Abstract
Arginine is an essential amino acid in neonates synthesized by gut epithelial cells and a precursor for NO that regulates vasodilatation and blood flow. Arginine supplementation has been shown to improve intestinal integrity in ischemia-reperfusion models and low plasma levels are associated with necrotizing enterocolitis. We hypothesized that enteral arginine is a specific stimulus for neonatal intestinal blood flow and mucosal growth under conditions of total parenteral nutrition (TPN) or partial enteral nutrition (PEN). We first tested the dose dependence and specificity of acute (3 h) enteral arginine infusion on superior mesenteric artery (SMA) blood flow in pigs fed TPN or PEN. We then determined whether chronic (4 d) arginine supplementation of PEN increases mucosal growth and if this was affected by treatment with the NO synthase inhibitor, NG-nitro-l-arginine methyl ester (L-NAME). Acute enteral arginine infusion increased plasma arginine dose dependently in both TPN and PEN groups, but the plasma response was markedly higher (100–250%) in the PEN group than in the TPN group at the 2 highest arginine doses. Baseline SMA blood flow was 90% higher in the PEN (2.37 ± 0.32 L⋅kg−1⋅h−1) pigs than in the TPN pigs (1.23 ± 0.17 L⋅kg−1⋅h−1), but was not affected by acute infusion individually of arginine, citrulline, or other major gut fuels. Chronic dietary arginine supplementation in PEN pigs induced mucosal growth in the intestine, but this effect was not prevented by treatment with L-NAME. Intestinal crypt cell proliferation, protein synthesis, and phosphorylation of mammalian target of rapamycin and p70S6 kinase were not affected by dietary arginine. We conclude that partial enteral feeding, but not acute enteral arginine, increases SMA blood flow in the neonatal pig. Furthermore, supplementing arginine in partial enteral feeding modestly increases intestinal mucosal growth and was NO independent.
Introduction
Arginine is a nutritionally essential amino acid in neonates and is required for protein synthesis and growth (1). Although arginine production in adults occurs mainly in the kidneys, in the neonate arginine is exclusively synthesized in gut epithelial cells from amino acid precursors like citrulline, glutamine, glutamate, and proline (2). Except for serving as a building block for proteins, arginine has been shown to exert beneficial effects on intestinal integrity and function, because it is the major amino acid precursor of polyamines essential for gut healing (3), an enhancer of cell migration and activator of protein synthesis (4–6), and the sole physiological precursor for NO (2).
NO is a signaling molecule that plays a central role in regulating vascular resistance and hence blood flow in the newborn intestinal circulation (7, 8). NO is a potent vasodilator that is produced by NO synthase (NOS)7 that catalyzes the production of NO from arginine (2). There are 3 different isoforms of NOS: neuronal NOS (nNOS), which was first discovered in neuronal tissues; inducible NOS (iNOS), which is inducible under inflammatory conditions; and endothelial NOS (eNOS), which was first identified in endothelial cells. nNOS and eNOS are expressed constitutively at low levels in a variety of cell types and tissues, whereas iNOS is normally not expressed at a significant level in cells or tissues. NO synthesis is regulated by the availability of arginine (9). Low plasma levels of arginine lead to decreased NO synthesis and subsequently diminished blood flow in the small intestine in low-grade endotoxemia in rats (10).
Clinical studies have shown low levels of arginine in preterm infants and arginine deficiency associated with an increased incidence of necrotizing enterocolitis (NEC) (11–14). NEC is the most common intestinal emergency in the preterm infant, with reported mortality rates of up to 50% (15). A detrimental series of pathophysiological events involving intestinal inflammation and ischemia leads to mucosal eruption, invasion of bacteria into the intestinal wall, necrosis, and, subsequently, sepsis. Low circulating levels of arginine or arginine precursors result in a shortage of arginine and arginine-derived products like NO, which subsequently may contribute to the actual development of NEC (16).
In neonatal intensive care, most preterm infants receive full total parenteral nutrition (TPN) or TPN with minimal or partial enteral nutrition (PEN), because of enteral feeding intolerance. However, arginine synthesis is diminished in TPN fed pigs (17). More importantly, we have previously shown in pigs that TPN significantly reduced iNOS activity, decreased superior mesenteric artery (SMA) blood flow, and induced mucosal atrophy (17). Lack of enteral substrate and mucosal atrophy might decrease arginine synthesis by epithelial cells, leading to inadequate production of NO by the intestinal vasculature, and predispose the preterm infant to vasoconstriction and tissue injury.
Several investigators have evaluated arginine administration in animal models of ischemia-reperfusion and experimental NEC. Increased bioavailability of arginine caused a significant increase in NO production and tissue perfusion in rat microcirculation (18). Arginine administration enhanced serum NO production and decreased mucosal injury in rat models of ischemia-reperfusion (19–21). Actual blood flow measurements during intestinal ischemia-reperfusion showed an increase in blood flow upon arginine administration in both mice and pigs (22, 23). Moreover, administration of arginine has been shown to decrease the incidence of experimental NEC in an acidified casein piglet model (24) and a hypoxia-reoxygenation model in young mice (25). Arginine administration has also been evaluated in a randomized, double-blind, placebo-controlled study where arginine supplementation to infants < 28 wk of gestation increased plasma arginine levels and significantly decreased the incidence of NEC (26).
Several possibilities may explain how arginine supplementation reduces intestinal injury and NEC in the neonate. First, supplemental arginine may lead to increased local NO production via eNOS in the intestinal vasculature, leading to vasodilatation and preservation and/or restoration of intestinal blood flow. Second, the ability of arginine to prevent NEC and gut injury may be due to the trophic physiological effects on the intestinal epithelium. Arginine supplementation in in vitro and in vivo models stimulated intestinal protein synthesis and increased epithelial cell survival via enhanced mammalian target of rapamycin (mTOR) and p70S6 kinase (p70S6K) signaling (4–6). However, it is unknown whether this trophic effect of arginine on the epithelium is NO and/or blood flow dependent in the neonate. We hypothesized that enteral arginine is a specific stimulus for neonatal intestinal blood flow and subsequent mucosal growth. The objectives of this study were to establish the dose dependency and specificity of enteral arginine infusion on SMA blood flow in neonatal pigs fed TPN or partial enteral nutrition (PEN) and to test whether enteral arginine supplementation increases intestinal mucosal growth and protein synthesis by a NO-dependent mechanism.
Materials and Methods
Pigs.
The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the Guide for the Care and Use of Laboratory Animals [Department of Health and Human Services publication no. 85–23, revised 1985, Office of Science and Health Reports, NIH, Bethesda, MD]. Three-d-old crossbred pigs were obtained from the Texas Department of Criminal Justice transported to the animal facility at the Children’s Nutrition Research Center and immediately placed in cages in a heated room (30°C) until surgery the following day.
Study protocol: in vivo blood flow studies.
Pigs underwent surgery under isoflurane general anesthesia. Silastic catheters were inserted into the jugular vein, carotid artery, and gastric fundus as previously described (27). An ultrasonic blood flow probe was placed around the SMA (17). Pre-operatively, pigs received enrofloxacin (2.5 mg⋅kg−1; Baytril, Bayer) and this was continued on each postoperative day. After surgery, each piglet received 1 dose of analgesic (0.1 mg butorphenol tartrate⋅kg−1; Torbugesic, Fort Dodge Laboratories). During the initial 24 h postoperatively, all pigs received TPN at 50% of full intake; the 100% TPN intake provided (in g⋅kg−1⋅d−1) 25 glucose, 13 l-amino acids, 5 lipid, and 452 kJ⋅kg−1⋅d−1 at a volume of 120 mL⋅kg−1⋅d−1. The l-arginine intake during 100% TPN was 0.630 g⋅kg−1⋅d−1.
On d 2, pigs were assigned to receive either TPN (n = 5; 240 mL⋅kg−1⋅d−1), or PEN (n = 9; 40% enteral at 96 mL⋅kg−1⋅d−1) with a liquid cow milk-replacer formula (Litter Life, Merrick) fed in 3 oral meals/d. Pigs were weighed daily to adjust their intake. On postoperative d 3–5, blood flow was measured in pigs of both groups (TPN and PEN). Pigs were given a continuous, i.g. infusion in a randomly assigned cross-over design with saline (0.9% NaCl; 4 mL∙kg−1∙h−1) for 1 h (baseline) followed by a primed, continuous 3-h i.g. infusion with different doses of arginine at 50, 100, 200, 400, and 800 μmol⋅kg−1⋅h−1. The priming dose equaled the amount of amino acid infused within 2 h for the respective treatments. During enteral arginine infusions, pigs were also given TPN infused i.v. at 10 mL⋅kg−1⋅h−1, such that pigs received parenteral arginine at a rate of 150 μmol⋅kg−1⋅h−1. Pigs received only 1 arginine dose per day and were placed back on their respective basal diet of either TPN or PEN during the rest of the day. A 3rd group of pigs were fed PEN (n = 8, 40% enteral at 96 mL⋅kg−1⋅d−1) and were infused enterally with citrulline, glutamate, glutamine, or glucose as a control at 800 μmol⋅kg−1⋅h−1. In pigs given PEN, parenteral nutrition was infused i.v. at 6 mL⋅kg−1⋅h−1 and enteral formula was infused i.g. as a priming bolus (8 mL/kg) followed by a continuous infusion at 2 mL⋅kg−1⋅h−1. The PEN pigs received a parenteral and enteral arginine intake of 150 and 45 μmol⋅kg−1⋅h−1, respectively. In all 3 infusion groups, SMA blood flow was monitored continuously throughout the 4-h infusion protocol. Arterial blood samples (1 mL) were collected at 1 and 4 h for measurement of plasma amino acid concentrations. After completion of the protocol, pigs were killed with a venous injection of pentobarbital sodium (50 mg/kg) and sodium phenytoin (5 mg/kg, Beuthanasia-D, Schering-Plough Animal Health).
Study protocol: chronic arginine supplementation.
Pigs (n = 32) underwent surgery as described above without the implantation of a gastric catheter and SMA blood flow probe followed by 24 h of TPN as above. Beginning on postoperative d 2, pigs were weighed and assigned to 1 of 3 treatments based on equal body weights. Pigs received PEN (20% enteral at 48 mL⋅kg−1⋅d−1) with a cow's milk-replacer formula (Litter Life) via an orogastric bolus 5 times/d for 4 d supplemented with 1) arginine (ARG; 800 μmol⋅kg−1⋅h−1; n = 11); 2) arginine plus a NOS inhibitor NG-nitro-l-arginine methyl ester (ARG+L-NAME, ARG 800 μmol⋅kg−1⋅h−1 + L-NAME 200 μmol⋅kg−1⋅h−1; n = 9); or 3) l-alanine (CO, 800 μmol⋅kg−1⋅h−1; n = 11) as a control. At the end of the 4-d treatment period, 4 h prior to termination, each pig was injected with an i.v. bolus of 5-bromodeoxyuridine (BrdU; 50 mg/kg; Sigma Aldrich) to measure the in vivo crypt cell proliferation index (27). Additionally, 30 min prior to euthanasia, each animal received an i.v. flooding dose of l-phenylalanine (1.5 mmol/kg, containing 0.15 mmol/kg l-[ring-13C6] phenylalanine; Cambridge Isotope Laboratories) to measure the rate of tissue protein synthesis (17). Arterial blood samples were taken at 0 and 30 min of l-phenylalanine infusion. Pigs were then killed with an i.v. injection of pentobarbital sodium and sodium phenytoin. The small intestine was excised, flushed with saline, and divided into 2 segments, designated as jejunum and ileum, and weighed. Tissue sections were fixed in 10% buffered formalin for morphological and BrdU analysis. An aliquot of each segment was snap-frozen in liquid nitrogen and stored at −80°C until analysis for protein and DNA content and isotopic tracer enrichment for protein synthesis analysis.
Plasma and tissue analyses.
Plasma samples were assayed for amino acids by reverse-phase HPLC (Pico Tag, Waters), glucose by glucose oxidase (Sigma-Aldrich), and insulin by RIA as previously described (28). Frozen intestinal and liver tissue samples were homogenized and assayed for protein and DNA content (17).
Histology and Immunohistochemistry.
Morphometry analysis was performed on formalin-fixed, paraffin-embedded, hematoxylin and eosin-stained intestinal sections (5 μm) as described previously (28). Villus height, crypt depth, and muscularis thickness were measured by using an Axiophot microscope (Carl Zeiss) and NIH IMAGE software, version 1.60. In vivo crypt cell proliferation was measured by BrdU crypt-cell labeling (29).
Tissue protein synthesis.
Samples of jejunum, ileum, liver, muscle, and pancreas were homogenized and deproteinized with 2 mol/L perchloric acid as described previously (17). The perchloric acid-soluble (free amino acid pool) and acid-insoluble (protein-bound amino acid pool) fractions were subjected to mass spectrometric analysis. The acid-insoluble fraction was hydrolyzed with 6 mol/L HCl for 24 h before GC-MS analysis. To measure the enrichment of [13C6] phenylalanine in the tissue protein-bound pool, hydrolyzed samples were derivatized to form N-pivaloyl-i-propyl esters and measured by GC-combustion-isotope ratio MS (Thermo Finnigan Deltaplus XL GC-C-IRMS; Thermo Electron) (30).
Fractional protein synthesis rates (FSR; %⋅d−1) of jejunum, ileum, liver, and muscle were calculated as follows:
where IEbound and IEfree are the isotopic enrichments (mol% excess) of [13C6]phenylalanine of the perchloric acid-insoluble and perchloric acid-soluble pool, respectively, t is the time of labeling (min), and 1440 is the number of minutes per day. Absolute synthesis rates (ASR; g⋅kg−1⋅d−1) of jejunum, ileum, and liver were calculated as follows:
where protein is the protein content of the organ in g/kg body weight.
Tissue immunoblotting.
The tissue abundance of phosphorylated mTOR and p70s6k was measured by immunoblotting. Frozen muscle and intestinal tissue samples (200 mg) were homogenized in buffer A containing: 50 mmol/L HEPES (pH 7.4), 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 5 mg/L phenylmethylsulfonyl-fluoride, 5 mg/L aprotinin, 5 mg/L chymostatin, and 5 mg/L pepstatin. The homogenate was then sonicated and centrifuged at 12,000 g for 15 min at 4°C. The resulting extracts were separated via 7–15% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat milk in Tris-buffered saline (20 mmol/L Tris, 150 mmol/L NaOH, pH 7.4). Membranes were incubated with a primary antibody diluted in 5% nonfat milk in Tris-buffered saline + 0.1% Tween-20. Membranes were incubated with a secondary antibody (goat anti-rabbit IgG-HRP, or goat anti-mouse IgG-HRP, 1:5000, Santa Cruz Biotech) and the bands were detected as described below. The membranes were probed with mTOR, phosphorylated mTOR, p70S6K, or phosphorylated p70S6K antibodies (1:1000–3000). Bands were detected with an enhanced chemiluminescence detection kit (ECL Plus, Amersham Biosciences) and semiquantitative data were obtained using a computer densitometer (Quantity One, Bio-Rad). Phosphorylated and total mTOR and p70S6K measurements were normalized to α-tubulin immunoreactivity.
Statistical analyses.
Minitab statistical software (Minitab) was used for statistical analysis. Data from acute arginine dose infusions were first analyzed by 1-way ANOVA and then linear regression. Differences in plasma arginine concentrations between TPN and PEN groups across arginine dose levels (50–800 μmol⋅kg−1⋅h−1) were tested by 2-way ANOVA, with feeding mode and arginine dose as main effects, followed by a Tukey’s means comparison test. Data are presented as the mean ± SEM and P < 0.05 was considered significant.
Results
In vivo blood flow studies: SMA blood flow response to acute infusion of arginine or arginine precursors.
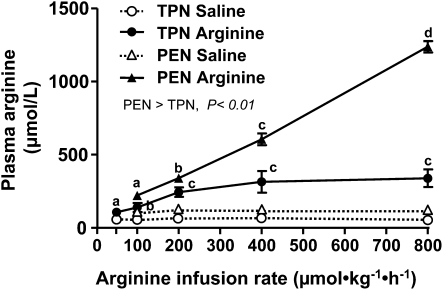
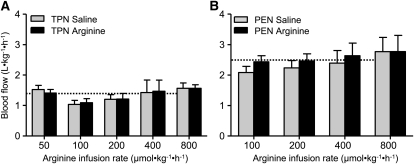
It is important to note that during both TPN and PEN protocols, that pigs were receiving parenteral arginine at a rate of 150 and 90 μmol⋅kg−1⋅h−1, respectively. Plasma arginine concentrations were measured during the saline basal infusion and not significantly different among the dose levels and thus have been averaged for simplicity. Plasma arginine and ornithine concentrations increased upon arginine infusion in both TPN (P < 0.05) and PEN (P < 0.05) (Fig. 1; Table 1). In addition, the plasma arginine and ornithine concentrations in PEN pigs were significantly higher compared with TPN pigs during both the basal saline and with arginine infusion (Fig. 1; Table 1). The plasma arginine and ornithine concentrations during the basal saline infusion were 80–100% higher in PEN compared TPN pigs; this may have been due to either the arginine absorbed from the formula or endogenous synthesis by enterocytes. Plasma concentrations of citrulline were higher, whereas those of glutamine, glutamate, and threonine were lower in PEN pigs than in TPN pigs across all arginine doses. Plasma glutamine, glutamate, and lysine were unaffected, whereas concentrations of proline were decreased during arginine infusion compared with the basal saline infusion, but in PEN pigs only. Plasma glucose and insulin concentrations were measured in PEN pigs but were not affected by the enteral arginine infusion rate (Supplemental Table 1). In general, the SMA flow rates within a given arginine dose were not significantly different during the 3-h treatment period (Supplemental Table 2). SMA blood flow was continuously measured in TPN and PEN pigs during i.g. arginine infusion. Interestingly, baseline SMA blood flow during saline infusion was higher in PEN pigs (2.48 ± 0.32 L⋅kg−1⋅h−1) than in TPN pigs (1.35 ± 0.17 L⋅kg−1⋅h−1) (P < 0.05). However, despite increased plasma arginine concentrations, arginine infusion did not change SMA blood flow in either TPN or PEN pigs (Fig. 2A,B).
FIGURE 1.
Plasma arginine concentrations in TPN and PEN pigs i.g. infused with saline or arginine. Values are means ± SEM, n = 4–6 (TPN) or 9 (PEN). Plasma arginine concentrations increased (P < 0.05) upon arginine infusion in both TPN and PEN compared with the respective saline baseline values. Plasma arginine concentrations increased dose dependently (linear, P < 0.01) with arginine infusion concentrations in both the TPN and PEN groups. Within a group, labeled means without a common letter differ, P < 0.05. Plasma arginine concentrations in PEN pigs were higher than in TPN pigs (P < 0.01).
TABLE 1.
Selected plasma amino acid concentrations in TPN- and PEN-fed pigs acutely i.g. infused with saline or arginine1
| Amino acid group | Feeding group | Arginine infusion rate, μmol⋅kg−1⋅h−1 |
|||||
| Saline2 | 50 | 100 | 200 | 400 | 800 | ||
| μmol/L | |||||||
| Arginine | TPN3 | 66 ± 7a | 124 ± 25b | 186 ± 35b | 283 ± 37c | 336 ± 105c | 404 ± 87c |
| PEN3 | 119 ± 6a | nd4 | 252 ± 21b | 385 ± 30c | 685 ± 43d | 1394 ± 38e | |
| Ornithine5 | TPN3 | 25 ± 3a | 34 ± 7a | 47 ± 7b | 71 ± 10bc | 82 ± 20bc | 113 ± 25c |
| PEN3 | 51 ± 5a | nd | 75 ± 7b | 108 ± 7bc | 178 ± 42c | 252 ± 12d | |
| Citrulline5 | TPN | 113 ± 17 | 118 ± 17 | 105 ± 8 | 125 ± 12 | 113 ± 17 | 111 ± 14 |
| PEN | 142 ± 4 | nd | 152 ± 9 | 134 ± 9 | 153 ± 8 | 165 ± 12 | |
| Proline5 | TPN | 640 ± 100 | 574 ± 65 | 556 ± 37 | 610 ± 52 | 596 ± 61 | 677 ± 90 |
| PEN | 493 ± 14b | nd | 352 ± 9a | 397 ± 33a | 387 ± 26a | 389 ± 25a | |
| Glutamine5 | TPN | 811 ± 125 | 619 ± 61 | 665 ± 42 | 645 ± 81 | 666 ± 92 | 628 ± 74 |
| PEN | 447 ± 45 | nd | 392 ± 49 | 396 ± 45 | 410 ± 53 | 341 ± 34 | |
| Glutamate5 | TPN | 353 ± 82 | 302 ± 36 | 256 ± 39 | 334 ± 45 | 295 ± 35 | 310 ± 31 |
| PEN | 234 ± 7 | nd | 188 ± 12 | 198 ± 19 | 190 ± 17 | 194 ± 16 | |
| Threonine | TPN | 1161 ± 333 | 1151 ± 125 | 1188 ± 152 | 1196 ± 180 | 1121 ± 169 | 936 ± 198 |
| PEN | 1089 ± 32 | nd | 989 ± 67 | 905 ± 87 | 986 ± 85 | 937 ± 82 | |
| Lysine | TPN | 295 ± 85 | 318 ± 46 | 307 ± 33 | 305 ± 43 | 254 ± 41 | 261 ± 48 |
| PEN6 | 365 ± 10 | nd | 273 ± 27 | 237 ± 11 | 251 ± 22 | 250 ± 10 | |
Means ± SEM, = 4–6 (TPN) or 9 (PEN) unless otherwise noted. Means in a row with superscripts without a common letter differ, P < 0.05.
Values for saline represent overall mean from each arginine dose level, = 24 (TPN) or 36 (PEN) unless otherwise noted.
Significant effect of treatment (TPN vs. PEN), < 0.05
nd, Not determined.
Significant arginine dose effect, linear < 0.01.
Significant arginine dose effect, linear < 0.05.
FIGURE 2.
SMA blood flow during enteral arginine infusion for 3 h in TPN (A) and PEN (B) pigs i.g. infused with saline or arginine. Values are means ± SEM, n = 3–5 (TPN) or 5–7 (PEN). Baseline SMA blood flow in PEN pigs was 90% higher than in TPN pigs (P < 0.01). The mean baseline SMA flow in TPN and PEN groups is shown as dashed lines for comparison.
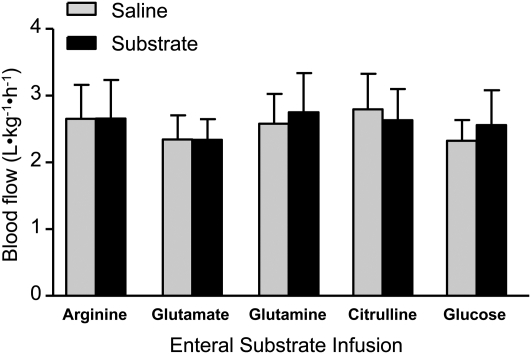
We also measured plasma arginine concentrations and SMA blood flow in PEN pigs during acute i.g. infusion of citrulline, glutamine, glutamate, or glucose. Plasma concentrations of glutamine, glutamate, and citrulline were increased when they were acutely infused i.g. (Table 2). Interestingly, the arterial plasma arginine concentration increased during citrulline and glutamine but not glutamate infusion. Equimolar infusion of citrulline and arginine resulted in plasma arginine concentrations of 561 and 1394 μmol/L (Tables 1 and 2). However, none of the amino acids or glucose affected SMA blood flow (Fig. 3) despite the increased plasma arginine concentrations.
TABLE 2.
Selected plasma amino acid and glucose concentrations in PEN pigs acutely infused i.g. with either saline or various arginine precursors and substrates12
| Substrate | Saline3 | Citrulline | Glutamine | Glutamate | Glucose |
| μmol/L | |||||
| Arginine | 115 ± 19a | 561 ± 32c | 206 ± 32b | 150 ± 10ab | 107 ± 9a |
| Citrulline | 125 ± 5a | 2481 ± 100c | 233 ± 22b | 134 ± 12a | 153 ± 12a |
| Glutamine | 291 ± 17a | 249 ± 10a | 842 ± 76c | 400 ± 15b | 311 ± 21a |
| Glutamate | 172 ± 16a | 178 ± 14a | 285 ± 18b | 282 ± 29b | 189 ± 12a |
| Ornithine | 48 ± 2a | 150 ± 11b | 68 ± 6a | 85 ± 7b | 46 ± 3a |
| Proline | 411 ± 12a | 432 ± 20ab | 483 ± 18b | 490 ± 27b | 447 ± 16a |
| Glucose, mmol/L | 6.27 ± 0.14 | 6.64 ± 0.39 | 6.01 ± 0.28 | 6.85 ± 0.18 | 6.54 ± 0.26 |
Means ± SEM, = 8. Means in a row with superscripts without a common letter differ, P < 0.05.
I.g. infusion rate of citrulline, glutamine, glutamate, and glucose was 800 mol⋅kg−1⋅h−1.
Values for saline represent overall mean from each arginine dose level ( = 32).
FIGURE 3.
SMA flow in PEN pigs acutely i.g. infused with citrulline, glutamine, glutamate, or glucose during the 3-h infusion period. Values are means ± SEM, n = 6–7.
In vivo response to chronic arginine supplementation.
In the ARG+L-NAME group, 1 piglet died postsurgery and 3 pigs were euthanized before the end of the study because of clinical signs of sepsis and breathing difficulties. In the remaining pigs, there were no complications, although occasional diarrhea was observed but not quantified. Arginine supplementation markedly increased plasma arginine and ornithine concentrations, whereas glutamate, glutamine, and lysine levels were decreased (Table 3). Concentrations of other amino acids were similar in all groups (data not shown).
TABLE 3.
Plasma amino acid concentrations in pigs fed PEN supplemented with CO, ARG, or ARG+L-NAME1
| Amino acid2 | CO | ARG | ARG+L-NAME |
| μmol/L | |||
| Alanine | 993 ± 84b | 434 ± 53a | 479 ± 64a |
| Arginine | 52 ± 7a | 647 ± 85c | 375 ± 66b |
| Citrulline | 60 ± 12 | 70 ± 22 | 75 ± 17 |
| Glutamine | 659 ± 70b | 371 ± 60a | 440 ± 93a |
| Glutamate | 238 ± 36b | 134 ± 9a | 121 ± 11a |
| Ornithine | 18 ± 2a | 290 ± 58b | 223 ± 50b |
| Proline | 524 ± 27 | 488 ± 89 | 497 ± 44 |
| Lysine | 254 ± 25b | 173 ± 15a | 228 ± 44ab |
Means ± SEM, = 9–11. Means in a row with superscripts without a common letter differ, P < 0.05.
Weight gain during the treatment period was less in the ARG and ARG+L-NAME groups compared with CO group (Table 4). However, ARG increased proximal intestinal weight, protein, and DNA mass compared with the CO group but not in the distal intestine. Interestingly, jejunal weight, protein mass, but not DNA, were higher in the ARG+L-NAME group than in the CO group. In contrast to ARG, ARG+L-NAME increased distal intestinal weight and protein mass compared with CO pigs. There was no treatment effect on liver growth, protein, and DNA mass (Table 4). Furthermore, the weights of kidney, stomach, or spleen were also not different among groups (data not shown). Despite the observed changes in jejunal weight, protein, and DNA mass, there were no differences in mucosal villous height, crypt depth, muscularis thickness, or crypt cell proliferation between any of the 3 groups (Table 4).
TABLE 4.
Weight gain, organ weights, and tissue analyses in pigs fed PEN supplemented with CO, ARG, or ARG+L-NAME1
| CO | ARG | ARG+L-NAME | |
| Body weight final, g | 3057 ± 120 | 2846 ± 114 | 2671 ± 173 |
| Weight gain, g⋅kg−1⋅d−1 | 80.6 ± 4.5b | 59.7 ± 4.0a | 49.5 ± 4.3a |
| Proximal small intestine | |||
| Weight, g/kg | 11.7 ± 0.5a | 14.7 ± 0.6b | 14.2 ± 0.9b |
| Protein mass, mg/kg | 1140 ± 54a | 1497 ± 63b | 1365 ± 60b |
| DNA mass, mg/kg | 80.5 ± 4.6a | 103.9 ± 5.4b | 89.6 ± 6.2a |
| Villus height, μm | 569 ± 46 | 590 ± 35 | 615 ± 65 |
| Villus area, mm | 42.8 ± 2.7 | 44.5 ± 2.1 | 45.7 ± 4.4 |
| Crypt depth, μm | 140.3 ± 3.9 | 143.5 ± 6.9 | 143.2 ± 9.6 |
| Muscularis thickness, μm | 149.4 ± 4.0 | 147.6 ± 6.7 | 153.6 ± 10.6 |
| BrdU-positive crypt cells, % | 18.0 ± 1.5 | 15.3 ± 1.4 | 16.8 ± 1.9 |
| Distal small intestine | |||
| Weight, g/kg | 14.4 ± 0.9a | 16.3 ± 1.0ab | 17.0 ± 1.3b |
| Protein mass, mg/kg | 1346 ± 106a | 1577 ± 115ab | 1609 ± 155b |
| DNA mass, mg/kg | 94.7 ± 5.2 | 113.5 ± 8.2 | 107.5 ± 12.7 |
| Liver | |||
| Weight, g/kg | 35.7 ± 0.6 | 33.8 ± 1.1 | 36.5 ± 1.4 |
| Protein mass, mg/kg | 5213 ± 160 | 5159 ± 197 | 5714 ± 203 |
| DNA mass, mg/kg | 172.5 ± 5.1 | 182.2 ± 7.7 | 181.3 ± 10.7 |
Means ± SEM, = 9–11. Means in a row with superscripts without a common letter differ, P < 0.05.
Protein FSR measured in the jejunum, ileum, liver, muscle, and pancreas and the protein ASR (data not shown) were not significantly different among the treatment groups (Table 5). We performed immunoblotting to determine whether arginine supplementation activated p70S6K and phospho-mTOR. However, levels of p70S6K and mTOR phosphorylation in gut and muscle tissue were similar among the groups (data not shown).
TABLE 5.
FSR in PEN pigs supplemented with CO, ARG, or ARG+L-NAME1
| CO | ARG | ARG+L-NAME | |
| %/d | |||
| Jejunum | 48.8 ± 3.1 | 44.2 ± 3.1 | 41.6 ± 6.1 |
| Ileum | 40.3 ± 3.0 | 42.9 ± 2.8 | 45.5 ± 7.9 |
| Liver | 69.1 ± 3.9 | 73.3 ± 3.6 | 71.2 ± 6.7 |
| Muscle | 12.5 ± 0.8 | 14.3 ± 1.1 | 13.9 ± 1.5 |
| Pancreas | 65.7 ± 3.5 | 80.8 ± 7.7 | 60.4 ± 6.1 |
Means ± SEM, = 9–11.
Discussion
In neonatal intensive care, full TPN or TPN combined with partial enteral feeding is standard treatment in preterm infants because of enteral feeding intolerance. However, TPN has shown to significantly decrease SMA blood flow and induce mucosal atrophy in neonatal pigs (17). During TPN, the absence of enteral nutrition reduces luminal arginine availability, which could limit NO synthesis and predispose the neonatal intestine to vasoconstriction and ischemia contributing to the development of mucosal atrophy and NEC. We hypothesized that provision of enteral arginine under conditions of TPN or PEN would increase small intestinal blood flow and mucosal growth and that these effects would be mediated by increased NO production. Our results demonstrate that over a wide range of enteral infusion rates, arginine does not acutely affect blood flow in neonatal pigs fed TPN or PEN. Moreover, we show that chronic dietary arginine supplementation during PEN only modestly increased intestinal growth and that this response was NO independent.
The main aim of our study was to address whether arginine stimulates blood flow in a clinically relevant situation of parenteral or PEN in neonatal pigs. We previously showed that a minimum of 40% enteral nutrition is necessary to stimulate intestinal growth (29). Interestingly, the current study showed that providing 40% PEN almost doubled the SMA blood flow compared with that in TPN pigs. However, despite markedly increased circulating arginine concentrations, we found that neither enteral arginine nor the arginine precursor citrulline, or glutamine, glutamate, or glucose acutely increased SMA blood flow in partially enterally fed pigs. The effect of enteral arginine on SMA blood flow has not yet been investigated in a neonatal model. Three studies performed in adult mice, rat, and pigs reported increased intestinal blood flow upon arginine administration (18, 23, 22). Studies in similar models of ischemia-reperfusion injury have reported a positive effect of arginine on NO synthesis thereby suggesting increased blood flow (19, 20). Thus, it seems that in a state of injury and influx of inflammatory cells, increased amounts of plasma arginine might increase arginine availability for NO production and as a consequence increase blood flow. However, these studies were performed in adult animals where intestinal circulation, NO concentration, NO-response, and eNOS expression is more developed than in neonates (8, 31).
Several factors may explain the lack of response of enteral arginine on SMA blood flow. First, blood flow stimulation may be limited by the availability of cofactors necessary to produce NO from arginine (9). Our partially enterally fed pigs were only fed 40% of total nutrient requirements enterally, so it might be possible that some factors were limiting for NO synthesis, namely tetrahydrobiopterin (BH4). BH4 is an essential cofactor for all 3 NOS isoforms and BH4 bioavailability within the endothelium is a critical factor in regulating the balance between NO and superoxide production by eNOS (32–34). Arginine can increase BH4 synthesis and thus given the marked increase in luminal and plasma arginine with enteral supplementation, BH4 availability would likely be increased. In support of this is the fact that SMA flow was higher in PEN compared with TPN, suggesting that the mucosal mechanisms that sense luminal nutrients were functional. Second, NO synthesis may be linked to the rate of arginine transport by cationic amino acid transporter-1 in endothelial cells and thus not dependent on plasma arginine concentration (35). Third, endogenous inhibitors of NOS isoforms such as asymmetric dimethylarginine may play a limiting role in NO production (36). Fourth, and most likely, endothelin-1 (vasoconstrictor) and NO (vasodilator) dysregulation might account for the lack of response. Insufficient supply of luminal nutrients undermining epithelial integrity might induce influx of proinflammatory cytokines, resulting in endothelin-1 mediated vasoconstriction (14, 37, 38). Interestingly, submucosal arterioles harvested from human intestine resected for NEC did not demonstrate evidence of eNOS function and showed vasoconstriction, presumably by lack of eNOS-derived NO (39).
Despite the lack of effect on SMA blood flow, we observed remarkable changes in circulating amino acid concentrations in response to enteral amino acid infusions in TPN- and PEN-fed neonatal pigs. Studies in neonatal pigs have shown that the small intestine is a major determinant of arginine synthesis and catabolism and these are substantially decreased in pigs fed TPN compared with enteral nutrition (40, 41). We show here that the circulating arterial arginine concentrations were dose dependently increased with increasing enteral arginine level. However, there were significantly greater increases in plasma arginine in pigs fed PEN than in TPN-fed pigs. We suspect that this occurred because of reduced arginine catabolism during the first-pass through the intestinal mucosa associated with PEN; we would not expect intestinal absorption of free arginine to be a limiting process. It is also possible that PEN increased the mucosal epithelial mass and/or capacity of enterocytes to synthesize arginine, which led to increased intestinal arginine release. It also appeared that the major product of intestinal arginine catabolism was ornithine based on the marked increase in circulating ornithine concentration, whereas citrulline and proline were unchanged, with increasing enteral arginine intake. We also found that citrulline and glutamine, but not glutamate, increased the plasma arginine concentration. Interestingly, the plasma arginine concentration was 1394 and 561 μmol/L during equal molar infusions of arginine and citrulline, respectively, suggesting that dietary arginine was a better precursor than citrulline for maintaining blood arginine. This finding is contrary to recent reports indicating that dietary citrulline may be more effective than arginine in increasing circulating arginine levels (42, 43). Yet, these results confirm the fact that citrulline is a more important precursor for de novo arginine synthesis compared with glutamine or glutamate and highlights the differences in first-pass splanchnic metabolism between arginine and citrulline.
In addition to acute effects of arginine, we next investigated the effects of longer term arginine administration on the intestinal growth. The ability of arginine to prevent NEC and gut injury may be due to trophic physiological effects on the intestinal epithelium. Arginine supplementation in in vitro and in vivo models stimulated intestinal protein synthesis and increased epithelial cell survival through the mTOR-mediated pathway (4–6). However, it is unknown whether this trophic effect of arginine on the epithelium is NO dependent. Our current results demonstrate that chronic arginine supplementation with partial enteral feeding only modestly stimulated gut growth.
Enteral arginine for 4 d increased the mass of tissue, protein, and DNA in the proximal small intestine but not the distal small intestine. Despite the increase in intestinal mass, we found no change in intestinal protein synthesis, villus height, or cell proliferation. In addition, we found no change in intestinal phosphorylation of mTOR or p70S6K. The fact that we did not find an effect on protein synthesis or cell proliferation suggests that the observed trophic effect was due to decreased intestinal protein breakdown. These results are in contrast to others that have found a positive effect of arginine on protein synthesis and mTOR signaling (5). Another important finding was that the addition of L-NAME in the formula did not reduce the trophic effect of enteral arginine on intestinal growth, suggesting that the effects were not NO dependent.
Finally, we found that body weight gain was reduced in arginine-supplemented pigs. This finding is in contrast to previously published studies in pigs where arginine resulted in increased weight gain in weanling pigs (44–46). These previous studies provided fully enterally fed weanling pigs with arginine, resulting in modest increases in plasma arginine concentrations within the physiological range. In the present study, we used supraphysiological concentrations (3 times the arginine requirement of 1.08 g⋅kg−1⋅d−1) (47), because endogenous arginine synthesis in our partially enterally fed pigs was expected to be greatly decreased (40, 41). The plasma arginine concentration in supplemented pigs was >10-fold higher than controls. This markedly high intake of arginine may have negatively affected protein metabolism. However, plasma concentrations of lysine were in the normal range, suggesting that lysine-arginine competition for cell entry did not occur and hence does not explain the decreased weight gain. Moreover, the protein synthesis rate in other organs, including the liver, pancreas, and skeletal muscle, were numerically higher but not statistically different from control pigs. This would imply pharmacological effects of arginine to suppress weight gain occurred by proteolytic mechanisms. It is not clear why supplementing L-NAME with arginine resulted in lower plasma arginine compared with arginine alone; it is possible that L-NAME blocked intestinal absorption or increased the catabolism of L-arginine.
In summary, this study aimed to further investigate the effects of enteral arginine on intestinal blood flow and mucosal growth in neonates fed TPN or PEN. Our results show that partial enteral feeding increased SMA blood flow compared with TPN. However, enteral arginine infusion did not affect SMA blood flow across a wide range of doses, including physiological and pharmacological. We also found that enteral infusion of arginine precursors, citrulline and glutamine, did not affect SMA flow despite the fact that they resulted in increased circulating arginine levels. Consistent with the lack of effect on SMA blood flow, we found that a pharmacological dose of enteral arginine only marginally increased intestinal mucosal growth and without significant changes in protein synthesis, cell proliferation, or activation on mTOR signaling. Our results were unexpected and contrary to considerable literature reports showing that increased circulating arginine via exogenous infusion results in increased blood flow. We postulate that the immature vascular mechanisms involved in intestinal NO synthesis and blood flow may explain our results; however, this question warrants further study in neonatal animals.
Supplementary Material
Acknowledgements
We thank Liwei Cui and Xiaoyan Chang for their expert technical assistance. B.S. and D.G.B. designed research; P.J.P., B.S., and D.G.B. conducted research; P.J.P., B.S., and D.G.B. analyzed data; J.B.v.G. provided essential materials and critically reviewed the paper; P.J.P. and D.G.B wrote the paper; and D.G.B. had primary responsibility for the final content. All authors read and approved the final version of this paper.
Footnotes
Supported by the USDA, Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001 (D.G.B.), the Ajinomoto Amino Acid Research Program (D.G.B.), and the Texas Medical Center Digestive Diseases Center (NIH grant no. P30 DK-56338) (D.G.B.).
Supplemental Tables 1 and 2 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: ARG, arginine diet; ASR, absolute synthesis rate; CO, control group; FSR, fractional protein synthesis rate; mTOR, mammalian target of rapamycin; NEC, necrotizing enterocolitis; NOS, nitric oxide synthase; L-NAME, NG-nitro-l-arginine methyl ester; p70S6K, p70S6 kinase; PEN, partial enteral nutrition; SMA, superior mesenteric artery; BH4, tetrahydrobiopterin; TPN, total parenteral nutrition.
Literature Cited
- 1.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17 [DOI] [PubMed] [Google Scholar]
- 2.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack SA, Johnson LR. Role of polyamines in gastrointestinal mucosal growth. Am J Physiol. 1991;260:G795–806 [DOI] [PubMed] [Google Scholar]
- 4.Rhoads JM, Niu X, Odle J, Graves LM. Role of mTOR signaling in intestinal cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;291:G510–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corl BA, Odle J, Niu X, Moeser AJ, Gatlin LA, Phillips OT, Blikslager AT, Rhoads JM. Arginine activates intestinal p70(S6k) and protein synthesis in piglet rotavirus enteritis. J Nutr. 2008;138:24–9 [DOI] [PubMed] [Google Scholar]
- 6.Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. Arginine and Leucine regulate p70 S6 kinase and 4E–BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537–43 [PubMed] [Google Scholar]
- 7.Nankervis CA, Reber KM, Nowicki PT. Age-dependent changes in the postnatal intestinal microcirculation. Microcirculation. 2001;8:377–87 [DOI] [PubMed] [Google Scholar]
- 8.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32:83–91 [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Meininger CJ. Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr. 2002;22:61–86 [DOI] [PubMed] [Google Scholar]
- 10.Prins HA, Houdijk AP, Wiezer MJ, Teerlink T, van Lambalgen AA, Thijs LG, van Leeuwen PA. The effect of mild endotoxemia during low arginine plasma levels on organ blood flow in rats. Crit Care Med. 2000;28:1991–7 [DOI] [PubMed] [Google Scholar]
- 11.Wilmore D. Enteral and parenteral arginine supplementation to improve medical outcomes in hospitalized patients. J Nutr. 2004;134:S2863–7 [DOI] [PubMed] [Google Scholar]
- 12.Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, Rhoads JM. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J Pediatr. 2000;137:785–93 [DOI] [PubMed] [Google Scholar]
- 13.Zamora SA, Amin HJ, McMillan DD, Kubes P, Fick GH, Butzner JD, Parsons HG, Scott RB. Plasma L-arginine concentrations in premature infants with necrotizing enterocolitis. J Pediatr. 1997;131:226–32 [DOI] [PubMed] [Google Scholar]
- 14.Richir MC, Siroen MP, van Elburg RM, Fetter WP, Quik F, Nijveldt RJ, Heij HA, Smit BJ, Teerlink T, et al. Low plasma concentrations of arginine and asymmetric dimethylarginine in premature infants with necrotizing enterocolitis. Br J Nutr. 2007;97:906–11 [DOI] [PubMed] [Google Scholar]
- 15.Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, Thompson WR, Scherer LR, Klein MD, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg. 2005;241:984–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G, Flynn NE, Flynn SP, Jolly CA, Davis PK. Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J Nutr. 1999;129:1347–54 [DOI] [PubMed] [Google Scholar]
- 17.Niinikoski H, Stoll B, Guan X, Kansagra K, Lambert BD, Stephens J, Hartmann B, Holst JJ, Burrin DG. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr. 2004;134:1467–74 [DOI] [PubMed] [Google Scholar]
- 18.Vukosavljevic N, Jaron D, Barbee KA, Buerk DG. Quantifying the L-arginine paradox in vivo. Microvasc Res. 2006;71:48–54 [DOI] [PubMed] [Google Scholar]
- 19.Fu TL, Zhang WT, Zhang L, Wang F, Gao Y, Xu M. L-arginine administration ameliorates serum and pulmonary cytokine response after gut ischemia-reperfusion in immature rats. World J Gastroenterol. 2005;11:1070–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward DT, Lawson SA, Gallagher CM, Conner WC, Shea-Donohue T. Sustained nitric oxide production via l-arginine administration ameliorates effects of intestinal ischemia-reperfusion. J Surg Res. 2000;89:13–9 [DOI] [PubMed] [Google Scholar]
- 21.Sukhotnik I, Helou H, Mogilner J, Lurie M, Bernsteyn A, Coran AG, Shiloni E. Oral arginine improves intestinal recovery following ischemia-reperfusion injury in rat. Pediatr Surg Int. 2005;21:191–6 [DOI] [PubMed] [Google Scholar]
- 22.Fukatsu K, Ueno C, Maeshima Y, Hara E, Nagayoshi H, Omata J, Mochizuki H, Hiraide H. Effects of L-arginine infusion during ischemia on gut blood perfusion, oxygen tension, and circulating myeloid cell activation in a murine gut ischemia/reperfusion model. JPEN J Parenter Enteral Nutr. 2004;28:224–30 [DOI] [PubMed] [Google Scholar]
- 23.Spanos CP, Papaconstantinou P, Spanos P, Karamouzis M, Lekkas G, Papaconstantinou C. The effect of L-arginine and aprotinin on intestinal ischemia-reperfusion injury. J Gastrointest Surg. 2007;11:247–55 [DOI] [PubMed] [Google Scholar]
- 24.Di Lorenzo M, Bass J, Krantis A. Use of L-arginine in the treatment of experimental necrotizing enterocolitis. J Pediatr Surg. 1995;30:235–40 [DOI] [PubMed] [Google Scholar]
- 25.Akisu M, Ozmen D, Baka M, Habif S, Yalaz M, Arslanoglu S, Kultursay N, Bayindir O. Protective effect of dietary supplementation with L-arginine and L-carnitine on hypoxia/reoxygenation-induced necrotizing enterocolitis in young mice. Biol Neonate. 2002;81:260–5 [DOI] [PubMed] [Google Scholar]
- 26.Amin HJ, Zamora SA, McMillan DD, Fick GH, Butzner JD, Parsons HG, Scott RB. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140:425–31 [DOI] [PubMed] [Google Scholar]
- 27.Stoll B, Price PT, Reeds PJ, Chang X, Henry JF, van Goudoever JB, Holst JJ, Burrin DG. Feeding an elemental diet vs a milk-based formula does not decrease intestinal mucosal growth in infant pigs. JPEN J Parenter Enteral Nutr. 2006;30:32–9 [DOI] [PubMed] [Google Scholar]
- 28.Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. Am J Physiol. 1997;273:E305–14 [DOI] [PubMed] [Google Scholar]
- 29.Burrin DG, Stoll B, Jiang RH, Chang XY, Hartmann B, Holst JJ, Greeley GH, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71:1603–10 [DOI] [PubMed] [Google Scholar]
- 30.Metges CC, Petzke KJ, Hennig U. Gas chromatography/combustion/isotope ratio mass spectrometric comparison of N-acetyl- and N-pivaloyl amino acid esters to measure 15N isotopic abundances in physiological samples: a pilot study on amino acid synthesis in the upper gastro-intestinal tract of minipigs. J Mass Spectrom. 1996;31:367–76 [DOI] [PubMed] [Google Scholar]
- 31.Reber KM, Su BY, Clark KR, Pohlman DL, Miller CE, Nowicki PT. Developmental expression of eNOS in postnatal swine mesenteric artery. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1328–35 [DOI] [PubMed] [Google Scholar]
- 32.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. Biofactors. 2009;35:21–7 [DOI] [PubMed] [Google Scholar]
- 34.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–20 [DOI] [PubMed] [Google Scholar]
- 35.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252 [DOI] [PubMed] [Google Scholar]
- 36.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang BY, Tsao PS, Kimoto M, Vallance P, et al. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–7 [DOI] [PubMed] [Google Scholar]
- 37.Woods M, Mitchell JA, Wood EG, Barker S, Walcot NR, Rees GM, Warner TD. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: evidence for intracellular endothelin-converting enzyme. Mol Pharmacol. 1999;55:902–9 [PubMed] [Google Scholar]
- 38.Yoshizumi M, Kurihara H, Morita T, Yamashita T, Oh-hashi Y, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, et al. Interleukin 1 increases the production of endothelin-1 by cultured endothelial cells. Biochem Biophys Res Commun. 1990;166:324–9 [DOI] [PubMed] [Google Scholar]
- 39.Nowicki PT, Dunaway DJ, Nankervis CA, Giannnone PJ, Reber KM, Hammond SB, Besner GE, Caniano DA. Endothelin-1 in human intestine resected for necrotizing enterocolitis. J Pediatr. 2005;146:805–10 [DOI] [PubMed] [Google Scholar]
- 40.Bertolo RF, Brunton JA, Pencharz PB, Ball RO. Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E915–22 [DOI] [PubMed] [Google Scholar]
- 41.Urschel KL, Evans AR, Wilkinson CW, Pencharz PB, Ball RO. Parenterally fed neonatal piglets have a low rate of endogenous arginine synthesis from circulating proline. J Nutr. 2007;137:601–6 [DOI] [PubMed] [Google Scholar]
- 42.Urschel KL, Shoveller AK, Uwiera RR, Pencharz PB, Ball RO. Citrulline is an effective arginine precursor in enterally fed neonatal piglets. J Nutr. 2006;136:1806–13 [DOI] [PubMed] [Google Scholar]
- 43.Lassala A, Bazer FW, Cudd TA, Li P, Li X, Satterfield MC, Spencer TE, Wu G. Intravenous administration of L-citrulline to pregnant ewes is more effective than L-arginine for increasing arginine availability in the fetus. J Nutr. 2009;139:660–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SW, McPherson RL, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134:625–30 [DOI] [PubMed] [Google Scholar]
- 45.Yao K, Yin YL, Chu W, Liu Z, Deng D, Li T, Huang R, Zhang J, Tan B, et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. 2008;138:867–72 [DOI] [PubMed] [Google Scholar]
- 46.Tan B, Yin Y, Liu Z, Li X, Xu H, Kong X, Huang R, Tang W, Shinzato I, et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2009;37:169–75 [DOI] [PubMed] [Google Scholar]
- 47.Wu G, Meininger CJ, Knabe DA, Bazer FW, Rhoads JM. Arginine nutrition in development, health and disease. Curr Opin Clin Nutr Metab Care. 2000;3:59–66 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.