melatonin as a neuroprotective drug relates to its bioavailability, its potential for exerting wide-spread neuroprotective actions, further amplified and prolonged by its metabolites [146], its ability to reduce inflammation, its capability to protect cytoskeleton organization, and its anti-apoptotic actions. Moreover, melatonin does not interfere with the thrombolytic and neuroprotective actions of other drugs. An adequate safety profile of the drug has been underscored.
EFFECT OF MELATONIN ON MIDDLE CEREBRAL ARTERY OCCLUSION (MCAO) MOUSE MODEL
Melatonin has proved effective not only in the cell-free purified mitochondrial system but also inhibits cytochrome c release in an middle cerebral artery occlusion (MCAO) mouse model [92, 97] and in primary cortical neurons (PCN) [97]. Thus, melatonin is likely to interfere with both caspase-dependent (cytochrome c) and independent (AIF) mitochondrial cell death pathways. In vitro and in vivo experiments have shown that melatonin prevents the activation of downstream caspase-3 in oxygen/glucose deprivation (OGD)-mediated PCN cell death [97], cerebral ischemia-induced mouse injury, and the MCAO rat model [82, 93, 97]. Other experiments demonstrate significantly fewer TUNEL-positive cells [88, 98], reduced levels of cleaved PARP, and less DNA fragments [92] are found with administration of melatonin in the rat MCAO model. In addition, melatonin prevents brain damage, with reduced TUNEL-positive cells following transient cerebral artery occlusion (CerAO) [187] and transient MCAO model [35], as well as attenuating kainic acid-induced neuronal death, and reduces the number of TUNEL-labeled DNA breaks [154].
The neuroprotective role of melatonin is also mediated through the enhancement of the PI3-K/Akt survival pathway [93, 98] and JNK pathway [93], and restores reduced phosphorylated Akt in a model of mouse intraluminal MCAO [93]. Melatonin protected neuronal cells from damage by enhancing the activation of Akt and its downstream target Bad, without affecting the expression of 14-3-3, which acts as an antiapoptotic factor through interaction with Bad, thus mediating antiapoptosis signals in a rat MCAO model [88]. Furthermore, in the same model, melatonin inhibits apoptotic signals by preventing the injury-induced decrease of phosphorylation of Raf-1, MEK1/2, and ERK1/2 and the downstream targets, including Bad and 90-kDa ribosomal S6 kinase [98].
CONCLUSIONS
Based on the data summarized herein, the multifunctional molecule melatonin may be a useful therapeutic agent for treatment of CNS injuries [154] (Table 1). There is a wealth of well-documented experimental research to support its use. Melatonin is safe, nontoxic, and available in pure form for human use as a drug. The results of experimental studies provide fundamental information for the effective design and execution of clinical trials using melatonin as a neuroprotective treatment for traumatic CNS injuries.
Table 1.
Efficacy of Melatonin in Animal Models of Neurodegenerative Disease and CNS Traumatic Injury
| Diseases/Models | Effect | References |
|---|---|---|
| Neurodegeneration |
|
[141, 142] |
| MCAO |
|
[221-223] [225] |
| Parkinson disease (PD) |
|
[128-130] |
| Huntington disease (HD) | Neuroprotective | |
| Amyotrophic lateral sclerosis (ALS) | Reduces ROS | |
| Alzheimer disease (AD) |
|
[110-124] |
| Brain ischemia/reperfusion |
|
[217-219] |
| Spinal cord injury (SCI) | [158, 161, 164, 167, 168, 207, 208] | |
| Traumatic brain injury (TBI) | [42, 45] |
REFERENCES
- 1.Agez L, Laurent V, Guerrero HY, Pévet P, Masson-Pévet M, Gauer F. Endogenous melatonin provides an effective circadian message to both the suprachiasmatic nuclei and the pars tuberalis of the rat. J. Pineal Res. 2009;46(1):95–105. doi: 10.1111/j.1600-079X.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 2.Altun A, Ugur-Altun B. Melatonin: therapeutic and clinical utilization. Int. J. Clin. Pract. 2007;61:835–845. doi: 10.1111/j.1742-1241.2006.01191.x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson AJ. Mechanisms and pathways of inflammatory responses in CNS trauma: spinal cord injury. J. Spinal Cord. Med. 2002;25:70–79. doi: 10.1080/10790268.2002.11753604. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DK, Hall ED. Pathophysiology of spinal cord trauma. Ann. Emerg. Med. 1993;22:987–992. doi: 10.1016/s0196-0644(05)82739-8. [DOI] [PubMed] [Google Scholar]
- 5.Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18:869–871. doi: 10.1096/fj.03-1031fje. [DOI] [PubMed] [Google Scholar]
- 6.Antolin I, Rodriguez C, Sain RM, Uría H, Kotler ML, Rodríguez-Colunga MJ, Tolivia D, Menéndez-Peláez A. Neurohormone melatonin prevents damage: effect on gene expression for antioxidative enzymes. FASEB J. 1996;10:882–890. doi: 10.1096/fasebj.10.8.8666165. [DOI] [PubMed] [Google Scholar]
- 7.Ates O, Cayli S, Gurses I, Yucel N, Altinoz E, Iraz M, Kocak A, Yologlu S. Does pinealectomy affect the recovery rate after spinal cord injury? Neurol. Res. 2007;29:533–539. doi: 10.1179/016164107X172121. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 9.Bar-Peled O, Knudson M, Korsmeyer SJ, Rothstein JD. Motor neuron degeneration is attenuated in Bax-deficient neurons in vitro. J. Neurosci. Res. 1999;55:542–556. doi: 10.1002/(SICI)1097-4547(19990301)55:5<542::AID-JNR2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol. Med. 2004;10:580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 12.Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994;269(46):28531–28534. [PubMed] [Google Scholar]
- 13.Benitez-King G, Anton-Tay F. Calmodulin mediates melatonin cytoskeletal effects. Experientia. 1993;49:635–641. doi: 10.1007/BF01923944. [DOI] [PubMed] [Google Scholar]
- 14.Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr. Opin. Neurol. 2002;15:355–360. doi: 10.1097/00019052-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Bilici D, Akpinar E, Kiziltunç A. Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol. Res. 2002;46(2):133–9. doi: 10.1016/s1043-6618(02)00089-0. [DOI] [PubMed] [Google Scholar]
- 16.Bogaerts V, Theuns J, van Broeckhoven C. Genetic finding in Parkinson’s disease and translation into treatment: a loading role for mitochondria? Genes Brain Behav. 2008;7:129–151. doi: 10.1111/j.1601-183X.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolanos JP, Almeida A, Stewart V, Peuchen S, Land JM, Clark JB, Heales SJ. Nitric oxidemediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J. Neurochem. 1997;68:2227–2240. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- 18.Bond WS. Toxic reactions and side effects of glucocorticoids in man. Am. J. Hosp. Pharm. 1977; 34:479–485. [PubMed] [Google Scholar]
- 19.Borlongan CV, Su TP, Wang Y. Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport. 2000;11:923–926. doi: 10.1097/00001756-200004070-00005. [DOI] [PubMed] [Google Scholar]
- 20.Brinckerho CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell. Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 21.Caballero B, Vega-Naredo I, Sierra V, Huidobro-Fernandez C, Soria-Valles C, De Gonzalo-Calvo D, Tolivia D, Pallas M, Camins A, Rodriguez-Colunga MJ, Coto-Montes A. Melatonin alters cell death processes in response to age-related oxidative stress in the brain of senescence accelerated mice. J. Pineal Res. 2009;46:106–114. doi: 10.1111/j.1600-079X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese V, Boyd-Kimball D, Scapagnini G, Butterfield DA. Nitric oxide and cellular stress response in brain aging and neurodegenerative disorders: the role of vitagenes. In Vivo. 2004;18:245–268. [PubMed] [Google Scholar]
- 23.Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB. Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem. Res. 2001;26:739–764. doi: 10.1023/a:1010955807739. [DOI] [PubMed] [Google Scholar]
- 24.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Age-related alterations in Ca2+signals and mitochondrial membrane potential in exocrine cells are prevented by melatonin. J. Pineal Res. 2008;45:191–198. doi: 10.1111/j.1600-079X.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- 25.Carlson GD, Gorden C. Current developments in spinal cord injury research. Spine J. 2002;2:116–128. doi: 10.1016/s1529-9430(01)00029-8. [DOI] [PubMed] [Google Scholar]
- 26.Carrillo-Vico A, Lardone PJ, Fernandez-Santos JM, Martín-Lacave I, Calvo JR, Karasek M, Guerrero JM. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin 2/ interleukin 2 receptor system. J. Clin. Endocrinol. Metab. 2005;90:992–1000. doi: 10.1210/jc.2004-1429. [DOI] [PubMed] [Google Scholar]
- 27.Carrillo-Vico A, Lardone PJ, Naji L, Fernández-Santos JM, Martín-Lacave I, Guerrero JM, Calvo JR. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and antiapoptotic effects. J. Pineal Res. 2005;39:400–408. doi: 10.1111/j.1600-079X.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 28.Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103 :203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 29.Cayli SR, Kocak A, Yilmaz U, Tekiner A, Erbil M, Ozturk C, Batcioglu K, Yologlu S. Effect of combined treatment with melatonin and methylprednisolone on neurological recovery after experimental spinal cord injury. Eur. Spine J. 2004;13:724–732. doi: 10.1007/s00586-003-0550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cervantes M, Morali G, Letechipia-Vallejo G. Melatonin and ischemia-reperfusion injury of the brain. J. Pineal Res. 2008;45:1–7. doi: 10.1111/j.1600-079X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 31.Chatham WW, Swaim R, Frohsin H, Heck LW, Miller EJ, Blackburn WD ., Jr Degradation of human articular cartilage by neutrophils in synovial fluid. Arthritis Rheum. 1993;36:51–58. doi: 10.1002/art.1780360109. [DOI] [PubMed] [Google Scholar]
- 32.Chattoraj A, Liu T, Zhang LS, Huang Z, Borjigin J. Melatonin formation in mammals: in vivo perspectives. Rev. Endocr. Metab. Disord. 2009;10(4):237–43. doi: 10.1007/s11154-009-9125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung RT, Tipoe GL, Tam S, Ma ES, Zou LY, Chan PS. Preclinical evaluation of pharmacokinetics and safety of melatonin in propylene glycol for intravenous administration. J. Pineal Res. 2006;41(4):337–43. doi: 10.1111/j.1600-079X.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 34.Chittenden T, Harrington EA, O’connor R, Flemington C, Lutz RJ, Evan GI, Guild BC. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995;374:733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 35.Chung SY, Han SH. Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J. Pineal Res. 2003;34:95–102. doi: 10.1034/j.1600-079x.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 36.Costa EJ, Shida CS, Biaggi MH, Ito AS, Lamy-Freund MT. How melatonin interacts with lipid bilayer: a study by fluorescence and ESR spectroscopies. FEBS Lett. 1997;416:103–106. doi: 10.1016/s0014-5793(97)01178-2. [DOI] [PubMed] [Google Scholar]
- 37.Costantino G, Cuzzocrea S, Mazzon E, Caputi AP. Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur. J. Pharmacol. 1998;363(1):57–63. doi: 10.1016/s0014-2999(98)00673-6. [DOI] [PubMed] [Google Scholar]
- 38.Crespo E, Macias M, Pozo D, Escames G, Martin M, Vives F, Guerrero JM, Acuna-Castroviejo D. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999;13:1537–1546. [PubMed] [Google Scholar]
- 39.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 40.Cuzzocrea S, Zingarelli B, Gilad E, Hake P, Salzman AL, Szabó C. Protective effect of melatonin in carrageenan-induced models of local inflammation: relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. J. Pineal Res. 1997;23(2):106–116. doi: 10.1111/j.1600-079x.1997.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 41.Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J. Chem. Neuroanat. 1996;10 :183–201. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 42.Seabra V, Bignotto M, Pinto LR, Jr, Tufik S. Randomised double blind clinical trial, controlled with placebo of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000;29:193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 43.Deng YQ, Xu GG, Duan P, Zhang Q, Wang JZ. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol. Sin. 2005;26:519–526. doi: 10.1111/j.1745-7254.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 44.Droge W. Oxidative stress and aging. Adv. Exp. Med. Biol. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- 45.Duchossoy Y, Arnaud S, Feldblum S. Matrix metalloproteinases: potential therapeutic target in spinal cord injury. Clin. Chem. Lab. Med. 2001;39:362–367. doi: 10.1515/CCLM.2001.057. [DOI] [PubMed] [Google Scholar]
- 46.Eddleston M, Mucke L. Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience. 1993;54:5–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global oxygen and glucose deprivation. Brain Res. 1994;651:92–100. doi: 10.1016/0006-8993(94)90683-1. [DOI] [PubMed] [Google Scholar]
- 49.Escames G, Leon J, Macias M, Khaldy H, Acuna-Castroviejo D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003;17:932–934. doi: 10.1096/fj.02-0692fje. [DOI] [PubMed] [Google Scholar]
- 50.Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin reduces stress-activated/mitogen-activated protein kinases in spinal cord injury. J. Pineal Res. 2009;46:79–86. doi: 10.1111/j.1600-079X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 51.Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin regulates matrix metalloproteinases after traumatic experimental spinal cord injury. J. Pineal Res. 2008; 45:149–156. doi: 10.1111/j.1600-079X.2008.00569.x. [DOI] [PubMed] [Google Scholar]
- 52.Feng Z, Chang Y, Cheng Y, Zhang BL, Qu ZW, Qin C, Zhang JT. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J. Pineal Res. 2004;37:129–136. doi: 10.1111/j.1600-079X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 53.Feng Z, Cheng Y, Zhang JT. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J. Pineal Res. 2004;37:198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 54.Feng Z, Qin C, Chang Y, Zhang JT. Early melatonin supplementation alleviates oxidative stress in a transgenic mouse model of Alzheimer’s disease. Free Radic. Biol. Med. 2006;40:101–109. doi: 10.1016/j.freeradbiomed.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Feng Z, Zhang JT. Protective effect of melatonin on beta-amyloid-induced apoptosis in rat astroglioma C6 cells and its mechanism. Free Radic. Biol. Med. 2004;37:1790–1801. doi: 10.1016/j.freeradbiomed.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 56.Floyd RA, Carney JM. The role of metal ions in oxidative processes and aging. Toxicol. Ind. Health. 1993;9:197–214. doi: 10.1177/0748233793009001-214. [DOI] [PubMed] [Google Scholar]
- 57.Floyd RA, Hensley K. Oxidative stress in braing aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 58.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 59.Fujimoto T, Nakamura T, Ikeda T, Takagi K. Potent protective effects of melatonin on experimental spinal cord injury. Spine. 2000;25:769–775. doi: 10.1097/00007632-200004010-00003. [DOI] [PubMed] [Google Scholar]
- 60.Garcia JJ, Reiter RJ, Pie J, Ortiz GG, Cabrera J, Sáinz RM, Acuña-Castroviejo D. Role of pinoline and melatonin in stabilizing hepatic microsomal membranes against oxidative stress. J. Bioenerg. Biomembr. 1999;31:609–616. doi: 10.1023/a:1005425213253. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Navarro A, Gonzalez-Puga C, Escames G, López LC, López A, López-Cantarero M, Camacho E, Espinosa A, Gallo MA, Acuña-Castroviejo D. Cellular mechanisms envolved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in culture. J. Pineal Res. 2007;43:195–205. doi: 10.1111/j.1600-079X.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- 62.Gegg ME, Beltran B, Salas-Pino S, Bolanos JP, Clark JB, Moncada S, Heales SJ. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/ neurodegeneration? J. Neurochem. 2002;86:228–237. doi: 10.1046/j.1471-4159.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- 63.Genovese T, Mazzon E, Crisafulli C, Esposito E, Di Paola R, Muià C, Di Bella P, Bramanti P, Cuzzocrea S. Effects of combination of melatonin and dexamethasone on secondary injury in an experimental mice model of spinal cord trauma. J. Pineal Res. 2007;43:140–153. doi: 10.1111/j.1600-079X.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- 64.Genovese T, Mazzon E, Mariotto S, Menegazzi M, Cardali S, Conti A, Suzuki H, Bramanti P, Cuzzocrea S. Modulation of nitric oxide homeostasis in a mouse model of spinal cord injury. J. Neurosurg. Spine. 2006;4:145–153. doi: 10.3171/spi.2006.4.2.145. [DOI] [PubMed] [Google Scholar]
- 65.Genovese T, Mazzon E, Muia C, Bramanti P, De Sarro A, Cuzzocrea S. Attenuation in the evolution of experimental spinal cord trauma by treatment with melatonin. J. Pineal Res. 2005;38:198–208. doi: 10.1111/j.1600-079X.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 66.Gilad E, Wong HR, Zingarelli B, Virág L, O'Connor M, Salzman AL, Szabó C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: role of inhibition of NF-kB activation. FASEB J. 1998;12:685–693. doi: 10.1096/fasebj.12.9.685. [DOI] [PubMed] [Google Scholar]
- 67.Gitto E, Reiter RJ, Sabatino G, Buonocore G, Romeo C, Gitto P, Buggé C, Trimarchi G, Barberi I. Correlation among cytokines, bronchopulmonary dysplasia and modality of ventilation in preterm newborns: improvement with melatonin treatment. J. Pineal Res. 2005;39:287–293. doi: 10.1111/j.1600-079X.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 68.Guenther AL, Schmidt SL, Laatsch H, Fotso S, Ness H, Ressmeyer AR, Poeggeler B, Hardeland R. Reactions of the melatonin metabolite AMK (N1-acetyl-5-methxykynuramine) with reactive nitrogen species: formation of novel compounds, 3-acetamidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J. Pineal Res. 2005;39:251–260. doi: 10.1111/j.1600-079X.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 69.Guinjoan SM, Vigo DE, Castro MN, Tateosian N, Chuluyan E, Costanzo E, Fahrer R, Grancelli H, Leiguarda R, Cardinali DP. Mood, Th1/Th2 cytokine profile, and autonomic activity in older adults with acute/decompensated Herat failure: preliminary observations. World J. Biol. Psychiatry. 2008;20:1–6. doi: 10.1080/15622970802432153. [DOI] [PubMed] [Google Scholar]
- 70.Gul S, Celik SE, Kalayci M, Tasyürekli M, Cokar N, Bilge T. Dose-dependent neuroprotective effects of melatonin on experimental spinal cord injury in rats. Surg. Neurol. 2005;64:355–361. doi: 10.1016/j.surneu.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 71.Gutierrez-Cuesta J, Tajes M, Jimenez A, Coto-Montes A, Camins A, Pallas M. Evaluation of potential pro-survival pathways regulated by melatonin in a murine senescence model. J. Pineal Res. 2008;45:497–505. doi: 10.1111/j.1600-079X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 72.Hagan RM, Oakley NR. Melatonin comes of age? Trends Pharmacol. Sci. 1995;16:81–83. doi: 10.1016/s0165-6147(00)88985-3. [DOI] [PubMed] [Google Scholar]
- 73.Hardeland R, Backhaus C, Fadavi A. Reactions of the NO redox forms BO+, *NO and HNO (protonated NO-) with the melatonin metabolite B1-acetyl-5-methoxykynurenamine. J. Pineal Res. 2007;43:382–388. doi: 10.1111/j.1600-079X.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 74.Harlan JM. Consequences of leukocytes-vessel wall interactions in inflammatory and immune reactions. Semin. Thromb. Hemost. 1987;13:425–433. doi: 10.1055/s-2007-1003520. [DOI] [PubMed] [Google Scholar]
- 75.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 76.Hill JM, Switzer RC. The regional distribution and cellular localization of iron in rat brain. Neuroscience. 1984;11:595–603. doi: 10.1016/0306-4522(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 77.Hirata F, Hayaishi O, Tokuyama T, Seno S. In vitro and in vivo formation of two new metabolites of melatonin. J. Biol. Chem. 1974;249:1311–1313. [PubMed] [Google Scholar]
- 78.Hsu JY, Mckeon R, Goussev S, Werb Z, Lee JU, Trivedi A, Noble-Haeusslein LJ. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J. Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huerto-Delgadillo L, Anton-Tay F, Benitez-King G. Effects of melatonin on microtubule assembly depend on hormone concentration: role of melatonin as a calmodulin antagonist. J. Pineal Res. 1994;17:55–62. doi: 10.1111/j.1600-079x.1994.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 80.Iacovitti L, Stull ND, Johnston K. Melatonin rescues dopamine neurons from cell death in tissue culture models of oxidative stress. Brain Res. 1997;768:317–326. doi: 10.1016/s0006-8993(97)00668-9. [DOI] [PubMed] [Google Scholar]
- 81.Iacovitti L, Stull ND, Mishizen A. Neurotransmitters, KCl and antioxidants rescue striatal neurons from apoptotic cell death in culture. Brain Res. 1999;816:276–285. doi: 10.1016/s0006-8993(98)00955-x. [DOI] [PubMed] [Google Scholar]
- 82.Jacob S, Poeggeler B, Weishaupt JH, Siren AL, Hardeland R, Bahr M, Ehrenreich H. Melatonin as a candidate compound for neuroprotection in amyotrophic lateral sclerosis (ALS): High tolerability of daily oral melatonin administration in ALS patients. J. Pineal Res. 2002;33 :186–187. doi: 10.1034/j.1600-079x.2002.02943.x. [DOI] [PubMed] [Google Scholar]
- 83.Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Res. 1999;50:271–274. doi: 10.1093/toxsci/50.2.271. [DOI] [PubMed] [Google Scholar]
- 84.Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon M. Clinical trials of controlled-release melatonin in children with sleep-wake cycle disorders. J. Pineal Res. 2000;29:34–49. doi: 10.1034/j.1600-079x.2000.290105.x. [DOI] [PubMed] [Google Scholar]
- 85.Jang MH, Jung SB, Lee MH, Kim CJ, Oh YT, Kang I, Kim J, Kim EH. Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neurosci. Lett. 2005;380:26–31. doi: 10.1016/j.neulet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 86.Jesudason EP, Baben B, Ashok BS, Masilamoni JG, Kirubagaran R, Jebaraj WC, Jayakumar R. Anti-inflammatory effect of melatonin on a beta vaccination in mice. Mol. Cell. Biochem. 2007;298 :69–81. doi: 10.1007/s11010-006-9353-x. [DOI] [PubMed] [Google Scholar]
- 87.Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br. J. Pharmacol. 2008;154:1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joo JY, Uz T, Manev H. Opposite effects of pinealectomy and melatonin administration on brain damage following cerebral focal ischemia in rat. Restor. Neurol. Neurosci. 1998;13:185–191. [PubMed] [Google Scholar]
- 89.Jou MJ, Peng TI, Reiter RJ, Jou SB, Wu HY, Wen ST. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res. 2004;37:55–70. doi: 10.1111/j.1600-079X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 90.Jou MJ, Peng TI, Yu PZ, Jou SB, Reiter RJ, Chen JY, Wu HY, Chen CC, Hsu LF. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal Res. 2007;43:389–403. doi: 10.1111/j.1600-079X.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 91.Kaptanoglu E, Tuncel M, Palaoglu S, Konan A, Demirpençe E, Kilinç K. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J. Neurosurg. 2000;93:77–84. doi: 10.3171/spi.2000.93.1.0077. [DOI] [PubMed] [Google Scholar]
- 92.Kilic E, Kilic U, Yulug B, Hermann DM, Reiter RJ. Melatonin reduces disseminate neuronal death after mild focal ischemia in mice via inhibition of caspase-3 and is suitable as an add-on treatment to tissue-plasminogen activator. J. Pineal Res. 2004;36:171–176. doi: 10.1046/j.1600-079x.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- 93.Kilic U, Kilic E, Reiter RJ, Bassetti CL, Hermann DM. Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J. Pineal Res. 2005;38:67–71. doi: 10.1111/j.1600-079X.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 94.Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-dose Glucocorticoid Study Group. N. Engl. J. Med. 1995;333:142–146. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- 95.Klusman I, Schwab ME. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res. 1997;762:173–184. doi: 10.1016/s0006-8993(97)00381-8. [DOI] [PubMed] [Google Scholar]
- 96.Kneisley LW, Moskowitz MA, Lynch HG. Cervical spinal cord lesions disrupt the rhythm in human melatonin excretion. J. Neural. Transm. Suppl. 1978;13:311–323. [PubMed] [Google Scholar]
- 97.Koh PO. Melatonin attenuates the cerebral ischemic injury via the MEK/ERK/p90RSK/bad signaling cascade. J. Vet. Med. Sci. 2008;70:1219–1223. doi: 10.1292/jvms.70.1219. [DOI] [PubMed] [Google Scholar]
- 98.Koh PO. Melatonin attenuates the focal cerebral ischemic injury by inhibiting the dissociation of pBad from 14-3-3. J. Pineal Res. 2008;44:101–106. doi: 10.1111/j.1600-079X.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 99.Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: an established antioxidant worthy of use in clinical trials. Mol. Med. 2009;15:43–50. doi: 10.2119/molmed.2008.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kostrzewa RM, Segura-Aguilar J. Novel mechanisms and approaches in the study of neurodegeneration and neuroprotection. A review. Neurotox. Res. 2003;5:375–383. doi: 10.1007/BF03033166. [DOI] [PubMed] [Google Scholar]
- 101.La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J. Exp. Med. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leon J, Acuna-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005;38:1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 103.Leon J, Escames G, Rodriguez MI, Lopez LC, Tapias V, Entrena A, Camacho E, Carrion MD, Gallo MA, Espinosa A, Tan DX, Reiter RJ, Acuna-Castroviejo D. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006;98:2023–2033. doi: 10.1111/j.1471-4159.2006.04029.x. [DOI] [PubMed] [Google Scholar]
- 104.Leonard BE, Song C. Stress and the immune system in the etiology of anxiety and depression. Pharmacol. Biochem. Behav. 1996;54:299–303. doi: 10.1016/0091-3057(95)02158-2. [DOI] [PubMed] [Google Scholar]
- 105.Li XC, Wang ZF, Zhang JX, Wang Q, Wang JZ. Effect of melatonin on calyculin A-induced tau hyperphosphorylation. Eur. J. Pharmacol. 2005;510:25–30. doi: 10.1016/j.ejphar.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 106.Li Y, Jiang DH, Wang ML, Jiao DR, Pang SF. Rhythms of serum melatonin in patients with spinal lesions at the cervical, thoracic or lumbar region. Clin. Endocrinol. 1989;30:47–56. doi: 10.1111/j.1365-2265.1989.tb03726.x. [DOI] [PubMed] [Google Scholar]
- 107.Limson J, Nyokong T, Daya S. The interaction of melatonin and its precursors with aluminum, cadmium, copper, iron, lead, and zinc: an absorptive voltammetry study. J. Pineal Res. 1998;24:15–21. doi: 10.1111/j.1600-079x.1998.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 108.Lin A, Ho LT. Melatonin suppresses iron-induced neurodegeneration in rat brain. Free Radic. Biol. Med. 2000;28:904–911. doi: 10.1016/s0891-5849(00)00169-6. [DOI] [PubMed] [Google Scholar]
- 109.Lindsey RW, Gugala Z, Pneumaticos SG. Injuries of the Vertebrae and Spinal Cord. In: Moore EE, Feliciano DV, Mattox KL, editors. “Trauma”. 5th. McGraw Hill; 2003. pp. 459–492. (ISBN0-07-137069-2) [Google Scholar]
- 110.Ling X, Zhang LM, Lu SD, Li XJ, Sun FY. Protective effect of melatonin on injuried cerebral neurons is associated with bcl-2 protein over-expression. Zhongguo Yao Li. Xue Bao. 1999;20:409–414. [PubMed] [Google Scholar]
- 111.Liu JB, Tang TS, Yang HL, Xiao DS. Antioxidation of melatonin against spinal cord injury in rats. Chin. Med J. 2004;117:571–575. [PubMed] [Google Scholar]
- 112.Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J. Clin. Endocrinol. Metab. 1999;84:323–327. doi: 10.1210/jcem.84.1.5394. [DOI] [PubMed] [Google Scholar]
- 113.Liu SJ, Wang JZ. Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacol. Sin. 2002;23:183–187. [PubMed] [Google Scholar]
- 114.Lopez A, Garcia JA, Escames G, Venegas C, Ortiz F, López LC, Acuña-Castroviejo D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J, Pineal Res. 2009;46:188–198. doi: 10.1111/j.1600-079X.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 115.Lu B, Wang L, Stehlik C, Medan D, Huang C, Hu S, Chen F, Shi X, Rojanasakul Y. Phosphatidylinositol 3-kinase/ Akt positively regulates Fas (CD95)-mediated apoptosis in epidermal Cl41 cells. J. Immunol. 2006;176:6785–6793. doi: 10.4049/jimmunol.176.11.6785. [DOI] [PubMed] [Google Scholar]
- 116.Mahlberg R, Walther S, Kalus P, Bohner G, Haedel S, Reischies FM, Kuhl KP, Hellweg R, Kunz D. Pineal calcification in Alzheimer’s disease: An in vivo study using computed tomography. Neurobiol. Aging. 2008;29:203–209. doi: 10.1016/j.neurobiolaging.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 117.Mailliet F, Ferry G, Vella F, Berger S, Cogé F, Chomarat P, Mallet C, Guénin SP, Guillaumet G, Viaud-Massuard MC, Yous S, Delagrange P, Boutin JA. Characterization of the melatoninergic MT3 binding site on the NRH: quinone oxidoreductase 2 enzyme. Biochem. Pharmacol. 2005;71(1-2):74–88. doi: 10.1016/j.bcp.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 118.Maldonado MD, Murillo-Cabezas F, Terron MP, Flores LJ, Tan DX, Manchester LC, Reiter RJ. The potential of melatonin in reducing morbidity-mortality after craniocerebral trauma. J. Pineal Res. 2007;42:1–11. doi: 10.1111/j.1600-079X.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 119.Maldonado MD, Siu AW, Sanchez-Hidalgo M. Melatonin and lipid uptake by murine fibroblasts: clinical implications. Neuroendocrinol. Lett. 2006;27:601–608. [PubMed] [Google Scholar]
- 120.Manda K, Ueno M, Anzai K. AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J. Pineal Res. 2002;42:386–393. doi: 10.1111/j.1600-079X.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 121.Marchetti B. Cross talk signals in the CNS: role for neurotrophic and hormonal factors, adhesion molecules and intercellular signalling agents in luteinizing hormone - releasing hormone (LHRH) - astroglial interactive network. Front Biosci. 1997;5:d88–d125. doi: 10.2741/a177. [DOI] [PubMed] [Google Scholar]
- 122.Martin M, Macias M, Escames G, Leon J, Acuna-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000;14:1677–1679. doi: 10.1096/fj.99-0865fje. [DOI] [PubMed] [Google Scholar]
- 123.Martin V, Sainz RM, Antolin I, Mayo JC, Herrera F, Rodríguez C. Several antioxidant pathways are involved in astrocyte protection by melatonin. J. Pineal Res. 2002;33:204–212. doi: 10.1034/j.1600-079x.2002.02113.x. [DOI] [PubMed] [Google Scholar]
- 124.Martinez GR, Almeida EA, Klitzke CF, Onuki J, Prado FM, Medeiros MH, Di Mascio P. Measurement of melatonin and its metabolites: importance for the evaluation of their biological roles. Endocrine. 2005;27:111–118. doi: 10.1385/endo:27:2:111. [DOI] [PubMed] [Google Scholar]
- 125.Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ. Antiinflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5- methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005;165:139–149. doi: 10.1016/j.jneuroim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 126.Mayo JC, Tan DX, Sainz RM, Lopoz-Burillo S, Reiter RJ. Oxidative damage to catalase induced by peroxy radicals: functional protection bymelatonin and other antioxidants. Free. Radic. Res. 2003;37 :543–553. doi: 10.1080/1071576031000083206. [DOI] [PubMed] [Google Scholar]
- 127.Mctigue DM, Tani M, Krivacic K., Chernosky A., Kelner G.S, Maciejewski D, Maki R, Ransohoff R.M, Stokes B.T. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J. Neurosci. Res. 1998;53:368–376. doi: 10.1002/(SICI)1097-4547(19980801)53:3<368::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 128.Menendez-Pelaez A, Howes KA, Gonzalez-Brito A, Reiter RJ. N-acetyltransferase activity, hydroxyindole-Omethyltransferase activity, and melatonin levels in the Harderian glands of the female Syrian hamster: changes during the light:dark cycle and the effect of 6-parachlorophenylalanine administration. Biochem. Biophys. Res. Commun. 1987; 145:1231–1238. doi: 10.1016/0006-291x(87)91569-5. [DOI] [PubMed] [Google Scholar]
- 129.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 130.Mohan N, Sadeghi K, Reiter RJ, Meltz ML. The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochem. Mol. Biol. Int. 1995;37:1063–1070. [PubMed] [Google Scholar]
- 131.Morera AL, Abreu P. Seasonality of psychopathology and circannual melatonin rhythm. J. Pineal Res. 2006;41:279–283. doi: 10.1111/j.1600-079X.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 132.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell. Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murphy MP. Nitric oxide and cell death. Biochim. Biophys. Acta. 1999;1411:401–414. doi: 10.1016/s0005-2728(99)00029-8. [DOI] [PubMed] [Google Scholar]
- 134.Nesic O, Lee J, Unabia GC, Johnson K, Ye Z, Vergara L, Hulsebosch CE, Perez-Polo JR. Aquaporin 1 - a novel player in spinal cord injury. J. Neurochem. 2008;105:628–640. doi: 10.1111/j.1471-4159.2007.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nesic-Taylor O, Cittelly D, Ye Z, Xu GY, Unabia G, Lee JC, Svrakic NM, Liu XH, Youle RJ, Wood TG, McAdoo D, Westlund KN, Hulsebosch CE, Perez-Polo JR. Exogenous Bcl-xL fusion protein spares neurons after spinal cord injury. J. Neurosci. Res. 2005;79:628–637. doi: 10.1002/jnr.20400. [DOI] [PubMed] [Google Scholar]
- 136.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J. Neurosci. 2002;22:7526– 7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Norrbrink Budh C, Hultling C, Lundeberg T. Quality of sleep in individuals with spinal cord injury: a comparison between patients with and without pain. Spinal Cord. 2005;43:85–95. doi: 10.1038/sj.sc.3101680. [DOI] [PubMed] [Google Scholar]
- 138.Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, Lefoulon F, Fauchere JL, Delagrange P, Canet E, Boutin JA. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000;275:31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 139.Onuki J, Almeida EA, Medeiros MH, Di Mascio P. Inhibition of 5-aminolevulinic acid-induced DNA damage by melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, quercetin or resveratrol. J. Pineal. Res. 2005;38:107–115. doi: 10.1111/j.1600-079X.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 140.Ozcankaya R, Delibas N. Malondialdehyde, superoxide dismutase, melatonin, iron, copper, and zinc blood concentrations in patients with Alzheimer disease: Cross-sectional study. Croat. Med. J. 2002;43:28–32. [PubMed] [Google Scholar]
- 141.Pajovic SB, Saicic ZS, Spasic MB, Petrovic MB. The effect of ovarian hormones on antioxidant enzyme activities in the brain of male rats. Physiol. Rev. 2003;52:189–194. [PubMed] [Google Scholar]
- 142.Paparrigopoulos T, Psarros C, Bergiannaki J, Varsou E, Dafni U, Stefanis C. Melatonin response to clonidine administration in depression: indication of presynaptic alpha2-adrenoceptor dysfunction. J. Affect. Disord. 2001;65:307–313. doi: 10.1016/s0165-0327(00)00270-6. [DOI] [PubMed] [Google Scholar]
- 143.Pappolla MA, Chyan YJ, Poeggeler B, Frangione B, Wilson G, Ghiso J, Reiter RJ. An assessment of the antioxidant and the antiamyloidogenic properties of melatonin implications for Alzheimer’s disease. J. Neural Transm. 2000;107:203–231. doi: 10.1007/s007020050018. [DOI] [PubMed] [Google Scholar]
- 144.Pappolla MA, Simovich MJ, Bryant-Thomas T, Chyan YJ, Poeggeler B, Dubocovich M, Bick R, Perry G, Cruz-Sanchez F, Smith MA. The neuroprotective activities of melatonin against the Alzheimer beta-protein are not mediated by melatonin membrane receptors. J. Pineal Res. 2002;32:135–142. doi: 10.1034/j.1600-079x.2002.1o838.x. [DOI] [PubMed] [Google Scholar]
- 145.Pappolla MA, Sos M, Omar RA, Bick RJ, Hickson-Bick DL, Reiter RJ, Efthimiopoulos S, Robakis NK. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 1997;17:1683–1690. doi: 10.1523/JNEUROSCI.17-05-01683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peyrot F, Ducroig C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J. Pineal Res. 2008;45:235–246. doi: 10.1111/j.1600-079X.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 147.Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F. Melatonin: a peroxyl free radical scavenger more effective than vitamin E. Life Sci. 1994;55:271–276. doi: 10.1016/0024-3205(94)00666-0. [DOI] [PubMed] [Google Scholar]
- 148.Pozo D, Reiter RJ, Calvo JR, Guerrero JM. Inhibition of cerebellar nitric oxide synthase and cyclic GMP production by melatonin via complex formation with calmodulin. J. Cell Biochem. 1997;65:430–442. doi: 10.1002/(sici)1097-4644(19970601)65:3<430::aid-jcb12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 149.Ravindra T, Lakshmi NK, Ahuja YR. Melatonin in pathogenesis and therapy of cancer. Indian J. Med. Sci. 2006;60:523–535. [PubMed] [Google Scholar]
- 150.Rebrin I, Zicker S, Wedekind KJ, Paetau-Robinson I, Packer L, Sohal RS. Effect of antioxidant- enriched diets on glutathione redox status in tissue homogenates and mitochondria of the senescence-accelerated mouse. Free Radic. Biol. Med. 2005;39:549–557. doi: 10.1016/j.freeradbiomed.2005.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reiter RJ. Melatonin: the chemical expression of darkness. Mol. Cell. Endocrinol. 1991;79:C153–C158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 152.Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995;9:526–533. [PubMed] [Google Scholar]
- 153.Reiter RJ, Acuna-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage: mechanisms for the protective actions of melatonin in the central nervous system. Ann. N.Y. Acad. Sci. 2001;939:200–215. [PubMed] [Google Scholar]
- 154.Reiter RJ, Cabrera J, Sainz RM, Mayo JC, Manchester LC, Tan DX. Melatonin as a pharmacological agent against neuronal loss in experimental models of Huntington’s disease, Alzheimer’s disease and parkinsonism. Ann. N.Y. Acad. Sci. 1999;890:471–485. doi: 10.1111/j.1749-6632.1999.tb08028.x. [DOI] [PubMed] [Google Scholar]
- 155.Reiter RJ, Tan DX, Leon J, Kilic U, Kilic E. When melatonin gets on your nerves: its beneficial actions in experimental models of stroke. Exp. Biol. Med. 2005;230:104–117. doi: 10.1177/153537020523000205. [DOI] [PubMed] [Google Scholar]
- 156.Reiter RJ, Tan DX, Manchester LC, Tamura H. Melatonin defeats neurally-derived free radicals and reduces the associated neuromorphological and neurobehavioral damage. J. Physiol. Pharmacol. 2007;58(Suppl. 6):5–22. [PubMed] [Google Scholar]
- 157.Reiter RJ, Tan DX, Qi W, Manchester LC, Karbownik M, Calvo JR. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol. Signals Recept. 2000;9:160–171. doi: 10.1159/000014636. [DOI] [PubMed] [Google Scholar]
- 158.Reiter RJ, Tan DX, Cabrera J, D'Arpa D, Sainz RM, Mayo JC, Ramos S. The oxidant/antioxidant network: role of melatonin. Biol. Signals Recept. 1999;8:56–63. doi: 10.1159/000014569. [DOI] [PubMed] [Google Scholar]
- 159.Reiter RJ, Tan DX, Jou MJ, Korkmaz A, Manchester LC, Paredes SD. Biogenic amines in the reduction of oxidative stress: melatonin and its metabolites. Neuro. Endocrinol. Lett. 2008;29:391–398. [PubMed] [Google Scholar]
- 160.Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S. Medical implications of melatonin: receptor-mediated and receptor independent actions. Adv. Med. Sci. 2007;52:11–28. [PubMed] [Google Scholar]
- 161.Reiter RJ. Melatonin: clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab. 2003;17:273–285. doi: 10.1016/s1521-690x(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 162.Reppert SM. Melatonin receptors: molecular biology of a new family of G protein coupled receptors. J. Biol. Rhythms. 1997;12(6):528–531. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- 163.Reppert SM, Perlow MJ, Tamarkin L, Klein DC. A diurnal melatonin rhythm in primate cerebrospinal fluid. Endocrinology. 1979;104(2):295–301. doi: 10.1210/endo-104-2-295. [DOI] [PubMed] [Google Scholar]
- 164.Ressmeyer AR, Mayo JC, Zelosko V, Sainz RM, Tan DX, Poeggeler B, Antolin I, Zsizsik BK, Reiter RJ, Hardeland R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003;8:205–213. doi: 10.1179/135100003225002709. [DOI] [PubMed] [Google Scholar]
- 165.Rodriguez MI, Carretero M, Escames G, López LC, Maldonado MD, Tan DX, Reiter RJ, Acuña-Castroviejo D. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic. Res. 2007; 41:15–24. doi: 10.1080/10715760600936359. [DOI] [PubMed] [Google Scholar]
- 166.Rodriguez MI, Escames G, Lopez LC, López A, García JA, Ortiz F, Acuña-Castroviejo D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J. Pineal Res. 2007;42:272–279. doi: 10.1111/j.1600-079X.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 167.Rodriguez MI, Escames G, Lopez LC, Lopez A, Garcia JA, Ortiz F, Sanchez V, Romeu M, Acuna-Castroviejo D. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp. Gerontol. 2008;43:749–756. doi: 10.1016/j.exger.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 168.Rosen J, Than NN, Koch D, Poeggeler B, Laatsch H, Hardeland R. Interactions of melatonin and its metabolites with the ABTS cation radical: extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006;41:374–381. doi: 10.1111/j.1600-079X.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 169.Rosenberg GA, Dencoff JE, Correa N, Jr, Reiners M, Ford CC. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: relation to blood-brain barrier injury. Neurology. 1996;46:1626–1632. doi: 10.1212/wnl.46.6.1626. [DOI] [PubMed] [Google Scholar]
- 170.Rosenberg GA, Estrada EY, Denco JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 171.Rosenbluth J. Central myelin in the mouse mutant shiverer. J. Comp. Neurol. 1980;194: 639–648. doi: 10.1002/cne.901940310. [DOI] [PubMed] [Google Scholar]
- 172.Samantaray S, Sribnick EA, Das A, Knaryan VH, Matzelle DD, Yallapragada AV, Reiter RJ, Ray SK, Banik NL. Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. J. Pineal. Res. 2008;44:348–357. doi: 10.1111/j.1600-079X.2007.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sanchez-Hidalgo M, Lu Z, Tan DX. Melatonin inhibits fatty acid induced triglyceride accumulation in ROS 17/2.8 cells: implications for osteoblast differentiation and osteoporosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:2208–2215. doi: 10.1152/ajpregu.00013.2007. [DOI] [PubMed] [Google Scholar]
- 174.Sanchez-Moreno C, Dorfman SE, Lichtenstein AH, Martín A. Dietary fat type affects vitamins C and E and biomarkers of oxidative status in peripheral and brain tissues of golden Syrian hamsters. J. Nutr. 2004;134:655–660. doi: 10.1093/jn/134.3.655. [DOI] [PubMed] [Google Scholar]
- 175.Scheer FA, Zeitzer JM, Ayas NT, Brown R, Czeisler CA, Shea SA. Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord. 2006;44:78–81. doi: 10.1038/sj.sc.3101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Seabra ML, Bignotto M, Pinto LR, Jr, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000;29:193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 177.Sewerynek E. Melatonin and the cardiovascular system. Neuro Endocrinol. Lett. 2002;23:79–83. [PubMed] [Google Scholar]
- 178.Shapiro SD. A concise yet informative stroll through matrix metalloproteinases and TIMPs. J. Cell. Sci. 2000;113:3355– 3356. [Google Scholar]
- 179.Smirnov AN. Nuclear melatonin receptors. Biochemistry. 2001;66(1):19–26. doi: 10.1023/a:1002821427018. [DOI] [PubMed] [Google Scholar]
- 180.Southgate G, Daya S. Melatonin reduces quinolinic acid-induced lipid peroxidation in rat brain homogenate. Metab. Brain Dis. 1999;14 :165–171. doi: 10.1023/a:1020610708637. [DOI] [PubMed] [Google Scholar]
- 181.Soybir G, Topuzlu C, Odabas O, Dolay K, Bilir A, Köksoy F. The effects of melatonin on angiogenesis and wound healing. Surg. Today. 2003;33:896–901. doi: 10.1007/s00595-003-2621-3. [DOI] [PubMed] [Google Scholar]
- 182.Srinivasan V, Spence DW, Trakh I, Pandi-Perumal SR, Cardinali DP, Maestroni GJ. Immunomodulation by melatonin: its significance for seasonally occurring diseases. Neuroimmunomodulation. 2008;15: 93–101. doi: 10.1159/000148191. [DOI] [PubMed] [Google Scholar]
- 183.Steinhilber D, Brungs M, Werz O, Wiesenberg I, Danielsson C, Kahlen JP, Nayeri S, Schrader M, Carlberg C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J. Biol. Chem. 1995;270(13):7037–7040. doi: 10.1074/jbc.270.13.7037. [DOI] [PubMed] [Google Scholar]
- 184.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stover SL, Fine PR. The epidemiology and economics of spinal cord injury. Paraplegia. 1987;25:225–228. doi: 10.1038/sc.1987.40. [DOI] [PubMed] [Google Scholar]
- 186.Sugden D, Davidson K, Hough KA, The MT. Melatonin, melatonin receptors and melanophores: a moving story. Pigment Cell. Res. 2004;17(5):454–460. doi: 10.1111/j.1600-0749.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 187.Sun FY, Lin X, Mao LZ, Ge WH, Zhang LM, Huang YL, Gu J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J. Pineal Res. 2002;33:48–56. doi: 10.1034/j.1600-079x.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 188.Swarnakar S, Mishra A, Ganguly K, Sharma AV. Matrix metalloproteinase- 9 activity and expression is reduced by melatonin during prevention of ethanol-induced gastric ulcer in mice. J. Pineal Res. 2007;43:56–64. doi: 10.1111/j.1600-079X.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 189.Szczepanik M. Melatonin and its influence on immune system. J. Physiol. Pharmacol. 2007;58(6):115–124. [PubMed] [Google Scholar]
- 190.Tan DX, Chen LD, Poeggeler B. Melatonin: a potent endogenous hydroxyl radical scavenger. Endocr. J. 1993;1:57–60. [Google Scholar]
- 191.Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR. Significance of melatonin in antioxidative defense system: reactions and products. Biol. Signals Recept. 2000;9:137–159. doi: 10.1159/000014635. [DOI] [PubMed] [Google Scholar]
- 192.Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003;34:75–78. doi: 10.1034/j.1600-079x.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- 193.Tan DX, Manchester LC, Reiter RJ, Qi WB, Zhang M, Weintraub ST, Cabrera J, Sainz RM, Mayo JC. Identification of highly elevated levels of melatonin in bone marrow: its origin and significance. Biochim. Biophys. Acta. 1999;1472:206–214. doi: 10.1016/s0304-4165(99)00125-7. [DOI] [PubMed] [Google Scholar]
- 194.Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, Weintraub ST, Qi W. Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radic. Biol. Med. 2000;29:1177–1185. doi: 10.1016/s0891-5849(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 195.Tan DX, Manchester LC, Terron MP, Flores LJ, Tamura H, Reiter RJ. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: hypothesis and significance. J. Pineal Res. 2007;43:317–320. doi: 10.1111/j.1600-079X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 196.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 197.Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 198.Taskiran D, Tanyalcin T, Sozmen EY, Peker GO, Gulmen V, Cagli S, Kanit L, Tekeli G, Barcin E, Zileli M, Kutay FZ. The effects of melatonin on the antioxidant systems in experimental spinal injury. Int. J. Neurosci. 2000;104:63–73. doi: 10.3109/00207450009035009. [DOI] [PubMed] [Google Scholar]
- 199.Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 200.Taupin P. Adult neurogenesis, neuroinflammation and therapeutic potencial of adult neural ıtem cells. Int. J. Med. Sci. 2008;5:127–132. doi: 10.7150/ijms.5.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tjong YW, Li MF, Hung MW, Fung ML. Melatonin ameliorates hippocampal nitric oxide production and large conductance calcium-activated potassium channel activity in chronic intermittent hypoxia. J. Pineal Res. 2008;44:234–243. doi: 10.1111/j.1600-079X.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 202.Tomas-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005;39:99–104. doi: 10.1111/j.1600-079X.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 203.Topsakal C, Kilic N, Ozveren F, Akdemir I, Kaplan M, Tiftikci M, Gursu F. Effects of prostaglandin E1, melatonin, and oxytetracycline on lipid peroxidation, antioxidant defense system, paraoxonase (PON1) activities, and homocysteine levels in an animal model of spinal cord injury. Spine. 2003;28:1643–1652. doi: 10.1097/01.BRS.0000083163.03910.B1. [DOI] [PubMed] [Google Scholar]
- 204.Tu Y, Sun RQ, Willis WD. Effects of intrathecal injections of melatonin analogs on capsaicin-induced secondary mechanical allodynia and hyperalgesia in rats. Pain. 2004;109:340–350. doi: 10.1016/j.pain.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 205.Tunez I, Montilla P, Del Carmen Munoz M, Feijoo M, Salcedo M. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington’s disease. J. Pineal Res. 2004;37:252–256. doi: 10.1111/j.1600-079X.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 206.Tyor WR, Avgeropoulos N, Ohlandt G, Hogan EL. Treatment of spinal cord impact injury in the rat with transforming growth factor beta. J. Neurol. Sci. 2002;200:33–41. doi: 10.1016/s0022-510x(02)00113-2. [DOI] [PubMed] [Google Scholar]
- 207.Vaillant C, Didier-Bazes M, Hutter A, Belin MF, Thomasset N. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J. Neurosci. 1999;19:4994–5004. doi: 10.1523/JNEUROSCI.19-12-04994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vane JR, Botting RM. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998;47:S78–S87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 209.Veneroso C, Tuñón MJ, González-Gallego J, Collado PS. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal Res. 2009;47(2):184–91. doi: 10.1111/j.1600-079X.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 210.Wang DL, Ling ZQ, Cao FY, Zhu LQ, Wang JZ. Melatonin attenuates isoproterenol-induced protein kinase A overactivation and tau hyperphosphorylation in rat brain. J. Pineal Res. 2004;37:11–16. doi: 10.1111/j.1600-079X.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 211.Wang JZ, Wang ZF. Role of melatonin in Alzheimerlike neurodegeneration. Acta Pharmacol. Sin. 2006;27:41–49. doi: 10.1111/j.1745-7254.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 212.Wang X, Figueroa BE, Stavrovskaya IG, Zhang Y, Zhu S, Day AL, Kristal BS, Friedlander RM. Methazolamide and melatonin inhibit mitochondrial cytochrome c release and are neuroprotective in experimental models of ischemic injury. Stroke. 2009;40:1877–1885. doi: 10.1161/STROKEAHA.108.540765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang XC, Zhang J, Yu X, Han L, Zhou ZT, Zhang Y, Wang JZ. Prevention of isoproterenol-induced tau hyperphosphorylation by melatonin in the rat. Sheng Li Xue Bao. 2005;57:7–12. [PubMed] [Google Scholar]
- 214.Weishaupt JH, Bartels C, Polking E, Dietrich J, Rohde G, Poeggeler B, Mertens N, Sperling S, Bohn M, Huther G, Schneider A, Bach A, Sirén AL, Hardeland R, Bähr M, Nave KA, Ehrenreich H. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 2006;41:313–323. doi: 10.1111/j.1600-079X.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 215.Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, Yong VW. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J. Neurosci. 2003;23:10107–10115. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Witt-Enderby PA, Bennett J, Jarzynaka MM, Firestine S, Melan MA. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. 2003;72:2183–2198. doi: 10.1016/s0024-3205(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 217.Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007;100:639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 218.Xu Z, Wang BR, Wang X, Kuang F, Duan XL, Jiao XY, Ju G. Erk1/2 and p38 mitogen activated protein kinase mediate inos-induced spinal neuron degeneration after acute traumatic spinal cord injury. Life Sci. 2006;79:1895–1905. doi: 10.1016/j.lfs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 219.Yeung HM, Hung MW, Fung ML. Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J. Pineal Res. 2008;45:373–382. doi: 10.1111/j.1600-079X.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 220.Yong W, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yun HY, Dawson VL, Dawson TM. Nitric oxide in health and diseases of the nervous system. Mol. Psychiatry. 1997;2:300–310. doi: 10.1038/sj.mp.4000272. [DOI] [PubMed] [Google Scholar]
- 222.Yune TY, Chang MJ, Kim SJ, Lee YB, Shin SW, Rhim H, Kim YC, Shin ML, Oh YJ, Han CT, Markelonis GJ, Oh TH. Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J. Neurotrauma. 2003;20:207–219. doi: 10.1089/08977150360547116. [DOI] [PubMed] [Google Scholar]
- 223.Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. J. Clin. Endocrinol. Metab. 2000;85:2189–2196. doi: 10.1210/jcem.85.6.6647. [DOI] [PubMed] [Google Scholar]
- 224.Zeitzer JM, Ayas NT, Wu AD, Czeisler CA, Brown R. Bilateral oculosympathetic paresis associated with loss of nocturnal melatonin secretion in patients with spinal cord injury. J. Spinal Cord. Med. 2005;28:55–59. doi: 10.1080/10790268.2005.11753798. [DOI] [PubMed] [Google Scholar]
- 225.Zhou J, Zhang S, Zhao X, Wei T. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-beta1-42. J. Pineal Res. 2008;45:157–165. doi: 10.1111/j.1600-079X.2008.00570.x. [DOI] [PubMed] [Google Scholar]