Abstract
α-Retinol (αR) is a structural isomer of retinol [vitamin A (VA)] that does not bind to serum retinol-binding protein (RBP). In this study, α-retinyl acetate (αRA) was synthesized and given orally (35 μmol) to VA-deficient lactating sows (n = 11) to assess its potential to trace RBP-independent retinol transport and tissue uptake. The αRA dose primarily appeared in sow serum as 4 α-retinyl esters (αRE) with peak serum total αR concentrations (the sum of the alcohol and ester forms) detected at 2 h (70 ± 23 nmol/L, mean ± SEM) postdose. From 0 to 40 h postdose, the percentage of serum total αR in the alcohol form did not increase. Rapid αR uptake into sow milk was observed with peak concentrations (371 ± 83 nmol/L) at 7.5 h postdose, consistent with the uptake of αRE from chylomicra. A high percentage of the αRA dose (62 ± 15%, mean ± SD) was present in the livers of sows (n = 6) killed 22–28 d postdose. Approximately 15–26% of the sow αRA dose was transferred to the livers of the nursing piglets (n = 17) after 3 d. In piglets and sows, a similar percentage of hepatic total αR was detected in the ester form as that of hepatic total retinol. Taken together, these data suggest that an oral dose of αRA effectively traces the uptake, esterification, chylomicron transport, and hepatic storage of retinol and may be useful for deciphering the role of RBP-independent delivery of retinol to other tissues.
Introduction
α-Retinol (αR)4 is an analogue of vitamin A (VA) formed naturally from the metabolism of α-carotene (1, 2), a prominent provitamin A carotenoid found in carrots as well as some other fruits and vegetables (3). αR differs from retinol only in a shift of the 5, 6 double bond to the 4, 5 position (2), but this structural alteration greatly modifies its bioactivity. Early studies indicated that, despite its ability to accumulate in liver (1) and bind to at least one type of cellular retinol-binding protein (4), αR possessed <2% of the biopotency of retinol as defined by its ability to support growth in VA-deficient rats (5). The low bioactivity of αR was at least partially explained by the finding that it could not bind to serum retinol-binding protein (RBP) (6), the major protein responsible for the physiological transport of retinol between tissues (7). RBP is sometimes referred to as RBP4 to distinguish it from cellular RBP.
More recently, αR was detected in the livers of Mongolian gerbils supplemented with α-carotene (2). The hepatic αR concentrations were very similar to the difference in hepatic retinol concentrations between α-carotene- and cottonseed oil-fed gerbils, suggesting that the α-retinal and retinal formed from α-carotene cleavage were metabolized, transported, and stored in the liver in a similar fashion. Additionally, no αR was detected in gerbil serum despite the presence of ample amounts of αR in the liver, supporting the previous in vitro findings that αR does not bind to RBP (6). From these data, we hypothesized that αR may be a useful chemical tracer for studying the RBP-independent transport and tissue uptake of retinol, which may occur primarily through its association with chylomicra and other lipoproteins (8, 9).
Therefore, the primary goal of the current study was to assess whether αR is an effective tracer of RBP-independent retinoid bioprocesses by examining the accumulation and clearance of αR and α-retinyl ester (αRE) in the serum, milk, and livers of VA-depleted lactating sows following a single, oral dose (35 μmol) of α-retinyl acetate (αRA). The lactating sow-piglet model has been previously utilized for studying various nutritional and biochemical aspects of VA supplementation to VA-deficient lactating women with extrapolation to nursing infants in developing nations (10–13). For comparative reasons, the size of the αRA dose was selected to be the same as the amount of 3, 4-didehydroretinyl acetate (DRA) administered as a tracer in a prior study (12). We also measured the hepatic and extrahepatic accumulation of αR in the nursing piglets from the αRA-dosed sows to estimate the percentage of the αRA dose transferred through the sow milk, which, presumably, could only occur through RBP-independent mechanisms.
Materials and Methods
Synthesis of αRA.
αRA was synthesized using a previously described method for the synthesis of 13C-retinyl acetate (14) except that α-ionone (Sigma Aldrich) instead of β-ionone was used as the starting reagent and no 13C was added. The synthesized αRA was purified (>95%) on 8%-water–deactivated alumina using hexanes and diethyl ether. Purity was confirmed via characterization by TLC, UV-VIS spectroscopy, and HPLC equipped with photodiode array (PDA) detection.
Animals and diet.
Approval for animal use was obtained from the University of Wisconsin (UW)-Madison Animal Care and Use Committee and all animal procedures adhered to the public health service policy on humane care and use of laboratory animals. The College of Agriculture and Life Sciences’ facilities are AAALAC accredited and frequently inspected. First-litter sows (gilts) (crossbreeds of Large White and Landrace) were housed at the Swine Research and Teaching Center in Arlington, WI. At 6.2 mo of age (31–47 d before breeding with Duroc boars), the gilts’ diet was changed from a standardized fortified diet to a diet that did not contain added preformed VA (Table 1). This diet was continued through gestation and after birth until the pigs were killed for liver analysis. Late-kill piglets were weaned at 7–10 d of age and were then provided a diet containing no preformed VA until they were killed.
TABLE 1.
Nutrient composition of sow diets for depleting VA stores
| Ingredient | Feed, g/kg |
| Wheat grain | 794 |
| Soybean meal, 48% crude protein | 126 |
| Dicalcium phosphate | 17.0 |
| Limestone, ground | 8.0 |
| Fat1 | 30.0 |
| Iodized sodium chloride | 5.0 |
| UW vitamin and mineral mix2 | 20.0 |
MaxFat (Maxco, Green Bay, WI), a blend of animal and vegetable fats.
A vitamin and mineral premix was developed that consisted of 62% wheat, 10% Vitamin Mix (Teklad, Madison, WI) (in g/kg Vitamin Mix: cholecalciferol, 0.26; dl-α-tocopheryl acetate, 72.0; vitamin B complex, 5.40; biotin, 7.50; folic acid 0.50; niacin, 5.53; pantothenic acid, 12.87; riboflavin, 7.84; vitamin B-12, 13.64; corn, 874.5), 13% choline, and 15% UW Mineral Mix (Mineral Mix resulted in the following minerals in mg/kg final feed: iron, 61; selenium, 0.3; zinc, 48; iodine 7.7; copper, 18).
Dosing and tissue collection.
On d 5 of lactation, each sow (n = 11) was given a single, oral dose (35 μmol) of αRA dissolved in corn oil after collecting a baseline sample of blood and milk. Blood (0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 10, 20, and 40 h postdose) was collected (8–10 mL/time) by placing jugular catheters into nonanesthetized sows as previously described (11). Milk (2.5, 5, 7.5, 10, 15, 20, 40, and 60 h postdose) was collected (10–15 mL/time) after oxytocin treatment (11). Serum and milk were placed on dry ice until being transferred to −80°C conditions. During the collection time, piglets were allowed to freely nurse. Piglets were killed 3 or 23 d after dosing and their liver, kidneys, spleen, lungs, and adrenal glands were collected. All piglet tissues were stored at −80°C until analysis. Sows (n = 6) were killed ∼25 d after αR dosing and their livers were stored at −20°C until analysis.
Serum analysis of αR.
Serum total αR (the sum of the alcohol and ester forms) was analyzed using an adaptation of a previously published method (2). Briefly, 1 mL serum was thawed and 10 μL of synthesized C23-alcohol (a β-apo-carotenol) (2) was added as an internal standard. Serum was deproteinized with 1 mL ethanol, and αR and αRE were extracted from serum 3 times with hexanes (1 mL). The pooled hexane extracts were dried under nitrogen. To each dried sample was added 0.75 mL ethanol + 0.1% BHT and 0.4 mL 50:50 KOH:H2O (wt:v) for saponification (15). Samples were placed in a 45°C water bath for 10 min followed by the addition of 0.5 mL water. αR was then immediately extracted 3 times with hexanes (1 mL) and the combined extracts were dried. The dried samples were redissolved in 75 μL methanol:dichloroethane (50:50, v:v) and 20 μL was injected into an HPLC system consisting of a Waters 717plus autosampler, a Waters 600E multisolvent delivery system, a guard column, a Waters Symmetry C18 column (3.5 μm, 4.6 × 75 mm) in series with a Waters Resolve C18 column (5 μm, 3.9 × 300 mm), a Shimadzu (Kyoto, Japan) SPD-10A UV-VIS detector, and a Shimadzu C-R7Aplus Chromatopac data processor. Samples were analyzed at 311 nm using an isocratic method (87.5:12.5, MeOH:H2O, v:v; with 10 mmol/L ammonium acetate at 0.8 mL/min). This method effectively separated αR from retinol (typical retention times of 21.5 and 22.8 min, respectively) and the C23-alcohol typically eluted at 38.7 min. A small peak was detected in baseline serum samples with a similar retention time to αR. The peak’s absorption spectra, as determined by HPLC equipped with a PDA detector, did not match that of αR. Therefore, for each sow, the area of this peak at baseline was subtracted from the area of the αR peak at all time points as a corrective measure. A standard curve was constructed from purified αR obtained from saponification of synthesized αRA standard to allow for peak quantification. The mass extinction coefficient (E1 cm1%) for αR is 1650 at 311 nm (1).
To determine the percentage of αR in ester form, 2 mL serum was thawed and 20 μL internal standard and 2 mL ethanol were added for deproteinization. Three hexane (2 mL) extracts were combined, split into 2 equal volumes, and dried. One of the divided extracts was analyzed in the saponified state (quantifies αR + αRE) as described above and the other was analyzed in the unsaponified state (quantifies αR). The percentage of αR in the ester form was then calculated using the following standard equation:
Serum analysis of retinyl ester and αRE.
To qualitatively assess the serum distribution of retinyl ester and αRE, serum from 3 different sows at 0 or 2 h postdose was thawed and pooled (3 mL/time point). Each sample was then deproteinized with 3 mL ethanol and extracted with 3 mL hexanes 3 times. The extracts were combined, split into 2 equal volumes, and dried; 1 portion was saponified. Samples were redissolved in 75 μL methanol:dichloroethane (50:50, v:v) and analyzed using a previously described HPLC method for retinyl ester analysis with slight modifications (2). The Waters HPLC system consisted of a 717plus autosampler, 1525 binary HPLC pump, guard column, Resolve C18 column (5 μm, 3.9 × 300 mm), and 996 PDA detector. The mobile phases were acetonitrile:water (95:5, v:v; solvent A) and acetonitrile:methanol:dichloroethane (85:10:5, v:v:v; solvent B), both containing 10 mmol/L ammonium acetate. Samples were analyzed at 2 mL/min using a gradient procedure: 1) 100% A for 3 min; 2) 7-min linear gradient to 100% B; 3) 12-min hold; and 4) 2-min reverse gradient to 100% A.
Milk analysis.
Milk (1 mL) was analyzed for total αR and retinol using an adaptation of a previously published method (12). To each milk sample, 20 μL C23-alcohol, 1.5 mL ethanol + 0.1% BHT, and 0.8 mL 50:50 KOH:H2O were added. After saponification for 1 h at 45°C, 3 hexane extractions, and extract drying, the residue was reconstituted in 100 μL methanol:dichloroethane (50:50, v:v) and 20 μL was injected onto a Waters HPLC system using the isocratic HPLC method previously described for separation of αR from retinol.
The fat content of individual milk samples was analyzed as previously described (12). Briefly, 2 mL 2:1 (v:v) dichloromethane:methanol and 1 mL ethanol was added to 1 mL milk. The sample was mixed on a vortex and centrifuged. The top layer was placed in a new test tube; 1 mL dichloromethane and 1 mL water (0.6% NaCl) were added, followed by mixing on a vortex and centrifuging. The bottom layer was transferred back to the original storage test tube; 1 mL 2:1 (v:v) dichloromethane:methanol and 1 mL ethanol were added followed by mixing on a vortex and centrifuging to form a delipidated pellet. The extract was transferred to a tared test tube; 1 mL water (0.6% NaCl) was added and the sample was mixed on a vortex and centrifuged. The aqueous layer was discarded and the extract dried under nitrogen overnight. When no solvents were visible, the test tube was weighed to determine the fat (g/L milk). The CV of this method was 4.0%.
Organ analyses.
Sow and piglet livers were analyzed using an adaptation of a previously published method (13). Samples were obtained from several sections of the liver and pooled. Briefly, liver (0.4–0.6 g) was ground with 3–5 g anhydrous sodium sulfate, extracted repeatedly with dichloromethane, and filtered into a 50-mL volumetric flask. After grinding, C23 alcohol (250 μL) was added as an internal standard for extraction efficiency. Aliquots (2 mL) were dried and analyzed in the saponified or unsaponified states as described previously. All residues were redissolved in 150 μL methanol:dichloroethane (50:50, v:v) and 20 μL was injected and analyzed using the isocratic HPLC method described above. The percents of retinol and αR in ester form were calculated using the formula described earlier.
Kidney (1.5–2.5 g), lung (1.5–2.5 g), spleen (1.5–2.5 g), and adrenal gland (0.4–0.6 g) extracts were analyzed as described above except that less C23-alcohol (25–50 μL) was added. For kidney, 8 mL aliquots of the 50-mL extracts was dried and analyzed. For the other organs, the 50-mL extract was split into two 25-mL portions that were dried and analyzed in the saponified and unsaponified states.
Statistical analysis.
A repeated-measures ANOVA test with spatial power error structure using time as a fixed effect and individual pigs as a random effect was applied using SAS PROC MIXED (version 8.2, SAS Institute) to determine the main effects of the αRA dose on total αR and retinol concentrations in serum and milk. The natural log transformation was used to ensure that the residuals were normally distributed. The least square means were calculated and the overall significance at specific time points was determined by least square means differences. Data normality was confirmed using a Shapiro-Wilk test. For serum and milk αR levels, the assumption of equal variance across all time points was confirmed using a likelihood ratio test. An unpaired t test was used to compare the percentage of piglet hepatic total αR and total retinol in the ester form with that of sows. Differences were considered significant at P < 0.05.
Results
Serum αR and retinol.
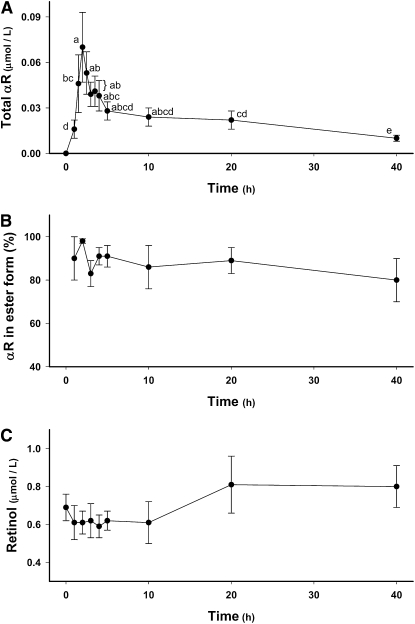
Serum samples were saponified to hydrolyze all αRE to αR. Following a single, oral dose of αRA (35 μmol) to sows, their serum total αR concentrations (the sum of the alcohol and ester forms) rose rapidly from undetectable levels to 70 ± 23 nmol/L (mean ± SEM) at 2 h (Fig. 1A). Serum total αR concentrations declined to greater than one-half that concentration by 5 h. A more gradual loss was observed from 5 to 40 h postdose, and αR could still be detected in saponified serum samples of most sows at 40 h. In unsaponified serum samples (0–40 h), little to no αR was detected. Using the αR concentrations in saponified and unsaponified serum samples, the mean percentage of serum αR in ester form was calculated to be 80–98% at all analyzed time points with no significant changes in this percentage detected from 0 to 40 h (Fig. 1B).
FIGURE 1.
Time course (0–40 h) of serum total αR concentration (A), the percentage of total αR in ester form (B), and retinol concentrations (C) in lactating sows administered αRA (35 μmol). Values are means ± SEM, n = 7–11. Means without a common letter differ, P < 0.05.
Chromatographic profiles of pooled and concentrated serum samples from several sows at 0 and 2 h post-dose (Supplemental Fig. 1A,B, respectively) revealed the appearance of αRE (peaks 1–4) at 2 h. These peaks were identified as αRE based on their nearly identical absorption spectra to αR, which has 3 distinct λ-maxima at 325, 311, and 298 nm and by their complete disappearance following saponification of the 2-h pooled serum sample (Supplemental Fig. 1C).
In contrast to serum αR, serum retinol was present almost completely in the alcohol form as indicated by the lack of detection of retinyl esters from 0 to 40 h by HPLC-PDA (data not shown). No changes in serum retinol concentrations were detected from 0 to 40 h postdose (Fig. 1C).
Milk αR and retinol.
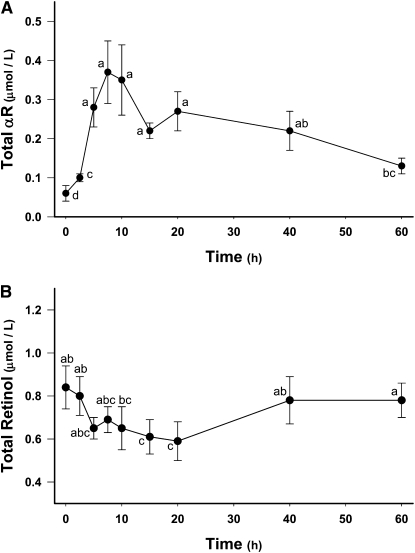
A significant accumulation of αR in sow milk was detected by 2.5 h (Fig. 2A). Compared with serum, peak total αR concentrations in milk were much higher (0.37 ± 0.08 μmol/L) and peaked later (7.5 h). From 7.5 to 60 h postdose, milk αR concentrations steadily declined but were still elevated at 60 h compared with baseline milk αR concentrations. Detection of small amounts of αR (0.05 ± 0.01 μmol/L) in baseline (0 h) milk samples was not anticipated; however, HPLC-PDA analysis revealed the presence of a peak matching both the retention time and the UV absorption spectrum of αR. Therefore, we concluded that the peak was indeed αR.
FIGURE 2.
Time course (0–60 h) of milk total αR (A) and retinol (B) concentrations in lactating sows administered αRA (35 μmol). Values are means ± SEM, n = 7–11. Means without a common letter differ, P < 0.05.
Milk total retinol concentrations (Fig. 2B) at 15 h (0.61 ± 0.08 μmol/L) and 20 h (0.59 ± 0.09 μmol/L) postdose were lower than baseline total retinol concentrations (0.82 ± 0.10 μmol/L). At 40 and 60 h postdose, milk total retinol concentrations were similar to baseline levels. Milk fat content (9.2 ± 1.9%) was similar at all time points. Correction of total αR and retinol concentrations in milk for milk fat content (data not shown) did not result in any significant changes in the data trends.
Tissue accumulation of αR in sows and piglets.
Total mean retinol concentrations in sow and piglet livers were <0.07 μmol/g liver, confirming VA deficiency (2). Of the 11 sows dosed with αRA, 6 were randomly chosen to be killed after 22–28 d to measure their total hepatic αR concentrations. A high percentage (62 ± 15%, mean ± SD) of the total αRA dose was detected in the sow livers as αR and αRE (Table 2). Randomly chosen piglets (n = 17) taken from the litters of the other 5 αRA-dosed sows contained 2.2 ± 0.7% of the αRA dose/liver after 3 d, whereas piglets (n = 6) killed 23 d postdose had very low hepatic αR concentrations. Analysis of the kidneys, spleens, and adrenal glands of the early-kill piglets revealed detectable but low amounts (<8 nmol/organ) of αR compared with their hepatic αR concentrations. In sows and piglets, the percentage of hepatic αR in the ester form was similar to that of retinol; however, piglet livers analyzed 3 d postdose had a higher percentage of total αR (83 ± 6%) and total retinol (87 ± 8%) in the ester form compared with sow livers (62 ± 7 and 52 ± 4%, respectively).
TABLE 2.
Sow and piglet hepatic αR concentrations following a single, oral dose of 35 μmol αRA to lactating sows
| Group | Time after dose, d | n | Total αR, nmol/g liver | Liver weight, g | Total αR, nmol/liver |
| Sows | 22–28 | 6 | 9.2 ± 2.31 | 2347 ± 306 | 21,550 ± 5190 |
| Piglets | 3 | 17 | 9.9 ± 3.2 | 77 ± 13 | 754 ± 232 |
| 23 | 6 | 0.06 ± 0.02 | 90 ± 25 | 4.9 ± 2.3 |
Values are means ± SD.
To estimate the percentage of the αRA dose transferred from each of the 5 dosed sows to their litters, the percentage (mean ± SD) of the sow αRA dose present in the livers of individual littermates was multiplied by the total number of piglets in the litter (Table 3). Approximately 22% of the sow αRA dose was transferred to the livers of their nursing piglets after 3 d.
TABLE 3.
Estimated total transfer of the α-RA dose administered to 5 lactating sows to the livers of their piglets after 3 d
| Piglets analyzed, n | αRA dose in liver, % | Piglets in litter, n | Estimated αRA dose transferred to litter,2% |
| 4 | 3.0 ± 0.71 | 7 | 21 ± 5 |
| 4 | 2.4 ± 0.2 | 11 | 26 ± 2 |
| 3 | 1.4 ± 0.4 | 11 | 15 ± 4 |
| 3 | 2.2 ± 0.2 | 12 | 26 ± 2 |
| 3 | 1.6 ± 0.2 | 12 | 21 ± 2 |
Values are means ± SD.
Percentage of the sow αRA dose present in the livers of individual littermates multiplied by the total number of piglets in the litter.
Discussion
The total αR concentrations in the livers of αRA-dosed lactating sows and their nursing piglets accounted for the majority of the sow αRA dose (62 and 22%, respectively). This implies that >80% of the αRA dose given to sows was bioavailable, a value similar to that reported for retinyl acetate (RA) (70–90%) (16, 17). The αRA dose predominantly appeared as αRE in sow serum, which was similar to RA-dosed sows where retinyl palmitate, oleate, sterate, and linoleate accounted for >90% of the serum retinyl ester content (11). Based on these observations as well as the typical chromatographic retention times of these retinyl esters (2), the identities of the 4 αRE peaks (Supplemental Fig. 1B) were likely α-retinyl linoleate, oleate, palmitate, and sterate, in order of retention time. Serum αRE was mostly cleared by 40 h, primarily via uptake into the liver and milk; however, there was no corresponding increase in serum αR. This was in contrast to previous results from sows dosed with DRA (12), where the serum concentration of didehydroretinol (DR), which binds to RBP, rapidly increased from 0–10 h. The lack of serum αR provided additional confirmation of the inability of αR to bind to RBP. The decline in milk retinol concentrations observed 15 and 20 h after αRA dosing may have been due to replacement with the newly delivered αR and suggests that αR and retinol may interact with similar physiological and biochemical pathways. In the liver, a similar percentage of total hepatic αR was stored in the ester form as that of total hepatic retinol in both sows and piglets. Collectively, these observations indicate that the αRA dose was absorbed into enterocytes, converted to αRE, transported into the circulation bound to chylomicra, taken up by tissues, and hepatically stored in a similar fashion as postprandial retinol but was not recirculated bound to RBP.
The major RBP-independent mechanism by which retinol may be physiologically distributed is via chylomicron-mediated transport and tissue uptake of retinyl esters (18–20). This has been well illustrated in RBP knockout mice, which, although viable, must derive their VA tissue requirements in this manner (21). Approximately 75% of chylomicron-bound postprandial retinol is cleared by the liver, with the remaining 25% cleared extrahepatically (19). A similar distribution pattern was observed for the αRA dose, because 40–76% of ingested αRA was detected in sow livers analyzed 22–28 d postdose. Sow livers were not analyzed at any earlier time points, because they were nursing the piglets; however, these data suggest that αR clearance from sow liver was slow, largely due to the inability of hepatic RBP to release it for transport to extrahepatic tissues. In contrast, piglet hepatic clearance of αR appeared to be more efficient with very little hepatic αR detected 23 d postdose. This could indicate that hepatic RBP-independent clearance mechanisms, such as excretion into the bile (22), are more active in growing piglets than in sows.
The rapid and significant accumulation of αR in the sow milk is consistent with previous findings that chylomicron-bound retinyl esters are an important source of milk total VA. In lactating rats, the uptake of retinyl esters from chylomicra by the mammary tissue is rapid (23) and is more significant compared with nonlactating rats (24, 25), likely due to elevated lipoprotein lipase expression (26, 27). Supplementation studies in lactating rats have also indicated a major role for dietary retinol in the milk and mammary tissue VA concentrations (28). In this study, based on the hepatic αR stores of analyzed piglets, 15–26% of the sow αRA dose was transferred to their litters after 3 d, which, at a minimum, also represents the percentage transferred into the milk. When sows were given the same dose (35 μmol) of DRA, which leads to the formation of the RBP-DR complex, an estimated 10–20% of the dose was irreversibly lost in milk in 2 d, and peak milk concentrations of total DR (0.35 ± 0.14 μmol/L) were similar to those of total αR in this study (0.37 ± 0.08 μmol/L) (12). Thus, for both αRA and DRA, the primary mechanisms by which their respective metabolites enter the milk in the first 2–3 d postdose appear to be RBP independent and chylomicron mediated. The αR content of other sow tissues was not analyzed; however, based on the very small αR amounts detected in piglet kidney, spleen, adrenal gland, and lung 3 d postdose, uptake into milk likely represented a sizable portion of the total extrahepatic αR tissue uptake in sows.
The detection of low amounts of αR in baseline milk samples was not anticipated and suggests that the very small amount of α-carotene present in both the standard fortified and VA-deficient diets (29, 30) was efficiently converted to αR. Low amounts of endogenous αR may also have been present in the swine livers; however, the detection of only trace amounts of hepatic αR in piglets 23 d postdose suggests that very little endogenous αR was present. In sows, it is not clear if endogenous hepatic αR concentrations were high enough to play an important inflationary role in the calculated percentage of the αRA dose recovered in their livers.
One goal of the present study was to provide some additional insights into the contribution of RBP-independent mechanisms in the transport, uptake, and transfer of retinol to milk in VA-deficient lactating women and their nursing infants following VA supplementation, which is commonly provided as large doses of retinyl ester (31, 32). Although such VA doses are comparatively much higher than the dose of αRA given to sows, the present study provides additional evidence that chylomicron-mediated uptake of retinyl esters into the milk accounts for a sizable percentage of the retinyl ester dose and the vast majority of its accumulation in tissue 2–3 d after VA supplementation. The rate of hepatic chylomicron clearance and the expression level of lipoprotein lipase in the mammary tissue likely play important roles in the amount of retinol transferred from mother to nursing child immediately following a dose of RA. The use of appropriately high αR tracer doses will be important for future work to better model high-dose VA supplementation.
In summary, these data establish αR as an effective tracer for RBP-independent retinoid accumulation and transfer in the lactating sow-nursing piglet model. They also provide additional evidence that chylomicron-derived retinyl esters rather than RBP-bound retinol are likely to be the major source of retinol in the milk of VA-deficient lactating mothers in the first 2–3 d following an oral dose of retinyl ester. To date, most knowledge regarding the precise contributions of RBP-independent mechanisms in retinol transport and tissue uptake has been obtained using RBP-knockout mice (7). The use of αR as a tracer now provides a method for effectively examining the biological role of RBP-independent retinol distribution in sophisticated, nontransgenic animal models.
Supplementary Material
Acknowledgments
We thank Peter Crump at the UW-Madison College of Agricultural and Life Sciences for assistance with data statistical analysis. S.A.T. designed research; J.T.D. and C.R.D. conducted research; R.L.S. and C.R.D. conducted analyses; J.T.D. analyzed data; J.T.D., S.A.T., and C.R.D. wrote the paper; and J.T.D. and S.A.T. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by USDA National Research Initiative 2007-35200-17729, NIH grant T32-DK007665 (J.T.D.), and the University of Wisconsin-Madison Graduate School 135-GV88.
Supplemental Figure 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: αR, α-retinol; αRA, α-retinyl acetate; αRE, α-retinyl ester; DR, 3,4-didehydroretinol; DRA, 3,4-didehydroretinyl acetate; PDA, photodiode array; RA, retinyl acetate; RBP, serum retinol-binding protein; VA, vitamin A.
Literature Cited
- 1.McAnally JS, Szymanski CD. Metabolism of alpha-carotene. Nature. 1966;210:1366. [DOI] [PubMed] [Google Scholar]
- 2.Tanumihardjo SA, Howe JA. Twice the amount of α-carotene isolated from carrots is as effective as β-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr. 2005;135:2622–6 [DOI] [PubMed] [Google Scholar]
- 3.Holden JM, Eldridge AL, Beecher GR, Buzzard IM, Bhagwat S, Davis CS, Douglass LW, Gebhardt S, Haytowitz D, et al. Carotenoid content in U.S. foods: an uptake of the database. J Food Compost Anal. 1999;12:169–96 [Google Scholar]
- 4.Ong DE, Chytil F. Specificity of cellular retinol-binding protein for compounds with vitamin A activity. Nature. 1975;255:74–5 [DOI] [PubMed] [Google Scholar]
- 5.Goodman DS, Smith JE, Hembry RM, Dingle JT. Comparison of the effects of vitamin A and its analogs upon rabbit ear cartilage in organ culture and upon growth of the vitamin A-deficient rat. J Lipid Res. 1974;15:406–14 [PubMed] [Google Scholar]
- 6.Muhilal H, Glover J. The affinity of retinol and its analogues for retinol-binding protein. Biochem Soc Trans. 1975;3:744–6 [DOI] [PubMed] [Google Scholar]
- 7.Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med. 2003;24:421–30 [DOI] [PubMed] [Google Scholar]
- 8.Paik J, Vogel S, Quadro L, Piantedosi R, Gottesman M, Lai K, Hemberger L, Vieira MM, Blaner WS. Vitamin A: overlapping delivery pathways to tissues from the circulation. J Nutr. 2004;134:S276–80 [DOI] [PubMed] [Google Scholar]
- 9.Berr F, Kern F., Jr Plasma clearance of chylomicrons labeled with retinyl palmitate in healthy human subjects. J Lipid Res. 1984;25:805–12 [PubMed] [Google Scholar]
- 10.Valentine AR, Tanumihardjo SA. One-time vitamin A supplementation in lactating sows enhances hepatic retinol in their offspring independent of dose size. Am J Clin Nutr. 2005;81:427–33 [DOI] [PubMed] [Google Scholar]
- 11.Penniston KL, Tanumihardjo SA. Elevated serum concentrations of β-glucuronide metabolites and 4-oxoretinol following treatment with vitamin A in lactating sows: a model for evaluating supplementation of lactating women. Am J Clin Nutr. 2005;81:851–8 [DOI] [PubMed] [Google Scholar]
- 12.Surles RL, Li J, Tanumihardjo SA. The modified-relative-dose-response values in serum and milk are positively correlated over time in lactating sows with adequate vitamin A status. J Nutr. 2006;136:939–45 [DOI] [PubMed] [Google Scholar]
- 13.Sun T, Surles RL, Tanumihardjo SA. Vitamin A concentrations in piglet extrahepatic tissues respond differently ten days after vitamin A treatment. J Nutr. 2008;138:1101–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanumihardjo SA. Synthesis of 10, 11, 14, 15-13C4- and 14, 15-13C2-retinyl acetate. J Labelled Comp Radiopharm. 2001;44:365–72 [Google Scholar]
- 15.Tanumihardjo SA, Penniston KL. Simplified methodology to determine breast milk retinol concentrations. J Lipid Res. 2002;43:350–5 [PubMed] [Google Scholar]
- 16.Sivakumar B, Reddy V. Absorption of labeled vitamin A in children during infection. Br J Nutr. 1972;27:299–304 [DOI] [PubMed] [Google Scholar]
- 17.Hollander D, Muralidhara KS. Vitamin A1 intestinal absorption in vivo: influence of luminal factors on transport. Am J Physiol. 1977;232:E471–7 [DOI] [PubMed] [Google Scholar]
- 18.Blaner WS, Obunike JC, Kurlandsky SB, Al-Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase hydrolysis of retinyl ester. J Biol Chem. 1994;269:16559–65 [PubMed] [Google Scholar]
- 19.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res. 1999;40:565–74 [PubMed] [Google Scholar]
- 20.Quadro L, Hamberger L, Gottesman ME, Colantuoni V, Ramakrishnan R, Blaner WS. Transplacental delivery of retinoid: the role of retinol-binding protein and lipoprotein retinyl ester. Am J Physiol Endocrinol Metab. 2004;286:E844–51 [DOI] [PubMed] [Google Scholar]
- 21.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zachman RD, Singer MB, Olson JA. Biliary secretion of metabolites of retinol and retinoic acid in the guinea pig and chick. J Nutr. 1966;88:137–42 [DOI] [PubMed] [Google Scholar]
- 23.Ross AC, Pasatiempo AMG, Green MH. Chylomicron margination, lipolysis, and vitamin A uptake in the lactating rat mammary gland: Implications for milk retinoid content. Exp Biol Med (Maywood). 2004;229:46–55 [DOI] [PubMed] [Google Scholar]
- 24.Mendelson CR, Zinder O, Blanchette-Mackie EJ, Chernick SS, Scow RO. Lipoprotein lipase and lipid metabolism in mammary gland. J Dairy Sci. 1977;60:666–76 [DOI] [PubMed] [Google Scholar]
- 25.Barber MC, Clegg RA, Travers MT, Vernon RG. Lipid metabolism in the lactating mammary gland. Biochim Biophys Acta. 1997;1347:101–26 [DOI] [PubMed] [Google Scholar]
- 26.Spooner PM, Garrison MM, Scow RO. Regulation of mammary and adipose tissue lipoprotein lipase and blood triacylglycerol in rats during late pregnancy. Effect of prostaglandins. J Clin Invest. 1977;60:702–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scow RO, Chernick SS, Fleck TR. Lipoprotein lipase and uptake of triacylglycerol, cholesterol and phosphatidylcholine from chylomicrons by mammary and adipose tissue of lactating rats in vivo. Biochim Biophys Acta. 1977;487:297–306 [DOI] [PubMed] [Google Scholar]
- 28.Akohoue SA, Green JB, Green MH. Dietary vitamin A has both chronic and acute effects on vitamin A indices in lactating rats and their offspring. J Nutr. 2006;136:128–32 [DOI] [PubMed] [Google Scholar]
- 29.Davis CR, Howe JA, Rocheford TR, Tanumihardjo SA. The xanthophyll composition of biofortified maize (Zea mays Sp.) does not influence the bioefficacy of provitamin A carotenoids in Mongolian gerbils (Meriones unguiculatus). J Agric Food Chem. 2008;56:6745–50 [DOI] [PubMed] [Google Scholar]
- 30.Kean EG, Hamaker BR, Ferruzzi MG. Carotenoid bioaccesibility from whole grain and degermed maize meal products. J Agric Food Chem. 2008;56:9918–26 [DOI] [PubMed] [Google Scholar]
- 31.WHO Micronutrient Initiative Safe vitamin A dosage during pregnancy and lactation: recommendation and report of a consultation. Geneva: WHO; 1998 [Google Scholar]
- 32.Surles RL, Mills JP, Valentine AR, Tanumihardjo SA. One-time graded doses of vitamin A to weanling piglets enhance hepatic retinol but do not always prevent vitamin A deficiency. Am J Clin Nutr. 2007;86:1045–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.